Abstract
Emerging evidence implicates the zinc importer ZIP4 as a critical factor that enhances pancreatic cancer proliferation; however, the role of ZIP4 in promoting pancreatic cancer progression by regulating apoptosis requires elucidation. To determine the effect of ZIP4 on apoptosis, we used cell lines where ZIP4 levels were upregulated or silenced in combination with Chelex 100 treatment to deplete intracellular zinc. Pancreatic cancer xenografts derived from those cells were also included. TUNEL and flow cytometry analysis were used to measure apoptosis and western blotting was used to analyze protein expression for PARP and multiple caspases. Cell cycle profiles were examined by flow cytometry. Zinc depletion by Chelex induced more apoptosis of pancreatic cancer cells in comparison to normal medium, where almost no apoptosis was observed. ZIP4 stably overexpressed MIA PaCa-2 (MIA-ZIP4) cells were more resistant to zinc depletion-induced apoptosis compared with vector control. Conversely, AsPC-1 (AsPC-shZIP4) cells with stable knockdown of ZIP4 were more sensitive to zinc deficiency than control. Resistance to apoptosis mediated by ZIP4 was accomplished by the caspase pathway. In vivo data also confirmed that ZIP4 overexpressed xenografts showed less apoptosis than controls. Cell cycle profiles indicate that silencing of ZIP4 leads to decreased cell population in S phase and G0/G1 arrest. These results described a previously uncharacterized role of ZIP4 in apoptosis resistance and elucidated a novel pathway through which ZIP4 regulates pancreatic cancer growth. This research provides additional evidence for ZIP4 and related signaling cascade as a molecular target for therapeutic intervention in pancreatic cancer.
Keywords: ZIP4, apoptosis, pancreatic cancer, cell cycle, caspase
Introduction
Pancreatic cancer is the fourth leading cause of solid cancer deaths with an overall 5-year survival rate of approximately 6%.1 The mortality associated with pancreatic cancer is due to inability to detect at early stage, high recurrence rate, resistance to chemotherapy and radiotherapy, and, lastly, the disease being typically metastatic at diagnosis.2 Therefore, great pressure still exists in understanding the biology and finding more effective treatments for this devastating disease.
Normally, cell growth is controlled through the balance between cell proliferation and programmed cell death, whereas cancer cells deregulate both by promoting cell proliferation and inhibiting apoptosis. The mechanism of pancreatic cancer cell proliferation, apoptosis, and cell cycle progression still requires further study and is a prerequisite for the development of early detection techniques and better treatments. A previous study has indicated that high-meat diet, especially red meat and double-cooked meat, which led to excessive zinc intake to human body, was regarded as a pivotal risk factor of pancreatic cancer development.3 And the essential trace element zinc plays an important role in regulating cancer cell growth.4 Zinc deficiency can induce apoptosis, growth retardation, and impair DNA synthesis.5,6 Conversely, overly high zinc concentration is toxic. Therefore, intracellular zinc concentrations are maintained within a fine range to preserve normal cell physiology.7,8 This balance is achieved by the antagonistic actions of zinc transporters, ZIPs and ZnTs, which are responsible for zinc import and export, respectively.8,9
Recent studies have shown that zinc transporters play important roles in cancer development. Downregulation of ZnT1, which results in low zinc efflux, has been observed in mammary gland tumor cells.10 ZIP6 is reportedly associated with estrogen-positive breast cancer development and metastasis.11 Similarly, ZIP10 was involved in the invasive behavior of breast cancer cells.12 These studies all suggest a positive correlation of excess intracellular zinc with cancer progression through either overexpression of a cellular zinc importer or loss of a zinc exporter.
Our previous studies indicate that the zinc importer ZIP4 promotes pancreatic cancer cell proliferation.13,14 ZIP4, encoded by SLC39A4, is responsible for dietary zinc uptake and maintains intracellular zinc level. Mutation of ZIP4 causes a genetic disorder of zinc deficiency acrodermatitis enteropathica (AE).15 Up until now, hepatocellular carcinoma and high-grade glioma have been reported to be associated with ZIP4 overexpression.16,17 However, whether ZIP4 can influence pancreatic cancer cell apoptosis and cell cycle remains unknown. To elucidate how ZIP4 may regulate pancreatic cancer cell progression, we investigated the apoptotic cell populations in pancreatic cancer cells and xenografts with overexpressed or silenced ZIP4, as well as the cell cycle progression in those cells. This study implicates an important apoptosis signaling pathway through which ZIP4 regulates pancreatic cancer growth.
