Abstract
Rationale
Traditional measures of anticoagulation, including activated clotting time (ACT), have been poorly correlated with circuit thrombosis in pediatric patients supported with extracorporeal membrane oxygenation (ECMO).
Objective
Investigate whether anti-Factor Xa levels are associated with the need for change of circuit/membrane oxygenator secondary to thrombus formation in pediatric patients.
Design and Settings
Retrospective single institution study.
Patients and Methods
Retrospective record review of 62 pediatric patients supported with ECMO from 2009-2011. Data on standard demographic characteristics, indications for ECMO, duration of ECMO, ACT measurements, anti-Factor Xa measurements, and heparin infusion rate (HIR) were collected. Generalized linear models were used to associate anti-Factor Xa concentrations and need for change of either entire circuit/membrane oxygenator secondary to thrombus formation.
Measurements and Main Results
Sixty two patients met study inclusion criteria. No circuit change was required in 45/62 (Group NC). 17/62 patients required change of circuit/membrane oxygenator due to thrombus formation (Group CC). Multivariate analysis of daily anti-Factor Xa measurements throughout duration of ECMO support estimated a mean anti-Factor Xa concentration of 0.20 IU/ml [95% CI: (0.16, 0.24)] in Group NC that was significantly higher than the estimated 0.13 IU/ml [95% CI: (0.12, 0.14)] in Group CC (p = 0.001). A 0.01 IU/ml decrease in anti-Factor Xa increased odds of need for circuit/membrane oxygenator change by 5% (OR = 1.105 95% CI: 1.00, 1.10), p=0.044). Based upon the observed anti-Factor Xa concentrations, Group CC had 41% increased odds for requiring circuit/ membrane oxygenator change compared to Group NC (OR=1.41, 95% CI: 1.01, 1.96, p = 0.044). Mean daily ACT measurement (p = 0.192) was not different between groups, but mean daily HIR (p <0.001) was significantly different between the two groups.
Conclusion
Higher anti-Factor Xa concentrations were associated with freedom from circuit/membrane oxygenator change due to thrombus formation in pediatric patients during ECMO support. ACT measurements did not differ significantly between groups with or without circuit/membrane oxygenator change. This is the first study to link anti-Factor Xa concentrations with a clinically relevant measure of thrombosis in pediatric patients during ECMO support. Further prospective study is warranted.
Keywords: ECMO, children, heparin, anti-Factor Xa
Introduction
Since the inception of ECMO support in the 1970's, unfractionated heparin (UFH) has been the most commonly used anticoagulant medication during extracorporeal support [1-3]. Traditionally, titration of UFH infusion rates has been based on bedside measurement of Activated Clotting Time (ACT) [4,5]. The ACT gained acceptance as the primary measurement of heparin effect in ECMO supported patients based on its ready bedside availability, rapid results, and ability to measure clotting time in whole blood. The use of ACT measurement in ECMO support evolved from its use during cardiopulmonary bypass and was never implemented on the basis of prospective investigations [6-8]. However, recent studies suggest that basing ECMO anticoagulation strategy on ACT measurement alone leads to sub-optimal anticoagulation in these patients [8-14].
Sub-optimal anticoagulation leads to increased clot formation within the extracorporeal circuit, which often necessitates ECMO circuit changes. The circuit/membrane oxygenator change procedure itself may be associated with an increased risk of complications and increased costs in an already critically ill patient. Furthermore, sub-optimal anticoagulation and increased clot formation in the extracorporeal circuit has been suggested to be associated with microembolic injury to end-organs and increased morbidity and mortality [8,15]. It has been proposed that additional monitoring of anticoagulation using anti-Factor Xa levels, a direct measurement of the plasma concentration of UFH and long considered the “gold standard” measure of heparin activity, may be warranted [9,16].
Since 2009, daily measurements of anti-Factor Xa levels have been performed at our institution in patients during ECMO support. The impact of these anti-Factor Xa measurements on clinical outcomes has been unclear because decisions regarding heparin titration have continued to be made based primarily on routine measurement of ACT. This retrospective study aimed to investigate whether sub-therapeutic anti-Factor Xa levels are associated with an increased need for ECMO circuit/membrane oxygenator changes in children during ECMO support.
