Abstract
Background
Many cannabinoid medications are approved in North America or in Phase III trials, such as Dronabinol, Nabilone, or Nabiximols. Little is known about their subjective psychoactive effects when used for pain management. We hypothesized that when used for pain, dronabinol has psychoactive effects in a dose response relationship, whose peak effects are comparable to smoking marijuana.
Methods
With IRB approval and written consent, this was a randomized controlled trial of single dose placebo, 10 or 20 mg dronabinol in 30 chronic non-cancer pain patients taking opioids and not using marijuana. Hourly, for 8 hours during 3 monitored sessions, subjects completed the Addiction Research Center Inventory (ARCI). Comparison sample was the ARCI ratings in a study population with no pain (N=20), monitored every 30 minutes after smoking a 1.99% THC (low) and a 3.51% (high strength) marijuana cigarette.
Results
The 10 and 20 mg dronabinol doses had significantly elevated scores on 4/5 subscales vs. placebo over time (p<.05). Average daily morphine use, total pain relief (TOTPAR), age, gender, and baseline pain level were not significant covariates. ARCI peak effects at 2 hours were similar to peak effects of smoked marijuana at 30 minutes (p=.80, 10 mg=low, 20 mg=high strength).
Conclusions
In pain patients, oral dronabinol has similar psychoactive effects to smoking marijuana. This risk must be considered in any decision to prescribe cannabinoid medications for pain.
Keywords: Cannabinoid, Dronabinol, Abuse potential, Addiction, Marijuana
INTRODUCTION
The psychoactive properties of cannabis have been recognized since early time and today, it remains the most widely used illicit drug globally1. Despite the possible stigma and legal issues associated with its use, Cannabis sativa has been widely used as a herbal remedy over the centuries for the treatment of various conditions such as migraine, spasticity, nausea and vomiting as well as a magnitude of other disorders2. The search for the active ingredients of the plant led to the first identification of its main psychoactive ingredient, δ-9-tetrahydrocannabinol (THC)3, and the subsequent development of cannabinoid medications.
Several cannabinoid medications are currently FDA approved in the United States or in phase III trials, such as Dronabinol, Nabilone, or Nabiximols. Research into their effects continues to be a field of increasing interest, particularly with regard to their use in a variety of therapeutic areas. Dronabinol is an oral synthetic stereoisomer of THC, and is currently approved as an appetite stimulant in patients with HIV/AIDS and as a treatment for chemotherapy-induced nausea and vomiting in patients who failed to respond adequately to conventional antiemetic treatments4. However, it has been widely studied for a number of other conditions including multiple sclerosis, Parkinson’s and Huntington’s disease, and disturbed behavior in Alzheimer’s disease and Tourette syndrome5.
Another area of study is the use of cannabinoid medications for the treatment of persistent intractable pain. With the discovery of the endocannabinoid system and its involvement with analgesia, recent interest has emerged regarding the pain-modulating effects of cannabinoids and the potential of their synergistic analgesic effect with opioids for pain management6–12. However, such research did not address the existing controversy about the psychoactive effects of medicinal cannabinoids when used for pain management, and whether such effects differ from those of the whole plant marijuana.
The primary objective of this study was to evaluate the subjective psychoactive effects of oral dronabinol in chronic pain patients on stable doses of opioids, and compare such effects with placebo and smoked marijuana. The Addiction Research Center Inventory (ARCI), which is considered a well validated and reliable measure to assess the subjective effects of certain classes of psychoactive drugs13, was used as the primary outcome measure for this study. We hypothesized that when used for pain, dronabinol has psychoactive effects in a dose response relationship, whose peak effects are comparable to smoking marijuana.
MATERIALS AND METHODS
Trial Design
The oral dronabinol study was a multi-dose, randomized, double-blind, placebo-controlled, crossover trial with three drug administration sessions conducted at Brigham and Women’s Hospital in the United States. The comparison sample study cohort with smoked marijuana was a four-visit, randomized-dosing trial conducted separately at McLean Hospital. The dronabinol cohort permitted quantitative determinations of the subjective psychoactive effects, while the smoked marijuana cohort allowed for additional qualitative comparisons.
Participants
The studies were reviewed and approved by the Institutional Review Boards at Brigham and Women’s Hospital and McLean Hospital, Boston, Massachusetts, USA. All subjects provided written informed consent before any study-related procedures were performed and were all compensated for their participation in the studies.
Table 1 summarizes the baseline characteristics of the oral dronabinol sample, which has previously been reported in depth in the publication of the analgesic data10. Thirty chronic non-cancer pain participants on opioid therapy were recruited through Brigham and Women’s Hospital. Subjects ranged in age from 21 to 67 years (median age, 43.5 ± 11.8), 53% were female and the majority self-identified as Caucasian (96.7%). Most subjects (66.7%) reported chronic pain more than 5 years, and low back pain (66.7%) was the most prevalent. The mean morphine equivalent dose per day was 68.1 (ranging from 7.5 to 228.0 mg). Baseline pain intensity was 6.9/10 (± 1.3). Nineteen participants (63.3%) reported using marijuana in the past, 8 of whom within the past year. All participants were required to abstain from use for 1 month before and during enrollment. Subjects were also required to have stable opioid analgesic dose for more than 6 months, and report pain of at least 4 on a 0–10 numeric rating scale. They were asked to reschedule their visit if their average pain over the past 24 hours was less than 4/10, assessed on the morning of the anticipated treatment.
Table 1.
