Abstract
Objective
Cognitive impairments are a feature of bipolar disorder (BD) and could be worsened by inflammatory cytokines. We determined whether (i) serum interleukin-1 receptor antagonist (IL-1RA) was increased in elderly BD subjects, (ii) whether IL-1RA was associated with worse neurocognitive function, and (iii) whether IL-1RA was associated with white matter integrity.
Methods
21 euthymic BD patients (65 +/− 9 years) with serum available for IL-1RA measures by enzyme-linked immunoassays were compared with 26 similarly aged control participants. Four factor analysis-derived z-scores and a global z-score were obtained from a battery of 21 neurocognitive tests. Diffusion Tensor Images were used to obtain fractional anisotropy (FA), and an Automated Labeling Pathway algorithm was used to obtain white matter hyperintensity (WMH) burden.
Results
IL-1RA was elevated in BD subjects compared to controls (439+/−326 pg/mL vs. 269+/−109 pg/mL; p=0.004). Moreover, IL-1RA was inversely correlated with three cognitive function factors and global cognition (r=−0.37; p=0.01). IL-1RA continued to correlate with global cognitive function even when co-varying for either IL-6 or brain-derived neurotrophic factor (BDNF). Although FA was lower in BD subjects (0.368 +/− 0.02 vs. 0.381 +/− 0.01; p=.02), IL-1RA was not associated with FA or WMH burden.
Conclusion
Elevated serum levels of IL-1RA in BD subjects, even during euthymic states, were associated with worse cognitive function. This association was not explained by co-occurring increases in IL-6, by decreased BDNF, nor by measures of white matter integrity. These cross-sectional findings support the possibility that the IL-1 family may contribute to cognitive impairments in BD.
Keywords: mood disorder, inflammation, cytokine, cognition, geriatric, white matter, diffusion tensor imaging, fractional anisotropy, white matter hyperintensities
INTRODUCTION
An association between bipolar disorder (BD) and cognitive dysfunction has been established in over seventy-five studies (Bearden et al., 2001, Arts et al., 2008, Robinson et al., 2006, Bora et al., 2010, Torres et al., 2007). These cognitive impairments are a major contributor to disability in BD, particularly with advancing age (Bearden et al., 2011, Bowie et al., 2010), and are distinct from normal age-related cognitive decline (Andreasen, 2010, Gildengers et al., 2012b, Gildengers et al., 2005). In fact, even with higher education and lower cardiovascular burden, BD patients have worse cognitive impairments than patients with major depression (Gildengers et al., 2012a). While it is now well-recognized that progressive cognitive impairments are a core feature of BD across mood states and start early in the disease (Mann-Wrobel et al., 2011, Adida et al., 2011, Malhi et al., 2007), their etiology has not been established (Savitz et al., 2005). Several possible etiologic pathways have been hypothesized including dysregulated dopaminergic and glutamatergic systems, glucocorticoid neurotoxicity, impaired neurotrophic support, mitochondrial dysfunction, and oxidative stress (Berk et al., 2010). In addition, there is a growing literature that inflammatory cytokines may be elevated in BD subjects (Drexhage et al., 2010, O’Brien et al., 2006, Kim et al., 2007, Ortiz-Domâinguez et al., 2007). It is thus plausible that elevated inflammatory cytokines contribute, in part, to the cognitive impairments in BD.
Even in young adults with BD, there is already evidence for elevated inflammatory cytokines (Goldstein et al., 2009). Cognitive impairments have been associated with inflammatory cytokines such as interleukin-6 (IL-6) in unipolar depression (Kuo et al., 2005, Chang et al., 2012, Grassi-Oliveira et al., 2011). Additionally, the interleukin-1 (IL-1) family has been implicated in mood disorders across a range of studies (Dantzer, 2001, Bluthe et al., 1999, Parnet et al., 2002, Zubareva et al., 2001, Lacosta et al., 1998, Lacosta et al., 2000, Bluthe et al., 1991, Larson, 2002, Larson and Dunn, 2001, Bluthe et al., 1997, Connor et al., 1998). This family includes IL-1α and IL-1β, two receptors (type I and type II), and an IL-1 receptor antagonist (IL-1RA) (Cartmell et al., 2001). IL-1β serum levels may be specifically associated with late-onset depressive episodes in older adults (Thomas et al., 2005, Diniz et al., 2010); and vulnerability for future mood disorder episodes may be predicted by pre-existing elevations in IL-1β and IL-1RA (Milaneschi et al., 2009) (van den Biggelaar et al., 2007). An IL-1β genetic polymorphism has been associated with age of onset of late-life major depression (Hwang et al., 2009). Moreover, the behavioral effects of inescapable shock in animals can be blocked by centrally administered IL-1RA (Maier et al., 1999). Similarly, social isolation can provoke depressive-like behaviors and decrease brain-derived neurotrophic factor (BDNF); and both these effects are blocked by injection of intra-hippocampal IL-1RA (Barrientos et al., 2003). Finally, mild cognitive impairment (MCI) has been associated with IL-1β (Trollor et al., 2010, Zhuang et al., 2012).
It is therefore plausible that the IL-1 family also could contribute to the cognitive impairments in BD. However, circulating levels of IL-1β (a localized mediator of inflammation) are typically beneath detection limits in the systemic blood (Burger et al., 2009), and thus serum IL-1β levels can be difficult to quantify accurately and to correlate with disease. On the other hand, IL-1RA is readily measured in the blood because it circulates at moderately high levels (Milaneschi et al., 2009) and crosses the blood-brain barrier (Gutierrez et al., 1994). We therefore sought to examine the cross-sectional relationship between serum IL-1RA and cognitive impairments in older adults with BD. We were specifically interested in two questions: (i) is serum IL-1RA elevated in BD vs. controls; (ii) and if so, is IL-1RA also correlated with cognitive dysfunction? Subsequently, we explored whether IL-1RA was additionally correlated with any measures of white matter integrity (inferred from fractional anisotropy and/or white matter hyperintensities), which could potentially explain its relationship with cognitive function. White matter changes have been associated with cognitive decline during aging (Madden et al., 2012, Lamar et al., 2010); and white matter changes may start early in the course of bipolar disorder (Lagopoulos et al., 2013, Emsell et al., 2013, Delaloye et al., 2011). For these analyses, we conducted an exploratory examination of cross-sectional data that was obtained as part of a larger project investigating the longitudinal course of cognitive function in older adults with BD. Although exploratory, we know of no prior studies that have co-examined cognition, inflammation, and neuroimaging in bipolar disorder.
METHODS
Subjects
We enrolled individuals with BD I or II from the outpatient clinics and inpatient units of Western Psychiatric Institute and Clinic as well as community referrals. All subjects provided written informed-consent, as required by the Institutional Review Board at the University of Pittsburgh, in accordance with the Helsinki Declaration of 1975, as revised in 1983. Diagnosis was established by the Structured Clinical Interview for Axis I DSM-IV Disorders (SCID-IV). Of these, 21 BD subjects had serum available for IL-1RA analysis. Comparator subjects (n=26) were individuals with no psychiatric or neurologic history selected to make the groups similar in age, education, gender, and cardiovascular burden. We recruited comparator subjects through health fairs, advertisements in local papers (Butters et al., 2004, Bhalla et al., 2009), and special efforts were directed at recruiting comparator subjects with general medical burden from primary care practices (Reynolds et al., 2011).
Inclusion criteria required age 50 years or older (although some cognitive changes are evident with aging even earlier in life) (Salthouse, 2009); clinical euthymia for four weeks preceding neurocognitive assessment with scores of 10 or less on the 17-item Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1967) and 10 or less on the Young Mania Rating Scale (YMRS) (Young et al., 1978), ability to comprehend and speak English fluently; corrected visual ability to read newspaper headlines, and hearing capacity adequate to respond to a raised conversational voice. Potential subjects were excluded if they had pre-existing history of dementia or neurologic disorder affecting the central nervous system (for example, Parkinson’s disease, traumatic brain injury, or multiple sclerosis); electro-convulsive therapy within the past six months; and substance abuse or dependence within the past twelve months.