Results
Overexpression of ZIP4 confers resistance to apoptosis in pancreatic cancer cells
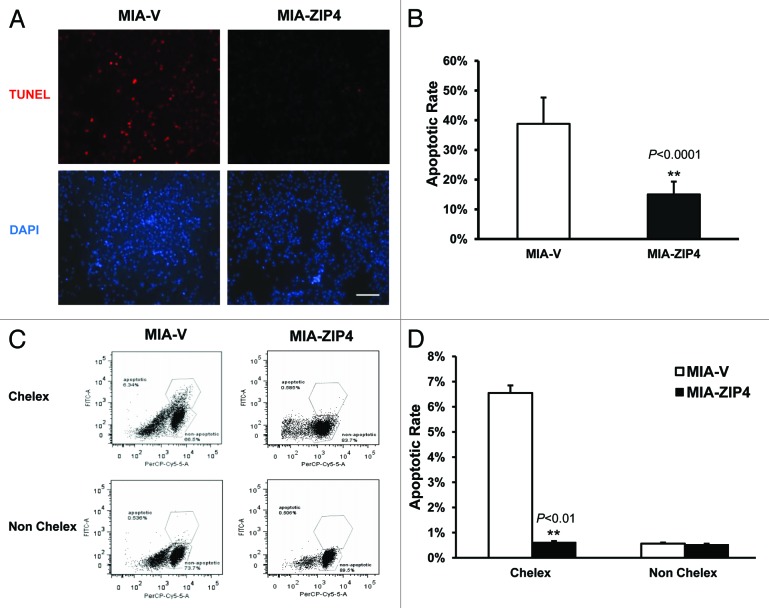
Our previous data have shown that ZIP4 promotes pancreatic cancer cell proliferation. Cell growth is controlled through the balance between cell proliferation and apoptosis; therefore, to further elucidate the impact of ZIP4 in pancreatic cancer cell growth, we examined the apoptosis in MIA PaCa-2 cells, which stably overexpressed ZIP4 (MIA-ZIP4) along with its vector control MIA-V cells. After serum starvation, the cells were released and incubated for 48 h in DMEM medium with 2% FBS with or without Chelex 100 pre-treatment, which serves to chelate and deplete zinc ions from the culture medium. Subsequent TUNEL assay showed that no apoptotic signal was detected for both cells that did not receive the Chelex treatment (Fig. S1); however, in Chelex-treated cells, 38.7% MIA-V control cells had undergone apoptosis, whereas only 15.0% MIA-ZIP4 cells showed apoptosis (P < 0.0001, Fig. 1A and B), indicating that ZIP4 overexpression confers resistance to apoptosis in pancreatic cancer cells under zinc-deficient conditions. To further validate this result, flow cytometry analysis was performed on those 2 MIA PaCa-2 cells where ZIP4 levels were modulated with and without Chelex pre-treatment (Fig. 1C and D). Under the Chelex pretreatment condition, the apoptotic cells in MIA-V and MIA-ZIP4 groups were 6.34%, and 0.585%, respectively (P < 0.01); however, without Chelex, apoptotic cells were only 0.536% and 0.506%, respectively. These results in combination with previously published data indicate that ZIP4 plays a critical role in regulating both cell proliferation and zinc deficiency-induced apoptosis in pancreatic cancer cells.
Figure 1.
ZIP4 confers resistance to zinc deficiency-induced apoptosis in MIA PaCa-2 cells. (A) MIA PaCa-2 cells were treated with 2% FBS Chelex media for 48 h; washed twice in warm PBS, then prepared for 3′-biotinylation of fragmented DNA using the TUNEL assay procedure. Individual fields of cells were captured for both DNA 3′-end labeling (red) or total cellular DNA (blue). Representative images of 3 independent experiments were shown. Scale bar represents 500 µm. (B) Quantitative data of panel (A) **P < 0.0001. (C) MIA PaCa-2 cells were treated with 2% FBS Chelex media and non-Chelex media for 48 h; prepared for 3′-biotinylation of fragmented DNA using the TUNEL assay procedure; then fluorescence was acquired by BD flow cytometer. Representative histograms of 3 independent experiments were shown. Scale bar represents 500 µm. (D) Quantitative data of flow cytometry analysis. **P < 0.01.
