Abstract
Growth charts for weight and height have provided the basis for assessment of children’s nutritional status for over half a century, with charts for body mass index (BMI) introduced in the 1990s. However, BMI does not provide information on the proportions of fat and lean mass; and within the past decade, growth charts for children’s body composition have been produced by using techniques such as skinfold thicknesses, body circumferences, bioelectrical impedance analysis (BIA), and dual-energy X-ray absorptiometry (DXA). For public health research, BIA and skinfold thicknesses show negligible average bias but have wider limits of agreement than specialized techniques. For patients, DXA is the best individual method, but multicomponent models remain ideal because they address perturbations in lean mass composition. Data can be expressed in age- and sex-specific SD scores, in some cases adjusting for height. Most such reference data derive from high-income countries, but techniques such as air-displacement plethysmography allow infant body composition growth charts to be developed in low- and middle-income settings, where the data may improve understanding of the effects of low birth weight, wasting, and stunting on body composition. Recent studies suggest that between-population variability in body composition may derive in part from genetic factors, suggesting a universal human body composition reference may not be viable. Body composition growth charts may be extended into adult life to evaluate changes in fat and lean mass through the entire life course. These reference data will improve the understanding of the association between growth, body composition, health, and disease.
Introduction
For well over a century, clinical assessment of children’s nutritional status has relied heavily on measurements of anthropometry. As early as 1835, the Belgian statistician Quetelet collected data on children’s weight and height and made use of the concept of the “normal distribution” to describe the pattern of human growth (1). In the 1870s, Bowditch collated anthropometric data on >24,000 schoolchildren from Boston, MA, and demonstrated differences in growth between the sexes and socioeconomic groups (1). Arguably the most influential contribution, however, came from the British auxologist Tanner (2, 3), who pioneered more sophisticated growth charts of a format that continues to be used today.
To construct growth charts, cross-sectional data are collected on a large representative sample of children, although, ideally, longitudinal data would be incorporated. The data are then subjected to statistical analysis, whereby not only the average size at each age is calculated but also the variability. Simplistically, any individual value can be expressed as a SD score (SDS)>4, calculated as follows: SDS = (measurement − population mean)/population SD where both the mean and SD of the population are calculated on an age- and sex-specific basis. Providing that the data are characterized by a normal distribution, any SDS can also be expressed as a percentile, whereby, for example, an individual on the 60th centile is taller than 60% of the population (2). If the data are skewed, however, then the simple relation between SD and percentile distributions is broken (2). For this reason, more advanced statistical approaches for assessing the population distribution of growth status were developed.
To address skewness, Cole (4, 5) developed a statistical approach that differentiates 3 different parameters of variability. Their “LMS” (Lambda Sigma Mu) method quantifies the median (M), the magnitude of variability (S), and the Box-Cox power (L) required to transform the data to achieve a normal distribution. This approach resolved the discrepancy between SDS and centiles and has become standard practice in the construction of growth charts. The approach has also been adapted to produce software that converts raw data to age- and sex-specific SDS, enabling the longitudinal assessment of an individual’s growth status relative to the reference population. Other statistical approaches can model a wider range of covariates and distributions (6).
Growth charts have remained fundamental to the assessment of basic nutritional status ever since. Most of these charts represent reference data but not growth standards. In other words, they describe the pattern of growth and its variability that is evident in a population at a given time point, but they do not assume that any particular level of growth is optimal. Longitudinal measurements showed that from early childhood onward, the majority of children do not cross up or down through the centiles but tend to track along a given centile, indicating that growth is self-regulating and target-seeking (7). Thus, regardless of whether a child is large or small at any given time point, centile crossing gives an indication of a clinical growth abnormality. On this basis, growth charts are used both in clinical monitoring to detect individual abnormalities in growth trajectory, but also in public health research and monitoring to understand variability and secular trends in children’s growth.
The logic of these growth charts is simple but very effective: at any age, a child can be ranked relative to others of the same age and sex to assess immediate growth status. Longitudinal data allow change in growth status to be assessed. Although early data prioritized weight and height, many other components of growth can be addressed in the same way.
Assessment of nutritional status.
Because weight and height are strongly associated in children, various efforts were made to produce charts for weight that took height into account. By the 1970s, opinion was converging on the use of BMI (calculated as weight in kilograms divided by the square of height in meters) as the optimal approach in adults. First developed by Quetelet in the 19th century (8), the utility of BMI is that it is highly correlated with weight and body fatness but has a relatively low correlation in adults with height (9). BMI thus became adopted as the primary index of adult overweight. In both sexes, the thresholds for overweight and obesity were defined as 25 and 30 kg/m2, respectively (10). Subsequently, an additional cutoff for chronic energy deficiency was added, at 18.5 kg/m2 (11).
In children, however, BMI has a characteristic curvilinear shape with age, a scenario for which growth charts provide the ideal solution. BMI growth charts were first produced for French children in 1982 (12) and for UK children in 1995 using the LMS method (13). The curvilinear association of BMI with age means that no simple invariant cutoff can be used to define overweight or obesity. Rather, British pediatricians adopted the 85th centile as the threshold for overweight and the 95th centile for obesity.
These charts allowed the nutritional status of children to be assessed over time and enabled a standardized approach to be used in early clinical monitoring of childhood obesity. Nevertheless, as the approach was replicated in other countries and individual national charts were produced, there was no international consensus on the BMI that is equivalent to obesity at any given age. To address this issue, Cole et al. (14) analyzed data from 6 countries, and in this large data set identified age-specific BMI cutoffs that were statistically equivalent to the adult BMI cutoffs of 25 and 30 kg/m2. Thus, international cutoffs for pediatric overweight and obesity were now available, and shortly thereafter they were followed by equivalent cutoffs for different degrees of pediatric underweight (15).
Limitations of BMI for body composition assessment.
These international pediatric BMI cutoffs have made a major contribution to the monitoring of nutritional status in children and adolescents worldwide and provide a template against which nutritional status can be assessed in the clinic. However, BMI is a global proxy of nutritional status. It is highly correlated with many different components of weight, such as lean mass (used here synonymously with fat-free mass), skeletal muscle mass, fat mass, and bone mass; yet, it cannot differentiate between them.
The primary evidence favoring BMI as an index of adiposity is that across a wide range of BMIs, there is a strong correlation between BMI and the proportion of fat in body weight, or percentage of fat (%fat) (16). However, this strong correlation emerges because of the tendency for low-BMI children to have low %fat and high-BMI children to have high %fat. In the middle of the range, children of a given BMI value can have very different %fat. This is shown clearly by disentangling BMI into its fat and lean components; however, before examining this issue it is first helpful to discuss the limitations of using %fat itself as an index of adiposity.