We hypothesized that lower average anti-Factor Xa levels per ECMO deployment were associated with increased frequency of circuit/membrane oxygenator change due to thrombus formation.
Materials and Methods
Patient Population
This retrospective single center study was conducted at Arkansas Children's Hospital, a 316-bed free-standing tertiary children's hospital in Little Rock, Arkansas and was approved by the Institutional Review Board at the University of Arkansas for Medical Sciences. The requirement for informed consent was waived. Subjects for this study were identified from a review of the institutional medical record as well as a clinical database of patients supported with ECMO from January 2009-March 2011. Inclusion criteria were children (1) supported with ECMO at Arkansas Children's Hospital, (2) between the ages of 0-21 years, and (3) who had daily measurement of anti-Factor Xa levels. Fifty patients were excluded from initial data analysis based on predetermined exclusion criteria [patients transferred from an outside hospital on ECMO (n=7), incomplete patient records after multiple attempts to locate missing data (n=14), repeat course of ECMO (n=14), and required manipulation of the circuit that did not involve changing of an entire circuit/membrane oxygenator (n=15)]. The 15 patients excluded for minor circuit manipulation did not experience cessation of ECMO flow and/or exposure to large blood volumes. The specific scenarios excluded by this criterion were: clot removal from a connector (n=7), clot removal from a bladder (n=1), bladder change secondary to clot formation (n=2), manifold change secondary to clot formation (n=1), clots removed from tubing (n=2), tubing rupture (n=1), and manifold requiring change secondary to a crack (n=1).
Study variables
Data abstracted included demographic variables, primary and secondary diagnoses, indication for ECMO support, duration of ECMO support, survival to hospital discharge, and complications. All ACT, anti-Factor Xa, aPTT, antithrombin III (AT3), and platelet measurements obtained during ECMO support were collected. Data on heparin infusion rates (HIR) and the number of times that either an entire circuit/membrane oxygenator required change due to thrombus formation were also collected. Data was stored and managed using Research Electronic Data Capture (REDCap) [17] hosted by the Clinical and Translational Center at the University of Arkansas for Medical Sciences (support through NCRR/NIH Grant 1 UL1 RR02988).
Clinical practice
Standard practice at our institution for anticoagulation of a patient during ECMO support includes administration of a bolus of approximately 100 units/kg of UFH at the time of ECMO cannula insertion, along with 200 units of heparin for each unit of PRBCs used to prime the circuit. The ECMO circuit prime also includes 120 mls of FFP per unit of blood. After the initiation of ECMO, 20 ml/kg of platelets are administered. Shortly after initiation of ECMO support, an initial ACT measurement is obtained using the Medtronic ACT Plus®. Once the ACT measurement falls below 250 seconds, a heparin infusion is initiated at a dose of approximately 20 units/kg/hour. Subsequently, ACT measurements are performed hourly and the heparin infusion rate is titrated to achieve ACT measurements within the desired range as ordered by the attending physician (typically 180-220 seconds). The HIR can then be titrated up to 4 units/kg/hour by the bedside ECMO specialist to achieve targeted ACT range before a physician is notified. At times of anticipated acute changes in anticoagulation, such as platelet infusions, large volume diuresis, or circuit changes, the attending physician or on-duty ECMO coordinator may authorize the bedside specialist to administer a UFH bolus of 5-10 units/kg, followed by repeat ACT assessment within 15 minutes. Anti-coagulation parameters are evaluated and target ACT ranges are stringently maintained. Standard practice at our institution also includes measurement of platelet count every 4 hours with administration of 10 mL/kg of platelets to maintain the platelet count within the range as ordered by the attending physician (typically above 100,000 /cu mm). Prothrombin time, fibrinogen, and AT3 concentration are measured once daily and more often as clinically indicated. Beginning in 2009, anti-Factor Xa concentrations were measured once daily and as clinically indicated. However, decisions regarding HIR titration continued to be made based primarily on ACT measurement.