Silencing of ZIP4 induces apoptosis in pancreatic cancer cells
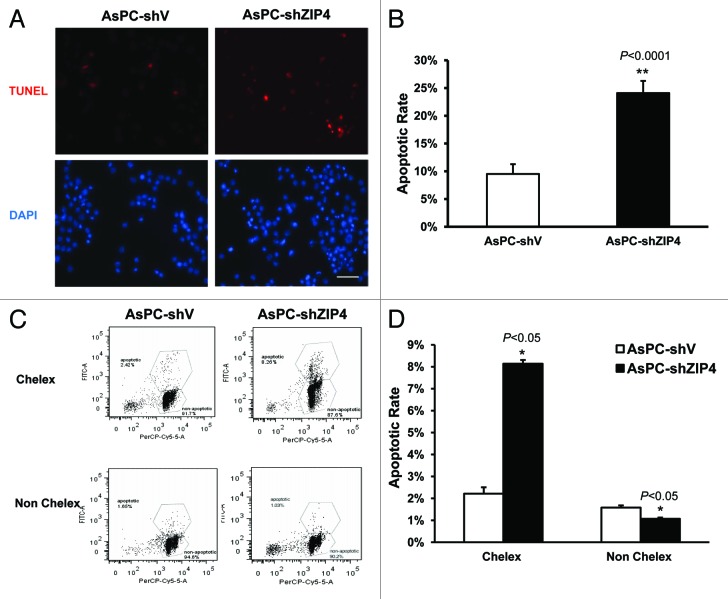
To further confirm ZIP4-mediated apoptosis resistance, we performed similar experiment in a second pancreatic cancer cell line AsPC-1 cells. Specifically, we compared apoptosis in stable ZIP4 knockdown cells (AsPC-shZIP4) and the corresponding vector control (AsPC-shV) cells. Similar to the MIA PaCa-2 cells, little to no apoptosis was observed in AsPC-shV and AsPC-shZIP4 cells in normal (zinc-adequate) media (Fig. S1), but under zinc-deficient conditions, apoptosis was detected by TUNEL assay in 24.1% AsPC-shZIP4 cells, but only in 9.5% AsPC-shV cells (Fig. 2A and B, P < 0.0001). As shown in Figure 2C and D, the flow cytometry data also confirmed these results, implicating ZIP4 as a key factor conferring resistance to apoptosis, and knocking down of ZIP4 sensitizes pancreatic cancer cells to zinc deficiency-induced apoptosis.
Figure 2.
Silencing of ZIP4 induces apoptosis in pancreatic cancer cells. (A) AsPC-1 cells were treated with 2% FBS Chelex media for 48 h, washed twice in warm PBS, then prepared for 3′-biotinylation of fragmented DNA using the TUNEL assay procedure. Individual fields of cells were captured for both DNA 3′-end labeling (red) or total cellular DNA (blue). Representative images of 3 independent experiments were shown. Scale bar represents 500 µm. (B) Quantitative data of panel (A) **P < 0.0001. (C) AsPC-1 cells were treated with 2% FBS Chelex media and non-Chelex media for 48 h; prepared for 3′-biotinylation of fragmented DNA using the TUNEL assay procedure; then fluorescence was acquired by BD flow cytometer. Representative histograms of 3 independent experiments were shown. (D) Quantitative data of flow cytometry analysis. *P < 0.05.
ZIP4 regulates apoptosis through the cleavage of caspases in pancreatic cancer cells
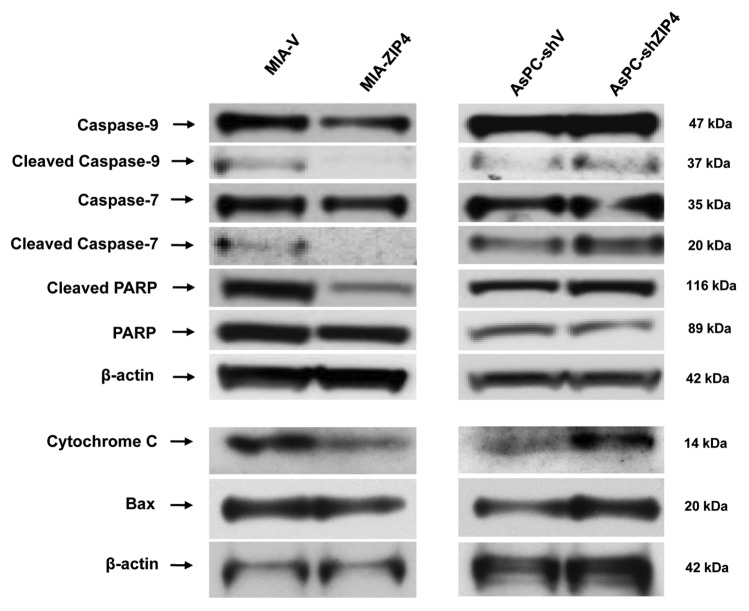
To further elucidate the molecular mechanism of ZIP4-mediated resistance to apoptosis, we examined the expression of several key caspases implicated in apoptosis (pro-protein and cleavage products). As shown in Figure 3, under the Chelex pre-treatment condition, overexpression of ZIP4 led to substantial decrease of cleaved caspase-9 and caspase-7. As anticipated, the reduction in cleaved caspases was mirrored by the decrease in PARP cleavage. In AsPC-1 cells, ZIP4 knockdown caused a dramatic increase in the cleavage of caspase-9, caspase-7, and PARP, indicating ZIP4 as an apoptotic regulator through the endogenous caspase pathway in pancreatic cancer cells under zinc-deficiency conditions. In order to elucidate how caspase-9 was activated, we examined the expression of cytochrome C and Bcl-2 family members Bax and Bak. We found that cytochrome C and Bax were downregulated when ZIP4 was overexpressed, and were upregulated when ZIP4 was silenced (Fig. 3), while there was no difference in Bak expression, indicating that caspase-9 might be activated through cytochrome C and Bax pathway.