The logic of %fat is that it adjusts fat mass for an index of body size—in this case, weight. Clearly, 3 kg of fat mass is a substantial amount for a 3 y old weighing 15 kg but very little for an adolescent weighing 60 kg. However, dividing fat mass by weight is statistically problematic, because the fat is present in both numerator and denominator (17, 18). As absolute fat mass increases, %fat rises increasingly slowly, eventually trending toward an asymptote at ~60% fat. In obese individuals, even large gains or losses in adipose tissue mass may induce only small changes in %fat. A second conceptual problem with %fat is that it is not an index of adiposity that is fully independent of body size. High %fat values might reflect high adiposity or low lean mass as, for example, in some patient groups (17, 19). The use of %fat as the primary body composition outcome therefore directs attention to fat at the expense of lean mass. Historically, this approach has resulted in extensive interest in height as the primary index of growth and similar interest in %fat as the primary index of body composition, with minimal interest being directed to lean mass, despite the fact that it comprises multiple functional tissues.
To resolve this problem in adults, VanItallie et al. (20) proposed splitting BMI into 2 components: the lean mass index (LMI; lean mass/height2) and the fat mass index (FMI; fat mass/height2), each expressed in the same kg/m2 units as BMI. This approach has 2 benefits: it adjusts tissue masses for an independent component of body size while keeping fat and lean outcomes separate.
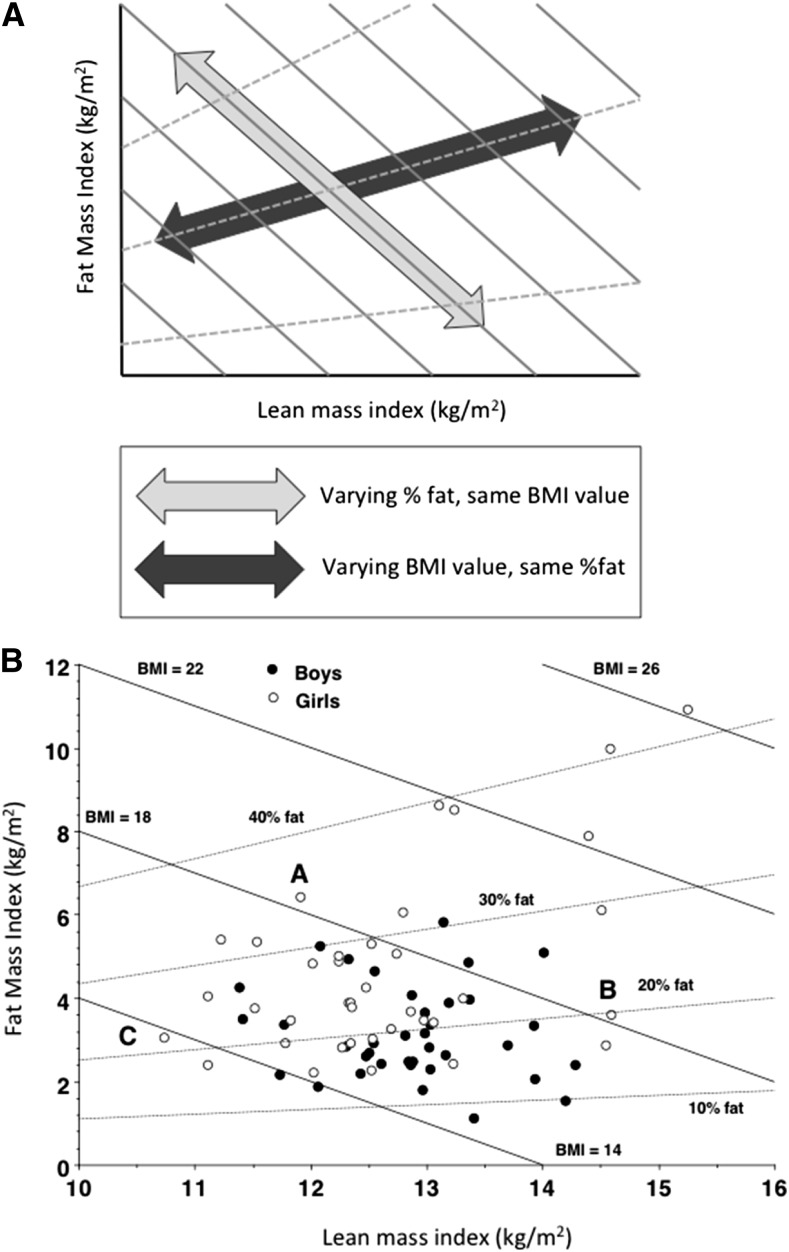
The same approach can be applied in children (21). The value of this approach is most clearly seen by using a graphic approach developed by Hattori et al. (22), who plotted FMI on the y-axis against LMI on the x-axis. Figure 1A illustrates the conceptual approach encapsulated by this chart, and Fig. 1B shows a scatterplot of body composition data from children aged 8 y on a Hattori chart. It can be seen that 2 children with the same BMI value can differ markedly in their adiposity, whether this is expressed as %fat or FMI. Equally, 2 children of the same %fat value can differ markedly in their BMI (21, 23). These charts highlight substantial variability in lean mass, an issue that has received little attention in pediatric clinical practice or research.
FIGURE 1.
(A) The contribution of lean mass and fat mass to BMI illustrated using a “Hattori chart” that plots fat mass/height2 on the y-axis against lean mass/height2 on the x-axis (21). Continuous lines represent constant BMI values; dotted lines represent constant %fat values. (B) The distribution of fat mass/height2 and lean mass/height2 in a sample of children aged 8 y. Children with the same BMI value may vary in their %fat (“A” vs. “B”), whereas those with the same %fat value may vary substantially in their BMI (“B” vs. “C”). Reproduced from reference 23 with permission. %fat, percentage of fat.
The limitations of BMI as an index of body composition are further highlighted if data from different ethnic groups are compared. Many studies have now reported varying amounts of adiposity for a given BMI value across ethnic groups (24–26). The largest contrast appears to be between South Asians and Europeans. Compared with the latter group, South Asians have been described as having a “thin-fat” phenotype, evident at birth (27), with relatively less lean mass and more fat mass at any given BMI value (24–26, 28).
The limitations of BMI are perhaps starkest when considering data from children with specific diseases associated with alterations in body composition. In a study in young children receiving artificial ventilation, the patients tended to have low LMI relative to healthy controls, but they had high FMI (19, 29). Because the children had normal BMI values, each of these clinical problems was concealed. One patient with myofibromatosis, a condition with many small tumors, showed the opposite pattern. He had high LMI due to the tumors, but low FMI. Dietetic management of these patients had focused specifically on maintaining BMI similar to that of healthy children, and the abnormalities in body composition were undetected and hence not able to be addressed.
