Abstract
Calcific aortic stenosis (AS) is a progressive disease with no effective medical therapy that ultimately requires aortic valve replacement (AVR) for severe valve obstruction. Echocardiography is the primary diagnostic approach to define valve anatomy, measure AS severity and evaluate the left ventricular (LV) response to chronic pressure overload. In asymptomatic patients, markers of disease progression include the degree of leaflet calcification, hemodynamic severity of stenosis, adverse LV remodeling, reduced LV longitudinal strain, myocardial fibrosis and pulmonary hypertension. The onset of symptoms portends a predictably high mortality rate unless AVR is performed. In symptomatic patients, AVR improves symptoms, improves survival and, in patients with LV dysfunction, improves systolic function. Poor outcomes after AVR are associated with low-flow low-gradient AS, severe ventricular fibrosis, oxygen dependent lung disease, frailty, advanced renal dysfunction and a high comorbidity score. However, in most patients with severe symptoms, AVR is lifesaving. Bioprosthetic valves are recommended for patients over the age of 65 years. Transcatheter AVR is now available for patients with severe comorbidities, is recommended in patients who are deemed inoperable and is a reasonable alternative to surgical AVR in high risk patients.
Keywords: Aortic stenosis, valve replacement, outcome, pressure overload
The prevalence of calcific aortic valve disease approaches 25% of all adults over 65 years of age. Most of these patients have only mild focal valve thickening, or aortic valve sclerosis, with normal valve function, but a significant number, ranging from 2-5% of all older adults, have significant aortic stenosis (AS) with obstruction to left ventricular (LV) outflow.1-3 Aortic sclerosis progresses to mild AS in less than 15% of patients over 2-7 years.4,5 However, once even mild valve obstruction is present, hemodynamic progression is common, leading to severe symptomatic AS that requires aortic valve replacement (AVR).6,7 An increasing number of older adults with calcific AS will be seen over the next few decades given worldwide demographics of an aging population.
Diagnosis
Clinical presentation
The initial diagnosis of calcific AS typically is based on detection of a systolic murmur followed by echocardiographic confirmation; in some cases, AS is first recognized on echocardiography requested for other indications. Most patients are diagnosed long before the onset of symptoms and are followed prospectively on a regular basis until AVR is indicated.8 Others present with symptoms, including exertional dyspnea, heart failure, angina or syncope, and require intervention soon after diagnosis. A smaller subset of patients presents with advanced disease with critical valve obstruction resulting in severe LV systolic dysfunction due to the high afterload imposed by the stenotic valve.
Valve anatomy
Transthoracic echocardiography is the primary diagnostic modality for evaluating aortic valve anatomy, AS severity and the LV response to chronic pressure overload. A congenital bicuspid valve is diagnosed accurately when short axis 2D or 3D images show two leaflets in systole; diastolic images are unreliable as a bicuspid valve with a raphe in one leaflet may be mistaken for a trileaflet valve and vice versa. Determination of the number of valve leaflets is more problematic once significant calcification is present.9 Rheumatic AS is distinguished by commissural fusion and mitral valve involvement.
Hemodynamic severity
Standard measures of AS severity are the maximum velocity (Vmax) across the stenotic valve, the mean transaortic pressure gradient (ΔPmean) calculated with the Bernoulli equation, and the functional aortic valve area (AVA), calculated with the continuity equation (Table 1 and Figure 1). Echocardiographic ΔPmean and AVA calculations have been well validated against invasive measurements and are now the clinical standard of care.8,10 AVA calculation is especially important when transaortic volume flow rate is higher than normal (as with coexisting aortic regurgitation) or lower than normal (as with LV dysfunction or a small normally functioning left ventricle) because transaortic velocities and gradients vary with volume flow rate. In patients with mixed stenosis and regurgitation, the diagnosis of severe valve disease will be evident based on a high transaortic gradient. However, with a low transaortic flow rate, severe AS might be missed if only velocity or pressure gradient data are considered.
Table 1. Classification of Aortic Stenosis Severity.
