Abstract
The typical diet of individuals in Western societies results in metabolic responses associated with fed-state fat metabolism for most of the daily life of the individual. This fat metabolism is characterized specifically by an increase in the concentration of plasma lipids, primarily triglycerides. Increased postprandial lipemia, which is typically observed in older individuals (i.e., >65 y old), has now emerged as an important correlate of cardiovascular disease risk. An understanding of the mechanisms contributing to the increased postprandial lipemia in older individuals becomes, therefore, of particular clinical importance in any effort to explain and address the well-documented increase in cardiovascular disease risk as individuals age. Current evidence points to an increase in the accumulation of ingested lipid in lipoprotein particles of hepatic origin, together with an overall accumulation of lipid in these lipoproteins during the postprandial period, as primary contributors to the postprandial lipemia in older persons. When this evidence is considered together with the evidence suggesting large atherogenic potential of lipoproteins of hepatic origin, this can, at least in part, explain the increased risk of cardiovascular disease in older individuals. Understanding changes in the metabolism of ingested fat in the immediate postprandial period with advancing age, and how lifestyle interventions such as diet and physical exercise can ameliorate the increase in postprandial lipemia in older individuals, is important in order to address the increased cardiovascular disease risk in this particularly affected and growing segment of the population.
Introduction
Cardiovascular disease (CVD),3 secondary to atherosclerosis, is the leading cause of death and a major cause of disability in developed societies (1). At least 70% of people aged between 60 and 79 y and 83% of those >80 y have CVD (2). Furthermore, more than half of cardiovascular procedures are currently performed on individuals aged ≥65 y (2), a number that will grow even larger as the number of older people increases in the near future. This evidence highlights the reduced quality of life for a large segment of the population and the increased adverse socioeconomic impact of CVD.
The specific role of plasma TG concentrations measured in the fasted state in determining the risk of CVD has been a matter of debate (3). However, when plasma TG concentrations are evaluated in the postprandial/fed state, evidence accumulated over the past decade strongly supports a positive association between postprandial lipemia (PPL) and CVD (4–6). The clinical importance of this association can be appreciated even more when considering that postprandial, but not fasting, TGs dominate the plasma TG pool during the course of the day (7). It is therefore imperative to study the metabolism of postprandial, rather than postabsorptive/fasting, TGs in any effort to understand the role of plasma TGs and associated lipoprotein changes in increasing the risk of CVD. In this regard, describing the metabolic processes by which dietary lipid contributes to the accumulation of plasma TGs in the immediate postprandial period is of particular physiologic and clinical importance.
It has been known for some time that older individuals have exaggerated PPL compared with young controls (8–13). This increase in PPL as individuals age may explain the increased incidence of CVD observed in the older segment of the population (1, 14). However, most of the studies indicated above are largely descriptive in nature. Despite the large amount of evidence documenting increased PPL in older persons, to date there have been only few studies that have attempted to investigate the metabolic pathways by which the ingested fat mediates an exaggerated response in plasma TG concentrations during the postprandial period in older individuals. Understanding the metabolic pathways implicated in the postprandial fate of ingested fat and how this fat contributes to the increase in PPL in older persons can lead to specific and targeted interventions that address the increased PPL in these individuals. For the purpose of this article, PPL responses studied in individuals ≥60 y of age have been reviewed.
Clinical Considerations of PPL in Older Individuals
CVD is a major contributor to disability among individuals ≥65 y of age, and CVD even at the subclinical level contributes to increased rates of frailty, hospitalization, and institutionalization in older adults (15), which underlines the burden and the impact of CVD in society, especially because this segment of the population continuous to expand. Plasma cholesterol concentrations have been traditionally measured to evaluate the risk of CVD. However, there is no adequate evidence to support that hypercholesterolemia and/or low cholesterol concentrations in HDL are accurate predictors of heart disease in older individuals (16). In reports that found a negative association between concentrations of HDL cholesterol and all-cause mortality, the predictive value of HDL cholesterol on mortality is not apparent for individuals over the age of 75 y (17). Such evidence suggests diminished clinical importance of traditional markers of lipid metabolism in determining adverse prognosis for CVD in the older segment of the population. On the other hand, characteristically low PPL reported in the case of octogenarians compared with middle-aged individuals (18) suggests that low PPL may be a better marker of longevity and/or reduced CVD risk in individuals of older age.
It has been suggested that evaluating plasma TG concentrations up to 4 h after the fat meal ingestion, a time period during which plasma TG concentrations typically reach a peak, can sufficiently describe the magnitude of PPL in young and middle-aged adults (19). However, in the case of older individuals, the prolonged presence of TGs in the circulation, rather than the peak concentration of plasma TGs, appears to be the primary determinant of the magnitude of PPL (20). Such evidence points to the need for obtaining several measurements of plasma TG concentrations during the postprandial period (i.e., up to 8 h) when evaluating PPL in older adults and in any effort to evaluate postprandial plasma TG responses that are clinically relevant to this population. At the same time, and as the clinical importance of postprandial plasma TG concentrations is becoming more recognized, studies performed specifically in older individuals (i.e., ≥65 y of age) will help to identify the clinical link between increased PPL and CVD in this population.
General Characteristics of PPL in Older Individuals
In a recent study (21), no differences in PPL between groups of young and older healthy individuals were reported. In addition, a single report found lower plasma TG concentrations during the postprandial period in older individuals (18), a finding that may be skewed by the fact that the younger control group was composed of middle-aged individuals rather than young participants. The overwhelming amount of evidence, however, clearly demonstrates that PPL is greater in older individuals (8–13, 20). The response of plasma TGs during the postprandial period in older individuals is characterized by a higher peak (observed typically 3–4 h after ingesting high-fat meal) and a more prolonged presence of these TGs in plasma compared with that in young individuals (8, 10).
Prolonged PPL is probably the most prominent age-related feature of postprandial TG metabolism when comparing responses between old and young adults. In this regard, when healthy older participants were matched to young controls in terms of their fasting plasma TG concentrations, which is a major determinant of the increase in plasma TG concentrations in the postprandial period (9, 22), plasma TG concentrations, including peak response, during the first 4 h of PPL were not different between age groups (20). However, the older individuals still demonstrated greater PPL compared with young individuals when PPL was evaluated during the 8-h period, a difference that was primarily due to the greater increase in PPL after 4 h after the fat ingestion (20). Measurement of plasma TG concentrations during only the first 4 h after meal ingestion may explain the lack of differences in PPL reported previously between old and younger individuals (21).
