Abstract
Within-session habituation and extinction learning co-occur as do subsequent consolidation of habituation (i.e., between-session habituation) and extinction memory. We sought to determine if, as we predicted: (1) between-session habituation is greater across a night of sleep vs. a day awake; (2) time-of-day accounts for differences; (3) between-session habituation predicts consolidation of extinction memory; (4) sleep predicts between-session habituation and/or extinction memory. Participants (N=28) completed 4–5 sessions alternating between mornings and evenings over 3 successive days (2 nights) with session 1 in either the morning (N=13) or evening (N=15). Twelve participants underwent laboratory polysomnography. During 4 sessions, participants completed a loud-tone habituation protocol while skin-conductance response (SCR), blink-startle electromyography (EMG), heart-rate acceleration (HRA) and deceleration (HRD) were recorded. For sessions 1 and 2, between-session habituation of EMG, SCR and HRD was greater across sleep. SCR and HRD were generally lower in the morning. Between-session habituation of SCR for sessions 1 and 2 was positively related to intervening (first night) slow wave sleep. In the evening before night 2, participants also underwent fear conditioning and extinction learning phases of a second protocol. Extinction recall was tested the following morning. Extinction recall was predicted only by between-session habituation of SCR across the same night (second night) and by intervening REM. We conclude that: 1) sleep augments between-session habituation, as does morning testing; 2) extinction recall is predicted by concurrent between-session habituation; and 3) both phenomena may be influenced by sleep.
Keywords: Sleep, Habituation, Extinction, Startle, Orienting
Introduction
Habituation is a phylogenetically ancient mechanism of neuronal plasticity and non-associative learning whereby behavioral and physiological responses to a stimulus diminish with its repeated presentation (Grissom and Bhatnagar 2009, Leussis and Bolivar 2006, Thompson and Spencer 1966). In animal models, between- and within-session habituation are dissociable processes as demonstrated by their responses to experimental lesions and pharmacological manipulations as well as by their genetic determinants (Bolivar 2009, Leussis and Bolivar 2006). Since between-session habituation represents neuroplasticity that, like other forms of neuroplasticity (Stickgold 2005), may undergo consolidation during sleep (Pace-Schott et al. 2011), the current study sought to examine whether such sleep-dependency may pertain to between-session habituation of responses to a startling acoustic stimulus.
Similarly, fear conditioning and its extinction are the behavioral manifestations of neuroplastic processes that underlie primitive forms of associative learning (Hermans et al. 2006, Milad and Quirk 2012). Fear conditioning associates a neutral stimulus with an inherently aversive or “unconditioned” stimulus (US). The previously neutral stimulus thereby becomes a conditioned stimulus (CS) capable, on its own, of eliciting a fearful response. Extinction involves learning that this stimulus that once signaled danger no longer does so. Extinction is new learning that co-exists with and competitively inhibits, but does not erase, the conditioned fear (Craske et al. 2008, Milad and Quirk 2012, Vervliet et al. 2013b). Because extinction is an emotional memory, it may, like other forms of memory, be augmented by sleep (Stickgold 2005). Our earlier work has shown that sleep promotes consolidation and generalization of extinction memory (Pace-Schott et al. 2009, Pace-Schott et al. 2012). In the present study, we sought to explore which elements of sleep might be important in these processes.
Extinction is the neurobiological basis of exposure therapy for anxiety disorders (Craske et al. 2008, McNally 2007, Foa et al. 2007). Extinction learning and within-session habituation, although theoretically dissociable (Craske et al. 2008), take place simultaneously in the context of exposure therapy and both are measured by declines in physiological and subjective reactivity (Craske et al. 2008, McSweeney and Swindell 2002). Similarly, following exposure, consolidation of within-session habituation and consolidation of extinction memory co-occur (Craske et al. 2008, McSweeney and Swindell 2002). Means to strengthen this therapeutic learning and more effectively prevent return of fear are of great scientific and clinical interest (Vervliet et al. 2013a, Vervliet et al. 2013b) and strategically timed sleep is one potential way to do so (Pace-Schott et al. 2012).
Our prior research suggests a role for sleep in the retention and generalization of extinction memory (Pace-Schott et al. 2009, Pace-Schott et al. 2012) as well as between-session habituation to aversive visual stimuli (Pace-Schott et al. 2011). Here we present preliminary findings of a study testing the following 4 hypotheses that: 1) there is superior retention of acoustic habituation across a 12-hr delay with a normal night’s sleep compared to an equal duration of a day’s continuous waking; 2) time of day will account for some of these differences; 3) individual differences in overnight consolidation of acoustic habituation predict such differences in the consolidation of extinction memory; 4) across a night’s sleep, there will be a relationship between sleep physiology and the degree of consolidation of extinction memory and/or between-session habituation.
Methods
Participants
28 healthy young adult males (mean age 25, SD 5.2, range 19–35) were studied exclusively because extinction memory varies with sex as well as with estradiol levels and cycle phase in women (Graham and Milad 2013, Milad et al. 2010). Exclusion criteria included any current neurological, psychiatric or medical conditions or history of seizures, significant head trauma, diagnosed DSM IV Axis-I mental disorder or sleep disorder. Excluded also were smokers and those self-reporting >5 cups caffeine/day or >12 alcoholic drinks/week. A telephone screening followed by a more extensive on-line screening addressed each exclusion criterion. This study accorded with the principles of Declaration of Helsinki, procedures were approved by Partners Healthcare Institutional Review Board and all participants provided written informed consent.
Procedure
Pre-study week
During the week prior to the experiment, participants were instructed to keep a regular sleep schedule consisting of a minimum of 7 hours in bed each night, bedtime no later than 2:00 AM and no daytime napping. Compliance with these instructions was monitored with the Evening-Morning Sleep Questionnaire (EMSQ) diary (Pace-Schott et al., 2013) and actigraphy. Participants were also asked to abstain from alcohol, recreational drugs and, on study days, caffeine. Participants also completed a battery of psychological and retrospective surveys detailed in Table 1.
Table 1.
Demographic, psychological trait, and subjective sleep duration in the PAPA and APAP groups.
| Characteristic | PAPA (SD) | APAP (SD) | p (unpaired t) |
|---|---|---|---|
| N | 15 | 13 | |
| Age | 24.9 (5.2) | 24.9 (5.1) | .98 |
| ESS | 4.87 (3.40) | 5.38 (3.62) | .70 |
| PSQI | 3.13 (1.85) | 3.25 (1.91) a | .87 |
| MEQ | 49.00 (11.53) | 50.46 (10.06) | .73 |
| CTQ (total) | 28.00 (4.02) c | 34.90 (9.72) d | .04* |
| THQ (total) | 2.08 (1.44) c | 3.00 (1.49) d | .16 |
| NEO-PI-R Neuroticism | 69.57 (21.46) | 73.54 (20.16) b | .63 |
| NEO-PI-R Extraversion | 118.07 (20.66) | 118.46 (18.73) b | .96 |
| NEO-PI-R Openness | 126.57 (26.84) | 112.62 (19.77) b | .67 |
| NEO-PI-R Agreeableness | 117.29 (17.89) | 110.31 (16.30) b | .30 |
| NEO-PI-R Conscientiousness | 128.71 (17.04) | 118.15 (16.64) b | .12 |
| STAI-Trait | 30.00 (7.91) | 32.15 (6.93) | .45 |
| Self-report average TST | 7.55 (0.57) | 7.96 (0.75) | .11 |
| Self-report caffeine/day (cups) | 0.93 (0.59) | 0.62 (0.42) | .12 |
| Self-report EtOH/wk (drinks) | 2.97 (2.48) | 2.93 (2.78) | .97 |
| Diary sleep Day -2 (min) | 503 (80) | 540 (73) a | .23 |
| Diary sleep Day -1 (min) | 465 (82) | 429 (76) a | .25 |
| Diary sleep Day 1 (min) | 449 (20) | 462 (23) a | .12 |
| Diary sleep Day 2 (min) | 451 (20) d | 421 (101) e | .34 |
p<.05,
N=12,
N=13,
N=12,
N=10,
N=11, TST=total sleep time.
ESS: Epworth Sleepiness Scale (Johns 1991), PSQI: Pittsburgh Sleep Quality Index (Buysse et al., 1989), MEQ: Morningness-Eveningness Questionnaire Horne & Ostberg 1976), STAI-T: Spielberger State-Trait Anxiety Inventory-Trait portion (Spielberger et al 1990, CTQ: Childhood Trauma Questionnaire (Bernstein et al 2008), THQ: Trauma History Questionnaire (Hooper et al 2011), NEO-PI-R: Revised NEO Personality Inventory (Costa & McCrae 1992).
