Abstract
Recent advances in the understanding of the molecular, genetic, neural, and physiologic basis for the generation and organization of circadian clocks in mammals have revealed profound bidirectional interactions between the circadian clock system and pathways critical for the regulation of metabolism and energy balance. The discovery that mice harboring a mutation in the core circadian gene circadian locomotor output cycles kaput (Clock) develop obesity and evidence of the metabolic syndrome represented a seminal moment for the field, clearly establishing a link between circadian rhythms, energy balance, and metabolism at the genetic level. Subsequent studies have characterized in great detail the depth and magnitude of the circadian clock’s crucial role in regulating body weight and other metabolic processes. Dietary nutrients have been shown to influence circadian rhythms at both molecular and behavioral levels; and many nuclear hormone receptors, which bind nutrients as well as other circulating ligands, have been observed to exhibit robust circadian rhythms of expression in peripheral metabolic tissues. Furthermore, the daily timing of food intake has itself been shown to affect body weight regulation in mammals, likely through, at least in part, regulation of the temporal expression patterns of metabolic genes. Taken together, these and other related findings have transformed our understanding of the important role of time, on a 24-h scale, in the complex physiologic processes of energy balance and coordinated regulation of metabolism. This research has implications for human metabolic disease and may provide unique and novel insights into the development of new therapeutic strategies to control and combat the epidemic of obesity.
Introduction
Circadian rhythms, from the Latin circa dies for “about a day,” are fundamental biologic processes, nearly ubiquitous in living systems, that enable organisms to anticipate and prepare for predictable changes to the environment that occur as the earth rotates on its axis every 24 h. These rhythms are generated and sustained by a genetically encoded molecular pacemaker (i.e., the circadian clock) that synchronizes, or entrains, to daily cycles of light and darkness and imposes temporal organization to ongoing biochemical, molecular, and physiologic processes. This circadian clock system enables organisms to preferentially sequester reactions, pathways, and behaviors to particular times to optimize functioning and increase fitness. For example, cyanobacteria, single-celled organisms that derive energy from the sun, can use their circadian clock to synthesize, assemble, and activate the photosynthetic machinery immediately before sunrise to maximize energy harvest during the day. Later in the day, the clock can direct the deactivation of photosynthesis to avoid inefficiency and energy wasting during darkness. In animals, including mammals, the circadian clock system performs a similar function in regulating cyclic energy harvest from the environment, because energy intake occurs in the context of daily feeding/fasting and sleep/wake cycles. Recent studies have revealed the profound interactions between the circadian clock system and energy regulation and metabolism at many levels of organization, which have substantial implications for human metabolic diseases, such as obesity and diabetes.
This review summarizes key experimental findings linking the circadian clock to energy balance and metabolism in mice, beginning with the initial description of the development of obesity and metabolic syndrome in mice harboring a mutation in the core circadian gene circadian locomotor output cycles kaput (Clock)4. This genetic linkage between the core molecular oscillator and metabolic function has contributed to a growing interest in the relations between circadian rhythms and metabolism at biochemical and molecular levels, which has led to a number of important discoveries and advances in understanding the role of the circadian clock and its complex, bidirectional interactions with critical metabolic pathways. Next, the role of nutrient composition of the diet on circadian rhythms is discussed, as well as the finding that many nutrient- and dietary ligand–binding nuclear hormone receptors in peripheral metabolic organs exhibit robust diurnal and circadian patterns of expression, potentially representing part of the interface linking diet and the circadian clock. In addition to dietary composition, the timing of food intake has recently emerged as an important factor mediating body weight regulation and the organization of metabolism in mice. Finally, evidence from clinical, observational, and epidemiologic studies in humans is presented, along with a discussion of the implications of the findings in mice for human health and metabolic disease, with particular emphasis on obesity.
Current Status of Knowledge
Genetic link between the clock and metabolism.
Perhaps the initial evidence suggestive of a connection between circadian rhythms and energy balance came from epidemiologic studies describing increased risk of obesity, as defined by a BMI ˃30 kg/m2, in shift workers compared with day workers [reviewed in (1)]. However, these studies remain complicated by a variety of other unhealthy behaviors that are common in shift workers, such as poor diet, lack of physical activity, and insufficient sleep. Therefore, the description of the first genetic evidence linking the circadian clock system to energy regulation and metabolism provided a critical step forward for the field, enabling a flurry of biochemical, genetic, molecular, and physiologic studies capable of addressing the underlying mechanisms and pathways. In the initial report, mice harboring a mutation in the core circadian gene Clock (termed Clock mutant mice) were fed a high-fat (HF) diet and observed to develop obesity at a young age, as well as a variety of metabolic and endocrine abnormalities consistent with the metabolic syndrome (2). In addition, the normal diurnal feeding rhythms present in mice were significantly blunted in the mutant mice: on a standard laboratory 12-h light:12-h dark (12:12 LD) cycle, nocturnal mice typically consume ~75–80% of their total daily calories during the dark phase; in contrast, Clock mutant mice consume ~50% (2). The Clock mutants were also shown to exhibit reduced overall expression levels and a blunted diurnal rhythm of orexin mRNA, a hypothalamic neuropeptide involved in energy regulation (2). Taken together, these results indicate profound metabolic dysfunction and energy imbalance in these mice that have a genetically defective circadian clock.
