Abstract
Fear conditioning (inescapable shock training (ST)) and fearful context re-exposure (CR) alone can produce significant fear indicated by increased freezing and reductions in subsequent REM sleep. Damage to or inactivation of the basolateral nucleus of the amygdala (BLA) prior to or after ST or prior to CR generally has been found to attenuate freezing in the shock training context. However, no one has examined the impact of BLA inactivation on fear-induced changes in sleep. Here, we used the GABAA agonist, muscimol (MUS), to inactivate BLA prior to ST, the period when fear is learned, and assessed sleep after ST and sleep and freezing after two CR sessions. Wistar rats (n=14) were implanted with electrodes for recording sleep and with cannulae aimed bilaterally into BLA. After recovery, the animals were habituated to the injection procedure (handling) over 2 consecutive days and baseline sleep following handling was recorded. On experimental day 1, the rats were injected (0.5ul) into BLA with either MUS (1.0uM; n = 7) or vehicle (distilled water (dW), n = 7) 30 min prior to ST (20 footshocks, 0.8mA, 0.5s duration, 60s interstimulus interval). On experimental days 7 and 21, the animals experienced CR (CR1 and CR2, respectively) alone. EEG and EMG were recorded for 8h on each day and the recording was scored for non-rapid eye movement sleep (NREM), rapid eye movement sleep (REM), and wakefulness. Freezing was examined during CR1 and CR2. MUS microinjections into BLA prior to ST blocked the post-training reduction in REM sleep seen in vehicle treated rats. Furthermore, in MUS treated rats, REM sleep after CR1 and CR2 was at baseline levels and freezing was significantly attenuated. Thus, BLA inactivation prior to ST blocks the effects of footshock stress on sleep and reduces fear memory, as indicated by the lack of freezing and changes in sleep after CR. These data indicate that BLA is an important regulator of stress-induced alterations in sleep, and an important site for forming fear memories that can alter sleep.
Keywords: basolateral amygdala, fear conditioning, muscimol, REM sleep
Introduction
Posttraumatic stress disorder (PTSD) is a neuropsychiatric disorder which develops in a significant subset of the population following psychological trauma and is a significant concern of the military (Breslau et al. 1998; Weiss et al. 1992). Symptomology includes re-experiencing the traumatic event, avoidance of traumatic cues and hyperarousal. Interestingly, it is often sleep complaints that bring military personnel into the clinic for treatment which ultimately leads to a PTSD diagnosis and not the daytime symptomology. Furthermore, subjective sleep disturbances have been linked to the genesis of PTSD (Koren et al. 2002) and may contribute to its daytime symptoms (due to the role sleep plays in cognitive functioning).
PTSD is viewed as a disorder of the brain’s fear system (Shvil et al. 2013). As such, experimental fear conditioning is one of our most important research models related to PTSD, as well as of other anxiety disorders (Davis 1990; Davis 1992 ; Foa et al. 1992; Grillon et al. 1996; Shalev et al. 1992). Fear conditioning occurs when a neutral stimulus or context becomes associated with the occurrence of a significant aversive emotional event, and subsequently the previously neutral stimuli and contexts can elicit behavioral and physiological fear responses similar to those induced by the event itself. This linkage and subsequent behavioral manifestation is mediated through the amygdala, an area of the brain central in current concepts of fear conditioning (e.g., (Myers and Davis 2007)). The amygdala also is hyperactive in PTSD (Bremner et al. 2005) and it has an established, but generally underappreciated, role in mediating fear- and stress-induced alterations in sleep (Liu et al. 2009; Wellman et al. 2013; Liu et al. 2011).
Changes in sleep can be fear-conditioned; i.e., evoking fearful memories produce changes in sleep similar to those produced by the initial fearful stressor. However, the relationship of fear conditioning to sleep is quite complex. Brief fear training such as that used in the majority of studies of fear conditioning produces minimal alterations in sleep (Hellman and Abel 2007; Sanford et al. 2003) whereas extensive training using inescapable shock (IS) as the aversive stimulus significantly reduces rapid eye movement (REM) sleep and training with escapable shock (ES) can produce significant increases in REM sleep (Yang et al. 2011; Sanford et al. 2010). Indices of fear (freezing) and stress (stress-induced hyperthermia (SIH)) are similar for both IS and ES training (Yang et al. 2011). REM sleep is also significantly increased in animals after behavioral fear is extinguished compared to REM sleep in those that continue to show behavioral fear (Wellman et al. 2008).
Most of the work on the role of the amygdala in mediating fear- and stress-induced alterations in sleep has focused on the central nucleus of the amygdala (CNA). Previously, we demonstrated that microinjections of the GABAA antagonist, bicuculline, into the CNA attenuated IS-induced reductions in REM whereas microinjections of the GABAA agonist muscimol did not (Liu X et al. 2006). Microinjections of bicuculline, but not muscimol, also reduced IS-induced Fos expression, a marker of neuronal activity (Chaudhuri et al. 2000; Farivar et al. 2004), in the locus coeruleus (LC), a brain region implicated in the regulation of REM (Steriade and McCarley 1990) and stress (Abercrombie and Jacobs 1987b, a). As muscimol produces GABAergic mediated inhibition and bicuculline blocks GABAergic inhibition, these studies suggest that inactivation of CNA is involved in the reductions in REM produced by IS. Pre-context microinjections of the corticotropin releasing factor (CRF) receptor 1 antagonist, antalarmin (ANT), into CNA also attenuated the reductions in REM that occur after exposure to a fearful context and reduced fear-induced Fos expression in PVN, LC and DRN (Liu et al. 2011). These effects of ANT were independent of fear-induced freezing. That is the major behavioral index of fear memory, freezing, was observed even though ANT microinjected into CNA blocked the effects of fear on REM. This suggests that CRF in CNA is an important regulator of post-stress-alterations in REM as well as stress-induced activation in the brain, but not of actual fear memory.
