Summary
When competition for sex-specific resources overlaps in time with offspring production and care, trade-offs can occur. Steroids hormones, particularly testosterone (T), play a crucial role in mediating such trade-offs in males, often increasing competitive behaviors while decreasing paternal behavior. Recent research has shown that females also face such trade-offs; however, we know little about the role of T in mediating female phenotypes in general, and the role of T in mediating trade-offs in females in particular. Here we examine the relationship between individual variation in maternal effort and endogenous T in the dark-eyed junco, a common songbird. Specifically, we measure circulating T before and after a physiological challenge (injection of gonadotropin releasing hormone, GnRH), and determine whether either measure is related to provisioning, brooding, or the amount of T sequestered in egg yolk. We found that females producing more T in response to a challenge spent less time brooding nestlings, but provisioned nestlings more frequently, and deposited more T in their eggs. These findings suggest that, while T is likely important in mediating maternal phenotypes and female life history tradeoffs, the direction of the relationships between T and phenotype may differ from what is generally observed in males, and that high levels of endogenous T are not necessarily as costly as previous work might suggest.
Keywords: life history tradeoffs, endogenous steroids, testosterone, gonadotropin releasing hormone (GnRH), maternal care, yolk hormones, dark-eyed junco (Junco hyemalis)
Introduction
Because time and energy can be spent only once, animals are often functionally constrained from maximally investing in all potentially beneficial traits (Lessells, 2008). Such constraints produce tradeoffs, which are key to understanding the evolution of life histories (Roff et al., 2002; Stearns, 1992). One of the most important behavioral tradeoffs occurs when competition for crucial sex-specific resources overlaps in time with the need to care for offspring (Magrath and Komdeur, 2003). This tradeoff between resource acquisition and offspring care is often portrayed as a continuum with males at one end, investing more in competition for mates, and females at the opposite end, investing mainly in offspring production and care (Rosvall, 2013; Shuster and Wade, 2003; Trivers, 1972). However, recent research has shown that females also benefit from increased competitive ability in reproductive contexts (Clutton-Brock, 2009; Langmore, 1998; LeBas, 2006; Rosvall, 2011; Stockley and Bro-Jørgensen, 2011; Tobias et al., 2012). Less is known about whether, and to what extent, females trade-off competitive ability with parental care, but research has shown that increased investment in traits that appear important for competitive ability (i.e. competitive traits: ornaments, armaments, aggression, etc. (Cain & Ketterson, 2012; West-Eberhard, 1983)) is often associated with decreased maternal effort (Bell et al., 2011; Dantzer et al., 2011; Fite et al., 2005; Fitzpatrick et al., 1995; Nordeide et al., 2006; Packer et al., 1995; Rosvall, 2011).
In vertebrates, the sex steroids are often important in mediating this tradeoff. In particular, among males, high levels of testosterone (T) are generally associated with increased investment in traits used to compete for reproductive resources (e.g., mates, territories), at the expense of parental care and self-maintenance (Adkins-Regan, 2005; Ketterson and Nolan, 1999; Wingfield et al., 2001); but see (Lynn et al., 2002). Relative to males, we know considerably less about the role of T in mediating female phenotypes in general and life history tradeoffs in particular (Ketterson et al., 2005; Staub and De Beer, 1997). However, the available data suggest that T may affect female trait expression in a manner similar to males. For example, experimentally elevating plasma T levels in females often increases the expression of traits used in same-sex competition, e.g. aggression (Rosvall, 2013; Sandell, 2007; Veiga et al., 2004; Zysling et al., 2006), and often decreases some forms of maternal care (Clotfelter et al., 2004; O’Neal et al., 2008; Rosvall, 2013; Rutkowska et al., 2005; Veiga and Polo, 2008). However, some components of maternal care are unaffected by experimentally elevated T (Clotfelter et al., 2004; DeVries and Jawor, 2013; Ketterson et al., 2005; O’Neal et al., 2008), and endogenous measures of T are often unrelated to female competitive traits (Elekonich, 2000; Hau et al., 2004; Jawor et al., 2006b) and maternal care (DeVries and Jawor, 2013). As a result, the relative importance of T in mediating female phenotypes and life history tradeoffs is currently unclear.
In addition to shaping a female’s parental phenotype, female T production may also have important effects on offspring phenotype via maternal effects. In oviparous vertebrates, individual females vary in the amount of T that they deposit in eggs (Gil, 2003; Groothuis et al., 2005; Groothuis and Schwabl, 2008). Experimental studies have shown that variation in the amounts of steroid hormone in the egg can have large effects on developing offspring both in the short-term and long-term, facilitating rapid growth and begging, and shaping adult morphology and competitive ability, but can also be costly in terms of immune function (Groothuis et al., 2005; Groothuis and Carere, 2005; Navara et al., 2005). Further, T has been implicated as a factor influencing primary sex ratios in birds (Correa et al., 2011; Rutkowska and Cichoń, 2006). Despite these potentially important consequences, less is known about the proximate mechanisms controlling the amount of T sequestered, and it is unclear whether the transfer of hormones is actively controlled by the female, or due to passive transfer (Groothuis and Schwabl, 2008; Müller et al., 2011; Schwabl, 1993). Previous studies have failed to find a relationship with circulating T and yolk T (Navara et al., 2005). However, other work has shown that individual ability to produce T predicts the amount of T a female deposits in her eggs (Jawor et al., 2007; Müller et al., 2011). Understanding the relationship between individual T profiles and yolk T is crucial to developing a greater understanding of the role of steroid-mediated maternal effects in shaping offspring development and phenotypic evolution.
Currently, the majority of studies examining the role of T in mediating female phenotypes, particularly in birds, have utilized a phenotypic engineering approach, in which T is experimentally altered (Adkins-Regan, 2005; Ketterson et al., 2005; Williams, 2008). Experimental studies of this type are crucial for revealing the causal relationship between T and phenotype. However, there are limits to what we can learn from phenotypic engineering (McGlothlin et al., 2008; 2007; 2010). We currently have very little information regarding the role of individual variation in endogenous testosterone in mediating female behavior or adjusting offspring phenotype via maternal effects (Groothuis et al., 2005; Williams, 2012; 2008). To fully understand the evolution of hormonally mediated phenotypes we must also examine the relationship between naturally existing hormonal and behavioral variation (Cain and Ketterson, 2012; DeVries and Jawor, 2013; McGlothlin et al., 2007; Moore et al., 2002).
Here we examine the relationships between endogenous T and maternal effort (behavior and yolk hormone deposition), in the dark-eyed junco (Junco hyemalis carolinensis), a common songbird often used as a model for exploring the relationships between hormones and behavior in free-living animals (e.g. (Deviche et al., 2001; Holberton et al., 2008; Ketterson and Nolan, 1999; Ketterson et al., 1992; McGlothlin et al., 2010; Raouf et al., 1997). Previous work has shown that experimentally elevated T in female juncos leads to increased aggression (Zysling et al., 2006), and reduced maternal care in some, but not all, measures (Clotfelter et al., 2004; O’Neal et al., 2008). More recently, we found that endogenous T production ability is positively related to female-female aggression (Cain and Ketterson, 2012), and that females tradeoff some forms of maternal care (brooding and egg size) with intra-sexual aggression (Cain & Ketterson, in press). To determine whether T may play a role in mediating this tradeoff or maternal phenotype in general, we examined the relationships between two measures of endogenous T, circulating T before and after a physiological challenge in the form of an injection of gonadotropin releasing hormone (or GnRH), and three measures of maternal effort: brooding, provisioning, and the amount of T sequestered in the yolk. Based on previous studies using experimentally elevated T, we predicted that endogenous T measures would be negatively related to maternal behavior, and positively related to yolk T. Alternatively, other work suggests that the relationships between T and maternal care may be more complicated (Dantzer et al., 2011; DeVries and Jawor, 2013; Spinney et al., 2006; Swett and Breuner, 2009; Veiga and Polo, 2008).
