Abstract
Infections with influenza and respiratory syncytial virus (RSV) rank high among the most common human respiratory diseases worldwide. Previously, we developed a replication-incompetent influenza virus by replacing the coding sequence of the PB2 gene, which encodes one of the viral RNA polymerase subunits, with that of a reporter gene. Here, we generated a PB2-knockout recombinant influenza virus expressing the F protein of RSV (PB2-RSVF virus) and tested its potential as a bivalent vaccine. In mice intranasally immunized with the PB2-RSVF virus, we detected high levels of antibodies against influenza virus, but not RSV. PB2-RSVF virus-immunized mice were protected from a lethal challenge with influenza virus but experienced severe body weight loss when challenged with RSV, indicating that PB2-RSVF vaccination enhanced RSV-associated disease. These results highlight one of the difficulties of developing an effective bivalent vaccine against influenza virus and RSV infections.
Introduction
Influenza virus and respiratory syncytial virus (RSV) infections are among the most common human respiratory diseases worldwide [1]. Influenza annually accounts for 3 to 5 million cases, 250,000 to 500,000 of which are fatal, while RSV is a major cause of severe respiratory diseases in infants throughout the world, with annual morbidity and mortality rates estimated at 64 million and 160,000, respectively [1,2].
Considerable effort has been expended to develop effective vaccines against influenza virus and RSV infections. However, in the 1960s, the formalin-inactivated RSV (FI-RSV) vaccine unexpectedly exacerbated respiratory diseases that subsequently resulted from RSV infection [3]. This adverse effect related to FI-RSV vaccination is termed enhanced respiratory disease (ERD); it is characterized by pulmonary eosinophilia and is associated with a substantial inflammatory response [4]. Importantly, it has hampered the development of an effective RSV vaccine.
Neutralizing antibodies against RSV, as well as a specific T-cell response, play a major role in virus clearance and in the clinical outcome of the disease [5-9]. Studies have shown the importance of individual RSV proteins as targets of protective immunity against RSV infection [10-12]. In fact, the passive transfer of palivizumab, a neutralizing monoclonal antibody against the RSV F envelope protein, inhibits the disease development associated with RSV infection in animal models [13,14] and significantly reduces the hospitalization rate of RSV patients [13,14]. Therefore, the F protein, which is a major envelope protein of RSV and mediates membrane fusion for both viral penetration and cell-to-cell spread [15], has been evaluated as an immunogen for RSV vaccines [11,12,15,16].
Influenza remains one of the biggest public health problems worldwide [17], and recombinant influenza viruses designed to help control influenza virus infection can now be generated [18]. Previously, by using a reverse genetics system [19], we generated a PB2-knockout (PB2-KO) influenza virus with a reporter gene in the coding region of the PB2 gene segment; this virus efficiently replicated in cells expressing PB2 protein, but not in normal cells, and stably maintained the reporter gene when it infected cells [20]. We also demonstrated that the PB2-KO virus elicited high levels of antibody and protected mice from a lethal challenge with influenza virus [20,21]. Notably, antibody against the reporter protein was detected in the vaccinated mice likely due to the viral RNA (vRNA) transcription mediated by incoming virus-derived PB2 protein [20,21], suggesting that the PB2-KO virus system had the potential to be used as a multivalent vaccine. More recently, PB2-KO viruses possessing the HA genes of either a pandemic (H1N1) 2009 or a highly pathogenic H5N1 strain have been shown to confer protective immunity against lethal challenge with the pandemic (H1N1) 2009 or a highly pathogenic H5N1 strain in mice [22]. We therefore hypothesized that the PB2-KO virus encoding the RSV F protein in its PB2 gene would confer protective immunity against both influenza and RSV infections. Here, we generated a PB2-KO influenza virus expressing the RSV F protein (PB2-RSVF virus) and assessed its efficacy as a bivalent vaccine against influenza virus and RSV infections in a mouse model.
Materials and methods
Ethics statement
All experiments were approved by the Institutional Animal Care and Use Committee of the United States Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services, National Wildlife Research Center (NWRC), Fort Collins, CO, USA. Approval number: V00806-0-02-12.
Cells
293T human embryonic kidney cells, a derivative of the 293 cell line into which the gene for simian virus 40 T antigen was inserted [23], were maintained in Dulbecco's modified Eagle medium (DMEM; Lonza) supplemented with 10% fetal calf serum (FCS; Invitrogen). Madin-Darby canine kidney (MDCK) cells were maintained in minimum essential medium (MEM; Invitrogen) supplemented with 5% newborn calf serum (NCS; Sigma). AX4 cells, a derivative of MDCK cells into which the gene for human α-2,6-sialyltransferase was inserted [24], were maintained in 5% NCS/MEM with 2 mg/ml puromycin (Sigma). AX4/PB2 cells, a derivative of AX4 cells into which the PB2 gene from the influenza A virus laboratory strain A/Puerto Rico/8/34 (H1N1, PR8) [20] was inserted [20,21], were maintained in 5% NCS/MEM with puromycin (2 μg/ml) and blasticidin (10 μg/ml) (InvivoGen). Human larynx carcinoma cells (HEp-2; ATCC CCL-23) were maintained in DMEM supplemented with 10% FCS, non-essential amino acids (Invitrogen), sodium pyruvate (Invitrogen), and 1% penicillin-streptomycin (Invitrogen). All cell lines were cultured at 37°C in 5% CO2.
Reverse genetics and virus propagation
Plasmid-based reverse genetics for influenza virus generation was performed by using plasmids containing the cDNAs for the influenza viral genes flanked by the human RNA polymerase I promoter and the mouse RNA polymerase I terminator (referred to as PolI plasmids) and plasmids for the eukaryotic protein expression of NP, PA, PB1, and PB2 under the control of the chicken β-actin promoter [25] as described previously [19]. Briefly, the wild-type PR8 virus was generated by using eight PolI plasmids for the eight gene segments of the wild-type PR8 virus [26]. To generate the PB2-RSVF virus, we constructed and used a PolI plasmid for the PB2(120)FRSV(336) gene that contained the A/WSN/33(H1N1)-derived 3′ PB2 non-coding region, 120 nucleotides (nt) of the PB2 coding sequence at the 3′ end, the full-length RSV F protein coding sequence, 336 nt of the PB2 coding sequence at the 5′ end, and the 5′ PB2 non-coding region instead of the wild-type PB2 gene. The initiation codon of the PB2 open reading frame (nucleotides 28-30) and the downstream ATG codon (nucleotides 58-60) were converted to GCG and ATC, respectively, by site-directed mutagenesis. The eight PolI plasmids and the four-protein expression plasmids were mixed with the transfection reagent TransIT-293 (Mirus), incubated at room temperature for 15 min, and added to 293T cells cultured in Opti-MEM I (Invitrogen). At 48 h post-transfection, the supernatant containing the wild-type PR8 or PB2-RSVF virus was harvested and inoculated into AX4 cells or AX4/PB2 cells, respectively, for propagation. The propagated recombinant viruses were titrated by use of plaque assays in AX4/AX4PB2 cells.
