Abstract
Citrate carrier (CIC) is an integral protein of the inner mitochondrial membrane that has a fundamental role in hepatic intermediary metabolism. Its primary function is to catalyze the transport of citrate from mitochondria, where this molecule is formed, to cytosol, where this molecule is used for fatty acid (FA) and cholesterol synthesis. Therefore, mitochondrial CIC acts upstream of cytosolic lipogenic reactions, and its regulation is particularly important in view of the modulation of hepatic lipogenesis. Although a great deal of data are currently available on the dietary modulation of cytosolic lipogenic enzymes, little is known about the nutritional regulation of CIC transport activity. In this review, we describe the differential effects of distinct FAs present in the diet on the activity of mitochondrial CIC. In particular, polyunsaturated FAs were powerful modulators of the activity of mitochondrial CIC by influencing its expression through transcriptional and posttranscriptional mechanisms. On the contrary, saturated and monounsaturated FAs did not influence mitochondrial CIC activity. Moreover, variations in CIC activity were connected to similar alterations in the metabolic pathways to which the transported citrate is channeled. Therefore, CIC may be considered as a sensor for changes occurring inside the hepatocyte and may represent an important target for the regulation of hepatic lipogenesis. The crucial role of this protein is reinforced by the recent discovery of its involvement in other cellular processes, such as glucose-stimulated insulin secretion, inflammation, tumorigenesis, genome stability, and sperm metabolism.
Introduction
Hepatic lipogenesis is an anabolic process leading to the de novo synthesis of FAs, which are generally distributed to other tissues by circulating lipoproteins, such as VLDL. Its main role is the conversion of excess energy introduced by food into the storage form of FAs, which are accumulated into adipose tissue or used by muscular tissues. It is also widely known that hepatic lipogenesis is strictly regulated by several nutritional and hormonal factors (1, 2). The FA composition of the diet is 1 of the nutritional factors influencing hepatic lipogenesis (3). Numerous studies indeed demonstrated that the qualitative composition of dietary fat, for example, a prevalence of PUFAs with respect to the saturated fats, reduces hepatic lipogenesis, thereby exerting a beneficial effect in the case of cardiovascular diseases (4). The quantitative aspect is also important in view of the fact that the total amount of dietary fat is able to influence hepatic lipogenesis (5). Moreover, the carbohydrate amount in the diet is another factor capable of modifying hepatic lipogenesis (1, 2, 6, 7).
Most of these studies were performed by analyzing the activities of enzymes involved in FA synthesis in the cytosol of hepatocytes, such as ATP-citrate lyase, acetyl-CoA carboxylase, and FA synthetase. It was found that the activity and the expression of these enzymes are modulated by FA composition of the diet. Acetyl-CoA carboxylase has also a regulatory role in hepatic FA synthesis because it represents the target of specific modulators, such as the metabolic intermediate citrate. Therefore, the attention of the researchers has been concentrated on these cytosolic processes, which, starting from the building blocks of acetyl-CoA, lead to the construction of palmityl-CoA and from this to other FAs through elongation or desaturation steps. In parallel, many experiments explored the hepatic biosynthesis of cholesterol, which follows an anabolic pathway different from that of FA synthesis by using the same starting molecule of acetyl-CoA. In this context, the function and the regulation of hydroxymethyl-CoA reductase, another hepatic cytosolic enzyme, was carefully investigated (8, 9).
However, in addition to these fundamental lipogenic reactions occurring in the cytosol of hepatocytes, there are other preliminary steps taking place in liver mitochondria. The main fuel for hepatic FA synthesis is indeed represented by the carbon units derived from carbohydrate and amino acid catabolism, which produce pyruvate or other ketoacids. These small molecules enter mitochondria and, in the mitochondrial matrix, can be completely oxidized when energy is required or can be converted into the molecule of citrate, an intermediate of the Krebs cycle. When this intermediate cannot be burned into the Krebs cycle (for example, for an excess of cellular energy level), it is exported from the mitochondrial matrix into the cytosol by the mitochondrial tricarboxylate carrier or the protein citrate carrier (CIC)2. This carrier protein is firmly inserted into the inner mitochondrial membrane in which it catalyzes the exit of mitochondrial citrate that otherwise would remain sequestered inside mitochondria (10). Citrate can then passively diffuse across the outer mitochondrial membrane into the cytoplasm through an anion selective channel. Cytosolic citrate can also be derived from the blood across the plasma membrane by a group of proteins known as plasma membrane citrate transporters (11).
It is evident that mitochondrial CIC represents the way by which carbohydrate (and amino acid) degradation, on one side, and lipid biosynthesis, on the other, are connected. As reviewed previously (12), CIC is regulated by diabetes, the thyroid, starvation, and PUFA supplementation. Therefore, the function and regulation of mitochondrial CIC is fundamental for the linkage between catabolic and anabolic pathways inside the cell.
In this review, we summarize and analyze the modulation by dietary fat of the activity of mitochondrial CIC that appears as a crucial sensor of metabolic changes occurring inside the hepatocyte. Very interestingly, recent studies revealed that this mitochondrial carrier is also involved in novel and distinct processes beyond hepatic lipogenesis, thereby positioning CIC at the intersection of many pathways inside the cell.
Current Status of Knowledge
Mitochondrial CIC.
Mitochondrial CIC is a hydrophobic inner membrane protein that belongs to the large family of mitochondrial metabolite carriers (10, 13). The members of this family of transport proteins, including mitochondrial CIC, are ~300 amino acids long and show common structural features, such as a tripartite structure in the inner membrane (Fig. 1). CIC, as well as the other members of the carrier family, is made up of 3 homologous repeats, each of them containing ~100 amino acids organized in the form of 2 α-helices across the inner mitochondrial membrane. In total, CIC contains 6 hydrophobic α-helices that are connected by short hydrophilic loops exposed to both the intermembrane space and the matrix (14, 15). Matrix loops are partially structured and contain short amphipatic helices parallel to the membrane plane on the matrix side (13). In addition, the amino and carboxy terminals of mitochondrial CIC are both exposed in the intermembrane space (16, 17). A short sequence, called the carrier signature [PX(D/E)XX(K/R)], has been identified at the end of the first, third, and fifth α-helices of mitochondrial CIC from rat (14) and yeast (18), as well as in the majority of other members of the carrier family (19, 20). This carrier signature plays a fundamental role in the tridimensional structure and the function of the mitochondrial metabolite carriers, as clearly demonstrated with the ADP/ATP carrier, the most abundant member of this family. It has also been proposed that the members of the carrier family are functional homodimers in the inner mitochondrial membrane (16, 21, 22), but this aspect has been questioned by others (23).
FIGURE 1.