The need for body composition data.
The limitations of weight and height as an index of body composition were already recognized when the first growth charts were produced (30). Similar charts for subcutaneous skinfold thicknesses were published for British children in the 1960s (30) and were updated 15 y later to address changes in children’s adiposity attributable to secular trends in nutritional status (31). As data on BMI revealed the emerging childhood obesity epidemic, interest in differentiating adiposity from lean mass grew, but a limitation of skinfold thicknesses is that they do not necessarily reflect the total amount of fat in the body, inasmuch as fat is internal and not indexed by skinfold measurements (32). Given this limitation, it is not possible to predict lean mass with accuracy from data on skinfold thickness (33), even though several such predictive equations have been published (34, 35).
In clinical practice, the value of assessing body composition is increasingly recognized (36). Whereas obesity and eating disorders currently remain defined by anthropometric criteria (weight relative to height, or BMI) (14, 37), these variables have poor sensitivity for monitoring response to treatment, and so body composition measurement could improve management. Second, body fat and its distribution merit monitoring more generally in patients in relation to the etiology of cardiovascular disease, hypertension, and type 2 diabetes, diseases now considered to have an ‘‘incubation period’’ during childhood and adolescence (38). Third, body composition is increasingly associated with clinical progress and outcome, such as growth status after liver transplantation and length of hospital stay in HIV patients given tube nutrition (39–41). Finally, measurements of lean mass may improve the capacity to tailor nutrition, treatment, and management to metabolic criteria—for example, predicting energy requirements or dialysis doses (29, 42, 43).
Yet, although research studies have increasingly demonstrated the value of body composition data for guiding clinical management, the measurement of body composition has remained rare in clinical practice, and BMI remains the most commonly used outcome. Until recently, 1 major challenge was the lack of methodologies suitable for routine use in younger age groups. However, from the 1990s, a large volume of research has addressed this issue, and a number of options are now available (33). Anthropometric measurement (e.g., skinfold thicknesses and girths) is the simplest option, whereas the spread of DXA instrumentation for osteoporosis monitoring has allowed its wider clinical application for body composition assessment. Most hospitals in industrialized populations can now provide some form of pediatric body composition assessment, and the limiting factor has shifted to the traditions of clinical practice.
Arguably the biggest remaining barrier to routine clinical body composition assessment in pediatrics has been the lack of reference data, hindering the interpretation of individual measurements. Until the 2000s, our understanding of the development of pediatric body composition was dominated by a classic study by Fomon et al. (44), which was published in 1982. This study merged a number of body composition data sets on to the U.S. National Center for Health Statistics growth centiles and modeled the development of fat mass, lean mass, and lean tissue components between birth and age 10 y. An additional article by Haschke (45), unfortunately not in the mainstream scientific literature, followed the same approach for adolescents. Subsequently, Lohman (46) updated this approach, providing the first description of body composition development from birth to adulthood. Although these studies represented invaluable pioneering work that stimulated the next generation of research, what was missing from this approach was an understanding of population variability.
Current Status of Knowledge
From the 2000s, an increasing number of research groups have published body composition reference data, with a number of examples summarized in Table 1 (31, 47–55). Going beyond anthropometry, techniques such as bioelectrical impedance analysis (BIA) and DXA have for the first time provided equivalent data on lean mass and total body fat mass (47, 49–51, 53–55). In public health research, it is now possible to examine secular changes not only in adiposity but also in lean mass and its functional correlates (56, 57), which may decline in association with sedentary behavior.
TABLE 1.
Examples of body composition reference data based on single techniques1
| Population | Methodology | Outputs | Sample size | Age range | Reference |
| n | y | ||||
| United Kingdom | Anthropometry | Triceps, subscap | 30,0002 | 0.1–19 | (31) |
| United States | BIA | TBW, LM,3 FM, %fat | 15,912 | 12–80 | (47) |
| United States | Anthropometry | Triceps, subscap | 32,783 | 1.5–20 | (48) |
| United States | DXA | LMI, FMI | 8961 | 8–20 | (49) |
| Holland | DXA | LM,3 %fat | 642 | 4–20 | (50) |
| Sweden | DXA | LM,3 FM | 1469 | 6–30 | (51) |
| Spain | Anthropometry | Triceps, subscap, waist, hip | 2160 | 13–18 | (52) |
| Japan | BIA | LMI,3 FMI | 1171 | 3–11 | (53) |
| Turkey | BIA | %fat | 4076 | 6–18 | (54) |
| India | DXA | FM, FMI, %fat | 888 | 5–18 | (55) |
BIA, bioelectrical impedance analysis; %fat, percentage of fat in body weight; FM, fat mass; FMI, fat mass index; hip, hip girth; LM, lean mass; LMI, lean mass index; subscap, subscapular skinfold; TBW, total body water; Triceps, triceps skinfold; waist, waist girth.
Mixed longitudinal sample.
LM used synonymously with fat-free mass.
Nevertheless, these data leave some challenges to be addressed, both in public health research and in clinical practice. Body composition techniques generate data in different ways, incorporating a variety of theoretical assumptions, and often predicting final values by using empirical “calibration” relations that are thus influenced by the nature of the population sample used for the calibration study (33). The result of this methodologic heterogeneity is that body composition data from different techniques cannot be used interchangeably; hence, existing reference data can only be used with confidence if additional data are collected using the same method.
One solution to this problem is to obtain reference data by using a multicomponent model. This approach reduces the need for theoretical assumptions when calculating body composition outputs by measuring several different body composition traits. The established approach is known as the 4-component model, in which information is collected on body weight, body volume, total body water (TBW), and bone mineral mass (58, 59). Because data on the water and mineral content of lean tissue are obtained empirically, the final differentiation of fat and protein is made more accurate. The method can also be adapted to incorporate other data.
The first such study by Butte et al. (60) followed 76 children through the first 2 y of life. In addition to quantifying age-related changes in fat and lean masses, this study also highlighted the rapid “chemical maturation” of the body during this developmental period as had been modeled by Fomon et al. (44). The hydration of lean tissue declines with age, whereas the mineral content increases. These changes alter the physical properties of lean tissue, which has implications for many body composition methodologies. For example, when predicting lean mass from TBW by hydrometry, age- and sex-specific values for the hydration of lean tissue must be used, whereas age- and sex-specific values for the density of lean tissue must also be used in densitometry (33, 44).
The 4-component model has recently been used to generate centiles and growth charts for fat and lean masses for the age range of 5–20 y (61), along with reference data for the hydration and density of lean tissue (62) and a range of individual anthropometric, prediction, and reference methods.
