Abstract
Sports-related concussions or mild traumatic brain injuries (mTBIs) are becoming increasingly recognized as a major public health concern; however, no effective therapy for these injuries is currently available. ω-3 (n–3) fatty acids, such as docosahexaenoic acid (DHA), have important structural and functional roles in the brain, with established clinical benefits for supporting brain development and cognitive function throughout life. Consistent with these critical roles of DHA in the brain, accumulating evidence suggests that DHA may act as a promising recovery aid, or possibly as a prophylactic nutritional measure, for mTBI. Preclinical investigations demonstrate that dietary consumption of DHA provided either before or after mTBI improves functional outcomes, such as spatial learning and memory. Mechanistic investigations suggest that DHA influences multiple aspects of the pathologic molecular signaling cascade that occurs after mTBI. This review examines the evidence of interactions between DHA and concussion and discusses potential mechanisms by which DHA helps the brain to recover from injury. Additional clinical research in humans is needed to confirm the promising results reported in the preclinical literature.
Mild Traumatic Brain Injury/Concussion
Concussion is a common injury among athletes, particularly those participating in contact sports such as football and hockey. Concussion, also referred to as mild traumatic brain injury (mTBI)3, is defined by the CDC as “a complex pathophysiologic process affecting the brain, induced by traumatic biomechanical forces secondary to direct or indirect forces to the head” (1). This injury often results in impairments in memory and orientation and may be accompanied by a loss of consciousness (2). Approximately 1.5 million concussion-related emergency room visits are reported annually in the United States (3). Because many of these injuries go undiagnosed, it is estimated that up to 3.8 million individuals may be affected annually by sports-related concussions (3). Public awareness of concussion has grown in recent years, and, consequently, the incidence of diagnosed concussions has also displayed a dramatic increase, particularly among those aged ≤18 y. The incidence of concussions among minors increased 57% between 2008 and 2009 (4), and among high school athletes, the incidence has increased by 4.2-fold over an 11-y consecutive period beginning in 1998 (5). Conservative estimates of the combined direct and indirect annual cost of concussion are approximately $12 billion (6).
The short-term symptoms of concussions are variable depending on the severity of the injury. Symptoms often include headache, cognitive impairment (i.e., diminished reaction times, or “feeling foggy”), sensitivity to light and sound, irritability, sleep disturbances, and loss of consciousness (1, 7). Symptoms typically resolve within 7–10 d for adults (7), but the presence of abnormal neurometabolic function may persist for up to 4 wk after injury (8). In children and adolescents, the recovery period from concussion may be longer (9, 10); furthermore, children and adolescents may be at an increased risk of long-term cognitive impairments after injury because the developing brain is believed to be more vulnerable to insult as a result of injury (8, 11). Postconcussion treatment and recovery is primarily limited to cognitive and physical rest until symptoms resolve. For athletes, returning to play after a concussion involves a gradual increase in the intensity of physical activity once cognitive and balance symptoms have resolved fully (12).
Ensuring adequate and full recovery for athletes before returning to play is critical. Athletes with a previous history of concussion have a significantly greater risk of receiving subsequent concussions (10), and the long-term consequences of repeated concussions can be severe. It has long been speculated that repeated TBIs can lead to chronic traumatic encephalopathy—a form of dementia classically associated with boxers—although a firm cause-and-effect relation remains to be established (13). In a recent examination of a cohort of retired professional football players, those with a history of ≥3 concussions were significantly more likely to be diagnosed with mild cognitive impairment (14) or depression (15), and the risk of depression increased with the number of concussions (16). Furthermore, the men in this cohort had an earlier age of onset of Alzheimer’s disease relative to the general population (14). The consequences of multiple concussions are not just a concern to professional athletes; the risk of multiple concussions is particularly high among younger athletes, with up to 36% of mTBI cases in this population being repeat injuries (11), underscoring the need for effective treatment and postinjury care.
Concussion Pathology
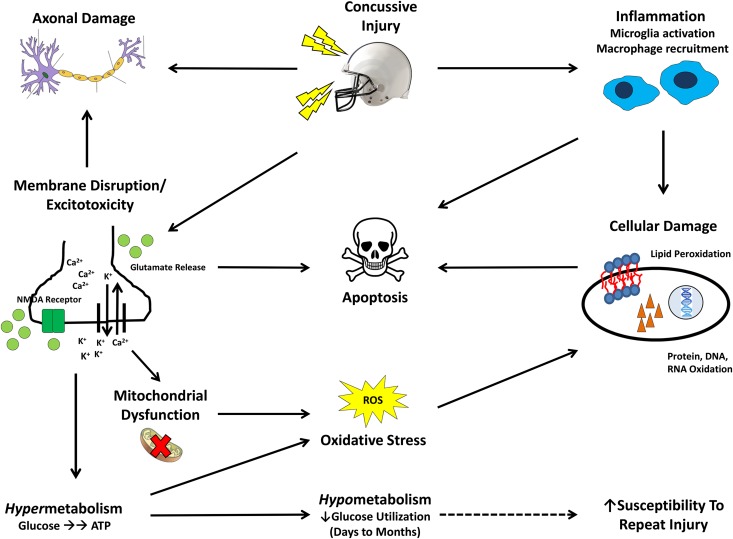
After an mTBI, gross pathologic changes are typically not observed, and diagnostic tools are primarily surveys that measure clinical symptomology (1, 17). Despite the absence of overt anatomical changes, a complex cascade of molecular events occurs in the brain as a result of mechanical trauma (Fig. 1) (17–20). Mild membrane disruption and stretching of the axons occurs secondary to the initial mechanical insult, resulting in loss of regulation of Na+/K+/Ca2+ flux. This loss of ionic regulation leads to a massive influx of Ca2+ and an efflux of K+. Membrane depolarization caused by these ion fluxes subsequently leads to the indiscriminant release of neurotransmitters, such as glutamate, resulting in elevated activation of N-methyl-d-aspartate receptors, causing additional ionic perturbations and nonspecific initiation of action potentials (19). These functional disturbances result in significant alterations to neuronal function, because critical components of cognitive processes, such as learning and memory, associated with the N-methyl-d-aspartate receptor are affected (19).
FIGURE 1.
Molecular cascade of events after a mild traumatic brain injury. The initial mechanical injury causes mild membrane disruption in the nerve, axonal damage, and indiscriminate neurotransmitter (glutamate) release and activation of ion channels, such as the NMDA receptor. Deregulation of Na+/K+/Ca2+ flux leads to excitotoxicity—a massive influx of calcium and an efflux of potassium and the release of the neurotransmitter glutamate. Ca2+ influx also exacerbates damage to the axonal structure and causes mitochondrial dysfunction. ATP-dependent Na/K pumps function at an elevated capacity, creating a hypermetabolic state generating oxidative stress that can result in cellular damage. Glucose stores become depleted due to the hypermetabolic state, resulting in a hypometabolic state, with low glucose utilization. This hypometabolic state may last for months in severe cases, and, during this time, the brain may be particularly vulnerable to repeated injury (dashed arrow). Concurrently, inflammation due to microglial activation occurs soon after the concussive injury, causing damage to cellular structures. Ultimately, the combination of oxidative stress, inflammation, excitotoxicity, mitochondrial dysfunction, and axonal damage results in neuronal apoptosis. The ω-3 FA DHA has been shown to address several of the hallmark pathologic features of this injury, such as excitotoxicity, oxidative stress, and inflammation. NMDA, N-methyl-d-aspartate; ROS, reactive oxygen species.