Figure 3.
ZIP4 regulates cell apoptosis through caspase pathway. The expression of caspase-9, caspase-7, PARP, cytochrome C, and Bax was validated by western blotting in MIA-ZIP4 and AsPC-shZIP4 cells.
ZIP4 confers apoptosis resistance in mouse models with pancreatic cancer xenografts
To validate the ZIP4-induced cell resistance to apoptosis in vivo, we determined the apoptosis in subcutaneous xenografts of nude mice that were implanted with MIA-ZIP4 and control MIA-V cells. As shown in Figure 4A and B, 4.2% of control MIA-V cells showed apoptosis signaling, whereas only 1.3% of MIA-ZIP4 cells were apoptotic (P = 0.04). These data indicate that ZIP4 confers apoptosis resistance not only in pancreatic cancer cells, but also in mouse models with pancreatic cancer xenografts. Further studies are warranted to investigate whether silencing of ZIP4 could sensitize pancreatic cancer to apoptosis in more pancreatic cancer mouse models, including both xenografts and spontaneous mouse models.
Figure 4.
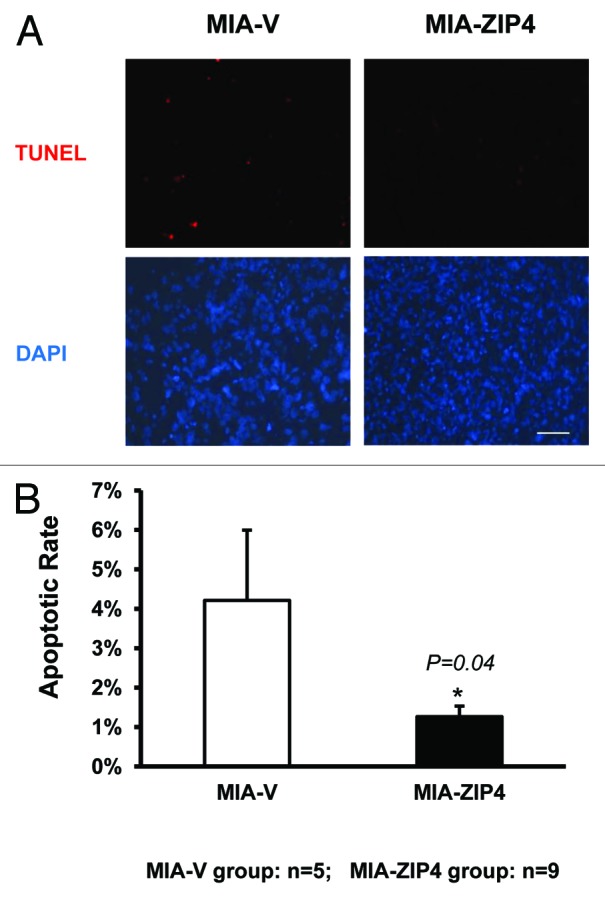
ZIP4 confers resistance to apoptosis in MIA PaCa-2 subcutaneous mouse xenografts. (A) Tissues were collected and processed into 5-μm thick slides. TUNEL assay was done according to the standard procedure. Individual fields of cells were captured for both DNA 3′-end labeling (red) or total cellular DNA (blue). Representative images from each group were shown. Scale bar represents 500 μm. (B) Quantitative data of (A) *P = 0.04.