Recently, we found that microinjections of ANT into the basolateral nucleus of the amygdala (BLA) in rats block both IS-induced reductions in REM sleep and the formation of memories that alter sleep without blocking fear memory as indicated by contextual freezing (Wellman et al. 2013). These findings suggest that BLA is critical for memories that produce fear-induced alterations in REM sleep, and that fear behavior in wakefulness and subsequent alterations in sleep may be regulated by separate neural processes. Understanding the processes that can link and separate fear behavior and sleep could provide significant insight into how dysfunction in the fear system can produce the sleep disturbances associated with PTSD.
Several studies have demonstrated that damage to, or inactivation of, BLA prior to or after fear conditioning (e.g., (Cousens and Otto 1998; Koo et al. 2004; Maren 1998; Maren et al. 1996; Sacchetti et al. 1999); for conflicting results see (Wilensky et al. 2000)) or prior to context re-exposure (Helmstetter and Bellgowan 1994; Muller et al. 1997) attenuates freezing in the fearful context. However, no one has determined whether pre-training global inactivation of BLA will reduce both behavioral fear and fear-induced alterations in sleep. In this study, we inactivated BLA with microinjections of the GABAA agonist, muscimol, prior to shock training and examined the relationship between fear behavior and sleep on two subsequent exposures to the fearful context alone. Our goal was to assess whether global inactivation of BLA blocked memories that produced fear behavior and alterations in sleep and to determine whether it was a critical region for linking the effects of fear on behavior and sleep.
Methods
Subjects
The subjects were 14 ninety-day-old Wistar rats obtained from Charles River laboratories (Wilmington, MA). Upon arrival, the rats were individually housed in polycarbonate cages and given ad lib access to food and water. The rooms were kept on a 12:12 light:dark cycle with lights on from 07:00 to 19:00 h. Light intensity during the light period was 100–110 lux and less than 1 lux during the dark period. Ambient room temperature was maintained at 24.5 ± 0.5 °C.
Surgery
Beginning one week following arrival, the rats were anesthetized with isoflurane (5% induction; 2% maintenance) and implanted with skull screw electrodes for recording their electroencephalogram (EEG) and stainless steel wire electrodes sutured to the dorsal neck musculature for recording their electromyogram (EMG). Leads from the recording electrodes were routed to a 9-pin miniature plug that mated to one attached to a recording cable. Bilateral guide cannulae (26 ga.) for microinjections into BLA were implanted with their tips aimed 1.0 mm above BLA (A 2.6, ML ±4.8, DV 8.0 (Kruger et al. 1995)). The recording plug and cannulae were affixed to the skull with dental acrylic and stainless steel anchor screws. Ibuprofen (15 mg/kg) was made available in their water supply for relief of post-operative pain. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by Eastern Virginia Medical School’s Animal Care and Use Committee (Protocol # 13-003).
Drugs
MUS (muscimol hydrobromide, 5-aminomethyl-3-hydroxyisoxazole) was obtained from Sigma–Aldrich, St. Louis, MO, USA. It was prepared in pyrogen-free distilled water (dW) and was sonicated for 20 min to ensure that the drug was dissolved completely. A fresh solution was prepared for each experimental day.
Procedures
All experimental manipulations were conducted during the fourth h of the light period such that sleep recording would begin at the start of the fifth h. This resulted in 8 h of light period recording on each experimental day.
Home cages were changed at least 3 days prior to injection day. The same room was used for animal housing and sleep recording. The microinjections and behavioral testing were conducted in a separate room from that used for recording.
Sleep Recording
For recording sleep, each animal, in its home cage, was placed on a rack outfitted for electrophysiological recording and a lightweight, shielded cable was connected to the miniature plug on the rat’s head. The cable was attached to a commutator that permitted free movement of the rat within its cage. EEG and EMG signals were processed by a Grass, Model 12 polygraph equipped with model 12A5 amplifiers and routed to an A/D board (Eagle PC30) housed in a Pentium class PC. The signals were digitized at 128 Hz and collected in 10 s epochs using a custom sleep data collection program.
The rats were allowed a post-surgery recovery period of 14 days prior to beginning the experiment. Once recovered, the animals were randomly assigned to one of two groups: MUS prior to shock training (MUS; n=7) or dW prior to shock training (dW; n=7) for studies of its effects on shock training and fear. All rats were habituated to the recording cable and chamber over 3 consecutive days. Then the rats were habituated to the 5 min handling procedure necessary for microinjections over 2 consecutive days and a baseline following handling (BH) was recorded.
Microinjections
For microinjections, injection cannulae (33 ga.) were secured in place within the guide cannulae, and projecting 1.0 mm beyond the tip of the guide cannulae for delivery of drug into the target region. The injection cannulae were connected to one end of a section of polyethylene tubing that had the other end connected to 5.0 ul Hamilton syringes. The injection cannulae and tubing were prefilled with the solution to be injected. Once the cannulae were in place, 0.5 uL of either drug or vehicle was bilaterally infused over 3 min. The cannulae were left in place one min pre- and post-injection to allow for maximal absorption of the solution.
Fear conditioning
Each shock-training session (ST) lasted 30 min. During this procedure, individual rats were placed in shock chambers (Coulbourn Habitest cages equipped with grid floors (Model E10-18RF) that were housed in Coulbourn Isolation Cubicles (Model H10-23)) and were allowed to freely explore for 5 min. Over the next 20 min, they were presented with 20 footshocks (0.8 mA, 0.5 s duration) at 1.0 min intervals. Shock was produced by Coulbourn Precision Regulated Animal Shockers (Model E13-14) and presented via the grid floor of the shock chamber. Five min after the last shock, the rats were returned to their home cages.
On days 7 and 21 after training, the rats were placed back in the shock chambers and allowed to explore freely for 30 min (no shock presented) before being returned to their home cage. These two context re-exposures (CR1 and CR2) were used to test for fear memory (assessed by behavioral freezing) and for post-exposure alterations in sleep.
The shock chamber was thoroughly cleaned with diluted alcohol following each session. Each session was videotaped using mini video cameras (Weldex, WDH-2500BS, 3.6 mm lens) attached to the center of the ceiling of the shock chamber for subsequent visual scoring of freezing.