Current Status of Knowledge on Mechanisms of Increased PPL in Older Adults
Considerations in the interpretation of findings related to PPL in older adults
As with any study investigating in vivo metabolic responses across subgroups of the population, experimental design–related factors can complicate the interpretation of the data describing the mechanisms contributing to an age-specific increase in PPL in older adults. Adipose tissue and muscle are the main tissues responsible for the clearance of plasma TGs, and quantitative and qualitative changes in these tissues in older individuals, such as loss of skeletal muscle and redistribution of fat storage toward visceral fat depots, if not taken into account, can mask specific effects of age, per se, on PPL. Visceral fat, in particular, plays an important role in the metabolism of postprandial TGs (23). From an experimental point of view, body fat and muscle changes that typically occur with aging make it more difficult to identify age-specific mechanisms that may contribute to the increased PPL observed in older adults and that are not the result of changes in body composition. Glucose intolerance with aging (21) can also confound the interpretation of postprandial TG responses in older individuals. Furthermore, because increased basal (i.e., fasting state) plasma TG concentrations can increase the magnitude of PPL (24), increased fasting plasma TG concentrations, a typical observation in older individuals, may by itself contribute to the increased PPL in some of these individuals (10). However, and because when older individuals have basal plasma TG concentrations comparable to those of young controls, they still demonstrate increased PPL (20), there are factors beyond the increase in fasting plasma TG concentrations that mediate the increase in PPL in older adults.
Dietary recommendations are often described according to an individual’s body weight. Therefore, the ingestion of an amount of fat according to an individual’s body mass is an applicable approach to study responses related to the effects of fat intake on PPL in real-life circumstances. However, when evaluating postprandial plasma TG responses from a mechanistic point of view, either accounting for the amount of fat ingested by the participant or ingesting fat according to the fat-free body mass/muscle of the individual to account for the primary role of skeletal muscle in determining plasma TG clearance (25) may be more relevant approaches. Under such conditions, although still present, increased PPL is less evident in older compared with younger individuals, and when PPL is adjusted to either the amount of ingested fat or the amount of ingested fat per unit of fat-free mass [unpublished observations from (20)]. The ingestion of fat according to the individual’s fat-free mass may skew the findings toward lower PPL in older individuals because it ignores the greater amount of adipose tissue typically observed in these individuals, and given that adipose tissue and its associated plasma volume play an important role in both the metabolism and the overall volume of distribution of circulating TGs. Specifically, a greater amount of adipose tissue can provide greater exposure of postprandial TGs to capillary sites for TG breakdown. Also, a greater amount of adipose tissue, which results in a relative increase in the plasma total volume, can contribute to lower plasma TG concentrations for any given amount of postprandial TG load entering the circulation. Therefore, adjusting the oral fat load to the lean body mass alone can underestimate the postprandial response of plasma TGs in older individuals relative to that in young controls. Although it is less practical, providing the fat load according to an individual’s plasma volume is probably a more experimentally relevant approach to evaluate responses in plasma TG concentrations during the postprandial period between populations that differ considerably in body composition, such as between young and older individuals.
Absorption of dietary fat
The absorption of dietary fat and its appearance in plasma chylomicron-TG initiates the increase in the concentration of plasma TGs during the postprandial period. The isolation of specific lipoprotein fractions participating in the metabolism of the dietary fat in plasma during the postprandial period after the ingestion of dietary fat labeled with stable isotopes of lipids, and analyses of the lipids in these lipoproteins using MS techniques, provides a powerful experimental approach to track the metabolism of ingested fat in plasma. By using this approach we have shown that neither the increase in the enrichment of postprandial chylomicron-TGs with stable isotopes over time nor the peak incorporation of labeled lipid into chylomicron-TGs is different between young and older individuals (20). This suggests that the capacity to absorb and accumulate dietary fat into plasma chylomicrons in older individuals is comparable to that in younger individuals. This argument is in line with more direct findings from other studies that indicate that the absorption of dietary fat is not affected by age (26–28).
Dose of the ingested fat
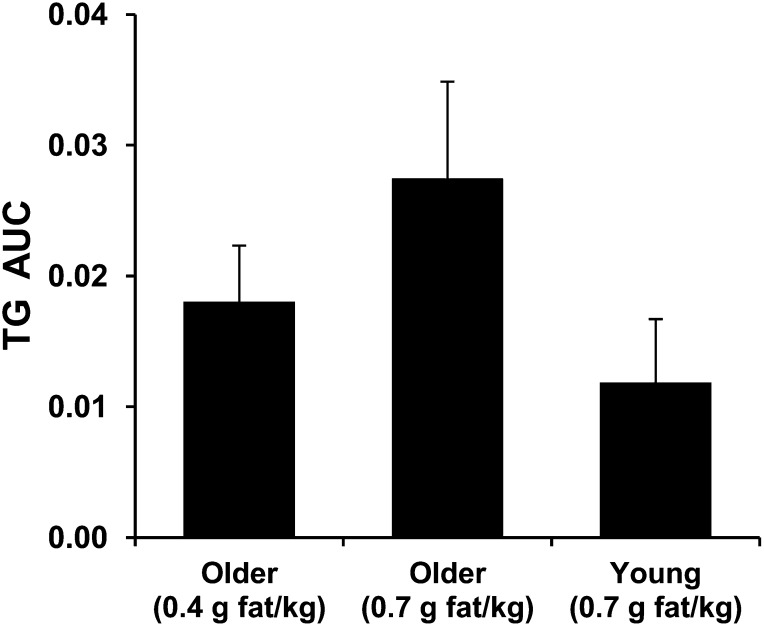
Among several factors that have the potential to modify the postprandial response of plasma TGs, the amount of ingested fat is probably the most potent physiologic factor influencing plasma TG concentrations. Increasing a relatively low dose of dietary fat (0.4 g/kg body weight) by 75% increases the average concentration of plasma TGs during the postprandial period in older persons by ~50% (29). In a series of experiments, we observed that apparently healthy older participants demonstrate PPL comparable to that of young participants when they ingest an amount of fat that is approximately half of that ingested by the young participants (20, 29) (Fig. 1).
FIGURE 1.
Plasma TG response calculated as the area under the plasma TG concentration curve over 8 h that was above the fasting plasma TG concentration (mmol/L × h) and adjusted to the fat-free body mass of the participants [TG AUC: (mmol/L × h)/Kg FFM)]. Participants, all men, ingested either moderate (0.7 g/kg) or low (0.4 g/kg) doses of fat in the form of whipping cream. The young (18–34 y old) and older (63–71 y old) participants had comparable lean body mass. The same older participants were studied according to low and moderate doses of fat. Values are means ± SEs; n = 6 per group of young or older participants. Adapted using data originally presented in references 20 and 63. FFM, fat-free mass.
Because until recently the main emphasis on postprandial lipid metabolism has been placed on plasma TGs, very little is known about the role of ingested fat in altering the postprandial metabolism of plasma FFAs in older individuals. This information is of clinical importance because increased concentrations of plasma FFAs have been linked to increased risk of CVD (30, 31). Current evidence shows that older individuals demonstrate an increase in plasma FFA concentrations during the postprandial period that is proportionally greater than that in plasma TG concentrations as a result of an increase in the amount of ingested fat. Specifically, increasing the amount of ingested fat by 75% increased postprandial plasma FFA concentrations by ~200% but increased postprandial plasma TG concentrations by only 50% in apparently healthy older adults (29).