The human RSV A2 strain obtained from ATCC (VR-1540) was propagated in HEp-2 cells. Briefly, HEp-2 cells (2.0 × 106 cells) were inoculated with 2.0 × 103 TCID50 of RSV and cultured for 2–3 days. The RSV-infected cells were then scraped with a rubber policeman and disrupted by pipetting several times. Supernatants were clarified by centrifugation at 3,000 rpm for 30 minutes and served as stock viruses. The propagated RSV was titrated by use of 50% tissue culture infective doses (TCID50) assays in HEp-2 cells as described previously [27].
Inactivated viruses
To prepare formalin-inactivated PR8 (FI-PR8) virus, we purified egg-propagated PR8 virus by ultracentrifugation through a 10%–50% sucrose density gradient and resuspended it in PBS. The purified virus was then mixed with formalin (final concentration, 0.1%) and incubated for one week at 4°C. Inactivation of the virus was confirmed by passaging viruses twice in MDCK cells. Heat-inactivated RSV (HI-RSV), which elicits neutralizing antibodies more efficiently than does formalin- or UV-inactivated RSV in mice [28], was prepared by purifying RSV as described above and incubating it at 80°C for 1 h. Virus inactivation was confirmed in HEp-2 cells.
Immunofluorescence assay
AX4/PB2 cells were infected with viruses at a multiplicity of infection (m.o.i.) of 0.2–1. At 16 h post-infection, cells were fixed with 4% paraformaldehyde in PBS and permeabilized with 0.1% Triton X-100. Cells were incubated with an anti-WSN HA antibody (clone WS 3-54) or an anti-RSV F protein goat polyclonal antibody (ABcam, ab20531) for 1 h at room temperature, followed by an incubation with Alexa Fluor 594–labelled anti-rabbit or Alexa Fluor 488–labelled anti-goat secondary antibodies (Invitrogen) for 1 h at room temperature. Cells were then observed under a fluorescence microscope.
Evaluation of the stability of the RSV F gene in the PB2-RSVF virus
AX4/PB2 cells were infected with 10-fold dilutions of the PB2-RSVF virus (undiluted to 10-10). The supernatants from the second-to-last well in which a cytopathic effect was observed were harvested and diluted for subsequent passages. After five serial passages, supernatants were subjected to plaque assays in AX4/PB2 cells. Total and anti-RSV F mAb-positive plaques were counted.
Growth kinetics and virus titration
AX4 and AX4/PB2 cells were infected with viruses at an m.o.i. of 0.001. Supernatants were collected at 12, 24, 48, and 72 h and subjected to virus titration by using plaque assays in AX4/PB2 cells.
Virus immunization and challenge
Four-week-old female BALB/c mice (Jackson Laboratories) were anesthetized with isoflurane and intranasally immunized with 50 μl of the PB2-RSVF virus (104,105, or 106 PFU), FI-PR8 (106 PFU), HI-RSV (106.5 TCID50), or PBS twice with a 2-week interval between immunizations. The PB2-RSVF virus used in these experiments was passaged once after being generated by reverse genetics, to ensure that most of the virus population expressed the RSV F protein (see Table 1). As controls for a natural virus infection, mice were infected with live PR8 virus (0.1 50% mouse lethal dose [MLD50]) or with RSV (106,5 TCID50). Two weeks after the boost immunization or infection, mice were intranasally challenged with 10 MLD50 of the PR8 virus or 106.5 TCID50 of RSV. For the PR8 challenge, lungs and nasal turbinates of mice were collected on days 3 and 6 post-challenge. For RSV, lungs were collected from mice on day 4 post-challenge. The collected organs were homogenized in 0.5 ml of PBS by using TissueLyser II (QIAGEN), and clarified by centrifugation at 5,000 rpm for 10 min at 4°C. Influenza virus and RSV titers in homogenates were determined by using plaque assays in MDCK cells and by using TCID50 assays in HEp-2 cells, respectively. The body weight of challenged mice was monitored daily for two weeks. The morbidity based on distress (e.g., abnormal coordination and posture or respiration) was monitored twice per day for two weeks. Mice with body weight loss of more than 25% of pre-infection values were humanely euthanized by isoflurane inhalation in an anesthesia induction chamber.
Table 1. Genetic stability of the PB2-RSVF virus.
| Percentage of plaques positive for RSV F protein* | |||||
|---|---|---|---|---|---|
| Passage 1 | Passage 2 | Passage 3 | Passage 4 | Passage 5 | |
| Exp. 1 | 100% | 100% | 90% | 100% | 90% |
| Exp. 2 | 100% | 100% | 100% | 90% | 80% |
| Exp. 3 | 100% | 90% | 90% | 100% | 90% |
The respective viral supernatants were subjected to standard virus plaque assays in confluent AX4/PB2 cells. Ten plaques were marked per well, which were then subjected to an immunodetection assay with an anti-RSV F protein antibody. The percentage of viral plaques that were positive for the RSV F protein among the 10 plaques is presented. The results of three independent experiments (Exp.) are shown.
Antibody detection
Sera were collected from immunized mice via mandibular vein bleeding under anesthesia two weeks after the boost immunization. Nasal wash and bronchoalveolar lavage (BAL) samples were also taken from mice humanely euthanized as described above at this point time. IgG and IgA antibodies in serum, nasal wash, and BAL samples were detected by using an enzyme-linked immunosorbent assays (ELISA) as described previously [29,30]. Briefly, 96-well plates were coated with the PR8 virus or RSV disrupted with a mixture of 0.5 M Tris-HCl (pH 7.8), 0.5% Triton X-100, and 0.6 M KCl overnight at 4°C. The virus-coated plates were incubated with the collected samples for 16 h at 4°C and then incubated for 1 h at room temperature with horseradish-peroxidase-conjugated goat anti-mouse IgG or IgA antibodies (Kierkegaard & Perry Laboratories, Inc., Gaithersburg, MD). Absorbance of the plates at 405 nm was then measured by using a plate reader (Tecan, Kirchheim).
Statistics analysis
ELISA, body weight and viral titer data were analyzed by repeated-measures ANOVA and non-parametric test. Statistical significance was calculated using GraphPad Prism version 5.00 for Macintosh (GraphPad software, San Diego, CA). P-values less than 0.05 were considered to be statistically significant.