Neurocognitive Assessment
The assessments, as previously described (Gildengers et al., 2012a, Gildengers et al., 2012b, Gildengers et al., 2007), consisted of 21 well-established and validated individual cognitive tests. These 21 tests measure multiple cognitive domains and therefore factor analysis was used to create four z-scores (Gildengers et al., 2012a, Gildengers et al., 2012b, Gildengers et al., 2007): (i) language, (ii) delayed memory, (iii) visuomotor ability, and (iv) information processing speed/executive function. A global z score was determined based on all 21 individual tests.
Neuroimaging
Brain imaging employed a Siemens 3 Tesla scanner and used the slice prescription method developed by Noll et al. (Noll et al., 1997). We obtained 3mm sections for (i) T1-weighted, (ii) fast spin-echo T2-weighted, (iii) fast spin-echo proton density-weighted, and (iv) T2-weighted interleaved fast Fluid-Attenuated Inversion Recovery (FLAIR). A volumetric Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence was performed in the coronal plane for morphometric analyses of 176 continuous slices.
We utilized the Automated Labeling Pathway (ALP) as previously described (Rej et al., 2013) to quantify regional gray and white brain volumes. ALP uses a fuzzy connected algorithm to segment white matter hyperintensities (WMH) (Wu et al., 2006) and to localize the WMH into the anatomical space (Wu et al., 2007). This method generates total WMH volumes, as well as WMH volumes for each frontal and subcortical white matter tract (Mori and Crain, 2005). Finally, Diffusion Weighted Images (DTI) were acquired using single-short spin-echo sequence with the following parameters: TR = 5300 ms; TE = 88 ms; TI = 2500 ms; flip angle=90; FOV=256*256mm; two diffusion values of b=0 and 1000 s/mm; 12 diffusion directions; four repeats; 40 slices; matrix size=128*128; voxel size=2 mm*2 mm; slice thickness=3 mm; and GRAPPA = 2. Fractional anisotropy (FA) maps used tract-based spatial statistics (Smith et al., 2006) to generate voxel-wise cross-registered skeletonized maps in MNI (Montreal Neurological Institute) space, and used ALP and WMH localization to generate white matter tract-specific summary scores for normal appearing white matter (i.e., excluding WMH), as previously described (Wu et al., 2007) (Rosano et al., 2007).
Cytokine assessments
Serum samples were stored in −80C freezers prior to examination. A high sensitivity and specific enzyme immunoassay (R&D Systems, Minneapolis, MN) was used to measure IL-1RA. All samples were run in duplicate. The average intra-assay and inter-assay coefficients of variation for IL-1RA were 5% and 8%, and sensitivity was 6.2 pg/mL. IL-6 was assessed using a similar assay (Diaclone, Besancon, France) as previously described (Prather et al., 2009), as was TNF-α (Alpco, Salem, NH) (Lotrich et al., 2010) and BDNF (R&D Systems, Minneapolis, MN) (Lotrich et al., 2013).
Statistical Analysis
Descriptive statistics (mean +/− standard deviation or %n) were used to describe the BD subjects and control participants. Continuous measures of the groups were compared using a t-test, or a Wilcoxon exact test when variables were non-normal and unable to be normalized with a transformation. Categorical variables were compared using a Chi-square test or when the expected count was small, a Fisher’s Exact test. Spearman correlations were then used to compare IL-1RA levels and other variables. Variables that were correlated with IL-1RA (p≤0.10) were considered for linear regression; potential meditational relationships were explored in multiple linear regressions considering both individual and combined contributions of the predictors. Power calculations were performed in SAS on the correlation between IL-1RA and white matter integrity.
RESULTS
Consistent with the literature, BD subjects performed notably worse than older adult control subjects across all cognitive domains (Table 1). Their white matter also had significantly lower fractional anisotropy (FA), though the WMH burden was similar to controls. Despite the slightly higher general medical burden, BD and control subjects had comparable levels of vascular disease. BD subjects were generally euthymic, but they did have mild residual symptoms of mania (YMRS) and depression (HRSD) (see Table 1).
Table 1.
| Control subjects Mean (SD) |
Bipolar subjects Mean (SD) |
Bipolar vs. controls | |
|---|---|---|---|
| Age | 65.5 (8.4) | 64.8 (9.1) | Wilcoxon Test p=0.51 |
| % Female | 53.9% | 61.9%) | χ2=0.31, p=0.58 |
| Race: | |||
| %White | 84.6% | 90.5% | Fisher’s Exact p=0.19 |
| %Black | 11.5% | 9.52% | |
| %Asian Pacific | 3.9% | 0% | |
| Education | 15.8 (2.7) | 15.0 (2.4) | t(45)= −1.0, p=0.32 |
| CIRS-G Total | 6.08 (3.9) | 8.24 (2.8) | t(45)=2.1, p=0.04 |
| CIRS-G Count | 3.96 (2.4) | 5.43 (1.8) | t(45)=2.3, p=0.02 |
| Framingham Risk Profile | 0.090 (0.09) | 0.098 (0.08) | Wilcoxon Test p=0.38 |
| Body Mass Index | 28.2 (5.8) | 29.5 (6.1) | t(45)=0.7, p=0.47 |
| Young Mania Scale | 0.500 (0.76) | 2.62 (2.0) | Wilcoxon Test p<0.0001 |
| 17-item Hamilton Scale | 1.62 (1.5) | 4.48 (2.5) | Wilcoxon Test p<0.0001 |
| Cognitive z scores | |||
| Global | 0.124 (0.44) | −0.787 (1.3) | Wilcoxon Test p<0.0001 |
| Visual | 0.056 (0.49) | −0.600 (0.92) | Wilcoxon Test p=0.006 |
| Memory | 0.221 (0.67) | −0.730 (0.90) | t(45)= −4.2, p=0.0001 |
| Language | 0.182 (0.81) | −0.454 (0.73) | t(45)= −2.8, p=0.008 |
| Speed/Executive | 0.007 (0.47) | −1.30 (3.4) | Wilcoxon Test p=0.002 |
| Labs | |||
| Eotaxin | 152.2 (37.7) | 166.2 (56.3) | t(45)=1.0, p=0.31 |
| BDNF | 21.6 (7.1) [n=25] | 21.3 (5.4) | t(44)= −0.17, p=0.87 |
| TNF-alpha | 4.41 (1.8) | 5.23 (1.9) | t(45)=1.5, p=0.15 |
| IL-10 | 1.12 (1.5) | 1.37 (2.4) | Wilcoxon Test p=0.84 |
| IL-6 | 1.50 (1.3) | 2.19 (1.7) [n=18] | Wilcoxon Test p=0.15 |
| Imaging | |||
| Whole brain gray volume | 34.5 (3.5) [n=20] | 32.0 (3.6) [n=13] | t(31)= −2.0, p=0.06 |
| Hippocampus volume | 0.613 (0.08) [n=20] | 0.581 (0.09) [n=13] | t(31)= −1.1, p=0.27 |
| Whole Brain Mean FA | 0.381 (0.01) [n=18] | 0.368 (0.02) [n=12] | t(28)= −2.5, p=0.02 |
| WMH volume | 0.0008 (0.001) [n=19] | 0.001 (0.002) [n=12] | Wilcoxon Test p=0.46 |
Comparison of control subjects (n=26) and bipolar subjects (n=21) on demographics, mood symptoms, cognition, cytokines levels (brain-derived neurotrophic factor, tumor necrosis factor-alpha, interleukin-10, interleukin-6), and structural neuroimaging (fractional anisotropy and white matter hyperintensity burden). Smaller ‘n’ are included in brackets.
I. Is IL-1RA increased in elderly BD subjects?
Supporting our first hypothesis, IL-1RA was elevated in BD subjects compared to age-matched controls (439+/−326 pg/mL vs. 269+/−109 pg/mL; t(45)= −3.0, p=0.004). There was one BD subject with extremely elevated IL-1RA levels (1713 pg/mL; confirmed with dilution) that was accounted for in the analysis using log transformed values.
