Abstract
Adoptively transferred T cells have the capacity to traffic to distant tumor sites, infiltrate even fibrotic tissue and kill antigen-expressing tumor cells. A variety of groups have investigated different genetic engineering strategies designed to enhance tumor specificity, increase T cell potency, improve proliferation, persistence, or migratory capacity, and increase safety. In this review we focus on recent developments in the T cell engineering arena, discuss the application of these engineered cell products clinically, and outline future prospects for this therapeutic modality.
Keywords: CAR T cells, cancer treatment, immunotherapy, genetic modification of T cells
Introduction
To date, monoclonal antibodies have been the most widely used form of immunotherapy for cancer. However, their limited, biodistribution, range of effector mechanisms recruited, and in vivo persistence have all restricted their clinical potency. In contrast, adoptively transferred effector T cells have the capacity to effectively traffic through multiple tissue planes to distant tumor sites (1), recruit multiple cellular and humoral effector mechanisms, and persist for many years, thereby producing complete and sustained disease remissions. Genetic engineering is a means by which we can further increase the potency of these tumor-targeted cellular products. In this review, we evaluate recent improvements in T cell engineering, describe their current clinical impact, and discuss the future prospects of this novel approach.
Adoptive transfer of T cells with native antigen specificity
T cells have the capacity to identify and eradicate malignant disease through native receptor recognition of tumor-associated antigens (TAAs), even without modification. Examples of such activity are well described in melanoma, where Rosenberg and others have reported that infusion of melanoma-specific tumor-infiltrating lymphocytes (TILs) and T cell clones targeting melanoma-associated antigens produces clinical responses in approx. 50% of patients(2–5). Similarly, we have infused over 100 hematopoietic stem cell transplant (HSCT) recipients with donor-derived polyclonal T cell lines targeting Epstein-Barr virus (EBV) to prevent and treat the often lethal EBV-associated lymphoproliferative disorder (post-transplant lymphoproliferative disease; PTLD) that frequently occurs in these severely immunocompromised patients(6,7). Small doses (circa 2x107 CTL/m2) of in vitro expanded EBV-specific cytotoxic T lymphocytes (CTLs) proved to be both safe and effective for the prophylaxis and treatment of EBV reactivation post-transplant(8). Subsequently this strategy was extended to EBV-associated malignancies that occur in immunocompetent individuals including Hodgkin disease (HD), non-Hodgkin lymphoma (NHL), and nasopharyngeal carcinoma (NPC). Although the viral antigen expression pattern in these patients is restricted to weakly immunogenic EBV proteins such as LMP1 and LMP2, the adoptively transferred CTLs trafficked to tumor sites, and produced complete remission in over half the subjects with refractory or relapsed disease(9–14).
In principle the successes described above should be extendable to any other TAAs that can be targeted by T cells. Unfortunately, however, most TAAs are self antigens and self-reactive T cells are largely anergized or deleted. Moreover, even if TAA-specific T cells can be generated and are then infused, these cells may fail to persist due to tumor immune evasion strategies such as (i) down-regulation of T cell target antigens, major histocompatibility complex (MHC) and co-stimulatory molecules; (ii) production of inhibitory/Th2-polarizing factors such as transforming growth factor (TGF) β, interleukin (IL) 10, IL13, and IL4, (iii) expression of pro-apoptotic molecules on the cell surface; and (iv) recruitment of regulatory T cells (Tregs) that inhibit the effector T cell response to tumor(15).
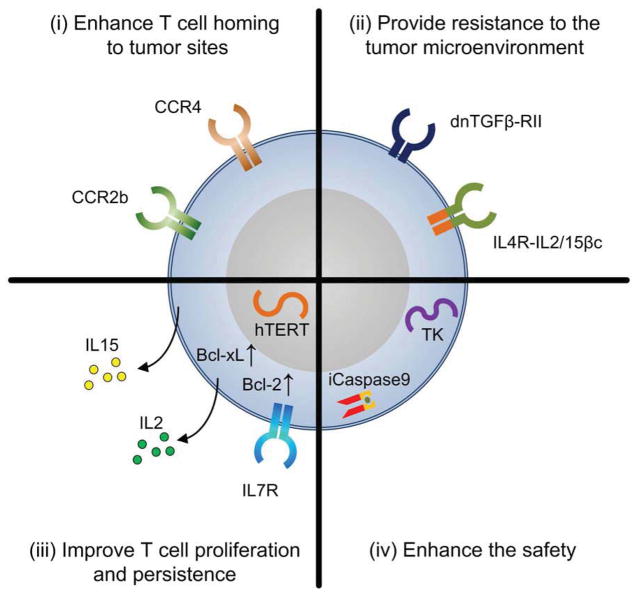
Nevertheless, advances in cell engineering technology has now allowed us to modify T cells with genes that can; increase the range of antigens they can recognize and/or augment their affinity for their targets; improve their homing to tumor sites; increase their resistance to tumor immune evasion strategies; enhance their proliferation and survival; and ensure their safety (Figure 1). Although it remains unclear as to which of these modifications, or combination thereof, will be most relevant in the clinical setting, in this review we will discuss the current status of T cell engineering.
Figure 1.
Examples of Genetic modifications that have been explored individually or in combination with the purpose of improving the function and safety of T cells. These modifications include the transgenic expression of proteins that (i) enhance T cell homing to tumor sites, (ii) provide resistance to the tumor microenvironment, (iii) improve their proliferation and persistence and (iv) enhance their safety.
Genetic modification of T cells
Effective genetic modification of T cells requires the use of systems that produce adequate gene transfer and expression of the desired transgene. The choice of gene transfer vector is dictated by the desired level and duration of expression necessary for the hoped-for therapeutic benefit.
Viral vectors have long been used as vehicles to deliver therapeutic genes to target cells. To permit sustained expression in a highly proliferative cell, such as the T cell, the majority of studies to date have used vectors that integrate in the host T cell genome, usually gammaretrovirus or lentivirus-based vectors, thereby avoiding the dilutional effect that would follow cell division if a non-integrating, non-replicating vector is used. Though gene-modified T cells have a long in vivo safety profile(16, 17) viral vectors are expensive to produce and test, and there is often a requirement for onerous and prolonged follow-up of treated patients that further adds to both cost and complexity(18). This has ensured continued interest in the development of efficient non-viral gene transfer.
RNA or DNA-based expression plasmids are much less expensive than viral vectors to produce and test, and can be used to alter T cell biology when efficient transgene integration (and hence long-term expression) is not required. More recently, transposon-based gene delivery systems have been developed that offer the practical advantages of plasmids coupled with the integrative capabilities of retroviruses. Most transposons are binary systems, incorporating two expression plasmids, one encoding the transposase and the other containing the gene of interest flanked by the transposon terminal repeat sequence required for transposition. After delivery to the target cell, the transposase binds to the terminal repeat sequences of the donor plasmid and the host genome, excises the gene of interest, and inserts it into the host genome. Transposons, unlike retroviral vectors, do not preferentially integrate close to transcription start sites in the host cell genome, potentially improving their safety profile. The Sleeping Beauty transposon is now being used to gene-modify T cells that are then adoptively transferred to patients with B cell malignancies, while the Piggybac system is being evaluated for similar application(19–22).
Modifications that enhance T cell targeting
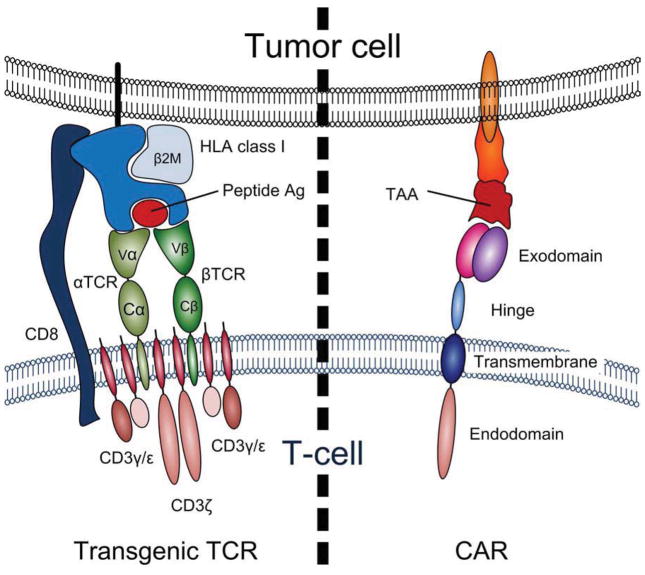
The generation of tumor-reactive T cells from cancer patients is often difficult due to the low immunogenicity of TAAs, which are either “self” antigens or “naïve” targets for the immune system. Therefore, investigators have explored genetic engineering approaches whereby autologous T cells are modified to express tumor-specific receptors. Two basic gene transfer approaches have been pursued clinically – (i) the transfer of antigen-specific receptor α and β chains, and (ii) the transgenic expression of chimeric antigen receptors composed of antibody-binding domains fused to T cell signaling domains (Figure 2).
Figure 2.
a schematic of transgenic α and β T cell receptors (αβT CRs) and chimeric antigen receptor (CARs) and shows the differences and similarities between these two most common strategies used to redirect the immune T cell response.
αβTCR gene transfer
In the above process, T cells are modified ex vivo to express TCR αβ heterodimers directed against a specific tumor target. These α and β chains may be isolated from T cell clones, from mice that are transgenic for the human TCR, or from phage/yeast/T cell displays(23,24). Once selected, the affinity of these TCRs can be further enhanced by mutation/selection. To date, transgenic α and β TCR chains targeting TAAs including melanoma antigens, minor histocompatibility antigens, and common oncoproteins have been generated and used to modify non-specifically activated T cells, rapidly producing a tumor-directed clinical grade product(25).
A number of factors have limited the broader introduction of this approach. Transferred α and β chains can cross-pair with endogenous TCR chains, forming hybrid TCRs with either loss of activity or gain of new and unwanted (autoimmune) specificity. The frequency of this problem can be reduced by incorporating a murine-derived transmembrane region in the transgenic TCR, though this is not ideal given the potential immunogenicity(26), or through the introduction of sequences encoding cysteine residues to form additional disulfide bonds that stabilize pairing of the transgenic TCR and minimize cross pairing with endogenous α and β chains(27–29). Alternatively, selective disruption of the endogenous αβTCR using zinc-finger nucleases (ZFNs)(30,31), or the substitution of γδ T cells as the platform for αβ transgenic TCR transduction may prevent this problem(32). At the moment we do not know how crucial these modifications will be or which will be most favorable for clinical use. Perhaps a more important general limitation of this strategy is that conventional TCRs recognize only single peptides presented in the context of individual HLA alleles, thus limiting their use to individuals with the appropriate HLA polymorphism. Hence, application to the broadest possible range of patients requires large panels of TCRs to be made and tested in large numbers of patients. Even then, loss of the single targeted peptide epitope may lead to tumor immune escape. Finally, as investigators select TCR clones with non-physiologically high levels of affinity, the risk of toxicity due to off-target binding to related epitopes present on normal tissue becomes an increasing concern.
Clinical studies with αβTCRs
Despite these concerns a number of clinical trials using engineered T cells expressing αβTCR have been initiated. These trials have focused on targeting well studied and extensively characterized self antigens including MART1 and gp100 (melanoma)(33,34), carcinoembryonic antigen (CEA) (colorectal cancer)(35), NY-ESO-1 (melanoma, synovial sarcoma and multiple myeloma)(36) and MAGE-A3 (melanoma, multiple myeloma, synovial sarcoma and esophageal cancer)(37). Though promising clinical responses support the therapeutic potential of this approach, there have also been a number of reported toxicities related to “on target” but “off tissue” effects. For example, investigators at the National Cancer Institute (NCI) reported the development of skin rashes, uveitis and hearing loss in patients treated with high affinity transgenic αβTCRs specific for MART1 or gp100(33), while the infusion of CEA-targeted T cells was associated with the development of severe inflammatory colitis(35). More recently, Morgan et al treated 9 patients with MAGE-A3 positive tumors with T cells modified with a high-avidity TCR directed against an HLA-A2-restricted MAGE-A3 epitope and though 5 patients experienced clinical regressions, 3 experienced mental status changes, two of whom lapsed into comas and subsequently died. Brain biopsies or postmortem brain autopsies revealed infiltration of CD8+ T-cells into the white matter and perivascular spaces. Furthermore, expanded CSF cells from an affected patient produced IFNγ in response to MAGE-A3+ tumor cell lines. It subsequently transpired that the TCR used in this study recognized not only MAGE-A3 but also MAGE-A9 and MAGE-A12, which was found to be expressed in human brain possibly explaining the neuronal cell destruction that precipitated post adoptive transfer(37).
Finally, “off target” toxicity has also been reported using MAGE-A3 TCR-modified T cells. In this case T cells were modified to express an affinity-enhanced αβTCRs targeting an HLA-A1 epitope from MAGE-A3 that was subsequently used as treatment for melanoma or relapsed multiple myeloma. The first patient, who received the cells as treatment for metastatic melanoma, died 4 days post-infusion of cardiac failure. Following extensive studies the cause of death was attributed to hemorrhagic myocardial infarction precipitated by demand ischemia and subsequently the trial was re-opened. However, the second patient, treated for relapsed multiple myeloma, also developed cardiogenic shock and died 5 days after infusion. Again, after ruling out the expression of MAGE-A3 or related MAGE proteins in cardiomyocytes/heart tissue, the group undertook a systematic investigation of TCR binding and reactivity, which revealed that their affinity-enhanced TCR recognized an unrelated peptide derived from Titin, which is highly expressed in muscle tissue and a target of auto-antibodies in some forms of myasthenia gravis, particularly in patients with thymomas(38). Subsequently, iPS cardiomyocytes were confirmed to express Titin. However, Maus et al also indicated that endogenous T cells with native specificity for the same epitope did not demonstrate any activity against Titin. Thus, in this case the cross-reactivity and subsequent toxicity appears to be a function of the engineering process that was designed to enhance affinity rather than the endogenous specificity(39,40 ).