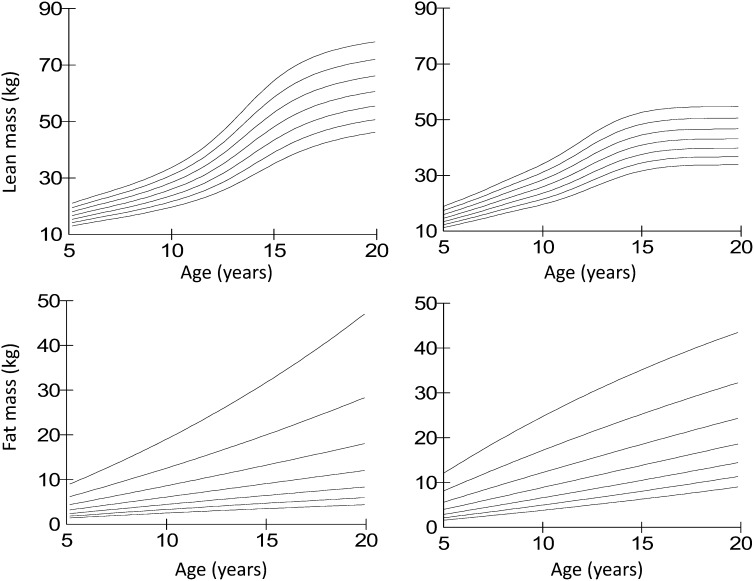
The 4-component growth charts are summarized in Fig. 2. Although lean mass has a characteristic curvilinear association with age, fat mass does not convey any noticeable pattern with age. However, it is possible that a larger sample size, stratifying by pubertal status, might have identified upward shifts in adiposity around the timing of puberty. When these data are expressed in size-adjusted format, in the form of LMI and FMI, the curvilinear association of lean mass with age is reduced but still evident, indicating that children gain lean mass disproportionately to height, especially in boys (61). The data for FMI indicate an increase in boys but not girls shortly before the main pubertal growth spurt in lean mass, as reported previously by Tanner and Whitehouse (31) for skinfold thicknesses. These growth charts thus help assess the degree of lean and fat tissue relative to others of the same age, gender, and height, and thereby separate to some extent the effects of age versus growth versus nutritional status.
FIGURE 2.
Body composition growth charts using the 4-component model in UK children and adolescents aged 5–20 y. The 2nd, 9th, 25th, 50th, 75th, 91st, and 98th percentiles are displayed in ascending order. Left panels: males; right panels: females. Reproduced from reference 61 with permission.
In children, the optimal power by which to raise height when correcting tissue masses for body size remains uncertain. Although lean mass scales with height-squared, fat tends to scale with height raised to a higher value (e.g., height) (17). Children maturing faster are not only taller for their age but also fatter, and this means that the FMI remains correlated with height (63). Nevertheless, there are many advantages to expressing body composition data in a format similar to that of BMI, which has been widely adopted in both clinical practice and public health research.
Although multicomponent reference data provide the most accurate growth charts, it is rarely possible to obtain such data in routine clinical practice or public health research. An important issue is therefore testing the level of agreement between body composition SDS from individual techniques and these multicomponent SDS. In the reference sample itself, between-technique agreement in SDS was high, with negligible indications of bias between methods (61).
Conducting the same exercise in both underweight and overweight patients, DXA SDS demonstrated the best agreement for fat mass and lean mass (64). BIA SDS demonstrated nonsignificant average bias for lean mass SDS but showed wider limits of agreement than DXA SDS. Skinfold-thickness SDS did not perform much better than BMI SDS for assessing adiposity; hence, at the present time, the use of DXA to measure body composition in patients and express the data in SDS format seems to be the best option. It is less likely that simple techniques will generate accurate body composition data in patients because of perturbations of physiology such as overhydration or loss of mineral mass.
These studies indicate that where the gold-standard 4-component model cannot be used to assess body composition, individual methods can be used with varying degrees of accuracy to generate body composition SDS. For public health research, BIA and skinfold thicknesses show negligible average bias but have wider limits of agreement than specialized techniques such as DXA, air displacement plethysmography, and isotope dilution for measurement of TBW. For clinical work, DXA is the best-performing individual method, but the 4-component model remains ideal because it is able to take into account perturbations in lean mass composition (64). Given the range of options now available, and evidence for the degree of agreement in SDS between methods, these body composition growth charts may therefore aid in monitoring individuals and populations over time.
Across populations.
As yet, the vast majority of research on children’s body composition, including the derivation of reference data and growth charts, has been conducted in high-income countries (HICs). There are, however, important potential applications for body composition reference data in other settings. In low- and middle-income countries (LMICs), chronic undernutrition and stunting remain major public health issues, and a significant minority of individuals suffer from moderate or acute malnutrition during early life. In clinical practice, patients need to be compared against healthy children from the same population, who may nevertheless differ substantially in their body composition from healthy children in HICs. Until recently, there were few opportunities to obtain body composition data in LMIC settings, especially in rural populations. Over the past decade, however, the first such reference data have begun to emerge [e.g., (55, 65–68)].
This issue has attracted particular interest in South Asian populations, who are well established to have greater adiposity for a given BMI value compared with Europeans (26, 28), even during early life (27). Even though average BMI values may be lower than in HIC populations, elevations in adiposity during childhood indicate the early origins of cardiovascular risk. Reference data for anthropometry and DXA have therefore been produced to aid in identifying those with high adiposity in South Asian populations (55, 65–68). However, less attention has so far been directed to lean mass in this context.
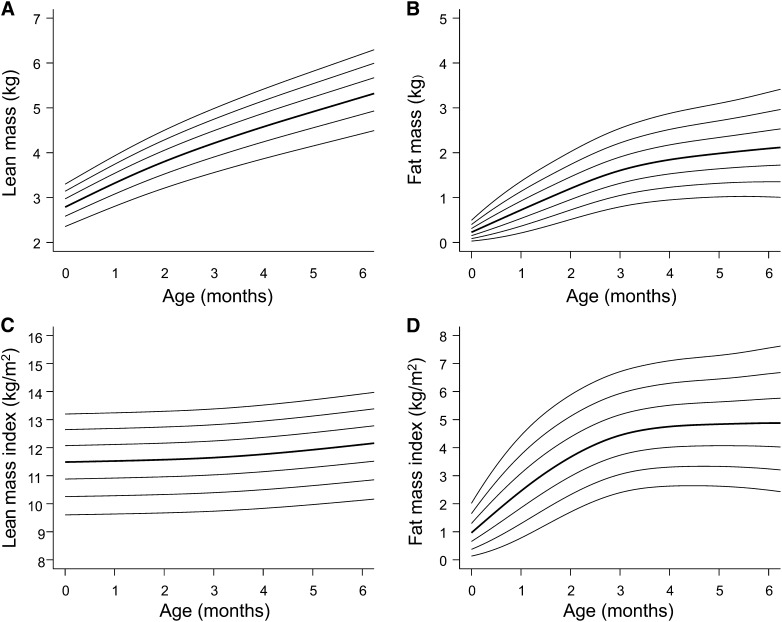
Despite the fact that much body composition variability emerges very early in life, and that both fat and lean masses may be influenced by stunting or wasting, our understanding of nutritional status in early life in LMIC populations remains dominated by data on weight, height, BMI, or mid–upper arm circumference, and more detailed data on body composition remain scarce. A recent study of infant body composition in an urban Ethiopian population by Andersen et al. (69) showed that neonates with low birth weight have reduced lean mass as well as lower fat mass. The study has provided novel reference data and growth charts for fat and lean masses between birth and age 6 mo with the use of air-displacement plethysmography (Fig. 3) (70). These data indicate subtle differences in the rate of fat and lean accretion relative to European populations from HICs.
