Abstract
Because obesity rates have increased dramatically over the past 3 decades, type 2 diabetes has become increasingly prevalent as well. Type 2 diabetes is associated with decreased pancreatic β-cell mass and function, resulting in inadequate insulin production. Conversely, in nondiabetic obesity, an expansion in β-cell mass occurs to provide sufficient insulin and to prevent hyperglycemia. This expansion is at least in part due to β-cell proliferation. This review focuses on the mechanisms regulating obesity-induced β-cell proliferation in humans and mice. Many factors have potential roles in the regulation of obesity-driven β-cell proliferation, including nutrients, insulin, incretins, hepatocyte growth factor, and recently identified liver-derived secreted factors. Much is still unknown about the regulation of β-cell replication, especially in humans. The extracellular signals that activate proliferative pathways in obesity, the relative importance of each of these pathways, and the extent of cross-talk between these pathways are important areas of future study.
Introduction
The rates of obesity worldwide continue to climb because of numerous social, environmental, and perhaps even genetic factors. In the United States, >35% of adults are obese (1). Along with obesity has come increasing prevalence of type 2 diabetes. Notably, however, only 11.3% of adults are estimated to have diagnosed or undiagnosed diabetes (2). These statistics reveal that secondary factors other than obesity must be required for diabetes to develop; the 2 are not inextricably linked. Obesity is associated with peripheral insulin resistance, a hallmark of the pathophysiology of type 2 diabetes. However, to develop hyperglycemia there must be a failure to produce sufficient insulin to meet this increased demand. This directs our attention to the pancreatic β-cell, where insulin is produced in response to circulating nutrients and other hormonal signals.
Pancreatic β-cells are found within the islets of Langerhans, small clusters of endocrine cells that make up ~1–2% of the total pancreatic mass. The islets are composed of multiple cell types, including insulin-producing β-cells, glucagon-producing α-cells, and several other less abundant cell types producing somatostatin, pancreatic polypeptide, and ghrelin. The amount of insulin secreted in response to metabolic demand is dependent on numerous factors, including proper sensing of nutrient and hormonal signals, adequate insulin synthesis and secretion, and the overall number of functional β-cells. Although all of these factors are important, this review will focus on the regulation of β-cell number. The number of β-cells in a pancreas can be quantified in several ways by using histologic analysis. Total β-cell volume can be estimated by measuring islet β-cell area on pancreatic sections. This β-cell area can then be corrected for total pancreatic section area, giving a fractional β-cell area or volume. Alternatively, β-cell area is corrected for total pancreatic weight, giving an estimate of β-cell mass. The terms β-cell mass and β-cell volume are used relatively interchangeably in the literature to indicate the overall number of β-cells in the pancreas. Individual β-cell size and number can also be measured as a way to distinguish between β-cell hyperplasia and hypertrophy. Although hypertrophy and neogenesis can contribute to changes in overall β-cell mass, this review will focus on the role of β-cell proliferation as a compensatory response to obesity in mice and humans.
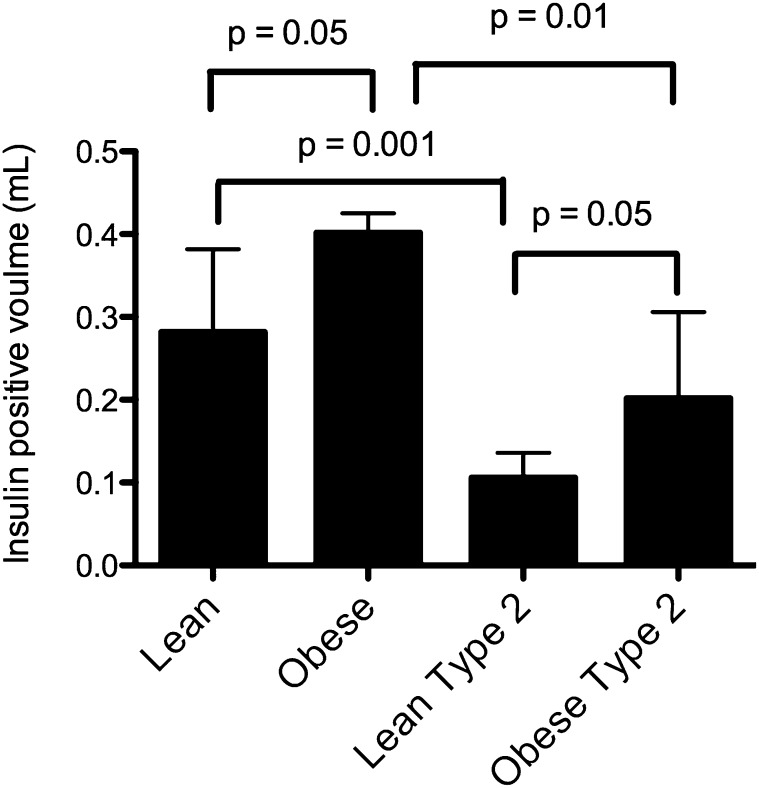
Individuals with type 2 diabetes have decreased β-cell mass compared with nondiabetic individuals of similar BMI. In fact, there seems to be a threshold effect whereby fasting blood glucose becomes elevated if β-cell mass is less than ~1.1% (3). More than 25 y ago, a study of autopsy specimens revealed 2 key points about β-cell mass in humans (4). First, β-cell mass is increased in nondiabetic obesity, and second, there is decreased β-cell mass in both lean and obese patients diagnosed with type 2 diabetes (Fig. 1). These findings have been replicated in more recent autopsy studies (5–8). Therefore, one can generate the hypothesis that an ability to increase β-cell mass in obesity is key to preventing type 2 diabetes.
FIGURE 1.
Differences in β-cell volume in human subjects. With obesity, there is an expansion of β-cells. However, individuals with type 2 diabetes have overall reduced β-cell numbers compared with their nondiabetic counterparts. Values are means ± SDs, n = 4–7. P values were calculated by using the Mann-Whitney test. Plotted from tabular data in reference 4 with permission from publisher S. Karger AG, Basel.
Unfortunately, we have very limited tools to study β-cell mass dynamics in humans. Because of the small size of the islet, the lack of good cell surface markers for β-cells, and the retroperitoneal position of the pancreas, it is not currently possible to image islet or β-cell mass in living people. Pancreatic biopsy is also not a reasonable method for 2 reasons. First, there are different islet densities in the various regions of the pancreas, so accurate characterization of β-cell mass would require multiple specimens throughout the entirety of the organ (9). Second, pancreatic biopsy is an invasive procedure that can lead to pancreatitis, which would be an unacceptable risk in healthy subjects. Therefore, we are left with postmortem samples of whole pancreas or isolated islet tissue to attempt to explain β-cell mass dynamics in humans. Because of these limitations, many have instead examined β-cell mass in rodent models. β-cell proliferative capacity tends to decrease from rat to mouse to human [(10–13) and DBD personal observations]. Therefore, despite significant differences between mouse and human β-cell proliferation, the mouse is the more relevant model. In addition, knockout and transgenic mice have allowed examination of the relevant pathways in β-cell proliferation. Therefore, most of the work we will discuss is from mouse models.
In rodents, there is a dramatic increase in β-cell mass with obesity (14). In mice specifically, β-cell mass expansion occurs in response to diet-induced obesity and genetic models of obesity. By using mouse models, details such as the timing of β-cell proliferation, the effects of various nutrients, and the requirement for various signaling pathways can be studied. In the ob/ob model of obesity, a mutation in the gene encoding the satiety hormone leptin results in significant weight gain and hyperglycemia in some mouse strains (15). In the relatively diabetes-resistant C57Bl/6J ob/ob mice, there is a 3.6-fold increase in β-cell volume without an increase in the total number of islets (16). This suggests that the increase in β-cell volume with obesity is primarily due to replication of existing β-cells within the islet, rather than the formation of new islets. There remains controversy about whether β-cell neogenesis or β-cell replication is the predominant mechanism of adaptive β-cell mass expansion. We will focus on β-cell replication as it contributes to changes in β-cell mass.