Results
Generation and characterization of the PB2–RSVF virus
Previously [20,21], to generate a recombinant influenza virus expressing a foreign protein from the PB2 gene, we constructed a recombinant PB2 gene segment encoding the GFP gene flanked by 120 nucleotides of each of the 3′ and 5&prime PB2 gene coding sequences (Figure 1A, PB2(120)GFP(120) gene [20]). The peak titer of the resultant PB2-KO virus (PB2(120)GFP(120) virus) in PB2-expressing cells (AX4/PB2 cells) was about one log lower than that of wild-type A/Puerto Rico/8/34 (H1N1) virus (PR8 virus) (Fig. 1C), and the number of infectious virus-like particles (VLPs) generated in the supernatants of plasmid-transfected cells increased with the length of the PB2 gene-derived coding sequence [20]. Hence, to obtain a higher titer of foreign-protein-expressing PB2-KO viruses, we constructed a recombinant PB2 gene with a longer PB2-gene-derived coding sequence at the 5′ end (Fig. 1B, PB2(120)GFP(336) gene). The resultant virus (PB2(120)GFP(336) virus) replicated more efficiently in AX4/PB2 cells than did PB2(120)GFP(120) virus, and the peak titer was comparable to that of the wild-type virus (Fig. 1C). Neither PB2-KO virus replicated in AX4 cells (Fig. 1C). On the basis of these findings, we constructed a recombinant PB2 gene segment encoding the RSV F gene flanked by 120 and 336 nucleotides of 3′ and 5′ coding sequence, respectively.
Fig. 1. Characterization of the PB2 (120)GFP(336) virus.
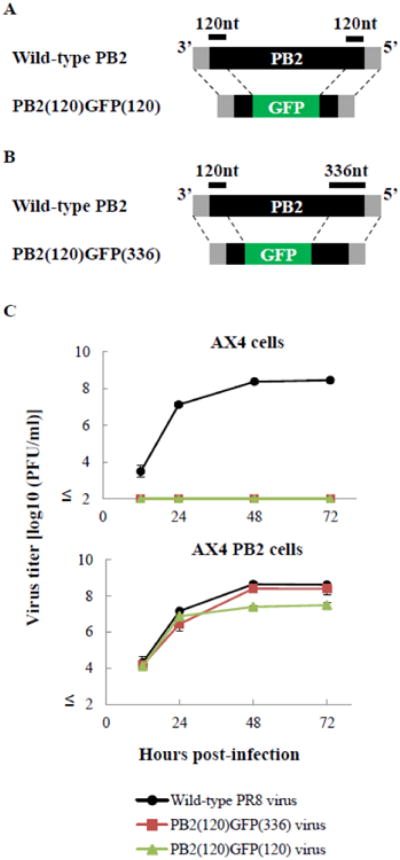
(A and B) Schematic diagram of wild-type PB2, PB2(120)GFP(120), and PB2(120)GFP(336) vRNAs. PB2(120)GFP(120) and PB2(120)GFP(336) vRNA possess 120 and 336 nucleotides (nt), respectively, of the coding sequence of the PB2 vRNA, along with both the 3′ and 5′ non-coding sequences, 120 nt of the 3′ coding sequence, and the GFP gene. The non-coding and coding sequences of PB2 vRNA are represented by the gray and black bars, respectively. The GFP gene is represented by the green bar. (C) Growth kinetics of PB2(120)GFP(120) and PB2(120)GFP(336) viruses. AX4 (upper panel) and AX4/PB2 (lower panel) cells were infected with the wild-type PR8, PB2(120)GFP(120), or PB2(120)GFP(336) virus at an m.o.i. of 0.001. Supernatants collected at the indicated time points were assayed for infectious virus by use of plaque assays in AX4/PB2 cells.
By replacing the GFP gene in the PB2(120)GFP(336) gene (Fig. 2B) with the full-length RSV F gene, we constructed the PB2(120)RSVF(336) gene (Fig. 2A). To eliminate the expression of the PB2-gene-derived peptide at the N-terminus of the F protein, two ATG codons in the 120-nucleotide PB2 protein-coding region were mutated (Fig. 2A). By using reverse genetics [19], we generated a PB2-KO virus possessing the PB2(120)RSVF(336) gene (PB2-RSVF virus). To confirm F protein expression in virus-infected cells, AX4/PB2 cells infected with PB2-RSVF virus were incubated with an F-protein-specific goat polyclonal antibody, followed by incubation with Alexa Fluor 488–labelled anti-goat antibody. We observed fluorescent signals for the F protein in the PB2-RSVF virus-infected cells, but no signal was detected in cells infected with the wild-type PR8 virus (Fig. 2B). The stability of the F gene in the PB2-RSVF virus was ascertained by serially passaging the virus in AX4/PB2 cells. F-protein-positive and total plaques were counted to determine the ratio of plaque-forming viruses expressing the F gene. After five serial passages, 80%–90% of the plaque-forming viruses expressed the F protein (Table 1). To confirm the replication-incompetent phenotype of the PB2-RSVF virus, we infected both AX4 and AX4/PB2 cells. We observed that the PB2-RSVF virus replicated in AX4/PB2 cells, although the peak titer was about one log lower than that of the wild-type PR8 virus. No virus was recovered from AX4 cells infected with PB2-RSVF virus. These results indicate that the F gene in the PB2-RSVF virus was stable and that replication of the PB2-RSVF virus was restricted to PB2-expressing cells (Fig. 2C).
Fig. 2. Characterization of the PB2-RSVF virus.
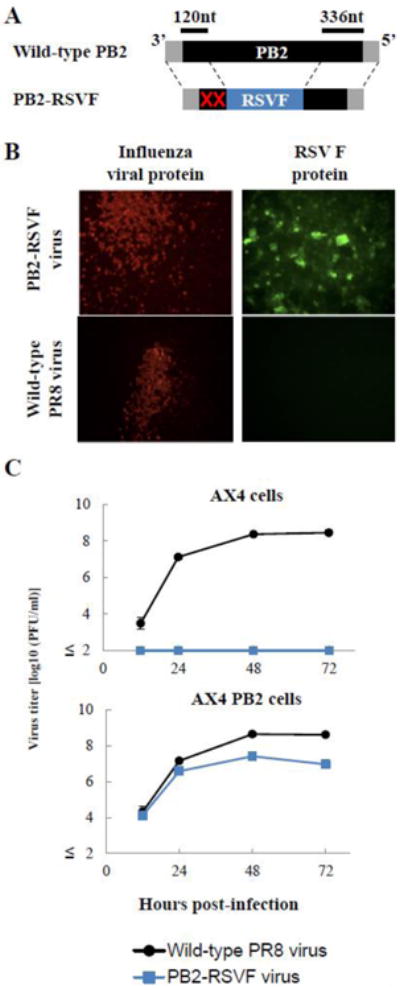
(A) Schematic diagram of wild-type PB2 and PB2-RSVF vRNAs. PB2(120)FRSV(336) vRNA possesses the 3′ non-coding sequence of the PB2 vRNA, 120 nt of the 3′ coding sequence of the PB2 vRNA with two ATG mutations (red crosses), the full-length RSV F gene, 336 nt of the 5′ coding region of the PB2 vRNA, and the 5′ non-coding sequence of PB2 vRNA. The non-coding and coding sequences of the PB2 vRNA are represented by the gray and black bars, respectively. The RSV F gene is represented by the blue bar. (B) Expression of RSV F protein in PB2-RSVF virus-infected cells. AX4/PB2 cells were infected with wild-type PR8 (top panels) or PB2-RSVF (bottom panels) virus at an m.o.i. of 0.1. At 16 h postinfection, the cells were fixed and stained with anti-influenza virus NP (left panels) and anti-RSV F (right panels) antibodies. (C) Growth kinetics of the PB2-RSVF virus. AX4 and AX4/PB2 cells were infected with wild-type PR8 or PB2-RSVF virus at an m.o.i. of 0.001. Supernatants collected at the indicated time points were assayed for infectious virus by use of plaque assays in AX4/PB2 cells.