Schedule of experimental sessions and total number of participants in analyses
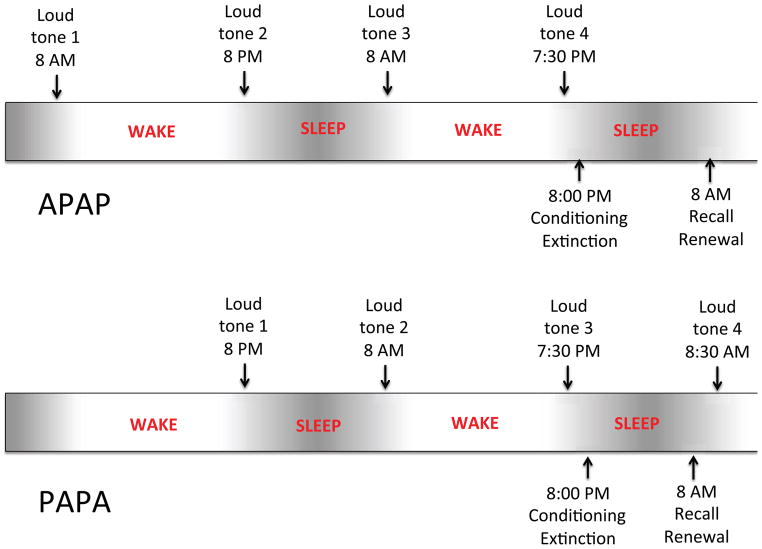
Participants completed 4 or 5 one-hour sessions, each 12 hours apart and alternating between approximately 8–9 AM and 8–9 PM, over the course of 3 successive days and 2 nights (see Figure 1). Of the 28 total subjects, 13 had their first of 5 sessions in the morning of the first day (AM-PM-AM-PM-AM or “APAPA” group) and 15 had their first of 4 sessions that evening (PM-AM-PM-AM or “PAPA” group). Both groups had their last session on the morning of the third day (i.e., 5th session for APAPA, 4th session for PAPA). In the APAPA group the interval between the first 2 sessions was one of continuous daytime wakefulness (AM to PM) whereas in the PAPA group it contained a normal night’s sleep (PM to AM). Three participants (1 APAPA, 2 PAPA) withdrew after completing only their first 2 sessions. Of the 28 participants, 14 participants completed a first night of laboratory polysomnography (PSG) between Sessions 1 and 2 (8 PAPA) or 2 and 3 (6 APAPA) in the Massachusetts General Hospital Division of Sleep Medicine Sleep Laboratory. Of these, 12 participants then completed a second PSG night between sessions 3 and 4 (8 PAPA) or 4 and 5 (4 APAPA). The remainder slept at home across both nights. Before and after each session, participants completed the self-assessments of sleepiness, mood and anxiety shown in Table 2.
Figure 1.
Experimental protocol. In both groups participants completed one-hour sessions, 12 hours apart, alternating between approximately 8–9 AM and 8–9 PM, over the course of 3 successive days and 2 nights. The APAPA group had the first of 5 sessions in the morning of the first day and the PAPA group had the first of 4 sessions in the evening of that day. Both groups had their last session on the morning of the third day. All participants completed the loud-tone protocol at their first 4 sessions and the fear conditioning and extinction protocol across the second night of the study.
Table 2.
Self–reported sleepiness, mood and anxiety at each session (mean of pre- and post-loud noise protocol ratings)
| Session | PAPA (SD)† | APAP (SD)† | p (Group) | p (Position) | P (interaction) |
|---|---|---|---|---|---|
| SSS | |||||
| 1 | 2.9 (1.2) a | 2.5 (1.0) c | .41 | .002 ** | .29 |
| 2 | 2.7 (1.7) a | 2.2 (1.3) c | .36 | .024 * | .61 |
| 3 | 2.9 (1.6) | 2.4 (1.5) c | .35 | .16 | .77 |
| 4 | 2.8 (1.5) d | 2.3 (0.7) b | .29 | .81 | .15 |
| PANAS (positive) | |||||
| 1 | 32.8 (9.2) c | 35.0 (9.3) c | .53 | .001 ** | .49 |
| 2 | 31.8 (13.3) a | 35.0 (6.9) d | .46 | .023 * | .69 |
| 3 | 30.6 (12.2) c | 33.7 (8.9) b | .50 | .70 | .44 |
| 4 | 31.7 (13.2) b | 34.1 (6.9) e | .62 | .18 | .86 |
| PANAS (negative) | |||||
| 1 | 12.6 (1.1) c | 13.0 (1.3) c | .32 | .003 ** | .74 |
| 2 | 12.4 (0.9) a | 12.5 (0.8) d | .71 | .35 | .06 |
| 3 | 14.1 (2.8) c | 12.6 (12.6) b | .05* | .78 | .28 |
| 4 | 13.5 (2.8) b | 12.8 (1.3) e | .36 | .87 | .07 |
| STAI-S | |||||
| 1 | 26.3 (4.2) c | 28.0 (4.8) c | .33 | .64 | .55 |
| 2 | 26.1 (7.1) a | 28.0 (4.6) d | .45 | .65 | .42 |
| 3 | 30.4 (10.2) a | 28.6 (7.3) b | .62 | .41 | .19 |
| 4 | 26.7 (7.5) c | 29.1 (6.9) e | .44 | .09 | .004 ** |
mean at session (collapses across Position),
p ≤. 05,
p ≤ .01,
No superscript, N=15,
N=14,
N=11,
N=13,
N= 12,
N= 10.
SSS: Stanford Sleepiness Scale (Hoddes et al 1973), PANAS: Positive and Negative Affect Schedule (Watson et al 1988), and the STAI-S: Spielberger State-Trait Anxiety Inventory-State portion (Spielberger et al 1990)
At all of 4 sessions (PAPA) or the first 4 of 5 sessions (APAPA), participants completed identical presentations of a validated loud-tone habituation protocol (Buhlmann et al. 2007, Carson et al. 2007). Therefore, in both groups, most participants completed the loud-tone protocol a total of 4 times. However, due to equipment problems or early withdrawal from study, 2 participants’ data were lost from certain channels in both of the 1st and 2nd loud-tone protocols and 8 participants’ data were lost from one or both of the 3rd and 4th loud-tone protocols. Across the second night, all participants completed a validated 5-phase fear conditioning and extinction protocol (Milad et al. 2007, Pace-Schott et al. 2009). Again, because of equipment problems or early withdrawal, 4 participants did not complete the fear conditioning and extinction protocol. Because of such variable attrition and data loss due to equipment problems, the total number of participants in each analysis is provided in Tables 1–3 or in Figure legends for the respective analyses (if analysis are not displayed in tables or figures, N is provided in the text of the Results).
Table 3.
Comparison of extinguished and unextiguished conditioned fear at the Extinction Recall phase and contextual fear renewal effect measured at the Fear Renewal phase using differential SCR and retrospective differential shock expectancy.
| mean 1st 2 (SD) | mean 1st 4 (SD) | |
|---|---|---|
| Differential SCR in μS (N=26) | ||
| Extinction Recall | ||
| CS+E | 0.22 (0.45) | 0.14 (0.27) |
| CS+U | 0.22 (0.54) | 0.09 (0.30) |
| p-value, paired t-test | 0.99 | 0.52 |
| Recall and Renewal | ||
| CS+E Recall | 0.22 (0.45) | 0.14 (0.27) |
| CS+E Renewal | 0.09 (0.53) | 0.01 (0.30) |
| p-value, paired t-test | 0.28 | 0.12 |
| Differential Retrospective Shock | ||
| Expectancy in mm (N=25) | ||
| Extinction Recall | ||
| CS+E | 20.84 (32.04) | |
| CS+U | 46.12 (36.90) | |
| p-value, paired t-test | 0.001 | |
| Recall and Renewal | ||
| CS+E Recall | 20.84 (32.04) | |
| CS+E Renewal | 53.06 (34.76) | |
| p-value, paired t-test | 0.002 | |
Loud-tone protocol
Participants were instrumented for recordings of skin conductance level (SCL), electromyography of the orbicularis oculi muscle (EMG) and heart rate (HR) as detailed below, and had headphones placed over both ears. They first completed 5 min of baseline recording while sitting quietly in a comfortable chair in a sound-attenuated subject room with cables to an adjacent control room where physiological signals were recorded and the subject was monitored by video. Participants were instructed to remain seated with eyes open and informed that they would hear a series of loud tones following a waiting period. After baseline recording, fifteen 500-ms, 97-dB, 1000-Hz pure tones with 0-msec rise and fall times were presented at intervals randomly varying between 27 and 52 sec.
Physiological recording and outcome variables
Left orbicularis oculi EMG, skin conductance level (SCL), and HR were measured during 5 min of continuous baseline recording. During the subsequent procedure, recording began 4 sec before each tone presentation and continued until 8.5 sec after tone offset. Response scores were calculated using the procedures of Carson et al. (2007) and Buhlmann et al. (2007). All physiological response scores were square-root transformed to reduce skewness and heteroscedasticity. All biosignals were recorded using the LabLinc V modular instrument system (Coulbourn Instruments, Allentown, PA). Analog signals were digitized at 1000 Hz using a Coulbourn Analog to Digital Converter.