Patient Demographic and Descriptive Characteristics for the Oral Dronabinol Sample (N = 30)
| Variable | |
|---|---|
| Age (median) | 43.5 (± 11.8; range, 21–67) |
| Gender (% female) | 53.3 |
| Race (% Caucasian) | 96.7 |
| Pain Site (% low back) | 66.7 |
| Pain duration (% > 5 y) | 66.7 |
| Opioid duration (% > 2 y) | 70 |
| Morphine equivalent (oral mg/d) | 68.1 (SD=57.2; range, 7.5–228) |
| Current Opioid medication (% of subjects) | |
| RTC | |
| Methadone | 30 |
| Morphine - long-acting | 30 |
| Oxycodone - long-acting | 16.7 |
| PRN | |
| Oxycodone - short acting | 36.7 |
| Morphine - short acting | 16.7 |
| Hydrocodone | 6.7 |
| Hydromorphone | 6.7 |
| Baseline levels: | |
| Prestudy Diary (0–10) | |
| Pain Intensity | 6.9 (± 1.3) |
| Pain Relief | 3.9 (± 1.7) |
| Pain Bothersomeness (0–4) | 2.7 (± 0.6) |
| Satisfaction Baseline (0–10) | 3.7 (± 2.0) |
| Brief Pain Inventory (0-0) | |
| Interference with sleep | 6.7 (± 3.2) |
| RAND-36 (0–100) | |
| Energy/Fatigue | 37.1 (± 20.8) |
| Pain | 26.6 (± 15.2) |
| Social Functioning | 47.8 (± 24.6) |
| HADS | |
| Anxiety | 6.7 (± 2.9) |
| Depression | 6 (± 3.3) |
Abbreviations: RTC, return to clinic; PRN, as needed; RAND-36, RAND 36-Health Survey; HADS, Hospital Anxiety and Depression Scale
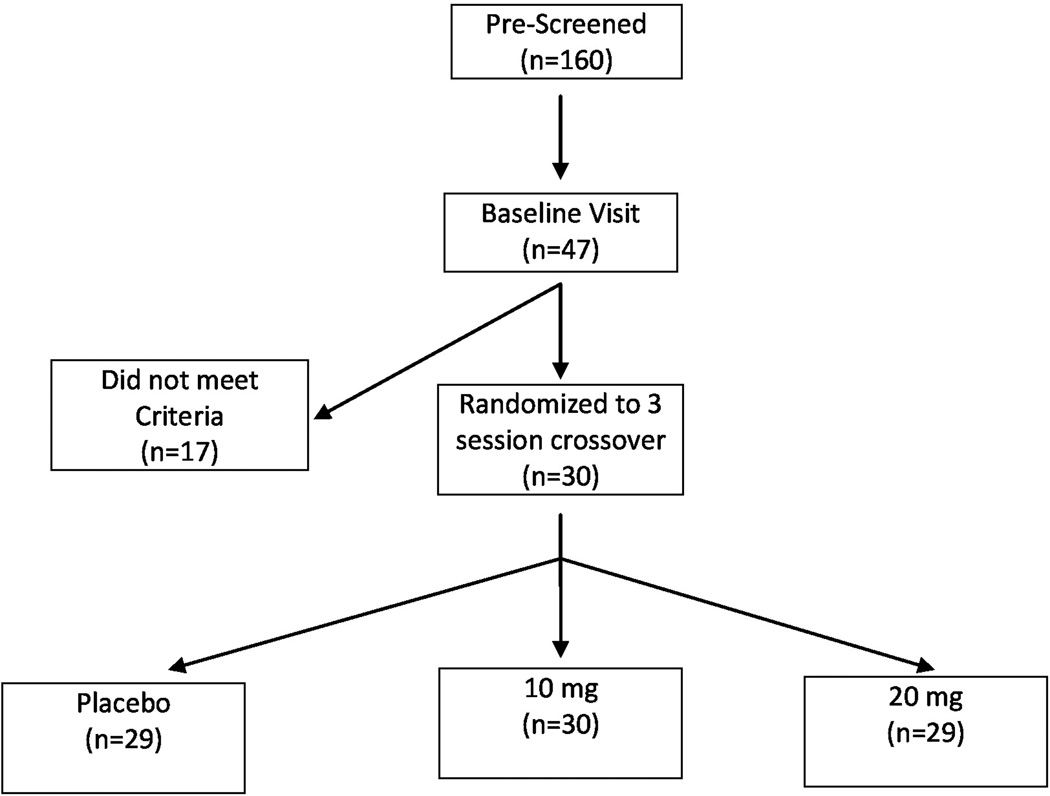
One hundred and sixty subjects were screened via telephone, and 47 subjects met preliminary inclusion criteria and presented for a baseline visit. Of these subjects, 30 met qualification and were randomized to receive the study drug. Twenty-nine subjects completed the study and one subject dropped out due to reported inability to concentrate (Fig. 1). Exclusion criteria included: pain of cancer origin; opioid dosing more frequently than every 8 hours; use of transdermal patch or intrathecal opioids (since it would be difficult to titrate their opioid dose during the trial); significant depression and/or anxiety symptoms (scores more than 11 on the Hospital Anxiety and Depression Scale); current substance abuse by self-report and psychosocial history, and any involvement in active litigation, compensation or disability issues.
Figure 1.
Research design and schema of the oral dronabinol sample
The comparison cohort consisted of twenty healthy participants with no pain who ranged in age from 19 to 30 years old (mean age, 21.7 ± 3). Ninety-five percent were Caucasian, reported using marijuana 1.6 ± 0.97 times per week, smoked 15.3 ± 4.2 tobacco cigarettes per day, and drank 9 ± 5.6 alcoholic drinks per week. They all showed normal physical examination, electrocardiogram and clinical laboratory tests before enrollment. They were all current tobacco smokers (average of 10–20 cigarettes daily), had no history of drug or alcohol dependence, and no diagnosis of any psychiatric disorders14.