A striking feature of the metabolic phenotype of the Clock mutant mice was the presence of hyperglycemia and hypoinsulinemia, a pattern suggestive of a defect along the insulin axis (2). Examination of isolated pancreatic islets, containing the insulin-secreting β-cells, from Clock mutant mice, as well as from mice carrying a null mutation of brain and muscle ARNT-like 1 (Bmal1), another core circadian clock gene that encodes the binding partner of the CLOCK protein, revealed profound defects in insulin secretion, both at basal levels and in response to glucose stimulation, compared with wild-type mice (3). Furthermore, mice lacking a functional circadian clock in the pancreatic islets were shown to develop frank diabetes at an early age due to insufficient insulin secretion (3). These mice were genetically engineered to eliminate the circadian clock machinery from the pancreatic islets alone, leaving normal clock function in remaining tissues intact. These results illustrate an important aspect of the circadian clock system: it acts cell-autonomously and exhibits specific functions limited to particular cell types and tissues. Those functions differ depending on the tissue, enabling the clock to regulate biologic processes in a tissue-specific manner, dependent on the needs of the individual tissue. For example, the clock in the liver has been shown to play a critical role in regulating blood glucose concentrations during feeding and fasting cycles throughout the diurnal cycle (4). At the level of the entire organism, these tissue-specific functions are coordinated temporally by the master circadian clock in the hypothalamic suprachiasmatic nucleus (SCN), which entrains to the solar cycle and synchronizes cellular clocks throughout the body (5).
In parallel to these studies, the molecular oscillator was shown to regulate the cellular rhythm of nicotinamide adenine dinucleotide (NAD+) (6, 7), a cofactor derived in part through metabolic flux that is necessary for the function of the histone deacetylase sirtuin (silent mating type information regulation 2 homolog) 1 (SIRT1). Through this regulatory role, the circadian clock mediates the temporal patterns of the downstream effects of SIRT1 (8, 9), establishing an additional link between the clock and cellular metabolism. Together, these findings facilitated the identification and characterization of an extended role for the clock in regulating critical cellular processes such as chromatin modification and protein acetylation/deacetylation. Through coordinated patterns of chromatin modification, the clock was then shown to regulate oscillations in metabolic gene expression (10), particularly in the liver. In hepatocytes, the oscillation of the core circadian clock component Rev-erbα directs a circadian rhythm of DNA binding by histone deacetylase 3 (HDAC3) across the genome, leading to global patterns of chromatin modification that contribute to coordinated cyclic expression of genes involved in lipid metabolism (11). This process enables the liver to properly regulate energy metabolism: disruption of the rhythmic binding and deacetylation by HDAC3 leads to excessive fat accumulation in hepatocytes, resulting in hepatic steatosis (11).
In human populations, genome-wide association studies have revealed associations between variants of the circadian clock–related gene Mntr1b, which encodes melatonin receptor 1B, fasting glucose concentrations, and the risk of type 2 diabetes (12–14). The pineal hormone melatonin is synthesized and released with a robust daily oscillation that is regulated by the master circadian clock in the SCN and ambient light exposure (15). The Mntr1b variants associated with increased type 2 diabetes risk appear to adversely affect pancreatic β-cell insulin secretory responses to glucose stimulation (16). Variants in the core circadian clock gene Crytochrome 2 (Cry2) have also been recently associated with fasting glucose concentrations and type 2 diabetes risk (17, 18). These results provide additional evidence, from human populations, for a genetic relation between the circadian clock system and metabolic function and the risk of metabolic diseases.
Together, these findings, as well as a number of others, have helped usher in a revolution in understanding the relations between circadian rhythms, metabolism, and energy balance at the biochemical, genetic, and molecular levels. In addition, they harkened back to a previous finding that the DNA binding activity of components of the core circadian clock machinery, CLOCK and BMAL1 (which together form a heterodimer), is regulated by redox state (19), suggesting that metabolic flux within the cell is capable of interacting with, and potentially regulating, the circadian clock. Interestingly, more recent studies have described a highly conserved circadian rhythm in the oxidation of peroxiredoxin proteins, which persists in the absence of transcription (20–22), raising intriguing questions related to the connections and relations between metabolic cycles and circadian rhythms within cells, as well as the evolutionary origins of the circadian clock itself.
Dietary impacts on circadian rhythms.