EMG
The blink-startle response was recorded using two 4-mm (sensor diameter) Sensor Medics Ag/AgCl surface electrodes filled with electrolytic conductive paste and attached beneath the left eye over the left orbicularis oculi muscle following Fridlund & Cacioppo (1986). EMG signals were amplified using a Coulbourn Bioamplifier, and were filtered for a range of 90 to 1000 Hz. These signals were then integrated using a 10-msec time constant with a Coulbourn Contour Following Integrator. To determine the magnitude of the blink startle response (Blumenthal et al. 2005), orbicularis oculi EMG response to each tone was calculated by subtracting the mean EMG level during the 1 s immediately preceding tone onset from the highest EMG level measured within 40 to 200 msec after tone offset. Two dependent variables were derived. The first, Mean Response, was the average of all 15 EMG responses to tones. The second, Relative Habituation, was calculated by computing the Pearson coefficient for the correlation of the natural logarithm of trial numbers 2 to 15 against the corresponding EMG response to tone at each trial.
SCR
Skin conductance level (SCL) was measured using two 9-mm (sensor diameter) Sensor Medics (Yorba Linda, CA) Ag/AgCl radiotranslucent electrodes (BioPac Systems Inc., Goleta, CA) filled with isotonic paste, attached with 14 mm of separation on the hypothenar surface of the non-dominant hand. SCL was recorded using a Coulbourn Isolated Skin Conductance coupler that applied a constant voltage of 0.5 V and was expressed in microSiemens. Skin conductance response (SCR) to each tone was calculated by subtracting the mean SCL during the 1 sec immediately preceding tone onset from the highest level occurring 1 to 4 sec following tone offset. Mean Response and Relative Habituation scores were calculated as for EMG.
HR
Heart rate (interbeat interval) was recorded from standard limb ECG leads connected to a Coulbourn High Gain Bioamplifier and then converted to heart rate (HR). Heart-rate accelerative (HRA) responses to each tone were calculated by subtracting the mean HR of the 2 heartbeats immediately preceding tone onset from the highest HR within 1 to 4 sec after tone offset. Heart-rate decelerative (HRD) responses to each tone were calculated by subtracting the lowest HR within 1 to 4 sec after tone offset from the mean HR of the 2 heartbeats immediately preceding tone onset. Mean Response but not Relative Habituation was calculated.
Fear conditioning and extinction protocol
The fear conditioning and extinction protocol included five experimental phases (Habituation, Fear Conditioning, Extinction Learning, Extinction Recall, Fear Renewal) that took place over 2 sessions occurring at either sessions 4 and 5 (APAPA group) or 3 and 4 (PAPA group). In either case, the first session (Habituation, Fear Conditioning, Extinction Learning) took place in the evening prior to the second night of the study and the second (Extinction Recall, Fear Renewal) occurred the following morning (Figure 1). Extinction memory was examined only overnight because comparisons of overnight to over-day extinction memory have previously been tested (Pace-Schott et al. 2009, Pace-Schott et al. 2013). Moreover the primary goals for the current study were to examine sleep correlates of extinction memory and determine whether overnight between-session habituation predicts overnight extinction memory.
Stimuli and methods for this procedure were identical to those described in previous publications (Linnman et al. 2011; Milad et al.. 2009; Milad et al.. 2007a; Milad et al.. 2007b; Pace-Schott et al.. 2009; Pace-Schott et al. 2013; Zeidan et al.. 2011), therefore only an abbreviated description follows. The unconditioned stimulus (US) was a mild electric shock delivered using a Coulbourn Transcutaneous Aversive Finger Stimulator. Before the Habituation phase, the subject was instructed to choose a level of shock that was “highly annoying but not painful” while receiving increasing intensities shock.
Conditioned stimuli (CS) consisted of digital photographs of three differently colored lamps displayed on a computer screen within the image of two different photographic environments (“contexts”). During the Habituation phase, participants viewed all 6 combinations of context and CS color without shocks. During the Fear Conditioning phase, in the “conditioning context”, the US accompanied 2 of the 3 colors (CS+) using a partial reinforcement schedule (5 of 8 of each CS+ color) while the third color remained unreinforced (CS−). During the Extinction Learning phase, one CS+ color (CS+E) but not the other CS+ (CS+U) was presented repeatedly in the second environment (“extinction context”) along with CS− but no US. The following morning, during the Extinction Recall phase, all 3 types of conditioned stimulus (CS+E, CS+U and CS−) were presented in the extinction context unaccompanied by any shocks. Immediately afterward, during the contextual Fear Renewal phase, all 3 types of stimuli were again presented but in the conditioning context, again without any shocks.
Following Fear Renewal, a retrospective rating of shock expectancy was obtained using a modification of methods in Vervliet et al. (2005) as detailed in Pace-Schott et al. (2013). Briefly, participants retrospectively rated their expectancy of being shocked by each CS color at its first 2 and last 2 presentations during each experimental phase using 100-mm visual analog scales. Differential shock expectancy score was then calculated by subtracting, from each CS+ expectancy (in mm), the expectancy for the CS− at the same point in time.
SCR served as the measure of conditioned response (CR) and was recorded using the procedures described above. SCR was calculated for each trial as the mean skin conductance level during the last 2 sec of context presentation (which preceded onset of the CS) subtracted from the maximum skin conductance level during the 6-sec CS presentation. SCRs were square-root transformed. The outcome variable was differential SCR calculated by subtracting the SCR to a CS− from the SCR to its sequentially corresponding CS+ trial.
Polysomnography (PSG)
PSG was measured on both nights following standard techniques. A trained sleep technician scored sleep stages in records from both nights using standard criteria (Iber et al. 2007, Rechtschaffen and Kales 1968). A full 10–20 electrode montage included 19 channels of encephalography (EEG) referenced to contralateral mastoids, two channels of electrooculography (EOG) (both outer canthi, one above and one below the eye), 2 channels of submental electromyography (EMG) and 1 channel of electrocardiography (ECG). Data were acquired at 250 Hz with high-pass filtering at 0.1 Hz and low-pass filtering at 100 Hz. The following sleep variables were computed: sleep onset latency, Total Sleep Time (TST), wake time after sleep onset, sleep efficiency, REM latency, and stages N1, N2, N3 and R as percentages of TST.
Statistical analyses
Self-report variables
Independent t-tests were used to compare APAPA and PAPA groups for retrospective self-report variables (Table 1). Repeated measures ANOVA were used to compare self-report variables between groups across sessions (Table 2).
Loud-tone protocol and self-report repeated measures
The following 3 methods were used to analyze: 1) baseline EMG, SCL and HR; 2) change in EMG, SCR, HRA and HRD from one session to the next (between-session habituation); 3) changes in Relative Habituation (within-session habituation) of EMG and SCR from one session to the next; 4) self-report repeated measures.
First Session-Pair Analyses. To avoid any confounding effects of interactions between the number of prior presentations and the nature of the intervening interval, data from only the first two sessions were initially analyzed. Analyses of Mean Response and Relative Habituation used 2-factor mixed-model ANOVA with the between-subjects factor Group (APAPA vs. PAPA) and the within-subject factor Session (first, second). Mean Response was also analyzed using independent t-tests of percentage change (i.e., Mean Response at session 1 minus Mean Response at session 2 expressed as percent of Mean Response at session 1). Among PAPA participants, those who slept in the laboratory and those who slept at home over the first night did not differ on any measure of Mean Response, Relative Habituation or percentage change. Therefore their data were pooled for comparison with the APAPA group all of whom remained awake between sessions 1 and 2. When analyzing self-report repeated measures, a second within-subject factor, Position (beginning of session vs. end of session), was nested in Session.
Analyses by Session-Order. The effects of session order on Mean Response (all variables) and Relative Habituation (EMG and SCR only) were analyzed using mixed-model ANOVA with the between-subjects factor Group and the within-subject factor Session (sessions 1–4). When analyzing self-report repeated measures, Position was again nested in Session. In separate analyses using Mean Response only, the outcome variable was the between-session change and the within-subject variable was Change Across Session Pairs (i.e., Mean Response at session 1 minus Mean Response at session 2, and Mean Response at session 3 minus Mean Response at session 4). For the PAPA group, both such changes were across a night’s sleep (PM to AM) whereas for APAPA group they were both across a day’s wakefulness (AM to PM).
Analyses by Time-of-Day. To analyze the effect of time-of-day on Mean Response (all variables) and Relative Habituation (EMG and SCR only), repeated-measures ANOVA collapsed across groups was performed. Within-subject factors were Time-of-Day (AM vs. PM) in which were nested Order (first or second test at each time-of-day). When analyzing self-report repeated measures Position was again nested in Session. Means comparisons were used for post-hoc comparison of within-subject measures.