Methods
Study Species, Site, And General Methods
This study took place on and around Mountain Lake Biological Station, in Giles Co., Virginia (37°22′N, 80°32′W), from April 15-August 10 2008, 2009, and 2010. Juncos are a socially monogamous, mildly dimorphic songbird (Nolan et al., 2002). Females build nests, incubate eggs, and brood nestlings; males assist in feeding and nest defense. General field methods are described in detail elsewhere (McGlothlin et al., 2010). Briefly, resident individuals were captured using baited potter traps and mist nets, and marked with numbered metal bands and unique combinations of color bands. Females were aged as young (first breeding season) or old (after first breeding season) using plumage, eye coloration and mark-recapture data from previous years (Nolan et al., 2002). We searched the study site daily for nests of all resident females. When located, nests were marked and then monitored to determine the social pair and the commencement of egg laying. Within 24h of clutch completion, the third laid egg of each female’s first nesting attempt was collected (2008, n= 42; 2009, n= 35; 2010, n= 31). If egg order was unknown we selected the largest egg, as the 3rd egg is often largest (Nolan et al., 2002).
Parental Behavior
In 2009 and 2010, we measured maternal behavior for all females that had both been administered GnRH challenges (see below), and had a nest that survived to the nestling stage, which limited the sample size (2009: n = 12; 2010: n= 16). To quantify maternal behavior we videotaped each nest for 4 consecutive hours (0900–1700) at day 3 post-hatching. A single observer watched all tapes at a later date and determined the number of female feeding trips, the number of brooding bouts, and the length of each brooding bout. Mean brooding bout was calculated by summing the total amount of time spent brooding and dividing by the number of brooding bouts. If the female was still brooding at the end of the tape that brooding event was excluded. Mean brood bouts ranged from 244s – 1588s, mean of 663s. We used a Campbell CR10 data logger located on the study site to record ambient temperature at 1400 (mid-point for most recordings). A female’s age category (young or old) had no effect on brooding or provisioning (mean brooding bout: ). To quantify maternal behavior we videotaped each nest for 4 consecutive hours (0900–1700) at day 3 post-hatching. A single observer watched all tapes at a later date and determined the number of female feeding trips, the number of brooding bouts, and the length of each brooding bout. Mean brooding bout was calculated by summing the total amount of time spent brooding and dividing by the number of brooding bouts. If the female was still brooding at the end of the tape that brooding event was excluded. Mean brood bouts ranged from 244s – 1588s, mean of 663s. We used a Campbell CR10 data logger located on the study site to record ambient temperature at 1400 (mid-point for most recordings). A female’s age category (young or old) had no effect on brooding or provisioning (mean brooding bout: t28 = −0.11, P= 0.91; provisioning rate: t28 = −1.06, P= 0.30), nor did year (provisioning rate; t28 = −1.27, P= 0.22, mean brooding bout; t28 = 1.15, P= 0.26), ambient temperature (mean brooding bout, R2 = 0.01, P= 0.62; provisioning rate, R2= 0.002, P=0.87), or date (mean brooding bout, R2 = 0.002, P= 0.79; provisioning rate, R2= 0.003, P= 0.77). There was a negative relationship between the two measures of parental behavior (R2= 0.28, P= 0.0047, n= 28); i.e. females that brooded most tended to feed less frequently.
GnRH Challenges
Testosterone production is regulated by the hypothalamic-pituitary-gonadal (HPG) axis. The hypothalamus responses to a variety of external and internal stimuli by releasing gonadotropin-releasing hormone (GnRH), stimulating the pituitary to release luteinizing hormones (LH), which then travels via the bloodstream to the gonads, which response by releasing sex steroids, including T (Adkins-Regan, 2005; (Jawor et al., 2006a)). To measure individual variation in ability to produce testosterone, we challenged females’ HPG axis by administering an injection of GnRH to produce a transient increase in circulating T (2008: n= 18; 2009: n= 26; 2010: n= 19). This procedure stimulates individuals to release maximal T levels (Cain and Ketterson, 2012; Jawor et al., 2007; 2006a; McGlothlin et al., 2007; Moore et al., 2002; Wingfield et al., 1991), and is repeatable in both sexes (Jawor et al., 2006a; Rosvall & Bergeon Burns unpublished). In female juncos the most robust response to GnRH is during the 7 days prior to oviposition, when females are rapidly yolking eggs (Jawor et al., 2007). We challenged females only during this stage, identifying females that were heavy and had the distinctive “torpedo-like” shape typical of yolking songbirds (Cain and Ketterson, 2012). Mean mass for female juncos early in the breeding season is 21.5g ± 0.18 (mean, s.e.) (Cain and Ketterson, 2012), mean mass for challenged females was 24.7 ± 1.44. Challenged females were captured during the pre-breeding season and transported to a central processing area. An initial blood sample (initial T) was taken from the wing, followed by an intramuscular injection of 50 μL of a solution containing 1.25 μg of chicken GnRH- I (Sigma L0637; American Peptide 54-8-23). After exactly 30 minutes a second blood sample was taken (post-challenge T). Samples were centrifuged and the plasma was drawn off and frozen at −20 C° until assayed. We recorded time at capture (capture time) and the total amount of time elapsed between capture and the initiation of the challenge (handling time, mean = 1696s).). This procedure stimulates individuals to release maximal T levels (Cain and Ketterson, 2012; Jawor et al., 2007; 2006a; McGlothlin et al., 2007; Moore et al., 2002; Wingfield et al., 1991), and is repeatable in both sexes (Jawor et al., 2006a; Rosvall & Bergeon Burns unpublished). In female juncos the most robust response to GnRH is during the 7 days prior to oviposition, when females are rapidly yolking eggs (Jawor et al., 2007). We challenged females only during this stage, identifying females that were heavy and had the distinctive “torpedo-like” shape typical of yolking songbirds (Cain and Ketterson, 2012). Mean mass for female juncos early in the breeding season is 21.5g ± 0.18 (mean, s.e.) (Cain and Ketterson, 2012), mean mass for challenged females was 24.7 ± 1.44. Challenged females were captured during the pre-breeding season and transported to a central processing area. An initial blood sample (initial T) was taken from the wing, followed by an intramuscular injection of 50 μL of a solution containing 1.25 μg of chicken GnRH- I (Sigma L0637; American Peptide 54-8-23). After exactly 30 minutes a second blood sample was taken (post-challenge T). Samples were centrifuged and the plasma was drawn off and frozen at −20 C° until assayed. We recorded time at capture (capture time) and the total amount of time elapsed between capture and the initiation of the challenge (handling time, mean = 1696s).