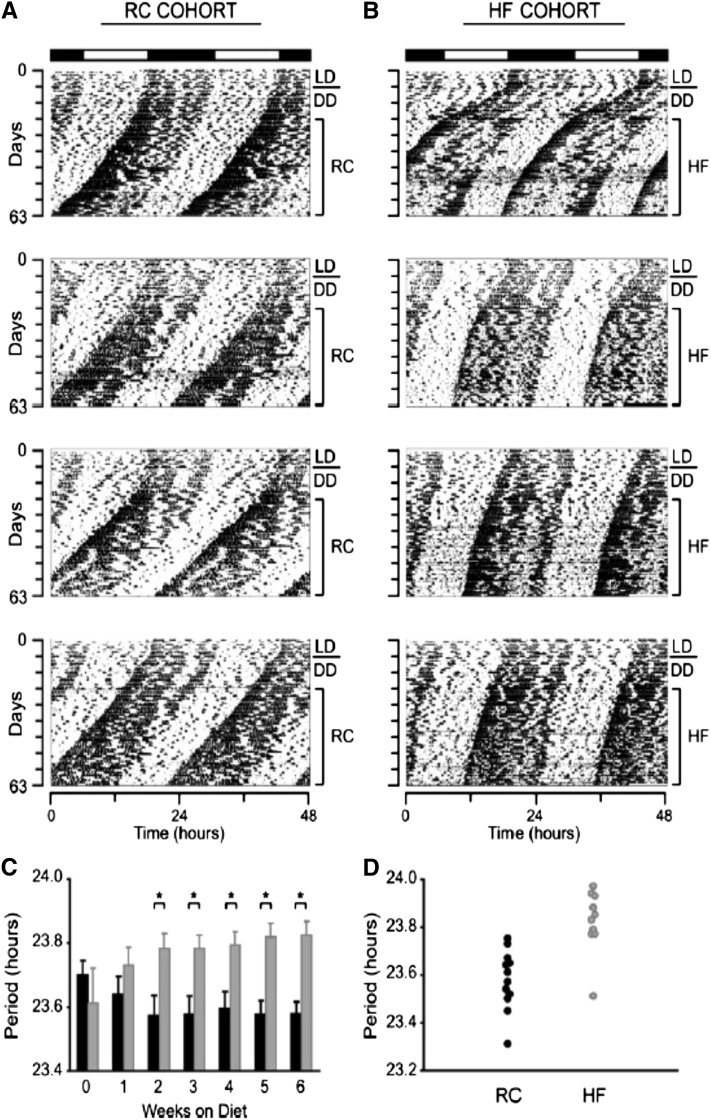
Although there has been a tremendous amount of interest in the role of the clock in regulating biochemical pathways and metabolic processes, relatively less effort has been expended examining how metabolic inputs themselves affect the clock. In an early study addressing this question, wild-type mice with a genetically intact circadian pacemaker were fed an HF diet and monitored carefully to determine how the properties of the circadian clock system were affected (23). The free-running period of the clock (i.e., the duration of 1 full circadian cycle in constant conditions without any external timing cues) was significantly lengthened with the HF diet [Fig. 1 (23)]. The robust effect on period length was observed after only 2 wk of feeding the diet and persisted for several weeks afterward. In addition, diurnal feeding rhythms were blunted: with the regular unpurified diet, mice consumed ~20% of their daily calories during the light phase, which was increased to ~30% with the HF diet (23). Finally, concentrations of circulating metabolic markers and the expression of circadian clock and metabolic genes were disrupted by the HF diet, typically leading to blunted rhythms (23). Taken together, these results demonstrate that an HF diet is capable of affecting circadian rhythms at both behavioral and molecular levels, suggesting that metabolic inputs can influence the functioning of the clock. One possibility is that certain nutritional components of the HF diet act through specific receptors to influence the circadian clock, although this hypothesis was not tested directly in the experiment. The mechanism(s) of the effects of the HF diet on the clock remains unknown, so additional studies are necessary to elucidate the specific impacts of dietary components on the circadian clock and its properties.
FIGURE 1.
A high-fat (HF) diet lengthens the circadian period in mice. Young adult, male, wild-type C57BL/6J mice were fed unpurified diet [regular chow (RC)] (A) or an HF diet (B) and released from entrained light:dark (LD) conditions to constant darkness (DD). Four representative activity records of individual mice are shown for each experimental group. (C) Comparison of free-running period (i.e., the length of time between the onset of activity of successive activity bouts) over time between mice fed RC (black bars; n = 12) and an HF diet (gray bars; n = 10). Week 0 represents the LD condition when all mice were fed RC. Week 1 is the first week being fed the respective experimental diet. (D) Distribution of free-running periods in mice fed RC or an HF after 6 wk being fed the diet. Values in panel C are means + SEMs. *P < 0.05. Reproduced from reference 23 with permission.
