Abstract
Gamma band activity participates in sensory perception, problem solving, and memory. This review considers recent evidence showing that cells in the reticular activating system (RAS) exhibit gamma band activity, and describes the intrinsic membrane properties behind such manifestation. Specifically, we discuss how cells in the mesopontine pedunculopontine nucleus (PPN), intralaminar parafascicular nucleus (Pf), and pontine Subcoeruleus nucleus dorsalis (SubCD) all fire in the gamma band range when maximally activated, but no higher. The mechanisms involve high threshold, voltage-dependent P/Q-type calcium channels or sodium-dependent subthreshold oscillations. Rather than participating in the temporal binding of sensory events as in the cortex, gamma band activity in the RAS may participate in the processes of preconscious awareness, and provide the essential stream of information for the formulation of many of our actions. We address three necessary next steps resulting from these discoveries, an intracellular mechanism responsible for maintaining gamma band activity based on persistent G-protein activation, separate intracellular pathways that differentiate between gamma band activity during waking vs during REM sleep, and an intracellular mechanism responsible for the dysregulation in gamma band activity in schizophrenia. These findings open several promising research avenues that have not been thoroughly explored. What are the effects of sleep or REM sleep deprivation on these RAS mechanisms? Are these mechanisms involved in memory processing during waking and/or during REM sleep? Does gamma band processing differ during waking vs REM sleep after sleep or REM sleep deprivation?
Keywords: Arousal, Calcium/calmodulin-dependent protein kinase II, Cyclic adenosine monophosphate, G-proteins, Neuronal calcium sensor, Schizophrenia
Gamma Band Activity
Gamma oscillations participate in sensory perception, problem solving, and memory (Eckhorn et al. 1988; Gray and Singer 1989; Jones 2007; Palva et al. 2009; Philips and Takeda 2009; Voss et al. 2009), and coherence at these frequencies occurs at cortical or thalamocortical levels (Llinas et al. 1991; Singer 1993). On the one hand, synchronous gamma band activation among thalamocortical networks (Llinas et al. 2002), and in other neuronal groups (i.e., hippocampal and striatal afferents and efferents) is thought to contribute to the merger, or “binding”, of information originating from separate regions (Llinas and Pare 1991). On the other hand, gamma oscillation deficits have been suggested as a pathophysiologic feature of diseases like schizophrenia and Alzheimer’s disease (Steriade and Llinas 1988; Ribary et al. 1991; Stam et al. 2002). Gamma band activity is present during waking and REM sleep, and REM sleep has been implicated in the consolidation of memories (Dickleman and Born 2010; Payne 2011). Interhemispheric coupling is increased during REM sleep compared to waking, suggesting differential functions during the two states (Corsi-Cabrera et al. 1987), that could be critical not only for memory consolidation, but also for the consolidation of emotional events (Walker 2009), and emotional reactivity (Gujar et al. 2011).
Gamma oscillations emerge from the dynamic interaction between intrinsic membrane and synaptic properties of thalamocortical networks (Steriade and Llinas 1988). The neuronal networks behind such activity include the presence of inhibitory cortical interneurons with intrinsic membrane potential oscillatory activity in the gamma range (Steriade and Llinas 1988; Llinas et al. 1991; Steriade 1999), many of which are electrically coupled (Gibson et al. 1999), as well as of fast rhythmic bursting pyramidal neurons (Cunningham et al. 2004). At the thalamic level, thalamocortical excitatory neurons have intrinsic properties needed to generate subthreshold gamma band membrane potential oscillations (Pedroarena and Llinas 1997).
While cortical interneurons can generate membrane potential gamma oscillations through the activation of voltage-dependent, persistent sodium channels (Llinas et al. 1991), and metabotropic glutamate receptors (Whittington et al. 1995), in thalamocortical neurons, the mechanism responsible for gamma band activity involves high threshold P/Q-type voltage-gated calcium channels located in the dendrites (Pedroarena and Llinas 1997; Whittington et al. 1995). Moreover, the same intrinsic properties mediating gamma band oscillations are present in the thalamus of several vertebrate species, indicating considerable evolutionary conservation (Llinas and Steriade 2006). However, very little is known about the effects of sleep disturbances, including sleep deprivation or REM sleep deprivation on these intrinsic mechanisms in the cortex. Even less is know about such effects on subcortical gamma band mechanisms.
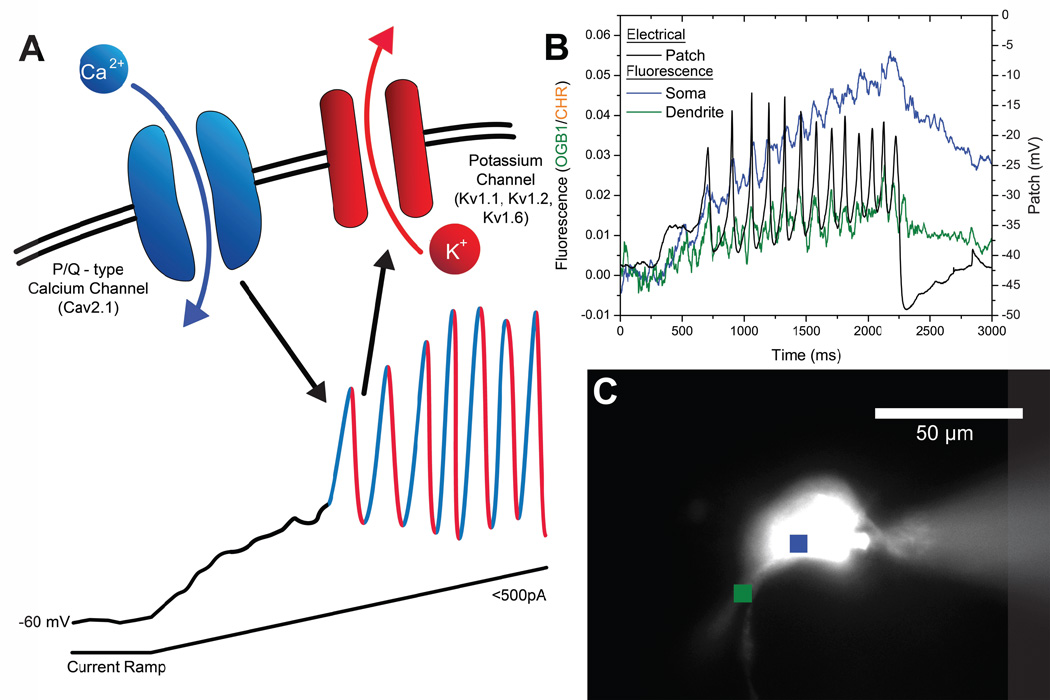
The reticular activating system (RAS) is known to control the manifestation of cortical gamma band activity during waking and during REM sleep. We recently described the presence of gamma band activity as part of the intrinsic properties of neurons in various nuclei of the RAS. We found that almost all cells in the mesopontine pedunculopontine nucleus (PPN) and its ascending target, the intralaminar thalamic parafascicular nucleus (Pf), as well as the descending target, the SubCoeruleus nucleus dorsalis (SubCD), were capped to fire at gamma frequencies (40–60 Hz), but no higher (Simon et al. 2010, 2011; Kezunovic et al. 2012). We found that this activity was subserved by voltage-dependent, high threshold N- and P/Q-type calcium channels in the PPN and Pf (Kezunovic et al. 2011, 2012), and by sodium-dependent subthreshold oscillations in the SubCD (Simon et al. 2011). We localized N- and P/Q-type calcium channel-mediated gamma oscillations to the dendrites of PPN and Pf cells (Hyde et al. 2013a, 2013b). Figure 1A provides a schematic of the mechanism described for the PPN, in which P/Q-type calcium channels mediate the depolarizing phase of each oscillation, while rectifier-like potassium channels mediate the repolarizing phase, all at gamma frequencies (Kezunovic et al. 2011). This figure also provides an example of calcium imaging of the dendrites (Fig. 1B) showing calcium transients in relation to electrophysiologically recorded oscillations in a PPN neuron (Fig. 1C).
Figure 1. Model of high threshold, voltage-dependent P/Q-type calcium channel oscillations, and visualization of calcium transients in the dendrite of a PPN neuron.
A) Application of a ramp stimulus in the presence of synaptic blockers and TTX slowly depolarizes the membrane, avoiding activation of potassium channels. Once the voltage-dependent high threshold of calcium channels is reached, ~ −30 mV, membrane oscillations are observed. Addition of the specific P/Q-type calcium channel blocker ω-agatoxin-IVA will eliminate the oscillations, demonstrating that the depolarizing phase is due to P/Q-type calcium channels (Cav2.1). Addition of the delayed rectifier-like potassium channel blocker dendrotoxin also blocks the oscillations, demonstrating that the repolarizing phase is due to these potassium channels (Kv1.1, Kv1.2, Kv1.6) (Kezunovic et al. 2012). B) Electrophysiological recording of a PPN cell during ramp-induced membrane oscillations (black record), while region of interest calcium transients were imaged from the cell body (blue record) and one of the dendrites (green record). The peaks of the calcium oscillations coincided with the peaks in the electrical recording, and different dendrites (not shown) manifested calcium transients coinciding with different of the peaks of the soma recording (Hyde et al 2013b). C) Locations of the regions of interest shown in B in a photomicrograph of a PPN cell injected with fluorescent dye, with the microelectrode evident on the right.