IL-1RA is an acute phase cytokine related to systemic inflammation. IL-1RA was accordingly also associated with increased IL-6 levels (rho=0.48, n=44, p=0.001) as well as lower BDNF levels (rho=−0.32, n=46, p=0.03), consistent with prior research in BD subjects (Goldstein et al., 2011). Despite these correlations, BD subjects and controls had similar BDNF and IL-6 levels (Table 1). It is therefore unlikely that BDNF or IL-6 could account for the relationship between IL-1RA and BD diagnosis. We further tested this by co-varying for BD diagnosis, BDNF, and IL-6 in the same analysis of IL-1RA. All three continue to be independently associated with IL-1RA (B=0.38 (s.e.=0.13), p=0.007, B=−0.02 (s.e.=0.01), p=0.03, B=0.13 (s.e.=0.05), p=0.007, respectively; and F(3,39)=8.54, p=0.0002, R2=0.40). Thus, there was no support for the possibility that either increased IL-6 or decreased BDNF accounted for the increased IL-1RA that we observed in BD.
II. Is IL-1RA associated with worse cognitive function?
IL-1RA was associated with worse performance across three cognitive domains that were defined by factor analysis (Table 2) as well as the global measure of cognitive function (Figure 1). Although the strongest finding was for a factor that loaded for processing speed/executive function/cognitive control tests, even the weakest non-significant correlation was a similar trend (p=0.08). Thus the global cognition score was selected for further analyses. Because BD was associated with both worse global cognitive function and elevated IL-1RA levels, it could be argued that the association between IL-1RA and cognitive deficits was simply because of diagnosis. However when both IL-1RA and BD diagnosis are co-included as predictors of global cognitive function, both remain associated with worse cognition (B=−0.57 (s.e.=0.28), p=0.048 and B=−0.67 (s.e.=0.29), p=0.025; respectively). Thus IL-1RA is associated with worse cognition, even when co-varying for BD diagnosis. Likewise, IL-1RA continued to be correlated with global cognitive impairments without covariates (B=−0.21 (s.e.=0.07), p=0.003) and also when co-varying for IL-6 (B=−0.31 (s.e=0.11), p=0.008) -- indicating that generalized systemic inflammation does not account the association between IL-1RA and cognition. And IL-1RA continued to be correlated with global cognitive impairments (B=−0.18 (s.e=0.07), p=0.01) when co-varying for BDNF -- indicating that decreased BDNF does not account the association. IL-1RA also correlated with Body Mass Index (BMI), but correlated strongly with cognitive impairments (p=0.001), when co-varying for BMI.
Table 2.
| Age | −0.102 (p=0.50) |
| Female | 0.165 (p=0.27) |
| Race | −0.159 (p=0.29) |
| Education | 0.091 (p=0.54) |
| CIRS-total | 0.285 (p=0.05) |
| CIRS-count | 0.272 (p=0.06) |
| YMRS Total | 0.410 (p=0.004) |
| HRSD (17 Items) | 0.150 (p=0.32) |
|
| |
| Global cognition Z score | −0.372 (p=0.01) |
| Visual cognition Z score | −0.312 (p=0.03) |
| Memory cognition Z score | −0.345 (p=0.02) |
| Language cognition Z score | −0.261 (p=0.08) |
| Speed/executive cognition Z score | −0.404 (p=0.005) |
|
| |
| Framingham Risk Profile | 0.097 (p=0.52) |
| Body Mass Index | 0.39 (p=0.007) |
| Eotaxin | −0.037 (p=0.80) |
| BDNF [n=46] | −0.320 (p=0.03) |
| TNF-alpha | 0.19 (p=0.22) |
| IL-10 | 0.008 (p=0.96) |
| IL-6 [n=44] | 0.48 (p=0.001) |
| Magnetic Resonance Imaging Measures | |
| Gray matter volume [n=33] | −0.226 (p=0.21) |
| Hippocampus volume [n=33] | −0.268 (p=0.13) |
| Whole Brain Mean FA [n=30] | −0.195 (p=0.30) |
| L anterior thalamic radiation FA | −0.223 (p=0.24) |
| R anterior thalamic radiation FA | −0.304 (p=0.10) |
| Corpus callosum FA | −0.253 (p=0.18) |
| L upper cingulate FA | −0.205 (p=0.28) |
| R upper cingulate FA | −0.279 (p=0.14) |
| L superior longitudinal fasciculus FA | −0.143 (p=0.45) |
| R superior longitudinal fasciculus FA | −0.194 (p=0.30) |
| Whole Brain WMH burden [n=32] | −0.112 (p=0.54) |
| L anterior thalamic radiation WMH | −0.109 (p=0.55) |
| R anterior thalamic radiation WMH | −0.126 (p=0.49) |
| Corpus callosum WMH | −0.246 (p=0.17) |
| L upper cingulate WMH | 0.098 (p=0.60) |
| R upper cingulate WMH | 0.163 (p=0.37) |
| L superior longitudinal fasciculus WMH | −0.039 (p=0.83) |
| R superior longitudinal fasciculus WMH | 0.065 (p=0.72) |
| Total WMH volume | −0.063 (p=0.74) |
Spearman Correlation Coefficients with IL-1RA (n=47 unless otherwise indicated in brackets).
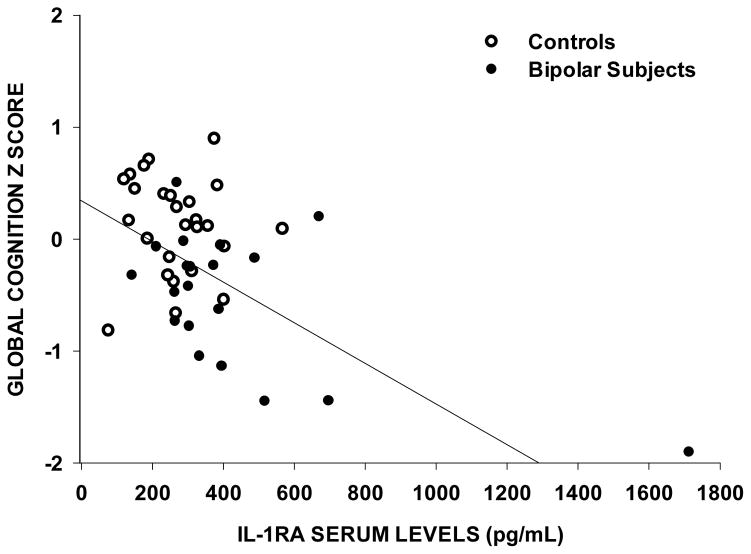
Figure 1.
Increased serum levels of interleukin-1 receptor antagonists (IL-1RA) as associate with worsening global cognition (r=−0.37, n=47, p=0.01). Cognitive z scores are obtained from 21 individual cognitive tests.
III. Is IL-1RA associated with white matter integrity?
IL-1RA was not associated with any measure of WMH burden either in the whole brain or in specific white matter tracts (Table 2). IL-1RA was also not associated with FA in any of the regions that we examined (Table 2). These findings therefore do not support the possibility that white matter integrity could account for the relationship between IL-RA and cognitive impairment. Nonetheless, there was a slight trend for IL-1RA being associated with decreased FA in the right anterior thalamic radiation (r = −0.30, n=30, p=0.10) and right upper cingulate (r= −0.28, n=30, p=0.14). Neither reached statistical significance (power=38% and 34%, respectively). Thus it is unlikely, but not definitively disproven, that decreased white matter integrity is the explanation for the association between IL-1RA and cognitive function.
DISCUSSION
We found that circulating IL-1RA levels were elevated in older adults with BD compared to similarly aged mentally healthy individuals; and this increased IL-1RA was associated with worse cognitive function across a variety of domains. This set of cross-sectional findings is consistent with the possibility that the IL-1 family of cytokines contributes to cognitive impairments in BD patients.