In support of the hypothesis that specific dietary components mediate the effects of the HF diet on the clock are experimental results demonstrating that the molecular pacemaker exhibits time-of-day sensitivity to circulating FAs. Cardiomyocytes, which derive the majority of their energy from FAs, express an intracellular circadian clock and are characterized by significant diurnal variation in their response to circulating FAs (24). Notably, this pattern in isolated cardiomyocytes matches that of the intact heart in vivo (25). Furthermore, disruption of the cardiomyocyte clock in vitro, through overexpression of the dominant-negative Clock mutant allele, was shown to cause a dramatic reduction in the expression of FA-responsive target genes during fasting, indicating that the molecular clock has a prominent role in mediating the appropriate genetic and biologic responses to FA exposure within cardiomyocytes (24). Additional studies demonstrated that a similar diurnal responsiveness to FAs exists in skeletal muscle, although in opposite phase to that of the heart (26). These results indicate that, at least in peripheral metabolic tissues, the molecular pacemaker recognizes and responds to circulating FAs with a time-of-day dependence. This raises the possibility that dietary-derived nutrients may act on the clock directly, leading to specific effects on the properties and organization of the clock, although the receptors responsible for mediating this connection to the circadian clock are unknown.
Potential candidates for such molecules linking nutrients to the core circadian molecular machinery are nuclear receptors (NRs), intracellular sensors of lipids and fat-soluble hormones capable of broadly regulating intracellular metabolic programs (27). In a comprehensive analysis of NR expression in 4 peripheral metabolic tissues in mice (white adipose tissue, brown adipose tissue, skeletal muscle, and liver), more than half of the NRs expressed were shown to exhibit robust transcriptional rhythms (25 of 45), with 3 of the remaining NRs displaying a single daily pulse of expression soon after light onset (28). The dynamic daily expression rhythms of these receptors and their downstream target genes represent a potential interface between the circulating nutrients and the molecular clockwork.
Within the liver, the circadian NR Rev-erbα provides an example of this connection. The abundance of the Rev-erbα gene product peaks in the light phase of the diurnal cycle (29), when it facilitates the recruitment of HDAC3 and NR co-repressor (NCoR) to the genome (11). This rhythm of DNA binding by HDAC3 in the mouse liver leads to a rhythm of histone acetylation/deacetylation across the diurnal cycle. HDAC3 binding sites are highly enriched near genes involved in lipogenesis, which leads to a coordinated daily expression rhythm of lipid metabolism genes (11). This expression rhythm is synchronized to the daily feeding/fasting cycle, such that the lipid metabolism genes are upregulated during the feeding phase of the LD cycle: REV-ERBα levels peak during the light phase (when nocturnal mice are fasting), bringing HDAC3 and NCoR to the genome. The deacetylase activity and repression of this complex turns off transcription during the light phase, therefore genes involved in lipid metabolism are not expressed while the animal is fasting. Later in the day, REB-ERBα levels drop, HDAC3 and NCoR are released from the genome, allowing histone acetylation to occur, which opens the chromatin to promote transcription. Thus, the lipid metabolism genes are expressed during the dark phase of the light cycle, when nocturnal mice are feeding (11). This coupling between pathways involved in lipid metabolism and feeding behavior mediated by the circadian clock optimizes energy balance in the liver. Importantly, when the interaction between HDAC3 and REV-ERBα is blocked, hepatic steatosis develops, underscoring the role of the clock in orchestrating proper local energy regulation (11).
Feeding rhythms, metabolic physiology, and energy regulation.
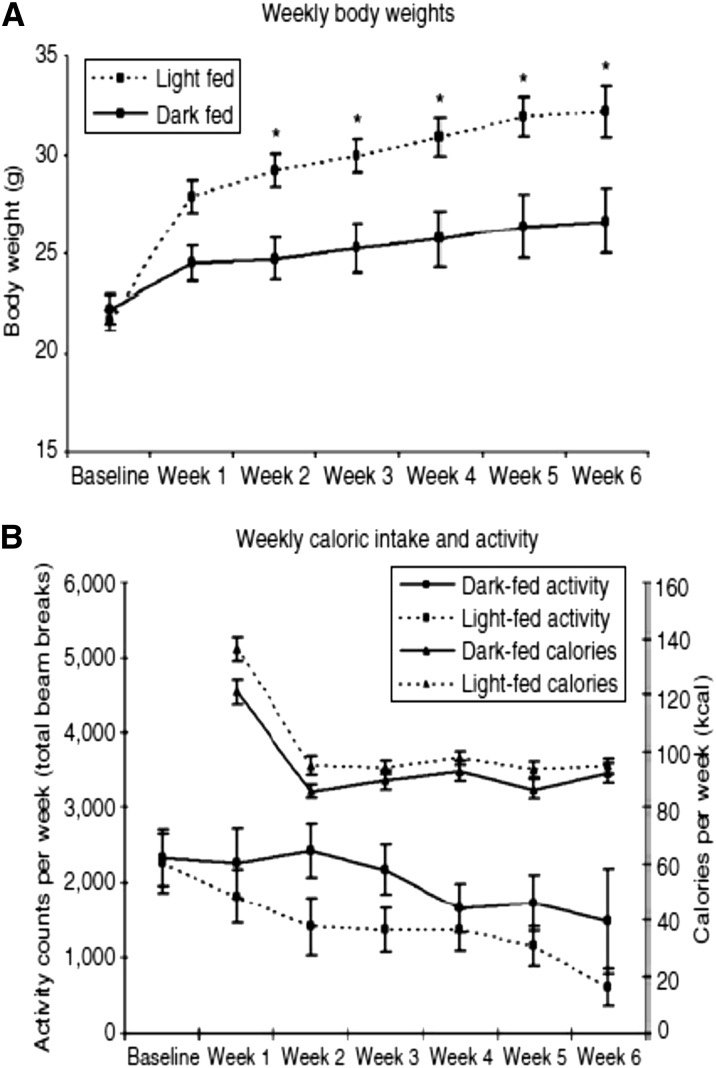
More recently, the role of feeding time itself on energy balance and metabolism has become an active area of investigation. In the initial study, mice provided with an HF diet were fed exclusively during the dark phase (i.e., the “right” time of day for nocturnal mice) or the light phase for 6 wk (30). The group fed during the light phase gained more weight, despite the absence of any significant differences in calorie intake or activity over the course of the experiment [Fig. 2 (30)]. This finding suggested that temporal rhythms in energy intake are relevant for energy balance, perhaps identifying approaches involving timed feeding schedules, designed according to the properties of the circadian clock system, as possible strategies for weight management and the treatment of obesity.
