Abstract
Bioactive compounds in foods have been gaining interest, and processes to consider them for public health recommendations are being discussed. However, the evidence base is difficult to assemble. It is difficult to demonstrate causality, and there often is not a single compound–single effect relation. Furthermore, health benefits may be due to metabolites produced by the host or gut microbiome rather than the food constituent per se. Properties that can be measured in a food may not translate to in vivo health effects. Compounds that are being pursued may increase gut microbial diversity, improve endothelial function, improve cognitive function, reduce bone loss, and so forth. A new type of bioactive component is emerging from epigenetic modifications by our diet, including microRNA transfer from our diet, which can regulate expression of human genes. Policy processes are needed to establish the level of evidence needed to determine dietary advice and policy recommendations and to set research agendas.
Introduction
A national and international dialog is underway on whether public health recommendations can be made on bioactive foods and ingredients that have health benefits. The Office of Dietary Supplements at the NIH has defined bioactive compounds as constituents in foods or dietary supplements, other than those needed to meet basic human nutritional needs, which are responsible for changes in health status (1). Recent publications from a conference (2) and symposium (3) evaluated the readiness of bioactive components for public health recommendations. Although existing processes for setting requirements for essential nutrients by the Food and Nutrition Board of the Institute of Medicine may not be practical for bioactive components of health, these reports set the stage for an evidence-based process. This process is yet to be defined for bioactive foods and ingredients. This article is a call for developing a process for establishing public health recommendations for bioactive foods and ingredients. Such a process is needed to be able to set a framework for research and the evidence base needed before consensus messaging can be given to health care professionals. The ultimate beneficiary of public health messages is the consumer.
Opportunities and Challenges
Opportunities for a bioactive food or component, or for diet more generally, begin with identifying a link between a bioactive food or ingredient and health or to a disease. Scientific evidence is accumulated. Claims can be formulated by food manufacturers and communicated directly to the consumer or for government approval of a claim, depending on the nature of the claim. Processes are developed for establishing claims. For developing public health recommendations, the process needs to be created. The interplay between scientists and policy makers is iterative because the process needs to be framed in order to know what evidence must be collected, and the quality of the evidence must be evaluated by policy makers before public health recommendations can be communicated to health care providers, food manufacturers, and ultimately consumers. Interest in bioactive foods and ingredients is high among consumers. The International Food and Information Council has conducted functional foods consumer surveys for 15 y. Among 1005 participants in the 2013 Functional Foods Consumer Survey, 45% said they were very interested and 86% said they were very or somewhat interested in learning more about foods that have benefits beyond basic nutrition.
Currently, bioactive foods and ingredients have almost no role in public policy. There are limited databases of these constituents. The flavonoid database recently developed by the USDA, an update of the isoflavone database in selected foods, is an encouraging example of recent strides in 1 class of bioactive compounds (4). Without such food composition databases, intakes of these compounds by groups and populations cannot be monitored in food intake surveys. Their content in foods is not included in nutrient labels. With no public health recommendations, there is no evidence-based process to provide consensus messages for training public health professionals and, in turn, for them or manufacturers to convey public health messages to consumers.
Reliable, science-based information is lacking for health benefits of bioactive foods and constituents. It is difficult to demonstrate causality for bioactive compounds and health. As with essential nutrients or food patterns, their role in the transition of healthy to unhealthy tissue or with development of a chronic disease can take decades. The highest quality of evidence is the randomized controlled trial (RCT)3, but this design is frequently very expensive and impractical for the long latency effects observed for many diet-disease links.
Additional challenges for studying the role of bioactive components and health are that they may effect rather small changes and affect multiple tissues. This can also be said of essential nutrients and diet. However, seemingly small reductions in blood pressure with diet alterations in an individual can result in significant benefits in reducing chronic disease at a population level. For example, 3-mm Hg reduction in systolic blood pressure was predicted to lower mortality due to stroke by 8% and coronary heart disease by 5% (5). Adding up the many small benefits to various tissues to produce a global index has been proposed for nutrients (6). This approach could easily apply to bioactive components as well.
The greatest need in accumulating evidence for bioactive components and their relation to health is the development of good biomarkers for both exposure and effect. A greater challenge for identifying biomarkers of exposure for bioactive components compared with essential nutrients is that the bioactivity of a food may not be attributable to a single constituent. If multiple constituents constitute the bioactive effector and multiple tissues respond, then monitoring the causal link between a bioactive source and health is indeed a challenge. But until we can monitor exposure and their health benefits, progress will be limited.
