Abstract
Previously we have shown that insertion of a LacZ reporter gene into the Col10a1 gene in the context of a bacterial artificial chromosome (BAC) drives strong and specific expression of LacZ in hypertrophic cartilage of transgenic mice [Gebhard S., Hattori T., Bauer E., Bosl M.R., Schlund B., Poschl E., Adam N., de Crombrugghe B., von der Mark K., 2007 Histochem. Cell Biol. 19 127:183–194]. BAC constructs in transgenic reporter mouse lines control efficient and specific LacZ expression in hypertrophic chondrocytes under the complete Col10a1 promoter. Here we report on the generation of Col10a1-specific Cre deleter mice using a BAC recombineering technique based on homologous recombination in E. coli. Sixteen BAC-Col10-Cre transgenic lines were generated containing between 1 and 5 copies of the BAC-Col10-Cre gene. All lines tested so far expressed Cre specifically in hypertrophic chondrocytes of E16.5 and P1 growth plates of long bones, ribs, vertebrae and sternum as examined by crossing with ROSA26 reporter mice. Cre activity was detected as early as E13.5 when hypertrophic cartilage develops in the diaphysis of femur and humerus. The data confirm that expression of Cre under the control of the complete BAC-Col10a1 promoter occurs with high efficiency and specificity in hypertrophic chondrocytes. The BAC-Col10-Cre lines should thus provide a valuable tool to get further insight into the role of genes involved in endochondral ossification by allowing their specific deletion in the hypertrophic zone of the growth plate.
Keywords: Collagen X, Hypertrophic cartilage, Bacterial Artificial Chromosome, Transgenic mouse, Cre-recombinase
1. Introduction
Replacement of cartilage by bone during endochondral ossification is a highly complex process which determines not only longitudinal growth of the vertebrate skeleton when occurring in the fetal and juvenile growth plate, but also plays a critical role in bone fracture callus healing and osteophyte formation in arthritic joints. Endochondral ossification in the growth plate runs down in a sequence of differentiation steps of chondrocytes from resting to proliferating, prehypertrophic, and hypertrophic chondrocytes, and becomes manifest in mineralization of hypertrophic cartilage, induction of bone marrow invasion, cartilage resorption, and formation of subchondral bone trabeculae (Poole, 1991). These events are tightly regulated in a most complex, synergistic network of several signalling pathways and transcriptions factors under the control of a multitude of hormones, growth factors, and their receptors (for reviews see Ballock and O’Keefe, 2003; Provot and Schipani, 2005; Goldring et al., 2006).
Hypertrophic chondrocytes which are located at the cartilage–bone transition zone in the growth plate of long bones, ribs, and vertebrae play a key role in the control of these events. They represent a specialized, highly active cell type in a distinct differentiation stage of the chondrogenic lineage and are responsible not only for the deposition of a transient, calcified cartilage matrix in the hypertrophic zone, but subsequently also for the destruction of this matrix by matrix metalloproteinases, for induction of bone marrow invasion and deposition of subchondral bone trabeculae. Thus, the hypertrophic zone seems to act as a switch point in gene expression from the chondrogenic to the osteogenic pathway. The pivotal role of hypertrophic chondrocytes is reflected by a substantial change in the expression pattern of extracellular matrix proteins and regulatory genes during differentiation of proliferating to hypertrophic cells (Lefebvre and Smits, 2005; Goldring et al., 2006): 1) The matrix of hyaline cartilage consisting of aggrecan, types II, VI and XI collagen and other cartilage matrix proteins is substituted by a calcifying matrix characterized by type X collagen, alkaline phosphatase and bone-typical glycoproteins such as osteopontin (Ballock and O’Keefe, 2003; Ortega et al., 2004). 2) Sox9, the major chondrogenic transcription factor is expressed continuously from the resting zone to the proliferating zone, reaches a maximum in the prehypertrophic zone, but disappears completely from the hypertrophic zone and the subchondral bone (Lefebvre and de Crombrugghe, 1998; de Crombrugghe et al., 2001; Akiyama et al., 2004). In contrast, Runx-2 which is also highly expressed in the prehypertrophic zone (Kim et al., 1999; Enomoto-Iwamoto et al., 2001) is upregulated in the subchondral bone forming zone. 3) MMP13 and VEGF which are downstream targets of Runx2 (Jimenez et al., 1999; Zelzer et al., 2001) are turned on in hypertrophic chondrocytes and play a critical role in cartilage resorption and bone marrow formation (Inada et al., 2004; Stickens et al., 2004). 4) In early embryonic stages, Indian hedgehog is strongly expressed in the prehypertrophic zone where it stimulates chondrocyte proliferation and prevents premature hypertrophic differentiation through the induction of PTHrP (Lanske et al., 1996; Vortkamp, 2000; Kronenberg, 2006); Ihh appears in hypertrophic cartilage in later fetal and postnatal stages (van der Eerden et al., 2000; Weisser et al., 2002). 5) β-catenin which is expressed in most cells of the growth plate including hypertrophic chondrocytes has been shown to stimulate the osteogenic pathway (Day et al., 2005; Hill et al., 2005; Tamamura et al., 2005) while inhibiting chondrogenic differentiation by direct interference with Sox9 gene expression (Akiyama et al., 2004). 6) Furthermore, BMP6 and -7 (Yoon and Lyons, 2004), FGFR1 (Ornitz and Marie, 2002; Ornitz, 2005), TSG (Schmidl et al., 2006) and numerous other growth factors and signaling molecules are expressed by hypertrophic chondrocytes (Provot and Schipani, 2005; Goldring et al., 2006). Yet, the specific role of many of these factors in the complex process of endochondral ossification remains to be elucidated.