Data Analyses
Sleep
Computerized EEG and EMG records were visually scored by trained observers blind to drug condition in 10 s epochs to determine wakefulness, NREM and REM. Wakefulness was scored based on the presence of low-voltage, fast EEG and high amplitude, tonic EMG levels. NREM was characterized by the presence of spindles interspersed with slow waves, lower muscle tone and no gross body movements. REM was scored continuously during the presence of low voltage, fast EEG, theta rhythm and muscle atonia. Data were collapsed into 4 h blocks (B1 & B2) and the total 8 h light period. The following sleep parameters were examined in the data analyses: total NREM (min), total REM (min); total sleep (REM + NREM), REM% (REM/total sleep*100) and number of NREM and REM episodes (defined as contiguous 10 s epochs of a given state).
The sleep data were analyzed with two-way mixed factors (Group (dW and MUS) X Treatment (days)) ANOVAs with repeated measures on Treatment. Post hoc Tukey Tests were used to determine differences among means as appropriate.
Freezing
Videotapes of the ST and CR sessions were scored for freezing, defined as the absence of body movement except for respiration (Blanchard and Blanchard 1969a; Doyere et al. 2000). Freezing was scored by a trained observer blind to condition in 5 s intervals during 1.0 min observation periods over the course of the 30 min of the CR trials. The percentage time spent freezing was calculated (FT%: freezing time/observed time × 100) for each animal for each observation period.
Freezing were scored during the five min pre-shock period to obtain baseline levels prior to ST. Freezing data for the CR days were analyzed for the entire 30 min exposure and compared to the pre-shock period on the ST day and across drug treatment groups on the CR test days. Post hoc comparisons were conducted with Tukey tests.
Histology
To localize the microinjection sites in, brain slices (40 μm) were made through the amygdala and the sections were mounted on slides and stained with cresyl violet. The sections were then examined in conjunction with a stereotaxic atlas (Kruger et al. 1995) to confirm cannulae placements. Though there were rostral-caudal variations in the placements among animals, the histology indicated that MUS or dW would have been infused into BLA and adjacent areas in all the rats, and all animals were used in the data analyses.
Results
Freezing
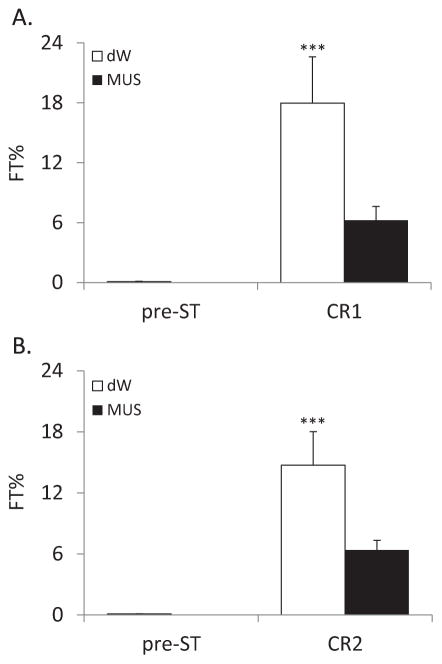
During the pre-ST period, there was essentially no freezing (<0.1% for each group) and no significant difference in FT% between the two groups (p=0.98). On CR1, FT% was significantly increased for the dW group compared to the MUS group (p=0.003; Figure 1A). Similarly, on CR2, FT% was significantly increased for the dW group compared to the MUS group (p=0.03; Figure 1B). Analyzing the data within groups, there were significant increases in FT% for the dW group in CR1 and CR2 (p=<0.001 and p=0.001, respectively) compared to the pre-ST condition. By comparison, FT% for the MUS group did not significantly differ across conditions.
Figure 1.
Percent time freezing (FT%) plotted for (A) the 5 min pre-shock period (Pre-ST) when the rats were naïve and for the first context re-exposure (CR1) and (B) for Pre-ST and the second context re-exposure (CR2). No shocks were presented during either CR. dW: distilled water; MUS: muscimol. Error bars are ± SEM. ***P<0.001 compared to Pre-ST.
Rapid Eye Movement Sleep
The analysis of total REM in 4 h blocks (B1-2) for the dW group (Figure 2A) found an effect of experimental condition (F3,18 = 8.784, p<0.001). Post hoc analyses for B1 found a significant decrease in REM compared to baseline recordings for ST (p<0.001), CR1 (p=0.012) and CR2 (p=0.018). Post hoc analysis for B2 also found REM significantly decreased when comparing BH to CR1 (p=0.007) and CR2 (p=0.03), but not for ST (p=0.075). The analysis of total REM for the entire 8 h light recording period of the dW group (Figure 2A) also revealed a significant effect of experimental condition (F3,18=8.784, p<0.001). Post hoc analyses across recordings days found REM was reduced compared to BH on ST (p=0.002), CR1 (p=0.003) and CR2 (p=0.007).
Figure 2.
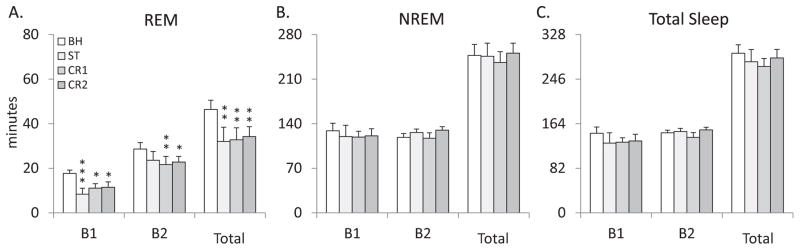
Comparisons of (A) total REM, (B) total NREM and (C) total sleep amounts for the dW (distilled water) control group plotted in 4 h blocks (B1 & B2) and for the total 8 h light period (total) across conditions. BH: baseline; ST: shock training; CR1: first context re-exposure; CR2: second context re-exposure. Error bars are ± SEM. *P<0.05; **P<0.01; and ***P<0.001 compared to BH.