To assess the pathogenicity of the PB2-RSVF virus, we inoculated mice intranasally with 104, 105, or 106 plaque-forming units (PFU) of the virus and monitored body weight for two weeks. All mice gained body weight and showed no signs or symptoms of disease (Fig. 3). These results suggest that the PB2-RSVF virus had low pathogenicity or was non-pathogenic.
Fig. 3. Body weight changes of mice infected with the PB2-RSVF virus.
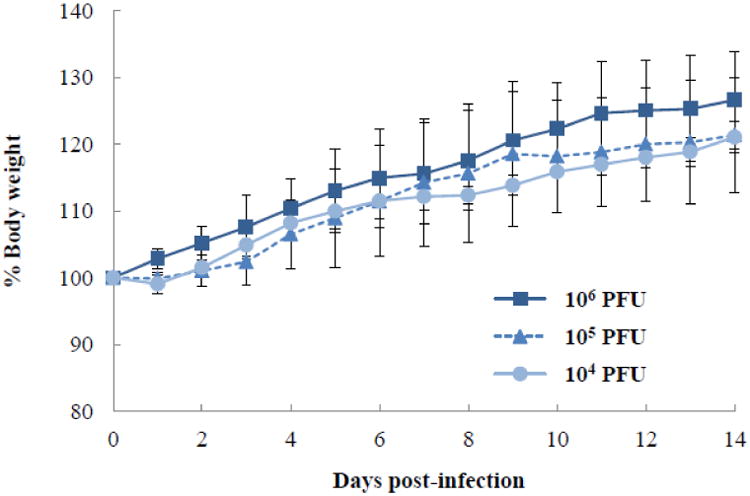
BALB/c mice (three mice per group) were inoculated intranasally with 104, 105, or 106 PFU of the PB2-RSVF virus (50 μl). Body weight was monitored daily for two weeks.
To confirm the replication incompetence of the PB2-RSVF virus in mice, we inoculated mice intranasally with 106 PFU of the virus and attempted to recover the virus from the nasal turbinates and lungs on day 3 post-inoculation. Although the PB2-RSVF viruses were recovered from the nasal turbinates and lungs of six and two mice, respectively, the virus titers were limited (37 to 138 PFU/g). These results suggest that the recovered PB2-RSVF viruses represented the remainder of the inocula and confirm that the PB2-RSVF virus was replication incompetent.
Humoral immune responses elicited by inoculation with PB2-RSVF virus
To evaluate the humoral immune responses elicited by the PB2-RSVF virus to influenza virus and RSV, mice were inoculated twice intranasally with 104, 105, or 106 PFU of PB2-RSVF virus with a 2-week interval between immunizations. We also prepared control mice that were inoculated with formalin-inactivated PR8 virus (FI-PR8 virus, equivalent to 106 PFU), live PR8 virus (0.1 50% mouse lethal dose [MLD50]; 50 PFU), heat-inactivated RSV (HI-RSV, equivalent to 105 50% tissue culture infectious dose [TCID50]), live RSV (106.5 TCID50), or phosphate-buffered saline (PBS). Two weeks after the boost immunization, antibody titers in serum, bronchoalveolar lavage (BAL), and nasal wash were evaluated using an enzyme-linked immunosorbent assay (ELISA). PR8-specific immunoglobulin G (IgG) titers in serum and IgA titers in serum, BAL, and nasal wash from the 106 PFU of PB2-RSVF virus-inoculated mice were significantly (P < 0.05) higher than those in PBS-inoculated mice (Fig. 4). The overall level of antibodies elicited by 106 PFU of the PB2-RSVF virus was higher than that elicited by the FI-PR8 virus (Fig. 4). These results indicate that the PB2-RSVF virus, particularly at the inoculation dose of 106 PFU, efficiently elicited humoral immune responses against influenza virus.
Fig. 4. Influenza virus-specific antibodies in immunized mice.
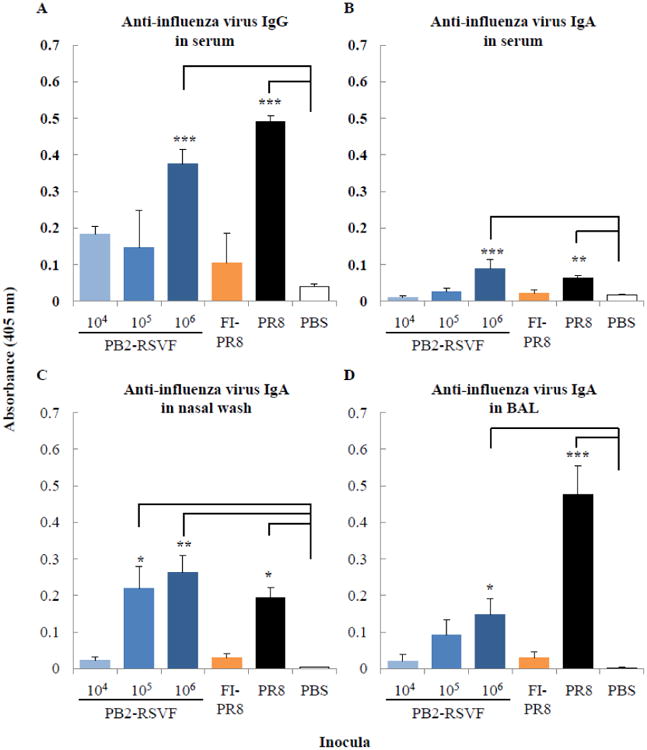
BALB/c mice (three mice per group) were immunized twice intranasally with 104, 105, or 106 PFU of the PB2-RSVF virus. Two weeks after the boost immunization, anti-influenza virus IgG (A) and IgA (B, C, and D) antibodies in 100-fold diluted serum (A and B), nasal wash (C), and BAL (D) samples were detected by using an ELISA. Mice immunized with formalin-inactivated PR8 virus (FI-PR8), infected with live PR8 virus (PR8), or mock immunized with PBS (PBS) served as controls. Values are expressed as the mean absorbance ± standard deviation (SD) (n=3). Statistical significance is indicated by asterisks (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001).