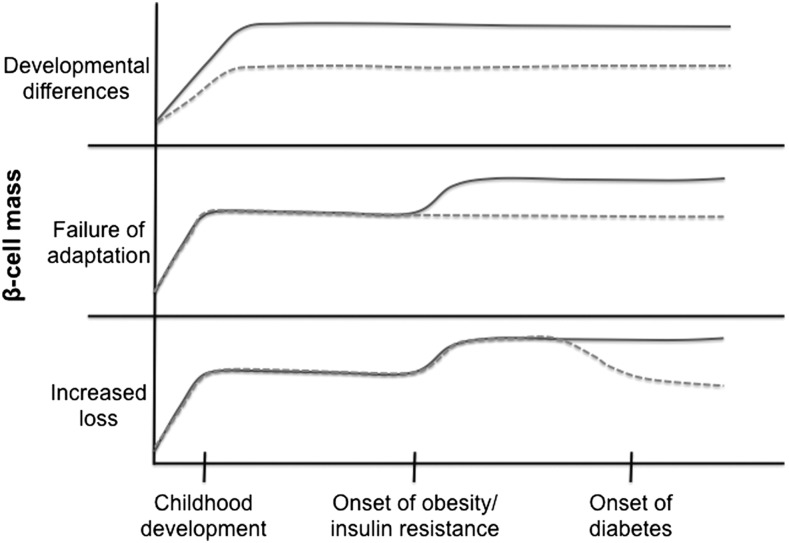
Again, it is challenging to understand what regulates β-cell mass expansion in obesity in humans. It is clear that in rodents there is an acute expansion of β-cell mass with the onset of insulin resistance and obesity. However, we do not have a complete understanding of when β-cell mass expansion occurs during the human life span and how it becomes dysfunctional in individuals with diabetes due to the limitations of postmortem studies. One hypothesis is that some individuals simply have higher initial β-cell mass after growth and development, giving them more reserve capacity to deal with insulin resistance later in life (Fig. 2, top panel). In fact, recent studies have shown that the β-cell mass in children/young adults is highly variable and establishment of adult β-cell mass likely occurs within the first few years of life (17, 18). Alternatively, some individuals may have a failure to initially expand β-cell mass in response to obesity and insulin resistance, predisposing them to eventual development of type 2 diabetes (Fig. 2, middle panel). These 2 hypotheses linking type 2 diabetes with inadequate development or expansion of β-cell mass are supported by the association of type 2 diabetes with single nucleotide polymorphisms near a number of cell cycle regulatory genes in genome-wide association studies (19, 20). A third hypothesis for the reduced β-cell mass in diabetic individuals is increased rates of β-cell death after the onset of obesity and hyperglycemia that ultimately reduces β-cell mass (Fig. 2, bottom panel). In this case, an initial proliferative expansion of β-cell mass in the prediabetic state would not be detectable in autopsy studies performed after the onset of diabetes. All 3 alternative timelines could be consistent with currently available autopsy data. It is probable that development of adequate β-cell mass, compensatory expansion of β-cell mass in response to insulin resistance, and rates of β-cell death all contribute to the overall determination of β-cell mass throughout the life span.
FIGURE 2.
Three possible timelines resulting in diminished β-cell mass in type 2 diabetes at the time of autopsy. In the top panel, a failure to achieve adequate β-cell mass during development or early childhood expansion results in decreased β-cell mass that persists throughout life, increasing susceptibility to type 2 diabetes. In the middle panel, a failure to expand β-cell mass in adult life in response to obesity and insulin resistance results in failure to produce adequate insulin and the development of type 2 diabetes. In the bottom panel, compensatory expansion occurs, but then there is increased loss of β-cells that results in lower β-cell mass when measured at autopsy. The dotted lines represent the timeline in an individual with increased susceptibility to diabetes.
Adaptive vs. Basal β-Cell Proliferation
To understand the mechanisms driving increased β-cell proliferation in response to obesity, it is necessary to draw a distinction between the basal β-cell replication rate and that of induced, or compensatory, proliferation. In adult mice, continuous long-term labeling of proliferating cells with 5-bromo-2-deoxyuridine (BrdU)6 showed that, relative to the total cell population, only ~1 in 1400 mature β-cells replicate per day (21). In adult humans, basal β-cell replication is even lower, with reported rates ranging from 0% to 0.4% (5, 6, 22, 23). This extremely low rate of cellular turnover suggests that, at least at baseline, adult β-cells do not have a high proliferative rate.
In the setting of nondiabetic obesity it appears that at least part of the expansion in β-cell mass comes from increased proliferation. Conversely, a failure to proliferate and/or increased apoptosis appear to be hallmarks of type 2 diabetes. This dichotomy can be modeled in the mouse, where leptin-deficient (ob/ob) C57BL/6 and BTBR (Black and Tan BRachyury) mice both become obese, but only the BTBR mice become diabetic (15, 24). The C57BL/6 mice display increased β-cell proliferation in response to obesity and can meet the increased demand for insulin, whereas the BTBR mice cannot. Differential genetic regulation of β-cell replication may explain the strain-specific differences in β-cell adaptive proliferation. In fact, differential expression of several key cell cycle genes important in β-cell proliferation has been found between these 2 strains (15, 25, 26).
In human autopsy studies there is conflicting evidence regarding an increase in β-cell proliferation with obesity. This is likely due to the challenges of studying β-cell dynamics in autopsy specimens and the different methodologies used to measure proliferation. Two studies by Butler and colleagues found no difference in β-cell replication between obese, lean, and type 2 diabetic individuals, as measured by Ki67 staining (5, 8). When proliferating cell nuclear antigen (PCNA) staining was used as a measure, Hanley et al. (6) found increased rates of replication in obese nondiabetic individuals compared with lean nondiabetic persons, along with decreased rates of proliferation in obese individuals with type 2 diabetes. However, a more recent study suggests the opposite, with increased numbers of PCNA-positive β-cells in specimens from individuals with type 2 diabetes compared with nondiabetic controls (27). These authors suggest the possibility of increased cell cycle entry (resulting in increased PCNA expression) but a failure to progress beyond the G1/S transition resulting in cell cycle arrest in islets of participants with type 2 diabetes. Our own work has shown that a number of key cell cycle genes are upregulated in human islets as a function of obesity, including Ki67 and others beyond the G1/S transition (25). However, at the protein level, many key cell cycle regulators are predominantly localized in the cytoplasm of the β-cell and only translocate to the nucleus under direct mitogenic stimulation (28, 29). The physiologic signals that stimulate nuclear localization and activation of key cell cycle molecules and initiate β-cell replication in human islets have not been clearly determined. Therefore, we will discuss some of the signaling pathways upstream of cell cycle regulation that stimulate adaptive β-cell proliferation.
Nutrient-Mediated Cell Growth and Replication
Intracellular signaling pathways activated by nutrients are well known to mediate cell growth and proliferation in many cell types, from yeast to humans. The mechanisms that translate nutrient availability to cellular proliferation have largely been studied in the context of cancer biology. Due to the expression of glucokinase, a lower affinity hexokinase, and the bidirectional glucose transporter, glucose transporter 2 (GLUT2), the pancreatic β-cell is uniquely designed to sense small changes in physiologic glucose concentrations and ultimately regulate intracellular ATP concentrations in response to glucose uptake and metabolism (30). The β-cells respond to increasing glucose through signaling pathways that lead to insulin secretion. However, these same metabolic and signaling pathways may also regulate other intracellular processes, including proliferation. In general, cell growth is inhibited under low-glucose/low-ATP conditions, primarily driven by increased AMP-activated kinase (AMPK) activity (31). However, when glucose concentrations are normal or high, numerous signaling molecules important in cell growth, including mammalian target of rapamycin complex 1 (mTORC1) and protein kinase A (PKA), are activated (32, 33). Therefore, glucose itself is 1 possible mediator of β-cell replication. However, these same downstream signaling molecules can also be activated by numerous other growth factors and hormonal signals. It remains unclear how or whether nutrients directly mediate β-cell replication.
High-Fat Diet Studies
A commonly used model of obesity is high-fat-diet feeding. Mice fed a high-fat diet had evidence of β-cell mass expansion (14). Mice fed high-fat diets also clearly had defects in β-cell function, despite increasing β-cell mass (14, 34). Numerous different types of high-fat diets have been used to characterize diabetes and glucose intolerance in mouse strains. As would be expected, the phenotype can be highly variable depending on the background strain, the macro- and micronutrient composition of the diet, and the timing of diet-induced obesity. Moderate-fat feeding (30–45% of total caloric intake; a 1:3 mixture of saturated and unsaturated fat from corn oil and coconut oil) in male C57BL/6J × DBA/2J hybrid mice for 1 y resulted in a dose-dependent increase in β-cell area due to β-cell hyperplasia. However, at the end of 1 y there was no detectable difference in β-cell replication or apoptosis as a function of diet or body weight (14). Similarly, high-fat feeding (60% of total caloric intake; fat from lard) in female C57BL/6JBomTac mice resulted in increased β-cell area and hyperplasia as early as 3 mo, persisting after 1 y of feeding (35). Male C57BL/6J fed a 60% fat diet for 8–12 wk also had increased β-cell mass, and at these earlier time points an increase in β-cell proliferation was evident (36, 37). Notably, a recent study reported that only the splenic region of the pancreas, not the duodenal or gastric regions, exhibited increased β-cell area and replication in response to 6 wk of high-fat diet feeding (38). Therefore, regional differences in β-cell proliferation responses may exist, leading to sampling error in histologic studies or confounding effects when using isolated islet tissue.