Plasma Testosterone Assays
Plasma T concentrations for 2009 and 2010 were determined using commercially available EIA kits (Assay Designs, Inc., #901-065) as described elsewhere (Clotfelter et al., 2004; Jawor et al., 2007). We added 2000cpm of tritiated T (H3-T) to calculate recovery efficiencies prior to two rounds of diethyl ether extractions. Samples were then dried down with N2 and re-suspended in 300ul of assay buffer and 50ul of ethanol. All samples were run in duplicate; a four-parameter logistic curve-fitting program (Microplate Manager; Bio-Rad Laboratories, Inc.) was used to determine concentrations. Plasma T concentrations for 2008 were determined using long-column chromatography followed by a radio-immunoassay as part of a separate experiment, described in Cain & Ketterson 2012. These values were used only for examining the relationship between plasma and yolk T. For all samples, values were corrected for incomplete recoveries; average recovery was 90%. Both initial and post-challenge T values were normal after a logit transformation (Shapiro-Wilk test: P>0.15 for both). Three samples were excluded because there was a problem with the challenge (bled at the wrong time) or the hormone assay (unusually high or low recoveries). The time of day the challenge occurred had no detectable relationship with initial or post-challenge T (all P > 0.35).
Yolk Testosterone Assays
The concentration of testosterone in yolk was determined using a testosterone enzyme-immunoassay kit (EIA), (Catalog # ADI-901-065) (Enzo Life Sciences Intl., Inc., Plymouth Meeting, PA), following a modified ethanol extraction (Kozlowski et al., 2009). Previously frozen eggs were allowed to semi-thaw to permit separation of the yolk from the albumin. We recorded the total egg mass and yolk mass and transferred yolks to 2ml Eppendorf tubes. Mean yolk mass was 0.504g ± 0.006 (mean, s.e.). After the yolk thawed fully, we added 500ul of distilled water and several glass mixing beads, then vortexed thoroughly until yolks were completely homogenized. For hormone extraction, 50ul of the yolk solution was transferred to a clean 1.5ml Eppendorf, and then further diluted with an additional 100ul of distilled water. Tritiated testosterone (2000 cpm H3-T) was added to the homogenate for calculation of extraction recoveries, and mean sample recovery was 82%. Homogenate was then vortexed and incubated at 37C for 1hr to allow the native and tritiated hormone to equilibrate. After incubation, 300ul of 100% ethanol was added to each sample. Samples were again vortexed and put on a shaker at 500rpm for 5min. Samples then were allowed to sit at room temperature for 10 min to incubate and settle. After incubation, samples were spun in a micro-centrifuge for 10min at 13,000 rpm. The supernatant was decanted into a 13×100 borosilicate culture tube and dried down using a forced air manifold and warm water bath. Dried samples were then rehydrated with 50ul of 100% ethanol and 300ul of assay buffer. Assays were run in accordance with kit directions and as described for plasma samples with two exceptions. First, because mean testosterone levels were above optimal levels for the assay, all samples were run at a 1:10 dilution. Second, kit standards were replaced by standards made from pooled and diluted yolk extract in order that samples and standards be of comparable matrices. Values were normally distributed after natural log transformation (Shapiro-Wilk W test, P> 0.15).
Assay Validation
To determine whether substances present in the extract interfered with the accuracy of the assay, or introduced bias (Engelhardt and Groothuis, 2005), we pooled extracts from 5 yolks prepared as above and performed a serial dilution of 1:4, 1:8, 1:16, 1:32, 1:64 and 1:128. Serial dilutions were parallel to the standard curve (ANCOVA, dilution by concentration interaction, P= 0.47). We also created five replicates of the diluted sample pool (1:50) and spiked each with a serial dilution of a known standard (i.e. 1000, 630, 320, 125, and 62.5 pg/ml) provided with the assay kit. Spiked recoveries were 91% of expected values.
This modified technique produced T concentration similar to what has been reported previously in this species. T concentration in yolks using this method ranged from 1.58 to 18.79 pg/mg, (mean, s.e.) 7.107 ± 0.454 pg/mg, and recoveries averaged 82.85%. Lipar et al. (1999) used long column RIAs to determine yolk T and report mean T value of 7.63 pg/mg, with recoveries of 71%; Jawor et al. (2007) also used RIAs and reported a mean T value of 1.71 pg/mg, with 58% recoveries.
Statistical Analysis
All analyses were done in JMP 10 (SAS Institute Inc.). To examine the relationship between hormone measures and parental behavior, we used the hormone measure as the dependent variable, allowing us to examine the relationship between T and the behavior of interest while holding the other variables constant (e.g. date, mass, year, capture time, handling time) (Jawor et al. 2006a; McGlothlin et al. 2007). However, we make no assumption about whether the relationship is causal, or in which direction any causality may operate. We used BIC (Bayesian Information Criterion) minimum scores to objectively select the predictive variables. The beginning model included the parental behavior of interest, year, and factors that have previously been shown to influence response (date, mass, capture time, handling time). For most of the females that received a GnRH challenge we also collected an egg (2008, n=26; 2009, n=11; 2010, n=19). Females were only included in this analysis if they received the GnRH challenge in the same year the egg was collected. The final model was run in a multiple regression analysis. To examine the relationships between yolk T, initial T and post-challenge T we used multiple regression models with yolk T as the dependent variable, with year and initial or post-challenge T as predictors.
To illustrate relationships between post-challenge T and behavior we calculated leverage plots pairs. Leverage pairs are derived from the residual error without the effect in the model and the actual residuals from the best-fit line, similar to a partial correlation (Sall, 1990). Finally, to visualize potential patterns of covariation between variables of interest we constructed a network model, see Figure 2 (Huffman et al., 2012). Specifically, we used the calculated coefficient of determination (adjusted R2) from each multiple regression model, i.e. tests examining relationship between hormones measures and maternal behavior/yolk T, to construct a correlation matrix between measures of hormonal phenotype and maternal care. We then used Cytoscape software (version 2.3) to construct a force-weighted network model to visualize the emerging network. Each node represents a variable of interest, colored according to type of trait (hormone measures are blue, maternal effort is orange, egg metrics are yellow). Lines connecting nodes denote the nature of the relationship between the variables (solid lines are positive; dashed lines are negative, faint lines indicate no detectable relationship). The length of the line is inversely related to the strength of the relationship; short lines indicate stronger relationships.
Figure 2.
Scatterplot illustrating the correlation between female ability to produce T in response to a GnRH challenge (post challenge T) and the concentration of yolk T (ng/g) deposited in eggs. Lines and symbols differ according to year; 2008, cross and dashed line; 2009, x and dotted line, 2010 square and solid line.
Results
Maternal Behavior And Testosterone
Initial T was negatively, but not significantly, related to brooding (F1, 16 = 2.74, R2= 0.16, P= 0.12), and unrelated to provisioning (P> 0.45). Post-challenge T was negatively related to brooding; females that produce more T brooded for shorter intervals (Fig. 1 & 3, Table 1, Overall model Adj. R2= 0.98, F6, 12= 33.03, P= 0.0007; mean brooding bout, b= −0.002, P= 0.0011). In the final model, date, year, mass, capture time and handling time, were all significant variables. Post-challenge T was positively related to provisioning behavior; females with higher T provisioned their young more often than females with lower T (Fig 1 & 3, Table 1. Overall model Adj. R2= 0.80, F4, 12= 12.27, P= 0.0028: provisioning rate, b= 3588, P= 0.0468). In the final model, year, mass, and handling time, were significant variables.