Silencing of ZIP4 induces cell cycle arrest in pancreatic cancer cells under zinc-deficiency conditions
To further assess the impact of ZIP4 on pancreatic cancer cell growth and apoptosis, we examined the cell cycle progression in pancreatic cancer cell lines with ZIP4 level modulated. Cells were starved in serum-free medium and then released in 2% FBS medium or 2% FBS medium pretreated with Chelex-100. Cells were then harvested 2 h later, stained with propidium iodide, and subjected to flow cytometry analysis. We found that under zinc-deficiency conditions, silencing of ZIP4 induced a G0/G1-phase arrest in AsPC-shZIP4 cells, indicated by accumulation of cells at this stage, with a corresponding decline of cell population in the S phase. In AsPC-shV cells pretreated with Chelex, 40.8% of cells were in G0/G1 phase, and 12.9% were in S phase. On the contrary, 47.9% of AsPC-shZIP4 cells were in G0/G1 phase, and only 8.1% of cells entered S phase. That is 17.4% increased cell population arrested at G0/G1 phase, and 37.2% decreased cells entering S phase, respectively (Fig. 5). Thus, silencing of ZIP4 in pancreatic cancer cells is associated with cell cycle arrest at G0/G1 phase. In a separate experiment, when ZIP4 is overexpressed in MIA PaCa-2 cells, under zinc-deficiency conditions, there is a significant increase of S-phase cells in MIA-ZIP4 cells compared with that in MIA-V cells (data not shown). These results demonstrate that ZIP4 plays an important role in pancreatic cancer cell growth, at least partially through cell cycle control.
Figure 5.
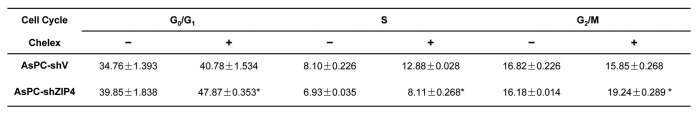
Silencing of ZIP4 induces cell cycle arrest in pancreatic cancer cells under zinc-deficiency condition. Cell cycle was analyzed by flow cytometry. Cells were starved for 48 h and then released by incubation with 2% RPMI media for 2 h. Then cells were stained with PI (BD Bioscience) before acquisition by a flow cytometer. The percentage of cells in G0/G1, S, and G2/M phases were measured and shown as mean ± SD of triplicate values. *P < 0.05.
Discussion
Our previous studies suggest that the zinc importer ZIP4 promotes pancreatic cancer cell proliferation. However, whether ZIP4 can influence pancreatic cancer cell apoptosis and cell cycle progression remains unknown. Our current study indicates that overexpression of ZIP4 confers resistance to apoptosis in pancreatic cancer, at least in part through the caspase pathway. Silencing of ZIP4 induces apoptosis and cell cycle arrest in pancreatic cancer cells under zinc-deficiency conditions. This study implicates an important apoptosis signaling pathway, through which ZIP4 regulates pancreatic cancer growth, and suggests a novel target for pancreatic cancer therapy.
Zinc is a crucial trace element and serves as a required cofactor in many metalloenzymes and transcription factors.13,18 Zinc is involved in pancreatic physiological processes such as glucagon metabolism, production of digestive enzymes, and insulin secretion. Moreover, disruption of zinc homeostasis is also linked to pancreatic cancer.13,19 Zinc deficiency causes apoptosis in multiple types of normal cells and tissues in animals, and also leads to apoptosis in cancer cells upon zinc depletion.20,21 Apoptosis plays an important role in development,22 and defects in apoptosis leads to various human disorders, including cancer.23 Pancreatic cancer ranks among one of the most lethal cancers, where defective apoptosis contributes to tumor progression and resistance to chemotherapy.24
Zinc transporters are surface proteins responsible for zinc uptake, efflux, and intracellular storage.8 Previous studies have indicated that several zinc transporters play important roles in the regulation of cell apoptosis. ZIP6 was reported to inhibit apoptosis induced by histone deacetylase inhibitors in cancer.25 ZnT2 has also been hypothesized to be involved in the regulation of apoptosis related with zinc. In islets and IL-1 cells (β-cell), cells overexpressing ZnT8 were more sensitive to IL-1β-induced apoptosis.26 Cao et al.27 found that the expression of ZIP2 in monocytes was altered upon zinc depletion, and that apoptosis is concurrent with these changes, which indicates that ZIP2 is involved in the regulation of apoptosis. ZIP4 is a key zinc transporter in pancreatic cancer cells, and previous research has focused primarily on its role in cell proliferation and tumor growth.13,14,28 To date, no studies have been done to investigate the impact of ZIP4 on apoptosis, especially in pancreatic cancer. Our study addresses this fundamental gap in the understanding on ZIP4 and pancreatic cancer, and shows that ZIP4 confers resistance to zinc deficiency-induced apoptosis in pancreatic cancer both in cell lines and in xenograft tumors.