Extinction memory
Memory for the differentiation of the CS+E and CS+U was analyzed using differential SCR data and repeated-measures ANOVA of the Extinction Recall phase. Within-subject factors were Trial nested in CS+ Type (CS+E or CS+U). In three separate analyses, Trial included all 8 CS+ of each type as well as just the first 4 and the first 2 presentations of each. Contextual renewal of conditioned fear was similarly measured using Trial nested in Phase (Extinction Recall or Fear Renewal). Trial consisted of the first 2 or first 4 CS+E presented at each phase. For purposes of regression analysis (see below), retention of extinction memory across the second night was measured using the Extinction Retention Index or ERI (Milad et al. 2007). ERI is defined as [100% − (100% × mean SCR of initial Extinction Recall trials/maximum SCR to a future CS+E during Fear Conditioning)]. In computing ERI, non-differential SCR was used and means were computed for both the first 2 and the first 4 presentations of the CS+E.
Relationships between sleep physiology and between-session habituation and extinction memory
Simple regression was used to perform an exploratory examination of relationships between sleep physiology and change in psychophysiological measures across the intervening first night (between-session habituation) or second night (extinction memory). Similar exploratory analyses were used to examine interrelationships between between-session habituation and extinction memory as well as between both forms of memory and psychological measures. To limit Type-1 error, alpha level was set at p < .05 and only a subset of variables were thus compared (see below).
Results
Retrospective self-report measures
Table 1 compares retrospective self-report measures between APAPA and PAPA groups. The only significant difference between groups was a larger total CTQ score in the APAPA group.
Baseline SCR, EMG and HR (preceding Loud-Tone protocol)
Baseline First Session-Pair Analyses
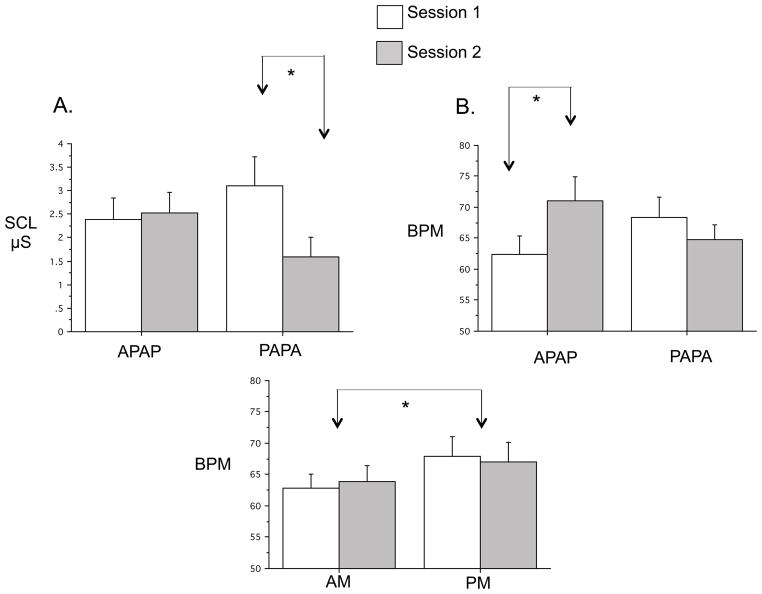
When comparing only sessions 1 and 2, there were no main effects of Group for baseline SCL, EMG and HR (APAPA N=12, PAPA N=14). However, there was a significant Group x Session interaction for SCL [F(1,24)=5.82, p=.024] reflecting a significant decrease from sessions 1 to 2 (PM to AM) in the PAPA group [F(1,13)=8.45, p=.012] but not the APAPA group (Fig. 2A)1. There was also a significant Group x Session interaction for HR [F(1,24)=9.59, p=.05] reflecting a significant increase in HR from the AM to PM in the APAPA group [F(1,11)=7.62, p=.019] but not from the PM to AM in the PAPA group (Fig. 1B)1.
Figure 2.
Comparison of baseline values of EMG, SCL and HR between groups. A. First Session-Pair Analyses of baseline SCL showing a significantly greater SCL in the first (PM) vs. second (AM) session of the PAPA group (N=12 APAPA, 14 PAPA). B. First Session-Pair Analyses of baseline HR showing significantly greater HR in the second (PM) vs. first (AM) session of the APAPA group (N=12 APAPA, 14 PAPA). C. Analyses by Time-of-Day of HR showing significantly greater HR in the PM vs. AM collapsing across Group (N=19). SCL: skin conductance level, μS: microSiemens, BPM: beats per minute. * p < .05. Error bars depict standard error of the mean.
Baseline Analyses by Session-Order
The above Group x Session interactions failed to reach significance across all 4 sessions.
Baseline Analyses by Time-of-Day
When AM and PM were compared, collapsing across Group, a significant Time-of-Day main effect was seen for HR [F(1,18)=4.46, p=.05] (Fig. 2C)1 but not for EMG or SCL. Baseline values therefore suggest a tendency for both SCL and HR to be greater in the evening.
EMG during Loud-tone protocol: orbicularis oculi blink-startle response
EMG First Session-Pair Analyses
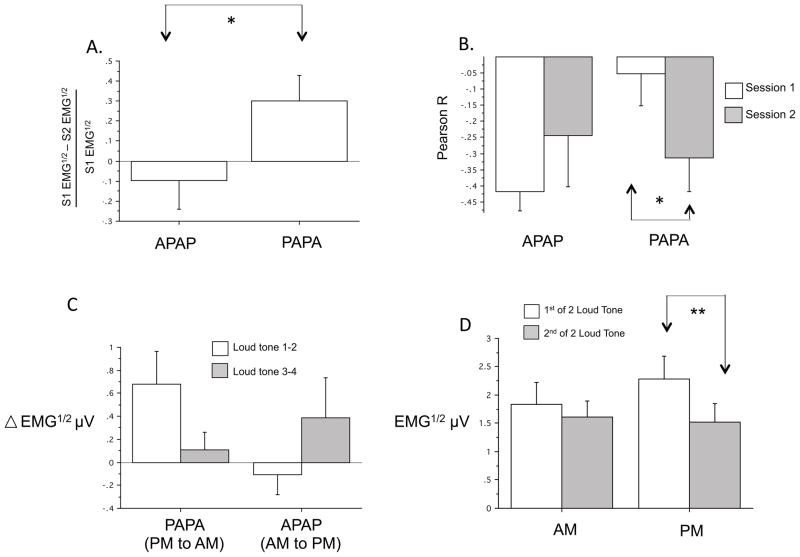
For EMG Mean Response to loud tones, there was no significant main effect of Group or Group x Session interaction. However, when the change in Mean Response from sessions 1 to 2 was expressed as percentage change (Fig. 3A)1, there was a significantly greater decrease from PM to AM (PAPA group) than from AM to PM (APAPA group) [F(1,24)=4.54, p=.044]. In addition, for Relative Habituation of the EMG response to tones (Fig. 3B)1, there was a significant Group x Session interaction [F(1,24)=4.54, p=.044]. This interaction resulted because, in the PAPA group, Relative Habituation across session 2 in the AM was significantly steeper than across session 1 in the PM [F(1,14)=5.70, p=.032] (Fig. 3B). In contrast, Relative Habituation did not differ between sessions when session 1 was in the AM and session 2 was in the PM (APAPA group).
Figure 3.
Comparisons of blink-startle response (orbicularis oculi EMG) to loud tones across sessions and between times-of-day. A. First Session-Pair Analyses of EMG Mean Response percentage change from session 1 to session 2 showing a significant difference between PAPA (PM to AM) and APAPA (AM to PM) groups (N=11 APAPA, 15 PAPA). B. First Session-Pair Analyses of EMG Relative Habituation showing significantly greater intra-session habituation (more negative correlation coefficient) in the second (AM) compared to first (PM) session of the APAPA group (N=11 APAPA, 15 PAPA). C. Analyses by Session-Order for Change Across Session Pairs across 2 PM to AM sessions in the PAPA group compared to 2 AM to PM changes in the APAPA group (p < .05 for Group x Session Pair interaction) (N=7 APAPA, 11 PAPA). D. Time-of-day analysis collapsing across groups showing lack of a Time-of-Day (AM vs. PM) main effect (N=18). EMG μV1/2 (or EMG1/2) square-root transformed electromyographic response in microVolts, * p < .05, ** p < .01, Error bars depict standard error of the mean.
EMG Analyses by Session-Order
When all 4 sessions were analyzed together using mixed-model ANOVA (N=11 PAPA, 7 APAPA), no main effect of Group or Session x Group interaction appeared for either EMG Mean Response or Relative Habituation. However, using Change Across Session Pairs as the within-subject variable, there was a significant Group x Session Pair interaction for Mean Response [F(1,16)=4.48, p=.05]. This interaction resulted because, in the PAPA group (in which both between-session changes were from PM to AM), the change in Mean Reaction from Sessions 1 to 2 was greater than from Sessions 3 and 4 (Fig. 3C) 1. However, the opposite was seen in the APAPA group (in which both changes were from AM to PM). Collapsing across Group, there was a main effect of Session for Mean Response [F(3,48)=5.92, p=.002] reflecting a clear decline in EMG Mean Response across the 4 sessions.