Hydrolysis of circulating lipoprotein-TGs
Endothelium-bound lipoprotein lipase (LPL) activity across body tissues is the rate-limiting step in the disposal of circulating TGs; therefore, LPL activity provides a direct mechanism to regulate (i.e., either increase or decrease) the concentration of plasma TGs during the postprandial period. Measurement of the LPL activity in plasma, which is collected immediately after the administration of i.v. heparin to release the LPL anchored in the endothelium, is a standard procedure to evaluate the capacity of an individual to hydrolyze circulating TGs. Although it is not always the case (18), postheparin LPL activity appears to decrease (32) with aging. Lower LPL activity can potentially explain the increase in PPL in older individuals, secondary to the decreased capacity for breaking down circulating TGs in these individuals. However, measurement of the activity of LPL in vitro cannot be translated directly into rates of breakdown of TGs taking place in vivo. When TG clearance is evaluated in vivo by using infusions of i.v. fat emulsions, plasma TG clearance does not differ between young and older individuals (33, 34).
Disposal of the ingested fat
Because the chylomicron-TG half-life is ~5 min (35), dietary lipid is liberated from chylomicrons relatively soon after the chylomicrons enter into the circulation. It is now recognized that not all of the lipid from chylomicrons delivered to tissues and hydrolyzed within their capillary walls is taken up locally in the form of FFAs. Some of the chylomicron-liberated FFAs are released back into the circulation and mix with the endogenous plasma FFA pool (36). Although the overall amount of dietary FAs that enter into the circulation depends largely on the amount of fat and the overall composition of the ingested meal, FFAs originating from the ingested fat can contribute as much as 35% to the total pool of postprandial plasma FFAs (37). These data have been largely obtained in young and middle-aged individuals. We have recently documented a substantially greater percentage of dietary FA contribution to the total FFA pool during the postprandial period in old (42%) compared with young (26%) participants (20). These age-related differences cannot be readily explained by methodologic considerations such as those discussed in the earlier paragraphs and related to body composition, because the 2 age groups were matched for fat-free body mass (20). In addition, the average absolute concentration of plasma FFAs originating from the ingested fat during the postprandial period in those studies was still 55% higher in the older individuals, even after accounting for the amount of ingested fat [unpublished observations from (20)].
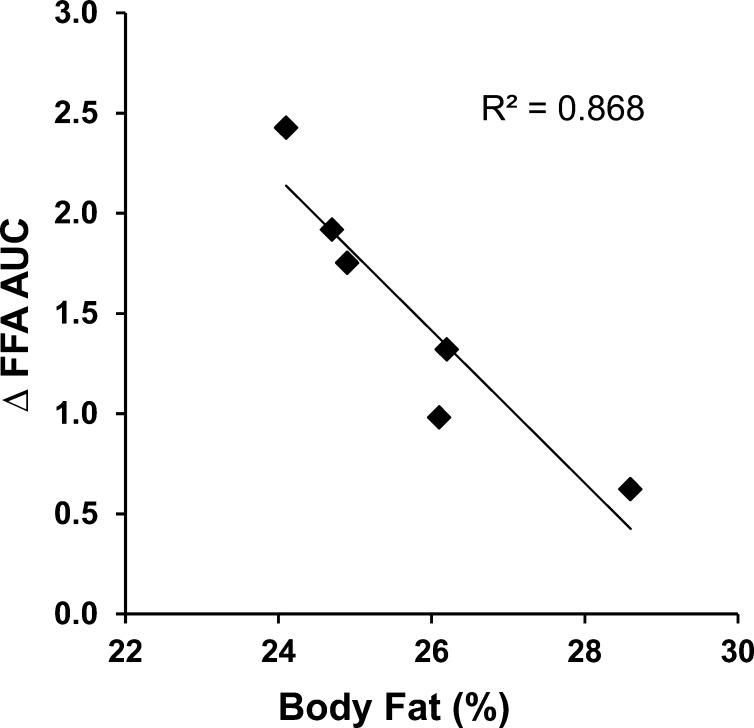
Age-related qualitative changes across tissues that are directly involved in the uptake of FFAs liberated from the hydrolysis of chylomicron-TGs may account for the greater accumulation of dietary lipid in plasma FFAs during the postprandial period in older individuals. There is evidence to suggest that older persons have reduced ability to store dietary fat in subcutaneous adipose tissue (38) and impaired capacity for FA uptake in skeletal muscle (39). An age-related dysfunction of adipose tissue cells in terms of their ability to store fat has been recently reported (40). When a relatively small amount of fat is ingested (i.e., 0.4 g/kg) by older individuals, postprandial accumulation of dietary FAs in plasma remains relatively constant across individuals. However, when a larger amount of fat is ingested (i.e., 0.7 g/kg), the accumulation of dietary FAs in plasma is lower in those individuals who have a greater percentage of body fat (29). We have not observed a similar relation in young individuals (unpublished results). As expected, the accumulation of dietary FAs in plasma during the postprandial period affects the concentration of total (i.e., endogenous + dietary) plasma FFAs in a way that, when a higher amount of fat is ingested, the difference in plasma FFA concentrations due to the increase in the concentration of FFAs originating from the higher dose of ingested fat is inversely related to the percentage of body fat in older individuals (Fig. 2). These observations are interpreted to suggest that increased subcutaneous adipose tissue in older persons may provide the potential to increase the disposal of dietary FAs during the postprandial period when large amounts of fat are consumed by possibly counterbalancing an age-related intrinsic dysfunction in adipose tissue. However, and because increased amounts of body fat contribute to well-known adverse health outcomes, preventing unfavorable qualitative changes in adipose tissue with aging that may impair the disposal of dietary lipid and/or decreasing the amount of ingested fat are more appropriate approaches to mitigate an increase in the accumulation of dietary FAs in plasma during the postprandial period in older adults.
FIGURE 2.
Relation between the difference in plasma FFA accumulations between low (0.4 g/kg) and moderate (0.7 g/kg) doses of ingested fat provided in the form of whipping cream (∆ FFA AUC) and the percentage of body fat in older (63–71 y old) men. FFA accumulation was calculated as the area under the plasma FFA concentration curve over 8 h that was above the fasting plasma FFA concentration (FFA AUC: mmol/L × h). ∆ FFA AUC between the moderate and low doses of ingested fat was calculated by subtracting the FFA AUC resulting from the low dose of ingested fat from that resulting from the moderate dose of ingested fat. Adapted using data originally presented in reference 29.