Although various pathways contribute to the initiation of apoptosis, most frequently apoptosis occurs via caspase activation, a common biomarker for programmed cell death.29,30 This study demonstrates that ZIP4 confers resistance to low zinc-induced apoptosis through caspase-9/caspase-7/PARP cascade. Caspase-9 is the upstream initiator of the intrinsic apoptosis pathway, which induces the activation of downstream caspase signaling cascades.22 We hypothesize that in zinc-deficiency conditions, ZIP4 overexpression inhibits caspase-9 and caspase-7 cleavage and blocks the apoptosis signal transduction by further inhibiting PARP cleavage, thereby preventing apoptosis. Cytochrome C was regarded as the activator of caspase-9, and Bax and Bak promote apoptosis via the mitochondrial and endoplasmic reticulum pathway.31 Our results showed that ZIP4-induced resistance to apoptosis in pancreatic cancer cells was mediated, at least in part, by cytochrome C and Bax. Other than intrinsic signaling pathways initiated by caspase-9, other key players in apoptosis signaling such as the TNF pathway,32 which was initiated by caspase-8, and caspase-independent pathway, which was mediated by AIF (apoptosis-inducing factor),33 may also participate in ZIP4-mediated resistance to apoptosis. Furthermore, we found that ZIP4 also regulates cell cycle progression in pancreatic cancer. Under zinc-deficiency conditions, silencing of ZIP4 induced a G0/G1 arrest, with a corresponding decline of cell population in the S phase. This may help to explain our previous finding on how ZIP4 promotes pancreatic cancer proliferation. In summary, our study suggests that ZIP4 promotes pancreatic cancer growth through controlling cell cycle and modulating cancer cell apoptosis. These findings provide new insights on ZIP4 function in pancreatic cancer and highlight the significance of ZIP4 as a potential molecular marker and therapeutic target in pancreatic cancer treatment.
Materials and Methods
Cell culture
Human pancreatic cancer cells MIA PaCa-2 and AsPC-1 cells were purchased from American Type Culture Collection (ATCC) in 2006, and were authenticated by DNA finger printing in April 2009. ZIP4-overexpressing and shRNA-silenced stable cell lines were selected in MIA PaCa-2 and AsPC-1 cells with retrovirus vectors (Origene) and propagated as previously described.13,14
Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay
200 000 cells/well were plated in 6-well plates and treated with 2% FBS Chelex medium (Chelex 100; Sigma C7901) or regular 2% FBS medium for 24 h when cells reached 65% confluence. The procedures were done according to the standard protocol (Roche In Situ Cell Death Detection Kit, Roche Applied Science). Cells were counterstained with Hoechst 33258 solution (2 μg/ml) for 30 min at room temperature, then covered with aqueous mounting medium. The images were captured by a Nikon fluorescence microscope. Objective magnification is 20×.
Flow cytometry analysis
Same concentration of cells were seeded in a 6-well plate at 200 000 cells/well and treated with 2% FBS Chelex medium or regular 2% FBS medium for 24 h when cells reached 65% confluence. Cells were trypsinized and fixed with 1% PFA on ice for 30–60 min, and then labeled and acquired by flow cytometry following the standard protocol (APO-DIRECT Apoptosis Detection Kit, BD Biosciences). Cells were stained by propidium iodide and FITC, and then acquired by a flow cytometer (BD LSRII, BD Biosciences) at 623 nm and 520 nm, respectively. Data were analyzed by FlowJo software Ver. 7.6.5 (Tree Star) and output as dot plots.
Western blotting
Cells were lysed with 600 µL lysis buffer (DL-Dithiothreitol, Sigma; LDS, Life Technologies) and loaded on a 4–12% Nupage Novex Bis-tris gel, and then transferred to nitrogen cellulose membrane. The membrane was probed with anti-PARP, anti-cleaved PARP; anti-caspase-7, anti-cleaved capase-7; anti-caspase-9, and anti-cleaved caspase-9 (1:1000; anti-rabbit; Cell Signaling Apoptosis Antibody Sampler Kit #9915S); anti-cytochrome C (1:1000; anti-rabbit; Cell Signaling Apoptosis Antibody Sampler Kit #4272S); anti-Bax (1:1000; anti-rabbit; Cell Signaling Apoptosis Antibody Sampler Kit #2772), or anti-β-actin (1:30 000; anti-mouse; Sigma A2288) antibody and detected by enhanced chemiluminescent (ECL) plus reagent kit or SuperSignal West Pico Chemiluminescent Substrate Kit (Thermo Fisher Scientific).