Figure 1.
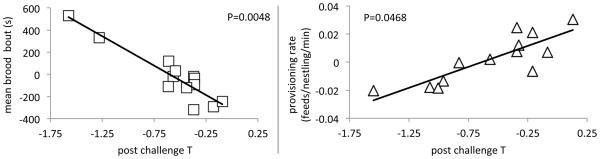
Scatter plots relating parental behavior (left: brooding behavior; right: provisioning behavior) to ability to produce T in response to GnRH challenge. Points in the scatter plots for T are leverage pairs, akin to partial correlation (see statistical methods), P values are from single effects in multiple regression.
Figure 3.

Network model illustrating the relationships between measures of hormonal phenotype and maternal behaviors. Each node represents a variable of interest, colored according to type of trait (hormone measures are blue, maternal effort is orange, egg metrics are yellow). Lines connecting nodes denote the nature of the relationship between the variables (solid lines are positive; dashed line is negative, faint lines indicate a trend that is not statistically significant). The length of the line is inversely related to the strength of the relationship; short lines are stronger. Notice tight clustering around post-challenge T as compared to initial T.
Table 1.
Final multiple regression models of the relationship between ability to produce testosterone in response to a GnRH challenge and measures of parental effort.
| Traits of interest | |||
|---|---|---|---|
|
| |||
| Full model results | Trait/Control variable | b (P) | |
| Post-challenge T | Adj. R2 = 0.80 F4, 12 = 12.27 P = 0.0028 |
provisioning rate | 3588 (0.0468) |
| Year | 1.73 (0.0021) | ||
| Mass | −0.51 (0.0023) | ||
| Handling time | −0.0005 (0.0012) | ||
|
| |||
| Post- challenge T | Adj. R2 = 0.98 F6, 12 = 33.03 P = 0.0007 |
mean brood bout | −0.002 (0.0011) |
| Date w/o year | −4.40e-7 (0.0061) | ||
| Year | 1.61 (0.0059) | ||
| Mass | −0.45 (0.0006) | ||
| Capture time | 4.04e-5 (0.0017) | ||
| Handling time | −0.002 (0.0048) | ||
Yolk T and Maternal T
Controlling for year, there was a positive correlation between yolk T and post-challenge T (Figure 2 & 3, Full model, F2, 47=3.20, Adj. R2= 0.13, P= 0.0324; post challenge T, P =0.0128; year, P=0.13). There was no detectable relationship between initial T and yolk T (P>0.25). Yolk mass and yolk T were not related (R2= 0.003, P= 0.88), and neither yolk mass nor yolk T differed between years (all P> 0.25). Yolk mass was unrelated to post-challenge T (P>0.50). However, there was a positive trend between yolk mass and initial T (Fig. 3, R2= 0.05, P=0.1244).
Discussion
We examined the relationships between endogenous T and three measures of maternal effort (provisioning, brooding and yolk T deposition) in females to determine whether endogenous T is potentially mediating female parental phenotypes and if so, whether the relationship in females is similar to that in males and previous experimental studies on females. We found that females capable of producing more T in response to a physiological challenge (injection of GnRH) spent less time brooding nestlings, congruent with predictions based on previous experimental studies. However, in contrast to experimental studies in both females and males, we found that high T females provisioned more frequently. Finally, we found that females producing more T in response to GnRH also deposited greater concentrations of T in the yolk.
Stronger relationships with post-challenge T
We found significant relationships between maternal effort and post-challenge T but not between behavior and initial T (Fig. 3). The lack of relationship between initial T and behavior is similar to other studies reporting no association between endogenous T and female behavior (DeVries and Jawor, 2013; Elekonich, 2000; Hau et al., 2004; Jawor et al., 2006a). This lack of association may be because circulating levels can rapidly change in response to uncontrollable sources of variation, thereby obscuring relationships, e.g. social interactions, time of day, etc. Consequently, the stronger relationships we observed with post-challenge T may have arisen because GnRH induced T, is less perturbable by the stimuli that may have influenced initial T, allowing us to detect patterns that would normally be obscured. Alternatively, the costs associated with high levels of circulating T (García-Vigón et al., 2008; Gerlach and Ketterson, 2013; Ketterson et al., 2005; O’Neal et al., 2008; Rosvall, 2013; Rutkowska et al., 2005; Veiga and Polo, 2008) may favor females that maintain circulating T at low levels, particularly once breeding has commenced. In support of this, research in juncos has shown that captive females did not elevate T after an aggressive interaction (Jawor et al., 2006a), circulating T measured immediately after a simulated intrusion during incubation was unrelated to aggression (Rosvall et al., 2012), and that incubating and brooding females do not respond to the GnRH challenge (Jawor et al., 2007).
However, females do increase T in response to GnRH before incubation begins, and individual ability to produce T during this period is related to fitness relevant behaviors weeks later (Cain & Ketterson 2012, this study). This suggests that individual GnRH responses during this critical period, when competitive ability may influence mate or territory selection, is likely to be an informative proxy for individual responsiveness to stimuli or sensitivity to steroids more generally, a possibility supported by several lines of research in juncos. Individual response to GnRH is repeatable in both sexes, and the source of individual variation appears to be individual differences in gonad function, suggesting that GnRH response is a property of the individual (Bergeon Burns & Rosvall, unpublished). Further, female aggression, which is positively related to post-challenge T (Cain and Ketterson, 2012), is also related to individual differences in the level of steroid receptor mRNA (Rosvall et al., 2012). Finally, it is important to note that in the final models examining these relationships, other factors were also significantly related to post-challenge T (see Table 1), as has been reported in previous work (Cain et al., 2013; Cain and Ketterson, 2012; Jawor et al., 2007; 2006a; McGlothlin et al., 2010; 2007). This suggests than individual responsiveness the GnRH may be a affected by downstream factors (Jawor et al., 2007; 2006a), which could also be important in regulating T and T-mediated phenotypes in females.
Testosterone & Maternal Care
Our finding that females capable of producing more T in response to the GnRH challenge also brood for less time is consistent with the generality that higher T levels are associated with reductions in parental care in both sexes (Ketterson et al., 2005; 1992; Rosvall, 2013; Stoehr and Hill, 2000; Trainor and Marler, 2001; Veiga and Polo, 2008; Wingfield et al., 2001). More specifically, it is congruent with previous work in juncos that found reduced brooding in females with T elevated experimentally (O’Neal et al., 2008). Together, these findings support the possibility that T may mediate tradeoffs between reproductive competition and maternal care, suggesting an important cost for high T in females. A recent examination of the costs of aggression in junco females found that more aggressive females, which tend to produce more T in response to GnRH (Cain and Ketterson, 2012), have hatchlings of lower mass than do less aggressive females (Cain and Ketterson, in press); mass is an important predictor of survival in songbird nestlings (Starck and Ricklefs, 1998). The negative relationship reported here between T and brooding may partially explain this pattern. Reduced brooding or incubation, may lead to slower developmental rates, forcing nestlings to devote more energy to thermoregulation and reducing the amount of energy available for growth (Ardia et al., 2010). Because only females brood in this species, reduced brooding may present an important cost for females with high T, and lead to selection favoring reduced T in females. If male and female T levels are genetically correlated, these costs could constrain male T (Ketterson et al., 2005; 2009).