Specific overexpression or deletion of genes under the control of a Col10a1 promoter should therefore be a powerful tool for elucidating the specific functions of known and unknown genes in the hypertrophic zone. Recently we have shown that a 4.6kb promoter sequence of the mouse Col1a1 gene including a 500bp enhancer element was sufficient to drive specific expression of a LacZ reporter gene in all hypertrophic cartilage zones of transgenic mice (Gebhard et al., 2004). Partial LacZ reporter gene expression in the lower hypertrophic zone of growth cartilage was also achieved under a 4.0kb Col10a1 promoter lacking the enhancer (Zheng et al., 2003). A transgenic mouse expressing the Cre recombinase under a 1kb Col10a1 promoter has recently been generated (Yang et al., 2005) which shows Cre expression in some but not all hypertrophic chondrocytes. A significantly enhanced level of specific LacZ transgene expression in hypertrophic cartilage was achieved when the reporter gene was cloned into a Col10a1-bacterial artificial chromosome (BAC) containing ~ 170kb upstream and 30kb downstream sequences of the mouse Col10a1 gene (Gebhard et al., 2007). Here we report on the generation of transgenic mouse lines expressing the bacterial Cre recombinase under that BAC-Col10a1 promoter. We demonstrate tissue-specific Cre activity of several of these lines by crossing with ROSA 26 reporter mice. The results indicate strong and specific expression of Cre in hypertrophic cartilage of all investigated BAC-Col10-Cre; ROSA26 lines independent from the BAC integration sites and transgene copy number.
2. Results
For construction of a Col10a1-Cre-Neo targeting vector, the lacZ cassette inserted into exon 2 of mouse Col10a in the previously described BAC targeting vector placH-Col10a1-frt-neo-frt (Gebhard et al., 2007) was replaced by Cre-cDNA. The Col10a1-Cre-neo vector was inserted into the BAC Clone RP23-192A7 by homologous recombination in E. coli (Lee et al., 2001) as described recently (Gebhard et al., 2007) (Fig. 1a). Injection of purified, linearized BAC DNA (Fig. 1b) into the pronuclei of oocytes resulted in the generation of 16 transgenic founders tested positive for Cre by PCR (Fig. 1c). Real-time PCR analysis of genomic Col10a1 including BAC-Col10a1 revealed that the founders contained between 1 and 5 copies of BAC-Col10-Cre DNA (Table 1). Seven of the founder lines were further characterized for Cre activity by crossing with ROSA26 reporter mice, or by crossing with the β1-integrinfl/fl;LacZ knock-in line (Potocnik et al., 2000) which expresses LacZ after deletion of floxed exons with Cre recombinase (Aszodi et al., 2003) (results not shown).
Fig. 1.
(a) Diagram showing the position of the Cre recombinase in the BAC-Col10-Cre-frtNeofrt DNA. For the insertion of Cre, the lacZ cassette of the pLacH-Col10a1-LacZ-frtNeofrt vector (Gebhard et al., 2007) was replaced by Cre, and the resulting vector was recombined with BAC-Col10a1 (RP23.192A7) by homologous recombination in E. coli. Correct insertion into exon 2 of Col10a1 was controlled by PCR using primers P1 (Col10a1-Int1) and P5 (“cre reverse”). PCR using primers P1 and P4 served as wt control for endogenous Col10a1. (b) Pulse field gel electrophoresis of BAC Col10a1-LacZ-frtNeofrt DNA after linearization and molecular sieve chromatography on Sepharose 4B. 1) Pulse field markers 10–250 kb; 2)–6) fractions 3–7 of the Sepharose chromatography. Fractions 4 and 5 contain the majority of the BAC DNA which was used for microinjection. (c) Detection of BAC-Col10a1-Cre transgenic offspring by PCR using Primers P1 and P5; 1) markers 2) founder #1466 3) wt 4) Founder #1427; 5) BAC- Col10-Cre DNA.