Interventions
The aim of this study was to compare the profile of acute effects on subjective measures of dronabinol at two doses (10 and 20mg capsules) with placebo in patients with chronic pain and smoked marijuana (1.99% and 3.51% Δ9 THC) in those without chronic pain.
At the baseline visit for those with chronic pain, enrollment criteria were verified, informed consent was obtained, a history and physical examination were completed and baseline questionnaires about the subjects’ pain, function, and mood were administered. During the three treatment sessions, each subject received identically appearing placebo, 10mg or 20mg dronabinol capsules in 1 of 6 randomly allocated sequences. The randomization scheme was generated by the Investigational Drug Service (IDS) hospital pharmacy, and both study personnel and participants were blinded until all subjects had completed the study. The 3 treatment sessions were separated by a minimum of three days between each visit. On arrival, subjects were asked to complete the Addiction Research Center Inventory (ARCI) questionnaire and answer questions about their pain and satisfaction levels. These questionnaires were subsequently administered every hour for 8 hours. A symptom checklist was administered at the initial and final visits to assess the occurrence of side effects. At the completion of each treatment session, subjects were asked to evaluate the blinding process and record their overall satisfaction with the treatment.
Primary Outcome Measure
The primary outcome measure of the study was the Addiction Research Center Inventory (ARCI). The ARCI is a true/false 49-item well-known, self rated, well validated and reliable measure that can be used to assess the subjective effects of certain classes of psychoactive drugs13. Elevated scores on its 5 subscales highly correlate with significant psychoactive effects that may potentially increase the likelihood that a substance will be abused in a vulnerable population: the Morphine-Benzedrine Group (MBG, a measure of euphoria and is considered as the hallmark subjective effect of abused drugs), the Benzedrine Group (BG, a measure of stimulant effects relating to intellectual efficacy and energy), the Pentobarbital-Chlorpromazine-Alcohol Group (PCAG, a measure of apathetic sedation), the Amphetamine Scale (A, a measure of amphetamine-like stimulant effects), and the Lysergic Acid Diethylamide (LSD-specific group, a measure of somatic discomfort and dysphoria). Participants were administered the ARCI via paper and pencil every hour in the dronabinol group, and electronically using a keypad every 30 min in the smoked marijuana group.
Study Procedures
A comparison sample of those with no pain who smoked marijuana was chosen to contextualize the findings regarding the psychoactive effects of dronabinol in patients with chronic pain. In other words, it was used to illustrate how the effects of dronabinol might compare to smoked cannabis, on a qualitative level primarily. This was a posthoc comparison, and thus would not be able to conclusively determine if there were significant differences between groups. As such, despite the statistical limitations in directly comparing cohorts, it was hypothesized that the comparison group would demonstrate similar psychoactive effects to those with chronic pain taking dronabinol. Study procedures in both cohorts were quite similar, and all data was collected in a monitored laboratory environment at individual sessions over the same period of time in two hospitals of the Partners Healthcare System (Brigham and Women’s and McLean Hospitals). The comparison sample was a healthy study population with no pain (n=20), monitored every 30 minutes with the ARCI for 3 hours after smoking a 1.99% THC (low strength) and a 3.51% THC (high strength) marijuana cigarette in random order, at different study sessions. Smoking sessions lasted for 15 min each through a water-cooled inhaler device, and participants were monitored continuously for the next 3 hours after smoking. The study visits were separated by a minimum of 3 days between each visit. Participants were required to abstain from all illicit drugs and alcohol for 72 and 24 hours, respectively, prior to the study. They could not smoke tobacco or have any caffeine from midnight prior to each study day. Breath samples to check for alcohol and carbon monoxide, as well as urine samples and blood samples (to test for THC and its metabolites, nicotine, cotinine) were collected when subjects arrived to the laboratory. Urine drug tests had to be negative for drugs of abuse excluding THC metabolites, or else study visit was re-scheduled. If the urine test was positive for THC metabolites, one of the blood samples was tested for the presence of THC and its metabolites to determine if there has been use within the past 72 hours. For more specific details on the experimental design of the comparison study, including the marijuana challenge session, see Penetar et al.14
Statistical analysis
All data were analyzed with SPSS (Statistical Package for the Social Services, v 13.0; Chicago, IL). A repeated measures analysis of covariance (ANCOVA) was used to assess hourly and peak subjective responses on the ARCI scales as a primary measurement of dronabinol subjective effects. This was supplemented by an ANOVA comparison between cohorts and doses at the time of peak psychoactive effects. This was determined by the highest mean of five ARCI subscale scores in both cohorts (t= 2 hours for the dronabinol group and t= 30 minutes for the smoked cannabis group). Tukey corrections were used for multiple dose comparisons within each ARCI subscale for the ANCOVA and peak effects analyses, since each subscale measures a separate construct. Potential covariates that were included in a sensitivity analysis were: age, gender, race, location, type and duration of pain, average morphine equivalent dose and duration of opioid treatment, baseline pain level, baseline HADS score, previous marijuana use and total pain relief reported over the course of the 8 hour sessions in the chronic pain group (TOTPAR). Statistical significance was set at p ≤ 0.05.