FIGURE 3.
Body composition reference data from birth to 6 mo of age for female infants from Jimma, Ethiopia, obtained by using air-displacement plethysmography. Individual charts are available for lean mass (A), fat mass (B), lean mass index (C), and fat mass index (D). The 2nd, 9th, 25th, 50th, 75th, 91st, and 98th percentiles are displayed in ascending order. Reproduced from reference 69 with permission.
These data may be valuable for understanding the early tissue accretion patterns that characterize stunted or wasted children in contrast to those who are spared malnutrition. They may also improve understanding of the early-life origins of chronic disease risk in LMIC populations, in whom chronic malnutrition and overweight may be experienced by individuals at different periods of the life course (71). A number of possible research questions where LMIC body composition reference data would prove valuable are highlighted in Table 2.
TABLE 2.
Key questions for body composition research in low- and middle-income countries
| Questions |
| Short-term questions |
| What is the association between low birth weight and neonatal body composition? |
| What is the association between neonatal and infant body composition and survival? |
| How does infant or childhood body composition change during treatment for severe acute malnutrition? |
| Long-term questions |
| What is the association between early body composition and adult obesity and chronic disease risk? |
| What is the association between early body composition and adult reproductive function? |
| What is the association between early body composition and longevity? |
Is a universal reference appropriate?
Data from adults indicate that body composition, proportions, and physique differ substantially between populations (72, 73). As reference data accumulate from different populations and settings, there is growing recognition that such population variability is already evident in early life.
This raises the important question of whether such differences derive from environmental factors or have at least some genetic basis. Compared with UK infants, those from Pune in India had not only ~800 g lower birth weight but also a preservation of adiposity in combination with extreme deficits in indices of lean tissue (27). This “thin-fat” phenotype appears therefore to emerge during fetal life. A study in Indian migrants to Surinam showed that even after 4 to 5 generations, the thin-fat phenotype was still evident at birth (74), and a reduced amount of lean mass in South Asian relative to European infants has also been reported in infants born in the United Kingdom (75). Nevertheless, these findings do not reveal whether the causes are environmental or genetic.
The source of growth variability within and between populations has long interested both biologists and clinicians. Some have assumed that individual and population variability derives primarily from genetic factors. For example, height appears to be highly heritable (76, 77), and many individual alleles have now been linked with height variability (78). Body composition components also have high heritability, although the coefficients are lower than those for height (79). However, it is increasingly recognized that heritability assessments are problematic and may conflate genetic and epigenetic sources of variability (80). Furthermore, substantial secular trends in growth and nutritional status indicate potent effects of living conditions (80).
Recently, the notion that humans have an “optimal” growth pattern has been promoted by the WHO, through their publication of anthropometric standards based on data collected from ~8500 children from widely different ethnic backgrounds and ecologic settings (Brazil, Ghana, India, Norway, Oman, United States) (81). Children of high socioeconomic status grew relatively consistently across these countries, suggesting that population differences primarily reflect differences in living conditions. These data convey a crucial public health message that all possible efforts must be made to minimize the effects of adverse environmental conditions on early growth patterns. However, few studies have explicitly considered the potential contribution of genetic factors to population variability, or the time scale over which chronic undernutrition develops in any given population. Not all growth faltering necessarily develops through ecologic exposures within a given life course; rather, growth reflects transgenerational exposures (80, 82).
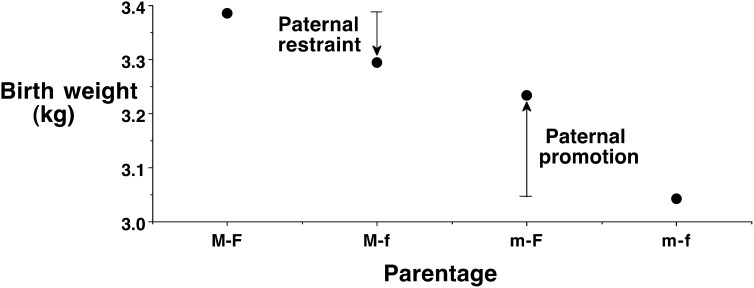
Recently, this issue was investigated by using a mixed-ethnicity study design (82). Birth weights were reported for neonates of 2 European parents or 2 Indian parents, and for neonates whose parents were of differing ethnicity (Fig. 4). Unsurprisingly, the neonates of the 2 Indian parents weighed substantially less at birth than those of the 2 European parents (Δ = −340g). This difference might reflect genetic factors, but it might also reflect contrasting size and nutritional status of Indian versus European adults and derive from contrasting parental exposure to environmental factors during development. These issues can be addressed by examining the birth weights of infants born to parents of contrasting ethnicity.
FIGURE 4.
Average birth weights of neonates categorized according to parental ethnicity. Compared with neonates of 2 European parents, those of 2 Indian parents had substantially lower birth weight. The weights of neonates of parents who differed in their ethnicity were intermediate. Indian mothers do not exert a fixed “constraint” on the fetal growth of their offspring; rather, European fathers can promote the growth of their offspring. Indian fathers contribute to the lower birth weights of their infants, shown by their restraining the growth of offspring of European mothers. Reproduced from reference 82 with permission. F, European father; f, Indian father; M, European mother; m, Indian mother.
Compared with the neonates of 2 Indian parents, those with an Indian mother and a European father weighed more at birth (Δ = +250g). This indicates that Indian mothers do not exert a fixed constraint on the fetal growth of their offspring and that European fathers can promote fetal growth in the offspring of Indian mothers. In the opposite direction, compared with the neonates of 2 European parents, those with a European mother and an Indian father weighed less at birth (Δ = −100g). This indicates a constraining effect on fetal growth in the offspring of European mothers by Indian fathers. Such an effect might reflect an epigenetic mechanism, but the most likely explanation is a polygenetic effect. In other words, this study suggests that growth differences between Indians and Europeans may derive in part from genetic differences, indicating contrasting growth potential (82).