Role of Metabolism
A further complication in understanding the benefits of bioactive components on health may be that their activity arises from the metabolites produced by the host or the gut microbiome rather than from their presence in the food. A recent example of the sometimes misleading characterization of a bioactive and predicting its relation to health is shown withantioxidants. Much effort was spent on assessing the antioxidant capacity of foods through oxygen radical antioxidant capacity (ORAC), fluorescence recovery after photobleaching (FRAP), trolox equivalent antioxidant capacity (TEAC), telomeric repeat amplification protocol (TRAP), ferrous oxidation-xylenol (FOX) orange, telomeric repeat amplification protocol assays to learn that they do not necessarily predict protection against protein, DNA, or lipid oxidation (7). Nor has it been established that plasma antioxidant status predicts a physiologic benefit. Rather, compounds that stimulate endogenous antioxidant and anti-inflammatory cell signaling in ways that promote health are more relevant to establishing bioactivity (7).
Recent discoveries validate what many cultures have traditionally believed that human gut microbial diversity responds to diet and can influence health (8). Human gut microbial diversity was greater in lean than in obese individuals in a study in Denmark (9). Low genetic microbial diversity was associated with a higher incidence of metabolic syndrome and an increased propensity to gain weight. Cotillard et al. (10) used metagenomic approaches to show that weight-loss interventions were less efficient for improving inflammatory biomarkers in individuals with low microbial gene diversity. In addition to affecting the gut, the gut microbiota affects other tissues such as the skeleton and immune system. For example, using germ-free mice, the role of the gut microbiome in regulating bone mass was demonstrated and bone mass was normalized by colonization with normal gut microbiota (11).
One of the first studies to reveal that consuming a bioactive ingredient can alter the gut microbiota with physiologic benefits in healthy people was related to enhanced mineral absorption. In healthy children, we showed that feeding prebiotics such as galacto-oligosaccharides and soluble corn fiber alters the human gut microbiome to favor the proportion of fermenting bacteria and to increase production of short-chain FAs. Galacto-oligosaccharides at 5 g/d and soluble corn fiber at 12 g/d increased calcium absorption by ~12% in pubertal children (12, 13).
What Constitutes a Successful Bioactive Food or Ingredient?
Many foods and constituents have been identified that confer health benefits affecting a diverse array of tissues. Bioactive ingredients have been derived from milk, eggs, meat, fish, soy, wheat, broccoli, rice, and more.
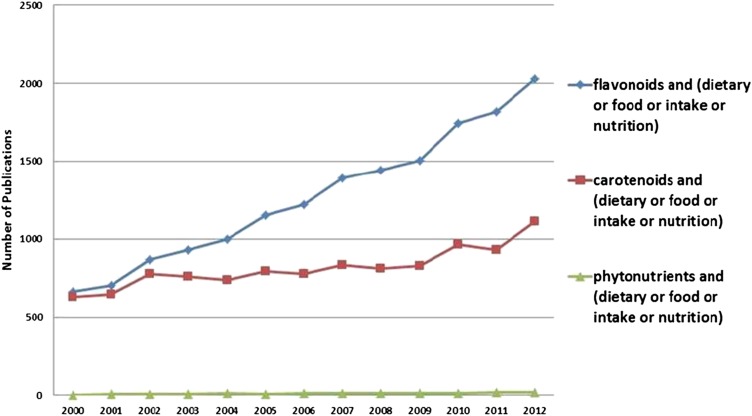
There is a substantial volume of evidence for some bioactive components that could be reviewed in a DRI-like process for making intake recommendation. A large evidence base exists for soy isoflavones, flavonoids, and carotenoids. Moreover, the rate of publications has been rapidly increasing for some categories, notably flavonoids (Fig. 1). But even with a large evidence base, determining public health messages will not be easy. For example, hundreds of studies were reviewed by the North American Menopause Society on the relation of soy isoflavones and menopausal health (14). Results were mixed for relieving menopausal symptoms and for bone and cardiovascular benefits. The relation of soy foods and breast and endometrial cancer was based on observational studies. The panel called for greater standardization and documentation of clinical trial data on soy. The lack of standardization and documentation of bioactive components will be a common problem for many bioactive trials. Similarly, systematic reviews and meta-analyses of dietary or blood antioxidants and diseases of the eye have not provided a clear path (15, 16). In 9 prospective cohort studies and 3 RCTs, there was insufficient evidence to support the role of antioxidants from diet or supplements (including vitamin A, vitamin C, vitamin E, zinc, lutein, zeaxanthin, and lycopene) on prevention of age-related macular degeneration (15). On the other hand, a meta-analysis of observational studies showed that blood concentrations of vitamin E, lutein, and zeaxanthin were inversely associated with age-related cataract (16).