To correct this excitotoxicity and restore ionic homeostasis, ATP-dependent pumping systems are forced to function at an increased capacity, resulting in a tremendous need for ATP, and these demands for ATP can be met through glycolytic pathways. Although neurons in this hypermetabolic state quickly produce ATP, this process is inefficient and results in excessive lactic acid production and the formation of oxygen radicals, which can damage key cellular components, such as DNA and phospholipid membranes. Furthermore, excessive Ca2+ influx reduces the polarization of mitochondrial membranes, resulting in mitochondrial dysfunction, which leads to oxidative stress, as well as impaired lactate metabolism (17–19).
Within a few hours, glucose stores become diminished in response to this hypermetabolic state, resulting in a hypometabolic state characterized by relatively low glucose utilization. This hypometabolic state may last for several days, or months in severe cases, and during this time, it is hypothesized that the brain is thought to be particularly vulnerable to a second injury (18, 19). Structural damage to the axons also occurs as a consequence of the initial injury and is exacerbated by the accumulation of Ca2+ ions and resulting oxidative stress, which is evidenced by the deposition of amyloid precursor protein.
An additional aspect of the concussion cascade is the role of inflammation related to microglial activation. Microglial activation, an integral part of the neuronal inflammatory response to the release of damage signals from the neurons, begins as early as 1 h after injury and can last up to 30 d after injury (20, 21). The resulting inflammation can result in a positive-feedback system, leading to a hyperactive inflammatory response that can damage cell membranes, as evidenced by the accumulation of lipid peroxidation products (20, 21). With repeated concussions, this inflammation may become very destructive to neurons, elevating the risk of future complications (20). Ultimately, the combination of oxidative stress, inflammation, hypermetabolism, mitochondrial dysfunction, and axonal damage induced by mTBI results in neuronal cell death via apoptosis, potentially impairing cognitive function (18, 19).
Despite our evolving understanding of the underlying molecular pathophysiology of concussion, efforts to develop pharmacologic treatments have been unsuccessful. These pharmacologic interventions focused on treating each of the distinct symptoms of concussion in isolation, and treatment is primarily individualized depending on the specific symptom set with which the patient presents (17, 22, 23). Given the multifactorial nature of concussive injury, the fact that each of these therapies typically addresses 1 specific aspect of the molecular concussion cascade may explain why pharmacologic intervention has been unsuccessful. At present, no intervention, pharmacologic or otherwise, has been identified that can enhance the recovery from a concussion.
Long-Chain PUFAs and Brain Health
DHA (22:6n–3) and EPA (20:5n–3) are long-chain ω-3 PUFAs (LCPUFAs) that are typically found in high concentrations in algae and fish. DHA in particular is recognized as being essential for brain development and normal brain function because it is the primary structural ω-3 LCPUFA present in the brain, constituting up to 97% of the ω-3 FAs in the brain, making it an integral component of neural membrane phospholipids (24). In addition to its structural role, DHA is involved in multiple brain functions, including cell membrane fluidity, receptor affinity, and modulation of signal transduction molecules (25).
Because of these prominent structural and functional roles in the brain, consumption of DHA has been shown to benefit brain development and cognitive function throughout the lifespan (24). As the brain develops during gestation and into early adulthood, DHA acts as a key structural component of the brain, preferentially accumulating in the frontal cortex and the hippocampus, areas of the brain recognized for their roles in executive function, learning, and memory (26, 27). Intake of DHA in early life has been linked to improvements in cognitive function and behavior in children in both epidemiologic (28, 29) and intervention (30–33) trials. Based in part on the role of DHA in neural function in early life, the Food and Agriculture Organization set forth specific dietary guidelines for ω-3 FA intake for infants and children (34), emphasizing the essential role of LCPUFAs in maintaining the health of the brain.
In the aging brain, epidemiologic studies observed repeatedly a beneficial association of DHA intake (either alone or as a component of seafood) and plasma concentrations of β-amyloid peptide (35–38) markers of accelerated structural aging, including brain volume and cognitive function (37, 39). Likewise, increased plasma DHA status has also been associated with improved cognitive outcomes (36, 39, 40), and elevations in both DHA intake and status are associated with a decreased risk of Alzheimer’s disease and a slower rate of age-related cognitive decline (41–45). Numerous human clinical trials demonstrated that supplementing specific subgroups of older adults with DHA (either alone or in conjunction with other nutritional compounds, such as EPA or lutein) results in improved cognitive outcomes, ranging from unimpaired adults (46) to adults with subjective memory complaints (47) or mild cognitive impairment (48–52), as well as very mild Alzheimer’s patients (51, 53) and ApoE4-negative Alzheimer’s patients (54).
The potential neuroprotective benefits of LCPUFAs, such as DHA for TBIs, were first highlighted in case studies detailing their use in the treatment of the lone survivor of the Sago Mine disaster and a teenager involved in a motor vehicle accident (55, 56). Both of these reports described the role of high doses of LCPUFAs in the recovery of these patients and suggested acute and chronic neuroprotective effects of LCPUFAs. The potential neuroprotective benefits of LCPUFAs are also evident from animal studies demonstrating that LCPUFA supplementation before (57–60) or after (60–65) TBI protects the brain by limiting structural damage to the axon and events such as neuronal apoptosis (57, 60, 63–65), restoring the expression of specific protective mediators (58, 59, 61, 62, 65), ultimately reducing injury-induced cognitive dysfunction (57, 59–61, 65). A list of published preclinical studies with reported outcomes is shown in Table 1.
TABLE 1.