When compared with adipose tissue and muscle, liver has traditionally received very little attention in terms of its role in dietary fat metabolism during the postprandial period. However, recognition of the liver as a tissue with an important role in the overall postprandial processes of uptake and delivery of dietary lipid in health and disease continues to grow. When considered per unit mass, the liver demonstrates a severalfold greater capacity for uptake of dietary lipid relative to that of either adipose tissue or muscle (41, 42). This suggests that the liver is an important player with great potential for the uptake of dietary lipid in the immediate postprandial period.
A well-established function of the liver, when compared with adipose tissue and muscle, is its ability to directly remove entire lipoprotein particles in the form of lipoprotein remnants, including their TG load. After i.v. administration of an experimental fat emulsion, removal of remnant-like particles was lower in older participants (34), which suggests decreased capacity for direct uptake of TGs via lipoprotein-remnant removal by the liver in older individuals. Morphologic changes in the vasculature of the liver combined with reduced hepatic perfusion with aging have been proposed as mechanisms for decreased hepatic lipoprotein-remnant removal with advancing age (43). However, the liver has the capacity to handle an increased flux of plasma FFAs. The uptake of plasma FFAs by the liver is directly proportional to their presentation to the liver (41, 44). Currently, there is no evidence to indicate that, similar to the metabolism of lipoprotein remnants, FFA uptake by the liver decreases with aging. This provides the potential for increased uptake of FFAs by the liver with aging, including FFAs liberated by LPL in adipose tissue and muscle during the postprandial period and that are not taken up directly by these tissues.
Metabolism of plasma lipoprotein-TGs of hepatic origin during the postprandial period
Concentrations of plasma lipoprotein-TGs of hepatic origin.
Lipoprotein particles originating from the liver can be divided both from a physiologic as well as a clinical perspective into 2 distinct pools: one that contains recently secreted large VLDL particles and another one that contains VLDL remnants that are formed after most of the TG content of the VLDL has been cleared. Although the concentration of intestinally derived chylomicrons increases greatly immediately after fat ingestion relative to the postabsorptive state, and as a result of the absorption of the dietary fat, in absolute terms the concentration of VLDL particles still dominates the TG-rich lipoprotein pool during the postprandial period (45, 46). The concentration of VLDL-TGs may even increase during the course of the postprandial period, a response that is linked, at least in part, to the preferential clearance of chylomicron-TGs during this period (47). VLDL-TG concentrations peak at ~4 h after fat meal ingestion in young individuals, but they peak at a later time (i.e., 6 h) and stay elevated for a longer period of time during the postprandial period in older individuals, a response that also mirrors that of overall plasma TG concentrations in the older individuals (8). Such evidence points not only to a greater role of VLDL-TGs in determining the overall increase in the concentrations of plasma TGs during the postprandial period in older individuals but also to the existence of some distinct mechanisms implicated in the metabolism of VLDL-TGs during the postprandial period in these individuals. Increased concentrations of both plasma VLDL-TGs and plasma total TGs in the postprandial period are positively linked to increased fat content in the liver in middle-aged individuals (48). Thus, greater concentrations of plasma VLDL-TGs and plasma total TGs manifested during the postprandial period in older individuals (8) may also be related to the liver fat content in these individuals, because liver fat content is typically higher in older people (49).
Older individuals demonstrate increased production of apo B-100 (50), a protein contained specifically in hepatic VLDL, and which is important for determining the rate of VLDL production in the liver (51). When considering that older individuals have higher concentrations of postprandial plasma FFAs (20), which stimulate VLDL-TG secretion (52), increased production of apo B-100 can facilitate greater secretion of postprandial VLDL-TGs in the older individuals. Because lipolysis of VLDL-TGs during the postprandial period is hindered by preferential lipolysis of chylomicron-TGs (47), an increased production rate of VLDL-TGs can contribute further to the increase in the VLDL-TG pool and explain the greater concentrations of VLDL-TGs observed in older individuals during the postprandial period (8).
The accumulation of lipoprotein remnants in plasma has long been suggested to increase the risk of CVD, and the accumulation of such remnants is a strong predictor of cardiovascular events when compared with traditional markers, such as LDL-cholesterol concentration (53, 54). Although the liver in older individuals displays a reduced capacity to remove lipoprotein remnants (34), currently there is no evidence that describes the extent to which lipoprotein remnants of intestinal versus hepatic origin dominate the plasma lipoprotein-remnant pool during the postprandial period in older persons. The accumulation of lipoprotein remnants, and their associated TGs, in the postprandial period has traditionally been interpreted as reduced capacity to remove plasma chylomicrons. However, there is limited quantitative evidence related to the nature of the lipoproteins that contribute to the total lipoprotein remnant pool in the postprandial period. The application of experimental approaches that distinguish between intestinal versus hepatic lipoprotein remnants are of particular importance for the study of PPL in older individuals when considering that VLDL-TG concentrations in the postprandial period increase more in older than in young individuals and that the metabolic product of VLDL, namely VLDL remnants, are important contributors to heart disease (55, 56). The extent to which PPL increases the risk of CVD may indeed depend on the extent to which (or whether) PPL involves an increase in VLDL-TGs during the postprandial period in older individuals. In support of this argument, accumulation of TG-rich lipoprotein particles originating from the liver is what characterizes hypertriglyceridemic patients with CVD (57). These VLDL-TG particles are also significantly related to the progression of atherosclerosis, independently of HDL-cholesterol concentrations (58).
Role of the liver in the recycling of the ingested fat.
Part of the ingested lipid appearing in the plasma lipid pool during the postprandial period is taken up by the liver and rapidly resecreted back into the circulation in the form of VLDL-TGs. On average, 17–20% of the ingested lipid is recycled into hepatic VLDL-TGs during the postprandial period in healthy individuals, and this amount is greater (i.e., up to 33–40%) in the later parts of the postprandial period (59, 60). After fat ingestion, 36% of the ingested lipid appears into the plasma VLDL-TG pool during the 8-h postprandial period in older individuals, a contribution that is significantly higher than that observed (i.e., 21%) in young controls (20). These differences occur in parallel with average contributions of the ingested lipid to the postprandial plasma FFA pool of 41% and 26% in old and young individuals, respectively. Because the liver is a major tissue involved in the uptake of circulating FFAs (41, 44), increased recycling of dietary fat into VLDL particles in older persons can be directly linked to the increase in the dietary FA concentrations in the postprandial plasma FFA pool in these individuals (20).