Table 1.
Copy number of BAC-Col10a1-Cre transgene in 16 founders, determined by real-time PCR for Col10a1
| Founder number | Transgene Col X Cop. number | BAC Cre copy number |
|---|---|---|
| 1396 | 3.97 | 4 |
| 1403 | 3.40 | 3 |
| 1404 | 1.10 | 1 |
| 1408 | 2.28 | 2 |
| 1410 | 1.75 | 2 |
| 1421 | 2.28 | 2 |
| 1426 | 1.02 | 1 |
| 1427 | 4.12 | 4 |
| 1433 | 0.87 | 1 |
| 1442 | 0.78 | 1 |
| 1443 | 1.93 | 2 |
| 1465 | 3.56 | 4 |
| 1466 | 4.84 | 5 |
| 1473 | 1.86 | 3 |
| 1476 | 4.06 | 4 |
| 1481 | 1.61 | 2 |
| Wt 1 | 0.42 | 0 |
| Wt 2 | 0.06 | 0 |
| Wt 3 | −0.30 | 0 |
Two copies of endogenous genomic Col10a1 have been subtracted from each value.
Fig. 2 shows strong β-galactosidase activity after X-gal staining of a newborn BAC-Col10-Cre; ROSA26 mouse (founder line #1427, 4 BAC copies) in all growth plates of the long bones, ribs and vertebrae. Similar staining pattern was seen in offspring of 5 other founder lines (#1396; #1408, #1481) analyzed by breeding with ItgB1 fl/fl;LacZ knock-in mice (K. von der Mark, in prep.). Further transgenic lines were not yet tested. The intensity of the ß-gal activity varied to some extent between founder lines depending on the BAC transgene copy number (see also Gebhard et al., 2007), while the staining pattern was independent of the BAC Copy number.
Fig. 2.
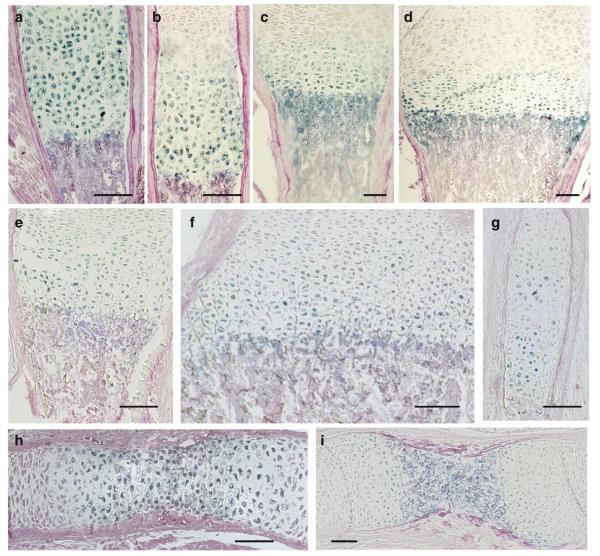
X-gal staining for β-galactosidase activity in BAC Col10a1-Cre;ROSA26 embryos and newborns demonstrates strong and specific expression of Cre in all hypertrophic cartilage zones. a–e: newborn (P1), founder line 1427; f–g: E15.5 , founder line 1465. (a) fore limb; (b) ulna and radius, (c) ribs, (d) vertebrae, (e) hindlimbs; f) whole embryo E15.5, g) ribs and fore limb, h) hand , ulna, radius.
Furthermore, β-galactosidase was expressed in sternebrae and hypertrophic cartilage zones in the skull (not shown). No β-galactosidase activity was seen in other connective tissues or internal organs. There was, however, significant staining in the subchondral bone marrow zone adjacent to the cartilage–bone border (see also Fig. 4). Generally, the lacZ expression pattern was similar or nearly identical to that observed recently in BAC Col10-LacZ transgenic mice as shown here for a P1 scapula (Fig. 3; see also Gebhard et al., 2007), indicating that Cre transgene expression under the BAC Col10a1 promoter is highly specific and sufficient for complete inactivation of floxed genes.
Fig. 4.
LacZ is expressed in all hypertrophic chondrocytes in growth plates of BAC-Col10a1-Cre;ROSA26 transgenic mice. a–d: Founder line 1427, P1; e–g. Founder line 1466, E16.5. h,i) founder line 1465, E 15.5. a) fibula; b,g) rib; c) ulna; d,e,i) humerus; h) radius. Paraffin sections, X-gal staining, counterstaining with Eosin. Scale bars: 100 μm.
Fig. 3.