| Valve anatomy | Aortic velocity (m/s) | Mean gradient (mm Hg) | Aortic valve area (cm2) | Other measures | |
|---|---|---|---|---|---|
| Aortic sclerosis | Focal leaflet thickening with normal leaflet motion | < 2.5 | < 10 | 3 -4 cm2 | |
| Mild AS | Mild leaflet thickening with mildly reduced motion | 2.5 – 3 | 10-20 | 1.5-3.0 | |
| Moderate AS | Mild-moderate Ca++ with moderately reduced leaflet motion | 3-4 | 20-40 | 1.0-1.5 | |
| Severe AS | Moderate-severe Ca++ with little leaflet motion | > 4 | > 40 | < 1.0 | Indexed AVA < 0.6 cm2/m2 Velocity ratio < 0.25 |
| Low-output low-gradient severe AS with reduced EF | Severe valve Ca++, reduced leaflet motion | ||||
| Rest | 3-4 | 20-40 | < 1.0 | ||
| Dobutamine stress | > 4 | > 40 | < 1.0 | ||
| Low-output low-gradient severe AS with normal EF | Severe valve Ca++, reduced leaflet motion | 3-4 | 20-40 | <1.0 | SVi <35 ml/m2, LVH, small LV chamber, reduced longitudinal systolic strain, increased Zva |
| Critical AS | Severe Ca++ with immobile leaflets | > 5 | > 60 | < 0.6 |
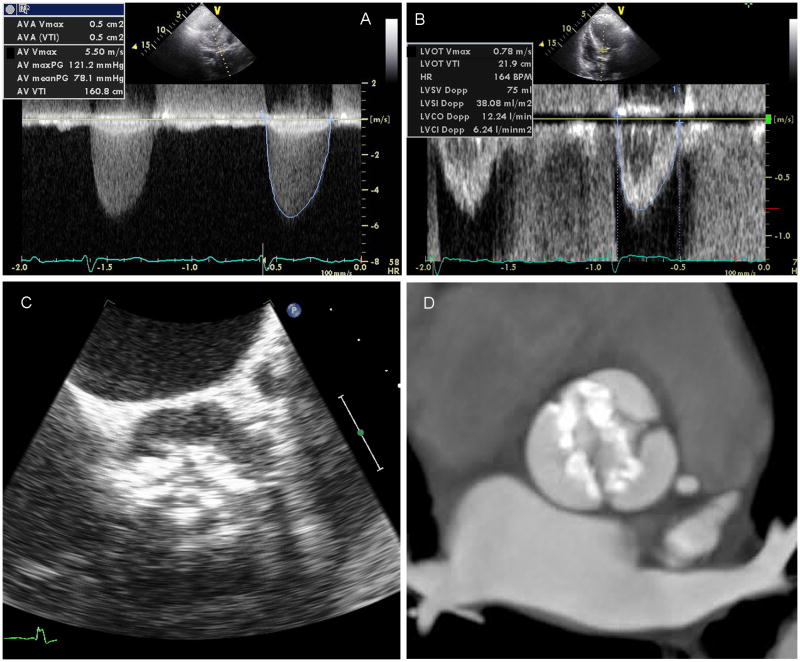
Figure 1. Diagnostic Imaging of Aortic Stenosis.
Transvalvular mean and peak gradients and the aortic valve VTI are obtained by continuous wave Doppler across the aortic valve (a). The LV outflow tract VTI is obtained from pulse wave Doppler in the LV outflow tract (b) and used along with the aortic valve VTI to calculate the AVA by the continuity equation. The valve morphology, opening, and calcification can be seen clearly on transesophageal echocardiographic imaging (c) and the amount of calcification in the valve leaflets quantified by CT (d).
Some clinicians index AVA to body size to account for expected smaller valve areas in smaller patients. However, indexing AVA to body size may not be appropriate in overweight or obese patients. Instead, the ratio of LV outflow to aortic velocity may be useful because this ratio is, in effect, indexed to the patient's own LV outflow tract size. A normal ratio is close to 1.0 with a ratio of 0.25 indicating a valve area 25% of normal. Direct planimetry of AVA is sometimes possible, particularly with transesophageal or 3D imaging, but overall accuracy of this approach is limited due to the nonplanar anatomy of the stenotic valve and to calcification-related acoustic shadowing and reverberations.
Cardiac magnetic resonance (CMR) imaging is useful when echocardiographic data are suboptimal or conflicting with clinical assessment of AS severity. CMR can distinguish between bicuspid or trileaflet valve anatomy and provide accurate assessment of peak jet velocity. CMR is also valuable for assessing aortic root and ascending aortic anatomy in patients with a bicuspid valve.
Computed tomography (CT) imaging provides an alternate approach for planimetry of valve area and provides quantitative measures of valve calcification. In patients undergoing transcatheter aortic valve replacement (TAVR), multimodality imaging includes CT assessment of aortic annulus size and shape, leaflet length, and the annular to coronary ostial distance.
Diagnostic cardiac catheterization is rarely needed for adults with AS but should be considered when echocardiographic and other noninvasive data are nondiagnostic or when there are discrepancies between noninvasive findings and other clinical data. When transaortic pressure measurements are made during catheterization, the phenomenon of pressure recovery—a higher pressure in the aorta distal to the valve than in the valve orifice itself—may create confusion if not recognized because Doppler measures the gradient at the orifice level. Pressure recovery is most likely to be seen in patients with a small aorta and systolic doming of a congenitally stenotic valve.