RESULTS
Dronabinol versus Placebo comparisons
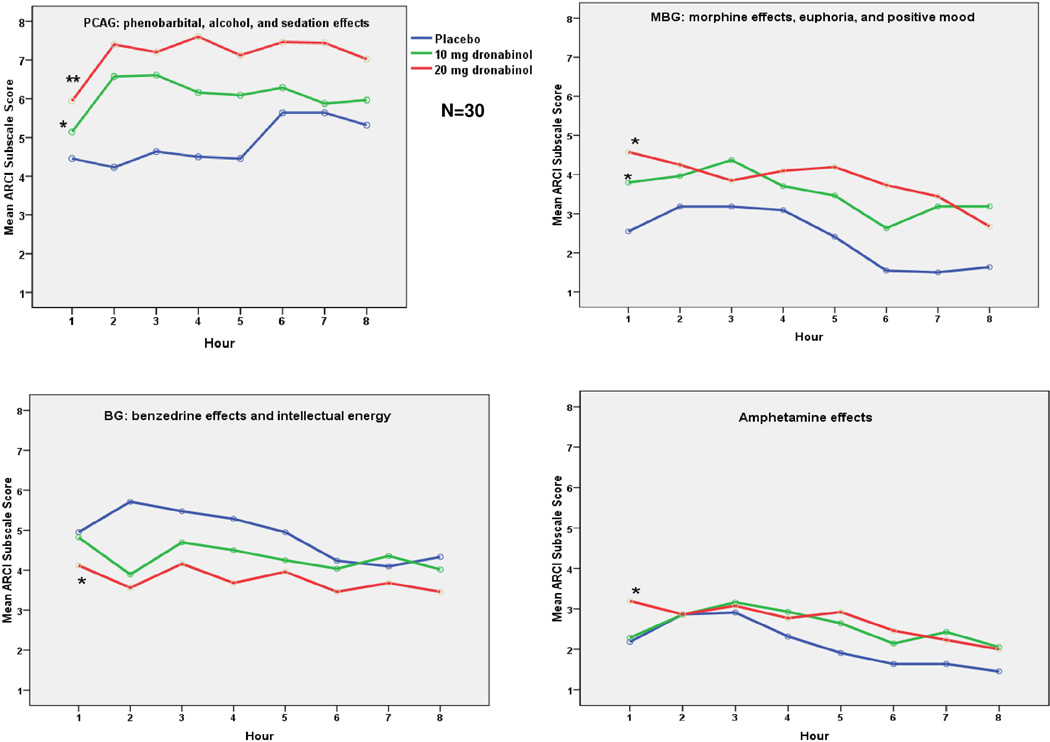
A repeated measures analysis found that the 10 and 20mg dronabinol doses resulted in significant changes, relative to placebo, on four of the five ARCI subscales over time (Fig. 2). Relative to placebo, dronabinol significantly increased Morphine-Benzedrine Group (MBG) [Drug effect: F = 5.5, p<0.05], Pentobarbital-Chlorpromazine-Alcohol Group (PCAG) [Drug effect: F = 8.0, p<0.01], and Amphetamine (A) [Drug effect: F = 3.3, p<0.05] scores, and decreased Benzedrine Group (BG) [Drug effect: F= 3.1, p<0.05] scores. There were no significant differences between the 10 mg vs. 20 mg doses. The overall trends, however, suggest that the 20 mg dose was associated with the greatest psychoactive effects. Benzedrine Group (BG) scores after dronabinol dose were lowest at 2 hours as compared to placebo, indicating a worsening of intellectual energy. For the other subscales, peak effects of the 2 doses of dronabinol vs. placebo were also found at 2 hours (P<.05 for MBG, PCAG, A, BG, and LSD). The effects of dose on Lysergic Acid Diethylamide (LSD) scores were non-significant over time (p>0.05), but the 10 and 20 mg doses were significantly greater than placebo at 2 hours.
Figure 2.
Hourly ARCI subscale scores for Dronabinol vs. Placebo, *=p<.05, **=p<.01 vs. placebo, LSD subscale comparisons nonsignificant (not shown)
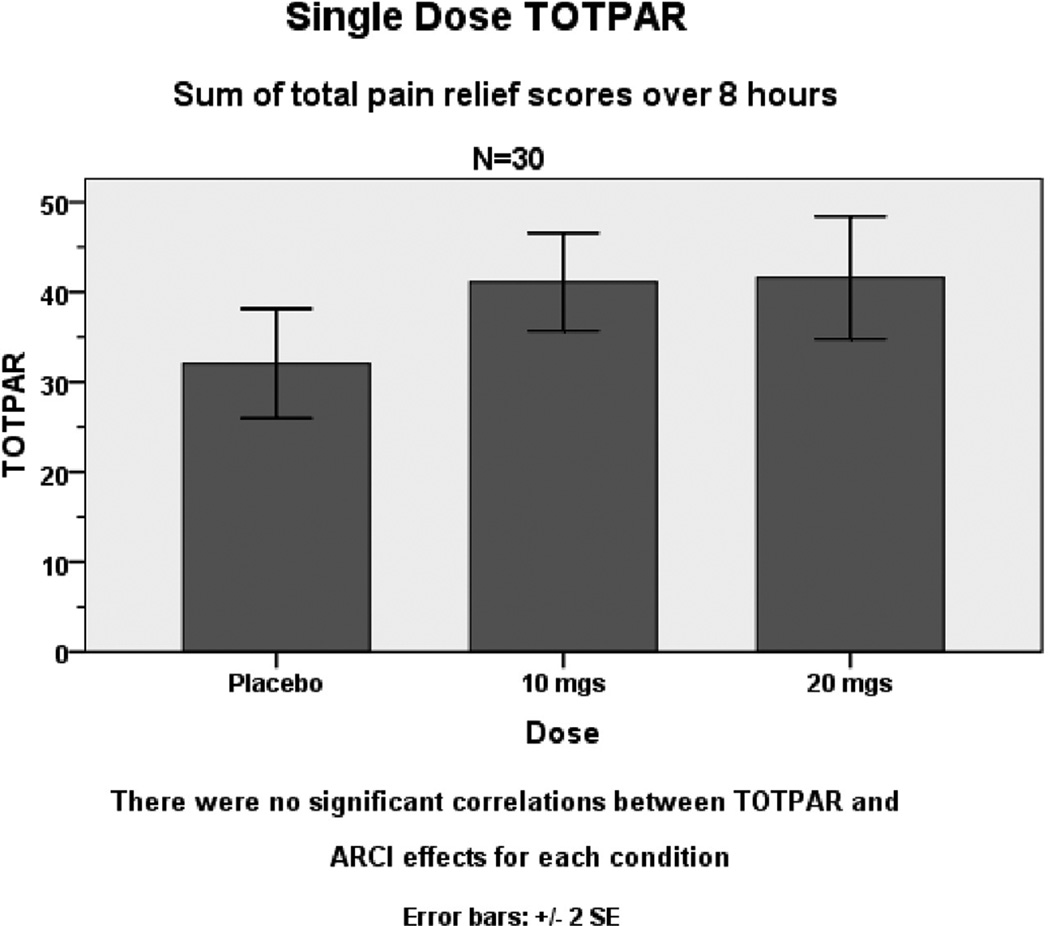