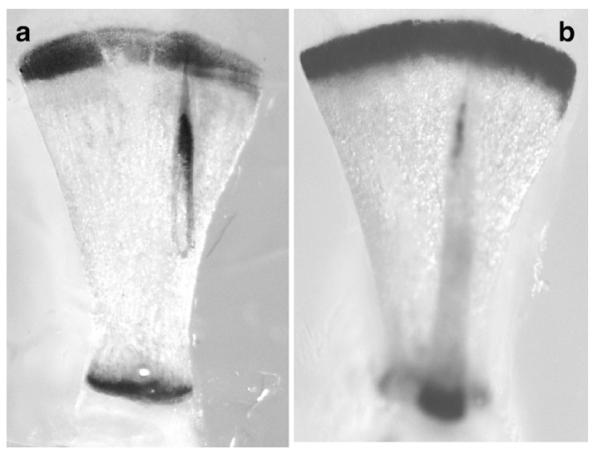
Comparison of β-galactosidase activity in the scapulae of BAC-Col10a1-Cre; ROSA26 transgenic line #1427 (a) , and of the BAC Col10a1 LacZ transgenic line #1520 (b) (Gebhard et al., 2007).
This was confirmed by analyzing β-galactosidase activity in paraffin sections of BAC-Col10-Cre, ROSA 26 tissues of two founder lines (Fig. 4). All hypertrophic chondrocytes in the growth plates of a newborn skeleton derived from founder #1427 showed β-galactosidase activity. Consistent β-gal activity was also observed in all hypertrophic chondrocytes of an E16.5 embryo (founder line #1466, 5 BAC copies) and E15.5 (founder line #1465) embryos (Fig. 4). The earliest indication of LacZ expression was seen at E13.5 in the diaphysis of humerus and femur (data not shown).
Frequently β-gal activity was seen in cells located in subchondral bone marrow and in endochondral bone trabeculae (e.g. Fig. 4c). This activity may be due to residual LacZ activity released from apoptotic chondrocytes, remaining in the subchondral part of the bone marrow. Alternatively or additionally, LacZ activity may remain in hypertrophic chondrocytes escaping resorption and apoptosis and surviving in the cartilaginous core of endochondral bone trabeculae (Fig. 5).
Fig. 5.
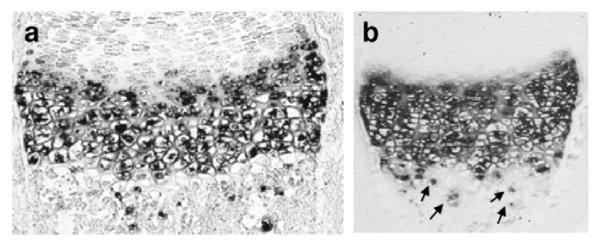
Demonstration of hypertrophic chondrocytes in subchondral bone trabeculae escaping resorption and apoptosis. Arrows indicate Col10a1 positive cells located below the resorption front. In situ hybridization with a Col10a1 probe. (a) tibia, (b) humerus of E18.5 wt embryos.
3. Discussion
For specific and substantial expression of transgenes in hypertrophic chondrocytes the 4.6 kb promoter of murine Col10a1 including a 500 bp enhancer element seems most suitable in light of the exclusive expression of this collagen in hypertrophic chondrocytes, and the high in vitro and in vivo transcriptional activity of reporter genes containing this enhancer (Thomas et al., 1995; Beier et al., 1997; Chambers et al., 2002; Gebhard et al., 2004)). Also the corresponding 2.5 kb promoter in the human COL10A1 gene which includes a highly homologous enhancer (Gebhard et al., 2004) is able to confer high transcriptional activity of reporter genes in hypertrophic chondrocytes (Riemer et al., 2002). Recently, the specific expression in hypertrophic chondrocytes of murine LacZ reporter genes containing only a 4 kb Col10a1 promoter without the enhancer has been reported (Zheng et al., 2003), but the expression of β-galactosidase was rather low and restricted to the lower hypertrophic zone. Similarly, a Col10-Cre transgenic mouse expressing Cre recombinase under a 1 kb proximal promoter of Col10a1 did not allow β-galactosidase expression in all hypertrophic chondrocytes after crossing with ROSA26 reporter mice (Yang et al., 2005).