In contrast to the typical pattern of negative relationships between parental care and testosterone, we found a positive relationship between provisioning and T. However, because the relationship between T and female maternal behavior is so rarely examined, it is difficult to say whether this relationship is unusual. In experimental studies, females with elevated T either show reduced maternal care relative to controls, or there is no effect, depending on the form of care and the species examined. In spotless starlings (Sturnus unicolor), T females showed delayed egg laying and reduced provisioning rates (Veiga and Polo, 2008); in tree swallows, T-females showed reduced incubation and hatching success (Rosvall, 2013); in juncos, T-females showed reduced brooding, nest defense, and nest success, but there was no effect on incubation, provisioning, nestling quality or extra-pair offspring production (Clotfelter et al., 2004; Gerlach et al., 2013; O’Neal et al., 2008). In contrast, though white-striped morph white-throated sparrow females (Zonotrichia albicollis) are more aggressive and engage less in maternal care relative to tan morphs, the morphs do not differ in circulating T levels (Spinney et al., 2006; Swett and Breuner, 2009). Our understanding of why within a species T affects some traits and not others, and why species differ in trait sensitivity, is currently quite limited. However, there is some indication that species’ trait sensitivity to T depends on female life histories and the relative importance of competition versus maternal care for those females (Rosvall, 2013).
Only one previous study has examined covariation between female T production ability (i.e. post-challenge T) and provisioning rates. That study addressed this question in female northern cardinals (Cardinalis cardinalis), and found no relationship between the two measures (DeVries and Jawor, 2013). The observed difference in the relationships between T and provisioning in cardinal and junco females likely stems from differences in female life histories as well as important differences in study methodology. The cardinal is a non-migratory resident that defends territories year-round (Halkin and Linville, 1999), while the junco migrates away from the breeding grounds and winters in flocks (Nolan et al., 2002). Further, in the cardinal study GnRH challenges were administered during the nestling period. However, cardinal females, like juncos females, did not elevate T in response to GnRH during the nestling period (DeVries and Jawor, 2013; Jawor et al., 2007). In contrast, we administered GnRH challenges in the pre-breeding season, during the period that female juncos exhibit the most robust response to a GnRH challenge (Jawor et al., 2007).
A final alternative explanation for the positive relationship between T and provisioning is that current definitions of maternal care, and of what makes a ‘good mother’, may be overly simplistic. While T does often lead to reductions in some forms of maternal care, high T levels may be important for other forms of maternal care, particularly when successful reproduction requires competition (Rosvall, 2013). In the junco, females capable of producing more T in the pre-breeding season may be more competitive, and acquire higher quality mates or territories than lower T females (Cain and Ketterson, 2012; Cain et al., 2011). As a result, though they brood less, they may be able to provision more and thus neutralize some negative effects of higher T (Cain & Ketterson, in press). Previous work in this population found that more aggressive females, which produce more T in response to GnRH, have greater nest success in some years (Cain & Ketterson, 2012; Cain & Ketterson, in press), suggesting that these high T females may actually be better mothers, if the metric used is greater offspring survival.
A similar pattern can be seen in other species. For example, in red squirrel females (Tamiasciurus hudsonicus), increased fecal androgens are associated with less time in the nest but more time devoted to territory defense and resource acquisition, which may increase juvenile survival and optimize female reproductive success (Dantzer et al., 2011). Experimentally elevating T in female tree swallows decreases incubation but increases aggression (Rosvall, 2013), which is important for acquiring a nest cavity, a necessary reproductive resource (Rosvall, 2008), and similar findings were reported in spotless starlings (Veiga and Polo, 2008). This pattern suggests that T may facilitate female ability to acquire resources important for indirect offspring care. This may generate tradeoffs with other forms of maternal care, but nevertheless may lead to improved reproductive success. Thus, caution is warranted when interpreting findings; a reduction in one form of maternal care or behavior may not inherently be poor mothering (Rosvall, 2013; Stiver and Alonzo, 2009).
Yolk Testosterone
In addition to maternal behavior, females can also affect offspring phenotype via the amount of steroid sequestered in the yolk (Gil, 2003; Groothuis et al., 2005; Schwabl, 1993). We found a positive relationship between T produced in response to a GnRH challenge and the amount of T deposited in yolk, replicating a finding from a previous study in juncos (Jawor et al., 2007), and in canaries (Serinus canaria) (Müller et al., 2011). In most species studied to date, yolk T functions as an anabolic steroid, stimulating growth and begging, although often at the cost of reduced immune function (Gil, 2003; Groothuis et al., 2005; Groothuis and Carere, 2005; Lipar and Ketterson, 2000; Schwabl, 1993); but see (Cox, 2005; Navara et al., 2005; Sockman and Schwabl, 2000). Exposure to T in the egg can also alter offspring ability to produce T later in life (Cain et al., 2013; Müller et al., 2011; Pfannkuche et al., 2011), and influence primary sex ratio (Correa et al., 2011; Rutkowska and Cichoń, 2006), suggesting another potential avenue by which females can influence offspring and grand-offspring fitness (Clutton-Brock et al., 1986; Trivers and Willard, 1973).
Previous studies on females in this population have found that aggressive females produce more T in response to GnRH (Cain and Ketterson, 2012), and in some years, produce smaller eggs and lighter hatchlings (Cain and Ketterson, in press). However, those nestlings gained mass faster than nestlings of less aggressive females, potentially neutralizing any negative effects that stem from the production of smaller eggs. The positive relationship between post-challenge T and yolk T suggests one way that nestlings of aggressive females may “catch-up”. If more aggressive females deposit more T in their eggs, this may stimulate greater growth. In tree swallows, higher aggressive interaction rates were positively correlated with yolk T (Whittingham and Schwabl, 2002), supporting this possibility. The faster growth rates of aggressive female nestlings may have also been further fueled by greater provisioning rates observed in high T females. Thus, individual variation in yolk T may be another way that females influence offspring quality and survival, outside of traditional measures of maternal care (Gil, 2003; Groothuis et al., 2005; Jawor et al., 2007; Ruuskanen et al., 2012). Another possibility is that high levels of yolk T may result in increased begging, motivating parents to provision more frequently. Thus, the positive relationship female T production and provisioning may be an indirect product of yolk T initiating behavioral differences in chicks, rather than a direct effect of T on female behavior. Differences in yolk T levels may also contribute to the year observed differences in nest success, e.g. females with high T levels may be more successful under competitive conditions, but not in conditions where resources are plentiful (Cain & Ketterson, in press). As the results reported here are correlative, determining which of these possibilities underlies the observed patterns in the junco will require further empirical study.
Conclusion
Steroid hormones play a key role in mediating life-history tradeoffs (Adkins-Regan, 2005; Ketterson and Nolan, 1999; Wingfield et al., 2001; Zera and Bottsford, 2001), however we know little about the tradeoff between competition and maternal effort in females. The physiological mechanisms underlying this tradeoff in females have rarely been examined directly, particularly in regards to endogenous steroid levels (DeVries and Jawor, 2013; Rosvall, 2013). The findings reported here support the possibility that T may play a similar role in females as has been reported in males, increasing competitive behavior at the expense of maternal effort. However, we also found unexpected positive relationships between some measures of maternal behavior and T. Together these results suggest that while T is likely important in maternal phenotypes and mediating tradeoffs, high transient levels of T are not necessarily as costly as previous work using experimental elevations of T might suggest (e.g. Gerlach et al., 2013, Veiga and Polo, 2008). These incongruent findings underscore the need for additional data on T in females, particularly on the relationships between endogenous T and behavior. Such data is essential if we are to further our understanding of how selection shapes T-mediated phenotypes in females in specific, and proximate regulation of life histories more generally.