Although these findings require replication, and extension to other ethnic groups, they have significant implications for our understanding of body composition variability. It is likely that ethnic groups differ in their body composition in part because of genetic factors, suggesting that population-specific reference data may be required. Already, studies have shown that the threshold at which adult BMI is associated with metabolic risk is not consistent across populations (83).
Extending into adulthood.
The value of body composition reference data does not stop at the end of adolescence, because body composition continues to change through adult life (84, 85). The causes of this variability include age-related changes in behavior, the impact of chronic diseases, and “cohort” effects, which refer to contrasting developmental exposures experienced by successive generations.
Some of these effects are evident in unpublished data from southern Italian hill villages, indicating a decline in lean mass from middle-age, along with increasing fat mass. It might appear that this represents the emergence of sarcopenia in middle age, in which there is a decline in muscle function and mass (86) that may be exacerbated by obesity (87). However, after correction for height, the decline in lean mass was much less evident and can be attributed largely to the older adults being shorter than younger adults due to having grown up in poorer economic conditions (although posture changes may also be relevant). This highlights the importance of adjusting body composition for height throughout the life course.
In conclusion, body composition reference data are essential in order to discern and interpret the physiologic basis of secular trends in nutritional status and to allow body composition to be monitored and managed in clinical practice. Over the past decade, such reference data have begun to emerge in both HIC and LMIC settings. Current evidence suggests that such data may need to be population specific, and caution is also needed when body composition assessment is performed with techniques differing from those used to collect the reference data. Through such reference data, the association between pediatric body composition and health is becoming clearer. This will benefit public health efforts to address chronic malnutrition and obesity while improving the management of pediatric diseases.
Acknowledgments
The sole author had responsibility for all parts of the manuscript.
Footnotes
Abbreviations used: BIA, bioelectrical impedance analysis; %fat, percentage of fat; FMI, fat mass index; HIC, high-income country; LMI, lean mass index; LMIC, low- and middle-income country; SDS, SD score; TBW, total body water.
Literature Cited
- 1.Bogin B. Patterns of human growth. Cambridge (UK): Cambridge University Press; 1988. [Google Scholar]
- 2.Tanner JM. The assessment of growth and development in children. Arch Dis Child. 1952;27:10–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child. 1976;51:170–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44:45–60 [PubMed] [Google Scholar]
- 5.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11:1305–19 [DOI] [PubMed] [Google Scholar]
- 6.Stasinopoulos DM, Rigby RA. Generalized additive models for location scale and shape (GAMLSS) in R. J Stat Softw. 2007;23:1–46 [Google Scholar]
- 7.Tanner JM. The regulation of human growth. Child Dev. 1963;34:817–47 [DOI] [PubMed] [Google Scholar]
- 8.Quetelet LAJ. Physiquesociale. Vol 2. Brussels: C. Muquardt; 1869. [Google Scholar]
- 9.Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. J Chronic Dis. 1972;25:329–43 [DOI] [PubMed] [Google Scholar]
- 10.Garrow JS, Webster J. Quetelet's index (W/H2) as a measure of fatness. Int J Obes. 1985;9:147–53 [PubMed] [Google Scholar]
- 11.Ferro-Luzzi A, Sette S, Franklin M, James WP. A simplified approach of assessing adult chronic energy deficiency. Eur J Clin Nutr. 1992;46:173–86 [PubMed] [Google Scholar]
- 12.Rolland-Cachera MF, Sempé M, Guilloud-Bataille M, Patois E, Péquignot-Guggenbuhl F, Fautrad V. Adiposity indices in children. Am J Clin Nutr. 1982;36:178–84 [DOI] [PubMed] [Google Scholar]
- 13.Cole TJ, Freeman JV, Preece MA. Body mass index reference curves for the UK, 1990. Arch Dis Child. 1995;73:25–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole TJ, Flegal KM, Nicholls D, Jackson AA. Body mass index cut offs to define thinness in children and adolescents: international survey. BMJ. 2007;335:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pietrobelli A, Faith MS, Allison DB, Gallagher D, Chiumello G, Heymsfield SB. Body mass index as a measure of adiposity among children and adolescents: a validation study. J Pediatr. 1998;132:204–10 [DOI] [PubMed] [Google Scholar]
- 17.Wells JC, Cole TJ. ALSPAC Study Team. Adjustment of fat-free mass and fat mass for height in children aged 8 y. Int J Obes (Lond). 2002;26:947–52 [DOI] [PubMed] [Google Scholar]
- 18.Wells JC, Victora CG. Indices of whole-body and central adiposity for evaluating the metabolic load of obesity. Int J Obes (Lond). 2005;29:483–9 [DOI] [PubMed] [Google Scholar]
- 19.Wells JC, Mok Q, Johnson AW. Nutritional status in children. Lancet. 2001;357:1293. [DOI] [PubMed] [Google Scholar]
- 20.VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body's fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52:953–9 [DOI] [PubMed] [Google Scholar]
- 21.Wells JC. A Hattori chart analysis of body mass index in infants and children. Int J Obes Relat Metab Disord. 2000;24:325–9 [DOI] [PubMed] [Google Scholar]
- 22.Hattori K, Tatsumi N, Tanaka S. Assessment of body composition by using a new chart method. Am J Hum Biol. 1997;9:573–8 [DOI] [PubMed] [Google Scholar]
- 23.Wells JC, Chomtho S, Fewtrell MS. Programming of body composition by early growth and nutrition. Proc Nutr Soc. 2007;66:423–34 [DOI] [PubMed] [Google Scholar]
- 24.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3:141–6 [DOI] [PubMed] [Google Scholar]