EMG Analyses by Time-of-Day
Although there was no main effect of Time-of-Day, a significant Session x Time-of-Day interaction [F(1,17)=5.14, p=.037] indicated that the difference between the 2 sessions was greater in the PM [F(1,21)=13.16, p=.002] than in the AM [F(1,19)=2.43, p=.14] (Fig. 3D) 1. For Relative Habituation, there was no main effect of Time-of-Day but there was a significant Session x Time-of-Day interaction [F(1,17)=4.62, p=.046]. This interaction reflected the fact that the greatest Relative Habituation occurred when the first presentation of the protocol took place in the AM. Means comparisons showed that such an AM first-session Relative Habituation was greater than the mean of Relative Habituation across the other 3 combinations of Time-of-Day and Order (F=5.06, p=.038). Collapsing across both Group and Time-of-Day, the main effect of Session remained significant for Mean Response [F(1,17)=12.93, p=.002] indicating that EMG to a second presentation of the protocol was smaller overall than to the first presentation (Fig. 3D).
In summary, the results of the First Session-Pair Analyses of Mean Response for EMG indicate that between-session habituation in the EMG blink startle response is greater across a 12-hr period containing a night’s sleep than across an equal duration of a day’s continuous wakefulness (Fig. 3A). Similarly, the results of the First Session-Pair Analyses of Relative Habituation suggest that within-session habituation becomes greater following a night’s sleep but not following a day’s wake (Fig. 3B). Analyses by Session-Order also suggested that the greatest between-session habituation took place across the first PM to AM interval (Fig. 3C). Notably, both of these habituation effects could also be attributed to a time-of-day effect whereby, in the morning, the EMG blink startle response is smaller and its habituation stronger. However, lack of a Time-of-Day main effect in the Time-of-day analysis (Fig. 3D) suggests that greater between-session habituation across the night cannot be attributed solely to a time-of-day effect.
SCR Loud-tone Protocol
SCR First Session-Pair Analyses
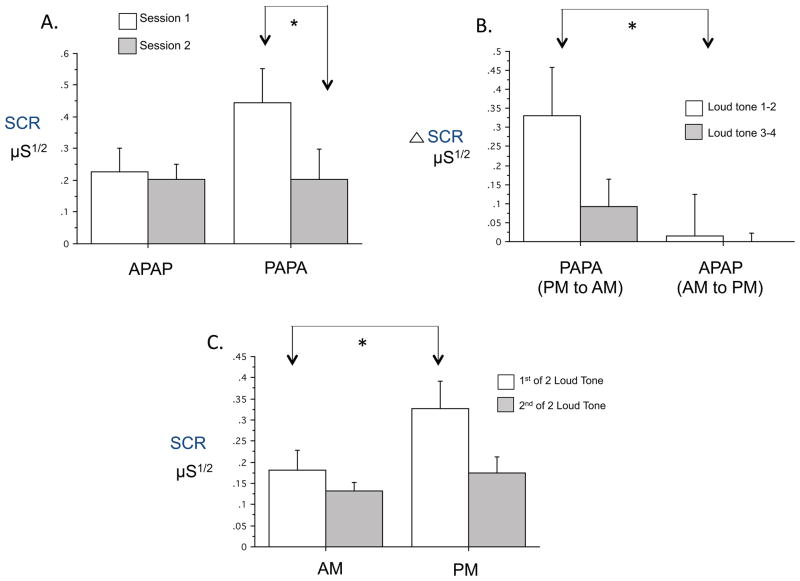
For SCR Mean Response, there was no significant main effect of Group. Although the Group x Session interaction failed to reach significance [F(1,14)=7.05, p=.08], there was a significant decrease from session 1 to 2 in the PAPA group [F(1,26)=7.05, p=.019] but not in the APAPA group (Fig. 4A) 1. Relative Habituation of SCR showed no Group main effect or Group x Session interaction. Both Mean Response and Relative Habituation showed significant main effects of Session [F(1,26)=4.91, p=.036 and F(1,26)=10.98, p=.003 respectively].
Figure 4.
Comparisons of SCR to loud tones across sessions and between times-of-day. A. First Session-Pair Analyses showing significantly lower SCR Mean Response in the second (AM) vs. first (PM) session of the PAPA group (N=13 APAPA, 15 PAPA). B. Analyses by Session-Order for Change Across Session Pairs across 2 PM to AM sessions in the PAPA group compared to 2 AM to PM changes in the APAPA group. There were significantly greater decreases across both PM to AM sessions combined in the PAPA group than across both AM to PM changes combined in the APAPA group (N=9 APAPA, 10 PAPA). C. Analyses by Time-of-Day collapsing across groups showing significantly greater SCR to the loud tones in the PM compared to AM (N=21). SCR μS1/2: square-root transformed skin conductance response in microSiemens, * p < .05, Error bars depict standard error of the mean.
SCR Analyses by Session-Order
When all 4 sessions were analyzed together using mixed-model ANOVA (N=11 PAPA, 10 APAPA), there again was no significant main effect of Group or Session x Group interaction for Mean Response or Relative Habituation. However, using Change Across Session Pairs as the within-subject variable, there was an near significant Group main effect [F(1,17)=4.38, p=.052] whereby, collapsing across Order, change across the PM to AM intervals in the PAPA group exceeded change across the AM to PM intervals in the APAPA group (Fig. 4B) 1. Mean Response showed a significant main effect of Session [F(3,57)=4.77, p=.017] reflecting a clear decline in SCR across the 4 sessions in both groups.
SCR Analyses by Time-of-Day1
The Time-of-Day main effect failed to reach significance [F(1,20)=3.51, p=.076] and there was no Session x Time-of-Day interaction. Nonetheless, means comparisons showed that SCR Mean Response in the first PM session was significantly larger than in the first AM session (F=6.23, p=.021, Fig. 4C) as well as greater than the mean of the other 3 combinations of Time-of-Day and Order (F=11.75, p=.003). Collapsing across both Group and Time-of-Day, the main effect of Session remained significant for both the SCR Mean Response [F(1,20)=6.63, p=.018] and SCR Relative Habituation [F(1,20)=7.54, p=.013]. This effect indicates a larger Mean Response and greater Relative Habituation at the first of 2 successive sessions.
In summary, the results of the First Session-Pair Analyses of Mean Response indicate that between-session habituation of the SCR to loud tones is greater across a 12-hr period containing a night’s sleep than across an equal duration of a day’s continuous wakefulness (Fig. 4A). Analyses by Session-Order also suggest that the change in Mean Response is greater over PM to AM intervals (Fig. 4B). Both of these effects could be attributed to a time-of-day effect whereby, in the morning, the SCR response is smaller. However, in the time-of-day analysis, lack of a Time-of-Day main effect after the first session (Fig. 4C) suggests that greater between-session habituation across the night cannot be attributed solely to a time-of-day effect.
Loud-tone Protocol: HRA and HRD
HRA and HRD First Session-Pair Analyses
For HRA Mean Response, there were no Group or Session main effects or interactions. Similarly, for HRD Mean Response, there were no Group or Session main effects. However, for HRD, a significant Group x Session interaction [F(1,26)=12.94, p=.001] reflected the fact that, whereas in the APAPA group, HRD strongly increased from the AM to the PM [F(1,12)=11.82, p=.005], HRD non-significantly decreased from PM to AM in the PAPA group (Fig. 5A)1.
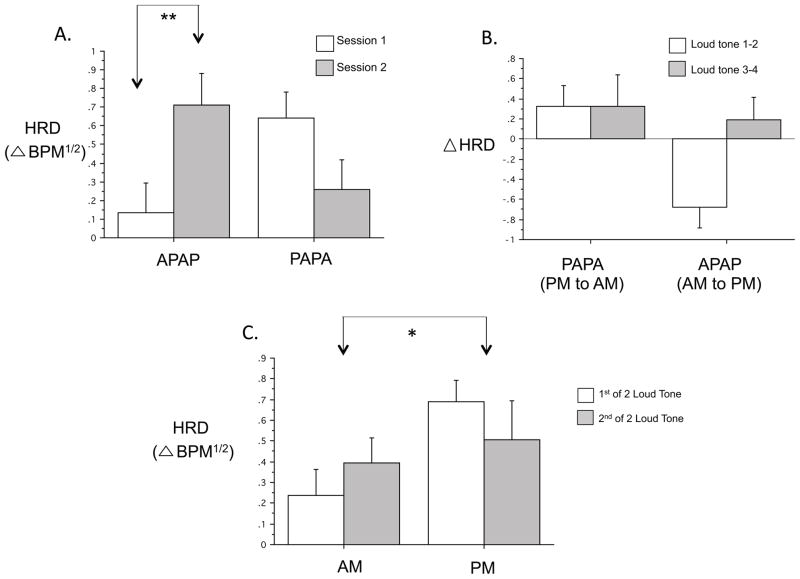
Figure 5.