All patients were supported with a centrifugal blood pump. All patients except for 2 patients in the NC group were supported with the Jostra Quadrox D (Surface Area (SA)= 0.8 M2) oxygenator, a polymethylpentene hollow fiber device. The two patients in the NC group supported with different oxygenators were supported with a Medtronic 0800 (SA= 0.8 M2), which is a silicone membrane device, in the case of one patient, and a Medtronic MiniMax Plus (SA = 0.8 M2), also a hollow fiber device, in the other patient.
Study definitions
It is standard practice at our institution for the bedside ECMO specialist to visibly inspect the ECMO circuit for signs of thrombus formation every hour. Formation of thrombus in the membrane oxygenator is assessed visibly and also by the measurement of “pre- and post-membrane” blood gases each day, as well as by the continuously monitored pre- and post-membrane circuit pressures. A decision that the circuit or membrane oxygenator requires change is at the discretion of the attending physician. Such a change typically is performed for the following reasons: (1) visible thrombus on the arterial limb of the circuit; (2) steadily worsening thrombus formation in components of the venous limb of the circuit; (3) worsening thrombus formation within the membrane oxygenator as assessed by worsening gas exchange across the oxygenator or increasing circuit pressures; or (4) development of the “sick circuit syndrome” [18-20] consisting of platelet and fibrinogen consumption, signs of worsening patient capillary leak, and increasing circuit visible thrombus or fibrin stranding.
Statistical Analysis
Differences between those patients not requiring a circuit/membrane oxygenator change during ECMO support (Group NC) and those patients requiring a change of an entire circuit/membrane oxygenator due to thrombus formation (Group CC) were estimated in both univariable and multivariable analysis. For univariable analysis, differences in patient characteristics, including demographic as well as clinical features and outcomes, between groups were estimated using Wilcoxon-Mann-Whitney test for continuous variables and Chi-square test for categorical variables (Fisher's exact used if Chi-square assumptions failed).
Linear regression and generalized linear models were both utilized to estimate any differences between groups for primary outcomes during the ECMO deployment. Generalized linear models included assuming a strictly positive distribution (Gamma distribution) for measures skewed towards zero as well as logistic regression for binary outcomes. All models accounted for the repeated daily measurements and restricted cubic splines in modeling days on ECMO. Restricted cubic splines relax the strict linear assumption of linear models, allowing the model to bend and curve to more accurately reflect changes in data [21]. Differences between groups were estimated using a multiplicative interaction between group and days on ECMO to accurately model changes over time within each group and an indicator of neonatal deployment. While only three variables were included in any one model, a total of 15 parameter estimates were modeled due to the complex interaction between group, ECMO run time and neonatal deployment. Additionally, a logistic regression model was used to estimate odds of circuit change adjusting for ECMO deployment and repeated observations on each subject. All statistical analysis was completed using Stata 13.0 (College Station, TX).
Results
Patient characteristics
Sixty-two patients met eligibility criteria for this study based on retrospective record review of 112 patients during ECMO support from 2009-2011. Group NC (45/62) required no circuit/membrane oxygenator change during ECMO support. Group CC (17/62) required change of circuit/membrane oxygenator due to thrombus formation.
Baseline demographic and clinical features of this patient population are shown in Table 1. The median age was 0.2 years for Group NC and 0.02 years for Group CC (p = 0.132). The Group NC had 47% neonates (age < 1 month) and Group CC had 65% neonates (p = 0.205). None of the demographic variables were significantly different between the groups (Table 1). Regarding the underlying indication for ECMO support, study patients were grouped into the following categories: ECMO for cardiopulmonary resuscitation (ECPR), septic shock, respiratory failure (both neonatal and pediatric), cardiac postoperative support, cardiac bridge- to- transplant, and other cardiac. Group NC contained 13/45 patients supported with ECMO for primary cardiac failure and Group CC 5/17 (p = 0.969). ECMO was used in the remainder for either respiratory failure or as rescue during ECPR (Table 1). No statistically significant difference existed between the groups based on indication for ECMO support. Survival to hospital discharge occurred in 27/45 (60%) Group NC and 12/17 (70%) Group CC (p = 0.627).
Table 1.