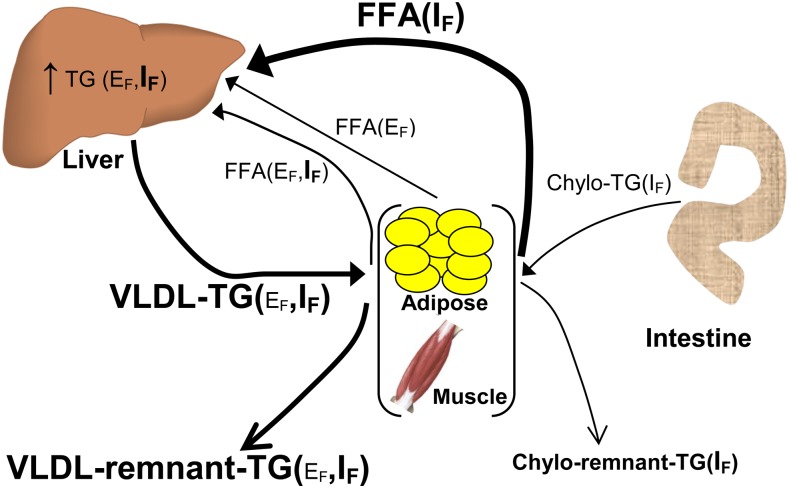
Compared with young controls, increased concentrations of plasma TGs during the postprandial period in older individuals are more evident after the first 4 h after fat ingestion and coincide with a greater increase in concentrations of dietary lipid in the plasma FFA pool (20). Such evidence adds indirect support for an increased contribution of the ingested fat to the greater plasma TG concentrations during the postprandial period in older individuals via increased hepatic TG secretion that is secondary to increased accumulation of ingested fat in the postprandial plasma FFA pool in these individuals. Given that the liver normally has a severalfold capacity for lipid storage (61), influx of dietary lipid to the liver in the postprandial period in young healthy individuals is expected to have a smaller effect in stimulating VLDL-TG production when compared with that in older people. This is due to an already increased fasting lipid content in the liver of older persons (49), and therefore a lower capacity to store increased amounts of lipid delivered to the liver during the postprandial period, which creates a physiologic circumstance that favors VLDL-TG production (as depicted in Fig. 3) Given also the recent evidence suggesting that TGs found in VLDL remnants, and not in chylomicron remnants, are the major contributor to the increase in TG concentrations during the postprandial period (55), dietary lipid carried in lipoprotein particles from hepatic origin may ultimately have a primary role in determining the magnitude of postprandial plasma TGs in older individuals. There is an apparent need for more studies that directly assess the quantitative importance of increased hepatic recycling of dietary lipid during the postprandial period in determining the magnitude of PPL with advanced age.
FIGURE 3.
Proposed mechanisms associated with altered metabolism of ingested fat in older individuals. Metabolic processes and metabolites that are increased in older compared with young individuals are indicated with bold lines and bold type, respectively. Chylo, chylomicron; EF, endogenous fat; IF, ingested fat.
Limitations of the Current Studies
A common observation among studies investigating the response of PPL in older individuals relative to that in young controls is the existence of a more prolonged increase in plasma TG concentrations in the older individuals (8, 10, 20). Therefore, evaluating the increase in plasma TG concentrations only during the early part of the postprandial period may not accurately describe the complete effects of fat meal ingestion on the perturbation of plasma lipids induced by fat meal ingestion in older people. This may explain the reported lack of difference in PPL between young and older individuals when the increase in plasma TG concentrations was evaluated during only the first 4 h after high-fat meal ingestion (21).
Considerable redistribution of body fat in the visceral area in some older individuals, which increases the exposure of the liver to lipids through the portal vein, may confound specific effects of aging on peripheral tissues (i.e., adipose tissue, muscle) in determining the magnitude of PPL in older persons. Studies that control for the specific effects of liver fat content on PPL are necessary to understand the specific role of extrahepatic tissues in regulating the PPL with aging. Because TG-rich lipoproteins from intestinal and hepatic origin share largely similar chemical properties in vitro, it is important to develop and use analytical procedures that can precisely quantify ingested lipid in these 2 biochemically similar but physiologically distinct lipoprotein pools, including their remnants, and to evaluate mechanisms implicated in the increased PPL with aging and the role of such lipoprotein remnants in determining the risk of CVD in older adults.
Future Directions
Table 1 summarizes major areas of investigation that deserve particular attention regarding the clinical aspects of PPL and the regulation of dietary lipid metabolism in older adults. The physiologic and clinical importance of postprandial lipid metabolism can be particularly appreciated when considering the increased fat content of a typical Western diet, which results in persons, including older individuals, spending most of the 24-h day in a postprandial state associated with increased plasma TG concentrations. Current evidence shows that TG concentrations in the postprandial state are positively linked to CVD.
TABLE 1.
Future research directions in the area of postprandial lipid metabolism in older adults1
| Describe the role of postprandial plasma TG versus FFA concentrations in the CVD risk in older adults |
| Understand how aging affects the handling of dietary lipid at the level of subcutaneous adipose tissue |
| Understand the postprandial partitioning of dietary lipid between hepatic TG storage and VLDL-TG secretion as a function of age |
| Quantify the contribution of lipoprotein remnants of intestinal versus hepatic origin to total postprandial plasma TG concentrations with aging |
| Identify specific and practical lifestyle interventions (e.g., nutrition, exercise) to ameliorate PPL in older adults |
CVD, cardiovascular disease; PPL, postprandial lipemia.
On the basis of current evidence it appears that the liver, which receives large amounts of dietary lipid relative to its size during the postprandial period, has a primary role in determining the magnitude of PPL in older individuals. This role is, in part, enhanced by already increased concentrations of postabsorptive lipid in the liver of older individuals. Increased hepatic lipid content, in combination with increased LPL-liberated dietary lipid present in the plasma during the postprandial period in older individuals, which exposes the liver to increased lipid, can stimulate hepatic TG secretion during the later parts of the postprandial period.
In addition to the need for studies that unravel the specific mechanisms contributing to the increased PPL with aging, parallel research efforts need to focus on establishing approaches that are effective in decreasing the magnitude of PPL in older individuals. Supplementation with long-chain n–3 FAs appears to attenuate the magnitude of PPL in young participants (62), and we have shown that in older individuals an acute increase in the plasma l-arginine concentration, a component of dietary protein, decreases the concentrations of plasma TGs and dietary FAs in the plasma FFA pool during the postprandial period (63). Although these lines of evidence provide promising support for approaches to decrease the magnitude of PPL, more direct evidence is necessary before establishing relevant dietary recommendations to ameliorate postprandial lipid responses associated with fat meal ingestion in older adults. Reducing the amount of ingested fat is the most direct approach in preventing large increases in PPL observed with aging. Evidence showing that decreasing the amount of ingested fat decreases the concentrations of both TGs and FFAs during the postprandial period (29) supports this notion. In this context, it is important to reconsider the extent to which general dietary practices are, in fact, appropriate to be applied in adults across the age spectrum. For example, when considering calls for increased dietary protein intake in older individuals (64), together with evidence discussed herein indicating the need to decrease meal-fat intake to prevent increases in PPL, the development of dietary recommendations related to meal macronutrient composition that address specifically the needs of older adults becomes of particular clinical importance for this segment of the population.