Acknowledgments
Research was supported by National Science Foundation (NSF) grants to EK (BSC 05-19211 and IOS 08-20055) and an NSF Doctoral Dissertation Improvement Grant to KC (0910036). KC was also supported by an NSF Graduate Research Fellowships Program fellowship and National Institute of Health training grant, Common Themes in Reproductive Diversity (NIH no. HD 049336-04). This research adhered to the Association for the Study of Animal Behavior/Animal Behavior Society Guidelines for the Use of Animals in Research, the legal requirements of the United States of America (USFWS special use permit number MB093279-2, USGS banding permit number 20261), the states of Indiana and Virginia, and was conducted in compliance with the University of Virginia and Indiana University Institutional Animal Care and Use Committee (protocol #09-037). The authors thank S. Wanamaker and A. Dapper for assistance with data collection; R. Stewart for guidance with steroid assays; the Ketterson Lab, C. Ziegenfus, and the United Junco Workers for the field assistance; S. Pryke, K.A. Rosvall and D.M. O’Neal for helpful discussions; S. Hoobler for additional support; and Mountain Lake Biological Station (B. Brodie III, Director and E. Nagy, Associate Director) and Mountain Lake Hotel for permission to work on their property.
Literature Cited
- Adkins-Regan E. Hormones And Animal Social Behavior. Princeton University Press; Princeton: 2005. [Google Scholar]
- Ardia DR, Pérez JH, Clotfelter ED. Experimental cooling during incubation leads to reduced innate immunity and body condition in nestling tree swallows. Proc Biol Sci. 2010;277:1881–1888. doi: 10.1098/rspb.2009.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MBV, Nichols HJ, Gilchrist JS, Cant MA, Hodge SJ. The cost of dominance: suppressing subordinate reproduction affects the reproductive success of dominant female banded mongooses. Proc Biol Sci. 2011;279:619–624. doi: 10.1098/rspb.2011.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain KE, Burns CMB, Ketterson ED. Testosterone production, sexually dimorphic morphology, and digit ratio in the dark-eyed junco. Behav Ecol. 2013;24:462–469. [Google Scholar]
- Cain KE, Ketterson ED. Competitive females are successful females; phenotype, mechanism and selection in a common songbird. Behav Ecol Sociobiol. 2012;66:241–252. doi: 10.1007/s00265-011-1272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain KE, Ketterson ED. Costs and benefits of competitive traits in females; aggression, maternal care and reproductive success. PLOS ONE. doi: 10.1371/journal.pone.0077816. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain KE, Rich MS, Ainsworth K, Ketterson ED. Two sides of the same coin? Consistency in aggression to conspecifics and predators in a female songbird. Ethol. 2011;117:786–795. doi: 10.1111/j.1439-0310.2011.01932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clotfelter ED, O’Neal DM, Gaudioso JM, Casto JM, Parker-Renga IM, Snajdr EA, Duffy DL, Nolan V, Ketterson ED. Consequences of elevating plasma testosterone in females of a socially monogamous songbird: evidence of constraints on male evolution? Horm Behav. 2004;46:171–178. doi: 10.1016/j.yhbeh.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH. Sexual selection in females. Anim Behav. 2009;77:3–11. [Google Scholar]
- Clutton-Brock TH, Albon SD, Guinness FE. Great expectations: dominance, breeding success and offspring sex ratios in red deer. Anim Behav. 1986;34:460–471. [Google Scholar]
- Correa SM, Horan CM, Johnson PA, Adkins-Regan E. Copulatory behaviors and body condition predict post-mating female hormone concentrations, fertilization success, and primary sex ratios in Japanese quail. Horm Behav. 2011;59:556–564. doi: 10.1016/j.yhbeh.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Cox RM. Testosterone has opposite effects on male growth in lizards (Sceloporus spp) with opposite patterns of sexual size dimorphism. J Exp Biol. 2005;208:4679–4687. doi: 10.1242/jeb.01948. [DOI] [PubMed] [Google Scholar]
- Dantzer B, McAdam AG, Palme R, Humphries MM, Boutin S, Boonstra R. Maternal androgens and behaviour in free-ranging North American red squirrels. Anim Behav. 2011;81:469–479. [Google Scholar]
- Deviche P, Breuner C, Orchinik M. Testosterone, corticosterone, and photoperiod interact to regulate plasma levels of binding globulin and free steroid hormone in dark-eyed juncos, Junco hyemalis. Gen Comp Endocrinol. 2001;122:67–77. doi: 10.1006/gcen.2001.7613. [DOI] [PubMed] [Google Scholar]
- DeVries MS, Jawor JM. Natural variation in circulating testosterone does not predict nestling provisioning rates in the northern cardinal, Cardinalis cardinalis. Anim Behav 2013 [Google Scholar]
- Elekonich MM. Female song sparrow, Melospiza melodia, response to simulated conspecific and heterospecific intrusion across three seasons. Anim Behav. 2000;59:551–557. doi: 10.1006/anbe.1999.1369. [DOI] [PubMed] [Google Scholar]
- von Engelhardt NK, Groothuis TG. Measuring steroid hormones in avian eggs. Ann NY Acad Sci. 2005;1046:181–192. doi: 10.1196/annals.1343.015. [DOI] [PubMed] [Google Scholar]
- Fite JE, French JA, Patera KJ, Hopkins EC, Rukstalis M, Ross CN. Elevated urinary testosterone excretion and decreased maternal caregiving effort in marmosets when conception occurs during the period of infant dependence. Horm Behav. 2005;47:39–48. doi: 10.1016/j.yhbeh.2004.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick S, Berglund A, Rosenqvist G. Ornaments or offspring: costs to reproductive success restrict sexual selection processes. Biol J Linnean Soc. 1995;55:251–260. [Google Scholar]
- García-Vigón E, Cordero PJ, Veiga JP. Exogenous testosterone in female spotless starlings reduces their rate of extrapair offspring. Anim Behav. 2008;76:345–353. [Google Scholar]
- Gerlach NM, Ketterson ED. Experimental elevation of testosterone lowers fitness in female dark-eyed juncos. Horm Behav. 2013 doi: 10.1016/j.yhbeh.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Gil D. Golden eggs: maternal manipulation of offspring phenotype by egg androgen in birds. Ardeola. 2003;50:281–294. [Google Scholar]
- Groothuis TGG, Carere C. Avian personalities: characterization and epigenesis. Neurosci Biobehav Rev. 2005;29:137–150. doi: 10.1016/j.neubiorev.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Groothuis TGG, Müller W, von Engelhardt NK, Carere C, Eising C. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci Biobehav Rev. 2005;29:329–352. doi: 10.1016/j.neubiorev.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Groothuis TGG, Schwabl H. Hormone-mediated maternal effects in birds: mechanisms matter but what do we know of them? Phil Trans Roy Soc B. 2008;363:1647–1661. doi: 10.1098/rstb.2007.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkin SL, Linville SU. Northern Cardinal (Cardinalis cardinalis) Cornell Lab of Ornithology; Ithaca: 1999. [Google Scholar]