Demographic and Clinical Features by Complete Circuit / Oxygenator Change
| Group NC | Group CC | |||
|---|---|---|---|---|
| Yes - Only One | Yes - Multiple | P* | ||
| N | 45 | 12 | 5 | |
| Age At ECMO (years) | 0.132† | |||
| Mean (SD) | 3.2 (5.6) | 0.2 (0.4) | 0.2 (0.3) | |
| Median [Min, Max] | 0.2 [0, 20.7] | 0.02 [0.01, 1.3] | 0.02 [0, 0.6] | |
| Neonate at ECMO | 0.434 | |||
| No | 24 (53%) | 4 (33%) | 2 (40%) | |
| Yes | 21 (47%) | 8 (67%) | 3 (60%) | |
| Gender | 0.333 | |||
| Male | 25 (55%) | 9 (75%) | 3 (60%) | |
| Female | 20 (44%) | 3 (25%) | 2 (40%) | |
| Race | 0.798‡ | |||
| Caucasian | 22 (49%) | 4 (33%) | 3 (60%) | |
| African-American | 16 (36%) | 6 (50%) | 2 (40%) | |
| Other | 7 (16%) | 2 (17%) | 0 | |
| Reason for ECMO | 0.327‡ | |||
| ECPR | 14 (31%) | 2 (17%) | 0 | |
| Septic Shock | 2 (4%) | 0 | 0 | |
| Neonatal Respiratory Failure | 13 (29%) | 6 (50%) | 3 (60%) | |
| Pediatric Respiratory Failure | 3 (7%) | 0 | 1 (20%) | |
| Cardiac / Post-Op Support | 5 (11%) | 3 (25%) | 1 (20%) | |
| Cardiac / Bridge to Transplant | 1 (2%) | 1 (8%) | 0 | |
| Cardiac / Other | 7 (16%) | 0 | 0 | |
| Survival at 24 Hours | 0.969 | |||
| No | 10 (22%) | 3 (25%) | 1 (20%) | |
| Yes | 35 (78%) | 9 (75%) | 4 (80%) | |
| Survival to Discharge | 0.627 | |||
| No | 18 (40%) | 3 (25%) | 2 (40%) | |
| Yes | 27 (60%) | 9 (75%) | 3 (60%) | |
Unless otherwise noted, N (Column %) and Chi-Square test reported.
Kruskal-Wallis test reported.
Fisher's Exact test reported.
Data was collected on the following ECMO-related complications: central nervous system (CNS) infarction, CNS hemorrhage, pulmonary hemorrhage, gastrointestinal (GI) hemorrhage, surgical/ cannula site hemorrhage, and need for continuous veno-venous hemodialysis (CVVHD). All hemorrhagic events were defined based on guidelines from the Extracorporeal Life Support Organization (ELSO) that indicate an event that required an intervention or PRBC transfusion. There was no statistically significant difference between the two groups for any of the pre-defined complications (Table 2).
Table 2.
Comorbidities by Complete Circuit / Oxygenator Change
| Group NC | Group CC | P | ||
|---|---|---|---|---|
| CNS Infarct by CT/US | 0.310 | |||
| No | 40 (89%) | 17 (100%) | ||
| Yes | 5 (11%) | 0 | ||
| CNS Hemorrhage by CT/US | 0.427 | |||
| No | 38 (84%) | 16 (94%) | ||
| Yes | 7 (16%) | 1 (6%) | ||
| Pulmonary Hemorrhage | 0.999 | |||
| No | 43 (96%) | 17 (100%) | ||
| Yes | 2 (4%) | 0 | ||
| GI Hemorrhage | ||||
| No | 45 (100%) | 17 (100%) | ||
| Yes | 0 | 0 | ||
| Surgical / Cannula Site Hemorrhage | 0.721 | |||
| No | 37 (82%) | 13 (76%) | ||
| Yes | 8 (18%) | 4 (24%) | ||
| Any Hemorrhage | 0.561 | |||
| No | 27 (60%) | 12 (71%) | ||
| Yes | 18 (40%) | 5 (29%) | ||
| Dialysis | 0.310 | |||
| No | 40 (89%) | 17 (100%) | ||
| Yes | 5 (11%) | 0 |
†N (Column %) and Fisher's Exact test reported.