Outline of preclinical studies evaluating the use of ω-3 FAs for recovery from mild traumatic brain injury1
| Results |
||||||
| Reference | mTBI model | Intervention | Timing | Pathophysiologic Events | Protective Mediators | Functional Outcomes |
| Mills et al., 2011 (57) | Impact acceleration injury | 0, 3, 12, 40 mg/kg DHA | 30 d before injury | ↓ Axonal damage (APP counts) in rats receiving high-dose DHA | — | ↓ Time latency to platform in rats receiving high-dose DHA on the Morris water maze |
| ↓ Number of apoptotic (caspase-3-positive) axons significantly reduced in rats receiving high-dose DHA | ↓ Errors in rats receiving high-dose DHA on the Morris water maze | |||||
| ↓ Inflammation (CD-68 positive cells) in rats receiving high dose DHA | ||||||
| Wu et al., 2007 (58) | Lateral fluid percussion injury | Control diet (0.9% DHA, 1% EPA) | 4 wk before injury | — | Fish oil normalized energy metabolism marker expression (SIR2, AMPK, p-AMPK, uMtCK) | — |
| Fish oil diet (12.4% DHA, 13.5% EPA) | ↓ Oxidized proteins in fish oil–fed rats | |||||
| Wu et al., 2004 (59) | Lateral fluid percussion injury | Control diet (0.9% DHA, 1% EPA) | 4 wk before injury | — | Fish oil diet normalized expression of BDNF signaling markers (synapsin I, CREB) | ↓ Time latency to platform in fish oil–fed rats on the Morris water maze |
| Fish oil diet (12.4% DHA, 13.5% EPA) | ↓ Oxidized proteins in fish oil–fed rats | |||||
| Wu et al., 2013 (65) | Lateral fluid percussion injury | 0% DHA, sedentary | 12 d after injury | ↓ Brain DHA content after injury | DHA normalized expression of BDNF signaling markers (BDNF, p-TrkB) | ↓ Slope of escape latency (↑ learning speed) after injury according to the Morris water maze test in DHA-fed rats |
| 1.2% DHA, sedentary | DHA feeding (with or without exercise) restored injury-induced reductions in brain DHA content | ↑ Antioxidant enzyme expression (SIR2) in DHA-fed rats | Exercise had an additive effect on learning performance according to the Morris water maze | |||
| 0% DHA, voluntary exercise | ↓ Lipid peroxidation (4-HHE) in DHA-fed rats | ↑ Membrane homeostasis marker expression (Acox1, 17β-HSD4, iPLA2, STX-3) in DHA-fed rats | ||||
| 1.2% DHA, voluntary exercise | Effects stronger when DHA was provided along with exercise | |||||
| Wu et al., 2014 (66) | Lateral fluid percussion injury | 0% DHA + 0 mg/kg curcumin | 14 d after injury | ↓ Brain DHA content after injury | ↑ Membrane homeostasis marker expression (17β-HSD4, FADS2) in DHA-fed rats | ↓ Time latency to complete the Barnes maze task in DHA-fed rats |
| 1.2% DHA + 0 mg/kg curcumin | DHA feeding (with or without curcumin) restored injury-induced reductions in brain DHA content | DHA normalized expression of BDNF signaling markers (BDNF, p-TrkB) | ||||
| 0% DHA + 500 mg/kg curcumin | ↓ Lipid peroxidation (4-HHE, 4-HNE) in DHA-fed rats | |||||
| 1.2% DHA + 500 mg/kg curcumin | ||||||
| Wu et al., 2011 (61) | Lateral fluid percussion injury | 0 or 1.2% DHA | 12 d after injury | — | DHA normalized expression of proteins related to learning/memory (BDNF, synapsin I, CREB, CaMKII) | ↓ Time latency to platform in DHA-fed rats on the Morris water maze |
| ↑ Antioxidant enzyme expression (SIR2, SOD) in DHA-fed rats | ||||||
| ↑ Membrane homeostasis marker expression (iPLA2, STX-3) in DHA-fed rats | ||||||
| Shin and Dixon, 2011 (62) | Controlled cortical impact injury | 1.5 mL of fish oil (360 mg/d EPA, 240 mg/d DHA) | 7 d after injury | — | Fish oil normalized dopamine release after injury | — |
| 1.5 mL of olive oil | ||||||
| Mills et al., 2011 (63) | Impact acceleration injury | 0, 10, 40 mg ⋅ kg−1 ⋅ d−1 fish oil (2:1 ratio of EPA/DHA) | 30 d after injury | ↓ Axonal damage (APP counts) in rats receiving fish oil | — | — |
| ↓ Number of apoptotic (caspase-3-positive) axons significantly reduced in rats receiving fish oil | ||||||
| Bailes and Mills, 2010 (64) | Impact acceleration injury | 0, 10, 40 mg ⋅ kg−1 ⋅ d−1 DHA | 30 d after injury | ↓ Axonal damage (APP counts) in rats receiving DHA | — | — |
| ↓ Number of apoptotic (caspase-3-positive) axons significantly reduced in rats receiving DHA | ||||||
| Wang et al., 2013 (60) | Lateral fluid percussion injury | 0 or 6% fish oil diet (0.8–1% EPA, 0.6% DHA) | 4 wk before injury and 2 wk after injury (6 wk total) | ↑ Higher density of hippocampal neurons in fish oil–fed rats (nonsignificant, P = 0.126) | — | ↑ Body weight recovery after injury in fish oil–fed rats |
| ↓ Time latency to platform in fish oil–fed rats on the Morris water maze | ||||||
| ↑ Time spent in target quadrant in fish oil–fed rats on the Morris water maze | ||||||
Acox1, acyl-coenzyme A oxidase 1; AMPK, AMP-activated protein kinase; APP, amyloid precursor protein; BDNF, brain-derived neurotrophic factor; CaMKII, calcium-dependent protein kinase II; CREB, cAMP response element-binding protein; FADS2, delta 6 fatty acid desaturase; iPLA2, calcium-independent phospholipase A2; mTBI: mild traumatic brain injury; p-AMPK: phosphorylated AMP-activated protein kinase; p-TrkB, p-neurotrophic tyrosine kinase receptor; SIR2, silent information regulator 2; SOD, superoxide dismutase; STX-3, syntaxin-3; uMtCK, ubiquitous mitochondrial creatine kinase; 17β-HSD4, 17β-hydroxysteroid dehydrogenase type 4; 4-HHE, 4-hydroxy-2-hexenal; 4-HNE, 4-hydroxy-2-nonenal; ↓, decreased; ↑, increased.
The potential neuroprotective benefits of LCPUFAs are also evident from animal studies demonstrating that LCPUFA supplementation before (57–60) or after (60–65) TBI protects the brain by limiting structural damage to the axon and events such as neuronal apoptosis (57, 60, 63–65), restoring the expression of specific protective mediators (58, 59, 61, 62, 65), ultimately reducing injury-induced cognitive dysfunction (57, 59–61, 65). Table 1 provides a list of published preclinical studies with reported outcomes.