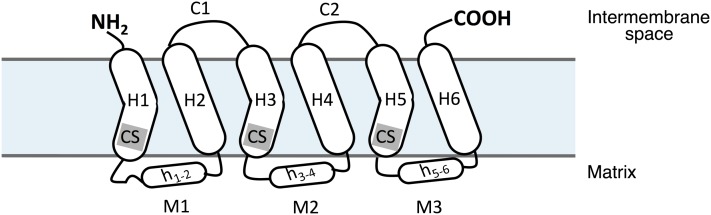
Schematic representation of the secondary structure of the citrate carrier, which consists of 6 transmembrane helices (H1–H6), connected by 3 loops on the matrix side (M1–M3) and 2 loops on the intermembrane space (C1, C2). Matrix loops contain short amphipatic helices (h1–2, h3–4, and h5–6). CS, carrier signature.
Mitochondrial CIC has been sequenced in several species, including animal, plant, and yeast. Human CIC is highly expressed in liver, adipocytes, prostate, lung, and thyroid but is present in low amount in other tissues, such as heart, skeletal muscle, brain, pancreas, and kidney. The protein is encoded by nuclear DNA (SLC25A1 gene) and is therefore synthesized on cytosolic ribosomes. After its cytosolic synthesis, the precursor protein is imported into mitochondria by a specialized import machinery, thereby reaching its final destination in the inner membrane. It is interesting to note that the CIC precursor protein from mammals and eels possess a short amino-terminal presequence that is absent in other organisms (24–26).
Mitochondrial CIC catalyzes an electroneutral exchange across the inner mitochondrial membrane of a tricarboxylate (citrate, isocitrate, cis-aconitate) plus a proton for another tricarboxylate, a dicarboxylate (malate), or phosphoenolpyruvate (27). In this way, the citrate synthesized in the matrix is transported outside mitochondria in exchange for l-malate (Fig. 2). Alternative pathways of exchange have also been proposed as illustrated in Figure 2 (dotted lines). CIC transport activity has been investigated both in native intact mitochondria and after functional reconstitution of the purified protein into liposomes (28–30). The kinetic properties and the molecular mechanism of the catalyzed reaction were characterized in detail previously (for a review, see reference 31).
FIGURE 2.
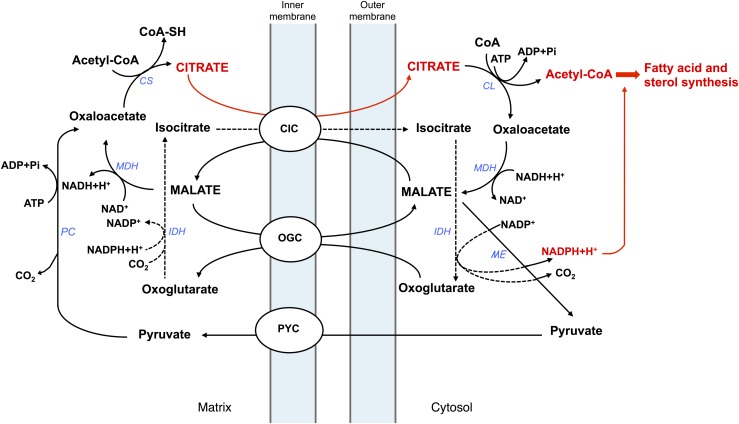
Schematic model depicting the key role of CIC in hepatic lipogenesis. CIC, citrate carrier; CL, ATP-citrate lyase; CS, citrate synthase; IDH, isocitrate dehydrogenase; MDH, malate dehydrogenase; ME, malic enzyme; NADH, reduced nicotinamide adenine dinucleotide hydrate; NAD+, oxidized nicotinamide adenine dinucleotide; OGC, oxoglutarate carrier; PC, pyruvate carboxylase; Pi, inorganic phosphate; PYC, pyruvate carrier.
Therefore, the main function of mitochondrial CIC is the transport of the molecule of citrate outside mitochondria to channel the carbon units derived from catabolism toward FA and cholesterol biosynthesis. Alternatively, mitochondrial CIC may catalyze the exit of phosphoenolpyruvate from mitochondria for the synthesis of glucose by gluconeogenesis (data not shown in Fig. 2). Very interestingly, other functions were associated recently with mitochondrial CIC, such as a role in inflammation (32), tumorigenesis (33), glucose-stimulated insulin secretion (34), sperm capacitation and acrosome reaction (35), and genome stability (36). All these findings underline the importance of this mitochondrial transporter that is involved not only in intermediary metabolism but also in apparently distant and distinct cellular processes.
n–6 and n–3 dietary PUFAs decrease the activity of mitochondrial CIC.
It is well known from the literature that dietary PUFAs of the n–3 and n–6 series are potent inhibitors of hepatic FA synthesis (3). The degree of inhibition depended on the amount of PUFAs added to the diet and on the length of dietary treatment (37–39). Most of these studies investigated the variations in the activity and expression of cytosolic lipogenic enzymes, acetyl-CoA carboxylase, and FA synthetase. In recent years, several experiments were performed to investigate the effects of PUFA dietary supplementation on the transport activity of mitochondrial CIC (Table 1).
TABLE 1.