Pancreatic cancer mouse models
Subconfluent pancreatic cancer cells such as MIA-ZIP4 or MIA-V cells were harvested by trypsinization and resuspended in DMEM. Only single-cell suspensions with >95% viability were used. The cells (3 × 106) were inoculated into the right flank (subcutaneous tumor model) of 5- to 6-wk-old male nude mice (NCI-Charles River). All mice were cared for in accordance with the OPRR and Animal Welfare Act guidelines, and were in accordance with the Helsinki Declaration of 1975. The tumor size was measured weekly using a digital caliper (VWR international), and the tumor volume was determined with the formula: tumor volume [mm3] = (length [mm]) × (width [mm])2 × 0.52. The animals were euthanized when their tumor size reached 2 cm in diameter, or the animals became moribund during the observation period, and the time of euthanization was recorded at the time of mortality.
Cell cycle analysis
Cells were synchronized by serum-free media for 24 h and then harvested at 6 h after release by incubation in 2% FBS medium with or without Chelex. Cells were then labeled with propidium iodide, and acquired by a flow cytometer (LSRII, BD Bioscience) at 623 nm. Data were analyzed by FlowJo software Ver. 7.6.5 (Tree Star, San Carlos) with the Watson Pragmatic Model.
Statistical analysis
All data are shown as mean ± SD of triplicates. Student t test was employed for statistical analysis. Percentages were tested by chi-square test. P < 0.05 was considered statistically significant.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Philippa Smith and Dr Charles S Cox, Jr at Department of Pediatric Surgery, UTHealth for their generous assistance in flow cytometry analysis. This work was supported in part by the National Institutes of Health (NIH) grant R21CA133604, R01CA138701, the William and Ella Owens Medical Research Foundation, and the MacDonald Research Fund (M.L.).
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/28111
References
- 1.Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–88. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long J, Zhang Y, Yu X, Yang J, LeBrun DG, Chen C, Yao Q, Li M. Overcoming drug resistance in pancreatic cancer. Expert Opin Ther Targets. 2011;15:817–28. doi: 10.1517/14728222.2011.566216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bracci PM. Obesity and pancreatic cancer: overview of epidemiologic evidence and biologic mechanisms. Mol Carcinog. 2012;51:53–63. doi: 10.1002/mc.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donadelli M, Dalla Pozza E, Scupoli MT, Costanzo C, Scarpa A, Palmieri M. Intracellular zinc increase inhibits p53(−/−) pancreatic adenocarcinoma cell growth by ROS/AIF-mediated apoptosis. Biochim Biophys Acta 2009; 1793:273-80. [DOI] [PubMed]
- 5.Chesters JK, Boyne R. Nature of the Zn2+ requirement for DNA synthesis by 3T3 cells. Exp Cell Res. 1991;192:631–4. doi: 10.1016/0014-4827(91)90085-9. [DOI] [PubMed] [Google Scholar]
- 6.King JC, Cousins RJ. Modern Nutrition in Health and Disease. In: M. E. SM, Ross A. C., Caballero B., Cousins R. J., ed. Modern Nutrition in Health and Disease. Baltimore: Lippincott Williams and Wilkins, 2005:271-85. [Google Scholar]
- 7.Kim BE, Wang F, Dufner-Beattie J, Andrews GK, Eide DJ, Petris MJ. Zn2+-stimulated endocytosis of the mZIP4 zinc transporter regulates its location at the plasma membrane. J Biol Chem. 2004;279:4523–30. doi: 10.1074/jbc.M310799200. [DOI] [PubMed] [Google Scholar]
- 8.Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr. 2004;24:151–72. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- 9.Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem. 2006;281:24085–9. doi: 10.1074/jbc.R600011200. [DOI] [PubMed] [Google Scholar]
- 10.Lee R, Woo W, Wu B, Kummer A, Duminy H, Xu Z. Zinc accumulation in N-methyl-N-nitrosourea-induced rat mammary tumors is accompanied by an altered expression of ZnT-1 and metallothionein. Exp Biol Med (Maywood) 2003;228:689–96. [PubMed] [Google Scholar]