In the sensitivity analysis, for each of the three treatment conditions (placebo, dronabinol 10 and 20mg) average daily morphine use equivalents, total pain relief (TOTPAR), age, gender, previous marijuana use, and baseline pain level did not show any significant correlations with ARCI effects on any subscale. In a repeated measures ANCOVA analysis, these factors were not significant univariate predictors of ARCI subscale scores; there was no significant interaction with dose; nor did they significantly alter the F value of dose as a primary predictor of outcome (Fig. 3). In particular, there was no significant relationship between the level of reported pain relief (TOTPAR) and ARCI subscale scores in correlational or ANCOVA analyses, indicating that dronabinol analgesia was reported by the subjects as a phenomenon distinct from its psychoactive effects.
Figure 3.
Comparisons of pain intensity differences (TOTPAR) with 10 mg and 20 mg dronabinol compared with placebo (P<0.05 and P<0.01).
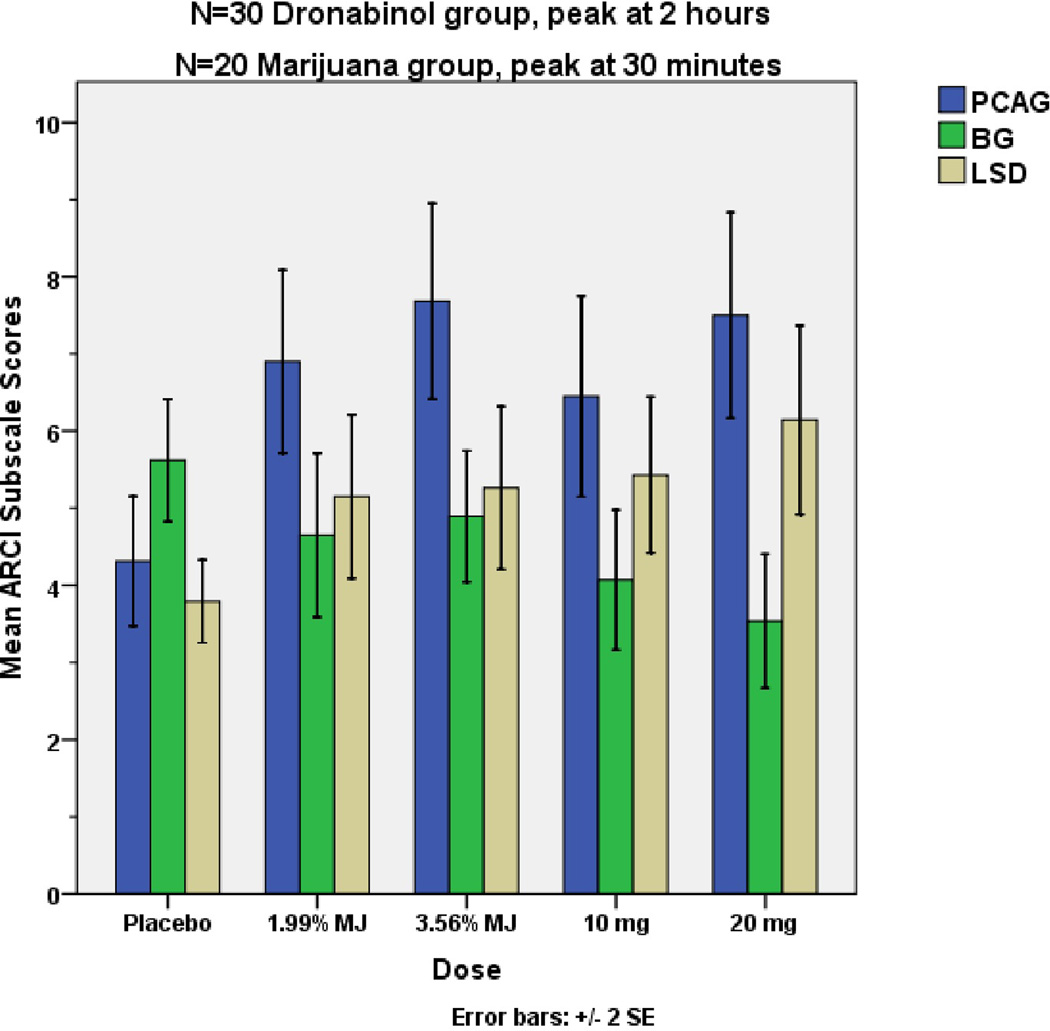
Dronabinol versus Marijuana smoking comparisons
Peak psychoactive effects of smoked cannabis were found at 30 minutes after completing the inhaling procedure. For three of the ARCI subscale scores, there were significant differences between the peak psychoactive effects of oral dronabinol (10 or 20 mg at 2 hours) and smoked marijuana (low and high dose at 30 minutes) as compared to placebo (Fig. 4). Pentobarbital-Chlorpromazine-Alcohol Group (PCAG) [Drug effect: F = 5.4, p<0.05] and Lysergic Acid Diethylamide (LSD) [Drug effect: F = 3.3, p<0.05] scores were significantly increased and Benzedrine Group (BG) [Drug effect: F = 3.5, p<0.05] score was significantly decreased in comparison to oral placebo. However, Morphine-Benzedrine Group (MBG) and Amphetamine (A) score differences were not statistically significant (p>0.05).