Effects of PUFA dietary supplementation on activity, kinetic variables, protein, and mRNA levels of mitochondrial CIC1
| References | Oil Source | FA composition | Amount | Experimental design | CIC activity | Km | Vmax | Protein concentrations | mRNA |
| n–6 | % | ||||||||
| Zara et al. (40) Siculella et al. (41) | Safflower | C16:0 | 7.7 | Rats fed a diet containing 15% safflower oil for 4 wk | ↓ 40% | ↔ | ↓ | ↓ 30% | ↓ 35% |
| C16:1 | 0.1 | ↓ 50% | ↓ 35% | ||||||
| C18:0 | 2.9 | ||||||||
| C18:1 | 15.2 | ||||||||
| C18:2 | 70.4 | ||||||||
| C18:3 | 0.3 | ||||||||
| C20:0 | 0.3 | ||||||||
| C20:1 | 0.2 | ||||||||
| C22:1 | 0.1 | ||||||||
| Ferramosca et al. (42) | Corn | C16:0 | 12.1 | Mice fed a diet containing 7.5% corn oil for 8 wk | ↓ 60% | ↓ 70% | |||
| C18:0 | 3.0 | ||||||||
| C18:1 | 27.4 | ||||||||
| C18:2 | 51.9 | ||||||||
| C18:3 | 2.7 | ||||||||
| Ferramosca et al. (43) | Pine nut | C16:0 | 8.0 | Mice fed a diet containing 7.5% pine nut oil for 8 wk | ↓ 40% | ||||
| C18:0 | 2.2 | ||||||||
| C18:1 | 23.2 | ||||||||
| C18:2 | 51.1 | ||||||||
| C18:3 | 2.1 | ||||||||
| C18:3 (5, 9, 12) | 8.8 | ||||||||
| Ferramosca et al. (51) | CLA | C16:0 | 12.0 | Mice fed a diet containing 1% CLA for 16 wk | ↑ 70% | ↔ | ↑ | ↑ 70% | ↑ 30% |
| C18:0 | 2.7 | ||||||||
| C18:1 | 42.5 | ||||||||
| C18:2 | 30.5 | ||||||||
| C18:3 | 2.9 | ||||||||
| CLA cis-9,trans-11 | 3.5 | ||||||||
| CLA trans-10,cis-12 | 3.7 | ||||||||
| n–3 | |||||||||
| Giudetti et al. (45) | Fish | C14:0 | 6.5 | Rats fed a diet containing 15% fish oil for 3 wk | ↓ 60% | ↔ | ↓ | ↓ 50% | ↓ 40% |
| C16:0 | 17.6 | ||||||||
| C16:1 | 9.1 | ||||||||
| C18:0 | 3.4 | ||||||||
| C18:1 | 17.9 | ||||||||
| C18:2 | 14.0 | ||||||||
| C18:3 | 2.4 | ||||||||
| C20:5 | 13.5 | ||||||||
| C22:5 | 2.3 | ||||||||
| C22:6 | 11.8 | ||||||||
| Ferramosca et al. (46) | Fish | C14:0 | 1.7 | Rats fed a diet containing 2.5% fish oil for 6 wk | ↓ 65% | ↔ | ↓ | ↓ 70% | ↓ 30% |
| C16:0 | 17.4 | Rats fed a diet containing 2.5% fish oil for 3 wk | ↓ 30% | ↔ | ↓ | ↓ 30% | ↔ | ||
| C18:0 | 3.7 | ||||||||
| C18:1 | 30.9 | ||||||||
| C18:2 | 54.0 | ||||||||
| C18:3 | 5.3 | ||||||||
| C20:5 | 3.5 | ||||||||
| C22:5 | 0.5 | ||||||||
| C22:6 | 5.0 | ||||||||
| Ferramosca et al. (46) | Krill | C14:0 | 2.5 | Rats fed a diet containing 2.5% krill oil for 6 wk | ↓ 65% | ↔ | ↓ | ↓ 70% | ↓ 30% |
| Ferramosca et al. (48) | C16:0 | 18.2 | Rats fed a diet containing 2.5% krill oil for 3 wk | ↓ 55% | ↔ | ↓ | ↓ 60% | ↓ 25% | |
| C18:0 | 2.8 | Rats fed a high-fat (35% fat) diet containing 2.5% krill oil for 12 wk | ↓ 60% | ↔ | ↓ | ↓ 55% | |||
| C18:1 | 25.8 | ||||||||
| C18:2 | 54.4 | ||||||||
| C18:3 | 4.9 | ||||||||
| C20:5 | 5.3 | ||||||||
| C22:5 | 2.3 | ||||||||
| C22:6 | 3.0 |
CIC, citrate carrier; CLA, conjugated linoleic acid; ↑, increase; ↓, decrease; ↔, no change.
The effects of n–6 PUFAs on the function of this mitochondrial transporter were investigated by feeding rats for 4 wk with a diet containing 15% safflower oil (40), an oil particularly rich (70.4%) in linoleic acid (C18:2) and containing only minor amounts of oleic (C18:1) and palmitic (C16:0) acid. To the best of our knowledge, the results obtained in this study demonstrated, for the first time, that the activity of CIC of liver mitochondria was noticeably inhibited (~40%) by n–6 PUFA administration in rats. The n–6 FA-mediated inhibition was due to a decrease in the CIC mRNA and, consequently, in the content of the protein in the inner mitochondrial membrane of treated rats (40, 41). Kinetic analysis of CIC activity showed a decrease in the Vmax of citrate transport but no change in the Km value, indicating that the dietary treatment did not modify the affinity of CIC for its substrate. Very interestingly, in animals fed a diet enriched in n–6 PUFAs, a parallel decrease in the activities of mitochondrial CIC and the cytosolic lipogenic enzymes was found (40), suggesting a coordinated modulation of the function of all these enzymes by PUFAs.
Similar results were obtained in mice fed a diet containing 7.5% corn oil (42) or 7.5% pine nut oil (43), 2 oils rich (about 50%) in linoleic acid, a PUFA of the n–6 series. However, differently from corn oil, pine nut oil contains pinolenic acid or all-cis-5,9,12-octadecatrienoic acid (Table 1). The latter is a quite unusual n–6 PUFA, characterized by polymethylene-interrupted double bonds (44), which seems to be able to reduce body weight gain of treated animals and to decrease lipid concentration in liver and plasma, without any influence on CIC or the enzymes of the de novo FA synthesis in mice (43). Indeed, the significant reduction of mitochondrial CIC activity found in mice fed both diets (supplemented with corn oil or pine nut oil) can be ascribed to the high content of linoleic acid present in both oils (Table 1) but not to pinolenic acid present only in pine nut oil.
The effects of dietary n–3 PUFAs on mitochondrial CIC activity were also investigated. Rats fed for 3 wk a diet containing 15% fish oil, rich in n–3 PUFAs such as EPA (C20:5) and DHA (C22:6), showed a strong reduction of CIC activity, CIC protein, and mRNA levels (45). No change in the affinity of the carrier protein for its substrate was observed in the same study. A significant decrease in citrate transport was also observed when rats were fed a diet contain a small percentage (2.5%) of fish oil (46). This concentration of fish oil added to a standard diet was sufficient to provide a daily amount of n–3 PUFAs comparable with the dietary intake of n–3 PUFAs in Western countries (47). Very interestingly, when rats were fed the same dose of n–3 PUFAs in the form of krill oil, a stronger decrease in the activities of mitochondrial CIC, cytosolic acetyl-CoA carboxylase, and FA synthetase was observed at relatively short periods of dietary treatment (2–3 wk) (46). Krill oil is a novel dietary supplement extracted from Antarctic krill (Euphausia superba), a shrimp-like zooplankton. This oil shows some peculiar characteristics, such as a high amount of phospholipids rich in long-chain n–3 PUFAs, which should guarantee a better bioavailability of these FAs, a different ratio of EPA-to-DHA, and the presence of specific antioxidants. Therefore, the krill oil–induced inhibition of CIC activity was more pronounced than that found in fish oil–fed rats, at least at short feeding times. It is also interesting to note that in krill oil–fed animals, the decrease in CIC activity was due to a reduced amount of the protein in the inner mitochondrial membrane, and this effect was obtained by both transcriptional and posttranscriptional mechanisms (46). Very recently, it was found that the potency of this effect is not lost in the presence of high fat (35%) in the diet, suggesting that krill oil has an intrinsic capability of reducing hepatic lipogenesis (48).
Overall, these findings indicate that dietary n–6 and n–3 PUFAs are potent inhibitors of mitochondrial CIC activity and consequently of hepatic lipogenesis. Accordingly, in cell culture experiments performed in H4IIE hepatoma cells, PUFAs, such as n–6 arachidonic acid (C20:4) and n–3 DHA (C22:6), reduced CIC mRNA content ~50% and 70%, respectively (49). However, as shown below, some studies performed in rodent models suggested that not all PUFAs exert the same effect. This is a particularly intriguing aspect, because the knowledge of molecular mechanisms responsible for the regulation of FA metabolism mediated by PUFAs highlights a possible role for some dietary fats as a potential cause of hepatic fat accumulation or as a potential treatment for non-alcoholic fatty liver disease (NAFLD) (50). Nevertheless, additional studies in humans are required to confirm this view.