The analysis of total REM for the MUS group (Figure 3A) found no significant alteration in total REM across experimental recording days for either blocks (F3,12 = 0.629, p=0.610) or the entire 8 h recording period (F3,16=0.637, p=0.602).
Figure 3.
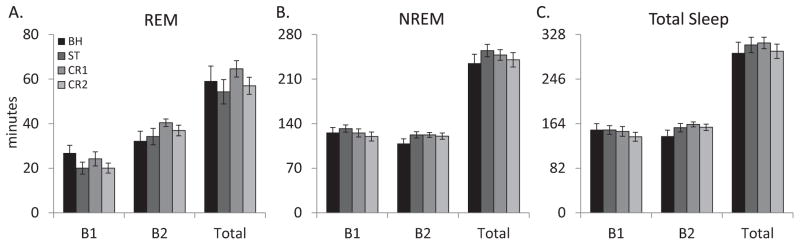
Comparisons of (A) total REM, (B) total NREM and (C) total sleep amounts for the MUS (muscimol) group plotted in 4 h blocks (B1 & B2) and for the total 8 h light period (total) across conditions. BH: baseline; ST: shock training; CR1: first context re-exposure; CR2: second context re-exposure. Error bars are ± SEM. There were no significant differences across conditions.
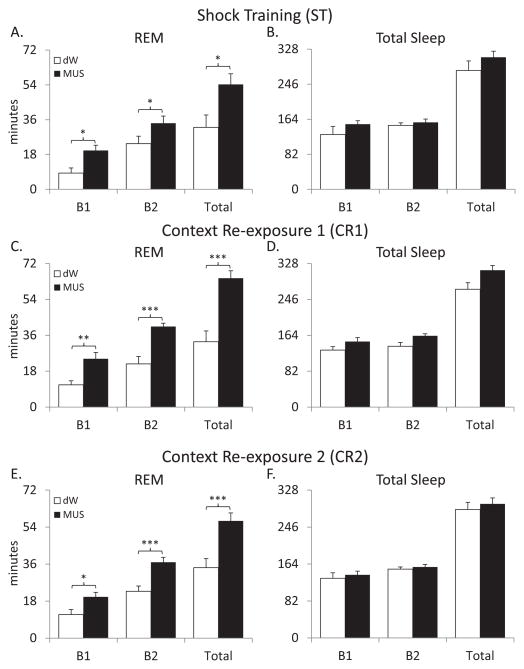
Comparing the dW and MUS groups revealed significant differences for total REM during B1 and B2 on ST (F1,12=6.964, p=0.022), CR1 (F1,11=21.274, p<0.001) and CR2 (F1,11=14.736, p<0.001). Total REM was significantly increased for the MUS group compared to the dW group on ST (B1 p=0.022, B2 p=0.036; Figure 4A), CR1 (B1 p=0.005, B2 p<0.001; Figure 4C) and CR2 (B1 p=0.021, B2 p<0.001; Figure 4E). An analysis between groups for total REM during the entire 8 h light period also found significant differences on ST (F1,12=6.964, p=0.022; Figure 4A), CR1 (F1,11=21.274, p<0.001; Figure 4C) and CR2 (F1,11=14.736, p<0.001; Figure 4E) with total REM being greater in the MUS group compared to the dW group.
Figure 4.
Comparisons between the dW (distilled water) control group and the MUS (muscimol) group for amounts of total REM and total sleep plotted in 4 h blocks (B1 & B2) and the total 8 h light period (total). (A) Total REM during shock training. (B) Total sleep during shock training. (C) Total REM during first context re-exposure. (D) Total sleep during first context re-exposure. (E) Total REM during second context re-exposure. (F) Total sleep during second context re-exposure. Error bars are ± SEM. *P<0.05; **P<0.01; and ***P<0.001, MUS compared to dW.
A complementary analysis of REM% showed virtually identical results to those observed for total REM (not shown).
An analysis of the number of REM episodes (Table 1) in blocks for the dW group found an effect of experimental condition (F3,18 = 6.076, p=0.005). Post hoc analyses of B1 found a significant decrease in the number or REM episodes in ST (p<0.001) and CR2 (p=0.005) compared to BH. There were no significant differences during B2.
Table 1.
Episodes of REM and NREM during 4 h blocks (B1-2) or total light period (Total) for the MUS and dW animals following BH, ST, CR1, & CR2.
| B1 | B2 | Total | |||
|---|---|---|---|---|---|
| REM episode | BH | dW | 10.6 ± 0.6 | 15.4 ± 1.6 | 26.0 ± 2.1 |
| MUS | 12.0 ± 1.0 | 15.7 ± 1.0 | 27.7 ± 1.5 | ||
| ST | dW | 5.1 ± 1.4 *** | 13.1 ± 1.8 | 18.3 ± 3.1 | |
| MUS | 8.4 ± 0.7 | 15.3 ± 0.9 | 23.7 ± 1.0 | ||
| CR1 | dW | 7.4 ± 1.1 | 13.6 ± 1.9 | 21.0 ± 2.7 | |
| MUS | 10.0 ± 1.2 | 15.8 ± 0.3 | 25.8 ± 1.2 | ||
| CR2 | dW | 6.1 ± 0.9 ** | 12.3 ± 1.5 + | 18.4 ± 2.1 | |
| MUS | 8.2 ± 0.8 | 15.8 ± 0.7 | 24.0 ± 0.8 |
| NREM episodes | BH | dW | 32.7 ± 1.6 | 38.0 ± 0.8 | 70.7 ± 1.8 |
| MUS | 36.9±2.9 | 34.9 ± 2.2 | 71.7 ± 4.9 | ||
| ST | dW | 26.1 ± 3.3 | 39 ± 2.3 | 65.1 ± 4.4 | |
| MUS | 33.3 ± 2.3 | 34.4 ± 2.8 | 67.7 ± 4.7 | ||
| CR1 | dW | 32.3 ± 2.4 | 43.1 ± 2.6 | 75.4 ± 3.1 | |
| MUS | 29.0 ± 1.5 | 30.0 ± 0.6 | 59.0 ± 1.7 | ||
| CR2 | dW | 29.1 ± 1.7 | 40.6 ± 3.4 | 69.7 ± 4.8 | |
| MUS | 26.2 ± 3.0 | 29.5 ± 3.2 | 55.7 ± 6.2 |
P<0.01 and
P<0.001 compared to BH.