By contrast, titers of the RSV-F-protein-specific antibody in the mice inoculated with PB2-RSVF virus were similar to those in the PBS-inoculated mice (Fig. 5). Of the inocula tested, only live RSV induced high levels of antibodies in inoculated mice. These results indicate that the PB2-RSVF virus did not elicit a strong humoral immune response against RSV.
Fig. 5. RSV-specific antibodies in immunized mice.
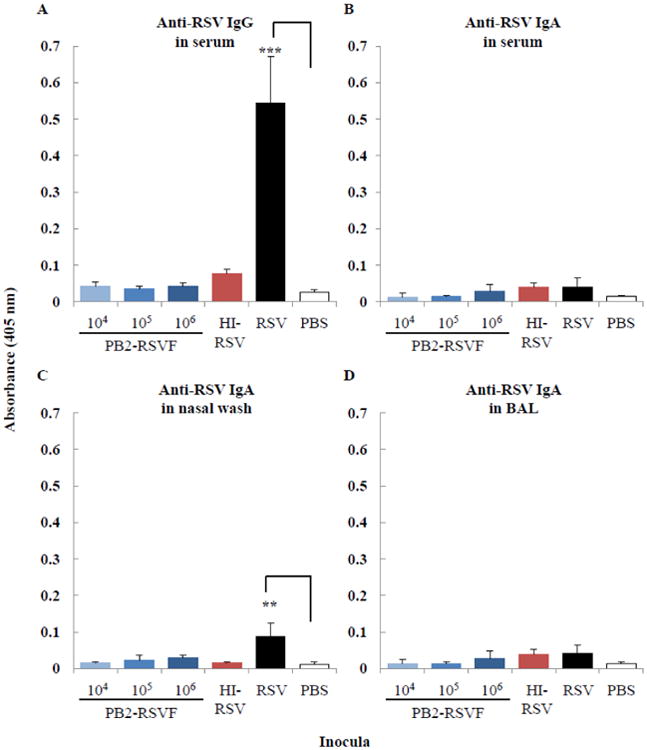
BALB/c mice (three mice per group) were immunized twice intranasally with 104, 105, or 106 PFU of the PB2-RSVF virus. Two weeks after the boost immunization, IgG (A) and IgA (B, C, and D) antibodies in undiluted serum (A and B), nasal wash (C), and BAL (D) samples were detected by using an ELISA. Mice immunized with heat-inactivated RSV (HI-RSV), infected with live RSV (values for 1:100 dilution), or mock immunized with PBS served as controls. Values are expressed as the mean absorbance ± SD (n=3). Statistical significance is indicated by asterisks (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001).
Vaccine efficacy of PB2-RSVF virus
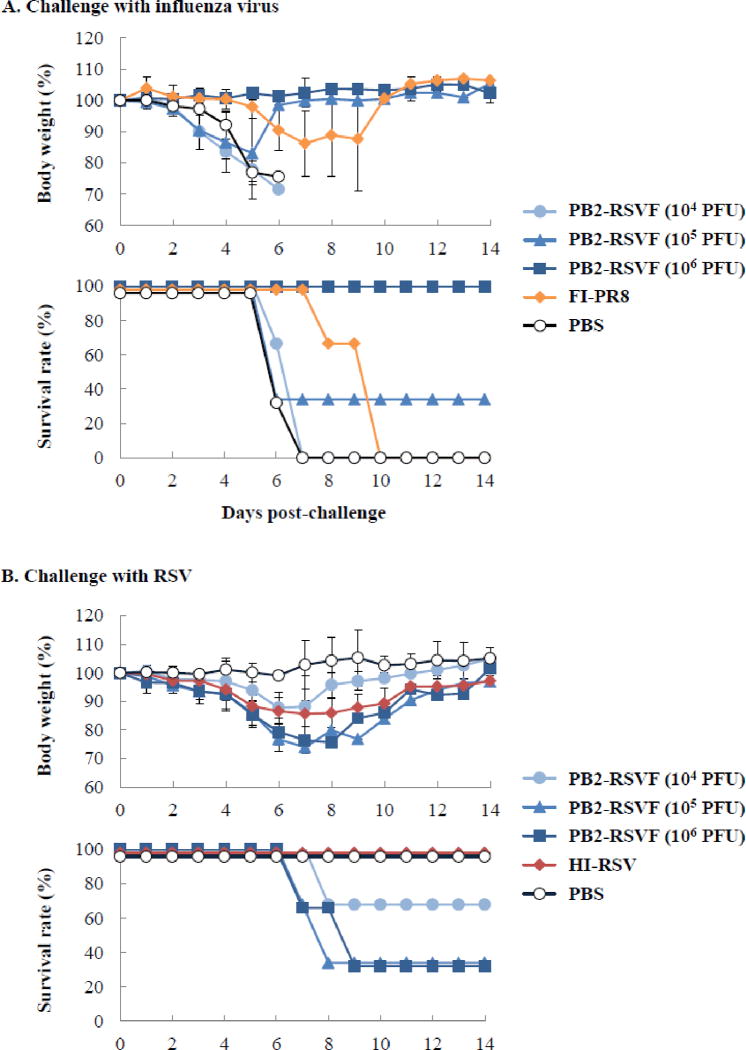
To investigate whether the PB2-RSVF virus could serve as a bivalent vaccine against influenza virus and RSV infections, we immunized mice twice intranasally with 104, 105 or 106 PFU of the PB2-RSVF virus with a 2-week interval between immunizations. As controls, the vaccine efficacy of the FI-PR8 virus (1 × 106 PFU), HI-RSV (105 TCID50), and PBS were also tested. Two weeks after the boost immunization, mice were challenged with 10 MLD50 of PR8 virus or 106.5 TCID50 of RSV. Body weight change and survival were monitored for two weeks. We observed that the mice that were immunized with 106 PFU of the PB2-RSVF virus and challenged with a lethal dose of PR8 virus showed no prominent changes in body weight, and they all survived (Fig. 6A). By contrast, all of the mice immunized with either PBS or 104 PFU of the PB2-RSVF virus died (Fig. 6A). Two mice in the FI-PR8 virus-inoculated group and one mouse in the group inoculated with 105 PFU of the PB2-RSVF virus also died (Fig. 6A). These results indicate that immunization with 105 PFU or a higher dose of the PB2-RSVF virus protected mice from lethal infection with influenza virus.
Fig. 6. Body weight changes of mice after virus challenge.