The SubCD, the descending target of the PPN, is different. The SubCD is critical to the generation of REM sleep and its characteristic atonia and ponto-geniculo-occipital (PGO) waves (P-waves in the rat) (Aserinsky and Kleitman 1953; Datta et al. 1998). The SubCD is most active during REM sleep (Boissard et al. 2002; Datta et al. 2009), and injection of the nonspecific cholinergic agonist carbachol induced a REM sleep like state with muscle atonia and PGO waves (Baghdoyan et al. 1987; Boissard et al. 2002; Mitler and Dement 1974). Lesion of this region produced REM sleep without atonia or PGO waves (Sanford et al. 1974; Mavanji et al. 2004). The SubCD receives cholinergic input from the PPN, and projects to many areas, including the lateral geniculate nucleus and hippocampus (Datta et al. 1998, 1999). We found that SubCD neurons were also capped to fire at gamma band frequencies, but the mechanism responsible for firing at these frequencies was identified as sodium-dependent subthreshold oscillations (Simon et al. 2011; Urbano et al. 2013), like those mediating gamma oscillations in some cortical interneurons (Llinas et al. 1991). Therefore, we speculate that the generation of gamma band activity during REM sleep may primarily include the PPN-SubCD pathway, while during waking it may primarily use the PPN-Pf/intralaminar thalamus pathway. There is another difference between the gamma band activity during waking and that during REM sleep in that the latter is generated during a relative decrease in frontal lobe blood flow (Maquet et al. 1966). This suggests that there is a lack of critical judgment during REM sleep and dreaming (Garcia-Rill et al. 2008). This is perhaps why, during the REM sleep state, in which we are relatively (to waking) disconnected from the external world, the brain manufactures dreams that are not bound by critical judgment, and can be considered cognitively “aberrant”. We explore below the issue of whether gamma band activity during waking is different from that during REM sleep.
These results suggest that a similar mechanism to that in the cortex for achieving temporal coherence at high frequencies is present in the PPN, and in its subcortical targets such as the Pf and SubCD nuclei. That is, the PPN generates and relays gamma band activity to its ascending target, the Pf, as well as to its descending target, the SubCD, and they each have the appropriate intrinsic membrane properties to in turn relay gamma frequency activity to their targets. We suggested that gamma band activity and electrical coupling in the PPN helps stabilize coherence related to arousal, providing a stable activation state during waking and paradoxical sleep (Kezunovic et al. 2010, 2011; Simon et al. 2010). We further proposed that sensory input induces gamma band activity in the RAS that participates in the process of preconscious awareness (Garcia-Rill et al. 2013; Urbano et al. 2013), that is, in the essential mechanism that allows the uninterrupted flow of afferent sensory information, the background tone, necessary for the “stream of consciousness”, as coined by William James. The RAS seems the ideal site for preconscious awareness since it is phylogenetically conserved, and modulates sleep/wake cycles, the startle response, and fight-vs-flight responses that include changes in muscle tone and locomotion.
Gamma Maintenance
It has been suggested that consciousness is associated with continuous gamma band activity, as opposed to an interrupted pattern of activity (Vanderwolf 2000a, b). The original description of the RAS specifically suggested that it participates in tonic or continuous arousal, and lesions of this region were found to eliminate tonic arousal (Moruzzi and Magoun 1949; Watson et al. 1974). This raises the question of how a circuit can maintain such rapid, recurrent activation for long periods. Expecting a circuit of 8–10 synapses to reliably relay 30–60 Hz cycling without failing is unrealistic. Without the intrinsic membrane properties afforded by rapidly opening channels such as those described for PPN, Pf, and subthreshold oscillations in SubCD, as well as the presence of electrically coupled neurons that help firing across different membrane potentials (Garcia-Rill et al. 2008), gamma band activity could not be maintained. The combination of a) channels capable of mediating fast membrane oscillations, and b) circuitry that involves activating these channels, is probably required for the maintenance of gamma band activity (Llinas 1988; Llinas et al. 1991, 2002, 2007; Kezunovic et al. 2011). RAS structures in which every cell in every nucleus exhibits gamma band activity, and in which a subgroup of cells manifest electrical coupling, then becomes a gamma-making machine. We speculate that it is the activation of the RAS during waking and REM sleep that induces coherent activity (through electrically coupled cells) and high frequency oscillations (through P/Q-type calcium channel and subthreshold oscillations). This leads to the maintenance of the background of gamma activity necessary to support a state capable of reliably assessing the world around us on a continuous basis. That is, these mechanisms may underlie preconscious awareness. However, we do not know how such a process is altered during REM sleep compared to waking, or its participation in memory consolidation and emotional responsiveness.
Three Questions
These suggestions raise additional complex questions, among others, which we have been pursuing to the next level of analysis, the intracellular mechanisms involved. What intracellular mechanism(s) mediate the maintenance of gamma band activity? Are the mechanisms behind gamma band activity different during waking than during REM sleep? What intracellular mechanisms are involved in pathological states such as schizophrenia?
A mechanism for gamma maintenance
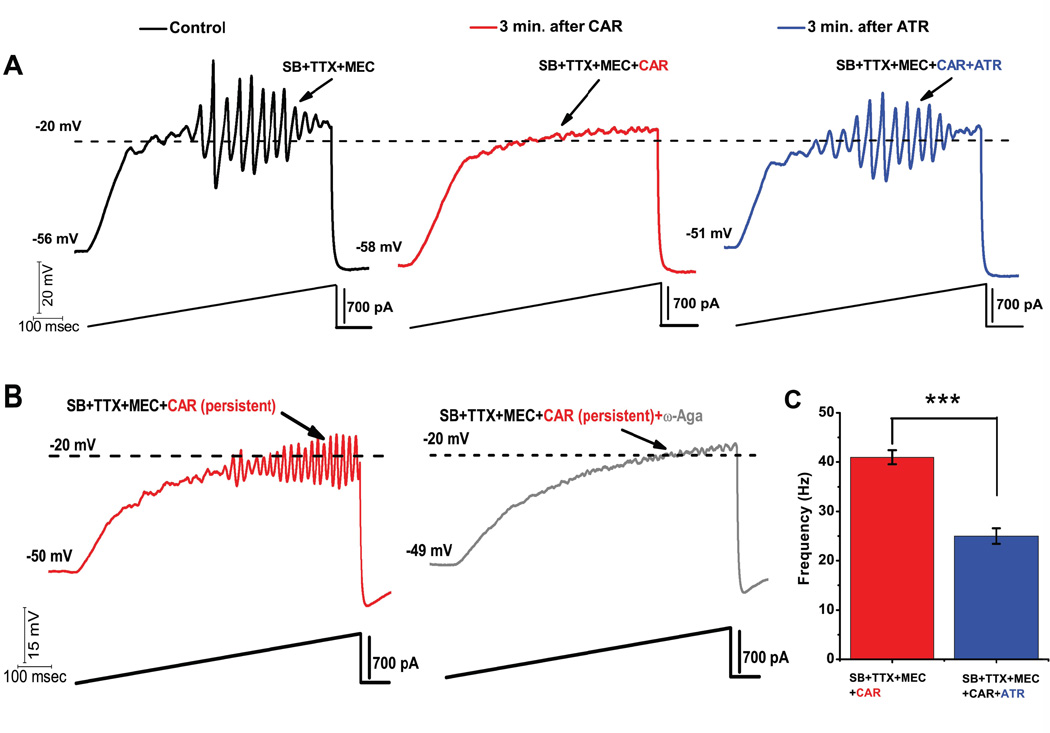
The PPN receives cholinergic input from the contralateral PPN and the laterodorsal tegmental nuclei (Semba and Fibiger 1992). PPN cholinergic neurons are hyperpolarized by the activation of postsynaptic muscarinic M2 cholinergic receptors (Luebke et al. 1993; Leonard and Llinas 1994). Studies from our laboratory showed that 73% of PPN output neurons were inhibited through M2 receptors, 13% were excited through M1 and nicotinic receptors, and 7% showed biphasic responses (Ye et al. 2010). The most abundant receptor type in the PPN is the M2 muscarinic receptor, which is Gi-protein coupled. Recently, we found that acute/short lasting application of the nonspecific cholinergic agonist carbachol (CAR) reversibly blocked PPN ramp-induced oscillations (Fig. 2A) (Kezunovic et al. 2013). This study showed that acute CAR application blocked P/Q-type calcium channel-mediated oscillations in the PPN through M2 muscarinic receptors, suggesting that phasic cholinergic input to PPN cells leads to an inhibition of high frequency oscillations (Kezunovic et al. 2013). We conclude that temporary membrane hyperpolarization caused by M2 receptor activation explains the acute inhibitory effect of CAR on the oscillatory activity in PPN neurons.
Figure 2. Acute vs persistent effect of CAR on the oscillatory behavior of PPN neurons.
A) Representative 1 sec long current ramp-induced oscillations of a PPN neuron in SB+TTX+MEC extracellular solution (left record, black). After 3 min of CAR in the extracellular solution, the oscillatory activity diminished (middle record, red). However, the acute effect of CAR on oscillations was reversed by adding ATR to the solution (after 3 min of perfusion with a solution containing CAR+ATR) (right record, blue). This established that cholinergic muscarinic receptors were responsible for the effect. B) Representative 1 sec long current ramp recording of a PPN neuron in the presence of SB+TTX+MEC+CAR (red record, top), recorded after persistent exposure to CAR (>20 min of exposure). Beside is the record of the same neuron showing that oscillations were blocked after adding ω-Aga (200 nM) to the extracellular solution (grey record, middle). These recordings confirm that P/Q-type calcium channel-mediated oscillatory activity in PPN neurons was induced by persistent activation by CAR. C) Bar graph showing the mean frequency of oscillatory activity in the presence of SB+TTX, the nicotinic receptor blocker MEC, persistent exposure to CAR (red column), and in the presence of SB+TTX+CAR and nonspecific cholinergic antagonist ATR (blue column). Note that the mean oscillatory frequency of PPN neurons was significantly higher during persistent exposure to CAR compared to the condition with ATR in the extracellular solution. This suggests that CAR increased the frequency of oscillations in PPN cells.
However, when CAR was applied for a prolonged period of time, CAR increased the frequency (but not the amplitude) of the oscillations (Fig. 2B). A specific P/Q-type calcium channel blocker, ω-agatoxin-IVA, abolished oscillatory activity in the PPN, suggesting that oscillations were mediated by P/Q-type calcium channels. The increase in frequency of oscillations indicates that CAR changed the kinetic properties of the channels. Our data showed that M2 (Gi-protein coupled) receptors can effectively block calcium channel mediated oscillations acutely, but also increase the frequency of oscillations by activating intracellular mechanisms chronically. This dual effect of the M2 muscarinic receptor had never been reported in the PPN nucleus. We propose that persistent cholinergic activation of the PPN through the M2 receptors plays a key role in maintaining high frequency activity among PPN neurons.