Chimeric antigen receptors (CARs)
T cell specificity can also be altered by expressing chimeric antigen receptors (CAR), which are artificial receptors composed of an extracellular domain that is responsible for antigen recognition, a transmembrane domain and one or more intracellular signaling domains. The extracellular domain is most commonly derived from the variable regions (i.e. antigen binding portion) of the heavy and light chains (VH and VL,) of a monoclonal antibody joined by a flexible linker. The intracellular signaling domain (endodomain) is most usually derived from the T cell receptor (CD3) zeta chain. CAR expression allows tumors to be targeted in an HLA-unrestricted manner, increasing the number of eligible patients, and extends the types of antigens that can be recognized by T cells to include carbohydrates and glycolipids. Second and third generation CARs incorporate additional endodomains that provide the necessary accessory signals or co-stimulation to allow T cells to pass through the multiple checkpoints that under physiological conditions regulate T cell activation, proliferation, differentiation and survival following receptor engagement(41).
Table I describes some of the CARs that have been developed for clinical use in solid tumors and hematological malignancies. Initial trials using T cells modified to express CARs that contained exclusively the CD3ζ signaling domain (so called first generation constructs) proved sub-optimal. Indeed, CAR engagement failed to induce either cytokine production or T cell expansion in vivo. Subsequently, second generation CARs, which contained additional co-stimulatory endodomains including CD27, CD28, 41BB, DAP10, OX40 or ICOS proved to confer greater strength of signaling and persistence to the T cells, resulting in improved potency. For example, in a head to head comparison, Savoldo and colleagues demonstrated that CAR-CD19 T cells encoding the costimulatory CD28 endodomain had strikingly enhanced expansion and persistence compared with their counterparts lacking this endodomain(42). Porter and colleagues used CAR-CD19-modified T cells expressing the 41BB endodomain to treat chronic lymphocytic leukemia (CLL) and saw significant in vivo expansion, and persistence for at least 6 months, which resulted in complete clinical responses in 2 of 3 treated patients(43,44). More recently the same group has applied this strategy for the treatment of patients with relapsed and refractory pre-B cell acute lymphoblastic leukemia (ALL)(45). Initial results from two treated patients confirmed the in vivo proliferative capacity of the infused cells in vivo, with detection in blood, bone marrow and cerebrospinal fluid post-infusion. Again, the transferred T cells produced initial clinical responses in both patients. However, this was sustained in only one while the second patient relapsed with a CD19 negative tumor two months post-treatment, demonstrating the potential for immune escape using a mono-specific therapy that targets a molecule that is not of direct pathogenic relevance(45). In addition, many of the B-CLL and ALL responders have developed acute toxicities during the expansion phase of the T cells, associated with fevers and the release of high levels of cytokines and cytokine receptors including soluble IL1Rα, IL2R, IL2, IL6, IL10, TNFα and IFNγ. Monoclonal antibodies to TNFα and the IL6R (etanercept and tocilizumab, respectively) were apparently able to rapidly reverse these toxicities. Other investigators using second and third generation CARs (incorporating 3 or more endodomains) have described similar toxicities(46–48).
Table I.
shows the different types of CARs that have been developed for the treatment of hematological and solid malignancies and their stage of preclinical and clinical development.
| Hematologic malignancies | CAR construct | Preclinical data | Clinical data | References | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Target Antigen | Exodomain (clone) | Hinge | Trans membrane | Endo domain 1 | Endo domain 2 | Endo domain 3 | |||
|
| |||||||||
| CD19 | ScFV(FMC63) | CH2CH3 | CD4 | CD3ζ | − | − | + | + | 53, 116–118 |
| ScFV(FMC63) | FcRγ | FcRγ | CD3ζ | − | − | + | − | 119,120 | |
| ScFV(FMC63) | CD8α | CD8 | CD3ζ | − | − | + | − | 121,122 | |
| ScFV(FMC63) | CH2CH3 | CD28 | CD3ζ | − | − | + | + | 42 | |
| ScFV(SJ25C1) | CD8α | CD8 | CD3ζ | − | − | + | − | 123,124 | |
| ScFV(FMC63) | CD28 | CD28 | CD28 | CD3ζ | − | + | + | 120,125–127 | |
| ScFV(FMC63) | CH2CH3 | CD28 | CD28 | CD3ζ | − | + | + | 19,42, 117,128 | |
| ScFV(FMC63) | CD8α | CD8 | 41BB | CD3ζ | − | + | + | 43–45,121,122 | |
| ScFV(FMC63) | CD8α | CD28 | CD28 | CD3ζ | − | + | − | 121,122 | |
| ScFV(SJ25C1) | CD28 | CD28 | CD28 | CD3ζ | − | + | + | 106,124,129–131 | |
| ScFV(FMC63) | CD8 | CD8 | CD28 | 41BB | CD3ζ | + | − | 125 | |
|
| |||||||||
| CD19 | ScFV(FMC63) | CD8α | CD28 | CD28 | 41BB | CD3ζ | + | − | 121,122 |
|
| |||||||||
| CD20 | ScFV(Leu16) | CH2CH3 | CD4 | CD3ζ | − | − | + | + | 53,132–137 |
| ScFV(Leu16) | CH2CH3 | CD4 | CD28 | CD3ζ | − | + | − | 137 | |
| ScFV(Leu16) | CH2CH3 | CD4 | CD28 | CD137 | CD3ζ | + | + | 137,138 | |
|
| |||||||||
| CD22 | ScFV(M791) | CH2CH3 | CD28 | CD28 | CD3ζ | − | + | − | 139 |
| ScFV(HA22) | CH2CH3 | CD28 | CD28 | CD3ζ | − | + | − | 139 | |
| ScFV(BL22) | CH2CH3 | CD28 | CD28 | CD3ζ | − | + | − | 139 | |
| ScFV(HA22SH) | CH2CH3 | CD28 | CD28 | CD3ζ | − | + | − | 139 | |
| ScFV(HA22SH) | CH2CH3 | CD8 | 41BB | CD3ζ | − | + | − | 139 | |
| ScFV(M791) | CH2CH3 | CD8 | CD28 | 41BB | CD3ζ | + | − | 139 | |
| ScFV(HA22) | CH2CH3 | CD8 | CD28 | 41BB | CD3ζ | + | − | 139 | |
| ScFV(BL22) | CH2CH3 | CD8 | CD28 | 41BB | CD3ζ | + | − | 139 | |
| ScFV(HA22SH) | CH2CH3 | CD8 | CD28 | 41BB | CD3ζ | + | − | 139 | |
|
| |||||||||
| CD30 | ScFV(HRS3) | CH2CH3 | FcεR1γ | CD3ζ | − | − | + | − | 62,140, 141 |
| ScFV(HRS3) | CH2CH3 | CD28 | CD28 | CD3ζ | − | + | − | 62 | |
|
| |||||||||
| Kappa | ScFV(CRL1785) | CH2CH3 | CD28 | CD3ζ | − | − | + | − | 142 |
| ScFV(CRL1785) | CH2CH3 | CD28 | CD28 | CD3ζ | − | + | − | 142 | |
|
| |||||||||
| CD70 | CD27 | CD27 | CD27 | CD3ζ | − | − | + | − | 143 |
|
| |||||||||
| CD123 | ScFV(7G3) | CH2CH3 | CD28 | CD3ζ | − | − | + | − | 144 |
|
| |||||||||
| NKG2D | mNKG2D | mNKG2D | mNKG2D | CD3ζ | − | − | + | − | 145–148 |
| Solid tumors | CAR construct | Preclinical data | Clinical data | References | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Target Antigen | Exodomain (clone) | Hinge | Trans membrane | Endo domain 1 | Endo domain 2 | Endo domain 3 | |||
|
| |||||||||
| GD2 | ScFV(14.G2a) | CH2CH3 | FcεR1γ | CD3ζ | − | − | + | − | 149, 150 |
|
| |||||||||
| GD2 | ScFV(7A4) | CH2CH3 | FcεR1γ | CD3ζ | − | − | + | − | 149, 150 |
| ScFV(14.G2a) | CH2CH3 | CD3 | CD3ζ | − | − | + | + | 78,79,149, 150 | |
|
| |||||||||
| HER2 | ScFV(NY29) | N/A | N/A | CD3ζ | − | − | + | − | 151 |
| ScFV(NY29) | CD28 | FcR1γ | CD3ζ | − | − | + | − | 152, 153 | |
| ScFV(FRP5) | CH2CH3 | FcεR1γ | CD3ζ | − | − | + | − | 154 | |
| ScFV(4D5) | CD28 | CD28 | CD3ζ | − | − | + | − | 155 | |
| ScFV(4D5) | CD8 | CD8 | CD3ζ | − | − | + | − | 155 | |
| ScFV(ICR12) | CD8α | CD3 | CD3ζ | − | − | + | − | 57 | |
| ScFV(FRP5) | N/A | CD28 | CD28 | CD3ζ | − | + | − | 156,157 | |
| ScFV(FRP5) | CH2CH3 | CD28 | CD28 | CD3ζ | − | + | − | 158 | |
| ScFV(FRP5) | CD8α | CD28 | CD28 | CD3ζ | − | + | − | 159 | |
| ScFV(4D5) | CD28 | CD28 | CD28 | CD3ζ | − | + | − | 155 | |
| ScFV(4D5) | CD8 | CD8 | CD28 | CD3ζ | − | + | − | 155 | |
|
| |||||||||
| HER2 | ScFV(4D5) | CD8 | CD8 | 41BB | CD3ζ | − | + | − | 155 |
| ScFV(ICR12) | CD28 | CD28 | CD28 | CD3ζ | − | + | − | 57 | |
| ScFV(4D5) | CD28 | CD28 | CD28 | 41BB | CD3ζ | + | − | 155 | |
| ScFV(4D5) | CD28 | CD28 | CD3ζ | CD28 | 41BB | + | − | 155 | |
| ScFV(4D5) | CD8α | CD8 | 41BB | CD28 | CD3ζ | + | − | 155 | |
| ScFV(4D5) | CD8α | CD8 | CD28 | 41BB | CD3ζ | + | + | 55,155 | |
| ScFV(4D5) | CD8α | CD8 | 41BB | CD3ζ | CD28 | + | − | 155 | |
|
| |||||||||
| FBP | ScFV(MOv18) | N/A | N/A | CD3ζ | − | − | + | − | 160, 161 |
| ScFV(MOv18) | N/A | FcRγ | CD3ζ | − | − | + | + | 52,162, 163 | |
|
| |||||||||
| CD171 | ScFV(CE7) | Fc | CD4 | CD3ζ | − | − | + | + | 164, 165 |
|
| |||||||||
| CAIX | ScFV(G250) | N/A | FcεR1γ | CD3ζ | − | − | + | − | 166, 167 |
| ScFV(G250) | CH2CH3 | CD4 | CD3ζ | − | − | − | + | 50,51,168 | |
|
| |||||||||
| PSMA | ScFV(J591) | CD8 | CD8 | CD3ζ | − | − | + | − | 169, 170 |
|
| |||||||||
| PSMA | ScFV(J591) | CD28 | CD28 | CD28 | CD3ζ | − | + | − | 170 |
|
| |||||||||
| IL13-zetakine | ScFV(E13Y) | Fcγ4 | CD4 | CD3ζ | − | − | + | − | 171–173 |
| ScFV(E13Y) | N/A | CD3 | CD28 | CD3ζ | − | + | − | 174 | |
| ScFV(E13Y) | CD8 | CD3 | CD28 | CD3ζ | − | + | − | 174 | |
|
| |||||||||
| EphA2 | ScFV(4H5) | CH2CH3 | CD28 | CD28 | CD3ζ | − | + | − | 175 |
|
| |||||||||
| FAP | ScFV(MO36) | Ch2CH3 | CD28 | CD28 | CD3ζ | − | + | − | 176 |
|
| |||||||||
| KDR | ScFV(P3S5) | CD28 | CD28 | CD3ζ | − | − | + | − | 177 |
| ScFV(mVEGFR-164) | cMyc-CD8 | CD8 | CD3ζ | − | − | + | − | 178 | |
| ScFV(mDC101) | CD8 | CD8 | CD28 | CD3ζ | − | + | − | 179,180 | |
| ScFV(KDR-1121) | CD28 | CD28 | CD28 | CD3ζ | − | + | − | 179, 180 | |
| ScFV(mDC101) | CD8 | CD8 | CD28 | 41BB | CD3ζ | + | − | 179, 180 | |
| ScFV(mSP6) | CD8 | CD8 | CD28 | 41BB | CD3ζ | + | − | 179, 180 | |
| ScFV(KDR-1121) | CD28 | CD28 | CD28 | 41BB | CD3ζ | + | − | 179, 180 | |
|
| |||||||||
| VEGFR-1 | ScFV(V-1) | CH2CH3 | CD4 | CD3ζ | − | − | + | − | 181 |
|
| |||||||||
| EGFRvIII | ScFV(3C10) | CD8 | CD8 | CD3ζ | − | − | + | − | 182 |
| ScFV(mab139) | CD8α | CD8 | CD3ζ | − | − | + | − | 183 | |
| ScFV(mab108) | FcIgG2 | CD28 | CD28 | CD3ζ | − | + | − | 184 | |
| ScFV(mab139) | CD8α | CD8 | ICOS | CD3ζ | − | + | − | 185 | |
|
| |||||||||
| Mesothelin | ScFV(SS1) | CD8α | CD8 | CD3ζ | − | − | + | − | 186 |
| ScFV(P4) | CD8α | CD8 | CD3ζ | − | − | + | − | 187 | |
| ScFV(SS1) | CD8α | CD8 | 41BB | CD3ζ | − | + | − | 63,186,188 | |
| ScFV(SS1) | CD8α | CD28 | CD28 | CD3ζ | − | + | − | 186 | |
| ScFV(P4) | CD8α | CD28 | CD28 | CD3ζ | − | + | − | 187 | |
| ScFV(SS1) | CD8α | CD28 | CD28 | 41BB | CD3ζ | + | − | 176 | |
|
| |||||||||
| CD44 v6/v7 | ScFV(1.1ASML) | CD8α | CD3 | CD3ζ | − | − | + | − | 189 |
| ScFV(VFF17) | cMyc-CD8α | CD3 | CD3ζ | − | − | + | − | 190 | |
|
| |||||||||
| TAG72 | ScFV(B72.3) | N/A | N/A | FCεR1γ | − | − | + | − | 140,191 |
| ScFV(CC49) | CH2CH3 | CH2CH3 | CD3ζ | − | − | + | − | 140,191 | |
| ScFV(CC49) | Fc1γ-CH3 | CD4 | CD3ζ | − | − | + | − | 192 | |
|
| |||||||||
| Lewis-Y | ScFV(Mluc1) | N/A | N/A | FcRγ | − | − | + | − | 193 |
| ScFV(Hu3S193) | CD8 | CD28 | CD28 | CD3ζ | − | + | − | 194–196 | |
|
| |||||||||
| MUC1 | ScFV(SM3) | CD28 | CD28 | CD3ζ | − | − | + | − | 197 |
| ScFV(SM3/HMFG2) | CH2CH3 | CD28 | CD3ζ | − | − | + | − | 61 | |
| ScFV(SM3) | IgD-CD28 | CD28 | CD28 | CD3ζ | − | + | − | 197 | |
| ScFV(SM3/HMFG2) | FcIgG1 | CD28 | CD28 | CD3ζ | − | + | − | 197 | |
| ScFV(HMFG2) | FcIgG1 | CD28 | CD28 | CD3ζ | − | + | − | 197 | |
| ScFV(HMFG2) | FcIgG1 | CD28 | CD28 | CD3ζ | − | + | − | 57 | |
| ScFV(HMFG2) | IgD-FcIgG1 | CD28 | CD28 | OX40 | CD3ζ | + | − | 60,197,198 | |
| ScFV(HMFG2) | FcIgG1 | CD28 | CD28 | 41BB | CD3ζ | + | − | 197 | |
|
| |||||||||
| FAR | ScFV(Fab35) | FcIgG1 | CD3 | CD3ζ | − | − | + | − | 199,200 |