FIGURE 2.
Light-phase feeding leads to increased weight gain in mice fed a high-fat diet. (A) Young adult, male, wild-type C57BL/6J mice were fed a high-fat diet exclusively during the dark phase (solid lines) or light phase (dashed lines) for 6 wk. Body weights were recorded weekly. Mice fed during the light phase (i.e., the “wrong” time of day for nocturnal mice) gained significantly more weight than did mice fed during the dark phase (*P < 0.05). (B) There were no significant differences in locomotor activity levels or food intake between the experimental groups. Values in panels A and B are means ± SEMs. Reproduced from reference 30 with permission.
Further evidence implicating abnormal feeding rhythms and positive energy balance came from experiments in mice genetically engineered to lack the circadian clock specifically in adipocytes. These mice were shown to exhibit a shift in their daily feeding behavior, with greater intake occurring during the light phase (31). There were no significant differences in overall caloric intake, only in the temporal pattern of consumption. This effect appeared to be specific to mice lacking an adipocyte cell clock, because mice with targeted deletions of the clock in the pancreas or hepatocytes have normal feeding rhythms. The mice without an adipocyte clock and shifted feeding rhythms developed obesity, as well as changes in the relative amounts of various TGs stored in adipocytes, circulating in the plasma, and present in hypothalamic neurons associated with energy balance (31). These findings demonstrate that the adipocyte circadian clock acts at the interface between the central nervous system and the predominant peripheral energy storage tissue to coordinate the temporal organization of feeding behavior and maintain energy balance.
In addition to regulating circulating and stored FAs, cyclic patterns of energy intake may organize metabolic processes through the regulation of expression of key metabolic genes. Although it has been appreciated for >10 y that the circadian clock regulates ~10% of the expressed transcripts in any given tissue (32–36), many of which are involved in metabolism and/or encode key, rate-limiting enzymes in various metabolic pathways (37–39), only more recently has it become evident that normal feeding rhythms also have a significant impact on gene expression, particularly in the liver. Under constant conditions, ~15% of transcripts in the liver cycle; however, only ~10% of these remain rhythmic during fasting, indicating that a large proportion of these genes are expressed rhythmically as a response to feeding, instead of in anticipation to it (40). Furthermore, in mice lacking a genetically intact circadian clock system, temporally restricted feeding schedules were used to re-establish rhythmic expression patterns of large numbers of these genes (40). Taken together, these findings demonstrate that both the circadian clock system and regular food intake rhythms work together to establish and maintain robust transcriptional rhythms of genes in the liver, a key metabolic tissue.
The physiologic impact of temporally restricted feeding rhythms and their effects on metabolic gene expression were recently demonstrated in a series of elegant experiments highlighting the importance of feeding rhythms in energy balance and metabolism. Compared with mice that consumed an HF diet ad libitum, mice with access to the same diet for a restricted 8-h window during the dark phase (i.e., 13 h after light onset until 21 h after light onset on a standard 12:12 LD cycle, conventionally termed “ZT13-ZT21”) were completely protected against diet-induced obesity and metabolic dysfunction, despite similar overall caloric intake (41). The appropriate energy balance and improved metabolic variables in these mice that received a temporally restricted feeding regimen appear to be due to optimized coordination in expression rhythms and metabolic cycles within the liver, which leads to beneficial changes in nutrient usage, energy expenditure, and liver metabolite composition (41). The stark metabolic improvements and protection against obesity in these mice that consume the same amount of calories as mice that consumed ad libitum complicate the traditional view of energy balance as a simple “calories in, calories out” equation. Instead, feeding rhythms and the circadian clock system exert a significant effect on energy balance and cardiometabolic health, a finding that has been replicated and extended in several independent studies (42–44).
Application to humans.