Figure 4.
Peak Psychoactive Effects of Dronabinol 10 or 20 mg vs. Low or High dose Marijuana Cigarettes
Peak psychoactive effects of dronabinol 10 and 20 mg at 2 hours were similar to peak effects of low and high strength smoked marijuana at 30 min in all five ARCI subscale scores, and there were no statistically significant differences between them (p>.45, Figure 4). Overall, as reported by the subjects on the ARCI, the subjective experiences of taking oral dronabinol or smoking marijuana do not appear to be significantly different than each other.
DISCUSSION
This study attempted to assess the subjective psychoactive effects of the cannabinoid medication, dronabinol, when used in combination with stable opioid dosage for pain management, as compared to placebo and smoked marijuana. Results of the study showed that the subjective effects of 10 and 20mg dronabinol were consistently and significantly greater than placebo on most ARCI subscales, and were equivalent to the psychoactive effects of smoking marijuana, although, as expected, it took longer to reach the same peak effects (2 hours with oral dronabinol as compared to 30 minutes with smoked marijuana). These findings imply that in our laboratory environment, dronabinol caused a “high” similar to smoking marijuana when used for pain management. However, this does not necessarily mean that dronabinol has the same abuse liability as marijuana, and other risk factors for addiction should also be considered before concluding that such a risk exists. For instance, the ARCI does not assess drug likability, which is also an important indicator of drug abuse potential.
In addition, the results of previous studies assessing the subjective effects and likelihood for abuse and diversion of dronabinol have differed according to the study setting. Several laboratory studies have shown that the subjective effects of smoked marijuana and oral cannabinoids are highly similar in subjects who are regular users of marijuana and do not have a diagnosis of chronic pain15–19. And yet, Phase IV drug surveillance observational clinical studies done primarily in patients with cancer or HIV/AIDS have reported a low abuse potential of such medications. Moreover, a review by Calhoun et al.20 found no evidence of abuse or diversion for dronabinol. The authors noted that marijuana users do not value the psychoactive effects of dronabinol because of its slow and gradual onset of action, weak reinforcing effect and dysphoric feeling. Similar conclusions were also reached with other cannabinoid medications, such as nabilone and nabiximols, with cases of abuse or diversion being rare and only occurring in a very small proportion of recipients21,22. It is important to recognize the distinct differences between these laboratory and clinical data since they should be interpreted independently. Our study bridges these gaps to some extent. The present study focuses on the subjective effects of dronabinol in a laboratory-based, randomized, double blinded, multiple dose, crossover trial in a specific patient population with pain, i.e. patients with non-cancer pain taking prescription opioids. Our results demonstrate that the subjective effects of dronabinol in a laboratory setting are comparable to smoking marijuana, which potentially applies to other oral cannabinoid medications as well in this environment. These laboratory findings may or may not translate clinically to an increased likelihood of dronabinol abuse and/or diversion in this vulnerable patient population (which requires further study).
Aside from these considerations, a special aspect in the case of prescribing cannabinoids for chronic pain is the expectation of long-term use and the possible combination with opioid therapy which may very well increase the abuse liability of both drugs due to their potentially additive addictive properties. In addition, evidence from the National Household Survey on Drug Abuse (NHSDA) suggests that the use of marijuana, especially at a young age, can potentially lead to the future use of other illicit substances23. In our study, although subjects were carefully screened prior to enrollment, THC was found in 4 of the serum samples of chronic pain subjects prior to receiving placebo, which suggests possible illicit use of marijuana by some subjects. Therefore, since substance use disorders are already highly prevalent in the chronic pain population24–26, potential candidates for cannabinoid therapy should be closely selected, educated about, and monitored27,28.