Comparisons of HRD to loud tones across sessions and between times-of-day. A. First Session-Pair Analyses showing significantly greater HRD Mean Response at the second (PM) compared to the first (AM) session in the APAPA group (N=13 APAPA, 15 PAPA). B. Analyses by Session-Order for Change Across Session Pairs across 2 PM to AM sessions in the PAPA group compared to 2 AM to PM changes in the APAPA group. There were significantly greater decreases across both PM to AM sessions combined in the PAPA group than across both AM to PM changes combined in the APAPA group (N=10 APAPA, 11 PAPA). C. Analyses by Time-of-Day collapsing across groups showing significantly greater HRD to the loud tones in the PM compared to AM (N=21). HRD BPM1/2: square-root transformed heart rate deceleration in beats-per-minute, * p < .05, Error bars depict standard error of the mean.
HRA and HRD Analyses by Session-Order
For HRA Mean Response, when all 4 sessions were analyzed together (N=11 PAPA, 10 APAPA), there again were no significant main effects of Group or Session or Session x Group interaction. Similarly, For HRA Mean Response, using the Change Across Session Pairs as the within-subject variable, there were no Group or Session-Pair main effects or interactions.
For HRD Mean Response, although there were no significant main effects of Group or Session, a near-significant Session x Group interaction [F(3,57)=2.92, p=.054] reflected the above Group difference in the change between sessions 1 and 2 (Fig. 5A)1. Using Session Pair as the within-subject variable, there were significant main effects for Group [F(1,19)=4.42, p=.049, PM larger], Session Pair [F(1,19)=4.91, p=.039, second session larger] and their interaction [F(1,19)=4.81, p=.041] (Fig 5B). This interaction resulted because, in the APAPA group, HRD increased session 1 to session 2 (Figs. 5A, 5B) and it decreased from session 3 to session 4 [F(1,9)=13.33, p=.005 for session 1–2 vs. 3–4 changes]. In contrast, HRD decreased across both sessions 1–2 and 3–4 in the PAPA group (Fig. 5B).
HRA and HRD Analyses by Time-of-Day
Collapsing across Group, for HRA Mean Response, there were neither main effects of Group or Time-of-Day nor a Group x Time-of-Day interaction. However, for HRD Mean Response, there was a significant Time-of-Day main effect [F(1,21)=4.52, p=.046] (Fig 5C).
Therefore, HRD (but not HRA) showed distinct Group differences especially when comparing the first 2 sessions. HRD decreased across a night’s sleep but increased markedly across a day’s wakefulness (Fig 5A). Strong evidence for a Time-of-Day component was the differing HR baselines (Fig., 2C), as well as the significant Time-of-Day main effect (Fig 5C). Nonetheless, the fact that the second between-session interval did not show the striking Group difference seen over the first between-session interval (Figs. 5B, 5C) suggests that factors other than time-of-day, such as the intervening behavioral state, can influence between-session habituation of HRD.
Pre and Post-Session Self-Report Measures
Self-report ratings of sleepiness, positive and negative mood, and state anxiety before and after each session showed no systematic differences between groups or times of day.
Self-report First Session-Pair analyses
Table 2 compares these measures between groups at each session. None of these measures showed a significant main effect of Group. However, at the end vs. the beginning of sessions participants were significantly sleepier [F(1,25)=13.05, p=.001] and reported less positive mood [F(1,23)=20.33, p=.0002].
Self-report Analyses by Session-Order
Analysis of all 4 sessions showed greater sleepiness [F(1,22)=8.75, p=.007] and less positive mood [F(1,16)=8.83, p=.009] at the end vs. beginning of sessions (Table 2). Increased negative emotion at the end of session 3 in the PAPA group and increased state anxiety at the end of session 4 in the APAPA group were likely due to the fact that fear conditioning took place during each of these sessions.
Self-report Analyses by Time-of-Day
Collapsing across Group, a Time-of-Day main effect [F(1,19)=9.32, p=.007] indicated significantly greater state anxiety in the PM.
Fear conditioning and extinction protocol
Using ANOVA, neither Extinction Learning nor Extinction Recall showed main effects for group (APAPA vs. PAPA) nor did home vs. laboratory sleep, and neither of these factors interacted with CS+Type (CS+E vs. CS+U) nor with Trial. Similarly, no ERI measure differed between groups or between home vs. laboratory sleep.
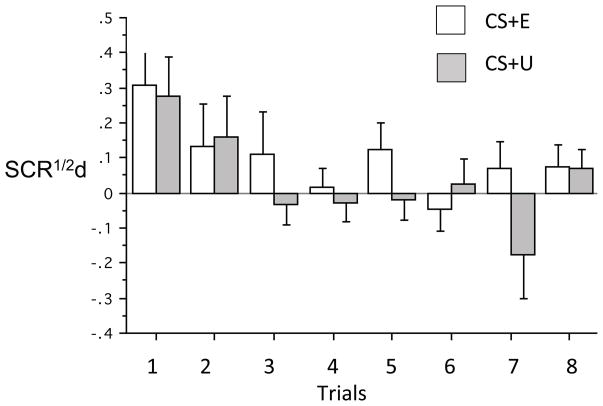
Extinction recall, expressed as suppressed differential SCR to previously fear conditioned stimuli, generalized across a night of sleep from a fear conditioned and extinguished stimulus (CS+E) to one that was conditioned but not extinguished (CS+U). This generalization was manifested by lack of a CS+Type (CS+E vs. CS+U) main effect at Extinction Recall when comparing all 8 presentations of both CS+ [F(1,25)=1.73, p=.20), only the first 2 presentations or the first 4 presentations [F(1,25)=.002, p=.99 and F(1,25)=.42, p=.52 respectively] (Table 3, Figure 6) 1. Similarly, comparing differential SCR to the first 2 or first 4 CS+E presentations between the Extinction Recall and Fear Renewal phases showed no evidence of contextual fear renewal [F(1,25)=1.22, p=.28 and F(1,25)=2.57, p=.12 respectively] (Table 3). However participants were able to retrospectively recall the learning and memory phenomena elicited by the fear conditioning and extinction protocol, thereby replicating Pace-Schott et al. (2013). Both the distinction between the CS+E and CS+U and the contextual fear renewal were evident in retrospective ratings, but not psychophysiological measures (Table 3).
Figure 6.
Differential skin conductance response to the extinguished (CS+E) and unextinguished conditioned stimuli at the extinction recall phase occurring in the morning of Day 3 (N=12 APAPA, 13 PAPA). Neither a main effect of CS+ type nor a CS+ type x Trial interaction was noted indicating generalization of extinction memory from the CS+E to the CS+U. SCR1/2, differential skin conductance response in microsiemens.
Relationships of between-session habituation and extinction memory with each other and with retrospective self-report measures
In order to examine whether overnight between-session habituation was a predictor of overnight extinction memory on a different night (night 1 habituation compared to night 2 extinction) and/or on the same night (both processes across night 2) only the PAPA group was analyzed. This was because only in this group did initial (vs. a repetition of) between-session habituation take place over the first night (Fig. 1). Similarly, because the APAPA group completed their last (4th) loud-tone procedure on the evening of the second day, only the PAPA group had a night of concurrent between-session habituation and extinction memory consolidation (Fig. 1). Among between-session habituation measures, the only significant correlation with Extinction Recall Index (ERI), determined from the first 4 CS+E trials at Extinction Recall, was seen for the amount of reduction in SC Mean Response (percentage change) across the second night (R=.69, p=.012, N=12). No retrospective self-report measures correlated with between-session habituation or ERI measures.
Sleep physiology and relationships with between-session habituation and extinction memory
Average sleep physiological parameters for the two groups are provided in Table 4. There were no significant differences between the two groups except for slightly longer sleep latency in the PAPA group on night 2. Sleep variables related to between-session habituation were measured across night 1 in the 8 individuals with PSG between sessions 1 and 2 (PAPA group). Those related to extinction memory were measured across the 12 individuals with PSG on night 2.
Table 4.