LV response to chronic pressure overload
LV volumes, mass and systolic function can be measured using 2D or 3D quantitative echocardiography. AS results in increased LV systolic pressure leading to increased myocardial cell mass and interstitial fibrosis. In compensated disease, this increase in LV wall thickness allows wall stress to remain relatively normal with a normal ejection fraction (EF) and normal cardiac output.11 Reduced LVEF or cardiac output occurs only in end-stage disease and usually is preceded by clinical symptoms, although LV diastolic dysfunction occurs earlier in the disease course (Figure 2). Metabolic abnormalities adversely affect hypertrophic LV remodeling and LV function in patients with AS.12,13
Figure 2. Left Ventricular Response to Pressure Overload from Aortic Stenosis.
All images are from patients with AVA <1 cm2 and preserved ejection fraction (EF >50%). Hypertrophic remodeling of the LV frequently leads to marked concentric LVH (arrow) (a). Diastolic dysfunction commonly develops as shown by mitral valve inflow with shortened deceleration time of 130 msec indicating restrictive diastolic filling (b) and reduced tissue Doppler e′ (arrow) at the septal annulus of 3 cm/sec (c). Although EF may be preserved, systolic dysfunction is demonstrated by reduced LV longitudinal systolic strain shown as color coded speckle tracking in a long axis view (d) or in a map of the LV with the apex in the center and the base at the circumference of a circle (e) (normal LV longitudinal systolic strain is closer to - 20%). Myocardial fibrosis contributes to systolic and diastolic dysfunction, shown by picrosirius red staining of LV tissue from a patient with AS undergoing valve replacement (f).
Other diagnostic considerations
Aortic dilation associated with AS
Dilation of the aortic sinuses and ascending aorta frequently accompanies calcific AS, particularly in those with bicuspid aortic valve disease. Aortic dilation is not primarily due to abnormal valve hemodynamics—in bicuspid valve patients there likely are underlying genetic factors leading to abnormal cell signaling in the aortic wall and in those with a trileaflet valve, atherosclerotic risk factors, including hypertension, play a role. CT or CMR imaging of the aorta is recommended in bicuspid valve patients unless the ascending aorta distal to the sinotubular junction is well visualized on echocardiography. Additional imaging also is appropriate adults with calcific trileaflet AS if echocardiography shows an aortic diameter over 4 cm.14
Coronary artery disease associated with AS
Significant coronary artery disease (CAD) is present in about 50% of adults with severe symptomatic AS. Unfortunately, stress testing with perfusion imaging or echocardiography has a low accuracy for diagnosis of CAD and is contraindicated if any cardiac symptoms are present, so that coronary angiography is recommended when CAD is a concern.14 CT coronary angiography may be a reasonable alternative to invasive coronary angiography in some cases, particularly in younger patients.
Definition of Severe AS
Clinical implications
With progressive calcific AS, LV outflow obstruction eventually results in clinical symptoms particularly with exercise, due to inadequate forward output and/or increased LV filling pressures caused by hypertrophic LV remodeling. Symptom onset portends a high mortality rate unless outflow obstruction is relieved by AVR.8 However, the exact degree of valve obstruction associated with symptom onset is variable among patients. Thus, it is challenging to define “severe AS” using any single numerical value; instead, hemodynamic measures must be considered in the context of symptoms, valve anatomy, and the LV response to chronic pressure overload.
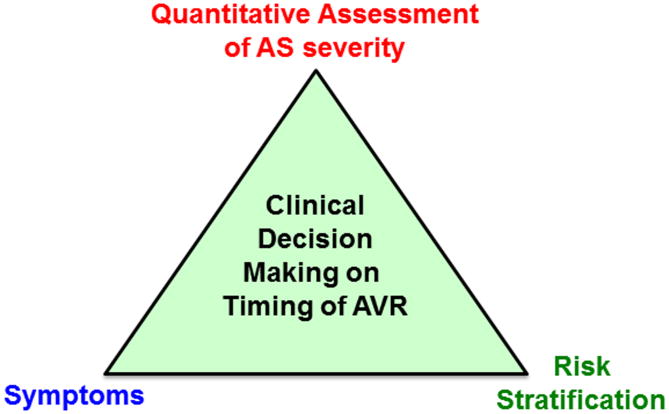
Even so, it is useful to provide a hemodynamic definition for severe AS to guide decision making in two clinical situations (Figure 3): (1) the asymptomatic patient with AS at high risk for adverse outcomes and progressive disease who might benefit from preemptive AVR prior to symptom onset; and (2) the patient with symptoms possibly due to AS to determine if symptoms are caused by valve obstruction so that AVR would be beneficial.
Figure 3. Clinical Decision Making on Timing of AVR.
Decisions about whether and when to recommend aortic valve replacement (AVR) are based upon integrating these factors.