Primary Outcomes
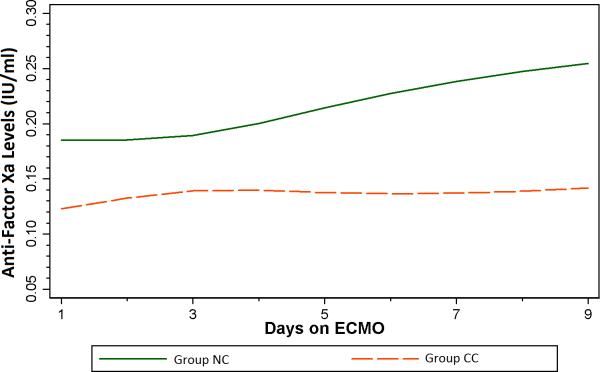
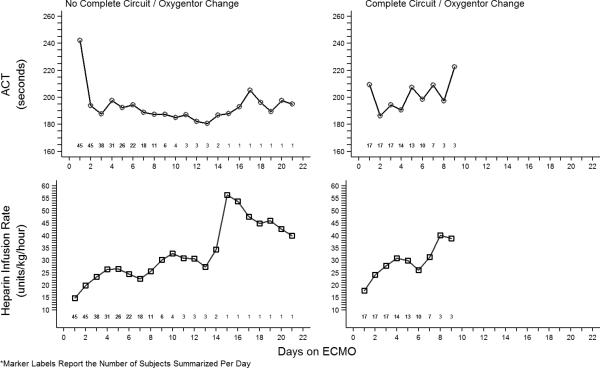
Table 3 summarizes the multivariate regression analysis for the primary outcome-the need for change of circuit or membrane oxygenator due to thrombus formation. The average anti-Factor Xa concentration of 0.20 IU/ml [95% CI: (0.16, 0.24)] in Group NC was significantly larger than the estimated 0.13 IU/ml [95% CI: (0.12, 0.14)] in Group CC (p = 0.001). The logistic regression model estimated that a 0.01 IU/ml decrease in anti-Factor Xa increased odds of need for circuit/membrane oxygenator change by 5% (OR = 1.05, 95% CI: 1.00, 1.10), p=0.044). (Figure 1) Based upon the observed anti-Factor Xa concentrations, Group CC had 41% increased odds of need for circuit/membrane oxygenator change compared to Group NC (OR=1.41, 95% CI: 1.01, 1.96, p=0.044). The average anti-Factor Xa level was 0.15 (95% CI: 0.09, 0.21) over all circuits for the 5 patients with multiple complete circuit/oxygenator changes. A total of 62 anti-Factor Xa observations were made on these five patients. Mean daily ACT measurement (p = 0.192) was not different between groups, but mean daily HIR (p =<0.001) was significantly different between the two groups. (Figure 2). A sensitivity analysis was performed to estimate change in anti-Factor Xa between groups using the previous model while additionally adjusting for mean daily HIR; however, this model did not provide different group estimates as compared to the original model.
Table 3.
Hemodynamic and Laboratory Measures by Complete Circuit / Oxygenator Change
| Group NC | Group CC | P | |
|---|---|---|---|
| ACT (seconds) | 195.26 (5.74) | 202.62 (3.96) | 0.192* |
| Prothombin Time | 75.25 (4.17) | 74.91 (5.66) | 0.962 |
| Anti-Thrombin III | 72.25 (2.82) | 60.42 (2.27) | <0.001* |
| Packed Red Blood Cell / kg | 33.40 (4.12) | 40.59 (5.78) | 0.311 |
| Platelet (thou/U) | 152.10 (12.10) | 135.09 (10.61) | 0.291 |
| Average Heparin Infusion Rate | 24.69 (1.34) | 20.79 (1.11) | <0.001* |
| Heparin Xa (IU/ml) | 0.20 (0.02) | 0.13 (0.01) | 0.001 |
Mean (SE) reported from Linear Model (Normal distribution) adjusting for repeated measurements and neonatal ECMO deployment. Otherwise, Strictly Positive (Gamma distribution) Generalized Linear Model is reported.
Figure 1.
Generalized linear regression model estimates of anti-Factor Xa values by need for circuit/membrane oxygenator change duing ECMO support
Figure 2.