The proliferative expansion of β-cells correlates with body weight gain and degree of insulin resistance in these high-fat-diet models, suggesting that β-cell replication is driven by the complex milieu of obesity and insulin resistance, and is not dependent solely on the intake of high amounts of dietary fat (14, 36). In fact, acute infusion of lipids does not increase β-cell replication in mice, although this has been seen in rats (11, 13). Elevated FFAs actually appear to inhibit glucose-stimulated β-cell proliferation, suggesting that the combination of dyslipidemia and hyperglycemia would lead to a reduced β-cell replication response (13). Interestingly, a high-fat diet (60% fat; lard-based) had a very early impact on β-cell mass and replication, with an increased amount of cyclin D2 after only 4–7 d of feeding. Some metabolic changes and weight gain were already evident in these short-term studies, although insulin resistance was not clearly present (34). Overall, in mouse models, high-fat-diet feeding seems to result in early β-cell replication leading to lasting increases in β-cell mass, but this effect is dependent on mild hyperglycemia and other metabolic changes, rather than direct effects of the dietary components.
Studies of human islets transplanted under the kidney capsule of immunodeficient mice can provide some insight into the response of human islets to controlled conditions of overnutrition. When mice transplanted with human islets are fed a high-fat diet for 12 wk, the human islet graft exhibited increased β-cell area and increased β:α-cell ratios, although β-cell proliferation was still exceedingly rare in the human islets (39). In contrast, when human islets were transplanted into ob/ob mice for 2 wk, they exhibited increased β-cell proliferation (40). The challenge in interpreting these results is the confounding impact of hypoxia and other stressors during islet transplantation and the non-native environment of the kidney capsule. In an ex vivo study with isolated human islets, there was no change in human islet diameter or cell number with short-term exposure to FFAs and elevated glucose concentrations, but cell cycle or proliferation was not directly measured (41). One could imagine that ex vivo treatment over just a few days is not sufficient to observe significant changes in β-cell number, but more subtle changes at the gene or protein level may be detectable to suggest enhanced proliferative capacity. In summary, there is little evidence to support a direct role of FFAs in β-cell replication. Instead, it appears that high-fat-diet feeding induces numerous metabolic changes that ultimately generate the necessary proliferative signals.
Hyperglycemia
Nutrient-mediated cell proliferation is a common growth mechanism in all cell types. As mentioned above, the β-cell is highly sensitive to changes in circulating glucose. Therefore, glucose has been considered as a potential mediator of β-cell hyperplasia. The role of increased carbohydrate intake has been examined using high-fructose or high-sucrose diets, leading to variable levels of obesity and insulin resistance. These diets are detrimental to islet function, but no direct measurement of β-cell mass was performed to delineate the role of high-carbohydrate feeding in β-cell mass dynamics (42, 43). In mice, short-term 4-d exposure to mild hyperglycemia through continuous glucose infusion led to increased β-cell replication, mediated at least partially by increases in cyclin D2 (44). Similarly, human islets transplanted into immunodeficient mice have increased β-cell replication rates in response to acute glucose infusion (45). Ex vivo, however, human islets do not exhibit increased proliferation in response to high glucose alone, but require additional inhibition of negative regulators of cell growth to replicate in response to glucose (12, 46).
All of these studies are confounded by the concomitant increase in insulin secretion that occurs when a β-cell is exposed to high glucose concentrations. Insulin itself may act as a mitogenic factor. When β-cells lack the insulin receptor, and therefore cannot respond to external insulin stimulation, they exhibit reduced proliferation (47). However, the response of β-cells lacking the insulin receptor to glucose-stimulated proliferation was not directly examined (47). Thus, it remains unclear whether the effect of glucose on β-cell proliferation is dependent on insulin signaling.
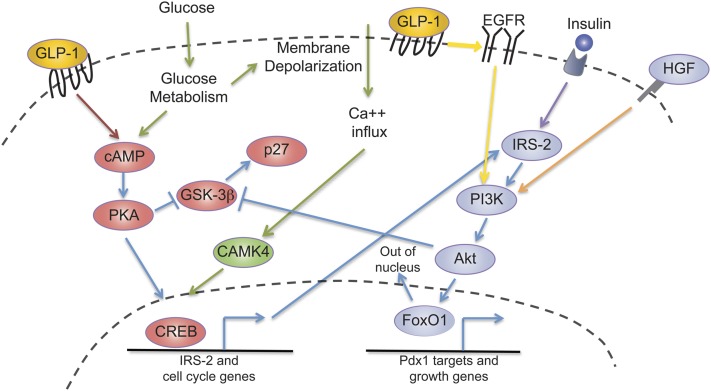
In an attempt to directly understand the role of glucose in stimulating β-cell replication in vivo, Yuval Dor’s group performed a series of elegant studies that suggest that β-cell glucose metabolism and membrane depolarization are critical for stimulation of β-cell proliferation. The stimulation of β-cell replication did not require overt hyperglycemia but rather was seen under conditions of increased demand for insulin secretion per β-cell. By using glucokinase activators or β-cell–specific knockout of glucokinase, these authors found that glucose metabolism was necessary and stimulatory for β-cell replication (48). After the metabolism of glucose results in increased concentrations of intracellular ATP, closure of the ATP-sensitive potassium channel leads to β-cell membrane depolarization. These same authors found that in addition to a requirement for glucokinase activity, glucose-stimulated β-cell replication is also dependent on closure of the ATP-sensitive potassium channel and membrane depolarization (48). Importantly, these were all short-term experiments, with changes in β-cell replication measured after just 24 h. It remains to be determined how these pathways affect overall β-cell mass in the setting of chronic increases in glucose and insulin demand. Intracellular glucose signaling also seems to be important in the maintenance of cyclin D2 expression in the mouse β-cell, perhaps priming β-cells for replication under conditions of increased insulin demand (49). Influx of calcium after membrane depolarization activates calcium/calmodulin-dependent kinase 4 (CAMK4), which can then phosphorylate and activate cAMP response element binding protein (CREB) (Fig. 3) (50). CREB is able to stimulate proliferation in the mouse MIN-6 β-cell line (51). Carbohydrate response element-binding protein (ChREBP) appears to be necessary for glucose-dependent proliferation in dispersed human β-cells, suggesting that glucose-sensing may also result in direct transcriptional activity to promote cell proliferation/replication (52).
FIGURE 3.
Multiple pathways can stimulate β-cell replication in obesity, and there is overlap of many of the downstream activators. GLP-1 signals through the GLPR (red arrow) and leads to increased intracellular cAMP, which activates PKA. PKA then phosphorylates and activates the transcription factor CREB. Glucose metabolism (green arrows) increases cAMP levels and can activate the same pathway. PKA also inhibits GSK3-β, which is an activator of the cell cycle inhibitor p27. Glucose metabolism (green arrows) results in production of ATP and membrane depolarization, which leads to an influx of calcium ions. These calcium ions activate CAMK4, which then also phosphorylates and activates CREB. CREB stimulates transcription of IRS-2, which then provides IRS-2 protein necessary for insulin signaling. Insulin signals through the insulin receptor to phosphorylate and activate IRS-2 (purple arrow), leading to activation of PI3K and Akt. Akt inhibits GSK3-β. Akt is also necessary to phosphorylate the inhibitory transcription factor FoxO1 and promote its exclusion from the nucleus. When FoxO1 inhibition is released, transcription of Pdx1 and other genes promoting cell growth can be expressed. GLP-1 signaling can also transactivate EGFR (yellow arrow) and lead to activation of PI3K, ultimately signaling through the same pathways. HGF signaling through its receptor c-Met also stimulates PI3K (orange arrow). Therefore, there is great overlap in the downstream pathways that are involved in signaling in response to GLP-1, glucose metabolism, insulin, and HGF. The cAMP/PKA, GSK3-β, CREB, and IRS-2/PI3K/Akt/FoxO1 pathways are used by multiple upstream mitogenic signals (blue arrows). This overlap suggests that perhaps multiple activators must be present to trigger β-cell proliferation physiologically, but additionally highlights the challenges of interpreting the importance of 1 upstream factor when a downstream target is manipulated experimentally. CAMK4, calcium/calmodulin-dependent kinase; CREB, cAMP response element binding protein; EGFR, epithelial growth factor receptor; FoxO1, forkhead box O1; GLP-1, glucagon-like peptide-1; GLPR, glucagon-like peptide-1 receptor; GSK3-β, glycogen synthase kinase 3-β HGF, hepatocyte growth factor; IRS-2, insulin receptor substrate 2; Pdx1, pancreatic and duodenal homeobox 1; PI3K, phosphoinositide-3 kinase; PKA, protein kinase A.