Hemodynamically severe AS
Current definitions of severe AS are based on prospective studies showing that the Vmax is the strongest predictor of symptom onset and clinical outcomes (Figure 4). In adults with calcific AS and an Vmax >4 m/s, 70 to 80% develop symptoms requiring AVR within 2 years compared to symptom onset in 25-35% of those with a Vmax between 3 and 4 m/s and only 15% of those with a Vmax <3 m/s.6,7,15-17 Higher Vmax values (>5 or 5.5 m/s) are associated with even higher rates of symptom onset.18 In symptomatic patients, other studies have shown that a Vmax >4 m/s or <3 m/s reliably identifies those who do or do not require AVR.19 Thus, a Vmax >4 m/s, corresponding to a mean gradient >40 mmHg, is generally considered severe AS, and a Vmax <3 m/s, corresponding to a mean gradient of 20 mmHg, denotes mild AS.
Figure 4. Outcomes for Patients with Aortic Stenosis by Jet Velocity.
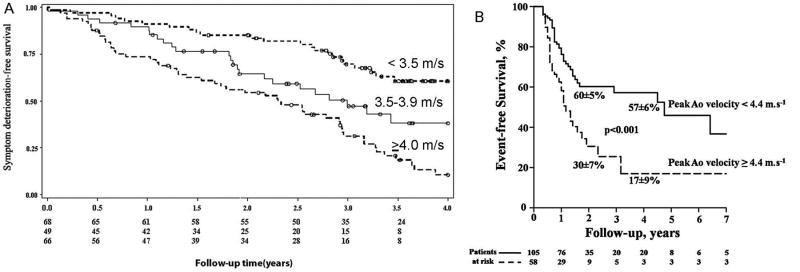
(A) Kaplan–Meier plot for survival free of symptoms of aortic stenosis by peak aortic velocity <3.50 m/s, 3.50–4.00 cm/s, and >4.00 cm/s (log rank P < 0.0001) in a study of 183 initially asymptomatic adults with moderate to severe aortic stenosis and normal left ventricular systolic function. From Stewart RA, et al.16 Reprinted with permission. (B) Kaplan–Meier event-free survival curves according to maximum aortic velocity in 163 initially asymptomatic aortic stenosis patients with a normal LV ejection fraction and an indexed AVA of 0.6 cm2/m2 or less. The mean±SD survival rates at two and 4 years are indicated. From Lancellotti et al.42 Reprinted with permission.
Although recommended in all AS patients, calculation of AVA is essential when Vmax is between 3 and 4 m/s (Figure 5). Most of these patients have moderate AS with an AVA between 1.0 and 1.5 cm2, but some have severe AS with an AVA <1.0 cm2 in the setting of a low transaortic volume flow rate.
Figure 5. Approach to the Diagnosis of Aortic Stenosis.
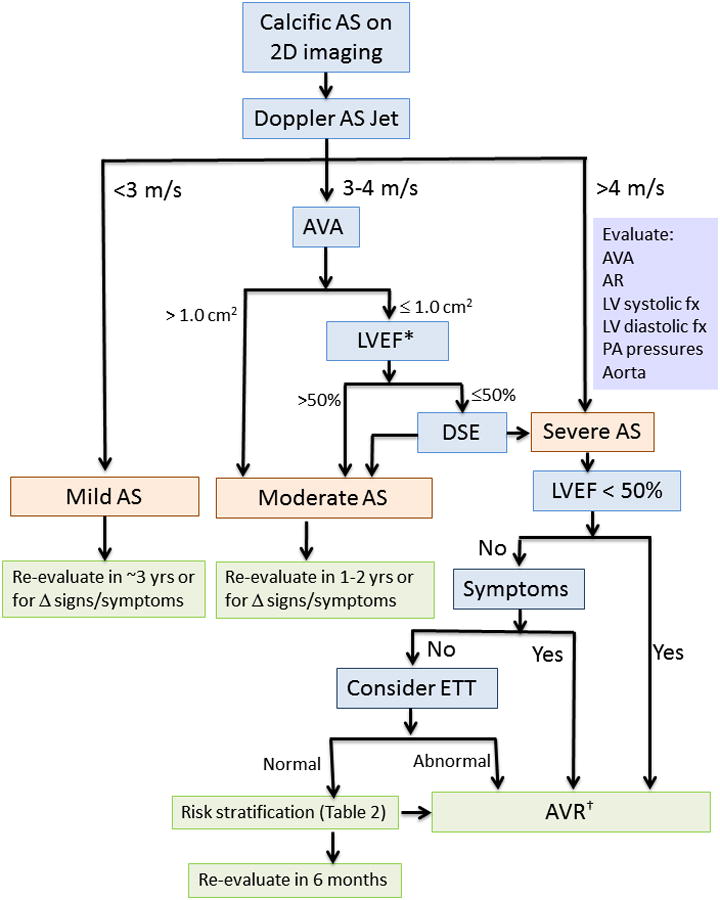
2D, two-dimensional; AS, aortic stenosis; AVA, aortic valve area; AR, aortic regurgitation; AVR, aortic valve replacement; DSE, dobutamine stress echocardiography; ETT, exercise treadmill testing; LV, left ventricular; LVEF, left ventricular ejection fraction; PA pulmonary artery.