| ScFV(Fab35) | FcIgG1 | CD28 | CD28 | CD3ζ | − | + | − | 200 | |
|
| |||||||||
| NCAM | ScFV(D29) | CD3 | CD3 | CD3ζ | − | − | + | − | 201,201 |
|
| |||||||||
| CEA | ScFV(MFE23,22) | CD3 | CD3 | CD3ζ | − | − | + | − | 201–203 |
| ScFV(MD45) | CD8 | FCγR | FCγR | − | − | + | − | 204 | |
| ScFV(pUC19) | CD8 | CD3 | CD3ζ | − | − | + | − | 205 | |
| ScFV(BW431/26) | FcIgG1 | CD3 | CD3ζ | − | − | + | − | 206 | |
| ScFV(MFE23) | CD8α | CD3 | CD3ζ | − | − | + | − | 207 | |
| ScFV(A3B3) | CD8α | N/A | CD3ζ | − | − | + | − | 208 | |
| ScFV(pUC19) | CD8 | CD3 | CD3ζ | − | − | + | − | 209 | |
| ScFV(C2-45) | CD8α | CD3 | CD3ζ | − | − | + | − | 210 | |
| ScFV(SCA431) | Fc | CD4 | CD3ζ | − | − | + | − | 211 | |
| ScFV(MFE(23,22) | CD28 | CD28 | CD28 | CD3ζ | − | + | − | 203 | |
|
| |||||||||
| CEA | ScFV(BW431/26) | FcIgG1 | CD28 | CD28 | CD3ζ | − | + | − | 159 |
| ScFV(MFE23) | CD8α | CD28 | CD28 | CD3ζ | − | + | − | 207 | |
| ScFV(F11-39) | CD8α | CD28 | CD28 | CD3ζ | − | + | − | 212,213 | |
| ScFV(C2-45) | CD8α | CD28 | CD28 | CD3ζ | − | + | − | 210,214 | |
| ScFV(pUC19) | CD8-CD28 | CD28 | CD28 | CD3ζ | − | + | − | 209 | |
| ScFV(SCA431) | Fc | CD4 | CD28 | CD3ζ | − | + | − | 215 | |
|
| |||||||||
| EGP2 | ScFV(GA733.2) | CD8α | FcRγ | FcRγ | CD3ζ | − | + | − | 216,217 |
|
| |||||||||
| EGP40 | ScFV(BR3E4/FG1) | N/A | N/A | FcRγ | − | − | + | − | 218 |
|
| |||||||||
| ERBB3/4 | ScFV(C11) | cMyc-CD8α | CD3 | CD3ζ | − | − | + | − | 219 |
| ScFV(FRP5) | cMyc-CD8α | CD3 | CD3ζ | − | − | + | − | 219 | |
| ScFV(RAK) | cMyc-CD8α | CD3 | CD3ζ | − | − | + | − | 219 | |
| ScFV(heregulinβ1) | cMyc-CD8α | CD3 | CD3ζ | − | − | + | − | 219 | |
| ScFV(hrgα) | CH2CH3 | CD34 | CD3ζ | − | − | + | − | 220 | |
|
| |||||||||
| ERBB3/4 | ScFV(hrgβ) | CH2CH3 | CD34 | CD3ζ | − | − | + | − | 220 |
|
| |||||||||
| ErbB | ScFV(T1E) | CD28 | CD28 | CD28 | CD3 ζ | − | + | − | 221 |
|
| |||||||||
| GD-3 | ScFV(MB3.6) | CD8α | CD3 | CD3ζ | − | − | + | − | 222,223 |
| ScFV(MB3.6) | CD8α | CD28 | CD28 | CD3ζ | − | + | − | 222 | |
|
| |||||||||
| PSCA | ScFV(7F5) | βC2 | CD3 | CD3ζ | − | − | + | − | 224 |
| ScFV(hu1G8) | CH2CH3 | CD28 | CD3ζ | − | − | + | − | 225 | |
| ScFV(hu1G8) | CD8α | CD8 | CD3ζ | − | − | + | − | 226 | |
| ScFV(7F5) | CD8α | CD8 | CD3ζ | − | − | + | − | 226 | |
| ScFV(MB1) | CD8α | CD8 | CD3ζ | − | − | + | − | 226 | |
|
| |||||||||
| HLA-A1+MAGE1 | ScFV(MZ2-82/30) | FcIgG1-CD3 | CD3 | CD3ζ | − | − | + | − | 227 |
| ScFV(G8) | N/A | CD4 | FcεR1γ | − | − | + | − | 228,229 | |
| ScFV(G8) | CD28 | CD28 | FcεR1γ | − | − | + | − | 229 | |
| ScFV(Hyb3) | CD28 | CD28 | FcεR1γ | − | − | + | − | 229 | |
|
| |||||||||
| NKG2D | mNKG2D | mNKG2D | mNKG2D | CD3ζ | − | − | + | − | 230–234 |
|
| |||||||||
| NKG2D | hNKG2D | hNKG2D | hNKG2D | CD3ζ | − | − | + | − | 235,236 |
| hNKG2D | FcIgG1 | CD28 | CD28 | CD3ζ | − | + | − | 237 | |
Immune responses to transgenic receptors
Most CARs and many transgenic TCRs contain novel sequences or sequences derived from other species. As a consequence, the recipient may generate an immune response that eliminates the modified T cells. For example, Lamers and colleagues reported the development of anti-scFv antibodies in three patients treated with T cells expressing a carbonic anhydrase IX (CAIX)-specific CAR(49–51), while Kershaw and colleagues observed a CAR-specific antibody response in a patient treated with CAR T cells modified to recognize the ovarian-associated α-folate receptor (αFR)(52). More recently, Jensen and colleagues reported lack of in vivo persistence due to the induction of endogenous cellular immune responses directed against a selection gene (neomycin phosphotransferase) incorporated in their CD20 CAR-containing plasmid(53). Although cellular and humoral responses have not been observed in all treated patients, the substitution of humanized single chain CARs may reduce the risk of premature deletion of T cells due to immune responses.
Safety concerns
As for αβTCR-modified T cells, a major concern with CAR T cell transfer relates to the potential for “on target antigen” but “off target tissue” toxicity – an effect associated with targeting TAAs that are not exclusively tumor-restricted in their expression profile. Lamers et al reported the development of cholestasis following the infusion of T cells modified with a CAR targeting carbonic anhydrase as treatment for renal cell carcinoma, which correlated with expression of carbonic anhydrase on biliary epithelial cells(49–51). Brentjens and colleagues reported renal and respiratory failure in a patient with CLL after a single infusion (3x107/kg) of T cells modified with a second generation CAR targeting CD19 that was given following high dose cyclophosphamide, administered to induce host lymphodepletion(54). The authors hypothesized the combination therapy may have led to a cytokine storm in vivo or to rapid tumor lysis. Finally, Morgan et al infused >1010 T cells modified with a third generation CAR targeting HER2 to a patient with widely metastatic colon cancer after intensive lymphodepletion. The subject rapidly developed pulmonary toxicity and died 4 days after infusion. After extensive analysis the investigators concluded that the toxicity might have been due to targeting of low levels of HER2 on pulmonary endothelium - a known site at which intravenously infused human CAR T cells accumulate(55,56).
Selective targeting
While it is now clear that genetically modified T cells can be targeted to tumors, it is equally apparent that targeting a single epitope or antigen alone has a high risk of leading to tumor immune escape or to toxicity due to “on target, off organ” effects if the targeted antigen is not uniquely expressed on target tumor cells. To improve safety Wilkie et al modified activated T cells with two CARs targeting the breast cancer-expressed TAAs HER2 (coupled with the CD3ζ endodomain) and MUC1 (coupled to CD28) and demonstrated that these dual-targeted T cells were able to deliver complementary signals, leading to potent cytotoxicity and synergistically-enhanced proliferation in the presence of tumor cells expressing both target antigens(57). Similar results have more recently been reported by Sadelain and colleagues(58). In addition, investigators are developing hybrid receptors which will induce T cell activation at the tumor site by inverting the inhibitory effects of cytokines, such as IL4, produced in the local environment(59,60). Finally, an alternate approach to promote potent anti-tumor effects while minimizing toxicity is to combine CAR and conventional therapies. The feasibility of such a strategy has recently been demonstrated by Sanchez and colleagues, who combined CAR T cells engineered to recognize MUC1 with anti-androgen therapy to provide additive anti-tumor effects in a prostate cancer model(61).
Genetic modification of T cells to improve in vivo migration, proliferation and survival
T cell migration
Once tumor-specific T cells are infused, they must migrate to distal tumor sites before exerting their cytotoxic effects. T cell migration occurs along a chemokine gradient so efficient trafficking requires that the chemokine receptors expressed by the infused T cells must match the chemokines produced by the tumor. In practice, however, tumor and surrounding stromal cells can produce a chemokine milieu that recruits T cell subsets such as Tregs that support rather than perturb the tumor microenvironment. In HD, for example, malignant Reed-Sternberg cells secrete chemokines (e.g. TARC) that attract immunoinhibitory/suppressive Th2 cells and Tregs, both of which contribute to the hostile immune microenvironment and directly impair the antitumor activity of effector T cells.
Gene transfer can alter the migration profile of infused, tumor-targeted (and pro-inflammatory) T cells through the forced expression of chemokine receptors that are matched with the chemokines produced by the target tumor, allowing the transferred cells to exploit the tumor’s own inhibitory mechanisms. Our group has expressed transgenic CCR4 receptors on T cells expressing CAR-CD30, an antigen that is highly expressed by many HD cells, allowing the tumor targeted effector cells to migrate toward the HD-generated TARC gradient, the cognate chemokine for CCR4(62). Similarly, Moon et al increased the migration of mesothelin-directed CAR T cells towards malignant pleural mesotheliomas, by modifying them to express CCR2b, the cognate receptor for the chemokine CCL2 that the tumors produce(63). CCL2 is secreted by many tumor types, and modifying CAR-GD2 T cells with the same chemokine receptor (CCR2b) produces a >10-fold increase in the homing capacity of the transgenic cells towards CCL2-secreting neuroblastoma with increased anti-tumor activity(64). A similar approach could be applied to other human malignancies in which a signature chemokine expression profile can be identified.
T cell proliferation and in vivo persistence
T cell proliferation requires continued antigenic stimulation, either via direct interaction with tumor cells or through professional APCs that present tumor antigens, as well as the presence of appropriate cytokines. Moreover, a proportion of the cells should enter the memory T cell compartment after infusion, so that protection can be assured long-term. Tumors have developed an array of strategies to prevent these events from occurring, necessitating countermeasures that will ensure T cell proliferation and survival.
Transgenic expression of growth factors/growth factor receptors
Recombinant IL2 has been systemically administered to support the expansion and persistence of adoptively-transferred T cells, but is associated with significant toxicity and the expansion of T regs, potentially offsetting the immunological benefits(65). Investigators are now exploring alternative methods to expand T cells in vivo, by genetically modifying them to express the growth factors IL2 or IL15, thereby producing effector T cells that are self-sustaining. Both IL2 and IL15-modified cells have been shown to retain their antigen specificity, phenotype and function. Importantly, they also retain their dependence on antigenic stimulation for continued expansion. For example, Quintarelli and colleagues genetically modified EBV-specific T cells with retroviral vectors encoding either IL15 or IL2 and showed that both promoted ex vivo and in vivo expansion and antitumor activity, confirming that this was achieved without induction of Tregs(66,67).
T cell growth and survival can also be increased by engineering cells to respond to cytokines, which do not normally induce proliferation of in vitro expanded T cells. Vera and colleagues have shown that transgenic expression of the IL7 receptor by antigen-specific T cells restores their responsiveness to the IL7 cytokine, and sustains their expansion in vitro and in vivo without affecting their antigen specificity or cytokine dependence(68). Since this cytokine has been safely administered to human subjects without apparent enhancement of Treg cell number and function, the infusion of a tumor-targeted T cell product engineered to express the IL7R could be followed by exogenous administration of clinical grade IL7 cytokine to promote transgenic cell proliferation and survival(69–72).