P<0.05, MUS compared to dW.
The analysis of REM episodes for the MUS group found no significant effect of experimental condition (F3,12 = 1.114, p=0.587).
Comparisons between groups showed a significant effect of experimental condition for number of REM episodes during blocks on CR2 (F1,11=5.579, p=0.039). Post hoc analysis found no significant difference for B1 but a significant increase in the number of REM episodes in the MUS group compared to the dW group during B2 (p=0.03). No other comparisons were significant.
Non-rapid Eye Movement Sleep and Total Sleep
The analysis did not reveal any significant differences in total NREM for the data considered in 4 h blocks or total 8 h period for either the dW (Figure 2B) or MUS (Figure 3B) groups. The analysis of NREM episodes (Table 1) in blocks also found no effect of experimental condition for the dW group or the MUS group. However, the between groups analysis showed a significant effect of experimental condition for number of NREM episodes during blocks on CR1 (F1,11=16.265, p=0.002). Post hoc analysis found no significant difference for B1 but found a significant decrease in NREM episodes for the MUS group compared to the dW group for B2 (p<0.001). No other comparisons were significant.
The analyses for total sleep amount did not reveal any significant effects during the 4 h light period blocks or the entire 8 h light period for either the dW (Figure 2C) or MUS (Figure 3C) groups or for the between groups comparisons (Figures 4B,D,F).
Discussion
The current results demonstrate that pre-training inhibition of BLA with the GABAA agonist, muscimol, attenuates the reductions in REM seen immediately following ST. Pre-training inhibition of BLA also significantly attenuates conditioned changes in both freezing in wakefulness and in subsequent sleep indicating an alteration in fear memory. Thus, our results show that the BLA is an important site for mediating the effects of a stressful experience on sleep as well for forming fearful memories that can impact future behavior and sleep.
The Amygdala and Footshock-induced Alterations in Sleep
The amygdala is central in current concepts of fear conditioning (e.g., (Myers and Davis 2007)) and it has an established role in regulating fear- and stress-induced alterations in sleep, especially REM sleep (Liu et al. 2009; Wellman et al. 2013; Liu et al. 2011). Most of the work examining its role in regulating sleep has focused on the CNA, the source (Peyron et al. 1998; Price et al. 1987; Semba and Fibiger 1992; Inagaki et al. 1983), along with the lateral division of the bed nucleus of the stria terminalis (BNST) (Amaral et al. 1992; Davis and Whalen 2001), of descending output of the amygdala to brainstem REM regulatory regions. BLA has output to both CNA and BNST (Amaral et al. 1992; Davis and Whalen 2001) and likely regulates the influence of fearful experiences and memories on REM via these descending pathways.
In a previous study, we microinjected muscimol, bicuculline, a GABAA antagonist, or saline vehicle into CNA prior to training rats with inescapable footshock using a paradigm similar to that used in the present study (Liu et al. 2009). After saline vehicle, training with inescapable footshock selectively reduced electrographically defined REM much as in the control condition in the present study. Rats treated with muscimol also showed reduced REM whereas microinjection of bicuculline into CNA prior to inescapable shock attenuated the reduction in REM. These results contrast with our current finding that inhibition of BLA blocks the effects of inescapable shock on REM and indicates that the relative activity of BLA and CNA is linked to changes in sleep produced by footshock stress. This suggestion is also consistent with the results of studies using Fos protein as a marker of neural activation. In mice trained with inescapable footshock, BLA shows a significant increase in Fos activation whereas CNA is virtually devoid of Fos (Liu et al. 2003). Thus, with inescapable footshock stress, reduced REM appears to be associated with activation of BLA and inactivation of CNA.
Fear Memory and Sleep
Behavioral freezing is a standard measure of fear memory and greater freezing has been interpreted as indicating stronger fear reactions (Blanchard and Blanchard 1969a, b; Doyere et al. 2000; Paylor et al. 1994 ; Phillips and LeDoux 1992). Damage to, or inactivation of, BLA prior to or after fear conditioning has generally been found to reduce freezing during CR (e.g., (Cousens and Otto 1998; Koo et al. 2004; Maren 1998; Maren et al. 1996; Sacchetti et al. 1999). Our study replicates this core finding and demonstrates that blocking or reducing the strength of fear memory (as indicated by freezing) associated with inescapable footshock can also attenuate reductions in REM. We also have demonstrated that successful fear extinction (new learning that reduces fear without erasing the original fearful memory (Myers and Davis 2007)) is followed by a return of REM amounts to baseline levels (Wellman et al. 2008). Together, these results suggest a straightforward relationship between the presence or absence of fear behavior and alterations in REM. However, the actual relationship of fear behavior and fear memory with sleep is quite complex. Training in a single shock training paradigm typical of studies seeking to understand the mechanisms underlying fear memory formation has been reported to produce approximately a one h increase in NREM over the 24 h period after training, but no significant alteration in REM amounts (Hellman and Abel 2007). We have also found that limited shock training produces much smaller changes in sleep and generally extinguish quite readily (Sanford et al. 2003). Additionally, although extensive training with and contextual reminders of inescapable shock significantly reduce REM, training with and contextual reminders of escapable shock can produce significant increases in REM sleep (Yang et al. 2011; Sanford et al. 2010). Indices of fear (freezing) and stress (stress-induced hyperthermia) are similar for both types of training (Yang et al. 2011). Thus, the actual impact of fear conditioning on sleep can vary with the amount of training and whether the unconditioned stimulus is uncontrollable (inescapable shock) or controllable (escapable shock).