* A subset of patients presents with low flow, low gradient severe AS with preserved EF, characterized by a stroke volume index <35 ml/m2 and usually accompanied by LVH, a very calcified valve, small LV chamber, and reduced longitudinal systolic strain. See text for details.
† Surgical AVR is appropriate in most patients. Transcatheter AVR is recommended in inoperable patients and may be reasonable in patients with high surgical risk.
Low flow, low gradient, low EF
In the symptomatic adult with AS who also has a low transaortic volume flow rate, the definition of severe AS is particularly problematic. When LVEF is reduced (<50%), heart failure symptoms may be due to primary LV dysfunction, valve obstruction, or their combination. Dobutamine stress echocardiography is helpful in this situation—specifically in patients with an EF <50%, and a small calculated AVA despite a Vmax <4 m/s—in order to separate patients with moderate AS and primary LV dysfunction from those with severe AS and a low EF due to afterload mismatch. AVR will not be helpful in relieving symptoms in the former patients, but will be lifesaving in the latter patients. Dobutamine is infused at incremental doses with the goal of increasing transaortic flow rate to the normal range. Severe AS is present if Vmax increases to over 4 m/s and AVA remains <1.0 cm2 at a normal flow rate; these patients benefit from AVR. Those with an increase in AVA to over 1.0 cm2 or with a maximum Vmax <4 m/s have moderate AS and medical therapy is appropriate. Lack of contractile reserve, defined as an increase in transaortic stroke volume or EF of <20% with dobutamine infusion, is associated with high cardiovascular mortality regardless of treatment,20,21 although outcomes appear to be somewhat better with AVR than with medical therapy22 (see risk stratification section).
Low flow, low gradient, preserved EF
Recent studies suggest that between 10 and 35% of adults with symptomatic AS present with paradoxical low flow and low gradient despite a normal EF. These patients have a calculated AVA <1 cm2, a Vmax between 3 and 4 m/s (mean gradient 20-40 mmHg), and a transaortic stroke volume <35 ml/m2 despite an EF >50%.23-25 This occurs in patients with increased LV hypertrophy, small chamber volumes, reduced longitudinal systolic function, and increased vascular afterload.25 A firm diagnosis of severe AS is challenging in these patients. Many of these patients have only moderate AS,26 a small body size, or the AVA calculation is erroneous. Some investigators recommend reclassifying AS severity based on transaortic volume flow rate along with pressure gradient.23,24 However, further studies are needed to clarify whether this classification system will aid in the clinical management of asymptomatic patients. In symptomatic patients with a small AVA, preserved EF, and lower aortic gradients, identifying patients who might benefit from AVR is aided by evaluation of severity of valve calcification, ensuring that echocardiographic measurements are made accurately and when normotensive, and careful assessment of other possible causes of symptoms.
Risk Stratification of the Asymptomatic Patient
The primary clinical marker for recommending AVR is the development of symptoms. However, additional diagnostic risk stratification can complement symptom assessment and guide management decisions about timing of AVR by predicting the rate of disease progression and/or event-free survival (Table 2).
Table 2. Risk Stratification of Patients with Severe AS.
| Asymptomatic Patients* | Symptomatic Patients† |
|---|---|
|
|
Markers of increased rate of disease progression and/or decreased event-free survival.
Markers of increased risk and/or potential futility in patients undergoing valve intervention.
Symptom onset
Exercise testing
Although the classical symptoms of AS—angina, heart failure, and syncope—are not subtle, they are late manifestations of disease. In the current era, the most common early symptom is simply exertional dyspnea. Patients who are knowledgeable about the disease process are reliable in promptly reporting a change in status, but symptom onset may be insidious and decline in exercise tolerance may be ascribed to normal aging changes or physical deconditioning. In adults with severe AS and uncertain symptoms, particularly in older and sedentary individuals, exercise testing offers a more objective assessment of functional capacity and symptom status than patient self-report. In apparently asymptomatic individuals, treadmill exercise testing is safe and helpful in risk stratification.27 An abnormal exercise test is defined as development of symptoms (angina, dyspnea, pre-syncope), decreased exercise tolerance, or an inadequate increase in systolic blood pressure (<20 mmHg).28 However, interpreting whether limiting symptoms on treadmill testing are truly cardiac in origin is not an exact science in patients who are elderly, overweight, and deconditioned.