Activated clotting time measurements and heparin infusion rates by circuit/membrane oxygenator change
Evaluating patient transfusion requirements as another means to assess hemorrhage demonstrated that transfusion volume of PRBCs, reported in ml/kg, is negatively associated with anti-Factor Xa levels. In a generalized linear model adjusting for day of ECMO run and neonatal deployment, a 31.7 ml/kg increase in PRBC (interquartile range) used led to a 0.05 IU/ml reduction in anti-Factor Xa (95% CI:-0.08, -0.01; P = 0.007). None of the 5 patients with a complete circuit/membrane oxygenator change secondary to circuit consumption had lab values consistent with DIC secondary to sepsis prior to ECMO initiation.
Discussion
In this study, we demonstrate that anti-Factor Xa concentrations were associated with need for circuit/membrane oxygenator change due to thrombus formation in pediatric ECMO patients. ACT measurements did not differ significantly between groups with or without circuit/ membrane oxygenator change. This is the first study to link anti-Factor Xa concentrations with a clinically relevant measure of thrombosis in pediatric ECMO patients.
The goal of therapeutic anticoagulation during ECMO support is to minimize thrombus formation within the patient and the circuit without increasing bleeding complications. Since 1966, the ACT has been the most common method for objective assessment of anticoagulation. However, it is known that ACT values can be affected by hypothermia, hemodilution, hypofibrinogenemia, and thrombocytopenia [5]. In addition, ACT standard ranges do not take into consideration maturational changes in the hemostatic system [9,22]. The risks of excessive anticoagulation include bleeding complications, which may be associated with increased morbidity and mortality. However, the risks associated with subtherapeutic anticoagulation are also great and include microthrombus formation and thromboembolization with resultant end-organ ischemia.
Additionally, the inherent risks of changing out a circuit or circuit component include increased exposure to blood products, air embolization, cessation of ECMO flow, and infection. A large retrospective study of pediatric ECMO patients found no relationship between ACT values and survival but noted an association between increased heparin infusion doses and improved survival [8]. Other investigators have noted the poor association between ACT measurements and heparin concentrations as measured by anti-Factor Xa concentration [9,11]. These lines of evidence suggest that ACT, while convenient, is not by itself adequate to guide optimal anticoagulation during ECMO support.
Our data suggest that higher mean anti-Factor Xa levels, measured once daily during ECMO support, are associated with freedom from circuit/membrane oxygenator change. During the time period of this study, anti-Factor Xa levels were routinely measured only once daily with other “morning labs” during ECMO support, and clinical decisions regarding heparin infusions continued to be based almost exclusively on ACT measurement. Thus, while a statistical difference in HIR is estimated between the two groups in this study, this is most likely a function of different sample sizes as the group averages do appear to be similar (Figure 2). Since treatment was not directed at achieving a “therapeutic” anti-Factor Xa level, many of the anti-Factor Xa measurements during the study period were below the limit of detection (< 0.1 IU/mL), particularly in Group CC. Of note, even in Group NC, which did not require circuit/membrane oxygenator change, most anti-Factor Xa measurements were < 0.3 IU/mL, which is considered to be in the “subtherapeutic” range. Despite the low absolute values for anti-Factor Xa measurements in this study, a significant difference was still apparent between groups with and without significant thrombosis of the extracorporeal circuit. It is thus tempting to speculate that a practice aimed at intentionally titrating heparin dosing to more frequent measurement of anti-Factor Xa levels might result not only in higher anti-Factor Xa values, but also in an even more dramatic difference in circuit thrombosis. In the current study, no differences in bleeding complications were noted between the two groups. It is not known if a more “liberal” heparin dosing strategy based primarily on anti-Factor Xa measurements would be associated with an increased risk of bleeding complications. Of note, in a recently published retrospective study, Bembea, et al noted an increased risk of hemorrhagic complications in pediatric ECMO patients who demonstrated high anti-Factor Xa levels with a concurrent low ACT measurement [9]. Prospective study of a treatment regimen primarily targeting anti-Factor Xa values is planned.