- 25.Hall LM, Moran CN, Milne GR, Wilson J, MacFarlane NG, Forouhi NG, Hariharan N, Salt IP, Sattar N, Gill JM. Fat oxidation, fitness and skeletal muscle expression of oxidative/lipid metabolism genes in South Asians: implications for insulin resistance? PLoS ONE. 2010;5:e14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nightingale CM, Rudnicka AR, Owen CG, Cook DG, Whincup PH. Patterns of body size and adiposity among UK children of South Asian, black African-Caribbean and white European origin: Child Heart and Health Study in England (CHASE study). Int J Epidemiol. 2011;40:33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yajnik CS, Fall CH, Coyaji KJ, Hirve SS, Rao S, Barker DJ, Joglekar C, Kellingray S. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes Relat Metab Disord. 2003;27:173–80 [DOI] [PubMed] [Google Scholar]
- 28.Haroun D, Taylor SJ, Viner RM, Hayward RS, Darch TS, Eaton S, Cole TJ, Wells JC. Validation of bioelectrical impedance analysis in adolescents across different ethnic groups. Obesity (Silver Spring). 2010;18:1252–9 [DOI] [PubMed] [Google Scholar]
- 29.Wells JC, Mok Q, Johnson AW, Lanigan JA, Fewtrell MS. Energy requirements and body composition in stable pediatric intensive care patients receiving ventilatory support. Food Nutr Bull. 2002;23(3 Suppl):95–8 [PubMed] [Google Scholar]
- 30.Tanner JM, Whitehouse RH. Standards for subcutaneous fat in British children. BMJ. 1962;1:446–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanner JM, Whitehouse RH. Revised standards for triceps and subscapular skinfolds in British children. Arch Dis Child. 1975;50:142–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies PS, Jones PR, Norgan NG. The distribution of subcutaneous and internal fat in man. Ann Hum Biol. 1986;13:189–92 [DOI] [PubMed] [Google Scholar]
- 33.Wells JC, Fewtrell MS. Measuring body composition. Arch Dis Child. 2006;91:612–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slaughter MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, Van Loan MD, Bemben DA. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60:709–23 [PubMed] [Google Scholar]
- 35.Deurenberg P, Pieters JJL, Hautvast JGA. The assessment of the body fat percentage by skinfold thickness measurements in childhood and young adolescence. Br J Nutr. 1990;63:293–303 [DOI] [PubMed] [Google Scholar]
- 36.Wells JC, Fewtrell MS. Is body composition important for paediatricians? Arch Dis Child. 2008;93:168–72 [DOI] [PubMed] [Google Scholar]
- 37.Nicholls D, Wells JC, Singhal A, Stanhope R. Body composition in early onset eating disorders. Eur J Clin Nutr. 2002;56:857–65 [DOI] [PubMed] [Google Scholar]
- 38.Rose G. Incubation period of coronary heart disease. BMJ Clin Med Ed. 1982;284(6329):1600–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klijn PH, van der Net J, Kimpen JL, Helders PJ, van der Ent CK. Longitudinal determinants of peak aerobic performance in children with cystic fibrosis. Chest. 2003;124:2215–9 [DOI] [PubMed] [Google Scholar]
- 40.Kelly DA. Nutritional factors affecting growth before and after liver transplantation. Pediatr Transplant. 1997;1:80–4 [PubMed] [Google Scholar]
- 41.Miller TL, Awnetwant EL, Evans S, Morris VM, Vazquez IM, McIntosh K. Gastrostomy tube supplementation for HIV-infected children. Pediatrics. 1995;96:696–702 [PubMed] [Google Scholar]
- 42.Roubenoff R, Kehayias JJ. The meaning and measurement of lean body mass. Nutr Rev. 1991;49:163–75 [DOI] [PubMed] [Google Scholar]
- 43.Mendley SR, Majkowski NL, Schoeller DA. Validation of estimates of total body water in paediatric dialysis patients by deuterium dilution. Kidney Int. 2005;67:2056–62 [DOI] [PubMed] [Google Scholar]
- 44.Fomon SJ, Haschke F, Ziegler EE, Nelson SE. Body composition of reference children from birth to age 10 years. Am J Clin Nutr. 1982;35 Suppl:1169–75 [DOI] [PubMed] [Google Scholar]
- 45.Haschke F. Body composition during adolescence. Columbus (OH): Ross Laboratories; 1989 [Google Scholar]
- 46.Lohman TG. Assessment of body composition in children. Pediatr Exerc Sci. 1989;1:19–30 [DOI] [PubMed] [Google Scholar]
- 47.Chumlea WC, Guo SS, Kuczmarski RJ, Flegal KM, Johnson CL, Heymsfield SB, Lukaski HC, Friedl K, Hubbard VS. Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord. 2002;26:1596–609 [DOI] [PubMed] [Google Scholar]
- 48.Addo OY, Himes JH. Reference curves for triceps and subscapular skinfold thicknesses in US children and adolescents. Am J Clin Nutr. 2010;91:635–42 [DOI] [PubMed] [Google Scholar]
- 49.Weber DR Moore RH Leonard MB Zemel BS Fat and lean BMI reference curves in children and adolescents and their utility in identifying excess adiposity compared with BMI and percentage body fat. Am J Clin Nutr. 2013;98:49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Sluis IM, de Ridder MA, Boot AM, Krenning EP, de Muinck Keizer-Schrama SM. Reference data for bone density and body composition measured with dual energy X ray absorptiometry in white children and young adults. Arch Dis Child. 2002;87:341–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alwis G, Rosengren B, Stenevi-Lundgren S, Düppe H, Sernbo I, Karlsson MK. Normative dual energy X-ray absorptiometry data in Swedish children and adolescents. Acta Paediatr. 2010;99:1091–9 [DOI] [PubMed] [Google Scholar]
- 52.Moreno LA, Mesana MI, González-Gross M, Gil CM, Ortega FB, Fleta J, Wärnberg J, León J, Marcos A, Bueno M. Body fat distribution reference standards in Spanish adolescents: the AVENA Study. Int J Obes (Lond). 2007;31:1798–805 [DOI] [PubMed] [Google Scholar]
- 53.Nakao T, Komiya S. Reference norms for a fat-free mass index and fat mass index in the Japanese child population. J Physiol Anthropol Appl Human Sci. 2003;22:293–8 [DOI] [PubMed] [Google Scholar]
- 54.Kurtoglu S, Mazicioglu MM, Ozturk A, Hatipoglu N, Cicek B, Ustunbas HB. Body fat reference curves for healthy Turkish children and adolescents. Eur J Pediatr. 2010;169:1329–35 [DOI] [PubMed] [Google Scholar]
- 55.Khadilkar AV, Sanwalka NJ, Chiplonkar SA, Khadilkar VV, Pandit D. Body fat reference percentiles on healthy affluent Indian children and adolescents to screen for adiposity. Int J Obes (Lond). 2013;37:947–53 [DOI] [PubMed] [Google Scholar]
- 56.Wells JC, Coward WA, Cole TJ, Davies PS. The contribution of fat and fat-free tissue to body mass index in contemporary children and the reference child. Int J Obes Relat Metab Disord. 2002;26:1323–8 [DOI] [PubMed] [Google Scholar]
- 57.Cohen DD, Voss C, Taylor MJ, Delextrat A, Ogunleye AA, Sandercock GR. Ten-year secular changes in muscular fitness in English children. Acta Paediatr. 2011;100:e175–7 [DOI] [PubMed] [Google Scholar]