Exercise echocardiography may provide more objective prognostic indicators, based on the concept that more severely stenotic, rigid valve leaflets will limit the ability to increase cardiac output with exercise. With a small, fixed valve area the rise in pressure gradient or velocity with exercise is greater following the mathematical relationship between flow rate and velocity across a flow limiting orifice.6 Accordingly, in patients with severe asymptomatic AS, a larger increase in mean transvalvular gradient with exercise independently predicts reduced event-free survival, even in those with a normal exercise test.29,30 Exercise-induced pulmonary hypertension (>60 mmHg) may provide further incremental prognostic value to the rest and exercise aortic valve gradients.31
BNP
Elevated levels of brain natriuretic peptide (BNP) in asymptomatic patients predict symptom onset and post-operative survival, functional status, and LV function, although the precise cut-off values vary between studies.32,33 Additional study of the role of BNP levels in clinical decision making is needed, but in elderly patients in whom the cause of dyspnea is unclear, BNP levels are helpful in determining whether symptoms are due to cardiac or non-cardiac causes. Similarly, in the apparently asymptomatic elderly patient with severe AS, an elevated BNP level suggests early clinical decompensation. A risk score comprising BNP, Vmax, and gender has been proposed as a means to incorporate biomarker and hemodynamic data into clinical decision-making,34 but further prospective studies of this concept are needed.
Valve anatomy
Valve calcification
In patients with severe asymptomatic AS, increased valve calcification is associated with reduced event-free survival.15,35,36 Rosenhek et al. reported that only 20% of patients with asymptomatic severe AS with moderate or severe valve calcification were free of death or symptoms at 3 years.15 Quantification of aortic valve calcification by CT correlates with AS severity and also provides independent prognostic information beyond echocardiographic indices of AS severity.35,36 CT assessment of valve calcification may also be useful in distinguishing truly severe from moderate AS in patients with a low flow state, but further studies are needed.37
Hemodynamic severity
Transvalvular velocity, gradients, and stroke volume
Vmax and rate of change in Vmax over time are independent predictors of clinical events in patients with severe asymptomatic AS and may help guide decisions about timing of AVR.6,17,18,38 Event-free survival of patients with Vmax >5 m/sec was 64%, 36%, and 25%, at 1, 2, and 3 years, respectively.18 Patients with Vmax >5.5 m/sec developed more severe symptoms, with half presenting with NYHA class III or IV symptoms.18 Among patients with severe asymptomatic AS with moderate to severe valve calcification and a rapid increase of Vmax (>0.3 m/sec in a year), there is a high likelihood of death or onset of symptoms.15,38
Recent studies have highlighted the adverse prognosis of patients with paradoxical low flow and/or low gradient AS,25,39 but these studies included both symptomatic and asymptomatic patients. Importantly, they highlight the potentially adverse consequences of not referring a symptomatic patient with AVA <1.0 cm2 to surgery due to uncertainty about AS severity caused by the lower transvalvular gradient. The impact of low flow and/or low gradients on outcomes in asymptomatic patients with AVA <1.0 cm2 remains controversial with conflicting results in clinical studies.26,24 This represents an area in need of further investigation.
Valvuloarterial impedance
Increased global LV load, or valvuloarterial impedance (Zva), measured as the ratio of systolic blood pressure plus mean transvalvular gradient to the stroke volume index [(SBP + MG)/SVi], integrates both valvular and vascular afterload. While Zva is a useful index for understanding pathophysiology and the effects of increased vascular load in patients with AS, it does not yet have a clear role in clinical decision making about timing of AVR. Increased Zva in adults with AS is associated with impaired LV systolic function, worse overall survival in symptomatic patients, and lower event-free survival rates in asymptomatic patients.25,40-42 However, when the increase in Zva in a given patient is due more to increased blood pressure and vascular stiffness (in the setting of moderate AS), the clinical response to the increased Zva should be aggressive blood pressure control rather than expedited AVR. The utility of Zva (beyond established indices of AS severity) as a guide to timing of AVR requires further study.
LV response to chronic pressure overload
Hypertrophic LV remodeling
Although LV hypertrophy can decrease wall stress, several studies have demonstrated the adverse impact of greater degrees of hypertrophic remodeling in patients with AS. Cioffi et al. showed that in asymptomatic patients with severe AS and inappropriately high LV mass—LV mass exceeding 10% of predicted based on height, sex, and stroke work—was associated with 56% and 29% event-free survival at 1 and 3 years, respectively, and was an independent predictor of an adverse outcome.43 Increased LV mass and concentric geometry are associated with increased peri-operative complications (e.g., low output syndrome) and mortality as well as worse long-term outcomes.44,45
LV longitudinal strain
More sensitive indices of systolic function can demonstrate LV dysfunction despite a normal EF. Reduced longitudinal strain is associated with increased myocardial fibrosis46 and predicts an abnormal exercise test and increased cardiac events in follow-up.47 Lancellotti et al. showed that longitudinal strain ≤15.9% is associated with a 2-year event-free survival of 29%.42
Myocardial fibrosis
Myocardial fibrosis is associated with an adverse prognosis.46,48,49 Increased midwall fibrosis (unrelated to previous myocardial infarction) is an independent predictor of mortality in patients with moderate and severe AS.49 Increased fibrosis is also associated with lower transvalvular gradients and less improvement in LV function after AVR.48,50 In addition to visually apparent fibrosis, CMR is capable of quantifying the extent of interstitial fibrosis.48 Whether an earlier operative strategy in asymptomatic patients with evidence of LV fibrosis will improve outcomes requires further study.