- Hau M, Stoddard ST, Soma KK. Territorial aggression and hormones during the non-breeding season in a tropical bird. Horm Behav. 2004;45:40–49. doi: 10.1016/j.yhbeh.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Holberton RL, Boswell T, Hunter MJ. Circulating prolactin and corticosterone concentrations during the development of migratory condition in the Dark-eyed Junco, Junco hyemalis. Gen Comp Endocrinol. 2008;155:641–649. doi: 10.1016/j.ygcen.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Huffman LS, Mitchell MM, O’Connell LA, Hofmann HA. Rising StARs: Behavioral, hormonal, and molecular responses to social challenge and opportunity. Horm Behav. 2012;61:631–641. doi: 10.1016/j.yhbeh.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Jawor JM, McGlothlin JW, Casto JM, Greives TJ, Snajdr EA, Bentley GE, Ketterson ED. Seasonal and individual variation in response to GnRH challenge in male dark-eyed juncos (Junco hyemalis) Gen Comp Endocrinol. 2006a;149:182–189. doi: 10.1016/j.ygcen.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Jawor JM, McGlothlin JW, Casto JM, Greives TJ, Snajdr EA, Bentley GE, Ketterson ED. Testosterone response to GnRH in a female songbird varies with stage of reproduction: implications for adult behaviour and maternal effects. Funct Ecol. 2007;21:767–775. [Google Scholar]
- Jawor JM, Young R, Ketterson ED. Females competing to reproduce: Dominance matters but testosterone may not. Horm Behav. 2006b;49:362–368. doi: 10.1016/j.yhbeh.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Ketterson ED, Atwell JW, McGlothlin JW. Phenotypic integration and independence: Hormones, performance, and response to environmental change. Integrative and Comparative Biology. 2009;49:365–379. doi: 10.1093/icb/icp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V. Adaptation, exaptation, and constraint: a hormonal perspective. Am Nat. 1999;154:S4–S25. doi: 10.1086/303280. [DOI] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V, Sandell M. Testosterone in females: mediator of adaptive traits, constraint on sexual dimorphism, or both? Am Nat. 2005;166:S85–S98. doi: 10.1086/444602. [DOI] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V, Wolf L, Ziegenfus C. Testosterone and avian life histories: effects of experimentally elevated testosterone on behavior and correlates of fitness in the dark-eyed junco (Junco hyemalis) Am Nat. 1992:980–999. doi: 10.1016/0018-506x(91)90016-b. [DOI] [PubMed] [Google Scholar]
- Kozlowski CP, Bauman JE, Caldwell Hahn D. A simplified method for extracting androgens from avian egg yolks. Zoo Biol. 2009;28:137–143. doi: 10.1002/zoo.20221. [DOI] [PubMed] [Google Scholar]
- Langmore NE. Functions of duet and solo songs of female birds. Trends Ecol Evol. 1998;13:136–140. doi: 10.1016/s0169-5347(97)01241-x. [DOI] [PubMed] [Google Scholar]
- LeBas NR. Female finery is not for males. Trends Ecol Evol. 2006;21:170–173. doi: 10.1016/j.tree.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Lessells CM. Neuroendocrine control of life histories: what do we need to know to understand the evolution of phenotypic plasticity? Phil Trans Roy Soc B. 2008;363:1589–1598. doi: 10.1098/rstb.2007.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipar JL, Ketterson ED. Maternally derived yolk testosterone enhances the development of the hatching muscle in the red-winged blackbird Agelaius phoeniceus. Proc Biol Sci. 2000;267:2005–2010. doi: 10.1098/rspb.2000.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn SE, Hayward LS, Benowitz-Fredericks ZM, Wingfield JC. Behavioural insensitivity to supplementary testosterone during the parental phase in the chestnut-collared longspur, Calcarius ornatus. Anim Behav. 2002;63:795–803. [Google Scholar]
- Magrath MJL, Komdeur J. Is male care compromised by additional mating opportunity? Trends Ecol Evol. 2003;18:424–430. [Google Scholar]
- McGlothlin JW, Jawor JM, Greives TJ, Casto JM, Phillips JL, Ketterson ED. Hormones and honest signals: males with larger ornaments elevate testosterone more when challenged. J Evol Biol. 2008;21:39–48. doi: 10.1111/j.1420-9101.2007.01471.x. [DOI] [PubMed] [Google Scholar]
- McGlothlin JW, Jawor JM, Ketterson ED. Natural variation in a testosterone-mediated trade-off between mating effort and parental effort. Am Nat. 2007;170:864–875. doi: 10.1086/522838. [DOI] [PubMed] [Google Scholar]
- McGlothlin JW, Whittaker DJ, Schrock SE, Gerlach NM, Jawor JM, Snajdr EA, Ketterson ED. Natural selection on testosterone production in a wild songbird population. Am Nat. 2010;175:687–701. doi: 10.1086/652469. [DOI] [PubMed] [Google Scholar]
- Moore IT, Perfito N, Wada H, Sperry TS, Wingfield JC. Latitudinal variation in plasma testosterone levels in birds of the genus Zonotrichia. Gen Comp Endocrinol. 2002;129:13–19. doi: 10.1016/s0016-6480(02)00563-4. [DOI] [PubMed] [Google Scholar]
- Müller W, Groothuis TGG, Goerlich VC, Eens M. GnRH - A missing link between testosterone concentrations in yolk and plasma and its intergenerational effects. PLoS ONE. 2011;6:e22675. doi: 10.1371/journal.pone.0022675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navara KJ, Hill GE, Mendonca MT. Variable effects of yolk androgens on growth, survival, and immunity in eastern bluebird nestlings. Physiol Biochem Zool. 2005;78:570–578. doi: 10.1086/430689. [DOI] [PubMed] [Google Scholar]
- Nolan V, Ketterson ED, Cristol DA, Rogers CM, Clotfelter ED, Schoech SJ, RCT, Snajdr EA. Dark-eyed Junco. Cornell Lab of Ornithology; Philadelphia: 2002. [Google Scholar]
- Nordeide JT, Rudolfsen G, Egeland ES. Ornaments or offspring? Female sticklebacks (Gasterosteus aculeatus L.) trade off carotenoids between spines and eggs. J Evol Biol. 2006;19:431–439. doi: 10.1111/j.1420-9101.2005.01018.x. [DOI] [PubMed] [Google Scholar]
- O’Neal DM, Reichard DG, Pavilis K, Ketterson ED. Experimentally-elevated testosterone, female parental care, and reproductive success in a songbird, the Dark-eyed Junco (Junco hyemalis) Horm Behav. 2008;54:571–578. doi: 10.1016/j.yhbeh.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Packer C, Collins DA, Sindimwo A, Goodall J. Reproductive constraints on aggressive competition in female baboons. Nature. 1995;373:60–63. doi: 10.1038/373060a0. [DOI] [PubMed] [Google Scholar]
- Pfannkuche KA, Gahr M, Weites IM, Riedstra B, Wolf C, Groothuis TGG. Examining a pathway for hormone mediated maternal effects--yolk testosterone affects androgen receptor expression and endogenous testosterone production in young chicks (Gallus gallus domesticus) Gen Comp Endocrinol. 2011;172:487–493. doi: 10.1016/j.ygcen.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Raouf SA, Parker PG, Ketterson ED, Nolan V, Ziegenfus C. Testosterone affects reproductive success by influencing extra–pair fertilizations in male dark–eyed juncos (Aves: Junco hyemalis) Proc Biol Sci. 1997;264:1599–1603. [Google Scholar]