FIGURE 1.
Numbers of publications on flavonoids, carotenoids, and phytonutrients and health outcomes. Data and figure are from the International Life Sciences Institute (ILSI) North America Emerging Science Trend Report, 2013.
Some bioactive foods and ingredients have regulatory approval for health claims and are already in the marketplace or are expected to be soon, which constitutes 1 form of success and allows a type of public health messaging. The number of bioactive components with regulatory approval are few. The European Food Safety Authority (EFSA) evaluates health claims under articles 13 (general function claims) and 14 (disease claims) of the 2007 European Union Regulation EC no. 1924/2006. In the United States, the FDA evaluates claims in 3 categories: health claims to describe a relation between a dietary entity and a disease, nutrient content claims that characterize the amount of nutrients in a food, and structure/function claims about the role of a nutrient or dietary ingredient to affect normal structure or function in humans under the 1990 Nutrition Labeling and Education Act, the 1992 Dietary Supplement Act, the 1994 Dietary Supplement Health and Education Act, the 1997 FDA Modernization Act, and the 2003 Committee Health Information for Better Nutrition Initiative, which provides for use of qualified health claims. Under the Dietary Supplement Health and Education Act, the FDA has approved ~12 health claims that describe a relation between a food, food component, or dietary supplement and reducing the risk of a disease or health-related condition that meets significant scientific agreement (17). There are an additional 17 qualified health claims subject to enforcement discretion (18).
Bioactive components that promote weight management and lower risk of obesity, diabetes, and cardiovascular disease have received the most attention. One product that has received EFSA approval is Slendesta (Kemin Industries, Inc.). Slendesta contains a patented active ingredient, P12, a peptide that increases secretion of cholecystokinin, a gut hormone that decreases food intake. Peptides that are released by specialized enteroendocrine cells are potential key targets for bioactive ingredients to increase satiety (19). The authors caution that the benefits of a bioactive component in controlling body weight are better measured chronically by a scale than acutely by satiety tests because satiety may adapt away.
Bioactive constituents that benefit heart health may be through their impact on endothelial function, which predicts cardiovascular events. Endothelial dysfunction can be assessed through brachial artery flow-mediated dilation and endothelial pulse amplitude testing (Endo-PAT). Chocolate and cocoa flavon-3-ols improve flow-mediated dilation both acutely (over 90–150 min) and chronically (2–18 wk) according to a recent meta-analysis (20).
EPA and DHA FA consumption and blood concentrations have been associated with increased flow-mediated dialysis (21) and decreased risk of sudden cardiac death (22). The consumption of fish (4 oz) twice a week to achieve intakes of 250–500 mg EPA and DHA is promoted by the 2010 Dietary Guidelines for Americans in order to decrease arrhythmias, blood clot formation, blood TGs, growth rate of atherosclerotic build-up, blood pressure, and inflammation and to improve arterial cell function (23). A recent health claim (article 13) for a whole food for its ability to improve blood vessel elasticity was awarded by EFSA for walnuts and is allowed in foods that provide ≥30 g of walnuts. In 1 study, walnut consumption improved flow-mediated dialysis by 367% (P = 0.009) (24).
On the Horizon
Bioactive components that alter the genetic code are now on the horizon and will undoubtedly lead to even more discussion and concern for policy makers. Epigenetic modifications by the environment are being explored. The recent exciting discovery of a cross-kingdom transfer of functionally active microRNA (miRNA) from ingested rice to blood in humans was very unexpected (25). miRNAs constitute one of the components of the epigenome. miRNAs (19–24 nucleotides) do not code for proteins but bind to target mRNAs to inhibit protein translation. The particular miRNA in rice (MIR168a) found in the blood of Chinese individuals can bind to the LDL receptor adaptor protein (LDLRAP1) mRNA to inhibit its expression in liver. This resulted in reduced LDL removal from mouse plasma. This is the first evidence that miRNAs of plant origin can epigenetically regulate expression of human genes. Thus, it is possible that exogenous miRNAs from the diet can regulate expression of target genes in humans to influence the risk of disease. In the example discussed above, altered LDL removal increased the risk of cardiovascular disease. This suggests that circulating miRNAs could function as biomarkers of disease as well as signaling molecules. Future research will likely reveal ways to harness miRNAs as bioactive components to benefit health.