LCPUFAs Mitigate Pathophysiologic Events after Injury
A major consequence of mTBI is the damage to neuronal structures, particularly damage to the axons, which can ultimately result in neuronal apoptosis (18, 19). DHA acts as an important structural component of the brain, and the DHA content of the brain has been shown to be reduced after injury (65, 66), suggesting that there may be an elevated requirement for DHA during recovery. Both prophylactic and therapeutic interventions with LCPUFAs have been shown to limit several pathophysiologic components of the concussion cascade. Using a rat model of mTBI, Mills et al. (57) found that DHA supplementation (40 mg ⋅ kg−1 ⋅ d−1) given before injury reduced both axonal injury and markers of cellular apoptosis relative to control fed animals. Furthermore, Bailes and Mills (64) reported that, when supplemental DHA (40 mg ⋅ kg−1 ⋅ d−1) was given after a TBI, both the number of injured (amyloid precursor protein–positive) axons and the number of apoptotic (caspase-3-positive) axons were reduced to amounts similar to those seen in uninjured animals. Postinjury supplementation with both DHA and EPA similarly reduced the injury response as measured by a similar set of brain health biomarkers (63). Despite unremarkable morphology in a rat model of repetitive mTBI, Wang et al. (60) reported that LCPUFAs reduced the injury response as well. A nonsignificant (P = 0.126) trend toward a higher density of hippocampal neurons was observed in rats supplemented with LCPUFAs both before and after repetitive brain injuries. In summary, both prophylactic and therapeutic supplementation with LCPUFAs appears to reduce the degree of axonal and neuronal damage, inflammation, and apoptosis after mTBI in rodent models.
LCPUFAs Increase the Expression of Protective Mediators
After mTBI, disruptions in the expression of several molecular systems necessary for proper neuronal and cognitive function are observed. Structural damage to the axon results in numerous biochemical alterations that can affect the release of neurotransmitters, as well as the expression of genes involved in neuronal function. Addressing the disrupted expression of these protective mediators by restoring these systems is integral in the recovery from concussive injury.
Oxidative stress occurs after mTBI as a byproduct of dysfunctional energy metabolism and mitochondrial dysfunction, resulting in damage to cellular structures and diminished expression of regulators of membrane homeostasis. Therefore, the restoration of energy metabolism and membrane homeostasis may reduce oxidative stress secondary to concussive injury, facilitating recovery. Indeed, postinjury supplementation with LCPUFAs appears to ameliorate oxidative stress secondary to mTBI, as evidenced by a reduction in the amount of 4-hydroxy-2-nonenal and 4-hydroxy-2-hexenal, markers of lipid peroxidation (65, 66). Furthermore, postinjury DHA supplementation has been reported to blunt head trauma–induced reductions in scavengers of oxidative stress and markers of membrane homeostasis and function, including manganese superoxide dismutase, Ca-independent phospholipase A2, and syntaxin-3 (61, 65). LCPUFA supplementation countered head trauma–induced reductions in energy metabolic markers, including AMP-activated protein kinase, phosphorylated AMP-activated protein kinase, and ubiquitous mitochondrial creatine kinase, indicating a normalization of energy metabolism related to hypermetabolism after injury. Therefore, it would appear that normalization of energy metabolism after mTBI in these models reduces damage to lipid membranes, likely as a result of reductions in oxidative stress.
Aside from oxidative stress, another major consequence of mTBI is the indiscriminant release of neurotransmitters that occurs secondary to the initial damage to the axons and the dysregulated flux of ions across neuronal membranes. This effect can persist well after the initial injury; reductions in dopamine release due to mTBI are observed even 1 week after injury (67). Animals supplemented with DHA/EPA after a TBI displayed a restoration of dopamine release relative to control fed animals (62). Neurotransmitters such as dopamine play important roles in learning and memory, and reductions in dopamine release may be partially responsible for functional deficits after mTBI; however, additional molecular pathways can influence cognitive function as well.
Brain-derived neurotrophic factor (BDNF) signaling is 1 such pathway that is critical for ensuring proper cognitive function, because it facilitates synaptic transmission and learning ability by modulating synapsin I and cAMP response element-binding protein (CREB) (68). As such, the restoration or maintenance of cognitive function after injury could in fact be influenced by BDNF signaling. Wu et al. (59) reported that LCPUFA supplementation before injury countered head trauma–induced reductions in BDNF, synapsin I, and CREB. Furthermore, multiple reports by the same investigators found that DHA supplementation given after injury similarly countered head trauma–induced reductions in BDNF, synapsin I, CREB, calcium-dependent protein kinase II, peroxisomal acyl-coenzyme A oxidase 1, and others (61, 65, 66). Given the established role of the BDNF system in modulating learning and cognitive function (68), the demonstrated impact of LCPUFA supplementation in restoring this system provides an additional mechanistic explanation to provide plausibility for facilitating the recovery of cognitive function after injury.
LCPUFAs Improve Functional Outcomes After Injury
Ultimately, the combination of neurometabolic insults and structural damage to axons results in disruptions in cognitive function, clinically manifesting in the form of headaches, loss of memory, decreased reaction times, and loss of consciousness (1, 7). Data from animal models of mTBI demonstrated consistently that the injury-induced reduction in cognitive function is diminished with either preinjury or postinjury supplementation with LCPUFAs, particularly DHA. Mills et al. (57) found that supplementation with DHA before a head injury improved memory as assessed by water maze testing. Wu et al. (59) similarly found that supplementation with DHA and EPA before TBI normalized spatial learning ability as assessed by water maze testing as well. Therapeutic supplementation with DHA after injury also improved learning ability through multiple measures of cognitive function in rodents, including the Morris water maze and the Barnes maze (61, 65, 66). Finally, when given both before and after repetitive mTBIs, Wang et al. (60) found that supplementation with DHA and EPA improved cognitive performance and spatial memory retention and improved the recovery of body weight after injury. In summary, LCPUFA supplementation, given before and/or after an mTBI, hinders the decline in cognitive function after injury in animal models. These data provide support for the hypothesis that LCPUFAs may facilitate recovery from, or possibly prevent, the cognitive decline observed after mTBI in humans.
Additional Mechanisms of DHA Action
Several additional mechanistic explanations regarding LCPUFA neuroprotection have been proposed because these FAs, particularly DHA, have diverse functions in neuronal tissue. Even outside the context of animal models of mTBI, DHA has demonstrated the ability to address several of the hallmark pathologic features of this injury, namely excitotoxicity, oxidative stress, and inflammation. DHA has been shown to reduce glutamate-induced excitotoxicity and both axonal and neuronal injury through modulation of ion channels (69–71). DHA has been shown to reduce oxidative stress by restoring/increasing the activity of antioxidant systems that enzymatically inactivate reactive oxygen species as well (58, 59, 70, 72). Because DHA is rapidly incorporated into neuronal phospholipids at the expense of arachidonic acid, DHA has also been shown to modulate the inflammatory response by shifting the inflammatory system toward less-inflammatory and pro-resolving mediators (73–75). In addition, DHA has been shown to modulate inflammation via gene transcription because DHA is a ligand for nuclear transcription factors (retinoid X receptor, PPAR) that regulate the inflammatory response (73). Finally, DHA metabolites, such as neuroprotectin D1, have been shown to reduce cell death via potentiation of cell signaling pathways that upregulate pro-survival regulators and downregulate pro-apoptotic molecules (76).