Another important finding in the study was the insignificant relationship between the level of total reported pain relief (TOTPAR) and ARCI subscale scores. This indicates that the psychoactive properties of dronabinol are independent of its synergistic analgesic effects with opioids (reported previously)10. It was believed from early studies that THC could only produce analgesia at doses that were high enough to cause other behavioral side effects. Noyes et al. compared single doses of placebo and 5,10,15 and 20 mg of THC in ten cancer pain subjects and found significant analgesia with the higher doses, but at the expense of significant sedation and mental clouding29. The first evidence that the antinociceptive effects of THC could be separated from its adverse behavioral effects was presented in 1994, when Smith and colleagues demonstrated that a kappa opioid receptor antagonist, nor-binaltorphimine (norBNI), blocked only the antinociceptive effect of THC in rodents without altering other pharmacological or behavioral effects30. Our study in humans illustrates this phenomenon of separate analgesic and psychoactive properties of cannabinoids in a clinical pain patient population. One could argue that the psychoactive effects of dronabinol were amplified by concomitant oral opioid usage in our pain cohort; however, baseline assessment of the study subjects did not reveal any patient reports of significant sedation, confusion, impairment, or other cognitive effects of opioids. Nevertheless, this is a potential confounder to generalizing that dronabinol has significant psychoactive effects comparable to smoking marijuana.
There are a number of additional limitations worth mentioning in the design of this study. First, the number of subjects in each group was limited, and the dronabinol cohort consisted of a heterogeneous sample on various doses of opioids. Although this may reflect a typical clinical pain population, future studies with less variability in opioid type and dosage and longer follow-up period are needed. Second, the comparison information was collected historically from a previous study that utilized the same primary outcome measure, and thus, between-study variations may have affected our findings. As noted in the methods, we recognize the statistical limitations in directly comparing the dronabinol and smoked marijuana groups. But these comparisons are still useful qualitatively to illustrate that the psychoactive effects of dronabinol are clinically meaningful. We cannot conclude that the effects of dronabinol and smoked cannabis are equivalent. In addition, only one measure (namely, the ARCI) was used to compare the acute dose-effects of oral dronabinol with smoked marijuana which is not sufficient to give definitive data on a drug’s abuse potential. Thus, additional data from physiological, psychomotor and cognitive measures should be collected in future studies to better understand the psychoactive effects and abuse liability of oral dronabinol. Also, urine drug testing was not performed prior to testing in the dronabinol group. We only assessed possible concurrent drug use with self-report which is a possible confounder to our results. Finally, we did not have an additional comparison group of chronic pain patients who were not taking opioids for pain, as opioids may share several of the subjective and psychoactive effects noted with cannabinoids31.
Despite these limitations, the results of this preliminary study suggest that in chronic pain patients on opioid therapy, dronabinol has similar psychoactive effects to smoking marijuana. This warrants further large-scale observational studies to assess a potential abuse and/or diversion risk. The subjective psychoactive effects of cannabinoids should be considered in any decision to prescribe these medications for chronic non-cancer pain. Moreover, our findings have implications for the analgesic development of all medicinal cannabinoids, and indicate that these safety issues should be carefully scrutinized in drug development. We were also able to determine that the psychoactive effects of dronabinol are distinct from their analgesic effects.
ACKNOWLEDGEMENTS
These studies were supported in part by an investigator-initiated grant from Solvay Pharmaceuticals, Inc. and by grants DA 12014 and DA 00343 from the National Institute on Drug Abuse.
Funding: Supported in part by an investigator-initiated grant from Solvay Pharmaceuticals, Inc. and by grants DA 12014 and DA 00343 from the National Institute on Drug Abuse.
Footnotes
Conflicts of interest disclosures: None
REFERENCES
- 1.World Drug Report 2011. Cannabis market. [Last accessed on May 30]; Available from: http://www.unodc.org/documents/data-and-analysis/WDR2011/The_cannabis_market.pdf. [Google Scholar]
- 2.Russo EB. History of cannabis and its preparations in saga, science, and sobriquet. Chem Biodivers. 2007 Aug;4(8):1614–1648. doi: 10.1002/cbdv.200790144. [DOI] [PubMed] [Google Scholar]
- 3.Gaoni Y, Mechoulam R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. [1964/04/01];Journal of the American Chemical Society. 1964 86(8):1646–1647. [Google Scholar]
- 4.Solvay Pharmaceuticals Inc; 2006. [Last accessed May 9]. Marinol® Prescribing Information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2006/018651s025s026lbl.pdf. [Google Scholar]
- 5. [Last accessed on May 30, 2012]; http://who.int/medicines/areas/quality_safety/4.2DronabinolCritReview.pdf. RotECoDDEAodais-iAf.