Some unusual PUFAs increase the activity of mitochondrial CIC.
Surprisingly, it was found recently that some PUFAs, such as conjugated linoleic acids (CLAs), are able to strongly increase CIC transport activity (51). A great deal of current interest in CLAs, mainly found in dairy products in the form of cis-9,trans-11-octadecadienoic acid, is due to their positive effects in cancer, cardiovascular diseases, diabetes, and obesity (52). However, these beneficial effects are in some instances associated with adverse effects, such as hepatic fat deposition and insulin resistance (53). Nevertheless, CLAs strongly prevent fat accumulation in adipose tissue.
Few clinical studies have been performed in humans, and the results available are not readily comparable. Therefore, the understanding of molecular mechanisms responsible for the regulation of lipid metabolism mediated by CLA administration in animal models could give new insights about the beneficial (on adiposity phenotypes) or negative (on hepatic syteatosis) effects derived from the use of this dietary supplement in humans. In this context, an interesting study was performed to investigate a possible modification of mitochondrial CIC activity in mice fed a diet supplemented with 1% CLAs and composed mainly of a 1:1 mixture of cis-9,trans-11-octadecadienoic acid and trans-10,cis-12-octadecadienoic acid (51). A strong time-dependent increase in the transport activity of mitochondrial CIC was found unexpectedly. An almost doubling of CIC activity was observed after 16 wk of CLA feeding. A parallel increase in the expression of CIC protein and in relative mRNA in CLA-treated animals was also observed. A strict correlation between the increase in CIC activity and expression and the stimulation of the cytosolic lipogenic enzymes was also found (51). Therefore, the stimulation of the citrate export from the mitochondrial matrix toward the cytosol and, consequently, of hepatic lipogenesis, may play a role in the onset of hepatic steatosis observed during CLA administration.
Specific combinations of PUFAs modulate CIC activity and hepatic lipogenesis.
Because specific PUFAs influence mitochondrial CIC activity in a unique manner, could a combination of appropriate FAs be used to modulate hepatic lipogenesis? In this context, an interesting study was performed to investigate the effects of a dietary combination of CLAs and pine nut oil on hepatic lipogenesis in mice (54). The starting point of this investigation was the finding that a 1:1 mixture of cis-9,trans-11-CLA and trans-10,cis-12-CLAs greatly increase de novo FA synthesis in mouse hepatocytes (51), whereas pine nut oil decreases lipid concentration in liver (43). Therefore, the rationale of this study was to check whether the association between these 2 dietary fats would be able to maintain the beneficial effects of CLAs, such as body fat reduction, but avoid adverse effects, such as liver steatosis and insulin resistance. To this end, mice were fed for 16 wk a diet enriched in a mixture of 1% CLAs (composed mainly of a 1:1 mixture of cis-9,trans-11-octadecadienoic acid and trans-10,cis-12-octadecadienoic acid) plus 7.5% pine nut oil. Very interestingly, a peculiar 2-phase behavior of CIC activity was found in these animals: at week 8 of dietary treatment, although mitochondrial CIC activity started to increase in CLA-fed mice, that of CLAs plus pine nut oil–fed mice started to decrease, thus leading, at week 16, to values even lower than the controls (25% decrease). Also, CIC protein expression showed the same trend. The changes in CIC mRNA were less evident than those found in CIC activity and in the amount of CIC protein, suggesting that dietary treatments influence not only the transcription of the CIC gene but also mRNA stability and/or translation efficiency. Moreover, the behavior of the lipogenic enzyme activities over time was consistent with that of mitochondrial CIC (54). Overall, these results indicate that the coadministration of CLAs with pine nut oil is able to positively modulate CIC activity and hepatic lipogenesis.
Some attempts were made in the past to overcome the adverse effects of CLA in hepatic lipogenesis (55, 56). However, these studies provided very limited information on the effects of the dietary combinations tested, because the length of treatments was limited to 3–4 wk and only cytosolic lipogenic enzymes activity and expression were investigated.
Effect of MUFAs and SFAs on CIC activity.
The effects of diets enriched with SFAs or MUFAs on the activity and expression of mitochondrial CIC were also investigated. When rats were fed for 3 wk a basal diet enriched with 15% coconut oil or 15% beef tallow, abundant in medium-chain and long-chain SFAs, respectively, no appreciable effect on both CIC activity and expression was found compared with control diet–fed animals (45, 57). No effect of SFAs on CIC mRNA content was also observed in H4IIE hepatoma cells (49).
Additional experiments demonstrated also that MUFAs, such as oleic acid (C18:1, n–9), do not inhibit either the activity or expression of mitochondrial CIC. In fact, the administration of a basal diet enriched with 15% of olive oil for 3 wk to rats or the addition of exogenous oleic acid to H4IIE cells had no effect on hepatic lipogenesis (49, 57). Conversely, some authors observed that an olive oil–enriched diet stimulated hepatic lipogenesis (58, 59) and induced fat accumulation in liver (60). However, in these studies, the activities of lipogenic enzymes in olive oil–fed animals were compared with those found in PUFA-treated animals. Therefore, the observed increase in hepatic lipogenesis was probably due to the type of diet fed to control animals. In this context, the results reported in a recent investigation (42) indicate a significant increase in the concentration of hepatic TGs in mice fed for 8 wk with an olive oil–enriched diet compared with corn oil–fed animals. Interestingly, this change in the hepatic lipid profile was not correlated with changes in the activity of mitochondrial CIC and the cytosolic lipogenic enzymes, which remained unaltered over time in olive oil–fed mice. On the contrary, a decrease in the activity of carnitine palmitoyltransferase I, the rate-limiting step for FA oxidation, was observed in olive oil–treated mice. Therefore, this impairment in the oxidation of FAs may play a role in the accumulation of TGs in the liver of mice fed a diet enriched in olive oil.
In another study (61), the effects of some C18 FAs on hepatic FA metabolism were analyzed. Male rats received a diet with stearic acid (C18:0), oleic acid (C18:1 cis), or elaidic acid (C18:1 trans) for 14 d. The experimental diets used in this study were formulated using hydrogenated soybean-oil preparations and olive oil in a concentration of 6% and 18%, respectively. The authors found that CIC activity was lower in the oleic acid–fed and C18:1 trans isomer–fed rats when compared with the rats fed stearic acid. However, it must be noted that these different results among nutritional studies could depend on the experimental conditions used in each investigation, such as dietary fat amount, relative concentration of different FAs, and duration of feeding. According to this last observation, dramatic changes in hepatic lipogenesis, consisting of a strong inhibition of CIC and lipogenic enzyme expression and activity, was found in rats fed a high-fat diet rich in SFAs and MUFAs. This inhibition, in agreement with recent studies (5), was time- and concentration-dependent (7, 48). Therefore, an excessive amount of dietary fat seems to be able to overtake the specific effect of single FAs on hepatic lipogenesis (7). This highlights the importance of the total amount of fat in the diet in addition to the molecular characteristics of each FA.