Given the diverse actions that LCPUFAs are demonstrated to play in ameliorating factors involved in concussion pathology, it is likely that these roles are not mutually exclusive and that any comprehensive mechanism will involve a combination of these effects. Considering the multifactorial nature of concussive brain injuries, the potential success of LCPUFAs as a therapy for concussions is supported by the fact that these FAs can address multiple aspects of the molecular concussion cascade.
Discussion
As our understanding of the pathogenesis and long-term effects of concussions increases, so too does our ability to design effective treatments for these injuries. There is a growing body of preclinical literature suggesting that ω-3 FAs, and DHA in particular, may play a therapeutic role in mTBI. At present, this is an emerging avenue for LCPUFA research, offering the potential for ameliorating or possibly even preventing the complications associated with concussions. This field, although exciting, remains in an early stage, and additional research to address several remaining questions is needed.
Trials conducted using animal models to date have used either DHA alone or DHA in combination with EPA in their respective study designs. Although EPA does play an important functional role in the body, the concentration of EPA in the brain is negligible (77–80), suggesting that EPA plays a limited role in mediating the beneficial effects of LCPUFA supplementation on mTBI pathology. It is worth noting that, to the best of our knowledge, no trials have been performed examining the effect of EPA alone in the context of mTBI. In contrast, DHA is the predominant ω-3 FA present in the brain, and, consistent with this finding, DHA, and not EPA, has been demonstrated to be critical for brain development and cognitive function throughout life (26, 27, 77). Additionally, there is evidence that the amount of DHA in brain tissue is decreased after mTBI (65, 66), suggesting an elevated need for DHA in mTBI recovery. Given our current understanding of the role of DHA in the brain, it is likely that DHA is more effective than EPA in addressing the pathophysiologic events related to concussion.
It is very important to note that the current state of the science regarding LCPUFA supplementation for the treatment of concussion is based primarily on animal models and that these animal models have specific limitations that preclude their ability to be adequately generalized to the human condition (17, 81). These limitations notwithstanding, the encouraging results obtained using these animal models of mTBI provided valuable mechanistic insights. Furthermore, the compelling nature of these animal trials data resulted in a 2011 recommendation from the Institute of Medicine to conduct human clinical trials to investigate the use of LCPUFAs as a treatment for TBI (23). Randomized, double-blind, placebo-controlled trials with humans are required before definitive clinical recommendations can be made, and, to that end, the first human clinical trials investigating DHA as an aid for concussion recovery are currently underway (registered at clinicaltrials.gov as NCT01903525 and NCT01814527). Although we await the results of these trials and others in the future, the well-established role of DHA in supporting the structure and function of the brain throughout the lifespan (26, 27, 46, 47, 53) provides encouragement that LCPUFAs may also prove beneficial in the context of concussion recovery.
Conducting well-designed clinical trials aimed at identifying therapies for concussive brain injuries will be challenging. Currently, there is no gold-standard measure to definitively diagnose concussions but rather only tools that measure symptomology, and these tools can be subjective in nature. Even the commonly used endpoint of return-to-play, defined as the amount of time that passes until an athlete receives medical clearance for unrestricted participation in his or her sport, is a somewhat subjective endpoint with a high potential to be influenced by external forces. Newer, more objective measures for concussion diagnosis and determinations of severity and recovery from mTBI are emerging, including blood markers and MRI scans (82–84), as well as sensors inside protective helmets that can determine impact severity (85). As these tools mature, their use should be considered to supplement existing methodologies to provide the greatest chance for establishing the therapeutic efficacy of interventions, such as LCPUFAs (17).
The consequences of concussion can be severe, and no therapies are currently available to aid the recovery from this injury. Supplementation with LCPUFAs, such as DHA, are a promising therapeutic possibility for concussive injuries. LCPUFAs represent an attractive option because they have a strong safety profile and have established benefits for the health of the brain, heart, and eyes that can still be realized by the concussed individual. The benefits of ω-3 FA intake have resulted in numerous intake recommendations ranging from 250 mg/d (34, 86, 87) to 500 mg/d (88–90) from various professional organizations, including the AHA and the International Society for the Study of Fatty Acids and Lipids, among others. Despite our increasing understanding of the importance of ω-3 FAs in nutrition, current estimates of dietary DHA and EPA intake for Americans are 90–120 mg/d for females and males aged ≥20 y (91), which is less than half of what is recommended by most authoritative organizations.
At present, estimating an amount of ω-3 FA intake that may be beneficial in mTBI recovery is difficult because we are awaiting the results of human clinical trials. Previously discussed reports outlining the use of ω-3 FAs in the recovery from severe TBIs (reviewed in Ref. 92) described the use of very-high doses of LCPUFAs (16.2 g/d EPA plus DHA) in the recovery of these patients, which would be an intake well above what would be advisably achieved through diet and supplementation for the general population. Within the context of mTBIs/concussions, translating a DHA intake used in several rat studies of mTBI recovery (40 mg ⋅ kg−1 ⋅ d−1 DHA) (57, 63, 64) using body surface area conversion methods (93) amounts to an estimated human intake of 387 mg/d DHA. This is certainly within the range of the majority of ω-3 FA intake recommendations for optimal intake, but well-designed human clinical trials are required before definitive intake recommendations for mTBI benefits can be made.
Although we are awaiting the results of randomized controlled trials to determine the efficacy of DHA for mTBI recovery, we may find a novel role for LCPUFA supplementation as a therapeutic or preventative measure against sports-related concussion in addition to the other well-established nutritional roles of these FAs.
Acknowledgments
The authors thank Connye Kuratko, Chris Butt, Chuck Rutkowski, and Karin Yurko-Mauro for their critical review of this manuscript and for their guidance and insightful comments. All authors have read and approved the final manuscript.
Footnotes
Abbreviations used: BDNF, brain-derived neurotrophic factor; CREB, cAMP response element-binding protein; LCPUFA, long-chain PUFA; mTBI, mild traumatic brain injury.