- 58.Fuller NJ, Jebb SA, Laskey MA, Coward WA, Elia M. Four-component model for the assessment of body composition in humans: comparison with alternative methods, and evaluation of the density and hydration of fat-free mass. Clin Sci. 1992;82:687–93 [DOI] [PubMed] [Google Scholar]
- 59.Wells JC, Fuller NJ, Dewit O, Fewtrell MS, Elia M, Cole TJ. Four-component model of body composition in children: density and hydration of fat-free mass and comparison with simpler models. Am J Clin Nutr. 1999;69:904–12 [DOI] [PubMed] [Google Scholar]
- 60.Butte NF, Hopkinson JM, Wong WW, Smith EO, Ellis KJ. Body composition during the first 2 years of life: an updated reference. Pediatr Res. 2000;47:578–85 [DOI] [PubMed] [Google Scholar]
- 61.Wells JC, Williams JE, Chomtho S, Darch T, Grijalva-Eternod C, Kennedy K, Haroun D, Wilson C, Cole TJ, Fewtrell MS. Body-composition reference data for simple and reference techniques and a 4-component model: a new UK reference child. Am J Clin Nutr. 2012;96:1316–26 [DOI] [PubMed] [Google Scholar]
- 62.Wells JC, Williams JE, Chomtho S, Darch T, Grijalva-Eternod C, Kennedy K, Haroun D, Wilson C, Cole TJ, Fewtrell MS. Pediatric reference data for lean tissue properties: density and hydration from age 5 to 20 y. Am J Clin Nutr. 2010;91:610–8 [DOI] [PubMed] [Google Scholar]
- 63.Cole TJ. Weight-stature indices to measure underweight, over- weight and obesity. In: Himes JH, editor. Anthropometric assessment of nutritional status. New York: Wiley Liss; 1991. p. 83–111. [Google Scholar]
- 64.Atherton RR, Williams JE, Wells JC, Fewtrell MS. Use of fat mass and fat free mass standard deviation scores obtained using simple measurement methods in healthy children and patients: comparison with the reference 4-component model. PLoS ONE. 2013;8:e62139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Virani N. Reference curves and cut-off values for anthropometric indices of adiposity of affluent Asian Indian children aged 3–18 years. Ann Hum Biol. 2011;38:165–74 [DOI] [PubMed] [Google Scholar]
- 66.Pandit D, Chiplonkar S, Khadilkar A, Khadilkar V, Ekbote V. Body fat percentages by dual-energy X-ray absorptiometry corresponding to body mass index cutoffs for overweight and obesity in Indian children. Clin Med Pediatr. 2009;3:55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pandey RM, Madhavan M, Misra A, Kalaivani M, Vikram NK, Dhingra V. Centiles of anthropometric measures of adiposity for 14- to 18-year-old urban Asian Indian adolescents. Metab Syndr Relat Disord. 2009;7:133–41 [DOI] [PubMed] [Google Scholar]
- 68.Mushtaq MU, Gull S, Abdullah HM, Shahid U, Shad MA, Akram J. Waist circumference, waist-hip ratio and waist-height ratio percentiles and central obesity among Pakistani children aged five to twelve years. BMC Pediatr. 2011;11:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andersen GS, Girma T, Wells JC, Kæstel P, Michaelsen KF, Friis H. Fat and fat-free mass at birth: air displacement plethysmography measurements on 350 Ethiopian newborns. Pediatr Res. 2011;70:501–6 [DOI] [PubMed] [Google Scholar]
- 70.Andersen GS, Girma T, Wells JC, Kæstel P, Leventi M, Hother AL, Michaelsen KF, Friis H. Body composition from birth to 6 mo of age in Ethiopian infants: reference data obtained by air-displacement plethysmography. Am J Clin Nutr. 2013;98:885–94 [DOI] [PubMed] [Google Scholar]
- 71.Wells JC. Obesity as malnutrition: the role of capitalism in the obesity global epidemic. Am J Hum Biol. 2012;24:261–76 [DOI] [PubMed] [Google Scholar]
- 72.Wells JC. The evolutionary biology of human body fatness: thrift and control. Cambridge (UK): Cambridge University Press; 2010. [Google Scholar]
- 73.Wells JC. Ecogeographical associations between climate and human body composition: analyses based on anthropometry and skinfolds. Am J Phys Anthropol. 2012;147:169–86 [DOI] [PubMed] [Google Scholar]
- 74.van Steijn L, Karamali NS, Kanhai HH, Ariëns GA, Fall CH, Yajnik CS, Middelkoop BJ, Tamsma JT. Neonatal anthropometry: thin-fat phenotype in fourth to fifth generation South Asian neonates in Surinam. Int J Obes (Lond). 2009;33:1326–9 [DOI] [PubMed] [Google Scholar]
- 75.Stanfield KM, Wells JC, Fewtrell MS, Frost C, Leon DA. Differences in body composition between infants of South Asian and European ancestry: the London Mother and Baby Study. Int J Epidemiol. 2012;41:1409–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perola M, Sammalisto S, Hiekkalinna T, Martin NG, Visscher PM, Montgomery GW, Benyamin B, Harris JR, Boomsma D, Willemsen G, et al. Combined genome scans for body stature in 6,602 European twins: evidence for common Caucasian loci. PLoS Genet. 2007;3:e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Silventoinen K, Sammalisto S, Perola M, Boomsma DI, Cornes BK, Davis C, Dunkel L, De Lange M, Harris JR, Hjelmborg JV, et al. Heritability of adult body height: a comparative study of twin cohorts in eight countries. Twin Res. 2003;6:399–408 [DOI] [PubMed] [Google Scholar]
- 78.Lettre G. Genetic regulation of adult stature. Curr Opin Pediatr. 2009;21:515–22 [DOI] [PubMed] [Google Scholar]
- 79.Schousboe K, Visscher PM, Erbas B, Kyvik KO, Hopper JL, Henriksen JE, Heitmann BL, Sørensen TI. Twin study of genetic and environmental influences on adult body size, shape, and composition. Int J Obes Relat Metab Disord. 2004;28:39–48 [DOI] [PubMed] [Google Scholar]
- 80.Wells JC, Stock JT. Re-examining heritability: genetics, life history and plasticity. Trends Endocrinol Metab. 2011;22:421–8 [DOI] [PubMed] [Google Scholar]
- 81.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wells JC, Sharp G, Steer PJ, Leon DA. Paternal and maternal influences on differences in birth weight between Europeans and Indians born in the UK. PLoS ONE. 2013;8:e61116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63 [DOI] [PubMed] [Google Scholar]
- 84.Schutz Y, Kyle UU, Pichard C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18–98 y. Int J Obes Relat Metab Disord. 2002;26:953–60 [DOI] [PubMed] [Google Scholar]
- 85.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS ONE. 2009;4:e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kamel HK. Sarcopenia and aging. Nutr Rev. 2003;61:157–67 [DOI] [PubMed] [Google Scholar]
- 87.Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M. Sarcopenic obesity: a critical appraisal of the current evidence. Clin Nutr. 2012;31:583–601 [DOI] [PubMed] [Google Scholar]