Even in transgenic mice expressing the 4.6 kb promoter/enhancer of Col10a1 which supported strong LacZ expression in hypertrophic cartilage zones of transgenic mice (Gebhard et al., 2004), β-galactosidase expression was not seen in all hypertrophic chondrocytes. By comparison, β-galactosidase activity was dramatically enhanced and evenly distributed in all hypertrophic chondrocytes of BAC-Col10a1-LAcZ transgenic mouse lines (Gebhard et al., 2007). The lacZ expression pattern was consistent in 5 different founder lines tested, indicating that the tissue specificity of the BACCol10a1-driven transgene expression is independent of the BAC integration site. The intensity of β-galactosidase staining correlated with the copy number of BAC-Col10a1-LacZ transgenes. This suggests that additional cis-regulatory elements may be located outside the 4.6 kb promoter region of Col10a1, but it is also possible that transgene expression in the context of a 220 kb BAC is superior as it is independent from chromosomal integration sites and from the influence of adjacent unspecific cis-regulatory elements.
Accordingly, the BAC-Col10-Cre transgenic lines presented here revealed strong and tissue-specific Cre expression in all hypertrophic cartilage zones of the fetal and newborn skeleton after breeding with ROSA26 reporter mice. The β-galactosidase activity seen in three founder lines tested (#1427, #1465 and #1466, containing between 4 and 5 copies of BAC-Col10-Cre) was comparable to that of the BAC-Col10-LacZ transgenic lines (Gebhard et al., 2007). Further BAC Col10-Cre lines mated to lacZ-expressing ItgB1fl/fl mice revealed a pattern of β-galactosidase activity with the same intensity and specificity (von der Mark, in prep). Cre expression remained stable in 5 BAC-Col10-Cre founder lines over at least 2 generations, some already in the third generation. Whether in founder lines containing 4 or 5 BAC copies all BAC copies have integrated as one concatameric cluster or were split into 2 or more integration sites, is still an open question, but may be irrelevant since one BAC-Col10-Cre copy may be sufficient for activation/deletion of some floxed genes, but insufficient for others.
Recently, a knock-in Col10a1-Cre deleter mouse was created by Cre insertion into exon 2 of the genomic Col10a1 locus by homologous recombination (Tsang et al., 2007). The data indicate even β-galactosidase activity in all hypertrophic cells of the growth plate as visualized after mating with a ROSA 26 reporter mouse (Tsang et al., 2007). While this Cre deleter line awaits further characterization, the general disadvantage of knock-in Cre lines is that such mice will be heterozygous for the gene into which Cre is inserted. Furthermore, only one Cre copy can be expressed, which may be sufficient in case the gene is driven by a strong promoter, but not sufficient for genes with weaker promoters.
Although LacZ expression was clearly seen in all hypertrophic chondrocytes, in most long bones substantial background activity was seen in the subchondral part of the bone marrow. Part of this may be due to residual LacZ activity released from hypertrophic chondrocytes after apoptosis at the cartilage–bone border. Additionally, a significant number of hypertrophic chondrocytes seems to survive as bone forming cells in the cartilaginous core of endochondral bone trabeculae (von der Mark and von der Mark, 1977). (Roach, 1998) described the transdifferentiation of hypertrophic chock chondrocytes in the growth plate into bone forming cells after asymmetric cell division. In most fetal and postnatal long bones of BAC-Col10a1-Cre; ROSA26 mice we observed significant β-galactosidase activity the subchondral bone marrow zone. Most likely, these cells represent such “post-hypertrophic” chondrocytes; some of these cells still express Col10a1 as seen by in situ hybridization; others may have completely turned off expression of cartilage genes, but express type I collagen, osteocalcin and other bone-specific genes, thus contributing to bone formation together with osteoblasts. Such a kind of switch in gene expression from Col10a1 to Col1a1 has been observed in long term cultures of human hypertrophic chondrocytes (Kirsch et al., 1992). The fact that the number of β-galactosidase positive cells exceeds that of Col10a1 positive cells in the subchondral zone indicates that LacZ expression under the ROSA26 promoter, once activated by BAC Col10a1-driven Cre in the hypertrophic zone, may continue in the progeny of hypertrophic chondrocytes in the subchondral zone even after Col10a1 expression is shut down. Thus, the BAC-Co10a1-Cre induced expression of LacZ reporter genes in transgenic mice may be used as a sensitive tracer to follow the cell fate of hypertrophic chondrocytes during endochondral ossification.
In conclusion, the availability of transgenic BAC Col10Cre deleter mouse lines capable of specific and efficient deletion of loxP-flanked genes in hypertrophic chondrocytes should facilitate the elucidation of the complex mechanism of endochondral ossification.