Aerobic exercise attenuates PPL in young individuals (65, 66), but similar evidence related to individuals of advanced age (i.e., >65 y) does not exist. The fact that physical exercise decreases postprandial TG concentrations in younger individuals by decreasing primarily the postprandial VLDL-TG pool (67), which appears to be main contributor to the overall increase in postprandial plasma TG concentrations in older persons (Fig. 3), constitutes promising evidence. Also, there is evidence for increased lipid oxidation, including increased hepatic lipid oxidation, with exercise (67, 68), suggesting that exercise may provide a targeted approach to decrease postprandial TG concentrations in lipoproteins of hepatic origin in older individuals by diverting lipids toward oxidation and away from recycling into VLDL-TGs. Future studies need to provide evidence for the existence of such exercise-mediated mechanisms in attenuating PPL in older individuals, which will encourage the incorporation of physical exercise as part of lifestyle interventions to ameliorate postprandial lipid responses in older persons.
The idea of developing a standardized fat-tolerance test in parallel with the establishment of relevant reference values of clinical importance (69, 70) to predict CVD risk is not different from the well-established application of the glucose tolerance test to predict the risk of type 2 diabetes. However, concerns related to the amount and type of dietary lipid(s) ingested, as well as the extent to which postprandial changes in plasma FFAs or lipoprotein remnants may be more relevant to CVD than changes in plasma TGs, have not permitted the development of such a test in a way that is applicable to clinical practice. Furthermore, practical concerns related to the duration of such a test and whether an oral versus an i.v. fat-tolerance test is more appropriate in this regard also have generally restricted any attempt to use such a test in a clinical setting. When such concerns have been addressed, a fat-tolerance test can become an invaluable clinical tool to diagnose risk of CVD early in older individuals and subsequently monitor this risk over time, in a way that allows developing and applying appropriate lifestyle and/or pharmacologic approaches that ensure reduced CVD risk as individuals grow older.
Acknowledgments
The sole author read and approved the final manuscript.
Footnotes
Abbreviations used: CVD, cardiovascular disease; LPL, lipoprotein lipase; PPL, postprandial lipemia.
Literature Cited
- 1.Mensah GA, Brown DW. An overview of cardiovascular disease burden in the United States. Health Aff (Millwood). 2007;26:38–48 [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. ; American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austin MA. Plasma triglyceride and coronary heart disease. Arterioscler Thromb. 1991;11:2–14 [DOI] [PubMed] [Google Scholar]
- 4.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308 [DOI] [PubMed] [Google Scholar]
- 5.Rivellese AA, Bozzetto L, Annuzzi G. Postprandial lipemia, diet, and cardiovascular risk. Curr Cardiovasc Risk Rep. 2009;3:5–11 [Google Scholar]
- 6.Kolovou GD, Mikhailidis DP, Kovar J, Lairon D, Nordestgaard BG, Ooi TC, Perez-Martinez P, Bilianou H, Anagnostopoulou K, Panotopoulos G. Assessment and clinical relevance of non-fasting and postprandial triglycerides: an expert panel statement. Curr Vasc Pharmacol. 2011;9:258–70 [DOI] [PubMed] [Google Scholar]
- 7.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassader M, Gambino R, Ruiu G, Marena S, Bodoni P, Pagano G. Postprandial triglyceride-rich lipoprotein changes in elderly and young subjects. Aging (Milano). 1996;8:421–8 [DOI] [PubMed] [Google Scholar]
- 9.Cohn JS, McNamara JR, Cohn SD, Ordovas JM, Schaefer EJ. Postprandial plasma lipoprotein changes in human subjects of different ages. J Lipid Res. 1988;29:469–79 [PubMed] [Google Scholar]
- 10.Issa JS, Diament J, Forti N. [Postprandial lipemia: influence of aging.] Arq Bras Cardiol. 2005;85:15–9 [DOI] [PubMed] [Google Scholar]
- 11.Jackson KG, Abraham EC, Smith AM, Murray P, O'Malley B, Williams CM, Minihane AM. Impact of age and menopausal status on the postprandial triacylglycerol response in healthy women. Atherosclerosis. 2010;208:246–52 [DOI] [PubMed] [Google Scholar]
- 12.Krasinski SD, Cohn JS, Schaefer EJ, Russell RM. Postprandial plasma retinyl ester response is greater in older subjects compared with younger subjects: evidence for delayed plasma clearance of intestinal lipoproteins. J Clin Invest. 1990;85:883–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nabeno Y, Fukuchi Y, Matsutani Y, Naito M. Influence of aging and menopause on postprandial lipoprotein responses in healthy adult women. J Atheroscler Thromb. 2007;14:142–50 [DOI] [PubMed] [Google Scholar]
- 14.Grundy SM, Pasternak R, Greenland P, Smith S, Jr, Fuster V. AHA/ACC scientific statement: assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. J Am Coll Cardiol. 1999;34:1348–59 [DOI] [PubMed] [Google Scholar]
- 15. Epidemiology of cardiovascular disease. In: Fuster V, Kelly BB, editors. Promoting cardiovascular health in the developing world: a critical challenge to achieve global health. Institute of Medicine. Washington: The National Academies Press; 2010. [PubMed]
- 16.Krumholz HM, Seeman TE, Merrill SS, Mendes de Leon CF, Vaccarino V, Silverman DI, Tsukahara R, Ostfeld AM, Berkman LF. Lack of association between cholesterol and coronary heart disease mortality and morbidity and all-cause mortality in persons older than 70 years. JAMA. 1994;272:1335–40 [PubMed] [Google Scholar]
- 17.Chyou PH, Eaker ED. Serum cholesterol concentrations and all-cause mortality in older people. Age Ageing. 2000;29:69–74 [DOI] [PubMed] [Google Scholar]
- 18.Weintraub M, Ringel Y, Rassin T, Arad J, Bodner G, Liron M. Octogenarian subjects have low postprandial levels of chylomicron remnants: a possible explanation for protection against atherosclerosis. J Gerontol. 1992;47:B209–13 [DOI] [PubMed] [Google Scholar]