Regulation of mitochondrial CIC expression by dietary FAs.
Therefore, it is clear that dietary FAs are able to influence differently the transport activity of mitochondrial CIC. This is a long-term effect that is mainly mediated by gene transcriptional regulation, thereby resulting in the modulation of the expression levels of this mitochondrial carrier. In particular, the analysis of CIC mRNA and protein expression levels, which varied according to the activity of the carrier protein, suggested the presence of complex mechanisms of regulation at the transcriptional and posttranscriptional levels. The mechanism by which FAs, and PUFAs in particular, affect CIC gene expression in liver is only partially known and involves distinct transcription factors, such as sterol regulatory element-binding protein-1 (SREBP-1) and peroxisome proliferator-activated receptors (PPARs). This does not exclude that other still unidentified transcription factors may regulate CIC gene expression, especially if we consider that this protein participates in a multiplicity of cellular processes beyond hepatic lipogenesis.
Recent studies indicate that PUFAs of the n–3 or n–6 series reduce the transcriptional activity of SREBP-1. Because CIC promoter activity is stimulated by this transcription factor, the presence of PUFAs, but not MUFAs or SFAs, reduces CIC gene expression in both HepG2 cells and hepatocytes (49, 62–64).
The structural and functional analysis of the rat CIC gene promoter revealed the presence of a PUFA response region, which is a cluster of putative binding sites for several transcription factors, composed of a nuclear factor-Y site, an E-box–like site, a sterol regulatory element-1–like site, and 4 stimulatory protein-1 sites (12). In particular, the E-box–like site seems to be responsible for both SREBP-1c transactivation and PUFA inhibition of the CIC promoter (49). Interestingly, SREBP-1 and nuclear factor-Y are also implicated in the PUFA inhibition of the transcription of several lipogenic genes. Furthermore, PUFAs cause a reduction of CIC gene expression by inhibiting the splicing process of CIC mRNA (49, 62–64). On the contrary, PUFAs activate CIC gene expression in hepatocytes and adipocytes by interacting with PPARα and PPARγ, respectively (63). A functional PPAR response element, conferring responsiveness to activation by PPARs, was indeed identified recently in the CIC promoter (63). Furthermore, PUFA-mediated PPARα activation stimulates FA oxidation in liver, in addition to CIC gene expression. The physiologic significance of these effects is currently unclear, because PPARα is the master regulator of β oxidation enzymes (64). Even if a role for transcriptional activation of CIC by PPARα in gluconeogenesis was proposed recently (63), many molecular aspects remain to be elucidated. In particular, it has been suggested that regulation of CIC expression by PPARα is highly controlled by FA cell content (64). Nevertheless, it seems that the main regulator of CIC gene expression is SREBP-1, which, as stated previously, is inhibited by very low concentrations of dietary PUFA.
Very recently, a new role was proposed for CIC in immune cells in the production of inflammatory mediators (32), whose amounts are controlled by PUFA. It seems that, during inflammation, PUFAs suppress the amount of the nuclear transcription factor NF-κB, which is a pivotal orchestrator of this event (65), and at the same time, control the expression of a subset of NF-κB–regulated genes, including the CIC gene. CIC gene expression is upregulated by NF-κB, leading to an increased availability of cytosolic acetyl-CoA and NAD(P)H plus H+, needed for the synthesis of several inflammation mediators (32). Therefore, PUFAs are able to reduce inflammation by influencing the expression and transport activity of CIC. These findings suggest that the transcriptional regulation of the CIC gene is very complex, because other factors that are not strictly related to lipid metabolism are involved.
The transcriptional factor forkhead box protein A1 (FOXA 1) is also a strong activator of CIC gene expression in pancreatic cells (66). This factor does not seem to be controlled directly by FAs; however, it plays a specific role in hormonal regulation, because it induces insulin secretion, which in turn controls SREBP amounts and consequently hepatic lipogenesis (3). It is noteworthy that insulin regulates CIC expression and function in liver (67) and that, conversely, mitochondrial CIC function is critical in pancreatic β-cells for insulin secretion, as demonstrated previously (34).
Mitochondrial CIC as a sensor of changes in energetic metabolism.
In this review, we focused our attention on the nutritional regulation of CIC, a mitochondrial transport protein that may serve multiple functions: 1) it supplies cytosol with the carbon units in the form of citrate for FA and cholesterol synthesis; 2) it may provide cytosol with phosphoenolpyruvate, an intermediate of gluconeogenesis; 3) it provides, via malic enzyme, NAD(P)H for lipogenesis or, via malate dehydrogenase, nicotinamide adenine dinucleotide (NAD+) for glycolysis; and 4) it modulates the amounts of citrate, which acts as an allosteric modulator of key enzymes involved in distinct metabolic pathways, such as phosphofruttokinase-1, acetyl-CoA carboxylase, the complex of the pyruvate dehydrogenase, and citrate synthase. Therefore, variations in the transport activity of mitochondrial CIC may have dramatic effects on cellular metabolism.
Overall, the molecule of citrate, whose amounts depend on the transport activity of mitochondrial CIC, represents an intermediate of metabolism with its carbon backbone and a fundamental regulator of energetic metabolism. In fact, an excess amount of citrate is able to decrease both glycolysis and the Krebs cycle by inhibiting phosphofruttokinase-1, the pyruvate dehydrogenase complex, and citrate synthase. Conversely, an excess amount of citrate stimulates FA synthesis and gluconeogenesis, thereby promoting the use of ATP and NAD(P)H plus H+. These last anabolic pathways are increased by a citrate-induced activation of both acetyl-CoA carboxylase, the first enzyme involved in FA synthesis, and fructose 1,6 bisphosphatase, an enzyme involved in the gluconeogenesis pathway.
As shown in Figure 2, citrate transferred into the cytosol is broken back to oxaloacetate and acetyl-CoA by ATP-citrate lyase. Oxaloacetate is reduced to malate, and this is converted to pyruvate, which reenters mitochondria by the pyruvate carrier. Malate may also be transported into the mitochondrial matrix (where it could give further oxaloacetate) using CIC. Therefore, this carrier catalyzes a proton-compensated electroneutral exchange of citrate with malate, which is at concentrations sufficient to react with the transport protein. A link between citrate export, malate import, and the pool of the Krebs cycle intermediates is then possible. Moreover, a separate cycle of malate export might use another mitochondrial carrier (68), thereby positioning CIC at the intersection of many pathways inside the cell. Because of the involvement of mitochondrial CIC in several cellular pathways, we can consider this transport protein as a sort of sensor of metabolic changes depending on the increase or decrease in the amount of the molecule of citrate or other metabolic intermediates in specific subcellular compartments.