We found no evidence that the relationship between IL-RA and cognitive function in BD subjects could be accounted for by generalized increases in other inflammatory cytokines. For example, the correlation between IL-RA and cognitive function continued to be present even when co-varying for IL-6. Likewise, lower serum BDNF levels, which can be associated with increased inflammatory cytokines in BD (Goldstein et al., 2011), did not account for the correlation between increased IL-1RA and worsening cognitive function. Thus, it is plausible that the IL-1 family, specifically, may play a key role in cognitive impairments in chronic mood disorders. And although there is accumulating evidence that several inflammatory cytokines are increased in BD (Drexhage et al., 2010, O’Brien et al., 2006, Kim et al., 2007, Ortiz-Domâinguez et al., 2007), we did not observe a significant increase in other cytokines such as IL-6 or TNF-α in these euthymic BD subjects. Likewise, and consistent with our findings in these euthymic subjects, BDNF may be associated with episodes of mania and depression but returns to normal during euthymic states (Kapczinski et al., 2008, Fernandes et al., 2011). Therefore, we did not further explore the role of other inflammatory cytokines or BDNF in the residual cognitive impairments that exist in these euthymic individuals with BD.
Increased mania scores also could not account for the correlation between IL-1RA and cognitive dysfunction. We did observe that IL-1RA was positively associated with mania, congruent with prior studies (Liu et al., 2004, Kim et al., 2007, Hope et al., 2011, Tsai et al., 2012). But by design, BD subjects were not in a manic episode, with average YMRS score of only 2.6.
Consistent with demonstrations that the IL-1 family are critical cytokines in neuroinflammatory processes (Basu et al., 2004), IL-1β has been previously associated with mild cognitive impairment (MCI) (Trollor et al., 2010), and an IL-1β polymorphism has been associated with MCI (Zhuang et al., 2012). Most animal studies also indicate that IL-1β, which is produced and released by astrocytes (Yazdi et al., 2010), adversely affects learning and memory (Huang and Sheng, 2010). Thus, although our findings are not evidence of a causal relationship between IL-1RA and cognitive function, there does appear to be support for this possibility. IL-1β may contribute to white matter repair following injury (Sato et al., 2012, Camara-Lemarroy et al., 2010, Herx et al., 2000, Allan and Rothwell, 2003), and neuronal IL-1β may actually have protective effects in Parkinson’s Disease (Parish et al., 2002). Conversely, IL-1β can be a critical mediator of tissue damage in demyelinating conditions such as multiple sclerosis, encephalomyelitis, and traumatic brain injury (Arend, 2002, Li et al., 2011, Zindler and Zipp, 2010, Allan and Rothwell, 2003) (Ferrari et al., 2004).
Given these potential dual roles for IL-1β, the specific role of IL-1RA in the brain is not clear. Increases in IL-1RA may prevent the adverse cognitive effects of IL-1β (Corbett et al., 2012), as well as block IL1β’s depressogenic effects (Maier et al., 1999) (Barrientos et al., 2003). IL1RA also mitigates the behavioral effects of peripheral inflammatory injections (Frank et al., 2012, Bluthe et al., 1992), which can have pronounced consequences in aged mice (Abraham and Johnson, 2009). Similarly, surgery in aged rats adversely affects hippocampal-dependent memory, an effect that can be blocked by central administration of IL-1RA (Barrientos et al., 2012). Despite these findings that IL-1RA can mitigate the adverse effects of IL1β, there is also evidence that IL-1RA may worsen cognition. For example, intracerebral injection of IL-1RA can worsen learning and memory in a variety of animal models (Yirmiya et al., 2002, Goshen et al., 2007). Blocking the IL-1 receptor through genetic knockouts also produces memory deficits (Avital et al., 2003, Goshen et al., 2007).
Thus, (i) it is possible that IL-1RA is increased in response to elevated inflammation, but that the increase is not sufficient to block the cognitive impairing effects of IL-1β; or (ii) the elevated IL-1RA is causing the impairment by blocking the possible beneficial role that normal physiological IL-1β plays in cognition and neuronal growth (Huang and Sheng, 2010, Parish et al., 2002, Sato et al., 2012, Camara-Lemarroy et al., 2010); or (iii) elevated peripheral IL-1RA could be associated with lower central IL-1RA production. For example, there can be decreased IL-1RA production by macrophages in the central nervous system of Alzheimer’s disease (Tarkowski et al., 2001) even when peripheral IL-1RA production is normal.
Interestingly, we found that IL-1RA was not associated with any measure of white matter integrity – either hyperintensity burden or fractional anisotropy. Thus, with the caveat that moderately low power (<40%) was a limitation, it is unlikely that white matter integrity mediated the association with cognitive impairment. It is also plausible that differences in white matter between BD patients and controls begin much earlier in life and are more related to the mood dysregulation than to cognitive decline with aging. Many white matter changes can be observed early in the course of BD (Lagopoulos et al., 2013, Emsell et al., 2013, Delaloye et al., 2011). In fact, some white matter tracts may have more fractional anisotropy in more severe pediatric-onset BD patients than in those that develop BD later in life (Lu et al., 2012). This cross-sectional study is unable to differentiate white matter changes that could have started early in life from those that worsen with age.
However, there are other potential mechanisms for plausible effects of the IL-1 family on cognitive function. IL-1β can drive neuroinflammatory events through both MAP kinase and proteosome pathways (Thornton et al., 2010), and this may be related to its ability to produce deficits in long term potentiation in the hippocampus (Imamura et al., 2011). Additionally, IL-1β behavioral effects may be mediated through the endocannibinoid system (Rossi et al., 2012). Moreover, IL-1β can increase the serotonin catabolite, 5-HIAAA, throughout the brain [235,236], regulate reuptake of serotonin by the synaptic transporter (Ramamoorty et al., 1995), as well as induce dopamine turnover in the hippocampus and hypothalamus (Connor et al., 1998) (Lacosta et al., 1998). And oxidative stress might further activate microglia-produced inflammation (Grande et al., 2012). Additionally, elevated glucocorticoids may worsen this ongoing neuroinflammatory response by rendering neurons less capable of removing synaptic glutamate and stopping free radical formation -- ultimately resulting in premature cell death (Sorrells et al., 2009). Finally, local IL-1β can exacerbate lowered BDNF signaling through overlapping and interacting intracellular second-messenger pathways (Tong et al., 2008). These various mechanistic explanations for the association of the IL-1 family with cognitive impairments are speculative at this point.
One limitation of the study is that we are unable to determine the source of the IL-1RA. IL-1RA production in hepatocytes is stimulated by IL-1β and interferon (Petrasek et al., 2011 ), inflammation stimulates myeloid cells production of IL-1RA (Lamacchia et al., 2010 ), and IL-1RA can arise from macrophages induced by IL-6 (Tilg et al., 1994). A second limitation is that this cross-sectional correlational study is unable to determine whether IL-1RA is causally related to either BD or cognitive deficits. We merely report associations. Thirdly, circulating levels of IL-1β are typically too low to be reliably detected (Burger et al., 2009), and thus we can’t address whether levels of local IL-1β in the brain are associated with cognitive function or not.
Potential effects of administering IL-1 related medications in BD patients to mitigate cognitive dysfunction are also speculative. IL-1 receptor antagonists have been proposed as a possible treatment for neurological disorders (Vezzani et al., 2010), and attenuation of central IL-1β activity has been one suggested approach for treating MDD (Koo and Duman, 2009). Blocking IL-1 with a commercially approved IL-1RA that can enter the CNS (Galea et al., 2011, Cawthorne et al., 2011) may decrease the fatigue seen in arthritis (Omdal and Gunnarsson, 2005) and Sjogren’s syndrome (Norheim et al., 2012). But clearly, additional studies are needed to better define the physiological role of IL-1RA in mood disorders.
In summary, this study provides evidence that the inflammatory cytokine IL-1RA is associated with the cognitive deficits seen in older adults with BD. Neither changes in white matter nor serum markers such as IL-6 and BDNF appeared to account for this association. Although consistent with pre-clinical evidence that the IL-1 family can affect brain function and cognition, our correlational findings are not evidence of causation. Also, IL-1RA only explained part of the relationship between BD and cognitive dysfunction. Cognitive impairments are very likely multi-factorial. Regardless, further delineating the specific role of IL-1RA in BD, with specific attention to the neuroprogressive cognitive impairments, will be an important direction for future study.