- 19.Rector RS, Linden MA, Zhang JQ, Warner SO, Altena TS, Smith BK, Ziogas GG, Liu Y, Thomas TR. Predicting postprandial lipemia in healthy adults and in at-risk individuals with components of the cardiometabolic syndrome. J Clin Hypertens (Greenwich). 2009;11:663–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puga GM, Meyer C, Everman S, Mandarino LJ, Katsanos CS. Postprandial lipemia in the elderly involves increased incorporation of ingested fat in plasma free fatty acids and small (Sf 20–400) triglyceride-rich lipoproteins. Am J Physiol Endocrinol Metab. 2011;301:E356–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez-Caballero AI, Alcala-Diaz JF, Perez-Martinez P, Garcia-Rios A, Delgado-Casado N, Marin C, Yubero-Serrano E, Camargo A, Caballero J, Malagon MM, et al. Lipid metabolism after an oral fat test meal is affected by age-associated features of metabolic syndrome, but not by age. Atherosclerosis. 2013;226:258–62 [DOI] [PubMed] [Google Scholar]
- 22.Syvänne M, Talmud PJ, Humphries SE, Fisher RM, Rosseneu M, Hilden H, Taskinen MR. Determinants of postprandial lipemia in men with coronary artery disease and low levels of HDL cholesterol. J Lipid Res. 1997;38:1463–72 [PubMed] [Google Scholar]
- 23.Nelson RH, Basu R, Johnson CM, Rizza RA, Miles JM. Splanchnic spillover of extracellular lipase-generated fatty acids in overweight and obese humans. Diabetes. 2007;56:2878–84 [DOI] [PubMed] [Google Scholar]
- 24.Couillard C, Bergeron N, Prud'homme D, Bergeron J, Tremblay A, Bouchard C, Mauriege P, Despres JP. Postprandial triglyceride response in visceral obesity in men. Diabetes. 1998;47:953–60 [DOI] [PubMed] [Google Scholar]
- 25.Rössner S, Eklund B, Kaijser L, Olsson AG, Walldius G. Removal of exogenous plasma triglycerides in forearm muscle and subcutaneous tissue of hyper-and normotriglyceridaemic men. Eur J Clin Invest. 1976;6:299–305 [DOI] [PubMed] [Google Scholar]
- 26.Borel P, Mekki N, Boirie Y, Partier A, Alexandre-Gouabau MC, Grolier P, Beaufrere B, Portugal H, Lairon D, Azais-Braesco V. Comparison of the postprandial plasma vitamin A response in young and older adults. J Gerontol A Biol Sci Med Sci. 1998;53:B133–40 [DOI] [PubMed] [Google Scholar]
- 27.Relas H, Gylling H, Rajaratnam RA, Miettinen TA. Postprandial retinyl palmitate and squalene metabolism is age dependent. J Gerontol A Biol Sci Med Sci. 2000;55:B515–21 [DOI] [PubMed] [Google Scholar]
- 28.Simko V, Michael S. Absorptive capacity for dietary fat in elderly patients with debilitating disorders. Arch Intern Med. 1989;149:557–60 [PubMed] [Google Scholar]
- 29.Puga GM, Meyer C, Mandarino LJ, Katsanos CS. Postprandial spillover of dietary lipid into plasma is increased with moderate amounts of ingested fat and is inversely related to adiposity in healthy older men. J Nutr. 2012;142:1806–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frayn KN, Williams CM, Arner P. Are increased plasma non-esterified fatty acid concentrations a risk marker for coronary heart disease and other chronic diseases? Clin Sci (Lond). 1996;90:243–53 [DOI] [PubMed] [Google Scholar]
- 31.Pilz S, Marz W. Free fatty acids as a cardiovascular risk factor. Clin Chem Lab Med. 2008;46:429–34 [DOI] [PubMed] [Google Scholar]
- 32.Brodows RG, Campbell RG. Effect of age on post-heparin lipase. N Engl J Med. 1972;287:969–70 [DOI] [PubMed] [Google Scholar]
- 33.Aberg W, Thorne A, Olivecrona T, Nordenstrom J. Fat oxidation and plasma removal capacity of an intravenous fat emulsion in elderly and young men. Nutrition. 2006;22:738–43 [DOI] [PubMed] [Google Scholar]
- 34.Vinagre CGC, Vinagre JCM, Pozzi FS, Maranhao RC. Influence of aging on chylomicron metabolism. Int J Atheroscler. 2007;2:284–8 [Google Scholar]
- 35.Cohen JC. Chylomicron triglyceride clearance: comparison of three assessment methods. Am J Clin Nutr. 1989;49:306–13 [DOI] [PubMed] [Google Scholar]
- 36.Barrows BR, Timlin MT, Parks EJ. Spillover of dietary fatty acids and use of serum nonesterified fatty acids for the synthesis of VLDL-triacylglycerol under two different feeding regimens. Diabetes. 2005;54:2668–73 [DOI] [PubMed] [Google Scholar]
- 37.Lambert JE, Parks EJ. Postprandial metabolism of meal triglyceride in humans. Biochim Biophys Acta. 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koutsari C, Ali AH, Nair KS, Rizza RA, O'Brien P, Khosla S, Jensen MD. Fatty acid metabolism in the elderly: effects of dehydroepiandrosterone and testosterone replacement in hormonally deficient men and women. J Clin Endocrinol Metab. 2009;94:3414–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blaak EE. Adrenergically stimulated fat utilization and ageing. Ann Med. 2000;32:380–2 [DOI] [PubMed] [Google Scholar]
- 40.Sepe A, Tchkonia T, Thomou T, Zamboni M, Kirkland JL. Aging and regional differences in fat cell progenitors—a mini-review. Gerontology. 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iozzo P, Turpeinen AK, Takala T, Oikonen V, Solin O, Ferrannini E, Nuutila P, Knuuti J. Liver uptake of free fatty acids in vivo in humans as determined with 14(R, S)-[18F]fluoro-6-thia-heptadecanoic acid and PET. Eur J Nucl Med Mol Imaging. 2003;30:1160–4 [DOI] [PubMed] [Google Scholar]
- 42.Labbé SM, Grenier-Larouche T, Croteau E, Normand-Lauziere F, Frisch F, Ouellet R, Guerin B, Turcotte EE, Carpentier AC. Organ-specific dietary fatty acid uptake in humans using positron emission tomography coupled to computed tomography. Am J Physiol Endocrinol Metab. 2011;300:E445–53 [DOI] [PubMed] [Google Scholar]
- 43.LE Couteur DG, Cogger VC, McCuskey RS, DE Cabo R, Smedsrod B, Sorensen KK, Warren A, Fraser R. Age-related changes in the liver sinusoidal endothelium: a mechanism for dyslipidemia. Ann N Y Acad Sci. 2007;1114:79–87 [DOI] [PubMed] [Google Scholar]
- 44.Bradbury MW. Lipid metabolism and liver inflammation. I. Hepatic fatty acid uptake: possible role in steatosis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G194–8 [DOI] [PubMed] [Google Scholar]