Therefore, the role of glucose in β-cell proliferation is likely a permissive one involving activation of multiple pathways. Glucose uptake and metabolism initiate signaling and transcriptional events that may prime the β-cell to respond to additional proliferative stimuli.
Mechanisms of Insulin-Induced β-Cell Proliferation
Increased caloric intake in obese individuals causes an increase in demand for insulin over both the short term (postprandial) and long term (due to the development of insulin resistance and increased fasting blood glucose). Both basal and postprandial glucose stimulate insulin production and release, and the insulin response is amplified by nutrient availability and/or metabolism (53), as well as the incretins glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) (54). In turn, insulin stimulates β-cell mass expansion both directly and indirectly through complex regulation of multiple signaling pathways.
Insulin indirectly modulates β-cell mass expansion in response to obesity via the nuclear hormone receptor peroxisome proliferator-activated receptor γ (PPARγ). In a mouse model, both insulin and obesity increase PPARγ concentrations in multiple tissues (55). Mice with a β-cell–specific PPARγ deletion have reduced β-cell mass expansion when fed a high-fat diet (56). Likewise, knockout of the PPARγ2 isoform in an ob/ob background results in failure to expand β-cell mass in response to obesity when compared with wild-type ob/ob littermates (57). Recent work suggests that this failure might be due to perturbation of an immune response that correlates with β-cell mass expansion in ob/ob islets (58).
β-Cells express all of the known components of the insulin-signaling pathway. Insulin directly binds to the insulin receptor (IR) and activates its tyrosine kinase activity. Isolated β-cells lacking the IR exhibit slow growth and decreased expression of numerous cell cycle proteins (27). Activation of the IR in β-cells induces phosphorylation of 1 of 4 known IR substrates (IRS 1–4). Tyrosine phosphorylated IRS family members are then able to interact with a variety of downstream signaling partners, most notably proteins of the phosphoinositide-3 kinase (PI3K) pathway (Fig. 3). Of the mice deficient for the various IRS family members, only mice deficient for IRS-2 had defects in β-cell mass. IRS-2-null mice had significant β-cell hypoplasia when fed a standard unpurified diet (59, 60). Heterozygous IRS-2 knockout mice also failed to induce compensatory β-cell proliferation when fed a high-fat diet (61). This appears to be due to increased nuclear localization of the inhibitory transcription factor forkhead box O1 (FoxO1), and thus insufficient β-cell proliferative response to hyperinsulinemia (62). Further support for the role of FoxO1 in regulating insulin-mediated cell cycle signaling is derived from recent studies also showing increased FoxO1 nuclear localization in β-cells lacking insulin receptor (27). However, β-cell–specific FoxO1 knockouts showed no alteration in β-cell replication, but rather exhibited β-cell dedifferentiation (63). Release of FoxO1 inhibition may be just 1 component of insulin-mediated β-cell replication: FoxO1 may not only inhibit proliferation but help to maintain β-cell–specific gene expression.
IRS proteins mediate Akt family activation through PI3K signaling. Once activated, Akt family members promote cell growth and survival through various pathways, including increased activity of mammalian target of rapamycin (mTOR). Transgenic overexpression of constitutively active Akt in mice causes an increase in β-cell mass (64, 65). Enhanced signaling through the Akt pathway is responsible for massively increased β-cell mass when glycogen synthase kinase 3-β (GSK3-β) is conditionally knocked out in mice fed a high-fat diet (66). GSK3-β is normally inhibited by insulin, demonstrating that insulin can promote β-cell proliferative response through both direct and indirect activation of the Akt signaling pathway. GSK3-β inactivation also promotes β-cell proliferation through downregulation of the cell cycle inhibitor p27 (Fig. 3) (67). Interestingly, p27 knockout mice have increased islet proliferation when made genetically obese or when fed a high-fat diet, whereas p27 overexpression in these scenarios reduces β-cell proliferation (68).
Wnt/β-catenin signaling, when combined with inhibition of RhoA and Rho-kinase (ROCK), has recently been shown to stimulate human β-cell proliferation ex vivo (69). In this unique system, Akt is phosphorylated due to inhibition of its inhibitor ROCK and CREB activity is increased leading to increased Irs2 expression. Similarly, activation of the cAMP/PKA/CREB pathway in human islets can result in phosphorylation and inactivation of GSK3β and increased expression of IRS-2. The overall idea is that multiple signaling pathways must be activated, including insulin/Akt, Wnt/β-catenin, and PKA/CREB to fully stimulate human β-cell replication with preservation of β-cell function. Whether these signaling pathways are important in adaptive human β-cell replication in vivo and how the pathways interact with one another under conditions of obesity and insulin resistance will be challenging to unravel.
Taken together, it is clear that the insulin-induced β-cell proliferative response to obesity is complex. The combination of direct and indirect signaling mechanisms and the overlap in multiple steps in the pathway make therapeutic targeting of specific molecular pathways difficult. Future work to target these pathways will require careful consideration of all potential pathways of action to ensure favorable outcomes.
Incretins and Their Role in β-Cell Proliferation
The incretin hormones, GLP-1 and GIP, are peptide hormones produced by the intestinal enteroendocrine L and K cells, respectively, in response to the presence of nutrients in the intestinal lumen or their absorption (54). GLP-1 may indirectly stimulate β-cell proliferation through its effects on insulin secretion. However, direct stimulation of β-cell replication has also been postulated. Although studies in rats and β-cell lines have demonstrated β-cell replication in response to incretin treatment, human β-cells fail to proliferate with these stimuli (70, 71).
Whether incretin signaling is important in adaptive β-cell proliferation during obesity remains unknown. Postprandial GLP-1 secretion is diminished in obese individuals and in those with type 2 diabetes (72, 73). The data on GIP concentrations in obesity are conflicting, and there is not overwhelming evidence to suggest increased circulating or postprandial concentrations in obese persons (72, 74, 75). Upon stimulation with glucose or amino acids, α-cells within the human and rat islet also secrete small amounts of GLP-1 (76, 77). Whether there is a local, intraislet incretin signaling pathway that is activated in obesity remains unclear. Current therapies for type 2 diabetes target the incretin pathway, including GLP-1 receptor (GLPR) agonists and inhibitors of the dipeptidyl peptidase 4 (DPP4) enzyme that rapidly degrades GLP-1 and GIP. Because of the availability of these medications, much interest remains in understanding the potential of incretin pathways to stimulate β-cell replication.
GLPR and GIP receptor (GIPR) are Gs protein–coupled receptors (GPCRs). Upon ligand binding to the GLPR, and in the presence of glucose, the α-stimulatory subunit of the G protein (Gαs) dissociates from the G protein complex and activates adenylate cyclase, which results in the formation of cAMP (78). cAMP activates PKA, which, in turn, stimulates the transcription factor CREB. CREB stimulates proliferation through simultaneous inhibition of the proliferation inhibitor p27 and activation of cyclins A2 and D1 (79). CREB also promotes transcription of IRS-2, promoting signaling through the IRS-2/PI3K/Akt pathway (50). GLP-1 signaling also results in transactivation of the epithelial growth factor receptor (EGFR). EGFR subsequently activates PI3K and its downstream effector Akt. Through this pathway, GLP-1 signaling inhibits FoxO1 nuclear translocation (80). Indeed, exendin-4 (a GLPR agonist) failed to stimulate β-cell expansion in transgenic mice expressing a constitutively nuclear FoxO1 in β-cells (80). These signaling pathways clearly overlap with glucose and insulin signaling in the β-cell (Fig. 3).