Whether or not fear behavior in wakefulness is linked to alterations in subsequent sleep appears to be regulated by the amygdala. In rats trained with inescapable shock, microinjections of the CRF antagonist, antalarmin, into CNA prior to CR attenuates subsequent REM reductions (Liu et al. 2011) and microinjections of antalarmin into BLA prior to shock training block both shock-induced reductions in REM sleep and subsequent reductions in REM that typically occur after CR (Wellman et al. 2013). However, freezing is not altered with microinjections of antalarmin into either CNA or BLA. By comparison, the present study demonstrates that global inactivation of BLA can block the formation of memories that can later produce both freezing and alterations in sleep. Together, these data indicate that the amygdala in involved in the expression of fear-related changes in sleep through CNA and that processes in BLA determine whether fear memories will alter sleep or not. A critical corollary of this, that fits the data from studies using differing amounts of shock training and inescapable and escapable shock as the unconditioned stimulus, is that fearful situations and fear responses are not all the same. They can produce similar overt behaviors and similar stress responses, but the differences in subsequent sleep indicates neurobiological differences that likely have significance for whether the experience and resultant memories have harmful consequences for the individual.
Fear Circuitry and Sleep
Because stress- and fear-related conditioning processes are thought to play significant roles in the development of emotional disorders (Charney and Deutch 1996; Pitman et al. 2001; Foa et al. 1992; Grillon et al. 1996; Shalev et al. 1992), there has been tremendous effort aimed at delineating the neural circuitry and mechanisms underlying fear conditioning. Most studies have measured immediate responses to fearful cues or contexts such as behavioral freezing (Blanchard and Blanchard 1969a; Paylor et al. 1994 ; Phillips and LeDoux 1992), autonomic responses (e.g., increased heart rate (Davis 1992)) or the ability of fearful cues or contexts to modify responses to other stimuli (e.g., fear-potentiated startle (Davis 1990; Davis 1992 )). Consequently, we now have a general understanding of the brain regions that regulate fearful behavior in wakefulness though there continues to be refinement of, and corrections to, models of fear circuitry as new findings become available (e.g., (Pare et al. 2004)). However, current concepts of fear circuitry and fear memory do not explain the effects of fear on sleep, nor do they explain the dissociation that can occur between fear behaviors in wakefulness and fear-induced alterations in sleep.
An example of the failure of current models to explain fear-induced changes in sleep is found in CNA regulation of fear behavior in wakefulness and subsequent reductions in REM produced by IS. In current fear models, activation of CNA induces the generation of fear behavior and physiological responses via descending brainstem projections (Duvarci et al. 2011). However, as discussed above, inhibition of the CNA suppresses REM whereas its activation (e.g., with electrical stimulation (Smith and Miskiman 1975)) can promote REM in some situations. Fortunately, recent studies may resolve some of this discrepancy. CNA neurons do, in fact, fire in response to footshock (Rosenkranz et al. 2006) and in response to conditioned stimuli (Duvarci et al. 2011). However, CNA is regulated by the BLA and lateral amygdala (Rosenkranz et al. 2006). Regulation of CNA output can be direct via excitatory glutamatergic projections from BLA and indirect through glutamatergic excitation of GABAergic neurons in the intercalated or lateral CNA neurons that project to descending outputs originating in the medial CNA (Reviewed in (Amano et al. 2010)). Thus, it is possible that CNA activation during fearful/stressful events regulates fear behavior in wakefulness, but afterward, with some stressors, can be inhibited to decrease REM in the post-stress period.
Other brain areas important for fear memory include the medial prefrontal cortex (mPFC) and the hippocampus. The mPFC has a role in mediating fear extinction (Quirk et al. 2006) and the effects of stressor controllability (Maier et al. 2006). The amygdala and mPFC interact with and influence each other (McEwen 2007) and they both can influence pontine regions involved with generating and regulating REM sleep (e.g., locus coeruleus, dorsal raphe and reticularis pontis oralis). Both also interact with the hippocampus which is important in contextual fear memory (LeDoux 2000). These interactions likely play significant roles in mediating the effects of fear and fearful memories on sleep and arousal, possibly via influences on the amygdala that vary with situational factors that modulate fearful experiences.
Conditioned Fear, Sleep and Models of Psychopathology
More than two-thirds of the general population is expected to be exposed to at least one traumatic event over their lifetime (Breslau et al. 1998) and there are estimates that the prevalence of PTSD in the general population ranges from 1% to 10% (Breslau et al. 1998) with higher estimates reported in victims of interpersonal violence (20–30%) (Breslau et al. 1998) and combat veterans (15–30%) (Weiss et al. 1992). Estimates for victims of rape are up to 80% (Javidi and Yadollahie 2012). Because PTSD is viewed as a disorder of the brain’s fear system (Shvil et al. 2013), experimental fear conditioning is one of our most important research models related to PTSD as well as to other anxiety disorders. However, it is important to note that although fear conditioning plays a role in psychopathology, it normally underlies adaptive behavior that extinguishes when the fear-inducing stimulus is withdrawn (Breslau et al. 1998). It is the failure of fear extinction that has been linked to persisting symptoms of PTSD (Myers and Davis 2007). Thus, experimental paradigms using brief stressors which have easily extinguished fear responses likely involve adaptive fear whereas significantly stronger stressors are likely needed to produce fear that is resistant to extinction. However, at present there is no clear way to distinguish adaptive from maladaptive fear responses.
Given that persisting post-trauma subjective sleep disturbances are linked to the development of PTSD (Koren et al. 2002), the potential for dissociation between fear behavior and sleep provides a unique opportunity to establish the functional role that sleep may have in processing emotional memories and in determining whether or not they lead to psychopathology. The increase in REM sleep after training with escapable shock (a controllable stressor) and after fear extinction suggests that it may play a positive functional role in the processing of fearful emotion and memories. This hypothesis is supported by the fact that controllable stress is typically associated with adaptive coping and neutral or positive outcomes (Foa et al. 1992) and that fear extinction is an adaptive modification of fear memory when the source of the fear is no longer present. It also is consistent with suggestions that REM plays roles in “decoupling” memory from its emotional charge (Walker and van der Helm 2009) and in the processing of traumatic memories (Mellman et al. 2002; Mellman et al. 2007). Considering alterations in sleep produced by different models of fear conditioning may thus aid in determining which experiences are likely to lead to pathological changes in the brain and behavior and could provide models for assessing the functional role of sleep in processing emotional experiences.