Closer at hand are bioactive constituents from food-derived peptides, modulators of the gut microbiome as pre- and probiotics, which stimulate endogenous antioxidant and anti-inflammatory signaling systems, modulators of food intake and satiety, etc. Some bioactive constituents derived from foods require novel technology to enrich them in foods (26). These can include nano-emulsions, nano-cages, or multilayer emulsions to encapsulate and disperse bioactive constituents to keep them stable and compatible with the food matrix. They also can improve their bioavailability. These products developed from new technologies to deliver bioactive components in the diet may also require regulatory approval.
What Constitutes Sufficient Evidence?
Many hurdles are being erected for determining recommendations for essential nutrients, let alone bioactive constituents. Many in the scientific community have adopted the evidence-based medicine model for nutrition. The prioritization of RCTs is justified because this study design allows causal inference. However, RCTs work best for drugs that are not naturally present in the diet and that have a single action, large effect, and work quickly. With nutrients (and staple foods) that are naturally present in the diet, an RCT cannot be ethically designed to evaluate the benefit of its presence compared with its absence. Moreover, health effects are typically modest, affect multiple tissues, and have a long latency effect. Too often, RCTs in nutrition use supplements to deliver a nutrient or compound to a population that are already above the amounts that confer health benefits and so are predestined to see no effect. The question being addressed changes from whether the constituent is health-promoting to whether more of the constituent than the participants were consuming at baseline is better.
The type of outcome measure expected to give dietary advice is also debated. Those supporting adaptation of the evidence-based medicine model to nutrition seek mortality data or at least an event such as a heart attack or fracture. Biomarkers for disease risk as surrogates for “hard outcome” data are hotly debated for establishing public health recommendations even for well-established biomarkers such as blood pressure, bone mineral density, and balance data. Most people do not seek dietary advice to extend their life. They are more interested in healthy eating for wellness and performance for an improved quality of life. Those supporting the “hard evidence” approach are sensitive to drugs that are approved on the basis of intermediary end points, which have occasionally had disastrous consequences when commercialized. But drugs are intended for select populations in contrast to diet for the whole population. Foods and essential nutrients (taken below the Tolerable Upper Level) generally have a history of safety. A global index for evaluating diet and health effects has been proposed that combines multiple changes in various tissues (blood pressure, glucose control, infection resistance, falls, bone status, etc., as relevant) (6). This approach has the advantages of increased power to find small effects and captures the multiplicity of effects that are characteristic of most dietary constituents.
Bioactive components can span the range from a foodstuff to an isolated, purified compound that could be ingested at amounts much higher than could be obtained through diet and may have properties more like a drug (i.e., more potent efficacy and higher safety risks). These should be considered on a case-by-case basis. For the FDA to suggest an Investigative New Drug approval for performing clinical research on every bioactive component stifles innovation (27).
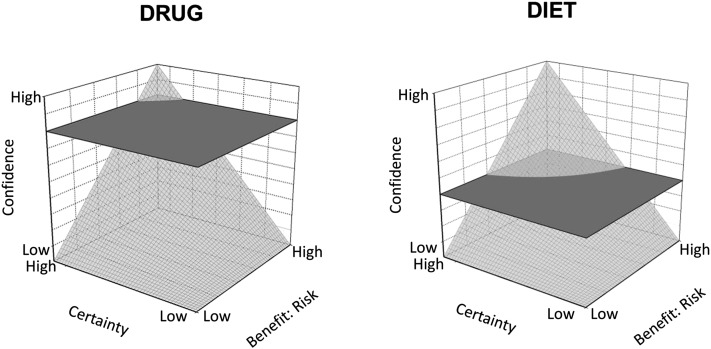
Many kinds of evidence are possible to establish the link between a dietary recommendation and health (i.e., basic research to establish how it works, translational research to determine relevance to humans, efficacy studies in controlled conditions to determine specific effects, and effectiveness studies to determine benefits in free-living populations). Achterberg (28) recently encouraged consideration of all of the evidence, rather than a single methodology (i.e., RCTs). The best recommendations use the totality of evidence with liberal doses of critical thinking and logic. Perhaps the most difficult decision is where to put the bar. Figure 2 suggests that the level of confidence to prompt action for advice for a nutrient and at least some bioactive components may require less confidence in demonstrated certainty and risk than for a drug. Do we need long-term dose-response RCTs in large study populations for every food and bioactive constituent? Or is biologic plausibility plus smaller clinical trials to demonstrate translation to humans sufficient? After all, in practice, current dietary advice is made on much less evidence than for drugs.