- 45.Schneeman BO, Kotite L, Todd KM, Havel RJ. Relationships between the responses of triglyceride-rich lipoproteins in blood plasma containing apolipoproteins B-48 and B-100 to a fat-containing meal in normolipidemic humans. Proc Natl Acad Sci USA. 1993;90:2069–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Björkegren J, Boquist S, Samnegard A, Lundman P, Tornvall P, Ericsson CG, Hamsten A. Accumulation of apolipoprotein C-I-rich and cholesterol-rich VLDL remnants during exaggerated postprandial triglyceridemia in normolipidemic patients with coronary artery disease. Circulation. 2000;101:227–30 [DOI] [PubMed] [Google Scholar]
- 47.Bjorkegren J, Packard CJ, Hamsten A, Bedford D, Caslake M, Foster L, Shepherd J, Stewart P, Karpe F. Accumulation of large very low density lipoprotein in plasma during intravenous infusion of a chylomicron-like triglyceride emulsion reflects competition for a common lipolytic pathway. J Lipid Res. 1996;37:76–86 [PubMed] [Google Scholar]
- 48.Matikainen N, Manttari S, Westerbacka J, Vehkavaara S, Lundbom N, Yki-Jarvinen H, Taskinen MR. Postprandial lipemia associates with liver fat content. J Clin Endocrinol Metab. 2007;92:3052–9 [DOI] [PubMed] [Google Scholar]
- 49.Cree MG, Newcomer BR, Katsanos CS, Sheffield-Moore M, Chinkes D, Aarsland A, Urban R, Wolfe RR. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab. 2004;89:3864–71 [DOI] [PubMed] [Google Scholar]
- 50.Millar JS, Lichtenstein AH, Cuchel M, Dolnikowski GG, Hachey DL, Cohn JS, Schaefer EJ. Impact of age on the metabolism of VLDL, IDL, and LDL apolipoprotein B-100 in men. J Lipid Res. 1995;36:1155–67 [PubMed] [Google Scholar]
- 51.Chen Z, Fitzgerald RL, Li G, Davidson NO, Schonfeld G. Hepatic secretion of apoB-100 is impaired in hypobetalipoproteinemic mice with an apoB-38.9-specifying allele. J Lipid Res. 2004;45:155–63 [DOI] [PubMed] [Google Scholar]
- 52.Lewis GF. Fatty acid regulation of very low density lipoprotein production. Curr Opin Lipidol. 1997;8:146–53 [DOI] [PubMed] [Google Scholar]
- 53.McNamara JR, Shah PK, Nakajima K, Cupples LA, Wilson PW, Ordovas JM, Schaefer EJ. Remnant-like particle (RLP) cholesterol is an independent cardiovascular disease risk factor in women: results from the Framingham Heart Study. Atherosclerosis. 2001;154:229–36 [DOI] [PubMed] [Google Scholar]
- 54.Nakajima K, Nakajima Y, Takeichi S, Fujita MQ. Plasma remnant-like lipoprotein particles or LDL-C as major pathologic factors in sudden cardiac death cases. Atherosclerosis. 2008;198:237–46 [DOI] [PubMed] [Google Scholar]
- 55.Nakajima K, Nakano T, Tokita Y, Nagamine T, Inazu A, Kobayashi J, Mabuchi H, Stanhope KL, Havel PJ, Okazaki M, et al. Postprandial lipoprotein metabolism: VLDL vs chylomicrons. Clin Chim Acta. 2011;412:1306–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakajima K, Nakajima Y, Takeichi S, Fujita MQ. ApoB-100 carrying lipoprotein, but not apoB-48, is the major subset of proatherogenic remnant-like lipoprotein particles detected in plasma of sudden cardiac death cases. Atherosclerosis. 2007;194:473–82 [DOI] [PubMed] [Google Scholar]
- 57.Karpe F. Postprandial lipoprotein metabolism and atherosclerosis. J Intern Med. 1999;246:341–55 [DOI] [PubMed] [Google Scholar]
- 58.Hodis HN, Mack WJ. Triglyceride-rich lipoproteins and progression of atherosclerosis. Eur Heart J. 1998;19 Suppl A:A40–4 [PubMed] [Google Scholar]
- 59.Heath RB, Karpe F, Milne RW, Burdge GC, Wootton SA, Frayn KN. Dietary fatty acids make a rapid and substantial contribution to VLDL-triacylglycerol in the fed state. Am J Physiol Endocrinol Metab. 2007;292:E732–9 [DOI] [PubMed] [Google Scholar]
- 60.Timlin MT, Barrows BR, Parks EJ. Increased dietary substrate delivery alters hepatic fatty acid recycling in healthy men. Diabetes. 2005;54:2694–701 [DOI] [PubMed] [Google Scholar]
- 61.Gibbons GF, Islam K, Pease RJ. Mobilisation of triacylglycerol stores. Biochim Biophys Acta. 2000;1483:37–57 [DOI] [PubMed] [Google Scholar]
- 62.Harris WS, Muzio F. Fish oil reduces postprandial triglyceride concentrations without accelerating lipid-emulsion removal rates. Am J Clin Nutr. 1993;58:68–74 [DOI] [PubMed] [Google Scholar]
- 63.Puga GM, Meyer C, Mandarino LJ, Katsanos CS. Increased plasma availability of L-arginine in the postprandial period decreases the postprandial lipemia in older adults. Nutrition. 2013;29:81–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gaffney-Stomberg E, Insogna KL, Rodriguez NR, Kerstetter JE. Increasing dietary protein requirements in elderly people for optimal muscle and bone health. J Am Geriatr Soc. 2009;57:1073–9 [DOI] [PubMed] [Google Scholar]
- 65.Katsanos CS, Grandjean PW, Moffatt RJ. Effects of low and moderate exercise intensity on postprandial lipemia and postheparin plasma lipoprotein lipase activity in physically active men. J Appl Physiol. 2004;96:181–8 [DOI] [PubMed] [Google Scholar]
- 66.Katsanos CS, Moffatt RJ. Acute effects of premeal versus postmeal exercise on postprandial hypertriglyceridemia. Clin J Sport Med. 2004;14:33–9 [DOI] [PubMed] [Google Scholar]
- 67.Gill JM, Frayn KN, Wootton SA, Miller GJ, Hardman AE. Effects of prior moderate exercise on exogenous and endogenous lipid metabolism and plasma factor VII activity. Clin Sci (Lond). 2001;100:517–27 [PubMed] [Google Scholar]
- 68.Malkova D, Evans RD, Frayn KN, Humphreys SM, Jones PR, Hardman AE. Prior exercise and postprandial substrate extraction across the human leg. Am J Physiol Endocrinol Metab. 2000;279:E1020–8 [DOI] [PubMed] [Google Scholar]
- 69.Jackson KG, Poppitt SD, Minihane AM. Postprandial lipemia and cardiovascular disease risk: interrelationships between dietary, physiological and genetic determinants. Atherosclerosis. 2012;220:22–33 [DOI] [PubMed] [Google Scholar]
- 70.Stalenhoef AF, de Graaf J. Association of fasting and nonfasting serum triglycerides with cardiovascular disease and the role of remnant-like lipoproteins and small dense LDL. Curr Opin Lipidol. 2008;19:355–61 [DOI] [PubMed] [Google Scholar]