Conclusion
GABAergic inactivation of BLA prior to ST attenuated reductions in REM that occur following inescapable footshock as well those following CR. Freezing during CR was also attenuated suggesting a significant weakening of fear memory. These results along with prior findings that antagonizing CRF1 receptors in BLA can block shock- and fear-induced reductions in REM without altering freezing indicate that BLA is a critical region for mediating the effects of stress on sleep as well as for the formation of fear memories that can impact sleep. Additional work is required to delineate the processes in BLA that enable fearful memories to alter sleep and to assess the potential role that sleep may have in mediating the long-term effects of emotional memories.
Acknowledgments
This work was supported by NIH research grant MH64827.
References
- Abercrombie ED, Jacobs BL. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. I. Acutely presented stressful and nonstressful stimuli. J Neurosci. 1987a;7 (9):2837–2843. doi: 10.1523/JNEUROSCI.07-09-02837.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abercrombie ED, Jacobs BL. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. II. Adaptation to chronically presented stressful stimuli. J Neurosci. 1987b;7 (9):2844–2848. doi: 10.1523/JNEUROSCI.07-09-02844.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T, Unal CT, Pare D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci. 2010;13 (4):489–494. doi: 10.1038/nn.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D, Price J, Pitkanen A, Carmichael S. Anatomical organization of the primate amydaloid complex. In: Aggleton J, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Wiley-Liss, Inc; New York: 1992. pp. 1–66. [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969a;67 (3):370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Passive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol. 1969b;68 (1):129–135. doi: 10.1037/h0027676. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med. 2005;35 (6):791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55 (7):626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Charney D, Deutch A. A functional neuroanatomy of anxiety and fear: implications for the pathophysiology and treatment of anxiety disorders. Critical Rev Neurobiol. 1996;10:419–446. doi: 10.1615/critrevneurobiol.v10.i3-4.70. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Zangenehpour S, Rahbar-Dehgan F, Ye F. Molecular maps of neural activity and quiescence. Acta Neurobiol Exp (Wars) 2000;60 (3):403–410. doi: 10.55782/ane-2000-1359. [DOI] [PubMed] [Google Scholar]
- Cousens G, Otto T. Both pre- and posttraining excitotoxic lesions of the basolateral amygdala abolish the expression of olfactory and contextual fear conditioning. Behav Neurosci. 1998;112 (5):1092–1103. doi: 10.1037//0735-7044.112.5.1092. [DOI] [PubMed] [Google Scholar]
- Davis M. Animal models of anxiety based on classical conditioning: the conditioned emotional response (CER) and the fear-potentiated startle effect. Pharmaco Therapeut. 1990;47:147–165. doi: 10.1016/0163-7258(90)90084-f. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in conditioned fear. In: Aggleton J, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Wiley-Liss, Inc; New York: 1992. pp. 255–305. [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Ann Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular psychiatry. 2001;6 (1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Doyere V, Gisquet-Verrier P, de Marsanich B, Ammassari-Teule M. Age-related modifications of contextual information processing in rats: role of emotional reactivity, arousal and testing procedure. Behav Brain Res. 2000;114 (1–2):153–165. doi: 10.1016/s0166-4328(00)00223-0. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Popa D, Pare D. Central amygdala activity during fear conditioning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31 (1):289–294. doi: 10.1523/JNEUROSCI.4985-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farivar R, Zangenehpour S, Chaudhuri A. Cellular-resolution activity mapping of the brain using immediate-early gene expression. Front Biosci. 2004;9:104–109. doi: 10.2741/1198. [DOI] [PubMed] [Google Scholar]
- Foa EB, Zinbarg R, Rothbaum BO. Uncontrollability and unpredictability in post-traumatic stress disorder: an animal model. Psychol Bull. 1992;112 (2):218–238. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- Grillon C, Southwick SM, Charney DS. The psychobiological basis of posttraumatic stress disorder. Molecular psychiatry. 1996;1 (4):278–297. [PubMed] [Google Scholar]
- Hellman K, Abel T. Fear conditioning increases NREM sleep. Behav Neurosci. 2007;121 (2):310–323. doi: 10.1037/0735-7044.121.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ, Bellgowan PS. Effects of muscimol applied to the basolateral amygdala on acquisition and expression of contextual fear conditioning in rats. Behav Neurosci. 1994;108 (5):1005–1009. doi: 10.1037//0735-7044.108.5.1005. [DOI] [PubMed] [Google Scholar]
- Inagaki S, Kawai Y, Matsuzaki T, Shiosaka S, Tohyama M. Precise terminal fields of the descending somatostatinergic neuron system from the amygdaloid complex of the rat. J Hirnforsch. 1983;24 (3):345–356. [PubMed] [Google Scholar]
- Javidi H, Yadollahie M. Post-traumatic Stress Disorder. Int J Occup Environ Med. 2012;3 (1):2–9. [PubMed] [Google Scholar]
- Koo JW, Han JS, Kim JJ. Selective neurotoxic lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J Neurosci. 2004;24 (35):7654–7662. doi: 10.1523/JNEUROSCI.1644-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren D, Arnon I, Lavie P, Klein E. Sleep complaints as early predictors of posttraumatic stress disorder: a 1-year prospective study of injured survivors of motor vehicle accidents. Am J Psychiatry. 2002;159:855–857. doi: 10.1176/appi.ajp.159.5.855. [DOI] [PubMed] [Google Scholar]