Literature Cited
- 1.Centers for Disease Control and Prevention. Heads up: facts for physicians about mild traumatic brain injury (MTBI) 2007. [cited 2013 July 19]. Available from: http://www.cdc.gov/concussion/headsup/pdf/Facts_for_Physicians_booklet-a.pdf
- 2.Giza CC, Kutcher JS, Ashwal S, Barth J, Getchius TS, Gioia GA, Gronseth GS, Guskiewicz K, Mandel S, Manley G, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil. 2006;21:544–8 [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Nonfatal traumatic brain injuries related to sports and recreation activities among persons aged ≤19 years–United States, 2001–2009. MMWR Morb Mortal Wkly Rep. 2011;60:1337–42 [PubMed] [Google Scholar]
- 5.Lincoln AE, Caswell SV, Almquist JL, Dunn RE, Norris JB, Hinton RY. Trends in concussion incidence in high school sports: a prospective 11-year study. Am J Sports Med. 2011;39:958–63 [DOI] [PubMed] [Google Scholar]
- 6.Finkelstein EA, Corso PS, Miller TR. The incidence and economic burden of injuries in the United States. New York: Oxford University Press; 2006. [Google Scholar]
- 7.McCrory P, Meeuwisse W, Aubry M, Cantu B, Dvorak J, Echemendia R, Engebretsen L, Johnston K, Kutcher J, Raftery M, et al. Consensus statement on Concussion in Sport–the 4th International Conference on Concussion in Sport held in Zurich, November 2012. J Sci Med Sport. 2013;16:178–89 [DOI] [PubMed] [Google Scholar]
- 8.Shrey DW, Griesbach GS, Giza CC. The pathophysiology of concussions in youth. Phys Med Rehabil Clin N Am. 2011;22:577–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J Pediatr. 2003;142:546–53 [DOI] [PubMed] [Google Scholar]
- 10.Guskiewicz KM, McCrea M, Marshall SW, Cantu RC, Randolph C, Barr W, Onate JA, Kelly JP. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290:2549–55 [DOI] [PubMed] [Google Scholar]
- 11.Prins ML, Giza CC. Repeat traumatic brain injury in the developing brain. Int J Dev Neurosci. 2012;30:185–90 [DOI] [PubMed] [Google Scholar]
- 12.Harmon KG, Drezner J, Gammons M, Guskiewicz K, Halstead M, Herring S, Kutcher J, Pana A, Putukian M, Roberts W. American Medical Society for Sports Medicine position statement: concussion in sport. Clin J Sport Med. 2013;23:1–18 [DOI] [PubMed] [Google Scholar]
- 13.McCrory P, Meeuwisse WH, Kutcher JS, Jordan BD, Gardner A. What is the evidence for chronic concussion-related changes in retired athletes: behavioural, pathological and clinical outcomes? Br J Sports Med. 2013;47:327–30 [DOI] [PubMed] [Google Scholar]
- 14.Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Cantu RC, Randolph C, Jordan BD. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719–26 [DOI] [PubMed] [Google Scholar]
- 15.Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Harding HP, Jr, Matthews A, Mihalik JR, Cantu RC. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39:903–9 [DOI] [PubMed] [Google Scholar]
- 16.Kerr ZY, Marshall SW, Harding HP, Jr, Guskiewicz KM. Nine-year risk of depression diagnosis increases with increasing self-reported concussions in retired professional football players. Am J Sports Med. 2012;40:2206–12 [DOI] [PubMed] [Google Scholar]
- 17.Institute of Medicine, National Research Council. Sports-related concussions in youth: improving the science, changing the culture. Washington, DC: National Academies Press; 2013 [PubMed] [Google Scholar]
- 18.Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train. 2001;36:228–35 [PMC free article] [PubMed] [Google Scholar]
- 19.Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clin Sports Med. 2011;30:33–48 [DOI] [PubMed] [Google Scholar]
- 20.Dashnaw ML, Petraglia AL, Bailes JE. doi: 10.3171/2012.10.FOCUS12284. An overview of the basic science of concussion and subconcussion: where we are and where we are going. Neurosurg Focus. 2012;33:E5:1–9. [DOI] [PubMed] [Google Scholar]
- 21.Blaylock RL, Maroon J. Immunoexcitotoxicity as a central mechanism in chronic traumatic encephalopathy—a unifying hypothesis. Surg Neurol Int. 2011;2:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petraglia AL, Maroon JC, Bailes JE. From the field of play to the field of combat: a review of the pharmacological management of concussion. Neurosurgery. 2012;70:1520–33 [DOI] [PubMed] [Google Scholar]
- 23.Institute of Medicine. Nutrition and traumatic brain injury: improving acute and subacute health outcomes in military personnel. Washington, DC: National Academies Press; 2011 [PubMed] [Google Scholar]
- 24.Lauritzen L, Hansen H, Jorgensen M, Michaelsen K. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog Lipid Res. 2001;40:1–94 [DOI] [PubMed] [Google Scholar]
- 25.Salem N, Litman B, Kim H, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–59 [DOI] [PubMed] [Google Scholar]
- 26.Ryan AS, Astwood JD, Gautier S, Kuratko CN, Nelson EB, Salem N., Jr Effects of long-chain polyunsaturated fatty acid supplementation on neurodevelopment in childhood: a review of human studies. Prostaglandins Leukot Essent Fatty Acids. 2010;82:305–14 [DOI] [PubMed] [Google Scholar]
- 27.Kuratko CN, Barrett EC, Nelson EB, Salem N., Jr The relationship of docosahexaenoic acid (DHA) with learning and behavior in healthy children: a review. Nutrients. 2013;5:2777–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montgomery P, Burton JR, Sewell RP, Spreckelsen TF, Richardson AJ. Low blood long chain omega-3 fatty acids in UK children are associated with poor cognitive performance and behavior: a cross-sectional analysis from the DOLAB study. PLoS One. 2013;8:e66697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNamara RK, Jandacek R, Tso P, Weber W, Chu WJ, Strakowski SM, Adler CM, Delbello MP. Low docosahexaenoic acid status is associated with reduced indices in cortical integrity in the anterior cingulate of healthy male children: a 1H MRS Study. Nutr Neurosci. 2013;16:183–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colombo J, Carlson SE, Cheatham CL, Shaddy DJ, Kerling EH, Thodosoff JM, Gustafson KM, Brez C. Long-term effects of LCPUFA supplementation on childhood cognitive outcomes. Am J Clin Nutr. 2013;98:403–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNamara RK, Able J, Jandacek R, Rider T, Tso P, Eliassen JC, Alfieri D, Weber W, Jarvis K, DelBello MP, et al. Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: a placebo-controlled, dose-ranging, functional magnetic resonance imaging study. Am J Clin Nutr. 2010;91:1060–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson AJ, Burton JR, Sewell RP, Spreckelsen TF, Montgomery P. Docosahexaenoic acid for reading, cognition and behavior in children aged 7–9 years: a randomized, controlled trial (the DOLAB Study). PLoS One. 2012;7:e43909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan AS, Nelson EB. Assessing the effect of docosahexaenoic acid on cognitive functions in healthy, preschool children: a randomized, placebo-controlled, double-blind study. Clin Pediatr (Phila). 2008;47:355–62 [DOI] [PubMed] [Google Scholar]