FIGURE 2.
What level of confidence is needed to prompt action? The relation between the ratio of benefit and risk of the intervention and the degree of certainty about the efficacy leads to a confidence outcome about a decision to recommend the intervention. The cut-plane is decided by a regulatory body or public policy committee; above the plane permits action. A high level of certainty is demanded by society for permitting approval of a drug because of high cost of medical treatment and high risk of inaction compared with side effects. Dietary recommendations to prevent disease demand less certainty than drugs used to treat disease. Isolated bioactive components with potentially higher risk of side effects may demand a higher level of confidence than a nutrient. Adapted with permission from reference 6.
Conclusions
Discoveries in bioactive components and health are escalating. Possibilities for improving human health for weight management, prevention and treatment of chronic disease, and wellness abound. On the horizon are bioactive components that influence the gut microbiome and genetic control. The process for evidence-based criteria to make public health statements lags behind. The sooner that a consensus among the scientific community for an acceptable process for dietary guidance on bioactive components for health can be formulated, the better will be the study designs and the more motivated will be researchers and sponsors to work on discoveries. The ultimate goal of policy makers, scientists, and professional societies is to improve human health, so if we work together to set up a reasonable process, we can make the most progress. Bioactive components are a challenging area, and multiple paths for the many types that span the range from whole foods to druglike isolated compounds will be needed.
Acknowledgments
The sole author had responsibility for all parts of the manuscript.
Footnotes
Abbreviations used: EFSA, European Food Safety Authority; LDLRAP, LDL receptor adaptor protein; miRNA, microRNA; RCT, randomized controlled trial.
Literature Cited
- 1. NIH, Office of Dietary Supplements. Federal Register Vol. 69 No. 179 FR Dec 04–20892, Sept. 16, 2004 [cited 10/6/19]. Available from: ods.od.nih/gov/Research/Bioactive_Food_Components_Initiative.aspx.
- 2.Biesalski HK, Erdman JW, Jr, Hathcock J, Ellwood K, Beatty S, Johnson E, Marchioli R, Lauritizen L, Rice HB, Shao A, et al. Nutrient reference values for bioactives: new approaches needed? A conference report. Eur J Nutr. 2013;52:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaine PC, Balentine DA, Erdman JW, Jr, Dwyer JT, Ellwood KC, Hu FB, Russell RM. Are dietary bioactives ready for recommended intakes. Adv Nutr. 2013;4:539–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhagwat S, Haytowitz DB, Wasswa-Kintu SI, Holden JM. USDA develops a database for flavonoids to assess dietary intakes. Procedia Food Science. 2013;2:81–6 [Google Scholar]
- 5.Stamler R. Implications of the INTERSALT study. Hypertension. 1991;17:I16–20 [DOI] [PubMed] [Google Scholar]
- 6.Blumberg J, Heaney RP, Huncharek M, Scholl T, Stampfer M, Vieth R, Weaver CM, Zeisel SH. Evidence-based criteria in the nutritional context. Nutr Rev. 2010;68:478–84 [DOI] [PubMed] [Google Scholar]
- 7.Finley JW, Kong A-N, Hintze KJ, Jeffery EH, Ji LL, Lei XG. Antioxidants in foods: state of the science important to the food industry. J Agric Food Chem. 2011;59:6837–46 [DOI] [PubMed] [Google Scholar]
- 8.Fang S, Evans RM. Wealth management in the gut. Nature. 2013;500:538–9 [DOI] [PubMed] [Google Scholar]
- 9.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildenbrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–6 [DOI] [PubMed] [Google Scholar]
- 10.Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N, et al. ; ANR MicroObes Consortium. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–8 [DOI] [PubMed] [Google Scholar]