In a similar fashion, GIP promotes β-cell proliferation through Gαs-mediated activation of PKA. It is less clear if GIP also promotes proliferation through the PI3K pathway (81). However, work on GIP’s role in proliferation has been conducted in neonatal rat islets and not replicated in adult mouse or human islets. Mice with a dominant negative mutation in the GIPR, expressed only in the β-cells, exhibit reduced β-cell mass. However, replication rates in these mice were not reduced during early life, when replication was highest in controls (82). Therefore, in mice, GIP signaling is not critical for adult β-cell mass expansion. However, dominant negative GIPR pigs had reduced β-cell proliferation resulting in reduced β-cell mass (83). Overall, the role of GIP in β-cell proliferation remains unclear.
The incretin hormones signal through similar pathways as glucose and insulin signaling and therefore may augment other proliferative signals sensed by the β-cell during obesity. There is not yet convincing evidence that the incretins are stimulatory for human β-cell proliferation, but given their widespread use as therapeutic agents for type 2 diabetes, this possibility remains intriguing.
Additional Evidence that GPCR Pathways May Regulate β-Cell Mass Proliferative Expansion in Obesity
Although the role of incretin signaling through the GLPR and the GIPR in mouse and human β-cell proliferation remains unclear, there is emerging evidence that GPCRs are generally important in β-cell mass expansion during obesity. Rgs16 and Rgs8 are regulators of GPCR signaling and can identify cell populations in which GPCR signaling is occurring. Rgs16 and Rgs8 are not expressed in adult islet tissue, except in the setting of β-cell damage or obesity (ob/ob model). Specifically, expression was correlated with elevated glucose and insulin concentrations. This suggests that GPCR signaling pathways are activated in β-cells with obesity and may mediate some of the adaptive proliferative effects of glucose and insulin. Whether these Rgs proteins themselves are playing a positive or negative role in the proliferative response remains unclear (84).
Recently, the miRNA miR-338-3p was identified as a potential inhibitor of β-cell mass expansion in response to obesity (85). Islet expression of miR-338-p was lower in high-fat-diet–fed and genetically obese mice, inversely correlating with β-cell mass expansion. Exogenous activation of the GLPR in isolated mouse and human islets led to reduced miR-338-p expression. Furthermore, inhibition of miR-338-p in β-cells induced proliferation through activation of cell cycle genes. This suggests that inhibition of miR-338-p by GLP1R signaling may be another mechanism driving the expansion of β-cell mass in obesity (85).
The inhibitory guanine nucleotide binding protein (G protein), Gαz, is also a potential inhibitor of β-cell proliferation in obesity (86). This G protein inhibits adenylate cyclase, leading to reduced cAMP production in the β-cell. This inhibitory G protein couples to the EP3-variant of the E prostanoid receptor (prostaglandin E receptor 3), which is activated by prostaglandin E2. Gαz knockout mice fed a high-fat diet had increased rates of β-cell proliferation and increased β-cell mass (86). Therefore, inhibition of cAMP signaling appears to be an important “brake” on β-cell proliferation. Release of this “brake” can lead to even greater β-cell proliferation in response to obesity. These results suggest that GPCR/cAMP pathways are able to drive β-cell proliferation, but perhaps cannot do so effectively when under inhibition by Gαz.
Overall, there are many separate lines of evidence to suggest that GPCR signaling is important in obesity-associated β-cell proliferation. Exactly which GPCRs and which agonists are the key regulators remains unclear.
Hepatocyte Growth Factor
Hepatocyte growth factor (HGF) is produced in cells of mesenchymal origin, such as adipocytes, vascular cells, and monocytes (87). HGF signals through the c-Met receptor, a tyrosine kinase receptor. c-Met signaling involves many downstream pathways, including PI3K (Fig. 3). In rat insulinoma cells (INS-1), HGF has been shown to stimulate proliferation via janus kinase 2/signal transducer and activator of transcription 5 (JAK2/STAT5) signaling under low-glucose conditions, presumably through activation of PI3K (88). HGF is a candidate factor for the regulation of β-cell replication during obesity. HGF production by adipocytes is positively correlated with BMI, and concentrations are elevated in mouse and human obesity (89, 90). The increase in HGF concentrations in high-fat-diet–induced obesity precedes the expansion of β-cell mass and the increase in insulin secretion, suggesting that it may act as an early signal of obesity/insulin resistance (90).
HGF appears to play a direct role in adaptive β-cell proliferation. Transgenic mice overexpressing HGF in the β-cell have increased β-cell mass and β-cell proliferation (91). However, under basal conditions, when β-cell proliferation is low, signaling through the HGF/c-Met pathway is not necessary for the maintenance of β-cell mass or glycemic control (92). The expansion of β-cell mass in response to diet-induced obesity was absent when rats were treated with HGF-receptor kinase inhibitor SU11274 (90). This effect was at least partially caused by reduced β-cell proliferation. Mice lacking the HGF receptor, c-Met, in the pancreas also failed to adequately increase proliferation and expand β-cell mass in response to the insulin-resistant state of pregnancy (93). However, the role of HGF/c-Met signaling in mice and humans under the conditions of obesity has not been directly assessed. HGF remains a strong candidate for a circulating signal of obesity that could stimulate adaptive β-cell proliferation.
Liver-Derived Circulating Factors in Insulin Resistance
Through studies using the liver-specific knockout of the IR, it became clear that insulin resistance in the liver results in increased β-cell replication. In a series of elegant studies, the Kulkarni group determined that the enhanced β-cell replication is due to a liver-derived factor found in the systemic circulation (94). This circulating factor derived from mouse hepatocytes was even able to stimulate human β-cell replication ex vivo; however, the exact factor was not determined. A few months later, Yi et al. (95) identified a new protein, betatrophin, as a circulating factor that promotes β-cell proliferation in response to insulin resistance. Betatrophin expression increases in adipose tissue and the liver in states of obesity and insulin resistance and is secreted into the circulation. When betatrophin is overexpressed in the liver, it leads to a dramatic increase in proliferation of pancreatic β-cells. Betatrophin signaling in the β-cell has not yet been determined, and its effects on human β-cell proliferation will need to be assessed. There may still exist other circulating factors from the liver that can have stimulatory effects on β-cell mass.
The Complexities of Therapeutic Intervention
In conclusion, with increasing rates of obesity, type 2 diabetes has also become increasingly prevalent. However, obesity and type 2 diabetes are not inextricably linked, and the ability to expand functional β-cell mass in response to insulin resistance is crucial in the prevention of diabetes. Understanding the extracellular and intracellular signals that regulate β-cell proliferation during this adaptive process will hopefully lead to new therapeutic targets for type 2 diabetes treatment and prevention.
We have discussed here recent advances in the molecular pathways controlling obesity-driven pancreatic β-cell proliferation. Multiple pathways converge within the β-cell to contribute to this compensatory proliferation (Fig. 3). A common theme that emerges is that rodent studies are not necessarily directly translatable to humans and there is a disconnect that is often complex. A prime example of this is glucose-mediated β-cell replication, in which additional metabolic pathways must be inhibited to promote glucose-driven proliferation in human islets. Likewise, whereas β-cell proliferation in response to a high-fat diet is readily observed in rodents, this phenomenon is much more difficult to measure and study at the molecular level in humans. Rodent studies and ex vivo human islet studies have, however, given us clues as to how the β-cell responds to hyperglycemia, increased insulin demand/presence, increased hormone concentrations, and liver-derived factors. Many of these extracellular signals appear to function through only a handful of known intracellular pathways mediated by cAMP or PI3K/Akt modulation of gene expression.
Currently, none of our therapies for type 2 diabetes directly stimulate β-cell proliferation in human islets. As discussed here, multiple extracellular signals have been identified that can activate proliferative pathways in obesity, but it seems likely that more have yet to be found. Understanding the importance and relative contribution of nutrient and hormonal signals will be challenging, given the complex interplay between glucose, insulin, and other hormonal signals in the setting of insulin resistance and obesity. Furthermore, although identifying common downstream signaling pathways may elucidate therapeutic targets, a lack of specificity will likely limit safety and efficacy of such therapies. We remain challenged by the inability to assess β-cell dynamics in living people. Although great progress has been made in recognizing the importance of the pancreatic β-cell in the pathogenesis of type 2 diabetes, ongoing efforts will hopefully reveal more about the specific effects of obesity on β-cell replication.