4. Materials and methods
4.1. Cloning of the placH+COL10A1-cre-frt-neo-frt targeting vector and preparation of BAC Col10a1-Cre DNA
From the placH+COL10A1-lacZ-frt-neo-frt the lacZ cassette (Gebhard et al., 2007) was excised with SmaI and EcoRI and cloned into pGL2basic vector (Promega) to form pGL+lacZ. The cre coding sequence with a nuclear localization sequence was excised from plasmid p102 (kindly provided by S. Henry, MD Anderson Cancer Center) by SmaI and PvuII digestion and substituted for the 5′ part of lacZ in pGL2+lacZ (cut by NcoI and EcoR, ends blunted with T4 DNA polymerase) to form pGL2+cre. From pGL2+cre the cre CDS and 3′ part of lacZ gene were cut out with SmaI and Bsu36I and substituted for the lacZ cassette of placH+COL10A1-lacZ-frt-neo-frt cut by SmaI and BstZ17I. The resulting plasmid placH+COL10A1-cre-frt-neo-frt was verified by sequencing. The 4.2 kb recombination cassette was excised by Xho I digestion, and 300 ng of gel-purified linear fragment was used for homologous recombination with BAC-RP23-192A7 (BACPAC Resources Center at Children’s Hospital Oakland Research Institute (CHORI) in EL250 E. coli according to Lee et al. (2001) as described previously (Gebhard et al., 2007).
Clones containing BAC Col10a1-Cre-frt-neo-frt DNA were tested for correct recombination by PCR analysis of 5′ and 3′ recombination sites using primer P1 located in Intron 1 of the Col10a1 gene and primer P5 (“cre reverse”) located in the cre coding sequence (see Fig. 1).
P1: 5′ TTTAGAGCATTATTTCAAGGCAGTTTCCA 3′ (Col10a1 Intron ); P5: 5′ AGGCAAATTTTGGTGTACGG 3′; size of amplicon: 305 bp
4.2. Generation of BAC Col10Cre transgenic mice
For microinjection BACCol10a1-cre DNA was linearized with PISceI enzyme (NEBiolabs) cleaving the unique site in the BACe3.6 vector part and purified by molecular sieve chromatography on Sepharose 4B equilibrated in microinjection buffer as described previously (Fig. 1b) (Gebhard et al., 2007). Purified BAC DNA was injected into the male pronucleus (1.5 ng/μl) of FVB mice and FV/C56Bl F1 hybrids. Offspring were tested for genomic BACCol10a1 Cre DNA by PCR using primers P1 and P5 (Fig. 1c). BAC-Col10a1-Cre gene copy numbers were analyzed both by Southern blotting using the 306 bp probe generated by the PCR reaction with Primers P1 and P5, and by quantitative real-time PCR for genomic Col10a1 using primers located in exon 3 and intron 2 of Col10a1 (Table 1). Primer Col10-5′=TGC TGC CCT GGT CTT ACT CT; Primer Col10-3′=GCC TTG GGA TCC TAA ACC TC.
4.3. Analysis of Cre activity in ROSA26 reporter mice
BAC-Col10a1-Cre male founders #1427 and #1466 were mated to female ROSA26 reporter mice (Soriano, 1999). Embryos of various stages and newborn offspring were tested for Cre activity by PCR; tissues were fixed, stained for β-galactosidase, and either embedded in paraffin, and sectioned, or clarified with KOH as described recently (Gebhard et al., 2007).
Acknowledgment
We wish to thank Dr. Reinhard Faessler for the generous support of the mouse breeding work done at the Max-Planck Institute of Biochemistry, Martinsried, and Dr. Rupec, University Hospital Munich, for providing the ROSA26 reporter mice. This work was supported by a grant to K.v.d.M. by the Deutsche Forschungsgemeinschaft (MA534/23-1).