- 34.Food and Agriculture Organization. Fats and fatty acids in human nutrition: Report of an Expert Consultation. Rome, Italy: Office of Knowledge Exchange, Research, and Extension, FAO; 2010 [PubMed] [Google Scholar]
- 35.Kalmijn S, van Boxtel M, Ocke M, Verschuren W, Kromhout D, Launer L. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology. 2004;62:275–80 [DOI] [PubMed] [Google Scholar]
- 36.Whalley LJ, Fox H, Wahle K, Starr J, Deary I. Cognitive aging, childhood intelligence, and the use of food supplements: possible involvement of n-3 fatty acids. Am J Clin Nutr. 2004;80:1650–7 [DOI] [PubMed] [Google Scholar]
- 37.Titova OE, Sijogren P, Brooks S, Kullberg J, Ax E, Kilander L, Riserus U, Cederholm T, Larsson EM, Johansson L, et al. Dietary intake of eicosapentaenoic and docosahexaenoic acids is linked to grey matter volume and cognitive function in elderly. Age (Dordr). 2013;35:1495–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu Y, Schupf N, Cosentino SA, Luchsinger JA, Scarmeas N. Nutrient intake and plasma beta-amyloid. Neurology. 2012;78:1832–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan ZS, Harris W, Beiser A, Au R, Himali J, Debette S, Pikula A, DeCarli C, Wolf P, Vasan R, et al. Red blood cell omega-3 fatty acid levels and markers of accelerated brain aging. Neurology. 2012;78:658–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muldoon MF, Ryan C, Sheu L, Yao J, Conklin S, Manuck S. Serum phospholipid docosahexaenoic acid is associated with cognitive functioning during middle adulthood. J Nutr. 2010;140:848–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez LB, Kritz-Silverman D, Barrett-Connor E. High dietary and plasma levels of the omega-3 fatty acid docosahexaenoic acid are associated with decreased dementia risk: the Rancho Bernardo Study. J Nutr Health Aging. 2011;15:25–31 [DOI] [PubMed] [Google Scholar]
- 42.Schaefer EJ, Bongard V, Beiser A, Lamon-Fava S, Robins S, Au R, Tucker K, Kyle D, Wilson P, Wolf P. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol. 2006;63:1545–50 [DOI] [PubMed] [Google Scholar]
- 43.Morris MC, Evans D, Bienias J, Tangney C, Bennett D, Wilson R, Aggarwal N, Schneider J. Consumption of fish and n-3 fatty acids and risk of incident alzheimer disease. Arch Neurol. 2003;60:940–6 [DOI] [PubMed] [Google Scholar]
- 44.Morris MC, Evans D, Tangney C, Bienias J, Wilson R. Fish consumption and cognitive decline with age in a large community study. Arch Neurol. 2005;62:1849–53 [DOI] [PubMed] [Google Scholar]
- 45.Gao Q, Niti M, Feng L, Yap K, Ng T. Omega-3 polyunsaturated fatty acid supplements and cognitive decline: Singapore Longitudinal Aging Studies. J Nutr Health Aging. 2011;15:32–5 [DOI] [PubMed] [Google Scholar]
- 46.Johnson EJ, McDonald K, Caldarella SM, Chung HY, Troen AM, Snodderly DM. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr Neurosci. 2008;11:75–83 [DOI] [PubMed] [Google Scholar]
- 47.Yurko-Mauro K, McCarthy D, Rom D, Nelson EB, Ryan AS, Blackwell A, Salem N, Jr, Stedman M, Investigators M. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 2010;6:456–64 [DOI] [PubMed] [Google Scholar]
- 48.Lee LK, Shahar S, Chin AV, Yusoff NA. Docosahexaenoic acid-concentrated fish oil supplementation in subjects with mild cognitive impairment (MCI): a 12-month randomised, double-blind, placebo-controlled trial. Psychopharmacology (Berl). 2013;225:605–12 [DOI] [PubMed] [Google Scholar]
- 49.Sinn N, Milte CM, Street SJ, Buckley JD, Coates AM, Petkov J, Howe PR. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: a 6-month randomised controlled trial. Br J Nutr. 2012;107:1682–93 [DOI] [PubMed] [Google Scholar]
- 50.Vakhapova V, Cohen T, Richter Y, Herzog Y, Korczyn AD. Phosphatidylserine containing omega-3 fatty acids may improve memory abilities in non-demented elderly with memory complaints: a double-blind placebo-controlled trial. Dement Geriatr Cogn Disord. 2010;29:467–74 [DOI] [PubMed] [Google Scholar]
- 51.Chiu CC, Su KP, Cheng TC, Liu HC, Chang CJ, Dewey ME, Stewart R, Huang SY. The effects of omega-3 fatty acids monotherapy in Alzheimer's disease and mild cognitive impairment: a preliminary randomized double-blind placebo-controlled study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1538–44 [DOI] [PubMed] [Google Scholar]
- 52.Kotani S, Sakaguchi E, Warashina S, Matsukawa N, Ishikura Y, Kiso Y, Sakakibara M, Yoshimoto T, Guo J, Yamashima T. Dietary supplementation of arachidonic and docosahexaenoic acids improves cognitive dysfunction. Neurosci Res. 2006;56:159–64 [DOI] [PubMed] [Google Scholar]
- 53.Freund-Levi Y, Eriksdotter-Jonhagen M, Cederholm T, Basun H, Faxen-Irving G, Garlind A, Vedin I, Vessby B, Wahlund LO, Palmblad J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006;63:1402–8 [DOI] [PubMed] [Google Scholar]
- 54.Quinn JF, Raman R, Thomas RG, Yurko-Mauro K, Nelson EB, Van Dyck C, Galvin JE, Emond J, Jack CR, Jr, Weiner M, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010;304:1903–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lewis M, Ghassemi P, Hibbeln J. Therapeutic use of omega-3 fatty acids in severe head trauma. Am J Emerg Med. 2013;31:273e5–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roberts L, Bailes J, Dedhia H, Zikos A, Singh A, McDowell D, Failinger C, Biundo R, Petrick J, Carpenter J. Surviving a mine explosion. J Am Coll Surg. 2008;207:276–83 [DOI] [PubMed] [Google Scholar]
- 57.Mills JD, Hadley K, Bailes J. Dietary supplementation with the omega-3 fatty acid docosahexaenoic acid in traumatic brain injury? Neurosurgery. 2011;68:474–81 [DOI] [PubMed] [Google Scholar]
- 58.Wu A, Ying Z, Gomez-Pinilla F. Omega-3 fatty acid supplementation restores mechanisms that maintain brain homeostasis in traumatic brain injury. J Neurotrauma. 2007;24:1587–95 [DOI] [PubMed] [Google Scholar]
- 59.Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma. 2004;21:1457–67 [DOI] [PubMed] [Google Scholar]
- 60.Wang T, Van K, Gavitt B, Grayson J, Lu T, Lyeth B, Pichakron K. Effect of fish oil supplementation in a rat model of multiple mild traumatic brain injuries. Restor Neurol Neurosci. 2013;31:647–59 [DOI] [PubMed] [Google Scholar]