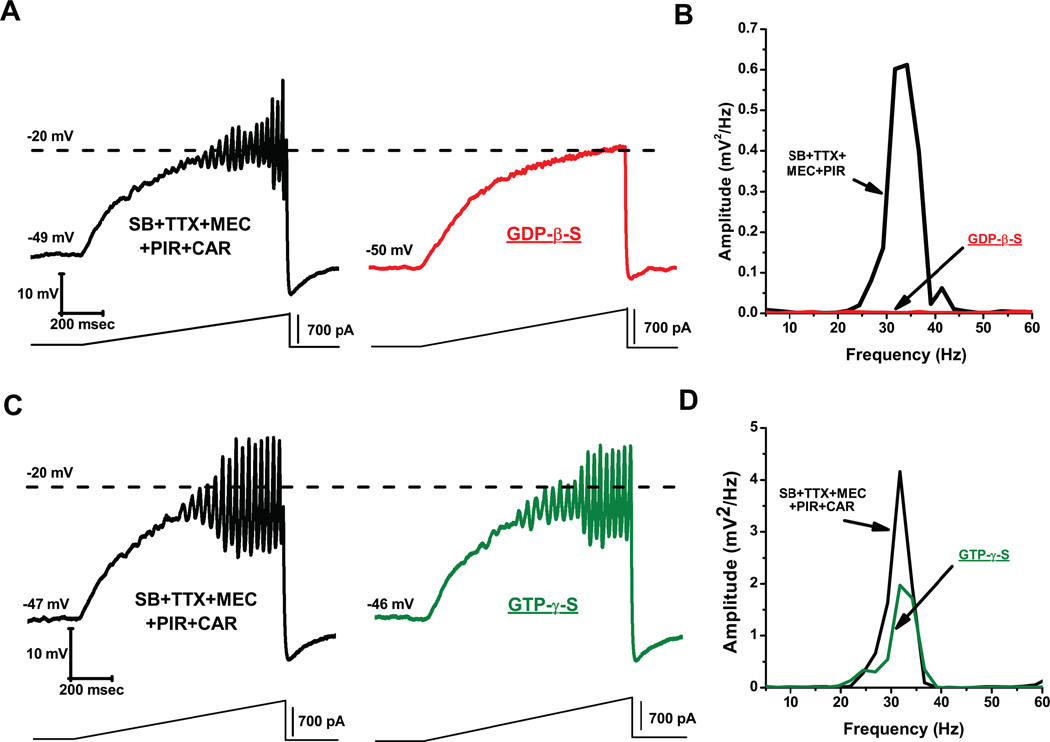
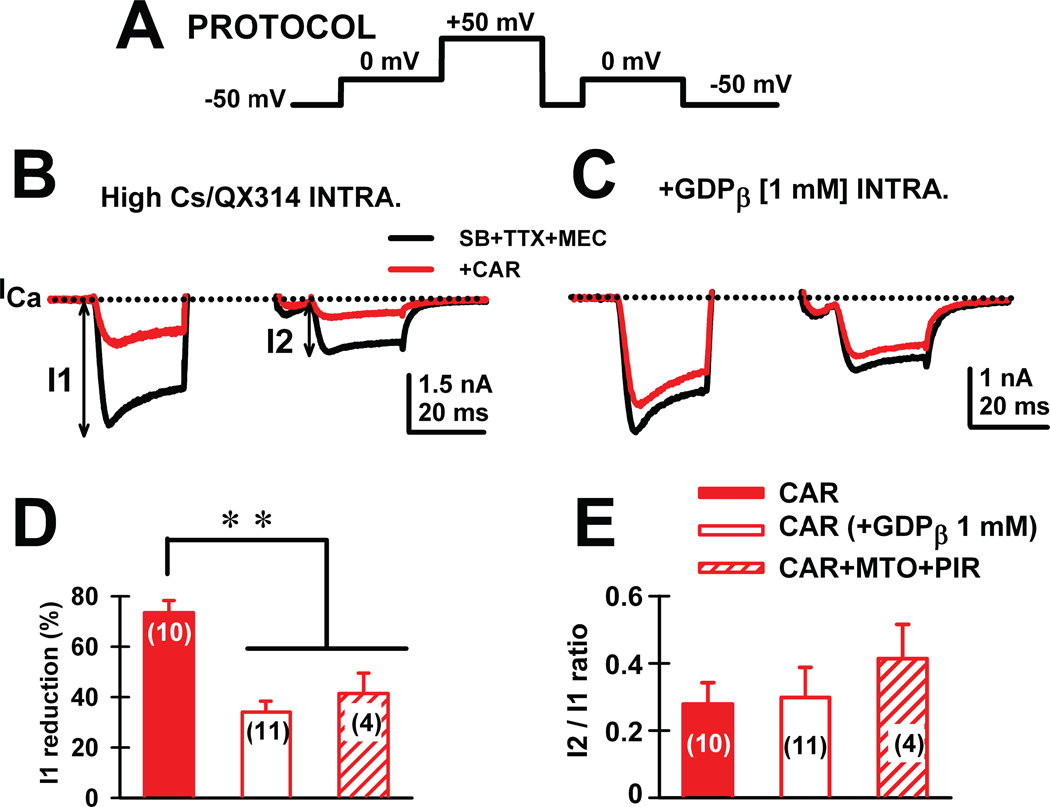
We then tested the participation of G-proteins in these effects. We found that no oscillations were observed in the presence of the G-protein blocker GDP-β-S, suggesting that availability of G-proteins is key to generating high frequency oscillations in the PPN (Fig. 3A, B). However, the G-protein stimulator GTP-γ-S did not change the ramp-induced oscillations (Fig. 3C, D). The fact that inactivation of G-proteins with GDP-β-S blocked oscillations, but stimulation of G-proteins with GTP-γ-S had no effect, suggests that G-protein binding can plateau, beyond which no blockade of oscillations is possible (Kezunovic et al. 2013). Furthermore, using a three-pulse protocol extensively used by other authors (Ikeda, 1996; Herlitze et al., 1996; Kammermeier et al., 2000), one can test the effects of specific currents while G-proteins are bound (prepulse) and compare them, after delivering a depolarizing step that removes G-protein binding, to the response during a postpulse (Fig. 4A). We found that CAR reduced calcium current amplitude of the prepulse more than the postpulse, suggesting a voltage-dependent Gprotein mechanism (Fig. 4B, D). In addition, acute CAR slowed the kinetics and reduced current amplitude of the calcium currents. The stimulatory effect of persistent CAR on calcium currents was prevented by adding GDP-β-S, and modulated similar effects on calcium currents only mediated by P/Q-type calcium channels (Fig. 4C, E). This novel result explains why the persistent CAR effect did not induce a total blockade of oscillations. Partial blockade of P/Q-type channels and slower activation/deactivation kinetics than in control conditions would provide a mechanism for inducing faster frequencies of oscillations in the presence of CAR. G-protein modulation of potassium channels might also provide the faster membrane potential repolarization necessary to sustain higher frequencies of oscillations (Kezunovic et al. 2013).
Figure 3. Blockade and stimulation of G-proteins. GDP-β-S blocks oscillatory activity in PPN neurons.
A) Representative membrane potential oscillation recorded during 1 sec ramp immediately after rupturing the membrane, in the presence of SB+TTX+MEC+PIR (left record, black). Note the blocking effect of GDP-β-S in the pipette (1 mM) on the oscillations in the same neuron after 10 min (right record, gray). B) Power spectrum of the records in A showing elimination of gamma band oscillations by GDP-β-S. GTP-γ-S does not amplify oscillatory activity in PPN cells. C) Representative membrane oscillations in the presence of SB+TTX+MEC+PIR+CAR following rupturing the membrane (left record, black). Note that activation of G-proteins by GTP-γ-S did not decrease or further increase the frequency of oscillations (right record, gray). D) Power spectrum of the records in C showing that high frequency oscillation frequency remained similar before (black line) vs after (gray line) GTP-γ-S.
Figure 4. Persistent voltage-independent G-protein modulation of voltage-gated calcium currents in PPN.
A) Three-pulse protocol was used to study the voltage dependence of G-protein modulation of calcium currents (ICa) in PPN neurons. B) In the presence of synaptic receptor blockers (SB: gabazine-GBZ+strychnine-STR+ NMDA blocker-AP5+AMPA/KA blocker-CNQX), TTX and Mecamylamine-MEC (nicotinic receptor antagonist), calcium currents (ICa; black record) were reduced in amplitude when the three-pulse protocol was applied. Indeed, the ICa observed after the second 0 mV pulse (I2) was always of lower amplitude than the one observed after the first pulse (I1), yielding a I2/I1 <1 in all neurons recorded. CAR (30 M) reduced the total amount of current during both pulses without affecting calcium current amplitude I2/I1 ratios (red record). C) The effects of CAR were prevented after adding GDP-β-S to the intracellular solution (1 mM). D) The effect of CAR (red bar) was significantly reduced when either intracellular GDP-β-S (blank bar) or extracellular muscarinic receptor antagonists (M2 antagonist methoctramine-MTO+ M1 antagonist pirenzepine-PIR) (hatched bar) were used. E) Calcium current I2/I1 ratio values (%) were unchanged by CAR under any of the experimental conditions. These results suggest that CAR reduces calcium currents through activation of muscarinic receptors that activate G-proteins.