Acknowledgments
Sponsored by NIH grants R01MH090250 (FL), R01MH084921 (AG), R01080240 (MB), P30MH90333 (CR) and the UPMC Endowment in Geriatric Psychiatry (CR)
Contributor Information
Dr. Francis E. Lotrich, Email: lotrichfe@upmc.edu, 3811 O’Hara Street, Pittsburgh, PA 15213, USA. Phone 412-246-6267; Fax 412-246-6260.
Dr. Meryl A. Butters, Email: buttersma@upmc.edu, 3811 O’Hara Street, Pittsburgh, PA 15213, USA. Phone 412-246-5280
Dr. Howard Aizenstein, Email: aizensteinhj@upmc.edu, 3811 O’Hara Street, Pittsburgh, PA 15213, USA. Phone 412-246-5465
Ms. Megan M. Marron, Email: marronmm@upmc.edu, 3811 O’Hara Street, Pittsburgh, PA 15213, USA. Phone 412-246-6442
Dr. Charles F. Reynolds, III, Email: reynoldscf@upmc.edu, 3811 O’Hara Street, Pittsburgh, PA 15213, USA. Phone 412-246-6414
Dr. Ariel G. Gildengers, Email: arielg@pitt.edu, 3811 O’Hara Street, Pittsburgh, PA 15213, USA. Phone 412-246-6002.
References
- Abraham J, Johnson R. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Br Behav Immunol. 2009;23:396–410. doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adida M, Jollant F, Clark L, Besnier N, Guillaume S, Kaladjian A, Mazzola-Pomietto P, Jeanningros R, Goodwin GM, Azorin JM, Courtet P. Trait-related decision-making impairment in the three phases of bipolar disorder. Biol Psychiatry. 2011;70:357–65. doi: 10.1016/j.biopsych.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Allan SM, Rothwell NJ. Inflammation in central nervous system injury. Philosophical Transactions of the Royal Society of London - Series B: Biol Sci. 2003;358:1669–1677. doi: 10.1098/rstb.2003.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. The lifetime trajectory of schizophrenia and the concept of neurodevelopment. Dialogues Clin Neurosci. 2010;12:409–15. doi: 10.31887/DCNS.2010.12.3/nandreasen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Gr Factor Rev. 2002;13:323–40. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- Arts B, Jabben N, Krabbendam L, van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychol Med. 2008;38:771–85. doi: 10.1017/S0033291707001675. [DOI] [PubMed] [Google Scholar]
- Avital AGI, Kamsler A, Segal M, Iverfeldt K, Richter-Levin G, et al. Impaired interleukin-1 signallng is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus. 2003;13:826–834. doi: 10.1002/hipo.10135. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Hein AM, Frank MG, Watkins LR, Maier SF. Intracisternal interleukin-1 receptor antagonist prevents postoperative cognitive decline and neuroinflammatory response in aged rats. J Neurosci. 2012;32:14641–14648. doi: 10.1523/JNEUROSCI.2173-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Sprunger DB, Campeau S, Higgins EA, Watkins LR, Rudy JW, Maier SF. Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neurosci. 2003;121:847–853. doi: 10.1016/s0306-4522(03)00564-5. [DOI] [PubMed] [Google Scholar]
- Basu A, Krady JK, Levison SW. Interleukin-1: a master regulator of neuroinflammation. J Neurosci Res. 2004;78:151–156. doi: 10.1002/jnr.20266. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Hoffman KM, Cannon TD. The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar Dis. 2001;3:106–50. doi: 10.1034/j.1399-5618.2001.030302.x. discussion 151–3. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Shih VH, Green MF, Gitlin M, Sokolski KN, Levander E, Marusak S, Hammen C, Sugar CA, Altshuler LL. The impact of neurocognitive impairment on occupational recovery of clinically stable patients with bipolar disorder: a prospective study. Bipolar Disord. 2011;13:323–33. doi: 10.1111/j.1399-5618.2011.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Conus P, Kapczinski F, Andreazza AC, Yucel M, Wood SJ, Pantelis C, Malhi GS, Dodd S, Bechdolf A, Amminger GP, Hickie IB, McGorry PD. From neuroprogression to neuroprotection: implications for clinical care. Med J Aust. 2010;193:S36–40. [PubMed] [Google Scholar]
- Bhalla RK, Butters MA, Becker JT, Houck PR, Snitz BE, Lopez OL, Aizenstein HJ, Raina KD, DeKosky ST, Reynolds CF., III Patterns of Mild Cognitive Impairment After Treatment of Depression in the Elderly. Am J Geriatr Psych. 2009;17:308–316. doi: 10.1097/JGP.0b013e318190b8d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthe R-M, Castanon N, Pousset F, Bristow A, Ball C, Lestage J, Michaud B, Kelley KW, Dantzer R. Central injection of IL-10 antagonizes the behavioural effects of lipopolysaccharide in rats. Psychoneuroendocrinol. 1999;24:301–311. doi: 10.1016/s0306-4530(98)00077-8. [DOI] [PubMed] [Google Scholar]
- Bluthe R-M, Dantzer R, Kelley KW. Interleukin-1 mediates behavioural but not metabolic effects of tumor necrosis factor a in mice. Eur J Pharmacol. 1991;209:281–283. doi: 10.1016/0014-2999(91)90184-r. [DOI] [PubMed] [Google Scholar]
- Bluthe R-M, Dantzer R, Kelly KW. Central mediation of the effects of interleukin-1 on social exploration and body weight in mice. Psychoneuroendocrinol. 1997;22:1–11. doi: 10.1016/s0306-4530(96)00042-x. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Dantzer R, Kelley KW. Effects of interleukin-1 receptor antagonist on the behavioral effects of lipopolysaccharide in rat. Br Res. 1992;573:318–320. doi: 10.1016/0006-8993(92)90779-9. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Cognitive Impairment in Affective Psychoses: A Meta-analysis. Schizophr Bull. 2010;36:112–125. doi: 10.1093/schbul/sbp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, Depp C, McGrath JA, Wolyniec P, Mausbach BT, Thornquist MH, Luke J, Patterson TL, Harvey PD, Pulver AE. Prediction of real-world functional disability in chronic mental disorders: a comparison of schizophrenia and bipolar disorder. Am J Psychiatry. 2010;167:1116–24. doi: 10.1176/appi.ajp.2010.09101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger D, Molnarfi N, Weber MS, Brandt KJ, Benkhoucha M, Gruaz L, Chofflon M, Zamvil SS, Lalive PH. Glatiramer acetate increases IL-1 receptor antagonist but decreases T cell-induced IL-1beta in human monocytes and multiple sclerosis. PNAS. 2009;106:4355–4359. doi: 10.1073/pnas.0812183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, Zmuda MD, Bhalla R, Meltzer CC, Pollock BG, Reynolds CF, III, Becker JT. The Nature and Determinants of Neuropsychological Functioning in Late-Life Depression. Arch Gen Psychiatry. 2004;61:587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- Camara-Lemarroy CR, Guzman-de la Garza FJ, Fernandez-Garza NE. Molecular inflammatory mediators in peripheral nerve degeneration and regeneration. Neuroimmunomodulation. 2010;17:314–324. doi: 10.1159/000292020. [DOI] [PubMed] [Google Scholar]
- Cartmell T, Luheshi GN, Hopkins SJ, Rothwell NJ, Poole S. Role of endogenous interleukin-1 receptor antagonist in regulating fever induced by localised inflammation in the rat. J Physiol. 2001;531:171–180. doi: 10.1111/j.1469-7793.2001.0171j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthorne C, Prenant C, Smigova A, Julyan P, Maroy R, Herholz K, Rothwell N, Boutin H. Biodistribution, pharmacokinetics and metabolism of interleukin-1 receptor antagonist (IL-1RA) using [(1)(8)F]-IL1RA and PET imaging in rats. Br J Pharmacol. 2011;162:659–672. doi: 10.1111/j.1476-5381.2010.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Lee I, Gean P, Lee SY, Chi M, Yang Y. Treatment response and cognitive impairment in major depression: Association with C-reactive protein. Br Behav Immunol. 2012;26:90–95. doi: 10.1016/j.bbi.2011.07.239. [DOI] [PubMed] [Google Scholar]