Acknowledgments
The authors thank Michelle Kimple for critical review of the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AMPK, AMP-activated kinase; BrdU, 5-bromo-2-deoxyuridine; BTBR, Black and Tan Brachyury; CAMK4, calcium/calmodulin-dependent kinase; ChREBP, carbohydrate response element binding protein; CREB, cAMP response element binding protein; DPP4, dipeptidyl peptidase 4; EGFR, epithelial growth factor receptor; EP3, prostaglandin E receptor 3; FoxO1, forkhead box O1; G protein; guanine nucleotide binding protein; GIP, glucose-dependent insulinotropic polypeptide; GIPR, glucose-dependent insulinotropic polypeptide receptor; GLP-1, glucagon-like peptide-1; GLPR, glucagon-like peptide-1 receptor; GLUT2, glucose transporter 2; GPCR, G protein–coupled receptor; GSK-3β, glycogen synthase kinase 3-β HGF, hepatocyte growth factor; INS-1, rat insulinoma cell line; IR, insulin receptor; IRS, insulin receptor substrate; JAK/STAT, janus kinase/signal transducer and activator of transcription; MIN-6, mouse insulinoma cell line; mTOR, mammalian target of rapamycin; mTORC1, mammalian target of rapamycin complex 1; PCNA, proliferating cell nuclear antigen; PI3K, phosphoinositide-3 kinase; PKA, protein kinase A; PPARγ, peroxisome proliferator-activated receptor γ Rgs; regulator of G protein receptor signaling; ROCK, rho kinase.
Literature Cited
- 1. Ogden CL, Carroll ME, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009#x20132010. NCHS Data Brief. 2012;82:1#x20138. [PubMed]
- 2.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. U.S. Department of Health and Human Services; 2011. [Google Scholar]
- 3.Ritzel RA, Butler AE, Rizza RA, Veldhuis JD, Butler PC. Relationship between beta-cell mass and fasting blood glucose concentration in humans. Diabetes Care. 2006;29:717–8 [DOI] [PubMed] [Google Scholar]
- 4.Klöppel G, Löhr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res. 1985;4:110–25 [DOI] [PubMed] [Google Scholar]
- 5.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–10 [DOI] [PubMed] [Google Scholar]
- 6.Hanley SC, Austin E, Assouline-Thomas B, Kapeluto J, Blaichman J, Moosavi M, Petropavlovskaia M, Rosenberg L. Beta-cell mass dynamics and islet cell plasticity in human type 2 diabetes. Endocrinology. 2010;151:1462–72 [DOI] [PubMed] [Google Scholar]
- 7.Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10 Suppl 4:32–42 [DOI] [PubMed] [Google Scholar]
- 8.Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC. β-Cell mass and turnover in humans: effects of obesity and aging. Diabetes Care. 2013;36:111–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Misawa R, Zielinski MC, Cowen P, Jo J, Periwal V, Ricordi C, Khan A, Szust J, Shen J, et al. Regional differences in islet distribution in the human pancreas–preferential beta-cell loss in the head region in patients with type 2 diabetes. PLoS ONE. 2013;8:e67454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulkarni RN, Mizrachi E-B, Ocana AG, Stewart AF. Human β-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes. 2012;61:2205–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steil GM, Trivedi N, Jonas JC, Hasenkamp WM, Sharma A, Bonner-Weir S, Weir GC. Adaptation of beta-cell mass to substrate oversupply: enhanced function with normal gene expression. Am J Physiol Endocrinol Metab. 2001;280:E788–96 [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Remedi MS, Pappan KL, Kwon G, Rohatgi N, Marshall CA, McDaniel ML. Glycogen synthase kinase-3 and mammalian target of rapamycin pathways contribute to DNA synthesis, cell cycle progression, and proliferation in human islets. Diabetes. 2009;58:663–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pascoe J, Hollern D, Stamateris R, Abbasi M, Romano LC, Zou B, O'Donnell CP, Garcia-Ocana A, Alonso LC. Free fatty acids block glucose-induced β-cell proliferation in mice by inducing cell cycle inhibitors p16 and p18. Diabetes. 2012;61:632–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hull RL, Kodama K, Utzschneider KM, Carr DB, Prigeon RL, Kahn SE. Dietary-fat-induced obesity in mice results in beta cell hyperplasia but not increased insulin release: evidence for specificity of impaired beta cell adaptation. Diabetologia. 2005;48:1350–8 [DOI] [PubMed] [Google Scholar]
- 15.Keller MP, Choi Y, Wang P, Davis DB, Rabaglia ME, Oler AT, Stapleton DS, Argmann C, Schueler KL, Edwards S, et al. A gene expression network model of type 2 diabetes links cell cycle regulation in islets with diabetes susceptibility. Genome Res. 2008;18:706–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bock T, Pakkenberg B, Buschard K. Increased islet volume but unchanged islet number in ob/ob mice. Diabetes. 2003;52:1716–22 [DOI] [PubMed] [Google Scholar]
- 17.Gregg BE, Moore PC, Demozay D, Hall BA, Li M, Husain A, Wright AJ, Atkinson MA, Rhodes CJ. Formation of a human β-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab. 2012;97:3197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57:1584–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, Zeggini E, Huth C, Aulchenko YS, Thorleifsson G, et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet. 2010;42:579–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris AP, Voight BF, Teslovich TM, Ferreira T, Segrè AV, Steinthorsdottir V, Strawbridge RJ, Khan H, Grallert H, Mahajan A, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44:981–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes. 2005;54:2557–67 [DOI] [PubMed] [Google Scholar]
- 22.Perl S, Kushner JA, Buchholz BA, Meeker AK, Stein GM, Hsieh M, Kirby M, Pechhold S, Liu EH, Harlan DM, et al. Significant human beta-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. J Clin Endocrinol Metab. 2010;95:E234–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butler AE, Cao-Minh L, Galasso R, Rizza RA, Corradin A, Cobelli C, Butler PC. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia. 2010;53:2167–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoehr JP, Nadler ST, Schueler KL, Rabaglia ME, Yandell BS, Metz SA, Attie AD. Genetic obesity unmasks nonlinear interactions between murine type 2 diabetes susceptibility loci. Diabetes. 2000;49:1946–54 [DOI] [PubMed] [Google Scholar]
- 25.Davis DB, Lavine JA, Suhonen JI, Krautkramer KA, Rabaglia ME, Sperger JM, Fernandez LA, Yandell BS, Keller MP, Wang I-M, et al. FoxM1 is up-regulated by obesity and stimulates beta-cell proliferation. Mol Endocrinol. 2010;24:1822–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krautkramer KA, Linnemann AK, Fontaine DA, Whillock AL, Harris TW, Schleis GJ, Truchan NA, Marty-Santos L, Lavine JA, Cleaver O, et al. Tcf19 is a novel islet factor necessary for proliferation and survival in the INS-1 β-cell line. Am J Physiol Endocrinol Metab. 2013;305:E600–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folli F, Okada T, Perego C, Gunton J, Liew CW, Akiyama M, D'Amico A, La Rosa S, Placidi C, Lupi R, et al. Altered insulin receptor signalling and β-cell cycle dynamics in type 2 diabetes mellitus. PLoS ONE. 2011;6:e28050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiaschi-Taesch NM, Kleinberger JW, Salim FG, Troxell R, Wills R, Tanwir M, Casinelli G, Cox AE, Takane KK, Srinivas H, et al. Cytoplasmic-nuclear trafficking of G1/S cell cycle molecules and adult human β-cell replication: a revised model of human β-cell G1/S control. Diabetes. 2013;62:2460–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiaschi-Taesch NM, Kleinberger JW, Salim FG, Troxell R, Wills R, Tanwir M, Casinelli G, Cox AE, Takane KK, Scott DK, et al. Human pancreatic β-cell G1/S molecule cell cycle atlas. Diabetes. 2013;62:2450–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuit FC, Huypens P, Heimberg H, Pipeleers DG. Glucose sensing in pancreatic beta-cells: a model for the study of other glucose-regulated cells in gut, pancreas, and hypothalamus. Diabetes. 2001;50:1–11 [DOI] [PubMed] [Google Scholar]