The differences between the manifestations of oscillations in PPN cells during acute vs persistent exposure to CAR may be related to the presence of phasic vs tonic cholinergic input. That is, short duration cholinergic input to PPN neurons may tend to block the capacity to oscillate at high frequencies, however, under persistent, long duration, or tonic cholinergic influence, PPN neurons could oscillate at higher frequencies, especially in the gamma range. Phasic cholinergic tone may be more characteristic of patterns observed during REM sleep, in which PPN cells burst more, while persistent cholinergic tone is more characteristic of waking, when PPN cells tend to fire more tonically. We hypothesize that, under phasic exposure to cholinergic input, G-proteins can still bind calcium channels, slowing their activation. This would reduce or prevent the induction of high frequency oscillations through P/Q-type calcium channels. However, under tonic cholinergic input, G-proteins may be bound to the persistent input, maximizing their utilization, thereby freeing calcium channels to become activated at their membrane potential threshold, leading to sustained gamma frequency oscillatory activity. This “permissive inhibition” of G-proteins by tonic cholinergic input may be a mechanism for the maintenance of gamma band activity for prolonged periods (Kezunovic et al. 2013). It would be of interest to determine if this mechanism differs between gamma band activity during waking compared to that during REM sleep, and if this mechanism is altered by total sleep or REM sleep deprivation.
Mechanisms for gamma activity during waking vs during REM sleep
Increased arousal and increased REM sleep drive are perhaps the most common symptoms in a number of psychiatric and neurological disorders, yet the mechanism for their increase is not known, and is not targeted for specific treatment. The recognition of the importance of these symptoms dates back over 50 years! Classic studies determined that, in schizophrenia, anxiety disorders, including posttraumatic stress disorder (PTSD), and bipolar and unipolar depression, increased vigilance and REM sleep drive (increased REM duration, decreased REM latency, hypervigilance, etc., usually coupled with decreases in slow wave sleep-SWS) are major, incapacitating symptoms (Braff et al. 1978; Butler et al. 1990; Caldwell and Domino 1967; Coble et al. 1976; Feinberg et al. 1969; Jus et al. 1973; Krupfer 1976; Ross et al. 1989; Shalev et al. 1992; Zarcone et al. 1975). Most patients with schizophrenia, bipolar depression, male obsessivecompulsive disorder, and panic attacks develop the disorder postpubertally (~80% between the ages of 15 and 25, during the normal decrease in REM sleep in humans (Roffwarg et al. 1966)), while unipolar depression in adolescents is very high (Garcia-Rill 1997). One group reported that neonates and endogenous depressives have the same distinctive features of baseline REM sleep, and suggested that the REM sleep abnormalities of endogenous depression represent an immature, underdeveloped REM sleep system (Vogel et al. 2000). We hypothesized that, if the developmental decrease in REM sleep does not occur or is reduced, lifelong increases in vigilance and REM sleep drive would ensue (Garcia-Rill et al. 2003). More recently, hypervigilance and REM sleep drive abnormalities have been confirmed in PTSD, nightmare disorder, ADHD, personality disorder, insomnia, depression, and Parkinson’s disease (PD) (Hasler et al. 2013; Kirov et al. 2012; Manni et al. 2011; Pillai et al. 2011; Rieman et al. 2012; Schredl et al. 2012; Simor et al. 2013).
The differences between gamma band activity during waking vs REM sleep are unknown and hardly acknowledged. Why is this important? Because it is high frequency, especially beta/gamma band activity that drives our cognitive function during waking but also our REM sleep, two obviously different states of awareness, and of memory consolidation. Our recent studies addressed the differential intracellular mechanisms assumed to subserve high frequency activity during waking vs REM sleep as a prelude to the selective pharmacological modulation of these states. We know that the two states are differentially regulated in the PPN. Injections of glutamate into the PPN were shown to increase both waking and REM sleep, but injections of NMDA increased only waking, while injections of kainic acid (KA) increased only REM sleep (Datta 2002; Datta and Siwek 1997; Datta et al. 2001a, b). Thus, the two states are independently activated by NMDA vs KA receptors. Moreover, the intracellular pathways mediating the two states appear to differ. For example, the CaMKII activation inhibitor, KN-93, microinjected into the PPN of freely moving rats resulted in decreased waking but not REM sleep (Datta et al. 2010). They also found that increased ERK1/2 signaling in the PPN is associated with maintenance of sleep via suppression of waking (Desarnaud et al. 2011), and that activation of intracellular protein kinase A (PKA) in the PPN instead contributed to REM sleep recovery following REM sleep deprivation (Datta and Desarnaud 2010). These authors showed that during REM sleep, pCREB activation in PPN cholinergic neurons was induced by REM sleep, and that PPN intracellular PKA activation, and a transcriptional cascade involving pCREB occurred in cholinergic neurons (Datta et al. 2009). These results suggest that waking is modulated by the CaMKII pathway while REM sleep is modulated by the cAMP-PKA pathway. In vivo recording studies have shown that PPN neurons manifest two major types of cellular activity in relation to waking and REM sleep in the form of “Wake/REM on” and “REM on” cells (Datta and Siwek 2002). This suggests that some PPN cells fire in relation to waking and REM sleep, and others only in relation to REM sleep, presumably through CaMKII and cAMP-PKA pathways vs only the cAMP-PKA pathway, respectively.
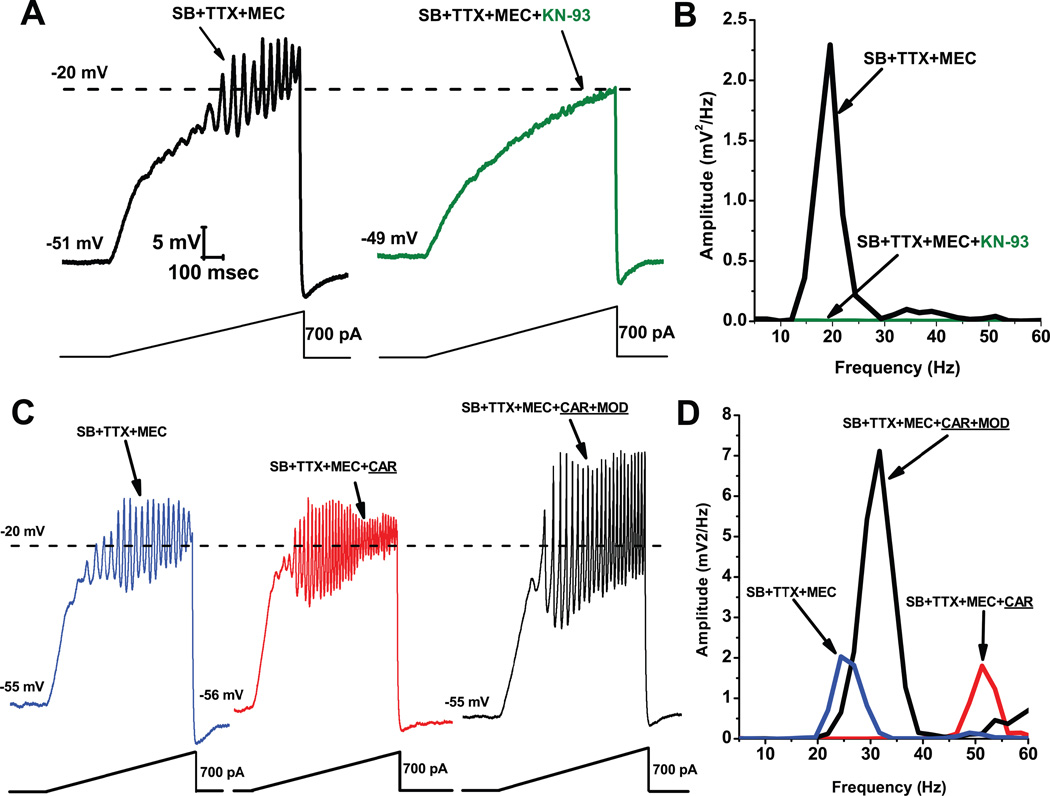
Figure 5 shows results demonstrating that KN-93 blocked the ability of ramps to induce oscillations in PPN neurons (Fig. 5A), an effect confirmed by the absence of oscillations in the power spectrum (Fig. 5B). Recordings were carried out in the presence of synaptic blockers (SB), tetrodotoxin (TTX), and mecamylamine (MEC), a nicotinic receptor antagonist. These findings suggest that blocking the activation of CaMKII using KN-93 blocked the ability of ramps to induce gamma band oscillations in PPN neurons. These data suggest that CaMKII is necessary for the manifestation of ramp-induced oscillations in at least some PPN neurons.
Figure 5. Effects of the CaMKII blocker KN-93.
A. Ramps induced oscillations in the beta range (black record). Following slice superfusion with KN-93 (10 M) for 10 min, ramps no longer elicited oscillations (green record). B. Power spectrum showing amplitude and frequency of ramp-induced oscillations before (black record, beta range) and after KN-93 (green record, no oscillations). Effects of MOD on CAR-induced oscillations. A. Recordings of a PPN neuron before administration of CAR (30 M) (blue record), 20 min after continuous superfusion with CAR (red record), and 20 min after continuous superfusion with MOD and CAR (black record). B. Power spectrum before CAR (blue line, beta range), after CAR (red line, gamma range), and after CAR+MOD (black line, beat/gamma range).
In collaboration with R. Llinas at NYU, we found that the effects of the stimulant modafinil (MOD) were also dependent on CaMKII, since its effects were blocked by the CaMKII activation blocker KN-93 (Urbano et al. 2007). Our previous studies using MOD extensively characterized its effects on, a) electrical coupling in the RAS and intralaminar thalamus, b) established the concentration dependence of its effects in vitro and in vivo, c) in animals and humans, and d) determined that its effects were blocked specifically by gap junction blockers (Beck et al. 2008; Garcia-Rill et al. 2007, 2008; Heister et al. 2007; Kezunovic et al. 2010). MOD, which typically takes 10–20 min to induce changes in electrical coupling, by itself does not appear to change gamma band oscillation frequency or amplitude. However, MOD was found to enhance CAR-induced oscillation amplitude. We used synaptic blockers plus the nicotinic receptor antagonist MEC to tonically activate only muscarinic receptors using CAR. Figure 5C, D shows ramp-induced oscillations in the presence of SB+TTX+MEC before CAR application (C blue record, D blue line showing beta frequency oscillations at 25 Hz), but the addition of CAR for 20 min led to a significant increase in oscillation frequency (but not amplitude) to 50 Hz (C red record, D red line in power spectrum). Superfusion of MOD for 20 min in the presence of CAR, significantly increased oscillation amplitude, but decreased oscillation frequency to 30 Hz (C black record, D black line in power spectrum). These results showed that MOD potentiated CAR-induced oscillation amplitude, but decreased their frequency from the gamma to the beta range in most cells, as was the case with MOD alone.