Selected T cell populations for gene transfer
The persistence of gene-modified T cells may be favored by infusing T cell subsets with stem cell-like properties since these should have superior in vivo longevity(73). One way to achieve this goal is to culture the cells ex vivo in cytokines, including IL15, IL7 and IL21, that have been shown to promote the expansion of T cells with a central memory phenotype(74,75). In non-human primate proof of concept studies Berger et al infused effector (CD62L−CD28−CD8+Fashi) and central (CD62L+CD28+CD8+ Fashi) memory-derived CMV-specific T cell clones and demonstrated that the central memory-derived T cells survived longer in vivo, suggesting that T cells isolated from different compartments have divergent fate(76). More recently Wang and colleagues isolated human melanoma-specific T cells from the central memory compartment, grew them in culture, and then showed that these highly differentiated and expanded effector T cell clones nonetheless effectively targeted skin melanocytes and persisted long-term in vivo(77).
Another means of ensuring in vivo persistence of tumor directed T cells may be to retarget T cells that have native receptor specificity for latent viruses and are known to be long-lived memory cells. For example, adoptively transferred EBV-specific CTLs have been shown to persist long-term (>10 years) in vivo, likely due to the fact that the infused cells derived both from central and effector memory subsets and were able to receive physiological co-stimulation in vivo via exposure to EBV-infected APCs(8). To assess whether the same was true if EBV-CTLs were used as a CAR platform, Pule et al compared the longevity of mitogen-activated T cells with that of polyclonal EBV-specific CTLs modified with a CAR targeting GD2 in patients with advanced neuroblastoma(78). Early after infusion, CAR-GD2-modified EBV-CTLs circulated at higher levels than activated T cells, but in extended follow-up studies, cells derived from both activated T cells and EBV-CTL populations were detected long-term (192 and 96 weeks, respectively), and the duration of persistence correlated with the percentage of CD4+ helper T cells within the infused product as well as with their expression of the central memory markers (CD45RO+CD62L+)(79). Importantly, in vivo persistence was associated with superior clinical outcome. Thus, future clinical studies using T cells that have been selected based on a central memory phenotype may extend the life span of adoptively-transferred cells and improve clinical efficacy, though the complexity, cost, and large blood volumes required for the up-front clinical grade selection of such cells must also be taken into consideration(80).
Co-stimulation
T cell proliferation and survival requires both antigenic stimulation and the sequential engagement of co-stimulatory molecules. Unlike “professional” APCs, which express both antigen and co-stimulatory molecules, tumor cells may express only the target antigen. Exposure to antigen in the absence of co-stimulation can lead to T cell apoptosis or anergy. One means of providing T cell co-stimulation is to force the expression of co-stimulatory ligands, such as CD80 and 41BBL by the engineered T cell that will engage their native co-stimulatory receptors in an autocrine or paracrine manner(81). Alternatively, the signaling portions of co-stimulatory molecules including CD27, CD28, OX40 and 41BB, have been incorporated into the intracellular portion of second and third generation CARs so that CAR engagement with the target antigen delivers both the antigen activation and co-stimulation signals simultaneously, which may substitute for the lack of co-stimulation from the tumor cells themselves(41). The effects of modifying CAR T cells with additional co-stimulatory endodomains have been summarized in Table I.
Increasing T cell resistance to the tumor
T cell survival can be increased by overexpressing pro-survival/anti-apoptotic genes. For example, T cells transduced with the human telomerase reverse transcriptase (hTERT) gene have increased longevity due to the prevention of telomere erosion. Unfortunately, this modification may cause genomic instability, limiting both safety and clinical value(82–84). An alternative means of increasing T cell persistence is to modify cells with anti-apoptotic genes, such as Bcl-2 and Bcl-xL(85,86), or to downregulate pro-apoptotic genes such as Fas, thus making the cells resistant to Fas/FasL-mediated apoptosis(87).
Counteracting the hostile tumor microenvironment
Genetic modification of T cells can also be used to counteract the immune-inhibitory tumor microenvironment that can neutralize adoptively transferred antigen-specific CTLs. One of the most widely used tumor evasion strategies is local secretion of TGFβ by the tumor or its stromal elements. TGFβ is a multifunctional cytokine that promotes tumor growth, limits effector T cell proliferation and function, activates Tregs, and induces tolerance. The detrimental effects of TGFβ can be negated by modifying cells to express a dominant-negative TGFβ receptor type II (dnTGFβ-RII), prolonging their persistence and enhancing tumor elimination in mice bearing TGFβ-expressing tumors(88–90), and we are now assessing the safety and efficacy of dnTGFβ-RII-modified tumor-specific CTLs in patients with relapsed/refractory HD or NHL.
Wilkie and colleagues took this approach one step further – they proposed not just negating the inhibitory effects of a tumor-produced immunosuppressive cytokine, but instead switching the signal into one that was activating for the transferred T cells. To accomplish this goal they modified CAR T cells to express a custom chimeric cytokine receptor, consisting of the exodomain of the IL4 receptor fused to the endodomain of the shared IL2/15 βc endodomain. They hypothesized that transgenic expression of this molecule on T cells would protect them from the inhibitory effects of IL4, a prototypic Th2-polarizing/inhibitory cytokine produced by a variety of tumors, whilst providing a pro-proliferative signal to T cells via the IL2/15 βc endodomain directly at the tumor site(60). This approach has yet to be clinically translated.
Genetic modification to improve safety
Suicide Genes
Along with enhancing potency, increasing longevity or conferring resistance to inhibitory signals genetic engineering approaches can also be employed to incorporate a “safety switch’ so that the infused cells can be eliminated should adverse effects occur. Transgenic expression of the B cell antigen CD20 by T cells has been proposed as a suicide gene strategy, and this is being currently being evaluated preclinically(91,92). In the clinical setting one of the most well-studied suicide systems utilizes the herpes simplex viral thymidine kinase (TK) gene, which converts the pro-drug ganciclovir (GCV) to a purine analog, inhibiting DNA polymerase. Thus, GCV can be administered as a means of eliminating actively proliferating cells and the activity of this approach is currently being tested in late phase clinical studies(93–96). There are, however, several shortcomings to using TK as a suicide gene(97). One is the inherent immunogenicity of this virus-derived gene, which might lead to premature clearance of infused cells. Second is the removal of a therapeutically valuable drug as an option for the treatment of viral infections post-transplant. Another concern is the time required to ablate infused cells - usually days to weeks. Even a recently developed codon-optimized HSV-TK required 3 days ganciclovir exposure to produce transgenic cell death(98) – a time frame that would be inadequate in cases where infused cells cause acute on- or off-target toxicity. An attractive alternative suicide strategy is the inducible Caspase9 transgene (iCaspase9)(99–100), which is non-immunogenic and rapidly (within 24 hours) produces apoptosis, even in non-dividing cells(101). iCaspase9 is trigged upon administration of a small molecule dimerizer, AP20187, and produces apoptosis in >95% of transgenic cells. Thus, incorporation of this safety switch in combination with other modifications may be prudent as T cell potency is increased.
Commercialization Strategies
Broader clinical use of complex biologics such as engineered T cells for human disease will require strategies that address limitations due to the personalized nature of the therapy and the lack of scalability of the complex manufacturing processes associated with the genetic modification and cell expansion process. Investigators have begun to explore strategies to generate “universal T cells,” which can be use in the allogeneic setting as an “off the shelf” product, as well as to develop simplified methodologies to generate modified T cells that use new, scalable and cost effective manufacturing processes.
Universal T cells
To develop a “one size fits all” CAR T cell therapy, Tamada and colleagues demonstrated that a variety of tumors could be targeted using fluorescein isothiocyanate (FITC)-labeled monoclonal antibodies in combination with T cells engineered to express a FITC-directed CAR(102). Powell and colleagues proposed a similar approach whereby T cells were modified with a universal immune receptor specific for biotinylated antigen-specific molecules (biotin binding immune receptor; BBIR). These BBIR T cells can specifically recognize and be activated by various biotinylated molecules, including ScFVs and antibodies. Although this is an interesting approach, the biodistribution and immunogenicity of the modified cells is unclear(103,104). Alternatively, ZFN technology can be used to delete endogenous HLA molecule expression facilitating the generation of a less allostimulatory T cell product(30), but it is not clear how such cells would avoid being killed by NK effector cells, which may be activated when they engage an HLA negative target(31).
Scalability of Process
Independent of advances in developing universal T cells, commercialization will require manufacturing platforms for engineered T cells to become more scalable and robust. Conventional processes rely on ex vivo cell propagation in plates, flasks or bags, all of which have limitations with respect to the availability of nutrients and oxygen (O2), and the accumulation of metabolic waste products including lactic acid and carbon dioxide (CO2). This occurs because conventional cultureware is restricted to the use of a shallow media volume to allow sufficient gas diffusion from above the cells, which restricts both available nutrients and the buffering capacity of the media. In addition, O2 and nutrient requirements progressively increase with cell concentration and rate of growth, so that cultures must be fed and split regularly. These frequent medium changes and cell manipulations are time consuming, expensive, reduce the reproducibility of the cell product manufacture and increase the risk of contamination.
Scale-up using hollow fiber or stirred tank bioreactors or plastic bags may overcome the above issues, but are not always easy to adapt for T cell culture(105–107). Hollyman and colleagues used a culture system based around the WAVE Bioreactor for the expansion of CAR-CD19 T cells, obtaining between 0.8–2.4×1010 T cells in 13–18 days of culture(108). Indeed the same platform was also shown to support the reliable expansion of tumor infiltrating lymphocytes, with no adverse effects on T cell phenotype or function(109,110). The major advantage of the WAVE bioreactor is the potential for large scale T cell production (>1010 cells). However, the system is expensive, requiring the purchase of the bioreactors themselves as well as ancillary equipment. Supported in part by the NHLBI - Production Assistance for Cellular Therapies (PACT) mechanism, we have taken a different approach by using a gas-permeable culture device (G-Rex: Wilson Wolf Manufacturing). In this G-Rex platform, O2 and CO2 are exchanged across a silicone membrane at the base of the flask, which allows for an increased depth of medium above, providing more nutrients and diluting waste products. This system supports the expansion of a range of suspension cell types including genetically-modified T cells(111–115). Importantly, the platform is highly scalable, GMP-compliant, and reduces the number of technician interventions approximately 4-fold while increasing the cell output by 3–20-fold compared with conventional methods. These and other manufacturing improvements will help gene-modified products have a broader clinical utility and become more attractive from a commercial perspective. Indeed, we are seeing the first evidence of such interest with the recently formed partnerships between Novartis and the University of Pennsylvania as well as between bluebirdbio, Celgene and Baylor College of Medicine to advance novel T cell therapies for the treatment of cancer.
Conclusions
T cell immunotherapy has the potential to cure patients with advanced cancer and has already had impressive successes. However, many obstacles remain before this approach can reach its full potential and become a standard of care. By using genetic modification to improve target recognition, enhance T cell persistence, improve migration, and increase safety, investigators are steadily increasing the range of cancers that can be treated and the potency of the benefits obtained. However, even if successful, broader implementation will also depend on the development of T cell manufacturing processes that are robust and scalable, which will enable T cell therapies to become more accessible, in part by attracting interest from commercial entities who will in turn ultimately transform adoptive T cell transfer from “boutique to chain store”.
Acknowledgments
U.A. is supported by the Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand. We are grateful for long-term support from the US National Institutes of Health (National Cancer Institute and National Institute of Health Heart, Lung and Blood Institute), the Department of Defense, and the Leukemia and Lymphoma Research Fund. We are also grateful to the foundations who have supported the centers investigators, including Alex’s Lemonade Stand, V Foundation, Dana Foundation, James S McDonnell Foundation, and Adrienne Helis Medical Research Foundation.