Therefore, the knowledge of the mechanisms by which different nutritional and hormonal factors control CIC expression and its function in the inner mitochondrial membrane are crucial. These studies may in fact provide insight into the interconnection existing between catabolic and anabolic pathways inside the cells and, in particular, in liver in which most of the reactions of the metabolism occurs and in which mitochondrial CIC is primarily expressed.
In conclusion, as it has been reported widely, different FAs exert distinct effects on the transcription of specific genes involved in hepatic lipid metabolism. Although a great deal of data are currently available on the hormonal and dietary modulation of the lipogenic enzymes, comparatively little is known about the regulation of mitochondrial CIC activity. Some studies performed in the past decade revealed that PUFAs were able to differently modulate CIC activity by influencing its expression by transcriptional and posttranscriptional mechanisms. Interestingly, a covariance of the activities of mitochondrial CIC and the cytosolic lipogenic enzymes was found. This indicates that an alteration of the metabolic flux across the inner mitochondrial membrane is strictly connected to a similar alteration of the metabolic pathway to which the substrate is channeled. Therefore, by acting on cytosolic citrate channeling, dietary fats may not only influence the pathogenesis of liver diseases but may also prevent and/or reverse their expression. We believe that understanding the biochemical mechanisms underlying fat accumulation in liver of animal models will lead to more targeted and effective therapeutics for hepatic steatosis in humans. In this context, it is important to note that, in some studies discussed in this review, PUFA administration to rodents was sufficient to provide a daily amount of n–3 PUFAs comparable with the dietary intake of n–3 PUFAs in Western countries (46–48). Nevertheless, additional studies in humans are needed to translate the results obtained in animal studies into the development of novel treatment strategies for NAFLD. This is a particularly intriguing topic because NAFLD is an increasingly prevalent disease that, to date, has no proven pharmacologic treatment to prevent or reverse its course.
Mitochondrial CIC may represent an important target for the regulation of hepatic lipogenesis, especially because this transport protein represents the necessary link between the 2 main fuels for energetic metabolism: carbohydrate and lipid. Moreover, the effects of a possible modulation of mitochondrial CIC function are not exclusively confined to the de novo FA synthesis but also extend to other cellular pathways, such as glucose-stimulated insulin secretion, inflammation, tumorigenesis, genome stability, and sperm metabolism.
Acknowledgments
Both authors read and approved the final manuscript.
Footnotes
Abbreviations used: CIC, citrate carrier; CLA, conjugated linoleic acid; NAFLD, non-alcoholic fatty liver disease; PPAR, peroxisome proliferator-activated receptor; SREBP-1, sterol regulatory element-binding protein-1.
Literature Cited
- 1.Girard J, Perdereau D, Foufelle F, Prip-Buus C, Ferré P. Regulation of lipogenic enzyme gene expression by nutrients and hormones. FASEB J. 1994;8:36–42 [DOI] [PubMed] [Google Scholar]
- 2.Strable MS, Ntambi JM. Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol. 2010;45:199–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jump DB. Fatty acid regulation of hepatic lipid metabolism. Curr Opin Clin Nutr Metab Care. 2011;14:115–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siriwardhana N, Kalupahana NS, Moustaid-Moussa N. Health benefits of n–3 polyunsaturated fatty acids: eicosapentaenoic acid and docosahexaenoic acid. Adv Food Nutr Res. 2012;65:211–22 [DOI] [PubMed] [Google Scholar]
- 5.Tovar AR, Díaz-Villaseñor A, Cruz-Salazar N, Ordáz G, Granados O, Palacios-González B, Tovar-Palacio C, López P, Torres N. Dietary type and amount of fat modulate lipid metabolism gene expression in liver and in adipose tissue in high-fat diet-fed rats. Arch Med Res. 2011;42:540–53 [DOI] [PubMed] [Google Scholar]
- 6.Kim TS, Freake HC. High carbohydrate diet and starvation regulate lipogenic mRNA in rats in a tissue-specific manner. J Nutr. 1996;126:611–7 [DOI] [PubMed] [Google Scholar]
- 7.Ferramosca A, Conte A, Damiano F, Siculella L, Zara V. Differential effects of high-carbohydrate and high-fat diets on hepatic lipogenesis in rats. Eur J Nutr. 2013;Nov 7. (Epub ahead of print; DOI:10.1007/s00394-013-0613-8) [DOI] [PubMed] [Google Scholar]
- 8.Istvan ES, Deisenhofer J. The structure of the catalytic portion of human HMG-CoA reductase. Biochim Biophys Acta. 2000;1529:9–18 [DOI] [PubMed] [Google Scholar]
- 9.Dong XY, Tang SQ. Insulin-induced gene: a new regulator in lipid metabolism. Peptides. 2010;31:2145–50 [DOI] [PubMed] [Google Scholar]
- 10.Palmieri F, Bisaccia F, Iacobazzi V, Indiveri C, Zara V. Mitochondrial substrate carriers. Biochim Biophys Acta. 1992;1101:223–7 [PubMed] [Google Scholar]
- 11.Inoue K, Zhuang L, Ganapathy V. Human Na+-coupled citrate transporter: primary structure, genomic organization, and transport function. Biochem Biophys Res Commun. 2002;299:465–71 [DOI] [PubMed] [Google Scholar]
- 12.Gnoni GV, Priore P, Geelen MJ, Siculella L. The mitochondrial citrate carrier: metabolic role and regulation of its activity and expression. IUBMB Life. 2009;61:987–94 [DOI] [PubMed] [Google Scholar]
- 13.Palmieri F. The mitochondrial transporter family SLC25: identification, properties and physiopathology. Mol Aspects Med. 2013;34:465–84 [DOI] [PubMed] [Google Scholar]
- 14.Kaplan RS, Mayor JA. Structure, function and regulation of the tricarboxylate transport protein from rat liver mitochondria. J Bioenerg Biomembr. 1993;25:503–14 [DOI] [PubMed] [Google Scholar]