These data suggest that MOD preferentially promotes high frequency activity through the CaMKII (“waking”) pathway, especially in the presence of tonic cholinergic input. Moreover, work on cocaine abusers (Morgan et al. 2010), and on an animal model of sleep-disordered breathing (Panckeri et al. 1996), suggest that MOD may also decrease REM sleep. Further work will be needed to determine if the cAMP/PK (“REM sleep”) pathway is preferentially activated during REM sleep compared to the CaMKII pathway. These findings also provide intracellular targets for the modulation of memory consolidation during REM sleep, and the effects of total vs REM sleep deprivation.
A mechanism for gamma modulation in schizophrenia
Reduced gamma band activity has been reported in bipolar disorder (Ozerdem et al. 2011), but most attention has been devoted to this mechanism in schizophrenia. Aberrant gamma band activity and coherence during cognitive tasks or attentional load have been reported in schizophrenic patients (Ulhass and Singer 2010). Several human studies demonstrated frequency-specific deficits in the coherence and maintenance of gamma oscillations in patients with schizophrenia (Spencer et al. 2003). Schizophrenia is characterized by RAS symptoms such as hyperarousal, increased REM sleep drive, decreased SWS, and hallucinations, among others. The hallucinations in schizophrenia were proposed to represent overactive REM sleep intrusion into waking (Dement 1967; Mamelak and Hobson 1989). That is, the states of waking and REM sleep, which are marked by gamma band activity, are both disturbed in schizophrenia. We found anatomical abnormalities specifically in RAS cholinergic neurons of the PPN in the brains of intractable inpatient (but not outpatient) schizophrenics (Garcia-Rill et al. 1995), which accounts for some of the profound hyperarousal and sleep-wake dysregulation in the disease. In addition, increased neuronal calcium sensor protein-1 (NCS-1) expression is present in schizophrenia and bipolar disorder (Bergson et al. 2003; Koh et al. 2003). That is, gamma band activity is reduced in precisely the same disorders that show NCS-1 over expression.
We hypothesize that over expression of NCS-1, and its regulation of intracellular calcium [Ca2+] dynamics and modulation of P/Q-type channels, underlie disorders that manifest decreased gamma band activity. Decreases in gamma band coherence and maintenance can account for many of the symptoms of schizophrenia. The positive symptoms include hallucinations, delusions, thought disorder, and agitation, while negative symptoms include lack of affect, anhedonia, and withdrawal. Cognitive symptoms include poor executive function, lack of attention, and disturbed working memory. All of these functions are associated with gamma band activity. However, despite known disturbances in gamma band coherence and maintenance in schizophrenia, none of the clinical trials completed or ongoing that tested modafinil (MOD) as a potential adjunct therapy assessed gamma band activity [Clinicaltrials.gov]. These trials assessed average safety (no safety issues, did not worsen positive symptoms), and average changes in cognitive function (not significant) or reduction in negative symptoms (reduced or unchanged) using neuropsychological testing, but none measured gamma band activity (Kane et al. 2010, 2012). However, the postmortem results described above by Koh et al. (2003) suggest that only some patients with schizophrenia may suffer from significant over expression of NCS-1, which may be manifested as decreased gamma band activity only in a subpopulation of patients. No human study has measured gamma band activity and correlated it with NCS-1 levels. Unfortunately, serum sampling does not reflect brain levels and, in fact, NCS-1 levels in leukocytes are actually decreased in schizophrenic patients (Torres et al. 2009). However, selecting patients on the basis of significantly reduced gamma band activity should provide a better test of our hypothesis. Before such testing can meet approval, we need specific information about the mechanisms at play in NCS-1 over expression and MOD on the manifestation of gamma band activity. We need to answer the questions: does NCS-1 modulate gamma band oscillations, and at what concentrations? How much of it begins to decrease intrinsic gamma oscillations? Such studies can only be performed in vitro.
Interaction of calcium with a large number of calcium-binding proteins represents one of the mechanisms by which this second messenger controls many biological processes. NCS-1 is neuron specific (Martone et al. 1999), and has an affinity for calcium of 300 nM, i.e. within physiological levels (Cox et al. 1994). NCS-1 regulates P/Q-type calcium channels (Tsujimoto et al. 2002), neurotransmitter regulation (Pan et al. 2002), intracellular protein trafficking (Taverna et al. 2002), and G-protein-coupled receptor phosphorylation in a calcium-dependent manner, partly through CaMKII, at least in the heart (Nakamura et al. 2011). NCS-1 also regulates the G-protein-coupled receptor kinase 1 (GRK1) gene that encodes G-proteins involved in modulating gamma band oscillations (Sallese et al. 2000). Importantly, NCS-1 regulates various types of calcium channels, including N- and P/Q-type, with differences across species and location (reviewed in Weiss et al. 2010). However, there was no information on the effects of NCS-1 on RAS neurons. We provided answers to the questions: do low levels of NCS-1 increase gamma band activity? Do high levels of NCS-1 decrease gamma band activity? Does NCS-1 elicit its effects through CaMKII? Does MOD compete with high levels of NCS-1 (as would be observed in over expression) to restore gamma band activity?
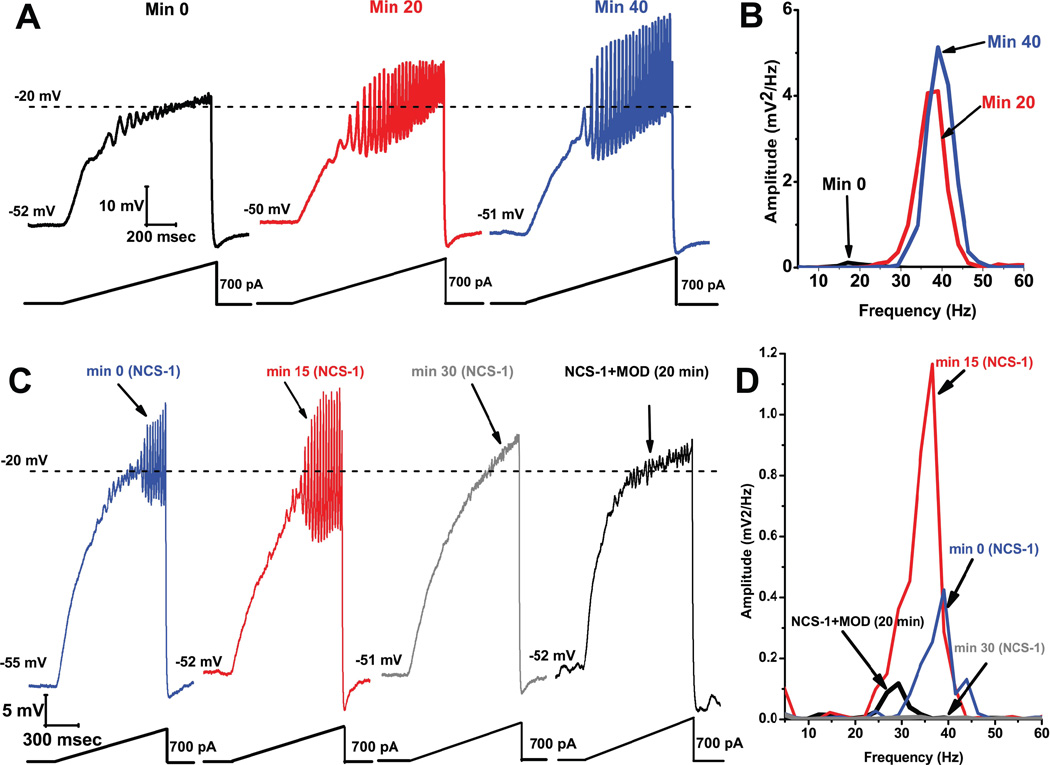
We carried out studies to determine the effects of including NCS-1 in the recording pipette in order to allow its diffusion intracellularly only in the recorded neuron. Recording of oscillations was as described above. Figure 6A, B shows that soon after patching (A black record) the ramp typically induced low amplitude oscillations (B black line in power spectrum). As the NCS-1 (1 M) diffused into the cell, the amplitude and frequency of the gamma band oscillations increased significantly with time. The red record in A shows that, after 20 min of exposure to NCS-1 at 1 µ oscillations increased in amplitude and frequency (B red line in power spectrum), and were maintained at these levels and higher after 40 min (blue record in A and blue line in the power spectrum in B). We recorded PPN cells for up to 1 hr without decrement in oscillation amplitude or frequency. These spectacular findings are the first to show that NCS-1 at 1 µ significantly amplifies gamma band oscillations in RAS neurons. These results suggest that this concentration of NCS-1 would appear to be in the range at which this protein can modulate gamma band oscillations. The question then becomes, if this concentration is increased, as would be expected in over expression, what is the effect on oscillations? We selected a concentration that would be quite high (and quite expensive), ten times or 10 µ, in the recording pipette. Figure 6C, D shows that soon after patching with NCS-1 at 10 µ (C blue record), oscillations were already of higher amplitude and frequency than without NCS-1 (D blue line in power spectrum). After 15 min, the amplitude (but not frequency) of the oscillations increased further (C red record, D red line in power spectrum). However, by 30 min, the oscillations were eliminated (C gray record, D gray line in power spectrum).
Figure 6. Effects of NCS-1 at 1 µM in the recording pipette on oscillations induced at −30 mV to −10 mV by a ramp (bottom record) applied to a PPN cell held at −50 mV.