(A) Body weight changes and survival rates of mice challenged with influenza virus. Mice were immunized twice with 104, 105, or 106 PFU of the PB2-RSVF virus or controls (formalin-inactivated PR8 [FI-PR8] virus and PBS). Two weeks after the boost immunization, mice were challenged with 10 MLD50 of PR8 virus. Body weight (upper panel) and survival (lower panel) were monitored daily for two weeks. (B) Body weight changes and survival rates of mice challenged with RSV. Mice were immunized twice with 104, 105, or 106 PFU of the PB2-RSVF virus or controls (heat-inactivated RSV [HI-RSV] and PBS). Two weeks after the boost immunization, mice were challenged with 106.5 TCID50 of RSV. Body weight (upper panel) and survival (lower panel) were monitored daily for two weeks. Values are expressed as mean changes in body weight (n=3). Bars represent SD.
For challenge with RSV, mice immunized with 105 or 106 PFU of the PB2-RSVF virus showed significantly (P < 0.001) severe body weight loss; two mice in each group lost over 25% of their body weight and were, therefore, euthanized (Fig. 6B). Notably, all of these mice exhibited symptoms such as tachypnea, inactivity, and ruffled fur. These results indicate that PB2-RSVF virus immunization enhanced RSV-associated disease. Further, mice immunized with 104 PFU of the PB2-RSVF virus also showed body weight loss, and one animal in this group had to be euthanized (Fig. 6B). Mice immunized with HI-RSV also lost body weight upon RSV infection (∼20%), although this weight loss was not as severe as that experienced by mice immunized with 105 and 106 PFU of the PB2-RSVF virus. By contrast, control mice mock-immunized with PBS showed no body weight loss (Fig. 6B). Thus, immunization with the PB2-RSVF virus aggravated RSV infection, and to a greater extent than did HI-RSV immunization.
Viral titers in respiratory organs of challenged mice
To further evaluate the vaccine efficacy of the PB2-RSVF virus against influenza virus infection, the nasal turbinates and lungs of mice immunized with the PB2-RSVF virus and challenged with PR8 virus were collected on days 3 and 6 post-challenge for virus titration (Table 2). FI-PR8 virus (106 PFU) and PBS served as control inocula for immunization. In mice mock-immunized with PBS, wild-type PR8 virus replicated to more than 107 and 108 PFU/g in the nasal turbinates and lungs, respectively, on day 3 post-infection; one out of three PR8 virus-challenged mice died on day 6 post-infection. Under these conditions, immunization with 106 PFU of the PB2-RSVF virus significantly (P < 0.05) reduced the virus titers in the nasal turbinates and lungs on day 3 post-challenge compared with the PBS mock immunization. Further, the viral titer in the lungs of mice immunized with 106 PFU of the PB2-RSVF virus was significantly lower than that in the lungs of FI-PR8 virus-immunized mice. These results attest to the vaccine efficacy of PB2-RSVF against influenza virus infection.
Table 2. Virus titers in the nasal turbinates and lungs of mice challenged with PR8 virusa.
| Immunogen (PFU) | Day after challenge | Virus Titer (mean log10PFU ± SD/g) in: | ||
|---|---|---|---|---|
| Nasal turbinates | Lungs | |||
| PB2-RSVF virus | 104 | 3 | 6.4±0.5 | 8.4±0.1 |
| 6 | 7.6±0.9 | 8.2±0.7 | ||
| 105 | 3 | 6.2* | 7.6±0.3 | |
| 6 | 5.8 | 7.9, 7.2 | ||
| 106 | 3 | 7.0* | 7.2*,** | |
| 6 | 5.0 | 4.3 | ||
| FI-PR8 virus | 106 | 3 | 6.5, 6.9 | 8±0.3 |
| 6 | 6.2, 7.6 | 5.8,7.8 | ||
| PBS | 3 | 7.3±0.6 | 8.5±0.2 | |
| 6 | 8.9, 7.8,b | 7.1,6.6,b | ||
Mice immunized with the indicated agents (104, 105, or 106 PFU of the PB2-RSVF virus or controls [formalin-inactivated PR8 (FI-PR8) virus and PBS) were challenged with 10 MLD50 of PR8 virus two weeks after the boost immunization. Three mice per group were euthanized on days 3 and 6 post-challenge for virus titration in the nasal turbinates and lungs. When virus was not detected (detection limit, 5 PFU/g organ) in all mice, individual titers were recorded.
One mouse died before this sampling time point; therefore, data could not be recorded for that mouse.
Statistically significant differences (P ≤ 0.05) compared with the PBS (*) and FI-PR8 (**) control groups are indicated by asterisks.
Similarly, we evaluated the vaccine efficacy of the PB2-RSVF virus against RSV infection by determining virus titers in the respiratory organs of PB2-RSVF-virus-immunized, RSV-challenged mice on day 4 post-infection (Table 3); HI-RSV (106.5 TCID50) and PBS served as immunization controls. In contrast to PR8 virus challenge, no significant difference in lung virus titer was detected between the PB2-RSVF-virus- and control-immunized mice. These results correlate with the limited levels of RSV-specific antibody induced by PB2-RSVF virus immunization (Fig. 5). In addition, these results suggest that the severe weight loss observed in the PB2-RSVF-virus-immunized, RSV-challenged mice (Fig. 6B) was not due to enhanced replication of the challenge virus.
Table 3. Virus titers in the lungs of mice challenged with RSVa.
| Immunogen (PFU) | Virus Titer (mean log10 PFU ± SD/g) |
|
|---|---|---|
| PB2-RSVF virus | 104 | 5.4 ± 0.07 |
| 105 | 5.6 ± 0.2 | |
| 106 | 5.4 ± 0.2 | |
| HI-RSV | 106 | 5.3 ± 0.02 |
| PBS | 5.4 ± 0.08 | |
Mice immunized with the indicated agents (104, 105, or 106 PFU of the PB2-RSVF virus or controls [heat-inactivated RSV (HI-RSV) and PBS) were challenged with 106.5 TCID50 of RSV two weeks after the boost immunization. Three mice per group were euthanized on day 4 post-challenge for virus titration in the lungs.
Discussion
Here, we generated a replication-incompetent influenza virus expressing the RSV F protein from the recombinant PB2 gene and assessed its efficacy as a bivalent vaccine against influenza virus and RSV infections in a mouse model. As demonstrated previously [21], the PB2-KO virus, encoding a foreign pathogen-derived antigen, elicited protective antibodies against influenza virus infection (Figures 4 and 6A). By contrast, the PB2-RSVF virus did not confer protective immunity against RSV infection (Figures 5 and 6B), as seen in other vaccine studies [3,11,16,31,32]. Instead, the immunization of mice with the PB2-RSVF virus caused enhanced respiratory disease (ERD) after exposure to RSV, likely due to the PB2-RSVF virus-induced expression of the RSV F protein in mouse cells. These results indicate that the immunity conferred by the RSV F protein expressed from this influenza replication-deficient vector aggravated RSV infection.