- 61.Wu A, Ying Z, Gomez-Pinilla F. The salutary effects of DHA dietary supplementation on cognition, neuroplasticity, and membrane homeostasis after brain trauma. J Neurotrauma. 2011;28:2113–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shin SS, Dixon C. Oral fish oil restores striatal dopamine release after traumatic brain injury. Neurosci Lett. 2011;496:168–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mills JD, Bailes J, Sedney C, Hutchins H, Sears B. Omega-3 fatty acid supplementation and reduction of traumatic axonal injury in a rodent head injury model. J Neurosurg. 2011;114:77–84 [DOI] [PubMed] [Google Scholar]
- 64.Bailes JE, Mills J. Docosahexaenoic acid reduces traumatic axonal injury in a rodent head injury model. J Neurotrauma. 2010;27:1617–24 [DOI] [PubMed] [Google Scholar]
- 65.Wu A, Ying Z, Gomez-Pinilla F. Exercise facilitates the action of dietary DHA on functional recovery after brain trauma. Neuroscience. 2013;248:655–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu A, Ying Z, Gomez-Pinilla F. Dietary strategy to repair plasma membrane after brain trauma: implications for plasticity and cognition. Neurorehabil Neural Repair. 2014;28:75–84 [DOI] [PubMed] [Google Scholar]
- 67.Shin SS, Bray ER, Zhang CQ, Dixon CE. Traumatic brain injury reduces striatal tyrosine hydroxylase activity and potassium-evoked dopamine release in rats. Brain Res. 2011;1369:208–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gómez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. 2008;9:568–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Danthi SJ, Enyeart J, Enyeart J. Modulation of native T-type calcium channels by omega-3 fatty acids. Biochem Biophys Res Commun. 2005;327:485–93 [DOI] [PubMed] [Google Scholar]
- 70.Wang X, Zhao X, Mao Z, Wang X, Liu Z. Neuroprotective effect of docosahexaenoic acid on glutamate-induced cytotoxicity in rat hippocampal cultures. Neuroreport. 2003;14:2457–61 [DOI] [PubMed] [Google Scholar]
- 71.Högyes E, Nyakas C, Killiaan A, Farkas T, Penke B, Luiten P. Neuroprotective effect of developmental docosahexaenoic acid supplement against excitotoxic brain damage in infant rats. Neuroscience. 2003;119:999–1012 [DOI] [PubMed] [Google Scholar]
- 72.Cao DH, Xu J, Xue R, Zheng W, Liu Z. Protective effect of chronic ethyl docosahexaenoate administration on brain injury in ischemic gerbils. Pharmacol Biochem Behav. 2004;79:651–9 [DOI] [PubMed] [Google Scholar]
- 73.Lu DY, Tsao Y, Leung Y, Su K. Docosahexaenoic acid suppresses neuroimflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: implications of antidepressant effects for omega-3 fatty acids. Neuropsychopharmacology. 2010;35:2238–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bazan NG. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer’s disease. J Lipid Res. 2009;50(Suppl):S400–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cole GM, Frautschy S. DHA may prevent age-related dementia. J Nutr. 2010;140:869–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bazan NG. Cellular and molecular events mediated by docosahexaenoic acid-derived neuroprotectin D1 signaling in photoreceptor cell survival and brain protection. Prostaglandins Leukot Essent Fatty Acids. 2009;81:205–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83(Suppl 6):1467S–76S [DOI] [PubMed] [Google Scholar]
- 78.Chen CT, Liu Z, Bazinet RP. Rapid de-esterification and loss of eicosapentaenoic acid from rat brain phospholipids: an intracerebroventricular study. J Neurochem. 2011;116:363–73 [DOI] [PubMed] [Google Scholar]
- 79.Chen CT, Liu Z, Ouellet M, Calon F, Bazinet RP. Rapid beta-oxidation of eicosapentaenoic acid in mouse brain: an in situ study. Prostaglandins Leukot Essent Fatty Acids. 2009;80:157–63 [DOI] [PubMed] [Google Scholar]
- 80.Chen CT, Domenichiello AF, Trepanier MO, Liu Z, Masoodi M, Bazinet RP. The low levels of eicosapentaenoic acid in rat brain phospholipids are maintained via multiple redundant mechanisms. J Lipid Res. 2013;54:2410–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O'Connor WT, Smyth A, Gilchrist MD. Animal models of traumatic brain injury: a critical evaluation. Pharmacol Ther. 2011;130:106–13 [DOI] [PubMed] [Google Scholar]
- 82.Manley GT, Maas AI. Traumatic brain injury: an international knowledge-based approach. JAMA. 2013;310:473–4 [DOI] [PubMed] [Google Scholar]
- 83.Yuh EL, Mukherjee P, Lingsma HF, Yue JK, Ferguson AR, Gordon WA, Valadka AB, Schnyer DM, Okonkwo DO, Maas AI, et al. Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann Neurol. 2013;73:224–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Okonkwo DO, Yue JK, Puccio AM, Panczykowski DM, Inoue T, McMahon PJ, Sorani MD, Yuh EL, Lingsma HF, Maas AI, et al. GFAP-BDP as an acute diagnostic marker in traumatic brain injury: results from the Prospective Transforming Research and Clinical Knowledge in Traumatic Brain Injury Study. J Neurotrauma. 2013;30:1490–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guskiewicz KM, Mihalik JP, Shankar V, Marshall SW, Crowell DH, Oliaro SM, Ciocca MF, Hooker DN. Measurement of head impacts in collegiate football players: relationship between head impact biomechanics and acute clinical outcome after concussion. Neurosurgery. 2007;61:1244–52 [DOI] [PubMed] [Google Scholar]
- 86.European Food Safety Authority. Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010;8:1461 [Google Scholar]
- 87.U.S. Department of Agriculture, U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th ed. Washington, DC: U.S. Government Printing Office; 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kris-Etherton PM, Innis S, American Dietetic Association, Dietitians of Canada. Position of the American Dietetic Association and Dietitians of Canada: dietary fatty acids. J Am Diet Assoc. 2007;107:1599–611 Erratum in: J Am Diet Assoc. 2007;107:2151 [PubMed] [Google Scholar]
- 89.Simopoulos AP, Leaf A, Salem N., Jr Workshop statement on the essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2000;63:119–21 [DOI] [PubMed] [Google Scholar]
- 90.Kris-Etherton PM, Harris WS, Appel LJ. American Heart Association Nutrition Committee. Omega-3 fatty acids and cardiovascular disease: new recommendations from the American Heart Association. Arterioscler Thromb Vasc Biol. 2003;23:151–2 [DOI] [PubMed] [Google Scholar]
- 91.U.S. Department of Agriculture, Agricultural Research Service. Nutrient intakes from food: mean amounts consumed per individual, by gender and age. What we eat in America, NHANES 2009–2010 [cited 2013 Nov 1]. Available from: http://www.ars.usda.gov/ba/bhnrc/fsrg
- 92.Sears B, Bailes J, Asselin B. Therapeutic uses of high-dose omega-3 fatty acids to treat comatose patients with severe brain injury. PhamaNutrition. 2013;1:86–9 [Google Scholar]
- 93.U.S. Department of Health and Human Services, Food and Drug Administration. Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers 2005 [cited 2013 Dec 18]. Available from: http://www.fda.gov/downloads/Drugs/Guidances/UCM078932.pdf