- 15.Walters DE, Kaplan RS. Homology-modeled structure of the yeast mitochondrial citrate transport protein. Biophys J. 2004;87:907–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capobianco L, Bisaccia F, Michel A, Sluse FE, Palmieri F. The N- and C-termini of the tricarboxylate carrier are exposed to the cytoplasmic side of the inner mitochondrial membrane. FEBS Lett. 1995;357:297–300 [DOI] [PubMed] [Google Scholar]
- 17.Capobianco L, Ferramosca A, Zara V. The mitochondrial tricarboxylate carrier of silver eel: dimeric structure and cytosolic exposure of both N- and C-termini. J Protein Chem. 2002;21:515–21 [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Kakhniashvili DA, Gremse DA, Wood DO, Mayor JA, Walters DE, Kaplan RS. The yeast mitochondrial citrate transport protein. Probing the roles of cysteines, Arg(181), and Arg(189) in transporter function. J Biol Chem. 2000;275:7117–24 [DOI] [PubMed] [Google Scholar]
- 19.Nelson DR, Felix CM, Swanson JM. Highly conserved charge-pair networks in the mitochondrial carrier family. J Mol Biol. 1998;277:285–308 [DOI] [PubMed] [Google Scholar]
- 20.Nury H, Dahout-Gonzalez C, Trézéguet V, Lauquin GJ, Brandolin G, Pebay-Peyroula E. Relations between structure and function of the mitochondrial ADP/ATP carrier. Annu Rev Biochem. 2006;75:713–41 [DOI] [PubMed] [Google Scholar]
- 21.Herzig S, Raemy E, Montessuit S, Veuthey JL, Zamboni N, Westermann B, Kunji ER, Martinou JC. Identification and functional expression of the mitochondrial pyruvate carrier. Science. 2012;337:93–6 [DOI] [PubMed] [Google Scholar]
- 22.Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen YC, Cox JE, Cardon CM, Van Vranken JG, Dephoure N, et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science. 2012;337:96–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunji ER, Crichton PG. doi: 10.1016/j.bbabio.2010.03.023. Mitochondrial carriers function as monomers. Biochim Biophys Acta. 2010;1797:817–31. [DOI] [PubMed] [Google Scholar]
- 24.Zara V, Ferramosca A, Palmisano I, Palmieri F, Rassow J. Biogenesis of rat mitochondrial citrate carrier (CIC): the N-terminal presequence facilitates the solubility of the preprotein but does not act as a targeting signal. J Mol Biol. 2003;325:399–408 [DOI] [PubMed] [Google Scholar]
- 25.Zara V, Dolce V, Capobianco L, Ferramosca A, Papatheodorou P, Rassow J, Palmieri F. Biogenesis of eel liver citrate carriCIC): negative charges can substitute for positive charges in the presequence. J Mol Biol. 2007;365:958–67 [DOI] [PubMed] [Google Scholar]
- 26.Ferramosca A, Zara V. Biogenesis of mitochondrial carrier proteins: molecular mechanisms of import into mitochondria. Biochim Biophys Acta. 2013;1833:494–502 [DOI] [PubMed] [Google Scholar]
- 27.Klingenberg M. Kinetic study of the tricarboxylate carrier in rat liver mitochondria. Eur J Biochem. 1972;26:587–94 [DOI] [PubMed] [Google Scholar]
- 28.Bisaccia F, De Palma A, Palmieri F. Identification and purification of the tricarboxylate carrier from rat liver mitochondria. Biochim Biophys Acta. 1989;977:171–6 [DOI] [PubMed] [Google Scholar]
- 29.Kaplan RS, Mayor JA, Johnston N, Oliveira DL. Purification and characterization of the reconstitutively active tricarboxylate transporter from rat liver mitochondria. J Biol Chem. 1990;265:13379–85 [PubMed] [Google Scholar]
- 30.Zara V, Palmieri L, Franco MR, Perrone M, Gnoni GV, Palmieri F. Kinetics of the reconstituted tricarboxylate carrier from eel liver mitochondria. J Bioenerg Biomembr. 1998;30:555–63 [DOI] [PubMed] [Google Scholar]
- 31.Kaplan RS. Structure and function of mitochondrial anion transport proteins. J Membr Biol. 2001;179:165–83 [DOI] [PubMed] [Google Scholar]
- 32.Infantino V, Convertini P, Cucci L, Panaro MA, Di Noia MA, Calvello R, Palmieri F, Iacobazzi V. The mitochondrial citrate carrier: a new player in inflammation. Biochem J. 2011;438:433–6 [DOI] [PubMed] [Google Scholar]
- 33.Catalina-Rodriguez O, Kolukula VK, Tomita Y, Preet A, Palmieri F, Wellstein A, Byers S, Giaccia AJ, Glasgow E, Albanese C, et al. The mitochondrial citrate transporter, CIC, is essential for mitochondrial homeostasis. Oncotarget. 2012;3:1220–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joseph JW, Jensen MV, Ilkayeva O, Palmieri F, Alárcon C, Rhodes CJ, Newgard CB. The mitochondrial citrate/isocitrate carrier plays a regulatory role in glucose-stimulated insulin secretion. J Biol Chem. 2006;281:35624–32 [DOI] [PubMed] [Google Scholar]
- 35.Cappello AR, Guido C, Santoro A, Santoro M, Capobianco L, Montanaro D, Madeo M, Andò S, Dolce V, Aquila S. The mitochondrial citrate carrier (CIC) is present and regulates insulin secretion by human male gamete. Endocrinology. 2012;153:1743–54 [DOI] [PubMed] [Google Scholar]
- 36.Morciano P, Carrisi C, Capobianco L, Mannini L, Burgio G, Cestra G, De Benedetto GE, Corona DF, Musio A, Cenci G. A conserved role for the mitochondrial citrate transporter Sea/SLC25A1 in the maintenance of chromosome integrity. Hum Mol Genet. 2009;18:4180–8 [DOI] [PubMed] [Google Scholar]
- 37.Hill R, Linazasoro JM, Chevallier F, Chaikoff IL. Regulation of hepatic lipogenesis: the influence of dietary fats. J Biol Chem. 1958;233:305–10 [PubMed] [Google Scholar]
- 38.Sabine JR, McGrath H, Abraham S. Dietary fat and the inhibition of hepatic lipogenesis in the mouse. J Nutr. 1969;98:312–8 [DOI] [PubMed] [Google Scholar]
- 39.Herzberg GR, Janmohamed N. Regulation of hepatic lipogenesis by dietary maize oil or tripalmitin in the meal-fed mouse. Br J Nutr. 1980;43:571–9 [DOI] [PubMed] [Google Scholar]
- 40.Zara V, Giudetti AM, Siculella L, Palmieri F, Gnoni GV. Covariance of tricarboxylate carrier activity and lipogenesis in liver of polyunsaturated fatty acid (n–6) fed rats. Eur J Biochem. 2001;268:5734–9 [DOI] [PubMed] [Google Scholar]
- 41.Siculella L, Damiano F, Sabetta S, Gnoni GV. n–6 PUFAs downregulate expression of the tricarboxylate carrier in rat liver by transcriptional and posttranscriptional mechanisms. J Lipid Res. 2004;45:1333–40 [DOI] [PubMed] [Google Scholar]
- 42.Ferramosca A, Savy V, Zara V. Olive oil increases the hepatic triacylglycerol content in mice by a distinct influence on the synthesis and oxidation of fatty acids. Biosci Biotechnol Biochem. 2008;72:62–9 [DOI] [PubMed] [Google Scholar]