A protective immune response against influenza virus was efficiently induced in mice intranasally immunized with the PB2-RSVF virus (Figures 4 and 6A), indicating that the inoculated PB2-KO virus successfully stimulated the immune system of the respiratory organs in mice. In the RSV challenge experiments, IgG and IgA were detected at significant levels in serum and nasal wash, respectively, collected from wild-type RSV-infected mice (Fig. 5A and C), indicating that the experimental settings (e.g., the animal model and ELISA) were functional. Yet, the PB2-RSVF virus, even dosed at 106 PFU per mouse, did not elicit RSV-specific antibodies (Fig. 5), suggesting that the expression level of the RSV F protein on the PB2-RSVF virions and/or in the PB2-RSVF virus-immunized mice may not have been sufficient to stimulate RSV F-specific antibody production. In the PB2-KO virus system [20], expression of the newly synthesized protein in virus-infected cells is expected to be low, because viral RNA transcription and replication in cells infected with the PB2-KO virus rely entirely on the limited number of incoming virus-derived RNA polymerase molecules. Optimization of the non-epitopic region of the RSV F protein (e.g., the transmembrane and intracellular domains could be replaced by their influenza viral envelope-protein-derived counterparts) may increase RSV F protein expression on the PB2-RSVF virions and thus improve the immunogenicity of the PB2-RSVF virus. Another possibility for the limited induction of RSV-F-specific immunity is the unique immune pathway involved in RSV clearance. Toll-like receptor 4 (TLR4), which is generally considered a key molecule for innate immunity against infection by Gram-negative bacteria, plays a critical role in the immune response to RSV infection [5,33,34]. It may be that the influenza-virus-based vaccine does not efficiently stimulate the TLR4 signaling pathway, given that wild-type influenza virus activates immune responses in both TLR4-deficient and TLR4-expressing mice [33]. Therefore, we may need to consider an alternative approach to delivering RSV antigens.
While PB2-RSVF virus immunization did not elicit RSV-specific antibodies at a detectable level (Fig. 5), RSV infection of mice immunized with PB2-RSVF virus caused ERD (Fig. 6B). Enhanced replication of the challenge RSV was not observed in the PB2-RSVF-virus-immunized mice (Table 3), suggesting that the development of ERD was not related to the extent of RSV replication, and probably not related to RSV-specific antibodies. In fact, similar results have been observed in animals immunized with alum adjuvant [35], empty DNA vector [16], and ovalbumin [36,37]. An excessive Th2-polarized immune response is believed to contribute to RSV-infection-triggered ERD [10,38-41], although the detailed mechanism remains unclear. To develop an efficacious RSV vaccine, therefore, an approach that stimulates the Th1-type immune system may be needed.
We observed that the PB2(120)GFP(336) virus with a longer PB2-gene-derived coding sequence at 5′ end of the recombinant PB2 gene segment replicated more efficiently than did the PB2(120)GFP(120) virus: the peak titers were comparable to that of the wild-type virus (Fig. 1C). The increased replication ability of PB2(120)GFP(336) virus was likely due to the higher packaging efficiency of the recombinant PB2 gene segment with the longer coding sequence into progeny virions.
In summary, immunization of mice with PB2-RSVF virus conferred protective immunity against a lethal dose of influenza virus but led to ERD in those mice upon RSV infection. These results demonstrate the difficulty of developing an RSV vaccine.
Acknowledgments
We thank Abigail Betanzos for her assistance during the project, and Susan Watson for editing the manuscript. This study was supported by a grant from the National Institute of Allergy and Infectious Disease, by a Grant-in-Aid for Specially Promoted Research, by a contract research fund for the Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases from the Ministry of Education, Culture, Sports, Science, and Technology, by grants-in-aid from the Ministry of Health, by ERATO (Japan Science and Technology Agency), and by the Advanced Research for Medical Products Mining Programme of the National Institute of Biomedical Innovation (NIBIO). This work was, in part, also funded by the Institute of Science and Technology of Federal District Icyt-DF (project number, ICyT-DF23/2011). W.F. received financial support from the National Council on Science and Technology of Mexico (Conacyt-Mexico) and from the Fulbright-García Robles program.
References
- 1.World healt organization. Acute Respiratory Infections. 2009 (Update September 2009) http://www.who.int/vaccine_research/diseases/ari/en/index2.html.
- 2.Girard MP, Cherian T, Pervikov Y, Kieny MP. A review of vaccine research and development: human acute respiratory infections. Vaccine. 2005;23:5708–5724. doi: 10.1016/j.vaccine.2005.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 4.Haynes LM, Jones LP, Barskey A, Anderson LJ, Tripp RA. Enhanced Disease and Pulmonary Eosinophilia Associated with Formalin-Inactivated Respiratory Syncytial Virus Vaccination Are Linked to G Glycoprotein CX3C-CX3CR1 Interaction and Expression of Substance P. Journal of Virology. 2003;77:9831–9844. doi: 10.1128/JVI.77.18.9831-9844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang J. Current progress on development of respiratory syncytial virus vaccine. BMB Rep. 2011;44:232–237. doi: 10.5483/BMBRep.2011.44.4.232. [DOI] [PubMed] [Google Scholar]
- 7.Shao HY, Yu SL, Sia C, Chen Y, Chitra E, et al. Immunogenic properties of RSV-B1 fusion (F) protein gene-encoding recombinant adenoviruses. Vaccine. 2009;27:5460–5471. doi: 10.1016/j.vaccine.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Singh SR, Dennis VA, Carter CL, Pillai SR, Jefferson A, et al. Immunogenicity and efficacy of recombinant RSV-F vaccine in a mouse model. Vaccine. 2007;25:6211–6223. doi: 10.1016/j.vaccine.2007.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olmsted RA, Elango N, Prince GA, Murphy BR, Johnson PR, et al. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc Natl Acad Sci U S A. 1986;83:7462–7466. doi: 10.1073/pnas.83.19.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bembridge GP, Lopez JA, Bustos R, Melero JA, Cook R, et al. Priming with a secreted form of the fusion protein of respiratory syncytial virus (RSV) promotes interleukin-4 (IL-4) and IL-5 production but not pulmonary eosinophilia following RSV challenge. J Virol. 1999;73:10086–10094. doi: 10.1128/jvi.73.12.10086-10094.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bembridge GP, Lopez JA, Cook R, Melero JA, Taylor G. Recombinant vaccinia virus coexpressing the F protein of respiratory syncytial virus (RSV) and interleukin-4 (IL-4) does not inhibit the development of RSV-specific memory cytotoxic T lymphocytes, whereas priming is diminished in the presence of high levels of IL-2 or gamma interferon. J Virol. 1998;72:4080–4087. doi: 10.1128/jvi.72.5.4080-4087.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bembridge GP, Rodriguez N, Garcia-Beato R, Nicolson C, Melero JA, et al. DNA encoding the attachment (G) or fusion (F) protein of respiratory syncytial virus induces protection in the absence of pulmonary inflammation. J Gen Virol. 2000;81:2519–2523. doi: 10.1099/0022-1317-81-10-2519. [DOI] [PubMed] [Google Scholar]