In addition to the low absolute anti-Factor Xa values noted above, other potential limitations to this study exist. First, as a retrospective study, it has the usual potential for bias associated with retrospective patient selection, particularly in light of the number of patients excluded from the analysis. In addition, the primary outcome variable, requirement for change of circuit/membrane oxygenator, was a clinical decision made by the attending physician and not subject to a strict protocol in any manner. However, clinical practice at our institution with regards to justifications for ECMO circuit/membrane oxygenator change did not vary during the study period, and the decision to change the circuit was made independently of any measure of anticoagulation.
Conclusions
Concentrations of anti-Factor Xa were significantly associated with need for circuit/membrane oxygenator change due to thrombus formation in pediatric patients during ECMO support. ACT measurements did not differ significantly between groups with or without circuit/membrane oxygenator change. This is the first study to link anti-Factor Xa concentrations with a clinically relevant measure of thrombosis in pediatric ECMO patients. We speculate that a strategy of anticoagulation that uses routine, frequent measurement of anti-Factor Xa levels as well as bedside ACT measurements may be beneficial. Prospective study with more frequent measurement of anti-Factor Xa is currently being conducted.
Acknowledgments
We gratefully acknowledge the entire ECMO team at Arkansas Children's Hospital for their outstanding dedication to clinical care and for their assistance in making this study possible.
Funding: This research was supported, in part, by Arkansas Children's Hospital Research Institute Student and Clinical Staff Research Intramural Grant Program and UAMS Traslational Research Institute support for REDCap (NCATS/NIH 1 UL1 RR029884)
Footnotes
Conflict of interest: None
References
- 1.Bartlett RH, Gazzaniga AB, Jefferies MR, Huxtable RF, Haiduc NJ, Fong SW. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Transactions - American Society for Artificial Internal Organs. 1976;22:80–93. [PubMed] [Google Scholar]
- 2.Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, Morris AH, Peirce EC, 2nd, Thomas AN, Proctor HJ, Drinker PA, Pratt PC, Bagniewski A, Miller RG., Jr. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. Jama. 1979;242(20):2193–2196. doi: 10.1001/jama.242.20.2193. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett RH, Roloff DW, Cornell RG, Andrews AF, Dillon PW, Zwischenberger JB. Extracorporeal circulation in neonatal respiratory failure: a prospective randomized study. Pediatrics. 1985;76(4):479–487. [PubMed] [Google Scholar]
- 4.Bembea MM, Annich G, Rycus P, Oldenburg G, Berkowitz I, Pronovost P. Variability in Anticoagulation Management of Patients on Extracorporeal Membrane Oxygenation: An International Survey. Pediatr Crit Care Med. 2013 doi: 10.1097/PCC.0b013e31827127e4. doi:10.1097/PCC.0b013e31827127e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green TP, Isham-Schopf B, Steinhorn RH, Smith C, Irmiter RJ. Whole blood activated clotting time in infants during extracorporeal membrane oxygenation. Critical care medicine. 1990;18(5):494–498. doi: 10.1097/00003246-199005000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Bull BS, Huse WM, Brauer FS, Korpman RA. Heparin therapy during extracorporeal circulation. II. The use of a dose-response curve to individualize heparin and protamine dosage. The Journal of thoracic and cardiovascular surgery. 1975;69(5):685–689. [PubMed] [Google Scholar]
- 7.Graves DF, Chernin JM, Kurusz M, Zwischenberger JB. Anticoagulation practices during neonatal extracorporeal membrane oxygenation: survey results. Perfusion. 1996;11(6):461–466. doi: 10.1177/026765919601100607. [DOI] [PubMed] [Google Scholar]