References
- Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, de Crombrugghe B. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aszodi A, Hunziker EB, Brakebusch C, Fassler R. Beta1 integrins regulate chondrocyte rotation, G1 progression, and cytokinesis. Genes Dev. 2003;17:2465–2479. doi: 10.1101/gad.277003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballock RT, O’Keefe RJ. The biology of the growth plate. J. Bone Joint Surg. Am. 2003;(85-A):715–726. [PubMed] [Google Scholar]
- Beier F, Vornehm S, Pöschl E, von der Mark K, Lammi MJ. Localization of silencer and enhancer elements in the human type X collagen gene. J. Cell. Biochem. 1997;66:210–218. doi: 10.1002/(sici)1097-4644(19970801)66:2<210::aid-jcb8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Chambers D, Young DA, Howard C, Thomas JT, Boam DS, Grant ME, Wallis GA, Boot-Handford RP. An enhancer complex confers both high-level and cell-specific expression of the human type X collagen gene. FEBS Lett. 2002;531:505–508. doi: 10.1016/s0014-5793(02)03606-2. [DOI] [PubMed] [Google Scholar]
- Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- de Crombrugghe B, Lefebvre V, Nakashima K. Regulatory mechanisms in the pathways of cartilage and bone formation. Curr. Opin. Cell Biol. 2001;13:721–727. doi: 10.1016/s0955-0674(00)00276-3. [DOI] [PubMed] [Google Scholar]
- Enomoto-Iwamoto M, Enomoto H, Komori T, Iwamoto M. Participation of Cbfa1 in regulation of chondrocyte maturation. Osteoarthritis Cartilage. 2001;9(Suppl A):S76–S84. doi: 10.1053/joca.2001.0448. [DOI] [PubMed] [Google Scholar]
- Gebhard S, Poschl E, Riemer S, Bauer E, Hattori T, Eberspaecher H, Zhang Z, Lefebvre V, de Crombrugghe B, von der Mark K. A highly conserved enhancer in mammalian type X collagen genes drives high levels of tissue-specific expression in hypertrophic cartilage in vitro and in vivo. Matrix Biol. 2004;23:309–322. doi: 10.1016/j.matbio.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Gebhard S, Hattori T, Bauer E, Bosl MR, Schlund B, Poschl E, Adam N, de Crombrugghe B, von der Mark K. BAC constructs in transgenic reporter mouse lines control efficient and specific LacZ expression in hypertrophic chondrocytes under the complete Col10a1 promoter. Histochem. Cell Biol. 2007;127:183–194. doi: 10.1007/s00418-006-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J. Cell. Biochem. 2006;97:33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev. Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Inada M, Wang Y, Byrne MH, Rahman MU, Miyaura C, Lopez-Otin C, Krane SM. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17192–17197. doi: 10.1073/pnas.0407788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez MJ, Balbin M, Lopez JM, Alvarez J, Komori T, Lopez-Otin C. Collagenase 3 is a target of Cbfa1, a transcription factor of the runt gene family involved in bone formation. Mol. Cell. Biol. 1999;19:4431–4442. doi: 10.1128/mcb.19.6.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IS, Otto F, Zabel B, Mundlos S. Regulation of chondrocyte differentiation by Cbfa1. Mech. Dev. 1999;80:159–170. doi: 10.1016/s0925-4773(98)00210-x. [DOI] [PubMed] [Google Scholar]
- Kirsch T, Swoboda B, von der Mark K. Ascorbate-independent differentiation of human chondrocytes in vitro: simultaneous expression of types I and X collagen and matrix mineralization. Differentiation. 1992;51:89–100. doi: 10.1111/j.1432-0436.1992.tb00503.x. [DOI] [PubMed] [Google Scholar]
- Kronenberg HM. PTHrP and skeletal development. Ann. N. Y. Acad. Sci. 2006;1068:1–13. doi: 10.1196/annals.1346.002. [DOI] [PubMed] [Google Scholar]
- Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, Karperien M, Defize LHK, Ho C, Mulligan RC, Abou-Samra AB, Juppner H, Segre GV, Kronenberg HM. PTH/PTHrP receptor in early development and Indian hedgehog- regulated bone growth [see comments] Science. 1996;273:663–666. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, de Crombrugghe B. Toward understanding SOX9 function in chondrocyte differentiation. Matrix Biol. 1998;16:529–540. doi: 10.1016/s0945-053x(98)90065-8. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Smits P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res. C Embryo Today. 2005;75:200–212. doi: 10.1002/bdrc.20048. [DOI] [PubMed] [Google Scholar]
- Ornitz DM. FGF signaling in the developing endochondral skeleton. Cytokine Growth Factor Rev. 2005;16:205–213. doi: 10.1016/j.cytogfr.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Marie PJ. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 2002;16:1446–1465. doi: 10.1101/gad.990702. [DOI] [PubMed] [Google Scholar]