To date, all of these experimental studies have been carried out in laboratory rodents in highly controlled studies. An important question is whether these data on the role of feeding rhythms on energy balance and metabolism will be applicable to humans and useful as potential treatment strategies themselves or as supplements to current approaches. On the basis of these consistent and robust data from animal models, a recent report in European dieters found a significant association between the timing of lunch and weight-loss effectiveness, with earlier lunch times linked to greater success (45). Further observational and epidemiologic studies are necessary to confirm this preliminary finding and to extend it to larger representative groups of people and a wider range of dietary schedules. In addition, clinical studies are necessary to rigorously test the hypothesis that daily rhythms of caloric intake influence energy balance and are relevant for metabolism in people.
A clinical entity termed “night eating syndrome” (NES) has been described in certain individuals that consume large proportions of their daily food intake during the night (46, 47). Perhaps not surprisingly, NES has been associated with obesity (48). Although purely speculative at present, it may be that some forms of NES represent primary disorders of circadian timing, with the energy intake rhythm being shifted to the “wrong” time of day, contributing to metabolic dysfunction at the molecular level, potentially leading to positive energy balance, weight gain, and other cardiometabolic abnormalities. More importantly, beyond this specific population of clinically defined NES patients, recent findings linking circadian rhythms and temporal patterns of food intake to energy regulation and metabolism are expected to be broadly applicable to many more people living in the 24/7 “modern” environment aligned against proper organization of circadian rhythms and sleep. Till Roenneberg and colleagues (49) at the Ludwig Maximilians University in Munich have surveyed the sleep behavior and circadian rhythms of hundreds of thousands of individuals around the world, describing and quantifying a shift in the timing of the midpoint of sleep between work days and free days, which they have termed “social jetlag”. This can be considered a form of chronic disruption of circadian rhythms for many people, akin to repeatedly experiencing jet lag associated with long-distance east-west travel. The magnitude of “social jetlag” has been positively associated with BMI in a large epidemiologic survey (50), suggesting that the degree of circadian misalignment may be related to amount of weight gain.
Given these findings from epidemiologic and observational studies in humans, as well as the widespread nature of circadian disruption in modern society, carefully designed clinical and interventional studies are necessary to more completely understand the mechanisms and characteristics of the metabolic dysfunction and energy imbalance that accompany circadian disruption and to determine whether behavioral approaches to align feeding rhythms and metabolic cycles to improve cardiometabolic health are feasible and capable of clinically meaningful benefits. In some cases, these approaches are already ongoing, and comprehensive analyses of individuals in a clinical research center have revealed that misalignment of circadian rhythms leads to metabolic, endocrine, and cardiovascular abnormalities that are consistent with increased disease risk (51). These experiments are complex, resource intensive, and logistically difficult to conduct; therefore, they are often restricted to relatively short durations. Thus, the longer-term effects of some of these adverse cardiometabolic changes are poorly understood. One possibility is that these disease risk factors may progress in magnitude over time with consistent or repeated exposure to circadian misalignment.
Additional research is necessary to address this and other important questions at the intersection of circadian rhythms, energy regulation, and cardiometabolic health. With the proliferation and widespread adoption of advanced technologies in everyday life by consumers, as well as physicians and researchers, it may be possible to design and use hand-held, portable applications and research tools to accurately measure and quantify circadian rhythm–related and metabolic data in real time in a natural environment, thus harnessing the power of technology while simultaneously expanding and leveraging the vast amount of potentially useful data to transform the understanding of circadian rhythms, metabolism, and their clinical relevance for obesity and metabolic disease.
Conclusions and Future Directions
The past 2 decades have witnessed a tremendous increase in understanding of the molecular, biochemical, neural, and genetic mechanisms that govern the generation, persistence, and organization of circadian rhythms. As discussed above, these ubiquitous, self-sustained, and cell-autonomous oscillations have been shown to be critically important for energy balance and the regulation of metabolism in mammals. Importantly, the connection is bidirectional: on one hand, the clock regulates energy intake and metabolic pathways throughout the organism, whereas on the other, feeding behavior and nutrient composition of the diet affect the clock itself, especially in peripheral metabolic organs, as well as its outputs.
As the specific mechanisms underlying these relations are uncovered, it may become possible to identify novel clock-related therapeutic targets and treatment strategies for preventing weight gain (or achieving weight loss) and improving cardiometabolic health. Furthermore, the findings linking abnormal feeding patterns to positive energy balance and metabolic dysfunction suggest that nonpharmacologic approaches based on regulating the timing of food intake to improve metabolic cycles may be useful as potential strategies in combating obesity and its associated comorbidities. Although future studies are required to confirm, validate, and extend the current experimental evidence, there is now a strong foundation upon which to build a greater understanding of the interconnections between circadian rhythms and metabolism and to use this information to expand and improve strategies for treating obesity and metabolic disease.