Abbreviations
- ALL
acute lymphoblastic leukemia
- APCs
antigen presenting cells
- BBIR
biotin binding immune receptor
- CARs
chimeric antigen receptors
- CLL
chronic lynphocytic leukemia
- CTLs
Cytotoxic T lymphocytes
- dnTGFβ-RII
dominant-negative TGFβ receptor type II
- EBV
Epstein-Barr virs
- GCV
ganciclovir
- G-Rex
Gas-permeable culture device
- HD
Hodgkin disease
- HSCT
hematopoietic stem cell transplant
- hTERT
human telomerase reverse transcriptase gene
- iCaspase9
inducible Caspase 9 transgene
- IL
interleukin
- MHC
major histocompatilibity complex
- NHL
non-Hodgkin lymphoma
- NPC
nasopharyngeal carcinoma
- PACT
Production Assistance for Cellular Therapies
- PTLD
post-transplant lymphoproliferative disease
- TAAs
tumor-associated antigens
- TGF
transforming growth factor
- TILs
tumor-infiltrating lymphocytes
- TK
thymidine kinase
- Tregs
regulatory T cells
- ZFNs
zinc-finger nucleases
- αFR
α-folate recptor
Footnotes
Disclosure of interest
UA has no COI to report, MKB is a scientific advisory board member of Jennerex and of Bluebird Bio. Present research in Center for Cell and Gene Therapy (of which he is director) receives support from CellMedica and the centre has a Research Collaboration with Celgene and Bluebird Bio. JFV is a scientific adviser for Wilson Wolf Manufacturing. AML, JFV and MKB have patent applications in the specialty of T cell and gene-modified T-cell therapy for cancer. All authors have disclosed any financial or personal relationship with organizations that could potentially be perceived as influencing the described research and all authors have read the journal’s policy on disclosure of potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009 Jul 16;114(3):535–46. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Wunderlich J, Nishimura MI, Yu D, Yang JC, Topalian SL, et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–73. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Dudley ME. Cancer regression in patients with metastatic melanoma after the transfer of autologous antitumor lymphocytes. Proc Natl Acad Sci U S A. 2004;101(Suppl 2):14639–45. doi: 10.1073/pnas.0405730101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rooney CM, Smith CA, Ng C, Loftin SK, Li C, Krance RA, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 7.Heslop HE, Ng CYC, Li C, Smith CA, Loftin SK, Krance RA, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nature Medicine. 1996;2:551–5. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 8.Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–35. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bollard CM, Gottschalk S, Leen AM, Weiss H, Straathof KC, Carrum G, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110:2838–45. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bollard CM, Straathof KC, Huls MH, Leen A, Lacuesta K, Davis A, et al. The generation and characterization of LMP2-specific CTLs for use as adoptive transfer from patients with relapsed EBV-positive Hodgkin disease. J Immunother. 2004;27:317–27. doi: 10.1097/00002371-200407000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Bollard CM, Aguilar L, Straathof KC, Gahn B, Huls MH, Rousseau A, et al. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin’s disease. J Exp Med. 2004;200:1623–33. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Straathof KC, Leen AM, Buza EL, Taylor G, Huls MH, Heslop HE, et al. Characterization of latent membrane protein 2 specificity in CTL lines from patients with EBV-positive nasopharyngeal carcinoma and lymphoma. J Immunol. 2005;175:4137–47. doi: 10.4049/jimmunol.175.6.4137. [DOI] [PubMed] [Google Scholar]
- 13.Straathof KC, Bollard CM, Popat U, Huls MH, Lopez T, Morriss MC, et al. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus--specific T lymphocytes. Blood. 2005;105:1898–904. doi: 10.1182/blood-2004-07-2975. [DOI] [PubMed] [Google Scholar]
- 14.Louis CU, Straathof K, Bollard CM, Gerken C, Huls MH, Gresik MV, et al. Enhancing the in vivo expansion of adoptively transferred EBV-specific CTL with lymphodepleting CD45 monoclonal antibodies in NPC patients. Blood. 2009;113:2442–50. doi: 10.1182/blood-2008-05-157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu Rev Immunol. 2007;25:243–65. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- 16.Scholler J, Brady TL, Binder-Scholl G, Hwang W, Plesa G, Hege KM, et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med. 2012;1:132ra53. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–35. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bear AS, Morgan RA, Cornetta K, June CH, Binder-Scholl G, Dudley ME, et al. Replication-competent retroviruses in gene-modified T cells used in clinical trials: is it time to revise the testing requirements? Mol Ther. 2012;20:246–9. doi: 10.1038/mt.2011.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh H, Manuri PR, Olivares S, Dara N, Dawson MJ, Huls H, et al. Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system. Cancer Res. 2008;68:2961–71. doi: 10.1158/0008-5472.CAN-07-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hackett PB, Jr, Aronovich EL, Hunter D, Urness M, Bell JB, Kass SJ, et al. Efficacy and safety of Sleeping Beauty transposon-mediated gene transfer in preclinical animal studies. Curr Gene Ther. 2011;11:341–9. doi: 10.2174/156652311797415827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izsvak Z, Hackett PB, Cooper LJ, Ivics Z. Translating Sleeping Beauty transposition into cellular therapies: victories and challenges. BioEssays. 2010;32:756–67. doi: 10.1002/bies.201000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakazawa Y, Huye LE, Dotti G, Foster AE, Vera JF, Manuri PR, et al. Optimization of the PiggyBac transposon system for the sustained genetic modification of human T lymphocytes. J Immunother. 2009;32:826–36. doi: 10.1097/CJI.0b013e3181ad762b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li LP, Lampert JC, Chen X, Leitao C, Popovic J, Muller W, et al. Transgenic mice with a diverse human T cell antigen receptor repertoire. Nat Med. 2010;16:1029–34. doi: 10.1038/nm.2197. [DOI] [PubMed] [Google Scholar]
- 24.Leisegang M, Wilde S, Spranger S, Milosevic S, Frankenberger B, Uckert W, et al. MHC-restricted fratricide of human lymphocytes expressing survivin-specific transgenic T cell receptors. J Clin Invest. 2010;120:3869–77. doi: 10.1172/JCI43437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stauss HJ, Cesco-Gaspere M, Thomas S, Hart DP, Xue SA, Holler A, et al. Monoclonal T-cell receptors: new reagents for cancer therapy. Mol Ther. 2007;15:1744–50. doi: 10.1038/sj.mt.6300216. [DOI] [PubMed] [Google Scholar]
- 26.Davis JL, Theoret MR, Zheng Z, Lamers CH, Rosenberg SA, Morgan RA. Development of human anti-murine T-cell receptor antibodies in both responding and nonresponding patients enrolled in TCR gene therapy trials. Clin Cancer Res. 2010;16:5852–61. doi: 10.1158/1078-0432.CCR-10-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen CJ, Li YF, El-Gamil M, Robbins PF, Rosenberg SA, Morgan RA. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 2007;67:3898–903. doi: 10.1158/0008-5472.CAN-06-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linnemann C, Schumacher TN, Bendle GM. T-cell receptor gene therapy: critical parameters for clinical success. J Invest Dermatol. 2011;131:1806–16. doi: 10.1038/jid.2011.160. [DOI] [PubMed] [Google Scholar]
- 29.Jorritsma A, Schotte R, Coccoris M, De Witte MA, Schumacher TN. Prospects and limitations of T cell receptor gene therapy. Curr Gene Ther. 2011;11:276–87. doi: 10.2174/156652311796150390. [DOI] [PubMed] [Google Scholar]
- 30.Provasi E, Genovese P, Lombardo A, Magnani Z, Liu PQ, Reik A, et al. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat Med. 2012;18:807–15. doi: 10.1038/nm.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torikai H, Reik A, Liu PQ, Zhou Y, Zhang L, Maiti S, et al. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood. 2012;119:5697–705. doi: 10.1182/blood-2012-01-405365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiasa A, Nishikawa H, Hirayama M, Kitano S, Okamoto S, Chono H, et al. Rapid alphabeta TCR-mediated responses in gammadelta T cells transduced with cancer-specific TCR genes. Gene Ther. 2009;16:620–8. doi: 10.1038/gt.2009.6. [DOI] [PubMed] [Google Scholar]
- 33.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. Gene therapy with human and mouse T cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–46. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19:620–6. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–24. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36:133–51. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He ZF, Lv W, Qiao J, Chen ZM, Pang LW, Chen XJ. Thymic expression of the main immunogenic region of titin in thymomatous myasthenia gravis. J Int Med Res. 2010;38:1324–32. doi: 10.1177/147323001003800414. [DOI] [PubMed] [Google Scholar]
- 39.Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, et al. Cardiovascular toxicity and titin cross-reactivity of affinity enhanced T cells in myeloma and melanoma. Blood. 2013 Jun 14;2013 doi: 10.1182/blood-2013-03-490565. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, et al. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med. 2013;5:197ra103. doi: 10.1126/scitranslmed.3006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramos CA, Dotti G. Chimeric antigen receptor (CAR)-engineered lymphocytes for cancer therapy. Expert Opin Biol Ther. 2011;11:855–73. doi: 10.1517/14712598.2011.573476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–6. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heslop HE. Safer CARS. Mol Ther. 2010;18:661–2. doi: 10.1038/mt.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buning H, Uckert W, Cichutek K, Hawkins RE, Abken H. Do CARs need a driver’s license? Adoptive cell therapy with chimeric antigen receptor-redirected T cells has caused serious adverse events. Hum Gene Ther. 2010;21:1039–42. doi: 10.1089/hum.2010.131. [DOI] [PubMed] [Google Scholar]
- 48.Peinert S, Kershaw MH, Prince HM. Chimeric T cells for adoptive immunotherapy of cancer: using what have we learned to plan for the future. Immunotherapy. 2009;1:905–12. doi: 10.2217/imt.09.69. [DOI] [PubMed] [Google Scholar]
- 49.Lamers CH, Willemsen R, van EP, van Steenbergen-Langeveld S, Broertjes M, Oosterwijk-Wakka J, et al. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2011;117:72–82. doi: 10.1182/blood-2010-07-294520. [DOI] [PubMed] [Google Scholar]
- 50.Lamers CH, Langeveld SC, Groot-van Ruijven CM, Debets R, Sleijfer S, Gratama JW. Gene-modified T cells for adoptive immunotherapy of renal cell cancer maintain transgene-specific immune functions in vivo. Cancer Immunol Immunother. 2007;56:1875–83. doi: 10.1007/s00262-007-0330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 52.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–15. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jensen MC, Popplewell L, Cooper LJ, DiGiusto D, Kalos M, Ostberg JR, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 2010;16:1245–56. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brentjens RJ, Riviere I, Hollyman D, Taylor C, Nikhamin Y, Stefanski J, et al. Unexpected Toxicity of Cyclophosphamide Followed by Adoptively Transferred CD19-Targeted T Cells in a Patient with Bulky CLL. Mol Ther. 9A.D;17:157. [Google Scholar]
- 55.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case Report of a Serious Adverse Event Following the Administration of T Cells Transduced With a Chimeric Antigen Receptor Recognizing ERBB2. Mol Ther. 2010;18:843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parente-Pereira AC, Burnet J, Ellison D, Foster J, Davies DM, van der Stegen S, et al. Trafficking of CAR-engineered human T cells following regional or systemic adoptive transfer in SCID beige mice. J Clin Immunol. 2011;31:710–8. doi: 10.1007/s10875-011-9532-8. [DOI] [PubMed] [Google Scholar]
- 57.Wilkie S, van Schalkwyk MC, Hobbs S, Davies DM, van der Stegen SJ, Pereira AC, et al. Dual Targeting of ErbB2 and MUC1 in Breast Cancer Using Chimeric Antigen Receptors Engineered to Provide Complementary Signaling. J Clin Immunol. 2012;32:1059–70. doi: 10.1007/s10875-012-9689-9. [DOI] [PubMed] [Google Scholar]