Pulmonary hypertension
Long-standing AS may result in increased pulmonary venous pressure, most likely due to diastolic dysfunction, and increased pulmonary vascular resistance. Among adults with severe AS, pulmonary hypertension is present in up to 65% and characterized as severe in 15-20% of patients.51 The presence of pulmonary hypertension is associated with worse heart failure symptoms, increased mortality with medical management, and increased peri-operative and late mortality in patients undergoing surgical or transcatheter AVR.51-54 Pulmonary hypertension with exercise impacts adversely on event-free survival in asymptomatic patients with severe AS31 in accord with its association with symptom onset in patients with asymptomatic mitral valve disease.
Medical Therapy
In patients with calcific AS, there are currently no medical therapies that delay the progression of disease. However, medical therapy plays an important role in the treatment of common co-morbidities in patients with AS.
Medical therapy for AS – valve, ventricle, vasculature
Although LV outflow obstruction at the valve level is the sine qua non of AS, increased vascular afterload (due to increased stiffness and/or resistance) exerts an additional load on the left ventricle. In fact, hypertrophic LV remodeling and dysfunction in response to this combined valvular and vascular chronic pressure overload drive much of the morbidity and mortality of AS.44,45,55,56 As such, potential targets for medical therapy to improve clinical outcomes in patients with AS include the valve, ventricle, and vasculature.
It is now clear that there is an active biology that leads to stiffening and calcification of the valve leaflets.57 Despite significant optimism that statins may help slow the progression of AS based on retrospective studies, prospective randomized trials have consistently shown that statins do not slow progression of disease or reduce aortic valve events in patients with mild to severe AS.58,59 Angiotensin converting enzyme (ACE) and angiotensin 2 are upregulated in diseased aortic valves,60 but retrospective studies have shown conflicting results regarding the impact of ACE-inhibitors on disease progression.61,62 Angiotensin receptor blockers (ARBs) may provide superior inhibition of the renin-angiotensin system (RAS) in the leaflets,63 however prospective studies are needed to determine the effectiveness of RAS inhibition on the progression of calcific AS. Ongoing efforts to elucidate the valvular biology should identify other therapeutic targets.
Hypertrophic LV remodeling that occurs due to chronic pressure overload leads to impaired coronary vasodilator reserve, LV dysfunction, and heart failure symptoms.56 No therapies are particularly effective for retarding and/or reversing this maladaptive remodeling in patients with AS. However, the RAS is upregulated in the pressure overloaded and failing heart, and preclinical studies of pressure overload show that ACE-inhibition attenuates (or reverses) hypertrophic remodeling and LV dysfunction.64 Small clinical studies have demonstrated that ACE/ARB medications are tolerated in patients with AS and even have favorable effects on symptoms and functional capacity in symptomatic patients awaiting surgery.65 A recent retrospective study showed that ACE/ARB medications were associated with improved survival and lower risk of cardiovascular events in patients with AS.66 Larger, prospective studies are needed to evaluate the impact of RAS inhibition on clinical outcomes in patients with AS and the mechanisms for these potential effects.
Hypertension
Because calcific AS is a disease of the elderly, concomitant hypertension is very common. In relatively young cohorts, the prevalence of hypertension was 30-40%,6 compared to 75% or higher in studies that included older patients undergoing transcatheter AVR.53 There has been an under-appreciation of the prevalence of hypertension in AS patients and reluctance to adequately treat it because of traditional teaching that AS is a disease with a “fixed afterload” and an emphasis on avoidance of vasodilators. However, it is misleading to think of AS as a disease with “fixed afterload.” Indeed, increased vascular afterload serves as an additional load on the left ventricle and is associated with increased hypertrophic remodeling and LV dysfunction in patients with AS.40,55 Increased global LV load—measured as the Zva (see above)—also portends a worse outcome.25,41 As such, it is important to recognize and treat hypertension in patients with AS. Uncontrolled hypertension may also mask the severity of AS so that AS severity should be re-evaluated after blood pressure control. There are no long-term prospective data supporting any specific anti-hypertensive agent, but given their potential favorable effects on hypertrophic LV remodeling, ACE/ARB medications may be considered preferentially.
Atrial fibrillation
AS patients may become quite symptomatic with atrial fibrillation (AF), particularly when the ventricular response is fast, because the atrial contribution to ventricular diastolic filling is particularly important in a small, hypertrophied ventricle with concomitant diastolic dysfunction. Rate and rhythm control as indicated by the clinical scenario is important, as is anticoagulation in accordance with management guidelines. The onset of AF in an otherwise asymptomatic patient with severe AS may be an early marker of symptom onset.