An important area of future interest for the field linking circadian rhythms, nutrition, and metabolism should be the intestinal microbiota. Interestingly, it was recently reported that intestinal epithelial cells interact with gut-resident bacteria through bacterial products and Toll-like receptor signaling, and that this communication is necessary to maintain homeostasis within the intestinal epithelium (52). Furthermore, a prediabetic syndrome arose when microbial signaling to host cells was blocked, due in part to increased local corticosterone production induced by disruption of the clock (52). These findings highlight the potential role that the circadian clock has in mediating interactions with the microbiota, which has recently been linked to nutrition and metabolic physiology as well as to several metabolic and inflammatory diseases (53–55), many of which are already known to be associated with disruption of circadian rhythms (38, 39).
Although premature, it is tempting to speculate that the circadian clock may serve at the interface between nutrition, the microbiota, and metabolism. In particular, it will be of interest to determine whether disruption of circadian rhythms in the host leads to changes in the structure, composition, and function of the microbiota. At present, little effort has been invested in this area. Future studies are expected to more completely characterize the nature and degree of the role that the clock has in mediating interactions between gut-resident bacteria and host cells, as well as to clarify the impact of dietary and nutritional interventions on these relations. With this information, it may be possible to manipulate the circadian clock system to preferentially favor beneficial commensal microorganisms or selectively minimize or eliminate potentially problematic species. Conversely, it may be possible to use specific probiotics or prebiotics to improve overall circadian organization within the host gastrointestinal tract (and possibly beyond). The ongoing revolutions in the understanding of the circadian clock system, as well as of the microbiota, offer an opportunity to synthesize knowledge and approach obesity and metabolic diseases from a more comprehensive, biologically based foundation.
Obesity and diabetes are prototypical examples of complex, polygenic diseases influenced by numerous environmental factors, including diet and physical activity, among others. As such, a variety of approaches and measures will likely be necessary for effective prevention and treatment. Given the growing body of evidence linking the circadian clock system to energy regulation and metabolic physiology, circadian organization emerges as a clinically significant factor that should be considered in the understanding of the pathophysiology of these diseases and in potential strategies for treating them.
Acknowledgments
Both authors wrote, reviewed, and approved the final manuscript.
Footnotes
Abbreviations used: BMAL1, brain and muscle ARNT-like 1 (also known as ARNTL); CLOCK, circadian locomotor output cycles kaput; Cry2, Cryptochrome 2; DD, constant darkness; FA, fatty acid; HDAC3, histone deacetylase 3; HF, high-fat; LD, light:dark; Mntr1b, melatonin receptor 1B; NAD+, nicotinamide adenine dinucleotide; NCoR, nuclear receptor co-repressor; NES, night eating syndrome; NR, nuclear receptor; SCN, suprachiasmatic nucleus; SIRT1, sirtuin (silent mating type information regulation 2 homolog) 1.
Literature Cited
- 1.Antunes LC, Levandovski R, Dantas G, Caumo W, Hidalgo MP. Obesity and shift work: chronobiological aspects. Nutr Res Rev. 2010;23:155–68 [DOI] [PubMed] [Google Scholar]
- 2.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA. 2008;105:15172–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–28 [DOI] [PubMed] [Google Scholar]
- 9.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alenghat T, Meyers K, Mullican SE, Leitner K, Adeniji-Adele A, Avila J, Bucan M, Ahima RS, Kaestner KH, Lazar MA. Nuclear receptor corepressor and histone deacetylase 3 govern circadian metabolic physiology. Nature. 2008;456:997–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng D, Liu T, Sun Z, Bugge A, Mullican SE, Alenghat T, Liu XS, Lazar MA. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331:1315–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouatia-Naji N, Bonnefond A, Cavalcanti-Proenca C, Sparso T, Holmkvist J, Marchand M, Delplanque J, Lobbens S, Rocheleau G, Durand E, et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. 2009;41:89–94 [DOI] [PubMed] [Google Scholar]
- 13.Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spegel P, Bugliani M, Saxena R, Fex M, Pulizzi N, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41:82–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, Loos RJ, Manning AK, Jackson AU, Aulchenko Y, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41:77–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pevet P, Challet E. Melatonin: both master clock output and internal time-giver in the circadian clocks network. J Physiol Paris. 2011;105:170–82 [DOI] [PubMed] [Google Scholar]