A) Oscillations were of low amplitude and frequency at the start (before NCS-1 diffused into the cell) but increased and were maintained after 20 min (red record) and 40 min (blue record). B. The power spectrum of the recordings in A showed that oscillations increased in amplitude and frequency for prolonged periods. Effects of NCS-1 at 10 µ in the recording pipette on ramp-induced oscillations in a PPN cell. C) Soon after patching oscillations were in the gamma range (blue record), and remained for 15 min (red record), but were eliminated after 30 min (gray record), and returned 20 min after adding MOD (black record). D) Power spectrum showing that NCS-1 at 10 mM had an immediate effect on oscillation amplitude and frequency (blue line), which persisted for 15 min (red line), but was then blocked by 30 min of exposure to high levels of NCS-1 (gray line), an effect partially reversed by MOD (black line).
These results showed that NCS-1 at high concentration (10 µ) at first increased, then blocked gamma band oscillations in PPN neurons as it diffused into the cell. These findings suggest that 10 µ NCS-1 is “abnormal”, presumably eliminating the activation of high threshold calcium channels. In the same cells, we then tested the effects of 300 µ MOD administration for 20 min. MOD was able to restore low amplitude, lower frequency oscillations, despite the presence of NCS-1 at 10 µ (C black record, D black line in power spectrum). These results suggest that MOD was able to compete successfully with very high concentrations of NCS-1. In some cells, we applied - agatoxin-IVA, a specific P/Q-type calcium channel blocker, 15 min after patching with NCS-1 (1 µ) in the pipette and the oscillations were blocked, suggesting that P/Q-type channels are involved in the effects of NCS-1.
These studies represent a logical progression of controlled research to identify one of the causal factors behind decreased gamma band activity that affects at least some schizophrenic patients. They are designed to identify the intracellular mechanisms responsible for the manifestation of over expression of NCS-1, the mechanisms behind a therapeutic agent that will compete for NCS-1 over expression-induced decreases in gamma band activity, and verify the dynamic effects of these treatments on cells that control waking and REM sleep. The information generated using MOD, a drug already approved for human use, is promising in the design of clinical trials for a novel treatment for schizophrenia, strongly suggesting that this novel therapy may be effective specifically in those patients known to suffer from reduced gamma band activity, and, by assumption, perhaps not in those not suffering from this symptom. Such information will be critical to the success of a clinical trial. We also need information on the effects of sleep and REM sleep deprivation on NCS-1 and its modulation of gamma activity. While serotonin reuptake inhibitors may reduce REM sleep in depression and REM sleep deprivation appears beneficial in depressives, schizophrenics do not appear to respond with REM sleep rebound to deprivation (Berger and Riemann 1993; Vogel 1975; Zarcone et al. 1975). These results need to be confirmed with more modern methods, but the differential responses of these two mood disorders points to a potentially informative research avenue.
Conclusions
The discovery of gamma band activity in the RAS, in which virtually all cells are geared to fire at gamma frequencies, suggests that sensory afferent information triggers such activity in the mesopontine region. Through both ascending projections to the intralaminar thalamus and descending projections to the SubCD, high frequency oscillations are relayed onto targets such as the cortex and hippocampus. These characteristics easily account for the proposal that the RAS is the critical agent in preconscious awareness. The RAS also possesses the necessary mechanism to maintain gamma band activity for prolonged periods. The fact that G-proteins must be occupied by cholinergic input in order to allow high threshold calcium channels to oscillate, a permissive inhibition, suggests that the maintenance of gamma band activity is hard work. The behavioral and cellular data indicate that separate intracellular pathways mediate high frequency activity during waking differentially from during REM sleep. These results point to potential novel targets for pharmacologically modulating each state differentially. One example of this approach is in the treatment of schizophrenia. Ten years ago, the over expression of NCS-1 in schizophrenia was described, followed by studies showing a dysregulation in gamma band activity in the disease. Recent results account for both of these findings. They help describe how NCS-1 at low concentrations promotes high threshold calcium channel-mediated gamma oscillations, which are reduced and blocked by high concentrations of NCS-1. Moreover, oscillations are restored by modafinil, suggesting a novel adjunct treatment, especially for negative symptoms in schizophrenia. Additional novel avenues for research make the RAS an exciting place to study, even after all these years.
Acknowledgments
This work was supported by NIH award R01 NS020246, and by core facilities of the Center for Translational Neuroscience supported by NIH award P20 GM103425 to Dr. Garcia-Rill. In addition, this work was supported by grants from FONCYT-Agencia Nacional de Promoción Científica y Tecnológica; BID 1728 OC.AR. PICT 2008-2019 and PICT-2012-1769 (to Dr. Urbano), and CONICET- PIP 2011-2013-11420100100072 and PICT-2012-0924 (to Dr. Bisagno).
References
- Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273–274. doi: 10.1126/science.118.3062.273. [DOI] [PubMed] [Google Scholar]
- Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. A neuroanatomical gradient in the pontine tegmentum for the cholinoceptive induction of desynchronized sleep signs. Brain Res. 1987;414:245–261. doi: 10.1016/0006-8993(87)90005-9. [DOI] [PubMed] [Google Scholar]
- Beck P, Odle A, Wallace-Huitt T, Skinner RD, Garcia-Rill E. Modafinil increases arousal determined by P13 potential amplitude; An effect blocked by gap junction antagonists. Sleep. 2008;31:1647–1654. doi: 10.1093/sleep/31.12.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M, Riemann D. Symposium: Normal and abnormal REM sleep regulation: REM sleep in depression- an overview. J Sleep Res. 1993;2:211–223. doi: 10.1111/j.1365-2869.1993.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Bergson C, Levenson R, Goldman-Rakic P, Lidow MS. Dopamine receptor-interacting proteins: the Ca2+ connection in dopamine signaling. Trends Pharmacol Sci. 2003;24:486–492. doi: 10.1016/S0165-6147(03)00232-3. [DOI] [PubMed] [Google Scholar]
- Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi PH. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci. 2002;16:1959–1973. doi: 10.1046/j.1460-9568.2002.02257.x. [DOI] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normal and schizophrenics. Psychophysiol. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Butler RW, Braff DL, Rausch JL, Jenkins MA, Sprock J, Geyer MA. Physiological evidence of exaggerated startle response in a subgroup of Vietnam veterans with combat-related PTSD. Am J Psychiat. 1990;147:1308–1313. doi: 10.1176/ajp.147.10.1308. [DOI] [PubMed] [Google Scholar]
- Caldwell DF, Domino EF. Electroencephalographic and eye movement patterns during sleep in chronic schizophrenic patients. Electroenceph Clin Neurophysiol. 1967;22:414–420. doi: 10.1016/0013-4694(67)90168-x. [DOI] [PubMed] [Google Scholar]
- Coble P, Foster FG, Kupfer DJ. Electroencephalographic sleep diagnosis of primary depression. Arch Gen Psychiat. 1976;33:1124–1127. doi: 10.1001/archpsyc.1976.01770090114012. [DOI] [PubMed] [Google Scholar]
- Corsi–Cabrera M, Meneses S, Molina E. Correlación Intrahemisférica y Acoplamiento Temporalde la Actividad Eléctrica Cortical Durante la Vigilia, la Etapa II y el Sueño Paradójico en el Hombre. Rev Mex Psicol. 1987;4:100–108. [Google Scholar]
- Cox JA, Durussel I, Comte M, Nef P, Lenz SE, Gundelfinger ED. Cation binding and conformational changes in VILIP and two neuron-specific calcium-binding proteins. J Biol Chem. 1994;269:32807–32813. [PubMed] [Google Scholar]
- Cunningham MO, Whittington MA, Bibbig A, Roopun A, LeBeau FE, Vogt A, Monyer H, Buhl EH, Traub RD. A role for fast rhythmic bursting neurons in cortical gamma oscillations in vitro. Proc Natl Acad Sci USA. 2004;101:7152–7157. doi: 10.1073/pnas.0402060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S. Evidence that REM sleep is controlled by the activation of brain stem pedunculopontine tegmental kainite receptor. J Neurophysiol. 2002;87:1790–1798. doi: 10.1152/jn.00763.2001. [DOI] [PubMed] [Google Scholar]
- Datta S, Desarnaud F. Protein kinase A in the pedunculopontine tegmental nucleus of rat contributes to regulation of rapid eye movement sleep. J Neurosci. 2010;30:12263–12273. doi: 10.1523/JNEUROSCI.1563-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Data S, O’Malley MW, Patterson EH. Calcium/calmodulin kinase II in the pedunculopontine tegmental nucleus modulates the initiation and maintenance of wakefulness. J Neurosci. 2010;31:1700–17016. doi: 10.1523/JNEUROSCI.3981-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Patterson EH, Siwek DF. Brainstem afferents of the cholinoceptive pontine wave generation sites in the rat. Sleep Res Online. 1999;2:79–82. [PubMed] [Google Scholar]