- 58.Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signallung promotes selective tumor eradication by engineered T cells. Nat Biotech. 2013;31:71–5. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lo ASY, Taylor JR, Farzanch F, Kemeny DM, Dibb NJ, Maher J. Harnessing the tumour-derived cytokine, CSF-1, to co-stimulate T-cell growth and activation. Mol Immunol. 2007;45:1276–87. doi: 10.1016/j.molimm.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 60.Wilkie S, Burbridge SE, Chiapero-Stanke L, Pereira AC, Cleary S, van der Stegen SJ, et al. Selective expansion of chimeric antigen receptor-targeted T-cells with potent effector function using interleukin-4. J Biol Chem. 2010;285:25538–44. doi: 10.1074/jbc.M110.127951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanchez C, Chan R, Bajgain P, Rambally S, Palapattu G, Mims M, et al. Combining T-cell immunotherapy and anti-androgen therapy for prostate cancer. Prostate Cancer Prostatic Dis. 2013;16:123–31. doi: 10.1038/pcan.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Di SA, De AB, Rooney CM, Zhang L, Mahendravada A, Foster AE, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113:6392–402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moon EK, Carpenito C, Sun J, Wang LC, Kapoor V, Predina J, et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin Cancer Res. 2011;17:4719–30. doi: 10.1158/1078-0432.CCR-11-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Craddock JA, Lu A, Bear A, Pule M, Brenner MK, Rooney CM, et al. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J Immunother. 2010;33:780–8. doi: 10.1097/CJI.0b013e3181ee6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–14. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Quintarelli C, Vera JF, Savoldo B, Giordano Attianese GM, Pule M, Foster AE, et al. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110:2793–802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–70. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vera JF, Hoyos V, Savoldo B, Quintarelli C, Giordano Attianese GM, Leen AM, et al. Genetic manipulation of tumor-specific cytotoxic T lymphocytes to restore responsiveness to IL-7. Mol Ther. 2009;17:880–8. doi: 10.1038/mt.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosenberg SA, Sportes C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006;29:313–9. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–14. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sportes C, Babb RR, Krumlauf MC, Hakim FT, Steinberg SM, Chow CK, et al. Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy. Clin Cancer Res. 2010;16:727–35. doi: 10.1158/1078-0432.CCR-09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perales MA, Goldberg JD, Yuan J, Koehne G, Lechner L, Papadopoulos EB, et al. Recombinant human interleukin-7 (CYT107) promotes T cell recovery following allogeneic stem cell transplantation. Blood. 2012;120:4882–91. doi: 10.1182/blood-2012-06-437236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–7. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003 Jun 1;101(11):4260–6. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 75.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005 Jan 3;201(1):139–48. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang A, Chandran S, Shah SA, Chiu Y, Paria BC, Aghamolla T, et al. The Stoichiometric Production of IL-2 and IFN-gamma mRNA Defines Memory T Cells That Can Self-Renew After Adoptive Transfer in Humans. Sci Transl Med. 2012;4:149ra120. doi: 10.1126/scitranslmed.3004306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–70. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Terakura S, Yamamoto TN, Gardner RA, Turtle CJ, Jensen MC, Riddell SR. Generation of CD19-chimeric antigen receptor modified CD8+ T cells derived from virus-specific central memory T cells. Blood. 2012;119:72–82. doi: 10.1182/blood-2011-07-366419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stephan MT, Ponomarev V, Brentjens RJ, Chang AH, Dobrenkov KV, Heller G, et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat Med. 2007;13:1440–9. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- 82.Dagarag M, Evazyan T, Rao N, Effros RB. Genetic manipulation of telomerase in HIV-specific CD8+ T cells: enhanced antiviral functions accompany the increased proliferative potential and telomere length stabilization. J Immunol. 2004;173:6303–11. doi: 10.4049/jimmunol.173.10.6303. [DOI] [PubMed] [Google Scholar]
- 83.Hooijberg E, Ruizendaal JJ, Snijders PJ, Kueter EW, Walboomers JM, Spits H. Immortalization of human CD8+ T cell clones by ectopic expression of telomerase reverse transcriptase. J Immunol. 2000;165:4239–45. doi: 10.4049/jimmunol.165.8.4239. [DOI] [PubMed] [Google Scholar]
- 84.Migliaccio M, Amacker M, Just T, Reichenbach P, Valmori D, Cerottini JC, et al. Ectopic human telomerase catalytic subunit expression maintains telomere length but is not sufficient for CD8+ T lymphocyte immortalization. J Immunol. 2000;165:4978–84. doi: 10.4049/jimmunol.165.9.4978. [DOI] [PubMed] [Google Scholar]
- 85.Charo J, Finkelstein SE, Grewal N, Restifo NP, Robbins PF, Rosenberg SA. Bcl-2 overexpression enhances tumor-specific T-cell survival. Cancer Res. 2005;65:2001–8. doi: 10.1158/0008-5472.CAN-04-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eaton D, Gilham DE, O’Neill A, Hawkins RE. Retroviral transduction of human peripheral blood lymphocytes with Bcl-X(L) promotes in vitro lymphocyte survival in pro-apoptotic conditions. Gene Ther. 2002;9:527–35. doi: 10.1038/sj.gt.3301685. [DOI] [PubMed] [Google Scholar]
- 87.Dotti G, Savoldo B, Pule M, Straathof KC, Biagi E, Yvon E, et al. Human cytotoxic T lymphocytes with reduced sensitivity to Fas-induced apoptosis. Blood. 2005;105:4677–84. doi: 10.1182/blood-2004-08-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bollard CM, Rossig C, Calonge MJ, Huls MH, Wagner HJ, Massague J, et al. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood. 2002;99:3179–87. doi: 10.1182/blood.v99.9.3179. [DOI] [PubMed] [Google Scholar]
- 89.Lacuesta K, Buza E, Hauser H, Granville L, Pule M, Corboy G, et al. Assessing the safety of cytotoxic T lymphocytes transduced with a dominant negative transforming growth factor-beta receptor. J Immunother. 2006;29:250–60. doi: 10.1097/01.cji.0000192104.24583.ca. [DOI] [PubMed] [Google Scholar]
- 90.Foster AE, Dotti G, Lu A, Khalil M, Brenner MK, Heslop HE, et al. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-beta receptor. J Immunother. 2008;31:500–5. doi: 10.1097/CJI.0b013e318177092b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Introna M, Barbui AM, Bambacioni F, Casati C, Gaipa G, Borleri G, et al. Genetic modification of human T cells with CD20: a strategy to purify and lyse transduced cells with anti-CD20 antibodies. 2000;11:611–20. doi: 10.1089/10430340050015798. [DOI] [PubMed] [Google Scholar]
- 92.Serafini M, Manganini M, Borleri G, Bonamino M, Imberti L, Biondi A, et al. Characterization of CD20-transduced T lymphocytes as an alternative suicide gene therapy approach for the treatment of graft-versus-host disease. Hum Gene Ther. 2004;15:63–76. doi: 10.1089/10430340460732463. [DOI] [PubMed] [Google Scholar]
- 93.Bonini C, Ciceri F, Marktel S, Magnani Z, Cazzaniga S, Zappone E, et al. Abrogation of GvHD and Early Immune Reconstitution after Infusion of HSV-TK Engineered Donor Lymphocytes after Haplo-Identical Hematopoietic Stem cell. Transplantation. 2002 Nov 16;:115a115a. [Google Scholar]
- 94.Bonini C, Bondanza A, Perna SK, Kaneko S, Traversari C, Ciceri F, et al. The suicide gene therapy challenge: how to improve a successful gene therapy approach. Mol Ther. 2007;15:1248–52. doi: 10.1038/sj.mt.6300190. [DOI] [PubMed] [Google Scholar]
- 95.Ciceri F, Bonini C, Marktel S, Zappone E, Servida P, Bernardi M, et al. Antitumor effects of HSV-TK-engineered donor lymphocytes after allogeneic stem-cell transplantation. Blood. 2007;109:4698–707. doi: 10.1182/blood-2006-05-023416. [DOI] [PubMed] [Google Scholar]
- 96.Ciceri F, Bonini C, Stanghellini MT, Bondanza A, Traversari C, Salomoni M, et al. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I–II study. Lancet Oncol. 2009;10:489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- 97.Traversari C, Marktel S, Magnani Z, Mangia P, Russo V, Ciceri F, et al. The potential immunogenicity of the TK suicide gene does not prevent full clinical benefit associated with the use of TK-transduced donor lymphocytes in HSCT for hematologic malignancies. Blood. 2007;109:4708–15. doi: 10.1182/blood-2006-04-015230. [DOI] [PubMed] [Google Scholar]
- 98.Marin V, Cribioli E, Philip B, Tettamanti S, Pizzitola I, Biondi A, et al. Comparison of different suicide-gene strategies for the safety improvement of genetically manipulated T cells. Hum Gene Ther Methods. 2012;23:376–86. doi: 10.1089/hgtb.2012.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Straathof KC, Pule MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–54. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramos CA, Asgari Z, Liu E, Yvon E, Heslop HE, Rooney CM, et al. An inducible caspase 9 suicide gene to improve the safety of mesenchymal stromal cell therapies. Stem Cells. 2010;28:1107–15. doi: 10.1002/stem.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Di SA, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–83. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tamada K, Geng D, Sakoda Y, Bansal N, Srivastava R, Li Z, et al. Redirecting gene-modified T cells toward various cancer types using tagged antibodies. Clin Cancer Res. 2012;18:6436–45. doi: 10.1158/1078-0432.CCR-12-1449. [DOI] [PubMed] [Google Scholar]
- 103.Urbanska K, Powell DJ. Development of a novel universal immune receptor for antigen targeting: To Infinity and beyond. Oncoimmunology. 2012;1:777–9. doi: 10.4161/onci.19730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Urbanska K, Lanitis E, Poussin M, Lynn RC, Gavin BP, Kelderman S, et al. A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor. Cancer Res. 2012;72:1844–52. doi: 10.1158/0008-5472.CAN-11-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carswell KS, Papoutsakis ET. Culture of human T cells in stirred bioreactors for cellular immunotherapy applications: shear, proliferation, and the IL-2 receptor. Biotechnol Bioeng. 2000;68:328–38. doi: 10.1002/(sici)1097-0290(20000505)68:3<328::aid-bit11>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 106.Hami LS, Green C, Leshinsky N, Markham E, Miller K, Craig S. GMP production and testing of Xcellerated T Cells for the treatment of patients with CLL. Cytotherapy. 2004;6:554–62. doi: 10.1080/14653240410005348. [DOI] [PubMed] [Google Scholar]
- 107.Klapper JA, Thomasian AA, Smith DM, Gorgas GC, Wunderlich JR, Smith FO, et al. Single-pass, closed-system rapid expansion of lymphocyte cultures for adoptive cell therapy. J Immunol Methods. 2009;345:90–9. doi: 10.1016/j.jim.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sadeghi A, Pauler L, Anneren C, Friberg A, Brandhorst D, Korsgren O, et al. Large-scale bioreactor expansion of tumor-infiltrating lymphocytes. J Immunol Methods. 2011;364:94–100. doi: 10.1016/j.jim.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 109.Sadeghi A, Pauler L, Annerén C, Friberg A, Brandhorst D, Korsgren O, et al. Large-scale bioreactor expansion of tumor-infiltrating lymphocytes. J Immunol Methods. 2011 Feb 1;364(1–2):94–100. doi: 10.1016/j.jim.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 110.Somerville RP, Devillier L, Parkhurst MR, Rosenberg SA, Dudley ME. Clinical scale rapid expansion of lymphocytes for adoptive cell transfer therapy in the WAVE® bioreactor. J Transl Med. 2012 Apr 4;10:69. doi: 10.1186/1479-5876-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hollyman D, Stefanski J, Przybylowski M, Bartido S, Borquez-Ojeda O, Taylor C, et al. Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy. J Immunother. 2009;32:169–80. doi: 10.1097/CJI.0b013e318194a6e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vera JF, Brenner LJ, Gerdemann U, Ngo MC, Sili U, Liu H, et al. Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion cultureware (G-Rex) J Immunother. 2010;33:305–15. doi: 10.1097/CJI.0b013e3181c0c3cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lapteva N, Vera JF. Optimization manufacture of virus- and tumor-specific T cells. Stem Cells Int. 2011;2011:434392. doi: 10.4061/2011/434392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lapteva N, Durett AG, Sun J, Rollins LA, Huye LL, Fang J, et al. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy. 2012;14:1131–43. doi: 10.3109/14653249.2012.700767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jin J, Sabatino M, Somerville R, Wilson JR, Dudley ME, Stroncek DF, et al. Simplified method of the growth of human tumor infiltrating lymphocytes in gas-permeable flasks to numbers needed for patient treatment. J Immunother. 2012;35:283–92. doi: 10.1097/CJI.0b013e31824e801f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cooper LJ, Topp MS, Serrano LM, Gonzalez S, Chang WC, Naranjo A, et al. T-cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003;101:1637–44. doi: 10.1182/blood-2002-07-1989. [DOI] [PubMed] [Google Scholar]
- 117.Cooper LJ, Al-Kadhimi Z, DiGiusto D, Kalos M, Colcher D, Raubitschek A, et al. Development and application of CD19-specific T cells for adoptive immunotherapy of B cell malignancies. Blood Cells Mol Dis. 2004;33:83–9. doi: 10.1016/j.bcmd.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 118.Singh H, Serrano LM, Pfeiffer T, Olivares S, McNamara G, Smith DD, et al. Combining adoptive cellular and immunocytokine therapies to improve treatment of B-lineage malignancy. Cancer Res. 2007;67:2872–80. doi: 10.1158/0008-5472.CAN-06-2283. [DOI] [PubMed] [Google Scholar]
- 119.Kowolik CM, Topp MS, Gonzalez S, Pfeiffer T, Olivares S, Gonzalez N, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66:10995–1004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 120.Loskog A, Giandomenico V, Rossig C, Pule M, Dotti G, Brenner MK. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia. 2006;20:1819–28. doi: 10.1038/sj.leu.2404366. [DOI] [PubMed] [Google Scholar]
- 121.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–64. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tammana S, Huang X, Wong M, Milone MC, Ma L, Levine BL, et al. 4-1BB and CD28 signaling plays a synergistic role in redirecting umbilical cord blood T cells against B-cell malignancies. Hum Gene Ther. 2010;21:75–86. doi: 10.1089/hum.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–86. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 124.Brentjens RJ, Santos E, Nikhamin Y, Yeh R, Matsushita M, La PK, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13:5426–35. doi: 10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- 125.Kochenderfer JN, Feldman SA, Zhao Y, Xu H, Black MA, Morgan RA, et al. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother. 2009;32:689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–20. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kebriaei P, Huls H, Jena B, Munsell M, Jackson R, Lee DA, et al. Infusing CD19-directed T cells to augment disease control in patients undergoing autologous hematopoietic stem-cell transplantation for advanced B-lymphoid malignancies. Hum Gene Ther. 2012;23:444–50. doi: 10.1089/hum.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brentjens R, Yeh R, Bernal Y, Riviere I, Sadelain M. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18:666–8. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–28. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.James SE, Orgun NN, Tedder TF, Shlomchik MJ, Jensen MC, Lin Y, et al. Antibody-mediated B-cell depletion before adoptive immunotherapy with T cells expressing CD20-specific chimeric T-cell receptors facilitates eradication of leukemia in immunocompetent mice. Blood. 2009;114:5454–63. doi: 10.1182/blood-2009-08-232967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jensen M, Tan G, Forman S, Wu AM, Raubitschek A. CD20 is a molecular target for scFvFc:zeta receptor redirected T cells: implications for cellular immunotherapy of CD20+ malignancy. Biol Blood Marrow Transplant. 1998;4:75–83. doi: 10.1053/bbmt.1998.v4.pm9763110. [DOI] [PubMed] [Google Scholar]