- 11.Sjögren K, Endhal C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Bäckhed F, Ohlsson Cl. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27:1357–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whisner CM, Martin BR, Schoterman MHC, Nakatsu CH, McCabe LD, McCabe GP, Wastney ME, van den Heuvel EGHM, Weaver CM. Galacto-oligosaccharides increase calcium absorption and gut bifidobacteria in young girls: a double blind crossover trial. Br J Nutr. 2013;110:1292–303 [DOI] [PubMed] [Google Scholar]
- 13.Whisner CM, Martin BR, McCabe GP, McCabe LD, Weaver CM. Soluble corn fiber effects on calcium absorption and retention in adolescent girls and boys. FASEB J. 2012;27:1056 [Google Scholar]
- 14.Clarkson TB, Utian WH, Barnes S, Gold EB, Basaria SS, Aso T, Kronenberg F, Frankenfeld CL, Cline JM, Landgren BM, et al. The role of soy isoflavones in menopausal health: report of The North American Menopause Society. Menopause. 2011;18:732–53 [DOI] [PubMed] [Google Scholar]
- 15.Chong EW, Wong TY, Kreis AJ, Simpson JA, Guymer RH. Dietary antioxidants and primary prevention of age related macular degeneration: systematic review and meta-analysis. BMJ 2007;335:755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui YH, Jing CX, Pan HW. Association of blood antioxidants and vitamins with risk of age-related cataract: a meta-analysis of observational studies. Am J Clin Nutr. 2013;98:778–86 [DOI] [PubMed] [Google Scholar]
- 17. The Chiropractic Resource Organization. FDA-authorized health claims [cited 2013 Sep 30]. Available from: http://www.chiro.org/nutrition/ABSTRACTS/FDA_Claims.shtml.
- 18. FDA. Summary of qualified health claims subject to enforcement discretion [cited 2014 Feb 11]. Available from: http://www.fda.gov/Food/IngredientsPackagingLabeling/LabelingNutrition/ucm073992.htm.
- 19.Bruen CM, O’Halloran F, Cashman KD, Giblin L. The effects of food components on hormonal signaling in gastrointestinal enteroendocrine cells. Food & Function. 2012;3:1131–43 [DOI] [PubMed] [Google Scholar]
- 20.Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, Cassidy A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr. 2012;95:740–51 [DOI] [PubMed] [Google Scholar]
- 21.Goodfellow J, Bellamy MF, Ramsey MW, Jones CJ, Lewis MJ. Dietary supplementation with marine omega-3 fatty acids improve systemic large artery endothelial function in subjects with hypercholesterolemia. J Am Coll Cardiol. 2000;35:265–70 [DOI] [PubMed] [Google Scholar]
- 22.Albert CM, Hennekens CH, O’Donnell CJ, Ajiani UA, Carey VJ, Willett WC, Ruskin JN, Mauson JE. Fish consumption and risk of sudden cardiac death. JAMA. 1998;279:23–8 [DOI] [PubMed] [Google Scholar]
- 23. USDA; U.S. Departments of Health and Human Services. Dietary guidelines for Americans 2010. 7th ed. Washington: U.S. Government Printing Office; 2010. [DOI] [PMC free article] [PubMed]
- 24.Katz DL, Davidhi A, Ma Y, Kavak Y, Bifulco L, Njike VY. Effects of walnuts on endothelial function in overweight adults with visceral obesity: a randomized, controlled, crossover trial. J Am Coll Nutr. 2012;31:415–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Hou D, Chen X, Li D, Zhu L, Zang Y, Li, Bian Z, Liang X, Cai X, et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res. 2012;22:107–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El SN, Simsek S. Food technological applications for optimal nutrition: an overview of opportunities for the food industry. Comp Rev Food Sci Food Safety. 2012;11:2–12 [Google Scholar]
- 27. Investigational New Drug Applications (INDs) determining whether human research studies can be conducted without an IND. Federal Register 78:2013. [cited 2014 Apr 1]. Available from https://federalregister.gov/a/2013-21889.
- 28.Achterberg C. A commentary on evidence-based analysis: is it right for the science of food- and nutrition-related behaviors? Nutr Today. 2013;48:153–60 [Google Scholar]