- 6.Smith FL, Cichewicz D, Martin ZL, Welch SP. The enhancement of morphine antinociception in mice by delta9-tetrahydrocannabinol. Pharmacol Biochem Behav. 1998 Jun;60(2):559–566. doi: 10.1016/s0091-3057(98)00012-4. [DOI] [PubMed] [Google Scholar]
- 7.Cichewicz DL, Martin ZL, Smith FL, Welch SP. Enhancement mu opioid antinociception by oral delta9-tetrahydrocannabinol: dose-response analysis and receptor identification. J Pharmacol Exp Ther. 1999 May;289(2):859–867. [PubMed] [Google Scholar]
- 8.Cichewicz DL, McCarthy EA. Antinociceptive synergy between delta(9)-tetrahydrocannabinol and opioids after oral administration. J Pharmacol Exp Ther. 2003 Mar;304(3):1010–1015. doi: 10.1124/jpet.102.045575. [DOI] [PubMed] [Google Scholar]
- 9.Cichewicz DL. Synergistic interactions between cannabinoid and opioid analgesics. Life Sci. 2004 Jan;74(11):1317–1324. doi: 10.1016/j.lfs.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 10.Narang S, Gibson D, Wasan AD, et al. Efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy. J Pain. 2008 Mar;9(3):254–264. doi: 10.1016/j.jpain.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Viganò D, Rubino T, Parolaro D. Molecular and cellular basis of cannabinoid and opioid interactions. Pharmacol Biochem Behav. 2005 Jun;81(2):360–368. doi: 10.1016/j.pbb.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Hohmann AG, Suplita RL. Endocannabinoid mechanisms of pain modulation. AAPS J. 2006;8(4):E693–E708. doi: 10.1208/aapsj080479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischman MW, Foltin RW. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br J Addict. 1991 Dec;86(12):1563–1570. doi: 10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- 14.Penetar DM, Kouri EM, Gross MM, et al. Transdermal nicotine alters some of marihuana's effects in male and female volunteers. Drug Alcohol Depend. 2005 Aug;79(2):211–223. doi: 10.1016/j.drugalcdep.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Hart CL, Haney M, Vosburg SK, Comer SD, Foltin RW. Reinforcing effects of oral Delta9-THC in male marijuana smokers in a laboratory choice procedure. Psychopharmacology (Berl) 2005 Sep;181(2):237–243. doi: 10.1007/s00213-005-2234-2. [DOI] [PubMed] [Google Scholar]
- 16.Kirk JM, Doty P, De Wit H. Effects of expectancies on subjective responses to oral delta9-tetrahydrocannabinol. Pharmacol Biochem Behav. 1998 Feb;59(2):287–293. doi: 10.1016/s0091-3057(97)00414-0. [DOI] [PubMed] [Google Scholar]
- 17.Chait LD, Zacny JP. Reinforcing and subjective effects of oral delta 9-THC and smoked marijuana in humans. Psychopharmacology (Berl) 1992;107(2–3):255–262. doi: 10.1007/BF02245145. [DOI] [PubMed] [Google Scholar]
- 18.Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H. Comparison of the subjective effects of Delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl) 2002 Jun;161(4):331–339. doi: 10.1007/s00213-002-1033-2. [DOI] [PubMed] [Google Scholar]
- 19.Hart CL, Ward AS, Haney M, Comer SD, Foltin RW, Fischman MW. Comparison of smoked marijuana and oral Delta(9)-tetrahydrocannabinol in humans. Psychopharmacology (Berl) 2002 Dec;164(4):407–415. doi: 10.1007/s00213-002-1231-y. [DOI] [PubMed] [Google Scholar]
- 20.Calhoun SR, Galloway GP, Smith DE. Abuse potential of dronabinol (Marinol) J Psychoactive Drugs. 1998 Apr-Jun;30(2):187–196. doi: 10.1080/02791072.1998.10399689. 1998. [DOI] [PubMed] [Google Scholar]
- 21.Ware MA, St Arnaud-Trempe E. The abuse potential of the synthetic cannabinoid nabilone. Addiction. 2010 Mar;105(3):494–503. doi: 10.1111/j.1360-0443.2009.02776.x. [DOI] [PubMed] [Google Scholar]
- 22.Robson P. Abuse potential and psychoactive effects of δ-9-tetrahydrocannabinol and cannabidiol oromucosal spray (Sativex), a new cannabinoid medicine. Expert Opin Drug Saf. 2011 Sep;10(5):675–685. doi: 10.1517/14740338.2011.575778. [DOI] [PubMed] [Google Scholar]
- 23.SAMHSA Fact sheet: National Household survey on Drug Abuse. [Last accessed June 06];2011 Sep; http://www.samhsa.gov/data/NSDUH/2k10NSDUH/2k10Results.htm#5.3.
- 24.Fishbain DA, Rosomoff HL, Rosomoff RS. Drug abuse, dependence, and addiction in chronic pain patients. Clin J Pain. 1992 Jun;8(2):77–85. doi: 10.1097/00002508-199206000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Maruta T, Swanson DW, Finlayson RE. Drug abuse and dependency in patients with chronic pain. Mayo Clin Proc. 1979 Apr;54(4):241–244. [PubMed] [Google Scholar]
- 26.Ready LB, Sarkis E, Turner JA. Self-reported vs. actual use of medications in chronic pain patients. Pain. 1982 Mar;12(3):285–294. doi: 10.1016/0304-3959(82)90160-9. [DOI] [PubMed] [Google Scholar]
- 27.Butler SF, Budman SH, Fernandez K, Jamison RN. Validation of a screener and opioid assessment measure for patients with chronic pain. Pain. 2004 Nov;112(1–2):65–75. doi: 10.1016/j.pain.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 28.Butler SF, Budman SH, Fernandez KC, et al. Development and validation of the Current Opioid Misuse Measure. Pain. 2007 Jul;130(1–2):144–156. doi: 10.1016/j.pain.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noyes R, Brunk SF, Baram DA, Canter A. Analgesic effect of delta-9-tetrahydrocannabinol. J Clin Pharmacol. 1975 Feb-Mar;15(2–3):139–143. doi: 10.1002/j.1552-4604.1975.tb02348.x. 1975. [DOI] [PubMed] [Google Scholar]
- 30.Smith PB, Welch SP, Martin BR. Interactions between delta 9-tetrahydrocannabinol and kappa opioids in mice. J Pharmacol Exp Ther. 1994 Mar;268(3):1381–1387. [PubMed] [Google Scholar]
- 31.Zacny JP, Gutierrez S. Subjective, psychomotor, and physiological effects profile of hydrocodone/acetaminophen and oxycodone/acetaminophen combination products. Pain Med. 2008 May-Jun;9(4):433–443. doi: 10.1111/j.1526-4637.2007.00359.x. 2008. [DOI] [PubMed] [Google Scholar]