- Connor TJ, Song C, Leonard BE, Merali Z, Anisman H. An assessment of the effects of central interleukin-1b, -2, -6, and tumor necrosis factor-a administration on some behavioural, neurochemical, endocrine and immune parameters in the rat. Neurosci. 1998;84:923–933. doi: 10.1016/s0306-4522(97)00533-2. [DOI] [PubMed] [Google Scholar]
- Corbett GT, Roy A, Pahan K. Gemfibrozil, a lipid-lowering drug, upregulates IL-1 receptor antagonist in mouse cortical neurons: implications for neuronal self-defense. J Immunol. 2012:2. doi: 10.4049/jimmunol.1102624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: where do we stand? Br Behav Immunol. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- Delaloye C, Moy G, de Bilbao F, Weber K, Baudois S, Haller S, Xekardaki A, Canuto A, Giardini U, Lovblad KO, Gold G, Giannakopoulos P. Longitudinal analysis of cognitive performances and structural brain changes in late-life bipolar disorder. Int J Geriatr Psychiatry. 2011;26:1309–1318. doi: 10.1002/gps.2683. [DOI] [PubMed] [Google Scholar]
- Diniz BS, Teixeira AL, Talib L, Gattaz WF, Forlenza OV. Interleukin-1beta serum levels is increased in antidepressant-free elderly depressed patients. Am J Geriatr Psychiatry. 2010;18:172–176. doi: 10.1097/JGP.0b013e3181c2947f. [DOI] [PubMed] [Google Scholar]
- Drexhage R, Knijff E, Padmos R, Heul-Nieuwenhuijzen L, Beumer W, Versnel M, et al. The mononuclear phagocyte system and its cytokine inflammatory networks in schizophrenia and bipolar disorder. Exp Rev Neurotherapeut. 2010;10:59–76. doi: 10.1586/ern.09.144. [DOI] [PubMed] [Google Scholar]
- Emsell L, Leemans A, Langan C, Van Hecke W, Barker GJ, McCarthy P, Jeurissen B, Sijbers J, Sunaert S, Cannon DM, McDonald C. Limbic and callosal white matter changes in euthymic bipolar I disorder: an advanced diffusion magnetic resonance imaging tractography study. Biol Psychiatry. 2013;73:194–201. doi: 10.1016/j.biopsych.2012.09.023. [DOI] [PubMed] [Google Scholar]
- Fernandes BS, Gama CS, Cereser KM, Yatham LN, Fries GR, Colpo G, de Lucena D, Kunz M, Gomes FA, Kapczinski F. Brain-derived neurotrophic factor as a state-marker of mood episodes in bipolar disorders: a systematic review and meta-regression analysis. J Psychiatric Res. 2011;45:995–1004. doi: 10.1016/j.jpsychires.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Ferrari CC, Depino AM, Prada F, Muraro N, Campbell S, Podhajcer O, Perry VH, Anthony DC, Pitossi FJ. Reversible demyelination, blood-brain barrier breakdown, and pronounced neutrophil recruitment induced by chronic IL-1 expression in the brain. Am J Path. 2004;165:1827–1837. doi: 10.1016/S0002-9440(10)63438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Thompson BM, Weber MD, Watkins LR, Maier SF. IL-1RA injected intra-cisterna magna confers extended prophylaxis against lipopolysaccharide-induced neuroinflammatory and sickness responses. J Neuroimmunol. 2012;252:33–39. doi: 10.1016/j.jneuroim.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea J, Ogungbenro K, Hulme S, Greenhalgh A, Aarons L, Scarth S, Hutchinson P, Grainger S, King A, Hopkins SJ, Rothwell N, Tyrrell P. Intravenous anakinra can achieve experimentally effective concentrations in the central nervous system within a therapeutic time window: results of a dose-ranging study. J Cer Blood Flow Metab. 2011;31:439–447. doi: 10.1038/jcbfm.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildengers AG, Butters MA, Chisholm D, et al. Cognitive functioning and instrumental activities of daily living in late-life bipolar disorde. Am J Geriatr Psychiatry. 2007;15:174–179. doi: 10.1097/JGP.0b013e31802dd367. [DOI] [PubMed] [Google Scholar]
- Gildengers AG, Butters MA, Chisholm D, Anderson SJ, Begley A, Holm M, Rogers JC, Reynolds CFr, Mulsant BH. Cognition in older adults with bipolar disorder versus major depressive disorder. Bipolar Dis. 2012a;14:198–205. doi: 10.1111/j.1399-5618.2012.00995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildengers AG, Chisholm D, Butters MA, Anderson SJ, Begley A, Holm M, Rogers JC, Reynolds CF, Mulsant BH. Two-year course of cognitive function and instrumental activities of daily living in older adults with bipolar disorder: evidence for neuroprogression? Psychol Med. 2012b:1–11. doi: 10.1017/S0033291712001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildengers AG, Houck PR, Mulsant BH, Dew MA, Aizenstein HJ, Jones BL, Greenhouse J, Pollock BG, Reynolds CFr. Trajectories of treatment response in late-life depression: psychosocial and clinical correlates. J Clin Psychopharmacol. 2005;25:S8–S13. doi: 10.1097/01.jcp.0000161498.81137.12. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Collinger KA, Lotrich FE, Marsland AL, Gill M-K, Axelson DA, Birmaher B. Preliminary Findings Regarding Pro-Inflammatory Markers and Brain-Derived Neurotrophic Factor among Adolescents with Bipolar Spectrum Disorders. J Child Adol Psychopharmacol. 2011;21:479–484. doi: 10.1089/cap.2011.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI, Kemp DE, Soczynska JK, McIntyre RS. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. J Clin Psychiatry. 2009;70:1078–90. doi: 10.4088/JCP.08r04505. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, et al. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinol. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Grande I, Magalhaes PV, Kunz M, Vieta E, Kapczinski F. Mediators of allostasis and systemic toxicity in bipolar disorder. Physiol Behav. 2012;106:46–50. doi: 10.1016/j.physbeh.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Grassi-Oliveira R, Bauer ME, Pezzi J, Teixeira A, Brietzke E. Interleukin-6 and verbal memory in recurrent major depressive disorder. Neuro Endocrinol Lett. 2011;32:540–544. [PubMed] [Google Scholar]
- Gutierrez EG, Banks WA, Kastin AJ. Blood-borne interleukin-1 receptor antagonist crosses the blood-brain barrier. J Neuroimmunol. 1994;55:153–160. doi: 10.1016/0165-5728(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc & Clin Psychol. 1967;6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Herx LM, Rivest S, Yong VW. Central nervous system-initiated inflammation and neurotrophism in trauma: IL-1 beta is required for the production of ciliary neurotrophic factor. J Immunol. 2000;165:2232–2239. doi: 10.4049/jimmunol.165.4.2232. [DOI] [PubMed] [Google Scholar]
- Hope S, Dieset I, Agartz I, Steen NE, Ueland T, Melle I, Aukrust P, Andreassen OA. Affective symptoms are associated with markers of inflammation and immune activation in bipolar disorders but not in schizophrenia. J Psychiatr Res. 2011;45:1608–1616. doi: 10.1016/j.jpsychires.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Huang Z-B, Sheng GQ. Interleukin-1 with learning and memory. Neurosc Bull. 2010;26:455–468. doi: 10.1007/s12264-010-6023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J-P, Tsai S-J, Hong C-J, Yang C-H, Hsu C-D, Liou YJ. Interleukin-1 beta -511C/T genetic polymorphism is associated with age of onset of geriatric depression. NeuroMolecular Med. 2009;11:322–327. doi: 10.1007/s12017-009-8078-x. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Wang H, Matsumoto N, Muroya T, Shimazaki J, Ogura H, Shimazu T. Interleukin-1 causes long-term potentiation deficiency in a mouse model of septic encephalopathy. Neurosci. 2011;187:63–69. doi: 10.1016/j.neuroscience.2011.04.063. [DOI] [PubMed] [Google Scholar]