- 43.Ferramosca A, Savy V, Einherand AWC, Zara V. Pinus koraiensis seed oil (PinnoThinTM) supplementation reduces body weight gain and lipid concentration of liver and plasma in mice. J Anim Feed Sci. 2008;17:621–30 [Google Scholar]
- 44.Imbs AB, Nevshupova NV, Pham LQ. Triacylglycerol composition of Pinus koraiensis seed oil. J Am Oil Chem Soc. 1998;75:865–70 [Google Scholar]
- 45.Giudetti AM, Sabetta S, Di Summa R, Leo M, Damiano F, Siculella L, Gnoni GV. Differential effects of coconut oil- and fish oil-enriched diets on tricarboxylate carrier in rat liver mitochondria. J Lipid Res. 2003;44:2135–41 [DOI] [PubMed] [Google Scholar]
- 46.Ferramosca A, Conte L, Zara V. A krill oil supplemented diet reduces the activities of the mitochondrial tricarboxylate carrier and of the cytosolic lipogenic enzymes in rats. J Anim Physiol Anim Nutr (Berl). 2012;96:295–306 [DOI] [PubMed] [Google Scholar]
- 47.Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, Fishell V, Hargrove RL, Zhao G, Etherton TD. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71(Suppl 1):179S–88S [DOI] [PubMed] [Google Scholar]
- 48.Ferramosca A, Conte A, Burri L, Berge K, De Nuccio F, Giudetti AM, Zara V. A krill oil supplemented diet suppresses hepatic steatosis in high-fat fed rats. PLoS One. 2012;7:e38797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Damiano F, Gnoni GV, Siculella L. Functional analysis of rat liver citrate carrier promoter: differential responsiveness to polyunsaturated fatty acids. Biochem J. 2009;417:561–71 [DOI] [PubMed] [Google Scholar]
- 50.Ferramosca A, Zara V. Modulation of hepatic steatosis by dietary fatty acids. World J Gastroenterol. 2014;20:1746–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferramosca A, Savy V, Conte L, Colombo S, Einerhand AW, Zara V. Conjugated linoleic acid and hepatic lipogenesis in mouse: role of the mitochondrial citrate carrier. J Lipid Res. 2006;47:1994–2003 [DOI] [PubMed] [Google Scholar]
- 52.Vyas D, Kadegowda AK, Erdman RA. Dietary conjugated linoleic acid and hepatic steatosis: species-specific effects on liver and adipose lipid metabolism and gene expression. J Nutr Metab. 2012;2012:932928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clément L, Poirier H, Niot I, Bocher V, Guerre-Millo M, Krief S, Staels B, Besnard P. Dietary trans-10, cis-12 conjugated linoleic acid induces hyperinsulinemia and fatty liver in the mouse. J Lipid Res. 2002;43:1400–9 [DOI] [PubMed] [Google Scholar]
- 54.Ferramosca A, Savy V, Conte L, Zara V. Dietary combination of conjugated linoleic acid (CLA) and pine nut oil prevents CLA-induced fatty liver in mice. J Agric Food Chem. 2008;56:8148–58 [DOI] [PubMed] [Google Scholar]
- 55.Yanagita T, Wang YM, Nagao K, Ujino Y, Inoue N. Conjugated linoleic acid-induced fatty liver can be attenuated by combination with docosahexaenoic acid in C57BL/6N mice. J Agric Food Chem. 2005;53:9629–33 [DOI] [PubMed] [Google Scholar]
- 56.Ide T. Interaction of fish oil and conjugated linoleic acid in affecting hepatic activity of lipogenic enzymes and gene expression in liver and adipose tissue. Diabetes. 2005;54:412–23 [DOI] [PubMed] [Google Scholar]
- 57.Siculella L, Sabetta S, Damiano F, Giudetti AM, Gnoni GV. Different dietary fatty acids have dissimilar effects on activity and gene expression of mitochondrial tricarboxylate carrier in rat liver. FEBS Lett. 2004;578:280–4 [DOI] [PubMed] [Google Scholar]
- 58.Portillo MP, Chávarri M, Durán D, Rodríguez VM, Macarulla MT. Differential effects of diets that provide different lipid sources on hepatic lipogenic activities in rats under ad libitum or restricted feeding. Nutrition. 2001;17:467–73 [DOI] [PubMed] [Google Scholar]
- 59.Takeuchi H, Nakamoto T, Mori Y, Kawakami M, Mabuchi H, Ohishi Y, Ichikawa N, Koike A, Masuda K. Comparative effects of dietary fat types on hepatic enzyme activities related to the synthesis and oxidation of fatty acid and to lipogenesis in rats. Biosci Biotechnol Biochem. 2001;65:1748–54 [DOI] [PubMed] [Google Scholar]
- 60.Ruiz-Gutiérrez V, Pérez-Espinosa A, Vásquez CM, Santa-Maria C. Effects of dietary fats (fish, olive and high-oleic-acid sunflower oil) on lipid composition and antioxidant enzymes in rat liver. Br J Nutr. 1999;82:233–41 [PubMed] [Google Scholar]
- 61.Giudetti AM, Beynen AC, Lemmens AG, Gnoni GV, Geelen MJ. Hepatic fatty acid metabolism in rats fed diets with different contents of C18:0, C18:1 cis and C18:1 trans isomers. Br J Nutr. 2003;90:887–93 [DOI] [PubMed] [Google Scholar]
- 62.Infantino V, Iacobazzi V, De Santis F, Mastrapasqua M, Palmieri F. Transcription of the mitochondrial citrate carrier gene: role of SREBP-1, upregulation by insulin and downregulation by PUFA. Biochem Biophys Res Commun. 2007;356:249–54 [DOI] [PubMed] [Google Scholar]
- 63.Damiano F, Gnoni GV, Siculella L. Citrate carrier promoter is target of peroxisome proliferator-activated receptor alpha and gamma in hepatocytes and adipocytes. Int J Biochem Cell Biol. 2012;44:659–68 [DOI] [PubMed] [Google Scholar]
- 64.Iacobazzi V, Infantino V, Palmieri F. Transcriptional regulation of the mitochondrial citrate and carnitine/acylcarnitine transporters: two genes involved in fatty acid biosynthesis and β-oxidation. Biology. 2013;2:284–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schumann J, Fuhrmann H. Impairment of NFkappaB activity by unsaturated fatty acids. Int Immunopharmacol. 2010;10:978–84 [DOI] [PubMed] [Google Scholar]
- 66.Iacobazzi V, Infantino V, Bisaccia F, Castegna A, Palmieri F. Role of FOXA in mitochondrial citrate carrier gene expression and insulin secretion. Biochem Biophys Res Commun. 2009;385:220–4 [DOI] [PubMed] [Google Scholar]
- 67.Gnoni GV, Giudetti AM, Mercuri E, Damiano F, Stanca E, Priore P, Siculella L. Reduced activity and expression of mitochondrial citrate carrier in streptozotocin-induced diabetic rats. Endocrinology. 2010;151:1551–9 [DOI] [PubMed] [Google Scholar]
- 68.Palmieri F. The mitochondrial transporter family (SLC25): physiological and pathological implications. Pflugers Arch. 2004;447:689–709 [DOI] [PubMed] [Google Scholar]