- Datta S, Patterson EH, Spoley EE. Excitation of pedunculopontine tegmental NMDA receptors induces wakefulness and cortical activation in the rat. J Neurosci Res. 2001b;66:109–116. doi: 10.1002/jnr.1202. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF. Excitation of the brain stem pedunculopontine tegmentum cholinergic cells induce wakefulness and REM sleep. J Neurophysiol. 1997;77:2975–2988. doi: 10.1152/jn.1997.77.6.2975. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J Neurosci Res. 2002;70:611–621. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF, Patterson EH, Cipolloni PB. Localization of pontine PGO wave generation sites and their anatomical projections in the rat. Synapse. 1998;30:409–423. doi: 10.1002/(SICI)1098-2396(199812)30:4<409::AID-SYN8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF, Stack EC. Identification of cholinergic and non-cholinergic neurons in the pons expressing phosphorylated cyclic adenosine monophosphate response element-binding protein as a function of rapid eye movement sleep. Neuroscience. 2009;163:397–414. doi: 10.1016/j.neuroscience.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Spoley EE, Patterson EH. Microinjection of glutamate into the pedunculopontine tegmentum induces REM sleep and wakefulness in the rat. A J Physiol Reg Integ Comp Physiol. 2001a;280:R752–R759. doi: 10.1152/ajpregu.2001.280.3.R752. [DOI] [PubMed] [Google Scholar]
- Dement WC. Studies on the effects of REM deprovation in humans and animals. Res Publ Assoc Res Nerv Ment Dis. 1967;43:456–467. [PubMed] [Google Scholar]
- Desarnaud F, Macone BW, Datta S. Activation of extracellular signal-regulated kinase signaling in the pedunculopontine tegmental cells is involved in the maintenance of sleep in rats. J Neurochem. 2011;116:577–587. doi: 10.1111/j.1471-4159.2010.07146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickelman S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitboeck HJ. Coherent oscillations: a mechanism of feature linking in the visual system? Biol Cybern. 1988;60:121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Braun M, Koresko RL, Gottlieb F. Stage 4 sleep in schizophrenia. Arch Gen Psychiat. 1969;21:262–266. doi: 10.1001/archpsyc.1969.01740210006002. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E. Disorders of the reticular activating system. Med Hypoth. 1997;49:379–387. doi: 10.1016/s0306-9877(97)90083-9. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Biedermann JA, Chambers T, Karson CN. Mesopontine neurons in schizophrenia. Neuroscience. 1995;66:321–335. doi: 10.1016/0306-4522(94)00564-l. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Charlesworth A, Heister DA, Ye M, Hayar A. The developmental decrease in REM sleep: the role of transmitters and electrical coupling. Sleep. 2008;31:1–18. doi: 10.1093/sleep/31.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Kobayashi T, Good C. The developmental decrease in REM sleep. Thal Rel Syst. 2003;46:1–17. [Google Scholar]
- Garcia-Rill E, Heister DS, Ye M, Charlesworth A, Hayar A. Electrical coupling: novel mechanism for sleep-wake control. Sleep. 2007;30:1405–1414. doi: 10.1093/sleep/30.11.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Kezunovic N, Hyde J, Beck P, Urbano FJ. Coherence and frequency in the reticular activating system (RAS) Sleep Med Rev. 2013;17:227–238. doi: 10.1016/j.smrv.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci USA. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujar N, McDonald A, Nishida M, Walker MP. A role for REM sleep in recalibrating the sensitivity of the human brain to specific emotions. Cereb Cortex. 2011;21:115–123. doi: 10.1093/cercor/bhq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Insana SP, James JA, Germain A. Evening-type veterans report worse lifetime posttraumatic stress symptoms and greater brainstem activity across wakefulness and REM sleep. Biol Psychiat. 2013 doi: 10.1016/j.biopsycho.2013.06.007. E-pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heister DS, Hayar A, Charlesworth A, Yates C, Zhou Y, Garcia-Rill E. Evidence for electrical coupling in the SubCoeruleus (SubC) nucleus. J Neurophysiol. 2007;97:3142–3147. doi: 10.1152/jn.01316.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- Hyde J, Kezunovic N, Urbano FJ, Garcia-Rill E. Visualization of fast calcium oscillations in the parafascicular nucleus. Pflügers Archiv. 2013a;465:1327–1340. doi: 10.1007/s00424-013-1264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde J, Kezunovic N, Urbano FJ, Garcia-Rill E. Spatiotemporal properties of high speed calcium oscillations in the pedunculopontine nucleus. J Appl Physiol. 2013b doi: 10.1152/japplphysiol.00762.2013. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda SR. Voltage-dependent modulation of N-type calcium channels by Gprotein beta gamma subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- Jones EG. Calcium channels in higher-level brain function. Proc Natl Acad Sci USA. 2007;14:17903–17904. doi: 10.1073/pnas.0709509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jus K, Bouchard M, Jus A, Villeneuve A, Lachance R. Sleep EEG studies in untreated long-term schizophrenic patients. Arch Gen Psychiat. 1973;29:286–290. doi: 10.1001/archpsyc.1973.04200030074011. [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ, Ruiz-Velasco V, Ikeda SR. A voltage-independent calcium current inhibitory pathway activated by muscarinic agonists in rat sympathetic neurons requires both Gα q/11 and Gβγ. J Neurosci. 2000;20:5623–5629. doi: 10.1523/JNEUROSCI.20-15-05623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JM, D’Souza DC, Patkar AA, Youakim JM, Tiller JM, Yang R, Keefe RS. Armodafinil as adjunctive therapy in adults with cognitive deficits associated with schizophrenia: a 4-week, double-blind, placebo-controlled study. J Clin Psychiat. 2010;71:1475–1481. doi: 10.4088/JCP.09m05950gry. [DOI] [PubMed] [Google Scholar]
- Kane JM, Yang R, Youakim JM. Adjunctive armodafinil for negative symptoms in adults with schizophrenia: a double-blind, placebo-controlled study. Schizophr Res. 2012;135:116–122. doi: 10.1016/j.schres.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Kezunovic N, Hyde J, Goitia B, Bisagno V, Urbano FJ, Garcia-Rill E. Muscarinic modulation of high frequency activity in the epdunculopontine nucleus (PPN) Frontiers Neurol: Sleep and Chronobiol. 2013 doi: 10.3389/fneur.2013.00176. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kezunovic N, Hyde J, Simon C, Urbano FJ, Garcia-Rill E. Gamma band activity in the developing parafascicular nucleus (Pf) J Neurophysiol. 2012;107:772–784. doi: 10.1152/jn.00677.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kezunovic K, Simon C, Hyde J, Smith K, Beck P, Odle A, Garcia-Rill E. Arousal from slices to humans: Translational studies on sleep-wake control. Transl Neurosci. 2010;1:2–8. doi: 10.2478/v10134-010-0003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kezunovic N, Urbano FJ, Simon C, Hyde J, Smith K, Garcia-Rill E. Mechanism behind gamma band activity in the pedunculopontine nucleus (PPN) Eur J Neurosci. 2011;34:404–415. doi: 10.1111/j.1460-9568.2011.07766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov R, Uebel H, Albrecht B, Banachewski T, Yordanova J, Rothenberg A. Attention-deficit/hyperactivity disorder (ADHD) and adaptation night as determinants of sleep patterns in children. Eur Child Adolesc Psychiat. 2012;21:681–690. doi: 10.1007/s00787-012-0308-3. [DOI] [PubMed] [Google Scholar]
- Koh PO, Undie AS, Kabbani N, Levenson R, Goldman-Rakic P, Lidow MS. Up-regulation of neuronal calcium sensor-1 (NCS-1) in the prefrontal cortex of schizophrenic and bipolar patients. Proc Natl Acad Sci USA. 2003;100:313–317. doi: 10.1073/pnas.232693499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupfer DL. REM latency: a psychobiologic marker for primary depressive disease. Biol Psychiat. 1976;11:159–174. [PubMed] [Google Scholar]
- Leonard CS, Llinas R. Serotonergic and cholinergic inhibition of mesopontine cholinergic neurons controlling REM sleep: an in vitro electrophysiological study. Neuroscience. 1994;59:309–330. doi: 10.1016/0306-4522(94)90599-1. [DOI] [PubMed] [Google Scholar]
- Llinas RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Grace AA, Yarom Y. In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc Natl Acad Sci USA. 1991;88:897–901. doi: 10.1073/pnas.88.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR, Leznik E, Urbano FJ. Temporal binding via cortical coincidence detection of specific and nonspecific thalamocortical inputs: a voltage-dependent dye-imaging study in mouse brain slices. Proc Natl Acad Sci USA. 2002;99:449–454. doi: 10.1073/pnas.012604899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR, Paré D. Of dreaming and wakefulness. Neuroscience. 1991;44:521–535. doi: 10.1016/0306-4522(91)90075-y. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Soonwook C, Urbano FJ, Hee-Sup S. γ-Band deficincy and abnormal thalamocortical activity in P/Q-type channel mutant mice. Proc Natl Acad Sci USA. 2007;104:17819–17824. doi: 10.1073/pnas.0707945104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95:3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- Luebke JI, McCarley RW, Greene RW. Inhibitory action of muscarinic agonists on neurons in the rat laterodorsal tegmental nucleus in vitro. J Neurosci. 1993;70:2128–2135. doi: 10.1152/jn.1993.70.5.2128. [DOI] [PubMed] [Google Scholar]
- Manni R, Terzaghi M, Ratti PL, Repetto A, Zangaglia R, Pacchetti C. Hallucinations and REM sleep behavior disorder in Parkinson’s disease: dream imagery intrusions and other hypotheses. Consc Cgn. 2011;20:1021–1026. doi: 10.1016/j.concog.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Mamelak AN, Hobson JA. Dream bizarreness as the cognitive correlate of altered neuronal brain in REM sleep. J Cog Neurosci. 1989;1 doi: 10.1162/jocn.1989.1.3.201. 2-1-222. [DOI] [PubMed] [Google Scholar]
- Maquet P, Peters JM, Aerts J, Delfiore G, Degueldre C, Luxen A, Franck G. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1966;383:163–166. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- Martone ME, Edelmann VM, Ellisman MH, Nef P. Cellular and subcellular distribution of the calcium-binding protein in the central nervous system of the rat. Cell Tissue Res. 1999;295:395–407. doi: 10.1007/s004410051246. [DOI] [PubMed] [Google Scholar]