- 16.Simonis-Bik AM, Nijpels G, van Haeften TW, Houwing-Duistermaat JJ, Boomsma DI, Reiling E, van Hove EC, Diamant M, Kramer MH, Heine RJ, et al. Gene variants in the novel type 2 diabetes loci CDC123/CAMK1D, THADA, ADAMTS9, BCL11A, and MTNR1B affect different aspects of pancreatic beta-cell function. Diabetes. 2010;59:293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C, Li H, Qi L, Loos RJ, Qi Q, Lu L, Gan W, Lin X. Variants in GLIS3 and CRY2 are associated with type 2 diabetes and impaired fasting glucose in Chinese Hans. PLoS ONE. 2011;6:e21464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293:510–4 [DOI] [PubMed] [Google Scholar]
- 20.Edgar RS, Green EW, Zhao Y, van Ooijen G, Olmedo M, Qin X, Xu Y, Pan M, Valekunja UK, Feeney KA, et al. Peroxiredoxins are conserved markers of circadian rhythms. Nature. 2012;485:459–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Neill JS, Reddy AB. Circadian clocks in human red blood cells. Nature. 2011;469:498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Neill JS, van Ooijen G, Dixon LE, Troein C, Corellou F, Bouget FY, Reddy AB, Millar AJ. Circadian rhythms persist without transcription in a eukaryote. Nature. 2011;469:554–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–21 [DOI] [PubMed] [Google Scholar]
- 24.Durgan DJ, Trexler NA, Egbejimi O, McElfresh TA, Suk HY, Petterson LE, Shaw CA, Hardin PE, Bray MS, Chandler MP, et al. The circadian clock within the cardiomyocyte is essential for responsiveness of the heart to fatty acids. J Biol Chem. 2006;281:24254–69 [DOI] [PubMed] [Google Scholar]
- 25.Durgan DJ, Hotze MA, Tomlin TM, Egbejimi O, Graveleau C, Abel ED, Shaw CA, Bray MS, Hardin PE, Young ME. The intrinsic circadian clock within the cardiomyocyte. Am J Physiol Heart Circ Physiol. 2005;289:H1530–41 [DOI] [PubMed] [Google Scholar]
- 26.Stavinoha MA, Rayspellicy JW, Hart-Sailors ML, Mersmann HJ, Bray MS, Young ME. Diurnal variations in the responsiveness of cardiac and skeletal muscle to fatty acids. Am J Physiol Endocrinol Metab. 2004;287:E878–87 [DOI] [PubMed] [Google Scholar]
- 27.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–70 [DOI] [PubMed] [Google Scholar]
- 28.Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–10 [DOI] [PubMed] [Google Scholar]
- 29.Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–60 [DOI] [PubMed] [Google Scholar]
- 30.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring). 2009;17:2100–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paschos GK, Ibrahim S, Song WL, Kunieda T, Grant G, Reyes TM, Bradfield CA, Vaughan CH, Eiden M, Masoodi M, et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med. 2012;18:1768–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Gant TW, Hastings MH, Kyriacou CP. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol. 2002;12:540–50 [DOI] [PubMed] [Google Scholar]
- 33.Duffield GE. DNA microarray analyses of circadian timing: the genomic basis of biological time. J Neuroendocrinol. 2003;15:991–1002 [DOI] [PubMed] [Google Scholar]
- 34.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–20 [DOI] [PubMed] [Google Scholar]
- 35.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83 [DOI] [PubMed] [Google Scholar]
- 36.Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, Takasugi T, Nagano M, Nakahama K, Suzuki Y, Sugano S, et al. A transcription factor response element for gene expression during circadian night. Nature. 2002;418:534–9 [DOI] [PubMed] [Google Scholar]
- 37.Bass J. Circadian topology of metabolism. Nature. 2012;491:348–56 [DOI] [PubMed] [Google Scholar]
- 38.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci USA. 2009;106:21453–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bray MS, Tsai JY, Villegas-Montoya C, Boland BB, Blasier Z, Egbejimi O, Kueht M, Young ME. Time-of-day-dependent dietary fat consumption influences multiple cardiometabolic syndrome parameters in mice. Int J Obes (Lond). 2010;34(11):1589–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salgado-Delgado R, Angeles-Castellanos M, Saderi N, Buijs RM, Escobar C. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology. 2010;151:1019–29 [DOI] [PubMed] [Google Scholar]
- 44.Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012;26:3493–502 [DOI] [PubMed] [Google Scholar]
- 45.Garaulet M, Gomez-Abellan P, Alburquerque-Bejar JJ, Lee YC, Ordovas JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond). 2013:37(4):604–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allison KC, Lundgren JD, O'Reardon JP, Geliebter A, Gluck ME, Vinai P, Mitchell JE, Schenck CH, Howell MJ, Crow SJ, et al. Proposed diagnostic criteria for night eating syndrome. Int J Eat Disord. 2010;43:241–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stunkard AJ, Grace WJ, Wolff HG. The night-eating syndrome; a pattern of food intake among certain obese patients. Am J Med. 1955;19:78–86 [DOI] [PubMed] [Google Scholar]
- 48.Gallant AR, Lundgren J, Drapeau V. The night-eating syndrome and obesity. Obes Rev. 2012;13:528–36 [DOI] [PubMed] [Google Scholar]
- 49.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23:497–509 [DOI] [PubMed] [Google Scholar]
- 50.Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22:939–43 [DOI] [PubMed] [Google Scholar]
- 51.Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell. 2013;153:812–27 [DOI] [PubMed] [Google Scholar]
- 53.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Musso G, Gambino R, Cassader M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu Rev Med. 2011;62:361–80 [DOI] [PubMed] [Google Scholar]
- 55.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–9 [DOI] [PubMed] [Google Scholar]