- Kapczinski F, Frey BN, Kauer-Sant’Anna M, Grassi-Oliveira R. Brain-derived neurotrophic factor and neuroplasticity in bipolar disorder. Ex pRev Neurotherapeut. 2008;8:1101–1113. doi: 10.1586/14737175.8.7.1101. [DOI] [PubMed] [Google Scholar]
- Kim YK, Jung HG, Myint AM, Kim H, Park SH. Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. J Affective Dis. 2007;104:91–95. doi: 10.1016/j.jad.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. Evidence for IL-1 receptor blockade as a therapeutic strategy for the treatment of depression. Cur Opin Invest Drugs. 2009;10:664–671. [PMC free article] [PubMed] [Google Scholar]
- Kuo HK, Yen CJ, Chang CH, Kuo CK, Chen JH, Sorond F. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: systematic review and meta-analysis. Lancet Neurol. 2005;4:371–380. doi: 10.1016/S1474-4422(05)70099-5. [DOI] [PubMed] [Google Scholar]
- Lacosta S, Merali Z, Anisman H. Influence of interleukin-1b on exploratory behaviors, plasma ACTH, corticosterone, and central biogenic amines in mice. Psychopharmacol. 1998;137:351–361. doi: 10.1007/s002130050630. [DOI] [PubMed] [Google Scholar]
- Lacosta S, Merali Z, Anisman H. Central monoamine activity following acute and repeated systemic interleukin-2 administration. Neuroimmunomodulation. 2000;8:83–90. doi: 10.1159/000026457. [DOI] [PubMed] [Google Scholar]
- Lagopoulos J, Hermens DF, Hatton SN, Tobias-Webb J, Griffiths K, Naismith SL, Scott EM, Hickie IB. Microstructural white matter changes in the corpus callosum of young people with Bipolar Disorder: a diffusion tensor imaging study. PLoS ONE. 2013;8:e59108. doi: 10.1371/journal.pone.0059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamacchia C, Palmer G, Bischoff L, Rodriguez E, Talabot-Ayer D, Gabay C. Distinct roles of hepatocyte- and myeloid cell-derived IL-1 receptor antagonist during endotoxemia and sterile inflammation in mice. J Immunol. 2010;185:2516–2524. doi: 10.4049/jimmunol.1000872. [DOI] [PubMed] [Google Scholar]
- Lamar M, Charlton RA, Morris RG, Markus HS. The impact of subcortical white matter disease on mood in euthymic older adults: a diffusion tensor imaging study. Am J Geriatr Psychiatry. 2010;18:634–642. doi: 10.1097/JGP.0b013e3181cabad1. [DOI] [PubMed] [Google Scholar]
- Larson SJ. Behavioral and motivational effects of immune-system activation. The Jl of Gen Psychol. 2002;129:401–414. doi: 10.1080/00221300209602104. [DOI] [PubMed] [Google Scholar]
- Larson SJ, Dunn AJ. Behavioral effects of cytokines. Br Behav Immun. 2001;15:371–387. doi: 10.1006/brbi.2001.0643. [DOI] [PubMed] [Google Scholar]
- Li Q, Powell N, Zhang H, Belevych N, Ching S, Chen Q, Sheridan J, Whitacre C, Quan N. Endothelial IL-1R1 is a critical mediator of EAE pathogenesis. Br Behav Immun. 2011;25:160–167. doi: 10.1016/j.bbi.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HC, Yang YY, Chou YM, Chen KP, Shen WW, Leu SJ. Immunologic variables in acute mania of bipolar disorder. J Neuroimmunol. 2004;150:116–122. doi: 10.1016/j.jneuroim.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Lotrich FE, Albusaysi S, Ferrell RE. Brain-derived neurotrophic factor serum levels and genotype: association with depression during interferon-alpha treatment. Neuropsychopharmacol. 2013;38:989–995. doi: 10.1038/npp.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE, Ferrell RE, Rabinovitz M, Pollock BG. Labile anger during interferon-alpha treatment is associated with a polymorphism in tumor necrosis factor-alpha. Clinical Neuropharmacology. 2010;33:191–197. doi: 10.1097/WNF.0b013e3181de8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LH, Zhou XJ, Fitzgerald J, Keedy SK, Reilly JL, Passarotti AM, Sweeney JA, Pavuluri M. Microstructural abnormalities of white matter differentiate pediatric and adult-onset bipolar disorder. Bipolar Dis. 2012;14:597–606. doi: 10.1111/j.1399-5618.2012.01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen NK, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochimica et Biophysica Acta. 2012;1822:386–400. doi: 10.1016/j.bbadis.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Nguyen KT, Deak T, Milligan ED, Watkins LR. Stress, learned helplessness, and brain interleukin-1b. Adv Exp Med and Biol. 1999;461:235–250. doi: 10.1007/978-0-585-37970-8_13. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Ivanovski B, Hadzi-Pavlovic D, Mitchell PB, Vieta E, Sachdev P. Neuropsychological deficits and functional impairment in bipolar depression, hypomania and euthymia. Bipolar Disord. 2007;9:114–25. doi: 10.1111/j.1399-5618.2007.00324.x. [DOI] [PubMed] [Google Scholar]
- Mann-Wrobel MC, Carreno JT, Dickinson D. Meta-analysis of neuropsychological functioning in euthymic bipolar disorder: an update and investigation of moderator variables. Bipolar Disord. 2011;13:334–42. doi: 10.1111/j.1399-5618.2011.00935.x. [DOI] [PubMed] [Google Scholar]
- Milaneschi Y, Corsi AM, Penninx BW, Bandinelli S, Guralnik JM, Ferrucci L. Interleukin-1 receptor antagonist and incident depressive symptoms over 6 years in older persons: the InCHIANTI study. Biol Psychiatry. 2009;65:973–8. doi: 10.1016/j.biopsych.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Crain BJ. MRI atlas of human white matter. Amsterdam ; Boston: Elsevier; 2005. [Google Scholar]
- Noll DC, Genovese CR, Nystrom LE, Vazquez AL, Forman SD, Eddy WF, Cohen JD. Estimating test-retest reliability in functional MR imaging. II: Application to motor and cognitive activation studies. Magn Reson Med. 1997;38:508–17. doi: 10.1002/mrm.1910380320. [DOI] [PubMed] [Google Scholar]
- Norheim K, Harboe E, Goransson L, Omdal R. Interleukin-1 inhibition and fatigue in primary Sjogren’s syndrome--a double blind, randomised clinical trial. PLoS ONE. 2012;7:e30123. doi: 10.1371/journal.pone.0030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien SM, Scully P, Scott LV, Dinan TG. Cytokine profiles in bipolar affective disorder: focus on acutely ill patients. Journal of Affect Dis. 2006;90:263–267. doi: 10.1016/j.jad.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Omdal R, Gunnarsson R. The effect of interleukin-1 blockade on fatigue in rheumatoid arthritis: A pilot study. Rheumatol Int. 2005;25:481–484. doi: 10.1007/s00296-004-0463-z. [DOI] [PubMed] [Google Scholar]
- Ortiz-Domâinguez A, Hernâandez ME, Berlanga C, Gutiâerrez-Mora D, Moreno J, Heinze G, et al. Immune variations in bipolar disorder: phasic differences. Bipolar Disord. 2007;9:596–602. doi: 10.1111/j.1399-5618.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- Parish CL, Finkelstein DI, Tripanichkul W, Satoska RAR, Drago J, Horne MK. The role of interleukin-1, interleukin-6, and glia in inducing growth of neuronal terminal arbors in mice. J Neurosci. 2002;22:8034–8041. doi: 10.1523/JNEUROSCI.22-18-08034.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnet P, Kelley KW, Bluthe R-M, Dantzer R. Expression and regulation of interleukin-1 receptors in the brain. Role in cytoknies-induced sickness behavior. J Neuroimmunol. 2002;125:5–14. doi: 10.1016/s0165-5728(02)00022-x. [DOI] [PubMed] [Google Scholar]