- 31.Yuan H-X, Xiong Y, Guan K-L. Nutrient sensing, metabolism, and cell growth control. Mol Cell. 2013;49:379–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci. 2013;126:1713–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costes S, Longuet C, Broca C, Faruque O, Hani EH, Bataille D, Dalle S. Cooperative effects between protein kinase A and p44/p42 mitogen-activated protein kinase to promote cAMP-responsive element binding protein activation after beta cell stimulation by glucose and its alteration due to glucotoxicity. Ann N Y Acad Sci. 2004;1030:230–42 [DOI] [PubMed] [Google Scholar]
- 34.Stamateris RE, Sharma RB, Hollern DA, Alonso LC. Adaptive β-cell proliferation increases early in high-fat feeding in mice, concurrent with metabolic changes, with induction of islet cyclin D2 expression. Am J Physiol Endocrinol Metab. 2013;305:E149–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahrén J, Ahrén B, Wierup N. Increased β-cell volume in mice fed a high-fat diet: a dynamic study over 12 months. Islets. 2010;2:353–6 [DOI] [PubMed] [Google Scholar]
- 36.Peyot M-L, Pepin E, Lamontagne J, Latour MG, Zarrouki B, Lussier R, Pineda M, Jetton TL, Madiraju SRM, Joly E, et al. Beta-cell failure in diet-induced obese mice stratified according to body weight gain: secretory dysfunction and altered islet lipid metabolism without steatosis or reduced beta-cell mass. Diabetes. 2010;59:2178–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, Azuma K, Hirose T, Tanaka K, Kominami E, et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 2008;8:325–32 [DOI] [PubMed] [Google Scholar]
- 38.Ellenbroek JH, Töns HA, de Graaf N, Loomans CJ, Engelse MA, Vrolijk H, Voshol PJ, Rabelink TJ, Carlotti F, de Koning EJ. Topologically heterogeneous beta cell adaptation in response to high-fat diet in mice. PLoS ONE. 2013;8:e56922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gargani S, Thévenet J, Yuan JE, Lefebvre B, Delalleau N, Gmyr V, Hubert T, Duhamel A, Pattou F, Kerr-Conte J. Adaptive changes of human islets to an obesogenic environment in the mouse. Diabetologia. 2013;56:350–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tyrberg B, Ustinov J, Otonkoski T, Andersson A. Stimulated endocrine cell proliferation and differentiation in transplanted human pancreatic islets: effects of the ob gene and compensatory growth of the implantation organ. Diabetes. 2001;50:301–7 [DOI] [PubMed] [Google Scholar]
- 41.Vernier S, Chiu A, Schober J, Weber T, Nguyen P, Luer M, McPherson T, Wanda PE, Marshall CA, Rohatgi N, et al. β-Cell metabolic alterations under chronic nutrient overload in rat and human islets. Islets. 2012;4:379–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Omar B, Pacini G, Ahrén B. Differential development of glucose intolerance and pancreatic islet adaptation in multiple diet induced obesity models. Nutrients. 2012;4:1367–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caton PW, Kieswich J, Yaqoob MM, Holness MJ, Sugden MC. Nicotinamide mononucleotide protects against pro-inflammatory cytokine-mediated impairment of mouse islet function. Diabetologia. 2011;54:3083–92 [DOI] [PubMed] [Google Scholar]
- 44.Alonso LC, Yokoe T, Zhang P, Scott DK, Kim SK, O'Donnell CP, Garcia-Ocana A. Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes. 2007;56:1792–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levitt HE, Cyphert TJ, Pascoe JL, Hollern DA, Abraham N, Lundell RJ, Rosa T, Romano LC, Zou B, O'Donnell CP, et al. Glucose stimulates human beta cell replication in vivo in islets transplanted into NOD-severe combined immunodeficiency (SCID) mice. Diabetologia. 2011;54:572–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rohatgi N, Aly H, Marshall CA, McDonald WG, Kletzien RF, Colca JR, McDaniel ML. Novel insulin sensitizer modulates nutrient sensing pathways and maintains β-cell phenotype in human islets. PLoS ONE. 2013;8:e62012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Assmann A, Ueki K, Winnay JN, Kadowaki T, Kulkarni RN. Glucose effects on beta-cell growth and survival require activation of insulin receptors and insulin receptor substrate 2. Mol Cell Biol. 2009;29:3219–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Porat S, Weinberg-Corem N, Tornovsky-Babaey S, Schyr-Ben-Haroush R, Hija A, Stolovich-Rain M, Dadon D, Granot Z, Ben-Hur V, White P, et al. Control of pancreatic β cell regeneration by glucose metabolism. Cell Metab. 2011;13:440–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salpeter SJ, Klochendler A, Weinberg-Corem N, Porat S, Granot Z, Shapiro AMJ, Magnuson MA, Eden A, Grimsby J, Glaser B, et al. Glucose regulates cyclin D2 expression in quiescent and replicating pancreatic β-cells through glycolysis and calcium channels. Endocrinology. 2011;152:2589–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Persaud SJ, Liu B, Sampaio HB, Jones PM, Muller DS. Calcium/calmodulin-dependent kinase IV controls glucose-induced Irs2 expression in mouse beta cells via activation of cAMP response element-binding protein. Diabetologia. 2011;54:1109–20 [DOI] [PubMed] [Google Scholar]
- 51.Liu B, Barbosa-Sampaio H, Jones PM, Persaud SJ, Muller DS. The CaMK4/CREB/IRS-2 cascade stimulates proliferation and inhibits apoptosis of β-cells. PLoS ONE. 2012;7:e45711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Metukuri MR, Zhang P, Basantani MK, Chin C, Stamateris RE, Alonso LC, Takane KK, Gramignoli R, Strom SC, O'Doherty RM, et al. ChREBP mediates glucose-stimulated pancreatic β-cell proliferation. Diabetes. 2012;61:2004–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Magnan C, Collins S, Berthault MF, Kassis N, Vincent M, Gilbert M, Pénicaud L, Ktorza A, Assimacopoulos-Jeannet F. Lipid infusion lowers sympathetic nervous activity and leads to increased beta-cell responsiveness to glucose. J Clin Invest. 1999;103:413–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–65 [DOI] [PubMed] [Google Scholar]
- 55.Vidal-Puig A, Jimenez-Liñan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Flier JS, Moller DE. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest. 1996;97:2553–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosen ED, Kulkarni RN, Sarraf P, Ozcan U, Okada T, Hsu CH, Eisenman D, Magnuson MA, Gonzalez FJ, Kahn CR, et al. Targeted elimination of peroxisome proliferator-activated receptor in beta cells leads to abnormalities in islet mass without compromising glucose homeostasis. Mol Cell Biol. 2003;23:7222–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Medina-Gomez G, Gray SL, Yetukuri L, Shimomura K, Virtue S, Campbell M, Curtis RK, Jimenez-Linan M, Blount M, Yeo GSH, et al. PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 2007;3:e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vivas Y, Martínez-García C, Izquierdo A, Garcia-Garcia F, Callejas S, Velasco I, Campbell M, Ros M, Dopazo A, Dopazo J. Early peroxisome proliferator-activated receptor gamma regulated genes involved in expansion of pancreatic beta cell mass. BMC Med Genomics. 2011;4:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–4 [DOI] [PubMed] [Google Scholar]
- 60.Kubota N, Tobe K, Terauchi Y, Eto K, Yamauchi T, Suzuki R, Tsubamoto Y, Komeda K, Nakano R, Miki H, et al. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes. 2000;49:1880–9 [DOI] [PubMed] [Google Scholar]
- 61.Terauchi Y, Takamoto I, Kubota N, Matsui J, Suzuki R, Komeda K, Hara A, Toyoda Y, Miwa I, Aizawa S, et al. Glucokinase and IRS-2 are required for compensatory cell hyperplasia in response to high-fat diet-induced insulin resistance. J Clin Invest. 2007;117:246–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takamoto I, Terauchi Y, Kubota N, Ohsugi M, Ueki K, Kadowaki T. Crucial role of insulin receptor substrate-2 in compensatory beta-cell hyperplasia in response to high fat diet-induced insulin resistance. Diabetes Obes Metab. 2008;10 Suppl 4:147–56 [DOI] [PubMed] [Google Scholar]