- Ortega N, Behonick DJ, Werb Z. Matrix remodeling during endochondral ossification. Trends Cell Biol. 2004;14:86–93. doi: 10.1016/j.tcb.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. Cartilage: “Molecular Aspects”. CRC Press; Boca Raton, Florida: 1991. The growth plate: cellular physiology, cartilage assembly and mineralization; pp. 179–211. [Google Scholar]
- Potocnik AJ, Brakebusch C, Fassler R. Fetal and adult hematopoietic stem cells require beta1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity. 2000;12:653–663. doi: 10.1016/s1074-7613(00)80216-2. [DOI] [PubMed] [Google Scholar]
- Provot S, Schipani E. Molecular mechanisms of endochondral bone development. Biochem. Biophys. Res. Commun. 2005;328:658–665. doi: 10.1016/j.bbrc.2004.11.068. [DOI] [PubMed] [Google Scholar]
- Riemer S, Gebhard S, Beier F, Poschl E, von der Mark K. Role of c-fos in the regulation of type X collagen gene expression by PTH and PTHrP: localization of a PTH/PTHrP-responsive region in the human COL10A1 enhancer. J. Cell. Biochem. 2002;86:688–699. doi: 10.1002/jcb.10260. [DOI] [PubMed] [Google Scholar]
- Roach HI. Trans-differentiation of hypertrophic chondrocytes into cells capable of producing a mineralized bone matrix. Bone Miner. 1998;19:1–20. doi: 10.1016/0169-6009(92)90840-a. [DOI] [PubMed] [Google Scholar]
- Schmidl M, Adam N, Surmann-Schmitt C, Hattori T, Stock M, Dietz U, Decrombrugghe B, Poschl E, von der Mark KC. Twisted gastrulation modulates BMP- induced collagen II and X expression in chondrocytes in vitro and in vivo. J. Biol. Chem. 2006;281:31790–31800. doi: 10.1074/jbc.M603419200. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stickens D, Behonick DJ, Ortega N, Heyer B, Hartenstein B, Yu Y, Fosang AJ, Schorpp-Kistner M, Angel P, Werb Z. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development. 2004;131:5883–5895. doi: 10.1242/dev.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamura Y, Otani T, Kanatani N, Koyama E, Kitagaki J, Komori T, Yamada Y, Costantini F, Wakisaka S, Pacifici M, Iwamoto M, Enomoto-Iwamoto M. Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J. Biol. Chem. 2005;280:19185–19195. doi: 10.1074/jbc.M414275200. [DOI] [PubMed] [Google Scholar]
- Thomas JT, Sweetman WA, Cresswell CJ, Wallis GA, Grant ME, Boot-Handford RP. Sequence comparison of three mammalian type-X collagen promoters and preliminary functional analysis of the human promoter. Gene. 1995;160:291–296. doi: 10.1016/0378-1119(95)00189-d. [DOI] [PubMed] [Google Scholar]
- Tsang KY, Chan D, Cheslett D, Chan WC, So CL, Melhado IG, Chan TW, Kwan KM, Hunziker EB, Yamada Y, Bateman JF, Cheung KM, Cheah KS. Surviving endoplasmic reticulum stress is coupled to altered chondrocyte differentiation and function. PLoS Biol. 2007;5:e44. doi: 10.1371/journal.pbio.0050044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Eerden BC, Karperien M, Gevers EF, Lowik CW, Wit JM. Expression of Indian hedgehog, parathyroid hormone-related protein, and their receptors in the postnatal growth plate of the rat: evidence for a locally acting growth restraining feedback loop after birth [In Process Citation] J. Bone Miner. Res. 2000;15:1045–1055. doi: 10.1359/jbmr.2000.15.6.1045. [DOI] [PubMed] [Google Scholar]
- von der Mark K, von der Mark H. Role of 3 genetically distinct collagen types in endochondral ossification and calcification of cartilage. J. Bone Joint Res. 1977;59:458–464. doi: 10.1302/0301-620X.59B4.72756. [DOI] [PubMed] [Google Scholar]
- Vortkamp A. The Indian hedgehog-PTHrP system in bone development. Ernst Schering Res. Found. Workshop. 2000. pp. 191–209. [DOI] [PubMed]
- Weisser J, Riemer S, Schmidl M, Suva LJ, Poschl E, Brauer R, von der Mark K. Four distinct chondrocyte populations in the fetal bovine growth plate: highest expression levels of PTH/PTHrP receptor, Indian hedgehog, and MMP-13 in hypertrophic chondrocytes and their suppression by PTH (1-34) and PTHrP (1-40) Exp. Cell Res. 2002;279:1–13. doi: 10.1006/excr.2002.5580. [DOI] [PubMed] [Google Scholar]
- Yang G, Cui F, Hou N, Cheng X, Zhang J, Wang Y, Jiang N, Gao X, Yang X. Transgenic mice that express Cre recombinase in hypertrophic chondrocytes. Genesis. 2005;42:33–36. doi: 10.1002/gene.20120. [DOI] [PubMed] [Google Scholar]
- Yoon BS, Lyons KM. Multiple functions of BMPs in chondrogenesis. J. Cell. Biochem. 2004;93:93–103. doi: 10.1002/jcb.20211. [DOI] [PubMed] [Google Scholar]
- Zelzer E, Glotzer DJ, Hartmann C, Thomas D, Fukai N, Soker S, Olsen BR. Tissue specific regulation of VEGF expression during bone development requires Cbfa1/Runx2. Mech. Dev. 2001;106:97–106. doi: 10.1016/s0925-4773(01)00428-2. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Zhou G, Morello R, Chen Y, Garcia-Rojas X, Lee B. Type X collagen gene regulation by Runx2 contributes directly to its hypertrophic chondrocytespecific expression in vivo. J. Cell Biol. 2003;162:833–842. doi: 10.1083/jcb.200211089. [DOI] [PMC free article] [PubMed] [Google Scholar]