- Kruger L, Saporta S, Swanson L. Photographic atlas of the rat brain. Cambridge University Press; New York: 1995. [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Liu X, Yang L. SL (2006) GABA antagonism of the central nucleus of the amygdala (CNA) attenuates reductions in rapid eye movement sleep (REM) after footshock stress. Sleep. 2006;29:A12. doi: 10.1093/sleep/32.7.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Tang X, Sanford LD. Fear-conditioned suppression of REM sleep: relationship to Fos expression patterns in limbic and brainstem regions in BALB/cJ mice. Brain Res. 2003;991 (1–2):1–17. doi: 10.1016/j.brainres.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Liu X, Wellman LL, Yang L, Ambrozewicz MA, Tang X, Sanford LD. Antagonizing corticotropin-releasing factor in the central nucleus of the amygdala attenuates fear-induced reductions in sleep but not freezing. Sleep. 2011;34 (11):1539–1549. doi: 10.5665/sleep.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yang L, Wellman LL, Tang X, Sanford LD. GABAergic antagonism of the central nucleus of the amygdala attenuates reductions in rapid eye movement sleep after inescapable footshock stress. Sleep. 2009;32 (7):888–896. doi: 10.1093/sleep/32.7.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Amat J, Baratta MV, Paul E, Watkins LR. Behavioral control, the medial prefrontal cortex, and resilience. Dialogues in clinical neuroscience. 2006;8 (4):397–406. doi: 10.31887/DCNS.2006.8.4/smaier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Overtraining does not mitigate contextual fear conditioning deficits produced by neurotoxic lesions of the basolateral amygdala. J Neurosci. 1998;18 (8):3088–3097. doi: 10.1523/JNEUROSCI.18-08-03088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Retrograde abolition of conditional fear after excitotoxic lesions in the basolateral amygdala of rats: absence of a temporal gradient. Behav Neurosci. 1996;110 (4):718–726. doi: 10.1037//0735-7044.110.4.718. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87 (3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159 (10):1696–1701. doi: 10.1176/appi.ajp.159.10.1696. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Pigeon WR, Nowell PD, Nolan B. Relationships between REM sleep findings and PTSD symptoms during the early aftermath of trauma. J Trauma Stress. 2007;20 (5):893–901. doi: 10.1002/jts.20246. [DOI] [PubMed] [Google Scholar]
- Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111 (4):683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12 (2):120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92 (1):1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Paylor R, Tracy R, Wehner J, Rudy J. DBA/2 and C57BL/6 mice differ in contextual fear but not auditory fear conditioning. Behav Neurosci. 1994;108:810–817. doi: 10.1037//0735-7044.108.4.810. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82 (2):443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106 (2):274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Shin LM, Rauch SL. Investigating the pathogenesis of posttraumatic stress disorder with neuroimaging. J Clin Psychiatry. 2001;62(Suppl 17):47–54. [PubMed] [Google Scholar]
- Price J, Russchen F, Amaral D. The limbic region. II: The amygdaloid complex. In: Swanson L, editor. Handbook of chemical neuroanatomy. Integrated systems of the CNA, Part I. Elsevier; New York: 1987. pp. 279–375. [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60 (4):337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Buffalari DM, Grace AA. Opposing influence of basolateral amygdala and footshock stimulation on neurons of the central amygdala. Biological psychiatry. 2006;59 (9):801–811. doi: 10.1016/j.biopsych.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Lorenzini CA, Baldi E, Tassoni G, Bucherelli C. Auditory thalamus, dorsal hippocampus, basolateral amygdala, and perirhinal cortex role in the consolidation of conditioned freezing to context and to acoustic conditioned stimulus in the rat. J Neurosci. 1999;19 (21):9570–9578. doi: 10.1523/JNEUROSCI.19-21-09570.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford LD, Fang J, Tang X. Sleep after differing amounts of conditioned fear training in BALB/cJ mice. Behav Brain Res. 2003;147 (1–2):193–202. doi: 10.1016/s0166-4328(03)00180-3. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Yang L, Wellman LL, Liu X, Tang X. Differential effects of controllable and uncontrollable footshock stress on sleep in mice. Sleep. 2010;33 (5):621–630. doi: 10.1093/sleep/33.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba K, Fibiger HC. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. The Journal of comparative neurology. 1992;323 (3):387–410. doi: 10.1002/cne.903230307. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Ragel-Fuchs Y, Pitman RK. Conditioned fear and psychological trauma. Biol Psychiatry. 1992;31 (9):863–865. doi: 10.1016/0006-3223(92)90113-e. [DOI] [PubMed] [Google Scholar]
- Shvil E, Rusch HL, Sullivan GM, Neria Y. Neural, psychophysiological, and behavioral markers of fear processing in PTSD: a review of the literature. Curr Psychiatry Rep. 2013;15 (5):358. doi: 10.1007/s11920-013-0358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CT, Miskiman DE. Increases in paradoxical sleep as a result of amygdaloid stimulation. Physiol Behav. 1975;15 (1):17–19. doi: 10.1016/0031-9384(75)90272-3. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCarley R. Brainstem Control of Wakefulness. Sleep Plenum Press; New York: 1990. [Google Scholar]
- Walker MP, van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135 (5):731–748. doi: 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss DS, Marmar CR, Schlenger WE, Fairbank JA, Jordan BK, Hough RL, et al. The prevalence of lifetime and partial post-traumatic stress disorder in Vietnam theater veterans. J Trauma Stress. 1992;5:365–376. [Google Scholar]
- Wellman LL, Yang L, Ambrozewicz MA, Machida M, Sanford LD. Basolateral amygdala and the regulation of fear-conditioned changes in sleep: role of corticotropin-releasing factor. Sleep. 2013;36 (4):471–480. doi: 10.5665/sleep.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman LL, Yang L, Tang X, Sanford LD. Contextual fear extinction ameliorates sleep disturbances found following fear conditioning in rats. Sleep. 2008;31 (7):1035–1042. [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, LeDoux JE. The Amygdala Modulates Memory Consolidation of Fear-Motivated Inhibitory Avoidance Learning But Not Classical Fear Conditioning. J Neurosci. 2000;20 (18):7059–7066. doi: 10.1523/JNEUROSCI.20-18-07059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Wellman LL, Ambrozewicz MA, Sanford LD. Effects of stressor predictability and controllability on sleep, temperature, and fear behavior in mice. Sleep. 2011;34 (6):759–771. doi: 10.5665/SLEEP.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]