Sleep quality and architecture of individuals receiving laboratory polysomnography.
| APAPA (N=6) | s.d. | PAPA (N=8) | s.d. | t-test | APAPA (N=4) | s.d. | PAPA (N=8) | s.d. | t-test | |
|---|---|---|---|---|---|---|---|---|---|---|
| TST (min) | 431.67 | 20.32 | 418.25 | 40.24 | 0.47 | 437.70 | 26.73 | 444.81 | 17.56 | 0.59 |
| Sleep Latency (min) | 7.42 | 7.19 | 6.81 | 5.11 | 0.86 | 1.88 | 1.11 | 5.19 | 2.66 | 0.04* |
| WASO (min) | 19.92 | 14.18 | 29.14 | 22.91 | 0.40 | 10.25 | 7.56 | 17.75 | 14.93 | 0.37 |
| REM Latency (min) | 119.75 | 66.71 | 125.25 | 48.85 | 0.86 | 139.00 | 46.22 | 101.25 | 29.20 | 0.11 |
| Sleep Efficiency (%) | 94.05 | 4.50 | 91.94 | 6.38 | 0.50 | 97.28 | 1.91 | 95.10 | 3.22 | 0.25 |
| Stage N1 (%TST) | 9.75 | 3.03 | 11.21 | 5.86 | 0.59 | 8.15 | 3.53 | 7.96 | 3.05 | 0.93 |
| Stage N2 (%TST) | 54.93 | 5.47 | 55.61 | 5.52 | 0.82 | 52.95 | 8.76 | 53.74 | 9.89 | 0.90 |
| Stage N3 (%TST) | 15.12 | 7.03 | 14.98 | 7.33 | 0.97 | 16.70 | 6.50 | 16.41 | 7.97 | 0.95 |
| Stage R (%TST) | 20.18 | 5.64 | 18.21 | 6.40 | 0.56 | 22.23 | 4.60 | 21.86 | 4.46 | 0.90 |
TST: Total sleep time, WASO: Wake time after sleep onset
Few significant relationships were seen between sleep physiological and psychophysiological variables. Across the first night in the PAPA group (who slept between their first 2 sessions), the amount of reduction in EMG blink-startle response to the loud tones from session 1 to session 2 was smaller as percentages of stage N1 light sleep increased across the first night (R=.72, p=.045, N=8). Conversely, the amount of reduction in the SCR Mean Response to the loud tones increased as the percentage of stage N3 slow wave sleep increased across this same night (R=.91, p=.002, N=8). This latter effect remained a trend when one individual who had no N3 sleep was removed (R=.67, p=.097, N=7). In addition, the amount of reduction in HRD between sessions 1 and 2 decreased with greater sleep onset latency (R=.85, p=.008, N=8).
Across the second night among all participants who received PSG, the degree to which they retained extinction memory to the extinguished CS increased with increased percent stage REM sleep (R=.72, p=.012, N=11). (This latter result employed the ERI measured using the mean of the first 2 CS+E at Extinction Recall after removal of one outlier in which ERI was 3 SD below the mean).
Discussion
The present study found that an auditory stimulus of sufficient intensity to evoke orienting and startle responses (but otherwise neutral) showed greater between-session habituation across a night of sleep than a day of wakefulness, thereby supporting our first hypothesis. In addition, for the EMG eyeblink-startle response, within-session habituation also increased relative to the prior session following a night of sleep, but decreased relative to the prior session across a day of wakefulness (Fig. 3B). Similarly, for skin conductance response (SCR), between-session habituation was greater from a first to second session when a night with sleep vs. a day awake intervened (Fig. 4A). Whereas heart rate acceleration to loud tones (HRA) showed no group effects, heart rate deceleration to these tones (HRD) increased markedly following a day’s wakefulness (between-session sensitization, Fig. 5A). When, in each group, changes across the second between-session interval of the same type as the first (i.e., PM to AM in PAPA, or AM to PM in APAPA) were included in analyses (Figs. 3C, 4B and 5B), overall group differences remained however absolute differences between between-session interval type were lessened (Figs. 4B and 5B) or reversed as was the case of EMG (Fig. 3C) across this second interval. When the groups were pooled, our second hypothesis regarding time-of-day was also partially supported. Specifically, time-of-day effects also emerged for SCR (as well as for baseline skin conductance level) and HRD (as well as for baseline heart rate) (Figs. 2, 4C, 5C). This suggests that a component of the between-session difference could be attributed to circadian and/or sleep-homeostatic factors that promote greater physiological reactivity in the evening than the morning. Notably this time-of-day effect was absent for blink-startle EMG and suggesting that, for this measure, sleep might consolidate or even augment neuroplasticity induced by within-session habituation (Pace-Schott et al. 2011). Results also provided limited support for our third hypothesis that between-session habituation would predict consolidation of extinction memory however this only emerged across concurrent (vs. sequential) nights and within a single physiological measure (SCR). Limited support is also provided for our fourth hypothesis which stated that sleep physiology during the consolidation period would influence between-session habituation (SWS promotes) and extinction memory (REM promotes).
In the fear conditioning and extinction procedure, across a 12-hr interval containing a normal night’s sleep, extinction memory generalized from a fear conditioned and extinguished stimulus (CS+E) to a similarly conditioned but unextinguished stimulus (CS+U), replicating findings from 2 previous studies (Pace-Schott et al. 2009, Pace-Schott et al. 2013). Notably, whereas such generalization eliminated differentiation of the CS+E and CS+U as measured by differential SCR, retrospective shock expectancy ratings maintained this distinction. Similarly shock expectancy demonstrated contextual fear renewal, an effect also absent in psychophysiological data. There was therefore a clear discrepancy between retrospective shock expectancy, which relied on declarative memory, and physiologically expressed fear and extinction memory that relied on both declarative and implicit learning.
This cognitive-physiological discrepancy may be adaptive in that healthy individuals are able to dampen anxious reactivity while maintaining awareness of potentially important contingencies should the need to respond arise. This capacity may be disturbed in anxiety disorder patients in whom physiological anxiety upon exposure to disorder-relevant cues persists even though the individual is cognitively aware of the unlikelihood of harm from the feared stimulus. One effect of sleep may be to enhance this ability to maintain physiological equanimity while remaining aware of past experience. In healthy individuals, this capacity diminishes slightly with increased homeostatic sleep pressure (i.e., in the evening) when physiological reaction to a conditioned but un-extinguished cue (i.e., the CS+U) returns (Pace-Schott et al. 2013).
Time-of-day can be an important determinant of physiological response magnitude (e.g., Hot et al. 2005, Miller and Gronfier 2006, Pace-Schott et al. 2013). In such cases, as for SCR and HRD in the current study, it is important to note that preceding sleep may remain as an influence on the expression of learning and memory. Whereas circadian factors are apparent even in a sleep-deprived state (Frey et al. 2004), reduction of homeostatic sleep need, without pharmacological intervention, requires preceding sleep. Hence, even if augmentation of between-session habituation or extinction memory is not specifically due to sleep-dependent memory consolidation (i.e., stabilization of acquired plastic changes in autonomic and limbic circuits), preceding sleep may still be required if expression of learned habituation or extinction requires the physiological conditions of the rested state. For example, buildup of extracellular adenosine contributes to increased homeostatic sleep pressure (Porkka-Heiskanen and Kalinchuk 2011) and, in the rat, adenosine A1 receptor activation has been shown to impair both fear conditioning (Corodimas and Tomita 2001) and extinction of appetitive conditioning (Kuzmin et al.. 1999).
Future research into the effects of normal sleep on the neurocircuitry responsible for the expression of emotional memory may provide important insights into the bases of normal and impaired emotion regulation. For example, following early morning awakenings in depression (Wehr and Wirz-Justice 1982), depressed mood (Murray 2007) and morning anxiety (Geraci and Uhde 1992) may result from deficits in sleep-dependent processes that normally restore homeostasis in limbic circuits. Such processes might involve REM sleep, which has been linked to normal mood regulation (Gujar et al. 2010, Rosales-Lagarde et al. 2012, van der Helm et al. 2011) as well as slow wave sleep (SWS) which has been linked to enhanced between-session habituation to aversive stimuli (Pace-Schott et al. 2011). Notably, REM and SWS are the two sleep stages characteristically altered in PTSD (Kobayashi et al. 2007). Although our PSG sample is small, these are the two sleep stages whose percentages correlated with extinction retention and between-session habituation of SCR respectively.
Several limitations apply to the current study. First, due to limited resources, the PSG sample was very small and future studies will need to examine these same psychophysiological and sleep parameters across a larger sample. Second the loud-tone procedure was carried out across the first laboratory night in which sleep quality is characteristically diminished relative to habitual sleep (Edinger et al. 1997). Therefore future studies should include a third night in the laboratory (or 3 nights of ambulatory PSG) to minimize this effect. However, among those whose first between-session interval fell across a night of sleep (PAPA group), having spent a first night in the sleep laboratory did not affect any between-session habituation measure in comparison to those who slept at home. Third, the total sample size for each group was less than ideal. All of these factors may have lessened the power needed to observe within-subject relationships, such as a relationship between extinction memory and between-session habituation. The PSG sample is especially small for examining correlations with extinction memory variables which tend toward high between-individual variability. Nonetheless, the fact that group differences emerged that were consistent with prior studies of habituation (Pace-Schott et al. 2011) and extinction (Pace-Schott et al. 2009, Pace-Schott et al. 2013, Pace-Schott et al. 2012) increases confidence in these findings. A final consideration involves the possibility that the APAPA group may have, on average, experienced greater childhood adversity (higher total CTQ score) and hence poorer between-session habituation. However, our finding that the CTQ did not correlate with the between-session habituation measures argues against this possibility.
Poor sleep in patients with certain anxiety disorders such as posttraumatic stress disorder (Kobayashi et al. 2007) may contribute to poor maintenance of emotional homeostasis (Mellman 2008). One way in which this may occur is that anxiety disorders patients remain physiologically reactive to any possibility, based upon declarative memory, that harm may re-occur. This might include exposure to related conditioned cues (i.e., enhanced generalization of fear or poor generalization of extinction) or exposure to fear-related cues in new environments (i.e., enhanced contextual fear renewal). Therefore, future examination of factors that may influence generalization of fear (Vervliet et al. 2013a) and extinction (Pace-Schott et al. 2009) as well as contextual fear renewal (Rougemont-Bucking et al. 2011) may be especially informative with regard to the effect of poor sleep on abnormal fear in anxiety disorders.