- 134.Jensen MC, Cooper LJ, Wu AM, Forman SJ, Raubitschek A. Engineered CD20-specific primary human cytotoxic T lymphocytes for targeting B-cell malignancy. Cytotherapy. 2003;5:131–8. doi: 10.1080/14653240310001028. [DOI] [PubMed] [Google Scholar]
- 135.Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–71. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang J, Press OW, Lindgren CG, Greenberg P, Riddell S, Qian X, et al. Cellular immunotherapy for follicular lymphoma using genetically modified CD20-specific CD8+ cytotoxic T lymphocytes. Mol Ther. 2004;9:577–86. doi: 10.1016/j.ymthe.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 137.Wang J, Jensen M, Lin Y, Sui X, Chen E, Lindgren CG, et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum Gene Ther. 2007;18:712–25. doi: 10.1089/hum.2007.028. [DOI] [PubMed] [Google Scholar]
- 138.Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119:3940–50. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Haso W, Lee DW, Shah NN, Stetler-Stevenson M, Yuan CM, Pastan IH, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood. 2013;121:1165–74. doi: 10.1182/blood-2012-06-438002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hombach A, Heuser C, Sircar R, Tillmann T, Diehl V, Pohl C, et al. An anti-CD30 chimeric receptor that mediates CD3-zeta-independent T-cell activation against Hodgkin’s lymphoma cells in the presence of soluble CD30. Cancer Res. 1998;58:1116–9. [PubMed] [Google Scholar]
- 141.Savoldo B, Rooney CM, Di SA, Abken H, Hombach A, Foster AE, et al. Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30zeta artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood. 2007;110:2620–30. doi: 10.1182/blood-2006-11-059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108:3890–7. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shaffer DR, Savoldo B, Yi Z, Chow KK, Kakarla S, Spencer DM, et al. T cells redirected against CD70 for the immunotherapy of CD70-positive malignancies. Blood. 2011;117:4304–14. doi: 10.1182/blood-2010-04-278218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tettamanti S, Marin V, Pizzitola I, Magnani CF, Giordano Attianese GM, Cribioli E, et al. Targeting of acute myeloid leukaemia by cytokine-induced killer cells redirected with a novel CD123-specific chimeric antigen receptor. Br J Haematol. 2013;161:389–401. doi: 10.1111/bjh.12282. [DOI] [PubMed] [Google Scholar]
- 145.Barber A, Zhang T, Megli CJ, Wu J, Meehan KR, Sentman CL. Chimeric NKG2D receptor-expressing T cells as an immunotherapy for multiple myeloma. Exp Hematol. 2008;36:1318–28. doi: 10.1016/j.exphem.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Barber A, Meehan KR, Sentman CL. Treatment of multiple myeloma with adoptively transferred chimeric NKG2D receptor-expressing T cells. Gene Ther. 2011;18:509–16. doi: 10.1038/gt.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Spear P, Barber A, Rynda-Apple A, Sentman CL. Chimeric antigen receptor T cells shape myeloid cell function within the tumor microenvironment through IFN-gamma and GM-CSF. J Immunol. 2012;188:6389–98. doi: 10.4049/jimmunol.1103019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Spear P, Barber A, Rynda-Apple A, Sentman CL. NKG2D CAR T-cell therapy inhibits the growth of NKG2D ligand heterogeneous tumors. Immunol Cell Biol 2013. 2013 Jul;91(6):435–40. doi: 10.1038/icb.2013.17. Epub 2013 Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rossig C, Bollard CM, Nuchtern JG, Merchant DA, Brenner MK. Targeting of G(D2)-positive tumor cells by human T lymphocytes engineered to express chimeric T-cell receptor genes. Int J Cancer. 2001;94:228–36. doi: 10.1002/ijc.1457. [DOI] [PubMed] [Google Scholar]
- 150.Rossig C, Bollard CM, Nuchtern JG, Rooney CM, Brenner MK. Epstein-Barr virus-specific human T lymphocytes expressing antitumor chimeric T-cell receptors: potential for improved immunotherapy. Blood. 2002;99:2009–16. doi: 10.1182/blood.v99.6.2009. [DOI] [PubMed] [Google Scholar]
- 151.Stancovski I, Schindler DG, Waks T, Yarden Y, Sela M, Eshhar Z. Targeting of T lymphocytes to Neu/HER2-expressing cells using chimeric single chain Fv receptors. J Immunol. 1993;151:6577–82. [PubMed] [Google Scholar]
- 152.Pinthus JH, Waks T, Malina V, Kaufman-Francis K, Harmelin A, Aizenberg I, et al. Adoptive immunotherapy of prostate cancer bone lesions using redirected effector lymphocytes. J Clin Invest. 2004;114:1774–81. doi: 10.1172/JCI22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pisarev V, Yu B, Salup R, Sherman S, Altieri DC, Gabrilovich DI. Full-length dominant-negative survivin for cancer immunotherapy. Clin Cancer Res. 2003;9:6523–33. [PubMed] [Google Scholar]
- 154.Ahmed N, Ratnayake M, Savoldo B, Perlaky L, Dotti G, Wels WS, et al. Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Cancer Res. 2007;67:5957–64. doi: 10.1158/0008-5472.CAN-06-4309. [DOI] [PubMed] [Google Scholar]
- 155.Zhao Y, Wang QJ, Yang S, Kochenderfer JN, Zheng Z, Zhong X, et al. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol. 2009;183:5563–74. doi: 10.4049/jimmunol.0900447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yoon SH, Lee JM, Woo SJ, Park MJ, Park JS, Kim HS, et al. Transfer of Her-2/neu specificity into cytokine-induced killer (CIK) cells with RNA encoding chimeric immune receptor (CIR) J Clin Immunol. 2009;29:806–14. doi: 10.1007/s10875-009-9308-6. [DOI] [PubMed] [Google Scholar]
- 157.Yoon SH, Lee JM, Cho HI, Kim EK, Kim HS, Park MY, et al. Adoptive immunotherapy using human peripheral blood lymphocytes transferred with RNA encoding Her-2/neu-specific chimeric immune receptor in ovarian cancer xenograft model. Cancer Gene Ther. 2009;16:489–97. doi: 10.1038/cgt.2008.98. [DOI] [PubMed] [Google Scholar]
- 158.Ahmed N, Salsman VS, Yvon E, Louis CU, Perlaky L, Wels WS, et al. Immunotherapy for osteosarcoma: genetic modification of T cells overcomes low levels of tumor antigen expression. Mol Ther. 2009;17:1779–87. doi: 10.1038/mt.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Birkholz K, Hombach A, Krug C, Reuter S, Kershaw M, Kampgen E, et al. Transfer of mRNA encoding recombinant immunoreceptors reprograms CD4+ and CD8+ T cells for use in the adoptive immunotherapy of cancer. Gene Ther. 2009;16:596–604. doi: 10.1038/gt.2008.189. [DOI] [PubMed] [Google Scholar]
- 160.Hwu P, Shafer GE, Treisman J, Schindler DG, Gross G, Cowherd R, et al. Lysis of ovarian cancer cells by human lymphocytes redirected with a chimeric gene composed of an antibody variable region and the Fc receptor gamma chain. J Exp Med. 1993;178:361–6. doi: 10.1084/jem.178.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hwu P, Yang JC, Cowherd R, Treisman J, Shafer GE, Eshhar Z, et al. In vivo antitumor activity of T cells redirected with chimeric antibody/T-cell receptor genes. Cancer Res. 1995;55:3369–73. [PubMed] [Google Scholar]
- 162.Kershaw MH, Westwood JA, Hwu P. Dual-specific T cells combine proliferation and antitumor activity. Nat Biotechnol. 2002;20:1221–7. doi: 10.1038/nbt756. [DOI] [PubMed] [Google Scholar]
- 163.Parker LL, Do MT, Westwood JA, Wunderlich JR, Dudley ME, Rosenberg SA, et al. Expansion and characterization of T cells transduced with a chimeric receptor against ovarian cancer. Hum Gene Ther. 2000;11:2377–87. doi: 10.1089/104303400750038480. [DOI] [PubMed] [Google Scholar]
- 164.Gonzalez S, Naranjo A, Serrano LM, Chang WC, Wright CL, Jensen MC. Genetic engineering of cytolytic T lymphocytes for adoptive T-cell therapy of neuroblastoma. J Gene Med. 2004;6:704–11. doi: 10.1002/jgm.489. [DOI] [PubMed] [Google Scholar]
- 165.Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15:825–33. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 166.Weijtens ME, Willemsen RA, van Krimpen BA, Bolhuis RL. Chimeric scFv/gamma receptor-mediated T-cell lysis of tumor cells is coregulated by adhesion and accessory molecules. Int J Cancer. 1998;77:181–7. doi: 10.1002/(sici)1097-0215(19980717)77:2<181::aid-ijc2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 167.Weijtens ME, Willemsen RA, Hart EH, Bolhuis RL. A retroviral vector system ‘STITCH’ in combination with an optimized single chain antibody chimeric receptor gene structure allows efficient gene transduction and expression in human T lymphocytes. Gene Ther. 1998;5:1195–203. doi: 10.1038/sj.gt.3300696. [DOI] [PubMed] [Google Scholar]
- 168.Lamers CH, Sleijfer S, van SS, van EP, van KB, Groot C, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21:904–12. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gong MC, Latouche JB, Krause A, Heston WD, Bander NH, Sadelain M. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia. 1999;1:123–7. doi: 10.1038/sj.neo.7900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002;20:70–5. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 171.Brown CE, Starr R, Aguilar B, Shami AF, Martinez C, D’Apuzzo M, et al. Stem-like tumor-initiating cells isolated from IL13Ralpha2 expressing gliomas are targeted and killed by IL13-zetakine-redirected T Cells. Clin Cancer Res. 2012;18:2199–209. doi: 10.1158/1078-0432.CCR-11-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kahlon KS, Brown C, Cooper LJ, Raubitschek A, Forman SJ, Jensen MC. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res. 2004;64:9160–6. doi: 10.1158/0008-5472.CAN-04-0454. [DOI] [PubMed] [Google Scholar]
- 173.Stastny MJ, Brown CE, Ruel C, Jensen MC. Medulloblastomas expressing IL13Ralpha2 are targets for IL13-zetakine+ cytolytic T cells. J Pediatr Hematol Oncol. 2007;29:669–77. doi: 10.1097/MPH.0b013e3181468c68. [DOI] [PubMed] [Google Scholar]
- 174.Kong S, Sengupta S, Tyler B, Bais AJ, Ma Q, Doucette S, et al. Suppression of human glioma xenografts with second-generation IL13R-specific chimeric antigen receptor-modified T cells. Clin Cancer Res. 2012;18:5949–60. doi: 10.1158/1078-0432.CCR-12-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chow KK, Naik S, Kakarla S, Brawley VS, Shaffer DR, Yi Z, et al. T Cells Redirected to EphA2 for the Immunotherapy of Glioblastoma. Mol Ther. 2012;21:629–37. doi: 10.1038/mt.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kakarla S, Chow KK, Mata M, Shaffer DR, Song XT, Wu MF, et al. Antitumor Effects of Chimeric Receptor Engineered Human T Cells Directed to Tumor Stroma. Mol Ther. 2013 Jun 4;2013 doi: 10.1038/mt.2013.110. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kershaw MH, Westwood JA, Zhu Z, Witte L, Libutti SK, Hwu P. Generation of gene-modified T cells reactive against the angiogenic kinase insert domain-containing receptor (KDR) found on tumor vasculature. Hum Gene Ther. 2000;11:2445–52. doi: 10.1089/10430340050207939. [DOI] [PubMed] [Google Scholar]
- 178.Niederman TM, Ghogawala Z, Carter BS, Tompkins HS, Russell MM, Mulligan RC. Antitumor activity of cytotoxic T lymphocytes engineered to target vascular endothelial growth factor receptors. Proc Natl Acad Sci U S A. 2002;99:7009–14. doi: 10.1073/pnas.092562399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chinnasamy D, Yu Z, Theoret MR, Zhao Y, Shrimali RK, Morgan RA, et al. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J Clin Invest. 2010;120:3953–68. doi: 10.1172/JCI43490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chinnasamy D, Tran E, Yu Z, Morgan RA, Restifo NP, Rosenberg SA. Simultaneous targeting of tumor antigens and the tumor vasculature using T lymphocyte transfer synergize to induce regression of established tumors in mice. Cancer Res. 2013;73:3371–80. doi: 10.1158/0008-5472.CAN-12-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang W, Ma Y, Li J, Shi HS, Wang LQ, Guo FC, et al. Specificity redirection by CAR with human VEGFR-1 affinity endows T lymphocytes with tumor-killing ability and anti-angiogenic potency. Gene Ther. 2013 May 2;2013 doi: 10.1038/gt.2013.19. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 182.Ohno M, Natsume A, Ichiro IK, Iwamizu H, Noritake K, Ito D, et al. Retrovirally engineered T-cell-based immunotherapy targeting type III variant epidermal growth factor receptor, a glioma-associated antigen. Cancer Sci. 2010;101:2518–24. doi: 10.1111/j.1349-7006.2010.01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Morgan RA, Johnson LA, Davis JL, Zheng Z, Woolard KD, Reap EA, et al. Recognition of Glioma Stem Cells by Genetically Modified T Cells Targeting EGFRvIII and Development of Adoptive Cell Therapy for Glioma. Hum Gene Ther 2012. 2012 Oct;23(10):1043–53. doi: 10.1089/hum.2012.041. Epub 2012 Sep 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhou X, Li J, Wang Z, Chen Z, Qiu J, Zhang Y, et al. Cellular Immunotherapy for Carcinoma Using Genetically Modified EGFR-Specific T Lymphocytes. Neoplasia. 2013;15:544–53. doi: 10.1593/neo.13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shen CJ, Yang YX, Han EQ, Cao N, Wang YF, Wang Y, et al. Chimeric antigen receptor containing ICOS signaling domain mediates specific and efficient antitumor effect of T cells against EGFRvIII expressing glioma. J Hematol Oncol. 2013;6:33. doi: 10.1186/1756-8722-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106:3360–5. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lanitis E, Poussin M, Hagemann IS, Coukos G, Sandaltzopoulos R, Scholler N, et al. Redirected antitumor activity of primary human lymphocytes transduced with a fully human anti-mesothelin chimeric receptor. Mol Ther. 2012;20:633–43. doi: 10.1038/mt.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70:9053–61. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hekele A, Dall P, Moritz D, Wels W, Groner B, Herrlich P, et al. Growth retardation of tumors by adoptive transfer of cytotoxic T lymphocytes reprogrammed by CD44v6-specific scFv:zeta-chimera. Int J Cancer. 1996;68:232–8. doi: 10.1002/(SICI)1097-0215(19961009)68:2<232::AID-IJC16>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 190.Dall P, Herrmann I, Durst B, Stoff-Khalili MA, Bauerschmitz G, Hanstein B, et al. In vivo cervical cancer growth inhibition by genetically engineered cytotoxic T cells. Cancer Immunol Immunother. 2005;54:51–60. doi: 10.1007/s00262-004-0569-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hombach A, Heuser C, Sircar R, Tillmann T, Diehl V, Kruis W, et al. T cell targeting of TAG72+ tumor cells by a chimeric receptor with antibody-like specificity for a carbohydrate epitope. Gastroenterology. 1997;113:1163–70. doi: 10.1053/gast.1997.v113.pm9322511. [DOI] [PubMed] [Google Scholar]