- Roff DA, Mostowy S, Fairbairn DJ. The evolution of trade-offs: testing predictions on response to selection and environmental variation. Evol. 2002;56:84–95. doi: 10.1111/j.0014-3820.2002.tb00851.x. [DOI] [PubMed] [Google Scholar]
- Rosvall KA. Sexual selection on aggressiveness in females: evidence from an experimental test with tree swallows. Anim Behav. 2008;75:1603–1610. [Google Scholar]
- Rosvall KA. Intrasexual competition in females: evidence for sexual selection? Behav Ecol. 2011;22:1131–1140. doi: 10.1093/beheco/arr106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvall KA. Life history trade-offs and behavioral sensitivity to testosterone: An experimental test when female aggression and maternal care co-occur. PLoS ONE. 2013;8:e54120. doi: 10.1371/journal.pone.0054120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvall KA, Burns CMB, Barske J, Goodson JL, Schlinger BA, Sengelaub DR, Ketterson ED. Neural sensitivity to sex steroids predicts individual differences in aggression: implications for behavioural evolution. Proc Biol Sci. 2012;279:3547–3555. doi: 10.1098/rspb.2012.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowska J, Cichoń M. Maternal testosterone affects the primary sex ratio and offspring survival in zebra finches. Anim Behav. 2006;71:1283–1288. [Google Scholar]
- Rutkowska J, Cichoń M, Puerta M, Gil D. Negative effects of elevated testosterone on female fecundity in zebra finches. Horm Behav. 2005;47:585–591. doi: 10.1016/j.yhbeh.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Ruuskanen S, Doligez B, Gustafsson L, Laaksonen T. Long-term effects of yolk androgens on phenotype and parental feeding behavior in a wild passerine. Behav Ecol Sociobiol. 2012;66:1201–1211. [Google Scholar]
- Sall J. Leverage plots for general linear hypotheses. Am Stat. 1990;44:308–315. [Google Scholar]
- Sandell MI. Exogenous testosterone increases female aggression in the European starling (Sturnus vulgaris) Behav Ecol Sociobiol. 2007;62:255–262. [Google Scholar]
- Schwabl H. Yolk is a source of maternal testosterone for developing birds. Proc Natl Acad Sci USA. 1993;90:11446–11450. doi: 10.1073/pnas.90.24.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster SM, Wade MJ. Mating Systems and Strategies: (Monographs in Behavior and Ecology) Princeton University Press; Princeton: 2003. [Google Scholar]
- Sockman KW, Schwabl H. Yolk androgens reduce offspring survival. Proc Biol Sci. 2000;267:1451–1456. doi: 10.1098/rspb.2000.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinney LH, Bentley GE, Hau M. Endocrine correlates of alternative phenotypes in the white-throated sparrow (Zonotrichia albicollis) Horm Behav. 2006;50:762–771. doi: 10.1016/j.yhbeh.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Starck JM, Ricklefs RE. Avian Growth and Development. Oxford University Press on Demand; 1998. [Google Scholar]
- Staub NL, De Beer M. The role of androgens in female vertebrates. Gen Comp Endocrinol. 1997;108:1–24. doi: 10.1006/gcen.1997.6962. [DOI] [PubMed] [Google Scholar]
- Stearns SC. The Evolution of Life Histories. Oxford University Press on Demand; 1992. [Google Scholar]
- Stiver KA, Alonzo SH. Parental and mating effort: Is there necessarily a trade-off? Ethol. 2009;115:1101–1126. [Google Scholar]
- Stockley P, Bro-Jørgensen J. Female competition and its evolutionary consequences in mammals. Biol Rev. 2011;86:341–366. doi: 10.1111/j.1469-185X.2010.00149.x. [DOI] [PubMed] [Google Scholar]
- Stoehr AM, Hill GE. Testosterone and the allocation of reproductive effort in male house finches (Carpodacus mexicanus) Behav Ecol Sociobiol. 2000;48:407–411. [Google Scholar]
- Swett MB, Breuner CW. Plasma testosterone correlates with morph type across breeding substages in male white-throated Sparrows. Physiol Biochem Zool. 2009;82:572–579. doi: 10.1086/605392. [DOI] [PubMed] [Google Scholar]
- Tobias JA, Montgomerie RD, Lyon BE. The evolution of female ornaments and weaponry: social selection, sexual selection and ecological competition. Phil Trans Roy Soc B. 2012;367:2274–2293. doi: 10.1098/rstb.2011.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Marler CA. Testosterone, paternal behavior, and aggression in the monogamous California mouse (Peromyscus californicus) Horm Behav. 2001;40:32–42. doi: 10.1006/hbeh.2001.1652. [DOI] [PubMed] [Google Scholar]
- Trivers R. Parental Investment and Sexual Selection. In: Campbell B, editor. Sexual Selection and the Descent of Man 1871 – 1971. Aldine; Chicago: 1972. pp. 136–179. [Google Scholar]
- Trivers RL, Willard DE. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- Veiga JP, Polo V. Fitness consequences of increased testosterone levels in female spotless starlings. Am Nat. 2008;172:42–53. doi: 10.1086/587850. [DOI] [PubMed] [Google Scholar]
- Veiga JP, Viñuela J, Cordero PJ, Aparicio JM, Polo V. Experimentally increased testosterone affects social rank and primary sex ratio in the spotless starling. Horm Behav. 2004;46:47–53. doi: 10.1016/j.yhbeh.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Whittingham LA, Schwabl H. Maternal testosterone in tree swallow eggs varies with female aggression. Anim Behav. 2002;63:63–67. [Google Scholar]
- Williams TD. Individual variation in endocrine systems: moving beyond the “tyranny of the Golden Mean. Phil Trans Roy Soc B. 2008;363:1687–1698. doi: 10.1098/rstb.2007.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TD. Hormones, life-history, and phenotypic variation: Opportunities in evolutionary avian endocrinology. Gen Comp Endocrinol. 2012;176:286–295. doi: 10.1016/j.ygcen.2011.11.028. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Hegner RE, Lewis DM. Circulating levels of luteinizing hormone and steroid hormones in relation to social status in the cooperatively breeding white-browed sparrow weaver, Plocepasser mahali. J Zool. 1991;225:43–58. [Google Scholar]
- Wingfield JC, Lynn S, Soma KK. Avoiding the “costs” of testosterone: ecological bases of hormone-behavior interactions. Brain Behav Evolut. 2001;57:239–251. doi: 10.1159/000047243. [DOI] [PubMed] [Google Scholar]
- Zera AJA, Bottsford JJ. The endocrine-genetic basis of life-history variation: the relationship between the ecdysteroid titer and morph-specific reproduction in the wing-polymorphic cricket Gryllus firmus. Evolution. 2001;55:538–549. doi: 10.1554/0014-3820(2001)055[0538:tegbol]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Zysling DA, Greives TJ, Breuner CW, Casto JM, Demas GE, Ketterson ED. Behavioral and physiological responses to experimentally elevated testosterone in female dark-eyed juncos (Junco hyemalis carolinensis) Horm Behav. 2006;50:200–207. doi: 10.1016/j.yhbeh.2006.03.004. [DOI] [PubMed] [Google Scholar]