Acknowledgments
Research supported by NIH/NIMH R21MH090357. We thank Margaret Merlino, RPSGT, Karen Gannon, RPSGT and the staff of the MGH Division of Sleep Medicine laboratory.
Footnotes
Sample size of this analysis is provided in the figure legend.
References
- Bernstein GA, Bernat DH, Davis AA, Layne AE. Symptom presentation and classroom functioning in a nonclinical sample of children with social phobia. Depress Anxiety. 2008;25:752–60. doi: 10.1002/da.20315. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ. Intrasession and intersession habituation in mice: from inbred strain variability to linkage analysis. Neurobiol Learn Mem. 2009;92:206–14. doi: 10.1016/j.nlm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhlmann U, Wilhelm S, Deckersbach T, Rauch SL, Pitman RK, Orr SP. Physiologic responses to loud tones in individuals with obsessive-compulsive disorder. Psychosom Med. 2007;69:166–72. doi: 10.1097/PSY.0b013e31802f2799. [DOI] [PubMed] [Google Scholar]
- Carson MA, Metzger LJ, Lasko NB, Paulus LA, Morse AE, et al. Physiologic reactivity to startling tones in female Vietnam nurse veterans with PTSD. J Trauma Stress. 2007;20:657–66. doi: 10.1002/jts.20218. [DOI] [PubMed] [Google Scholar]
- Corodimas KP, Tomita H. Adenosine A1 receptor activation selectively impairs the acquisition of contextual fear conditioning in rats. Behav Neurosci. 2001;115:1283–90. doi: 10.1037//0735-7044.115.6.1283. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO five-factor inventory (NEO-FFI) Professional Manual. Odessa FL: Psychological Assessment Resources, Inc; 1992. [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behav Res Ther. 2008;46:5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Fins AI, Sullivan RJ, Jr, Marsh GR, Dailey DS, et al. Sleep in the laboratory and sleep at home: comparisons of older insomniacs and normal sleepers. Sleep. 1997;20:1119–26. doi: 10.1093/sleep/20.12.1119. [DOI] [PubMed] [Google Scholar]
- Foa E, Hembree E, Rothbaum B. Therapist Guide. Oxford University Press; 2007. Prolonged Exposure Therapy for PTSD. Emotional Processing of Traumatic Experiences. [Google Scholar]
- Frey DJ, Badia P, Wright KP., Jr Inter- and intra-individual variability in performance near the circadian nadir during sleep deprivation. J Sleep Res. 2004;13:305–15. doi: 10.1111/j.1365-2869.2004.00429.x. [DOI] [PubMed] [Google Scholar]
- Fridlund AJ, Cacioppo JT. Guidelines for human electromyographic research. Psychophysiology. 1986;23:567–89. doi: 10.1111/j.1469-8986.1986.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Geraci MF, Uhde TW. Diurnal rhythms and symptom severity in panic disorder. A preliminary study of 24-hour changes in panic attacks, generalised anxiety, and avoidance behaviour. Br J Psychiatry. 1992;161:512–6. doi: 10.1192/bjp.161.4.512. [DOI] [PubMed] [Google Scholar]
- Graham BM, Milad MR. Blockade of Estrogen by Hormonal Contraceptives Impairs Fear Extinction in Female Rats and Women. Biol Psychiatry. 2013;73:371–78. doi: 10.1016/j.biopsych.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom N, Bhatnagar S. Habituation to repeated stress: get used to it. Neurobiol Learn Mem. 2009;92:215–24. doi: 10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujar N, McDonald SA, Nishida M, Walker MP. A Role for REM Sleep in Recalibrating the Sensitivity of the Human Brain to Specific Emotions. Cereb Cortex. 2010;21:115–23. doi: 10.1093/cercor/bhq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D, Craske MG, Mineka S, Lovibond PF. Extinction in human fear conditioning. Biol Psychiatry. 2006;60:361–8. doi: 10.1016/j.biopsych.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–6. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Hooper L, Stockton P, Krupnick J, Green BA. Development, Use, and Psychometric Properties of the Trauma History Questionnaire. Journal of Loss and Trauma. 2011;16:258–83. [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Hot P, Leconte P, Sequeira H. Diurnal autonomic variations and emotional reactivity. Biol Psychol. 2005;69:261–70. doi: 10.1016/j.biopsycho.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Iber C, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Kobayashi I, Boarts JM, Delahanty DL. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007;44:660–9. doi: 10.1111/j.1469-8986.2007.537.x. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Bolivar VJ. Habituation in rodents: a review of behavior, neurobiology, and genetics. Neurosci Biobehav Rev. 2006;30:1045–64. doi: 10.1016/j.neubiorev.2006.03.006. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Mechanisms of exposure therapy: how neuroscience can improve psychological treatments for anxiety disorders. Clin Psychol Rev. 2007;27:750–9. doi: 10.1016/j.cpr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- McSweeney FK, Swindell S. Common processes may contribute to extinction and habituation. J Gen Psychol. 2002;129:364–400. doi: 10.1080/00221300209602103. [DOI] [PubMed] [Google Scholar]
- Mellman TA. Sleep and Anxiety Disorders. Sleep Medicine Clinics. 2008;3:261–68. doi: 10.1016/j.jsmc.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–51. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–54. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, et al. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–8. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Gronfier C. Diurnal variation of the startle reflex in relation to HPA-axis activity in humans. Psychophysiology. 2006;43:297–301. doi: 10.1111/j.1469-8986.2006.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray G. Diurnal mood variation in depression: a signal of disturbed circadian function? J Affect Disord. 2007;102:47–53. doi: 10.1016/j.jad.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Milad MR, Orr SP, Rauch SL, Stickgold R, Pitman RK. Sleep promotes generalization of extinction of conditioned fear. Sleep. 2009;32:19–26. [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Shepherd E, Spencer RM, Marcello M, Tucker M, et al. Napping promotes inter-session habituation to emotional stimuli. Neurobiol Learn Mem. 2011;95:24–36. doi: 10.1016/j.nlm.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Spencer RMC, Vijayakumar S, Ahmed N, Verga PW, et al. Extinction of conditioned fear is better learned and recalled in the morning than in the evening. J Psychiatr Res. 2013;47:1776–84. doi: 10.1016/j.jpsychires.2013.07.027. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Verga PW, Bennett TS, Spencer RM. Sleep promotes consolidation and generalization of extinction learning in simulated exposure therapy for spider fear. J Psychiatr Res. 2012;46:1036–44. doi: 10.1016/j.jpsychires.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Kalinchuk AV. Adenosine, energy metabolism and sleep homeostasis. Sleep medicine reviews. 2011;15:123–35. doi: 10.1016/j.smrv.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology Techniques and Scoring System for Sleep Stages of Human Subjects. Brain Information Service/Brain Research Institute; University of California at Los Angeles: 1968. [Google Scholar]
- Rosales-Lagarde A, Armony JL, Del Rio-Portilla Y, Trejo-Martinez D, Conde R, Corsi-Cabrera M. Enhanced emotional reactivity after selective REM sleep deprivation in humans: an fMRI study. Frontiers in behavioral neuroscience. 2012;6:Article 25, 1–13. doi: 10.3389/fnbeh.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougemont-Bucking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, et al. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS neuroscience and therapeutics. 2011;17:227–36. doi: 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory (Self-Evaluation Questionnaire) Palo Alto: Consulting Psychologists Press; 1990. [Google Scholar]
- Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–8. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- van der Helm E, Yao J, Dutt S, Rao V, Saletin JM, Walker MP. REM sleep depotentiates amygdala activity to previous emotional experiences. Curr Biol. 2011;21:2029–32. doi: 10.1016/j.cub.2011.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervliet B, Baeyens F, Van den Bergh O, Hermans D. Extinction, generalization, and return of fear: a critical review of renewal research in humans. Biol Psychol. 2013a;92:51–8. doi: 10.1016/j.biopsycho.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Vervliet B, Craske MG, Hermans D. Fear extinction and relapse: state of the art. Annual review of clinical psychology. 2013b;9:215–48. doi: 10.1146/annurev-clinpsy-050212-185542. [DOI] [PubMed] [Google Scholar]
- Vervliet B, Vansteenwegen D, Baeyens F, Hermans D, Eelen P. Return of fear in a human differential conditioning paradigm caused by a stimulus change after extinction. Behav Res Ther. 2005;43:357–71. doi: 10.1016/j.brat.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Wirz-Justice A. Circadian rhythm mechanisms in affective illness and in antidepressant drug action. Pharmacopsychiatria. 1982;15:31–9. doi: 10.1055/s-2007-1019506. [DOI] [PubMed] [Google Scholar]