- 63.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell. 2012;150:1223–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA. Islet beta cell expression of constitutively active Akt1/PKB alpha induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Invest. 2001;108:1631–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tuttle RL, Gill NS, Pugh W, Lee JP, Koeberlein B, Furth EE, Polonsky KS, Naji A, Birnbaum MJ. Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat Med. 2001;7:1133–7 [DOI] [PubMed] [Google Scholar]
- 66.Liu Y, Tanabe K, Baronnier D, Patel S, Woodgett J, Cras-Méneur C, Permutt MA. Conditional ablation of Gsk-3β in islet beta cells results in expanded mass and resistance to fat feeding-induced diabetes in mice. Diabetologia. 2010;53:2600–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stein J, Milewski WM, Hara M, Steiner DF, Dey A. GSK-3 inactivation or depletion promotes β-cell replication via down regulation of the CDK inhibitor, p27 (Kip1). Islets. 2011;3:21–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uchida T, Nakamura T, Hashimoto N, Matsuda T, Kotani K, Sakaue H, Kido Y, Hayashi Y, Nakayama KI, White MF, et al. Deletion of Cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nat Med. 2005;11:175–82 [DOI] [PubMed] [Google Scholar]
- 69.Aly H, Rohatgi N, Marshall CA, Grossenheider TC, Miyoshi H, Stappenbeck TS, Matkovich SJ, McDaniel ML. A novel strategy to increase the proliferative potential of adult human β-cells while maintaining their differentiated phenotype. PLoS ONE. 2013;8:e66131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parnaud G, Bosco D, Berney T, Pattou F, Kerr-Conte J, Donath MY, Bruun C, Mandrup-Poulsen T, Billestrup N, Halban PA. Proliferation of sorted human and rat beta cells. Diabetologia. 2008;51:91–100 [DOI] [PubMed] [Google Scholar]
- 71.Rutti S, Sauter NS, Bouzakri K, Prazak R, Halban PA, Donath MY. In vitro proliferation of adult human beta-cells. PLoS ONE. 2012;7:e35801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carr RD, Larsen MO, Jelic K, Lindgren O, Vikman J, Holst JJ, Deacon CF, Ahrén B. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J Clin Endocrinol Metab. 2010;95:872–8 [DOI] [PubMed] [Google Scholar]
- 73.Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–23 [DOI] [PubMed] [Google Scholar]
- 74.Verdich C, Toubro S, Buemann B, Lysgård Madsen J, Juul Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety—effect of obesity and weight reduction. Int J Obes Relat Metab Disord. 2001;25:1206–14 [DOI] [PubMed] [Google Scholar]
- 75.Muscelli E, Mari A, Casolaro A, Camastra S, Seghieri G, Gastaldelli A, Holst JJ, Ferrannini E. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes. 2008;57:1340–8 [DOI] [PubMed] [Google Scholar]
- 76.Marchetti P, Lupi R, Bugliani M, Kirkpatrick CL, Sebastiani G, Grieco FA, Del Guerra S, D'Aleo V, Piro S, Marselli L, et al. A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets. Diabetologia. 2012;55:3262–72 [DOI] [PubMed] [Google Scholar]
- 77.Whalley NM, Pritchard LE, Smith DM, White A. Processing of proglucagon to GLP-1 in pancreatic α-cells: is this a paracrine mechanism enabling GLP-1 to act on β-cells? J Endocrinol. 2011;211:99–106 [DOI] [PubMed] [Google Scholar]
- 78.Buteau J. GLP-1 receptor signaling: effects on pancreatic beta-cell proliferation and survival. Diabetes Metab. 2008;34: Suppl 2:S73–7 [DOI] [PubMed] [Google Scholar]
- 79.Song W-J, Schreiber WE, Zhong E, Liu F-F, Kornfeld BD, Wondisford FE, Hussain MA. Exendin-4 stimulation of cyclin A2 in beta-cell proliferation. Diabetes. 2008;57:2371–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buteau J, Spatz ML, Accili D. Transcription factor FoxO1 mediates glucagon-like peptide-1 effects on pancreatic beta-cell mass. Diabetes. 2006;55:1190–6 [DOI] [PubMed] [Google Scholar]
- 81.Friedrichsen BN, Neubauer N, Lee YC, Gram VK, Blume N, Petersen JS, Nielsen JH, Møldrup A. Stimulation of pancreatic beta-cell replication by incretins involves transcriptional induction of cyclin D1 via multiple signalling pathways. J Endocrinol. 2006;188:481–92 [DOI] [PubMed] [Google Scholar]
- 82.Herbach N, Bergmayr M, Göke B, Wolf E, Wanke R. Postnatal development of numbers and mean sizes of pancreatic islets and beta-cells in healthy mice and GIPR(dn) transgenic diabetic mice. PLoS ONE. 2011;6:e22814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Renner S, Fehlings C, Herbach N, Hofmann A, von Waldthausen DC, Kessler B, Ulrichs K, Chodnevskaja I, Moskalenko V, Amselgruber W, et al. Glucose intolerance and reduced proliferation of pancreatic beta-cells in transgenic pigs with impaired glucose-dependent insulinotropic polypeptide function. Diabetes. 2010;59:1228–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Villasenor A, Wang ZV, Rivera LB, Ocal O, Asterholm IW, Scherer PE, Brekken RA, Cleaver O, Wilkie TM. Rgs16 and Rgs8 in embryonic endocrine pancreas and mouse models of diabetes. Dis Model Mech. 2010;3:567–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jacovetti C, Abderrahmani A, Parnaud G, Jonas J-C, Peyot M-L, Cornu M, Laybutt R, Meugnier E, Rome S, Thorens B, et al. MicroRNAs contribute to compensatory β cell expansion during pregnancy and obesity. J Clin Invest. 2012;122:3541–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kimple ME, Moss JB, Brar HK, Rosa TC, Truchan NA, Pasker RL, Newgard CB, Casey PJ. Deletion of GαZ protein protects against diet-induced glucose intolerance via expansion of β-cell mass. J Biol Chem. 2012;287:20344–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakamura T, Sakai K, Nakamura T, Matsumoto K. Hepatocyte growth factor twenty years on: much more than a growth factor. J Gastroenterol Hepatol. 2011;26: Suppl 1:188–202 [DOI] [PubMed] [Google Scholar]
- 88.Gahr S, Merger M, Bollheimer LC, Hammerschmied CG, Schölmerich J, Hügl SR. Hepatocyte growth factor stimulates proliferation of pancreatic beta-cells particularly in the presence of subphysiological glucose concentrations. J Mol Endocrinol. 2002;28:99–110 [DOI] [PubMed] [Google Scholar]
- 89.Bell LN, Ward JL, Degawa-Yamauchi M, Bovenkerk JE, Jones R, Cacucci BM, Gupta CE, Sheridan C, Sheridan K, Shankar SS, et al. Adipose tissue production of hepatocyte growth factor contributes to elevated serum HGF in obesity. Am J Physiol Endocrinol Metab. 2006;291:E843–8 [DOI] [PubMed] [Google Scholar]
- 90.Araújo TG, Oliveira AG, Carvalho BM, Guadagnini D, Protzek AOP, Carvalheira JBC, Boschero AC, Saad MJA. Hepatocyte growth factor plays a key role in insulin resistance-associated compensatory mechanisms. Endocrinology. 2012;153:5760–9 [DOI] [PubMed] [Google Scholar]
- 91.Garcia-Ocañ a A, Takane KK, Syed MA, Philbrick WM, Vasavada RC, Stewart AF. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J Biol Chem. 2000;275:1226–32 [DOI] [PubMed] [Google Scholar]
- 92.Mellado-Gil J, Rosa TC, Demirci C, Gonzalez-Pertusa JA, Velazquez-Garcia S, Ernst S, Valle S, Vasavada RC, Stewart AF, Alonso LC, et al. Disruption of hepatocyte growth factor/c-Met signaling enhances pancreatic beta-cell death and accelerates the onset of diabetes. Diabetes. 2011;60:525–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Demirci C, Ernst S, Alvarez-Perez JC, Rosa T, Valle S, Shridhar V, Casinelli GP, Alonso LC, Vasavada RC, Garcia-Ocana A. Loss of HGF/c-Met signaling in pancreatic β cells leads to incomplete maternal β-cell adaptation and gestational diabetes mellitus. Diabetes. 2012;61:1143–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.El Ouaamari A, Kawamori D, Dirice E, Liew CW, Shadrach JL, Hu J, Katsuta H, Hollister-Lock J, Qian W-J, Wagers AJ, et al. Liver-derived systemic factors drive β cell hyperplasia in insulin-resistant states. Cell Rep. 2013;3:401–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yi P, Park J-S, Melton DA. Betatrophin: a hormone that controls pancreatic β cell proliferation. Cell. 2013;153:747–58 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]