- Petrasek J, Dolganiuc A, Csak T, Kurt-Jones EA, Szabo G. Type I interferons protect from Toll-like receptor 9-associated liver injury and regulate IL-1 receptor antagonist in mice. Gastroenterol. 2011;140:697–708. doi: 10.1053/j.gastro.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather A, Rabinvitz M, Pollock B, Lotrich F. Cytokine-induced depression during IFN-α treatment: the role of IL-6 and sleep quality. Br Behav Immunol. 2009;23:1109–1116. doi: 10.1016/j.bbi.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorty S, Ramamoorty JD, Prasad PD, Bhat GK, Mahesh VB, Leibach FH, et al. Regulation of the human serotonin transporter by interleukin-1 beta. Biochemical and Biophysical Res Com. 1995;216:560–567. doi: 10.1006/bbrc.1995.2659. [DOI] [PubMed] [Google Scholar]
- Rej S, Butters MA, Aizenstein HJ, Begley A, Tsay J, Reynolds CF, Mulsant B, Gildengers AG. Neuroimaging and neurocognitive abnormalities associated with bipolar disorder in old age. Int J Geriatr Psychiatry. 2013 doi: 10.1002/gps.4021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CF, 3rd, Butters MA, Lopez O, Pollock BG, Dew MA, Mulsant BH, Lenze EJ, Holm M, Rogers JC, Mazumdar S, Houck PR, Begley A, Anderson S, Karp JF, Miller MD, Whyte EM, Stack J, Gildengers A, Szanto K, Bensasi S, Kaufer DI, Kamboh MI, DeKosky ST. Maintenance treatment of depression in old age: a randomized, double-blind, placebo-controlled evaluation of the efficacy and safety of donepezil combined with antidepressant pharmacotherapy. Arch Gen Psychiatry. 2011;68:51–60. doi: 10.1001/archgenpsychiatry.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LJ, Thompson JM, Gallagher P, Goswami U, Young AH, Ferrier IN, Moore PB. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J Aff Dis. 2006;93:105–115. doi: 10.1016/j.jad.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Rosano C, Aizenstein HJ, Studenski S, Newman AB. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007;62:1048–55. doi: 10.1093/gerona/62.9.1048. [DOI] [PubMed] [Google Scholar]
- Rossi S, Sacchetti L, Napolitano F, De Chiara V, Motta C, Studer V, Musella A, Barbieri F, Bari M, Bernardi G, Maccarrone M, Usiello A, Centonze D. Interleukin-1 causes anxiety by interacting with the endocannabinoid system. J Neurosci Res. 2012;32:13896–13905. doi: 10.1523/JNEUROSCI.1515-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. When does age-related cognitive decline begin? Neurobiol of Aging. 2009;30:507–514. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Ohtaki H, Tsumuraya T, Song D, Ohara K, Asano M, Iwakura Y, Atsumi T, Shioda S. Interleukin-1 participates in the classical and alternative activation of microglia/macrophages after spinal cord injury. J Neuroinflammation. 2012;9:65. doi: 10.1186/1742-2094-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Solms M, Ramesar R. Neuropsychological dysfunction in bipolar affective disorder: a critical opinion. Bip Disord. 2005;7:216–35. doi: 10.1111/j.1399-5618.2005.00203.x. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Sorrells SF, Caso JR, Munhoz CD, Sapolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64:33–9. doi: 10.1016/j.neuron.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkowski E, Liljeroth AM, Nilsson L, Minthon A, Blennow K. Decreased levels of intrathecal interleukin 1 receptor antagonist in Alzheimer’s disease. Dementia Geriatr Cognit Disord. 2001;12:314–317. doi: 10.1159/000051276. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Davis S, Morris C, Jackson E, Harrison R, O’Brien JT. Increase in interleukin-1beta in late-life depression. Am J Psychiatry. 2005;162:175–177. doi: 10.1176/appi.ajp.162.1.175. [DOI] [PubMed] [Google Scholar]
- Thornton P, McColl BW, Cooper L, Rothwell NJ, Allan SM. Interleukin-1 drives cerebrovascular inflammation via MAP kinase-independent pathways. Current Neurovasc Res. 2010;7:330–340. doi: 10.2174/156720210793180800. [DOI] [PubMed] [Google Scholar]
- Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83:113–118. [PubMed] [Google Scholar]
- Tong L, Balazs R, Soiampornkul R, Thangnipon W, Cotman CW. Interleukin-1 beta impairs brain derived neurotrophic factor-induced signal transduction. Neurobiol Aging. 2008;29:1380–1393. doi: 10.1016/j.neurobiolaging.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres IJ, Boudreau VG, Yatham LN. Neuropsychological functioning in euthymic bipolar disorder: a meta-analysis. Acta Psychiatr Scand Suppl. 2007:17–26. doi: 10.1111/j.1600-0447.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- Trollor JN, Smith E, Baune BT, Kochan NA, Campbell L, Samaras K, Crawford J, Brodaty H, Sachdev P. Systemic inflammation is associated with MCI and its subtypes: the Sydney Memory and Aging Study. Dementia & Geriat Cogn Disord. 2010;30:569–578. doi: 10.1159/000322092. [DOI] [PubMed] [Google Scholar]
- Tsai S-Y, Chung K-H, Wu J-Y, Kuo C-J, Lee H-C, Huang SH. Inflammatory markers and their relationships with leptin and insulin from acute mania to full remission in bipolar disorder. J Affect Disord. 2012;136:110–116. doi: 10.1016/j.jad.2011.08.022. [DOI] [PubMed] [Google Scholar]
- van den Biggelaar AHJ, Gussekloo J, de Craen AJM, Frolich M, Stek ML, van der Mast RC, Westendorp RGJ. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Exp Gerontol. 2007;42:693–701. doi: 10.1016/j.exger.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Balosso S, Maroso M, Zardoni D, Noé F, Ravizza T. ICE/caspase 1 inhibitors and IL-1beta receptor antagonists as potential therapeutics in epilepsy. Curr Op Invest Drugs. 2010;11:43–50. [PubMed] [Google Scholar]
- Wu M, Rosano C, Butters M, Whyte E, Nable M, Crooks R, Meltzer CC, Reynolds CF, 3rd, Aizenstein HJ. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. 2006;148:133–42. doi: 10.1016/j.pscychresns.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Rosano C, Lopez-Garcia P, Carter CS, Aizenstein HJ. Optimum template selection for atlas-based segmentation. Neuroimage. 2007;34:1612–8. doi: 10.1016/j.neuroimage.2006.07.050. [DOI] [PubMed] [Google Scholar]
- Yazdi AS, Drexler SK, Tschopp J. The role of the inflammasome in nonmyeloid cells. J Clin Immunol. 2010;30:623–627. doi: 10.1007/s10875-010-9437-y. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Winocur G, Goshen I. Brain interleukin-1 is involved in spatial memory and passive avoidance conditioning. Neurobiol Learning Mem. 2002;78:379–389. doi: 10.1006/nlme.2002.4072. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Zhuang L, Liu X, Xu X, Yue C, Shu H, Bai F, Yu H, Shi Y, Zhang Z. Association of the interleukin 1 beta gene and brain spontaneous activity in amnestic mild cognitive impairment. J Neuroinflammation. 2012;9:263. doi: 10.1186/1742-2094-9-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindler E, Zipp F. Neuronal injury in chronic CNS inflammation. Best Practice & Res Clin Anaesthesiol. 2010;24:551–562. doi: 10.1016/j.bpa.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Zubareva OE, Krasnova IN, Abdurasulova IN, Bluthe R-M, Dantzer R, Klimenko VM. Effects of serotonin synthesis blockade on interleukin-1b action in the brain of rats. Br Res. 2001;915:244–247. doi: 10.1016/s0006-8993(01)02910-9. [DOI] [PubMed] [Google Scholar]