- 8.Baird CW, Zurakowski D, Robinson B, Gandhi S, Burdis-Koch L, Tamblyn J, Munoz R, Fortich K, Pigula FA. Anticoagulation and pediatric extracorporeal membrane oxygenation: impact of activated clotting time and heparin dose on survival. The Annals of thoracic surgery. 2007;83(3):912–919. doi: 10.1016/j.athoracsur.2006.09.054. discussion 919-920. doi:10.1016/j.athoracsur.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 9.Bembea MM, Schwartz JM, Shah N, Colantuoni E, Lehmann CU, Kickler T, Pronovost P, Strouse JJ. Anticoagulation Monitoring during Pediatric Extracorporeal Membrane Oxygenation. Asaio J. 2013;59(1):63–68. doi: 10.1097/MAT.0b013e318279854a. doi:10.1097/MAT.0b013e318279854a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliver WC. Anticoagulation and coagulation management for ECMO. Seminars in cardiothoracic and vascular anesthesia. 2009;13(3):154–175. doi: 10.1177/1089253209347384. [DOI] [PubMed] [Google Scholar]
- 11.Nankervis CA, Preston TJ, Dysart KC, Wilkinson WD, Chicoine LG, Welty SE, Nelin LD. Assessing heparin dosing in neonates on venoarterial extracorporeal membrane oxygenation. Asaio J. 2007;53(1):111–114. doi: 10.1097/01.mat.0000247777.65764.b3. doi:10.1097/01.mat.0000247777.65764.b3. [DOI] [PubMed] [Google Scholar]
- 12.Gorlinger K, Bergmann L, Dirkmann D. Coagulation management in patients undergoing mechanical circulatory support. Best practice & research Clinical anaesthesiology. 2012;26(2):179–198. doi: 10.1016/j.bpa.2012.04.003. doi:10.1016/j.bpa.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Reed RC, Rutledge JC. Laboratory and clinical predictors of thrombosis and hemorrhage in 29 pediatric extracorporeal membrane oxygenation nonsurvivors. Pediatr Dev Pathol. 2010;13(5):385–392. doi: 10.2350/09-09-0704-OA.1. doi:10.2350/09-09-0704-OA.1. [DOI] [PubMed] [Google Scholar]
- 14.Maul TM, Wolff EL, Kuch BA, Rosendorff A, Morell VO, Wearden PD. Activated partial thromboplastin time is a better trending tool in pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2012;13(6):e363–371. doi: 10.1097/PCC.0b013e31825b582e. doi:10.1097/PCC.0b013e31825b582e. [DOI] [PubMed] [Google Scholar]
- 15.Muntean W. Coagulation and anticoagulation in extracorporeal membrane oxygenation. Artificial organs. 1999;23(11):979–983. doi: 10.1046/j.1525-1594.1999.06451.x. [DOI] [PubMed] [Google Scholar]
- 16.Sievert A, Uber W, Laws S, Cochran J. Improvement in long-term ECMO by detailed monitoring of anticoagulation: a case report. Perfusion. 2011;26(1):59–64. doi: 10.1177/0267659110385513. doi:10.1177/0267659110385513. [DOI] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. 2 DOI:10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urlesberger B, Zobel G, Zenz W, Kuttnig-Haim M, Maurer U, Reiterer F, Riccabona M, Dacar D, Gallisti S, Leschnik B, Muntean W. Activation of the clotting system during extracorporeal membrane oxygenation in term newborn infants. The Journal of pediatrics. 1996;129(2):264–268. doi: 10.1016/s0022-3476(96)70252-4. [DOI] [PubMed] [Google Scholar]
- 19.Adrian K, Skogby M, Friberg LG, Mellgren K. The effect of s-nitroso glutathione on platelet and leukocyte function during experimental extracorporeal circulation. Artificial organs. 2003;27(6):570–575. doi: 10.1046/j.1525-1594.2003.07106.x. [DOI] [PubMed] [Google Scholar]
- 20.Stammers AH, Fristoe LW, Christensen K, Deptula J, Sydzyik RT, Zavadil D, Willett L. Coagulopathic-induced membrane dysfunction during extracorporeal membrane oxygenation: a case report. Perfusion. 1997;12(2):143–149. doi: 10.1177/026765919701200209. [DOI] [PubMed] [Google Scholar]
- 21.Harrell FE. Regression modeling strategies. Springer; New York: 2001. [Google Scholar]
- 22.Nowak-Gottl U, Kosch A, Schlegel N. Neonatal thromboembolism. Seminars in thrombosis and hemostasis. 2003;29(2):227–234. doi: 10.1055/s-2003-38839. doi:10.1055/s-2003-38839. [DOI] [PubMed] [Google Scholar]