- Mavanji V, Ulloor J, Saha S, Datta S. Neurotoxic lesions of phasic pontine-wave generator cells impair retention of 2-way active avoidance memory. Sleep. 2004;27:1282–1292. doi: 10.1093/sleep/27.7.1282. [DOI] [PubMed] [Google Scholar]
- Mitler MM, Dement WC. Cataplectic-like behavior in cats after micro-injections of carbachol in pontine reticular formation. Brain Res. 1974;68:335–343. doi: 10.1016/0006-8993(74)90402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott E, Pittman B, Stickgold R, Malison RT. Normalizing effect of modafinil on sleep in chronic cocaine users. Am J Psychiat. 2010;167:331–340. doi: 10.1176/appi.ajp.2009.09050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moruzzi G, Magoun HW. Brainstem reticular formation and activation. Electroenceph Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- Nakamura TY, Jeromin A, Mikoshiba K, Wakabayashi S. Neuronal calcium sensor-1 promotes immature heart function and hypertrophy by enhancing Ca2+ signals. Circ Res. 2011;109:512–523. doi: 10.1161/CIRCRESAHA.111.248864. [DOI] [PubMed] [Google Scholar]
- Ozerdem A, Guntenkin B, Atagun I, Turp B. Reduced long distance gamma (28–48 Hz) coherence in euthymic patients with bipolar disorder. J Affect Disord. 2011;132:325–332. doi: 10.1016/j.jad.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Palva S, Monto S, Palva JM. Graph properties of synchronized cortical netwoks during visual working memory maintenance. Neuroimage. 2009;49:3257–3268. doi: 10.1016/j.neuroimage.2009.11.031. [DOI] [PubMed] [Google Scholar]
- Pan CY, Jeromin A, Lundstrom K, Yoo SH, Roder J, Fox AP. Alterations in exocytosis induced by neuronal Ca+2 sensor-1 in bovine chromaffin cells. J Neurosci. 2002;22:2427–2433. doi: 10.1523/JNEUROSCI.22-07-02427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panckeri KA, Schotland HM, Pack AI, Hendricks JC. Modafinil decreases hypersomnolence in the English bulldog, a natural animal model of sleep-disordered breathing. Sleep. 1996;19:626–631. doi: 10.1093/sleep/19.8.626. [DOI] [PubMed] [Google Scholar]
- Pedroarena C, Llinás RR. Dendritic calcium conductances generate high-frequency oscillation in thalamocortical neurons. Proc Natl Acad Sci USA. 1997;94:724–728. doi: 10.1073/pnas.94.2.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai V, Kalmbach DA, Ciesla JA. A meta-analysis of electroencephalographic sleep in depression: evidence for genetic markers. Biol Psychiat. 2011;70:919–919. doi: 10.1016/j.biopsych.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Ribary U, Ioannides AA, Singh KD, Hasson R, Bolton JP, Lado F, Mogilner A, Llinás RR. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc Natl Acad Sci USA. 1991;88:11037–11041. doi: 10.1073/pnas.88.24.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieman D, Spiegelhalder K, Nissen C, Hirscher V, Baglioni C, Feige B. REM sleep instability- a new pathway for insomnia? Pharmacopsychiat. 2012;45:167–176. doi: 10.1055/s-0031-1299721. [DOI] [PubMed] [Google Scholar]
- Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- Ross RJ, Ball WA, Sullivan KA, Caraff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiat. 1989;146 doi: 10.1176/ajp.146.6.697. 6976-6707. [DOI] [PubMed] [Google Scholar]
- Sallese M, Iacovelli L, Cumashi A, Capobianco L. Regulation of G protein-coupled receptor kinase subtypes by calcium sensor proteins. Biochim Biophys Acta. 2000;1498:112–121. doi: 10.1016/s0167-4889(00)00088-4. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Morrison AR, Mann GL, Harris JS, Yoo L, Ross RJ. Sleep patterning and behaviour in cats with pontine lesions creating REM without atonia. J Sleep Res. 1994;3:233–240. doi: 10.1111/j.1365-2869.1994.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Schredl M, Paul F, Reinhard I, Ebner-Priemer UW, Schmahl C, Bohus M. Sleep and dreaming in patients with borderline personality disorder: a polysomnographic study. Psychiat Res. 2012;200:430–436. doi: 10.1016/j.psychres.2012.04.036. [DOI] [PubMed] [Google Scholar]
- Semba K, Fibiger HC. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. J Comp Neurol. 1992;323:387–410. doi: 10.1002/cne.903230307. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Orr SP, Per T, Schreiber S, Pitman RK. Physiologic responses to loud tones in Israeli patients with posttraumatic stress disorder. Arch Gen Psychiat. 1992;49:870–875. doi: 10.1001/archpsyc.1992.01820110034005. [DOI] [PubMed] [Google Scholar]
- Simon C, Kezunovic N, Williams DK, Urbano FJ, Garcia-Rill E. Cholinergic and glutamatergic agonists induce gamma frequency activity in dorsal subcoeruleus nucleus neurons. Amer J Physiol Cell Physiol. 2011;301:C327–C335. doi: 10.1152/ajpcell.00093.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Kezunovic N, Ye M, Hyde J, Hayar A, Williams DK, Garcia-Rill E. Gamma band unit and population responses in the pedunculopontine nucleus. J Neurophysiol. 2010;104:463–474. doi: 10.1152/jn.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simor P, Horvath K, Ujma PP, Gombos F, Bodizs R. Fluctuations between sleep and wakefulness: wake-like features indicated by increased EEG alpha power during different stages in nightmare disorder. Biol Psychiat. 2013 doi: 10.1016/j.biopsycho.2013.05.022. E-pub. [DOI] [PubMed] [Google Scholar]
- Singer W. Synchronization of cortical activity and its putative role in informtion processing and learning. Annu Rev Physiol. 1993;55:349–374. doi: 10.1146/annurev.ph.55.030193.002025. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ, van Cappellen van Walsum AM, Pijnenburg YA, Berendse HW, de Munck JC, Scheltens P, van Dijk BW. Generalized synchronization of MEG recordings in Alzheimer’s disease: evidence for involvement of the gamma band. J Clin Neurophysiol. 2002;19:562–574. doi: 10.1097/00004691-200212000-00010. [DOI] [PubMed] [Google Scholar]
- Steriade M. Cellular substrates of oscillations in corticothalamic systems during states of vigilance. In: Lydic R, Baghdoyan HA, editors. Handbook of Behavioral State Control. Cellular and molecular mechanisms. New York: CRC Press; 1999. pp. 327–347. [Google Scholar]
- Steriade M, Llinás RR. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68:649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- Taverna E, Francolini M, Jeromin A, Hilfiker S, Roder J, Rosa P. Neuronal calcium sensor 1 and phosphatidylinositol 4-OH kinase beta interact in neuronal cells and are translocated to membranes during nucleotide-evoked exocytosis. J Cell Sci. 2002;115:3909–3922. doi: 10.1242/jcs.00072. [DOI] [PubMed] [Google Scholar]
- Torres KCL, Souza BR, Miranda DM, Sampiao AM, Nicolato R, Neves Expression of neuronal calcium sensor-1 (NCS-1) is decreased in leukocytes of schizophrenia and bipolar disorder patients. Prog Neuro-Pharmacol Biol Psychiat. 2009;33:229–234. doi: 10.1016/j.pnpbp.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Tsujimoto T, Jeromin A, Satoh N, Roder JC, Takahashi T. Neuronal calcium sensor 1 and activity-dependent facilitation of P/Q-type calcium currents at presynaptic nerve terminals. Science. 2002;295:2276–2279. doi: 10.1126/science.1068278. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Urbano FJ, Leznik E, Llinas R. Modafinil enhances thalamocortical activity by increasing neuronal electrotonic coupling. Proc Natl Acad Sci USA. 2007;104:12554–12559. doi: 10.1073/pnas.0705087104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano FJ, Kezunovic N, Hyde J, Simon C, Beck P, Garcia-Rill E. Gamma band activity in the reticular activating system (RAS) Front Neurol: Sleep and Chronobiol. 2013;3:1–16. doi: 10.3389/fneur.2012.00006. 6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH. What is the significance of gamma wave activity in the pyriform cortex? Brain Res. 2000a;877:125–133. doi: 10.1016/s0006-8993(00)02568-3. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH. Are neocortical gamma waves related to consciousness? Brain Res. 2000b;855:217–224. doi: 10.1016/s0006-8993(99)02351-3. [DOI] [PubMed] [Google Scholar]
- Vogel GW. A review of REM sleep deprivation. Arch Gen Psychiat. 1975;32:749–761. doi: 10.1001/archpsyc.1975.01760240077006. [DOI] [PubMed] [Google Scholar]
- Vogel GW, Feng P, Kinney GG. Ontogeny of REM sleep in rats: possible implications for endogenous depression. Physiol Behav. 2000;68:453–461. doi: 10.1016/s0031-9384(99)00207-3. [DOI] [PubMed] [Google Scholar]
- Vogel GW, Feng P, Kinney GG. Ontogeny of REM sleep in rats: possible implications for endogenous depression. Physiol Behav. 2000;68:453–461. doi: 10.1016/s0031-9384(99)00207-3. [DOI] [PubMed] [Google Scholar]
- Voss U, Holzmann R, Tuin I, Hobson JA. Lucid dreaming: a state of consciousness with features of both waking and non-lucid dreaming. Sleep. 2009;32:1191–1200. doi: 10.1093/sleep/32.9.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP. The role of sleep in cognition and emotion. Ann NY Acad Sci. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- Watson RT, Heilman KM, Miller BD. Neglect after mesencephalic reticulr formation lesions. Neurology. 1974;24:294–298. doi: 10.1212/wnl.24.3.294. [DOI] [PubMed] [Google Scholar]
- Weiss JL, Hui H, Burgoyne RD. Neuronal calcium sensor-1 regulation of calcium channels, seceretion, and neuronal outgrowth. Cell Mol Neurobiol. 2010;30:1283–1292. doi: 10.1007/s10571-010-9588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Ye M, Hayar A, Strotman B, Garcia-Rill E. Cholinergic modulation of fast inhibitory and excitatory transmission to pedunculopontine thalamic projecting neurons. J Neurophysiol. 2010;103:2417–2432. doi: 10.1152/jn.01143.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarcone V, Azumi K, Dement W, Gulevich G, Kraemer H, Pivik R. REM phase deprivation and schizophrenia. Arch Gen Psychiat. 1975;32:1431–1436. doi: 10.1001/archpsyc.1975.01760290099012. [DOI] [PubMed] [Google Scholar]