- 11.Taylor KM, Morgan HE, Johnson A, Hadley LJ, Nicholson RI. Structure-function analysis of LIV-1, the breast cancer-associated protein that belongs to a new subfamily of zinc transporters. Biochem J. 2003;375:51–9. doi: 10.1042/BJ20030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kagara N, Tanaka N, Noguchi S, Hirano T. Zinc and its transporter ZIP10 are involved in invasive behavior of breast cancer cells. Cancer Sci. 2007;98:692–7. doi: 10.1111/j.1349-7006.2007.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X, Zhang S, Liuzzi JP, Chang SM, Cousins RJ, et al. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci U S A. 2007;104:18636–41. doi: 10.1073/pnas.0709307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li M, Zhang Y, Bharadwaj U, Zhai QJ, Ahern CH, Fisher WE, Brunicardi FC, Logsdon CD, Chen C, Yao Q. Down-regulation of ZIP4 by RNA interference inhibits pancreatic cancer growth and increases the survival of nude mice with pancreatic cancer xenografts. Clin Cancer Res. 2009;15:5993–6001. doi: 10.1158/1078-0432.CCR-09-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang K, Pugh EW, Griffen S, Doheny KF, Mostafa WZ, al-Aboosi MM, el-Shanti H, Gitschier J. Homozygosity mapping places the acrodermatitis enteropathica gene on chromosomal region 8q24.3. Am J Hum Genet. 2001;68:1055–60. doi: 10.1086/319514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weaver BP, Zhang Y, Hiscox S, Guo GL, Apte U, Taylor KM, Sheline CT, Wang L, Andrews GK. Zip4 (Slc39a4) expression is activated in hepatocellular carcinomas and functions to repress apoptosis, enhance cell cycle and increase migration. PLoS One. 2010;5:5. doi: 10.1371/journal.pone.0013158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Y, Chen Y, Wang Y, Yang J, Zhu VF, Liu Y, Cui X, Chen L, Yan W, Jiang T, et al. ZIP4 is a novel molecular marker for glioma. Neuro Oncol. 2013;15:1008–16. doi: 10.1093/neuonc/not042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho E. Zinc deficiency, DNA damage and cancer risk. J Nutr Biochem. 2004;15:572–8. doi: 10.1016/j.jnutbio.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Kelleher SL, McCormick NH, Velasquez V, Lopez V. Zinc in specialized secretory tissues: roles in the pancreas, prostate, and mammary gland. Adv Nutr. 2011;2:101–11. doi: 10.3945/an.110.000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68(Suppl):447S–63S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 21.King JC. Zinc: an essential but elusive nutrient. Am J Clin Nutr. 2011;94:679S–84S. doi: 10.3945/ajcn.110.005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–58. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485–95. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- 24.Westphal S, Kalthoff H. Apoptosis: targets in pancreatic cancer. Mol Cancer. 2003;2:6. doi: 10.1186/1476-4598-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma X, Ma Q, Liu J, Tian Y, Wang B, Taylor KM, Wu P, Wang D, Xu G, Meng L, et al. Identification of LIV1, a putative zinc transporter gene responsible for HDACi-induced apoptosis, using a functional gene screen approach. Mol Cancer Ther. 2009;8:3108–16. doi: 10.1158/1535-7163.MCT-08-0772. [DOI] [PubMed] [Google Scholar]
- 26.Egefjord L, Jensen JL, Bang-Berthelsen CH, Petersen AB, Smidt K, Schmitz O, Karlsen AE, Pociot F, Chimienti F, Rungby J, et al. Zinc transporter gene expression is regulated by pro-inflammatory cytokines: a potential role for zinc transporters in beta-cell apoptosis? BMC Endocr Disord. 2009;9:7. doi: 10.1186/1472-6823-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao J, Bobo JA, Liuzzi JP, Cousins RJ. Effects of intracellular zinc depletion on metallothionein and ZIP2 transporter expression and apoptosis. J Leukoc Biol. 2001;70:559–66. [PubMed] [Google Scholar]
- 28.Zhang Y, Bharadwaj U, Logsdon CD, Chen C, Yao Q, Li M. ZIP4 regulates pancreatic cancer cell growth by activating IL-6/STAT3 pathway through zinc finger transcription factor CREB. Clin Cancer Res. 2010;16:1423–30. doi: 10.1158/1078-0432.CCR-09-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green D, Kroemer G. The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol. 1998;8:267–71. doi: 10.1016/S0962-8924(98)01273-2. [DOI] [PubMed] [Google Scholar]
- 30.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shankar S, Srivastava RK. Bax and Bak genes are essential for maximum apoptotic response by curcumin, a polyphenolic compound and cancer chemopreventive agent derived from turmeric, Curcuma longa. Carcinogenesis. 2007;28:1277–86. doi: 10.1093/carcin/bgm024. [DOI] [PubMed] [Google Scholar]
- 32.Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296:1635–6. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- 33.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–6. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.