- 13.Grimaldi M, Gouyon B, Sagot P, Quantin C, Huet F, et al. Palivizumab efficacy in preterm infants with gestational age < or = 30 weeks without bronchopulmonary dysplasia. Pediatr Pulmonol. 2007;42:189–192. doi: 10.1002/ppul.20503. [DOI] [PubMed] [Google Scholar]
- 14.Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- 15.Brock SC, Heck JM, McGraw PA, Crowe JE., Jr The transmembrane domain of the respiratory syncytial virus F protein is an orientation-independent apical plasma membrane sorting sequence. J Virol. 2005;79:12528–12535. doi: 10.1128/JVI.79.19.12528-12535.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bembridge GP, Rodriguez N, Garcia-Beato R, Nicolson C, Melero JA, et al. Respiratory syncytial virus infection of gene gun vaccinated mice induces Th2-driven pulmonary eosinophilia even in the absence of sensitisation to the fusion (F) or attachment (G) protein. Vaccine. 2000;19:1038–1046. doi: 10.1016/s0264-410x(00)00344-3. [DOI] [PubMed] [Google Scholar]
- 17.Wright PF, Neumann G, Kawaoka Y. Othomyxoviruses. In: Howley DMKPM, editor. Fields Virology. 5th. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 1691–1740. [Google Scholar]
- 18.Ozawa M, Kawaoka Y. Taming influenza viruses. Virus Research. 2011;162:8–11. doi: 10.1016/j.virusres.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neumann G, Watanabe T, Ito H, Watanabe S, Goto H, et al. Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci U S A. 1999;96:9345–9350. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozawa M, Victor ST, Taft AS, Yamada S, Li C, et al. Replication- incompetent influenza A viruses that stably express a foreign gene. J Gen Virol. 2011;92:2879–2888. doi: 10.1099/vir.0.037648-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Victor ST, Watanabe S, Katsura H, Ozawa M, Kawaoka Y. A replication- incompetent PB2-knockout influenza A virus vaccine vector. J Virol. 2012 doi: 10.1128/JVI.06232-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uraki R, Kiso M, Iwatsuki-Horimoto K, Fukuyama S, Takashita E, et al. A novel bivalent vaccine based on a PB2-knockout influenza virus protects mice from pandemic H1N1 and highly pathogenic H5N1 virus challenges. J Virol. 2013;87:7874–7881. doi: 10.1128/JVI.00076-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DuBridge RB, Tang P, Hsia HC, Leong PM, Miller JH, et al. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987;7:379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatakeyama S, Sakai-Tagawa Y, Kiso M, Goto H, Kawakami C, et al. Enhanced expression of an alpha2,6-linked sialic acid on MDCK cells improves isolation of human influenza viruses and evaluation of their sensitivity to a neuraminidase inhibitor. J Clin Microbiol. 2005;43:4139–4146. doi: 10.1128/JCM.43.8.4139-4146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 26.Horimoto T, Murakami S, Muramoto Y, Yamada S, Fujii K, et al. Enhanced growth of seed viruses for H5N1 influenza vaccines. Virology. 2007;366:23–27. doi: 10.1016/j.virol.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie C, He JS, Zhang M, Xue SL, Wu Q, et al. Oral respiratory syncytial virus (RSV) DNA vaccine expressing RSV F protein delivered by attenuated Salmonella typhimurium. Hum Gene Ther. 2007;18:746–752. doi: 10.1089/hum.2007.053. [DOI] [PubMed] [Google Scholar]
- 28.Moghaddam A, Olszewska W, Wang B, Tregoning JS, Helson R, et al. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat Med. 2006;12:905–907. doi: 10.1038/nm1456. [DOI] [PubMed] [Google Scholar]
- 29.Kohlmann R, Schwannecke S, Tippler B, Ternette N, Temchura VV, et al. Protective efficacy and immunogenicity of an adenoviral vector vaccine encoding the codon-optimized F protein of respiratory syncytial virus. J Virol. 2009;83:12601–12610. doi: 10.1128/JVI.01036-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kida H, Brown LE, Webster RG. Biological activity of monoclonal antibodies to operationally defined antigenic regions on the hemagglutinin molecule of A/Seal/Massachusetts/1/80 (H7N7) influenza virus. Virology. 1982;122:38–47. doi: 10.1016/0042-6822(82)90375-0. [DOI] [PubMed] [Google Scholar]
- 31.Openshaw PJ, Clarke SL, Record FM. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int Immunol. 1992;4:493–500. doi: 10.1093/intimm/4.4.493. [DOI] [PubMed] [Google Scholar]
- 32.Olszewska W, Suezer Y, Sutter G, Openshaw PJ. Protective and disease- enhancing immune responses induced by recombinant modified vaccinia Ankara (MVA) expressing respiratory syncytial virus proteins. Vaccine. 2004;23:215–221. doi: 10.1016/j.vaccine.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 33.Haynes LM, Moore DD, Kurt-Jones EA, Finberg RW, Anderson LJ, et al. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J Virol. 2001;75:10730–10737. doi: 10.1128/JVI.75.22.10730-10737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 35.Boelen A, Andeweg A, Kwakkel J, Lokhorst W, Bestebroer T, et al. Both immunisation with a formalin-inactivated respiratory syncytial virus (RSV) vaccine and a mock antigen vaccine induce severe lung pathology and a Th2 cytokine profile in RSV-challenged mice. Vaccine. 2000;19:982–991. doi: 10.1016/s0264-410x(00)00213-9. [DOI] [PubMed] [Google Scholar]
- 36.Schwarze J, Hamelmann E, Bradley KL, Takeda K, Gelfand EW. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J Clin Invest. 1997;100:226–233. doi: 10.1172/JCI119516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barends M, Van Oosten M, De Rond CG, Dormans JA, Osterhaus AD, et al. Timing of infection and prior immunization with respiratory syncytial virus (RSV) in RSV-enhanced allergic inflammation. J Infect Dis. 2004;189:1866–1872. doi: 10.1086/386341. [DOI] [PubMed] [Google Scholar]
- 38.Becker Y. Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy--a review. Virus Genes. 2006;33:235–252. doi: 10.1007/s11262-006-0064-x. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg HF, Dyer KD, Domachowske JB. Respiratory viruses and eosinophils: exploring the connections. Antiviral Res. 2009;83:1–9. doi: 10.1016/j.antiviral.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevens WW, Sun J, Castillo JP, Braciale TJ. Pulmonary eosinophilia is attenuated by early responding CD8(+) memory T cells in a murine model of RSV vaccine-enhanced disease. Viral Immunol. 2009;22:243–251. doi: 10.1089/vim.2009.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prince GA, Jenson AB, Hemming VG, Murphy BR, Walsh EE, et al. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactivated virus. J Virol. 1986;57:721–728. doi: 10.1128/jvi.57.3.721-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]