- 192.McGuinness RP, Ge Y, Patel SD, Kashmiri SV, Lee HS, Hand PH, et al. Anti-tumor activity of human T cells expressing the CC49-zeta chimeric immune receptor. Hum Gene Ther. 1999;10:165–73. doi: 10.1089/10430349950018968. [DOI] [PubMed] [Google Scholar]
- 193.Mezzanzanica D, Canevari S, Mazzoni A, Figini M, Colnaghi MI, Waks T, et al. Transfer of chimeric receptor gene made of variable regions of tumor-specific antibody confers anticarbohydrate specificity on T cells. Cancer Gene Ther. 1998;5:401–7. [PubMed] [Google Scholar]
- 194.Peinert S, Prince HM, Guru PM, Kershaw MH, Smyth MJ, Trapani JA, et al. Gene-modified T cells as immunotherapy for multiple myeloma and acute myeloid leukemia expressing the Lewis Y antigen. Gene Ther. 2010;17:678–86. doi: 10.1038/gt.2010.21. [DOI] [PubMed] [Google Scholar]
- 195.Westwood JA, Smyth MJ, Teng MW, Moeller M, Trapani JA, Scott AM, et al. Adoptive transfer of T cells modified with a humanized chimeric receptor gene inhibits growth of Lewis-Y-expressing tumors in mice. Proc Natl Acad Sci U S A. 2005;102:19051–6. doi: 10.1073/pnas.0504312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Westwood JA, Murray WK, Trivett M, Haynes NM, Solomon B, Mileshkin L, et al. The Lewis-Y carbohydrate antigen is expressed by many human tumors and can serve as a target for genetically redirected T cells despite the presence of soluble antigen in serum. J Immunother. 2009;32:292–301. doi: 10.1097/CJI.0b013e31819b7c8e. [DOI] [PubMed] [Google Scholar]
- 197.Wilkie S, Picco G, Foster J, Davies DM, Julien S, Cooper L, et al. Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. J Immunol. 2008;180:4901–9. doi: 10.4049/jimmunol.180.7.4901. [DOI] [PubMed] [Google Scholar]
- 198.Parente-Pereira AC, Burnet J, Ellison D, Foster J, Davies DM, van der Stegen S, et al. Trafficking of CAR-engineered human T cells following regional or systemic adoptive transfer in SCID beige mice. J Clin Immunol. 2011;31:710–8. doi: 10.1007/s10875-011-9532-8. [DOI] [PubMed] [Google Scholar]
- 199.Gattenlohner S, Marx A, Markfort B, Pscherer S, Landmeier S, Juergens H, et al. Rhabdomyosarcoma lysis by T cells expressing a human autoantibody-based chimeric receptor targeting the fetal acetylcholine receptor. Cancer Res. 2006;66:24–8. doi: 10.1158/0008-5472.CAN-05-0542. [DOI] [PubMed] [Google Scholar]
- 200.Simon-Keller K, Barth S, Vincent A, Marx A. Targeting the fetal acetylcholine receptor in rhabdomyosarcoma. Expert Opin Ther Targets. 2013;17:127–38. doi: 10.1517/14728222.2013.734500. [DOI] [PubMed] [Google Scholar]
- 201.Gilham DE, O’Neil A, Hughes C, Guest RD, Kirillova N, Lehane M, et al. Primary polyclonal human T lymphocytes targeted to carcino-embryonic antigens and neural cell adhesion molecule tumor antigens by CD3zeta-based chimeric immune receptors. J Immunother. 2002;25:139–51. doi: 10.1097/00002371-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 202.Sheen AJ, Irlam J, Kirillova N, Guest RD, Sherlock DJ, Hawkins RE, et al. Gene therapy of patient-derived T lymphocytes to target and eradicate colorectal hepatic metastases. Dis Colon Rectum. 2003;46:793–804. doi: 10.1007/s10350-004-6659-1. [DOI] [PubMed] [Google Scholar]
- 203.Bridgeman JS, Hawkins RE, Bagley S, Blaylock M, Holland M, Gilham DE. The optimal antigen response of chimeric antigen receptors harboring the CD3zeta transmembrane domain is dependent upon incorporation of the receptor into the endogenous TCR/CD3 complex. J Immunol. 2010;184:6938–49. doi: 10.4049/jimmunol.0901766. [DOI] [PubMed] [Google Scholar]
- 204.Darcy PK, Kershaw MH, Trapani JA, Smyth MJ. Expression in cytotoxic T lymphocytes of a single-chain anti-carcinoembryonic antigen antibody. Redirected Fas ligand-mediated lysis of colon carcinoma. Eur J Immunol. 1998;28:1663–72. doi: 10.1002/(SICI)1521-4141(199805)28:05<1663::AID-IMMU1663>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 205.Nolan KF, Yun CO, Akamatsu Y, Murphy JC, Leung SO, Beecham EJ, et al. Bypassing immunization: optimized design of “designer T cells” against carcinoembryonic antigen (CEA)-expressing tumors, and lack of suppression by soluble CEA. Clin Cancer Res. 1999;5:3928–41. [PubMed] [Google Scholar]
- 206.Hombach A, Wieczarkowiecz A, Marquardt T, Heuser C, Usai L, Pohl C, et al. Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. J Immunol. 2001;167:6123–31. doi: 10.4049/jimmunol.167.11.6123. [DOI] [PubMed] [Google Scholar]
- 207.Haynes NM, Trapani JA, Teng MW, Jackson JT, Cerruti L, Jane SM, et al. Rejection of syngeneic colon carcinoma by CTLs expressing single-chain antibody receptors codelivering CD28 costimulation. J Immunol. 2002;169:5780–6. doi: 10.4049/jimmunol.169.10.5780. [DOI] [PubMed] [Google Scholar]
- 208.Ma Q, DeMarte L, Wang Y, Stanners CP, Junghans RP. Carcinoembryonic antigen-immunoglobulin Fc fusion protein (CEA-Fc) for identification and activation of anti-CEA immunoglobulin-T-cell receptor-modified T cells, representative of a new class of Ig fusion proteins. Cancer Gene Ther. 2004;11:297–306. doi: 10.1038/sj.cgt.7700685. [DOI] [PubMed] [Google Scholar]
- 209.Emtage PC, Lo AS, Gomes EM, Liu DL, Gonzalo-Daganzo RM, Junghans RP. Second-generation anti-carcinoembryonic antigen designer T cells resist activation-induced cell death, proliferate on tumor contact, secrete cytokines, and exhibit superior antitumor activity in vivo: a preclinical evaluation. Clin Cancer Res. 2008;14:8112–22. doi: 10.1158/1078-0432.CCR-07-4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Shirasu N, Shibaguci H, Kuroki M, Yamada H, Kuroki M. Construction and molecular characterization of human chimeric T-cell antigen receptors specific for carcinoembryonic antigen. Anticancer Res. 2010;30:2731–8. [PubMed] [Google Scholar]
- 211.Chmielewski M, Rappl G, Hombach AA, Abken H. T cells redirected by a CD3zeta chimeric antigen receptor can establish self-antigen-specific tumour protection in the long term. Gene Ther. 2013;20:177–86. doi: 10.1038/gt.2012.21. [DOI] [PubMed] [Google Scholar]
- 212.Gyobu H, Tsuji T, Suzuki Y, Ohkuri T, Chamoto K, Kuroki M, et al. Generation and targeting of human tumor-specific Tc1 and Th1 cells transduced with a lentivirus containing a chimeric immunoglobulin T-cell receptor. Cancer Res. 2004;64:1490–5. doi: 10.1158/0008-5472.can-03-2780. [DOI] [PubMed] [Google Scholar]
- 213.Sasaki T, Ikeda H, Sato M, Ohkuri T, Abe H, Kuroki M, et al. Antitumor activity of chimeric immunoreceptor gene-modified Tc1 and Th1 cells against autologous carcinoembryonic antigen-expressing colon cancer cells. Cancer Sci. 2006;97:920–7. doi: 10.1111/j.1349-7006.2006.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Shibaguchi H, Luo NX, Kuroki M, Zhao J, Huang J, Hachimine K, et al. A fully human chimeric immune receptor for retargeting T-cells to CEA-expressing tumor cells. Anticancer Res. 2006;26:4067–72. [PubMed] [Google Scholar]
- 215.Chmielewski M, Hahn O, Rappl G, Nowak M, Schmidt-Wolf IH, Hombach AA, et al. T cells that target carcinoembryonic antigen eradicate orthotopic pancreatic carcinomas without inducing autoimmune colitis in mice. Gastroenterology. 2012;143:1095–107. doi: 10.1053/j.gastro.2012.06.037. [DOI] [PubMed] [Google Scholar]
- 216.Ren-Heidenreich L, Hayman GT, Trevor KT. Specific targeting of EGP-2+ tumor cells by primary lymphocytes modified with chimeric T cell receptors. Hum Gene Ther. 2000;11:9–19. doi: 10.1089/10430340050016111. [DOI] [PubMed] [Google Scholar]
- 217.Ren-Heidenreich L, Mordini R, Hayman GT, Siebenlist R, LeFever A. Comparison of the TCR zeta-chain with the FcR gamma-chain in chimeric TCR constructs for T cell activation and apoptosis. Cancer Immunol Immunother. 2002;51:417–23. doi: 10.1007/s00262-002-0301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Daly T, Royal RE, Kershaw MH, Treisman J, Wang G, Li W, et al. Recognition of human colon cancer by T cells transduced with a chimeric receptor gene. Cancer Gene Ther. 2000;7:284–91. doi: 10.1038/sj.cgt.7700121. [DOI] [PubMed] [Google Scholar]
- 219.Altenschmidt U, Kahl R, Moritz D, Schnierle BS, Gerstmayer B, Wels W, et al. Cytolysis of tumor cells expressing the Neu/erbB-2, erbB-3, and erbB-4 receptors by genetically targeted naive T lymphocytes. Clin Cancer Res. 1996;2:1001–8. [PubMed] [Google Scholar]
- 220.Muniappan A, Banapour B, Lebkowski J, Talib S. Ligand-mediated cytolysis of tumor cells: use of heregulin-zeta chimeras to redirect cytotoxic T lymphocytes. Cancer Gene Ther. 2000;7:128–34. doi: 10.1038/sj.cgt.7700100. [DOI] [PubMed] [Google Scholar]
- 221.Davies DM, Foster J, van der stegen SJC, Parente-Pereire AC, Chiapero-Stanke L, Delinassios GJ, et al. Flexible targeting of ErbB dimers that drive tumorigenesis by using genetically engineered T cells. Mol Med. 2012;18:565–76. doi: 10.2119/molmed.2011.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lo AS, Ma Q, Liu DL, Junghans RP. Anti-GD3 chimeric sFv-CD28/T-cell receptor zeta designer T cells for treatment of metastatic melanoma and other neuroectodermal tumors. Clin Cancer Res. 2010;16:2769–80. doi: 10.1158/1078-0432.CCR-10-0043. [DOI] [PubMed] [Google Scholar]
- 223.Yun CO, Nolan KF, Beecham EJ, Reisfeld RA, Junghans RP. Targeting of T lymphocytes to melanoma cells through chimeric anti-GD3 immunoglobulin T-cell receptors. Neoplasia. 2000;2:449–59. doi: 10.1038/sj.neo.7900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Morgenroth A, Cartellieri M, Schmitz M, Gunes S, Weigle B, Bachmann M, et al. Targeting of tumor cells expressing the prostate stem cell antigen (PSCA) using genetically engineered T-cells. Prostate. 2007;67:1121–31. doi: 10.1002/pros.20608. [DOI] [PubMed] [Google Scholar]
- 225.Katari UL, Keirnan JM, Worth AC, Hodges SE, Leen AM, Fisher WE, et al. Engineered T cells for pancreatic cancer treatment. HPB (Oxford) 2011;13:643–50. doi: 10.1111/j.1477-2574.2011.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2013;31:71–5. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Willemsen RA, Weijtens ME, Ronteltap C, Eshhar Z, Gratama JW, Chames P, et al. Grafting primary human T lymphocytes with cancer-specific chimeric single chain and two chain TCR. Gene Ther. 2000;7:1369–77. doi: 10.1038/sj.gt.3301253. [DOI] [PubMed] [Google Scholar]
- 228.Willemsen RA, Debets R, Hart E, Hoogenboom HR, Bolhuis RL, Chames P. A phage display selected fab fragment with MHC class I-restricted specificity for MAGE-A1 allows for retargeting of primary human T lymphocytes. Gene Ther. 2001;8:1601–8. doi: 10.1038/sj.gt.3301570. [DOI] [PubMed] [Google Scholar]
- 229.Willemsen RA, Ronteltap C, Chames P, Debets R, Bolhuis RL. T cell retargeting with MHC class I-restricted antibodies: the CD28 costimulatory domain enhances antigen-specific cytotoxicity and cytokine production. J Immunol. 2005;174:7853–8. doi: 10.4049/jimmunol.174.12.7853. [DOI] [PubMed] [Google Scholar]
- 230.Barber A, Zhang T, Sentman CL. Immunotherapy with chimeric NKG2D receptors leads to long-term tumor-free survival and development of host antitumor immunity in murine ovarian cancer. J Immunol. 2008;180:72–8. doi: 10.4049/jimmunol.180.1.72. [DOI] [PubMed] [Google Scholar]
- 231.Barber A, Rynda A, Sentman CL. Chimeric NKG2D expressing T cells eliminate immunosuppression and activate immunity within the ovarian tumor microenvironment. J Immunol. 2009;183:6939–47. doi: 10.4049/jimmunol.0902000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhang T, Lemoi BA, Sentman CL. Chimeric NK-receptor-bearing T cells mediate antitumor immunotherapy. Blood. 2005;106:1544–51. doi: 10.1182/blood-2004-11-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhang T, Barber A, Sentman CL. Chimeric NKG2D modified T cells inhibit systemic T-cell lymphoma growth in a manner involving multiple cytokines and cytotoxic pathways. Cancer Res. 2007;67:11029–36. doi: 10.1158/0008-5472.CAN-07-2251. [DOI] [PubMed] [Google Scholar]
- 234.Zhang T, Sentman CL. Mouse tumor vasculature expresses NKG2D ligands and can be targeted by chimeric NKG2D-modified T cells. J Immunol. 2013;190:2455–63. doi: 10.4049/jimmunol.1201314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Barber A, Zhang T, DeMars LR, Conejo-Garcia J, Roby KF, Sentman CL. Chimeric NKG2D receptor-bearing T cells as immunotherapy for ovarian cancer. Cancer Res. 2007;67:5003–8. doi: 10.1158/0008-5472.CAN-06-4047. [DOI] [PubMed] [Google Scholar]
- 236.Zhang T, Barber A, Sentman CL. Generation of antitumor responses by genetic modification of primary human T cells with a chimeric NKG2D receptor. Cancer Res. 2006;66:5927–33. doi: 10.1158/0008-5472.CAN-06-0130. [DOI] [PubMed] [Google Scholar]
- 237.Lehner M, Gotz G, Proff J, Schaft N, Dorrie J, Full F, et al. Redirecting T cells to Ewing’s sarcoma family of tumors by a chimeric NKG2D receptor expressed by lentiviral transduction or mRNA transfection. PLoS One. 2012;7:e31210. doi: 10.1371/journal.pone.0031210. [DOI] [PMC free article] [PubMed] [Google Scholar]