Coronary artery disease
CAD is common in patients with AS, and guidelines for primary and secondary prevention should be followed. These include the use of aspirin, statins, ß-blockers, ACE-inhibitors, and aldosterone antagonists as indicated. While nitrates may be used for anginal symptoms, an excessive decrease in preload and/or afterload should be avoided.
Heart failure
In patients with severe AS, initial symptoms of heart failure usually occur in the setting of preserved EF. Symptomatic patients require AVR, although diuretics are often used pre-operatively to decrease congestion and provide symptomatic relief.14 AVR is also indicated in patients with severe AS and a reduced LVEF, regardless of whether LV dysfunction is due to chronic pressure overload or primary myocardial disease.14 However, some patients present with heart failure symptoms in the setting of only mild or moderate AS and primary LV dysfunction. These patients should be treated with standard heart failure therapies including ß-blockers, ACE-inhibitors/ARBs, aldosterone antagonists, nitrates/hydralazine, and diuretics as clinically indicated.
Decompensated heart failure
Patients with severe AS may present with advanced heart failure symptoms characterized by pulmonary congestion, pulmonary hypertension, afterload mismatch, and reduced cardiac output. While AVR is indicated, these patients are at considerable surgical risk.67 In high risk surgical patients, balloon valvuloplasty can modestly reduce AS severity, albeit temporarily.68 Alternatively, systemic vascular resistance (SVR) may be targeted to unload the heart. In this regard, nitroprusside has been shown to decrease wedge pressure and improve cardiac output by decreasing SVR and increasing LV contractility, which may stabilize patients with low output.69 Recently, a single dose of sildenafil has been shown to unload both right and left ventricles in patients with severe AS and advanced heart failure by reducing pulmonary and systemic vascular afterload.70 These preliminary results raise the possibility that medical therapy may serve as a stabilizing bridge to definitive AVR in high-risk patients with advanced heart failure symptoms and low output.
Aortic Valve Replacement
AVR has transformed the outlook of patients with AS. Numerous studies have confirmed the concept of Ross and Braunwald71 that the onset of symptoms heralds a predictable decline in survival, with roughly 50% of patients dying within the next 3-5 years.17,72 AVR clearly is indicated in patients with symptomatic severe AS,14,73 and surgery in such patients improves symptoms and increases life expectancy.17,74,75 In patients with LV systolic dysfunction, reduction in afterload following AVR significantly improves, and often normalizes, LVEF76,77 which also translates into improved survival. As noted previously, some patients with severe AS have heart failure and LV systolic dysfunction with low stroke volume and low aortic valve gradient. In these patients, determination of AS severity and the presence of LV contractile reserve with dobutamine infusion are very helpful for operative risk stratification and identifying those for whom AVR will likely improve outcome.20-22
AVR for treatment of AS represents 50% of all operations for valvular heart disease in North America78 with an increasing use of bioprosthetic valves over mechanical valves.79 Isolated AVR can now be accomplished with a mini-sternotomy, although a full sternotomy is often required if extensive concomitant CABG is required. Bioprosthetic valves are generally recommended for patients over the age to 6514 because of greater durability in older individuals, but there is also greater usage in younger patients because of lifestyle considerations and lack of necessity of chronic anticoagulation. There are no definitive data favoring one bioprosthetic valve (porcine heterograft, bovine pericardial heterograft, or homograft) over another. The Ross operation (replacement of the aortic valve with the pulmonary autograft and replacement of the pulmonic valve with a homograft) is an alternative for adolescents and young adults, including women who wish to become pregnant. Due to complexity of the Ross procedure, it is not favored by the majority of surgeons, although in centers with expertise in this operation outcomes are excellent.80
The operative mortality associated with AVR is dependent both on patient risk factors and the skill and experience of the surgical team. Comorbidities associated with higher 30-day mortality include age, LV dysfunction, concomitant CAD, previous CABG, renal insufficiency, and chronic pulmonary disease. A number of readily available risk scores including the EuroSCORE, the Society of Thoracic Surgeons (STS) risk calculator, and the valve-specific risk calculator of Ambler, et al provide an estimate of surgical risk although none of these scores is optimal because other important variables, such as frailty and cognitive capacity, are not included.81-83 These same factors impact long-term survival after AVR.84
The mortality associated with AVR has decreased dramatically over the past two decades,78,79 and 30-day mortality in the STS Database is currently under 3% for isolated AVR and under 4.5% for combined AVR plus CABG, despite increasing age and comorbidities of those undergoing surgery.79 However, among more elderly patients, the risks are considerably higher, and a large dataset of 142,488 patients enrolled in Medicare indicate that the average in-hospital mortality for AVR is 8.8%.85 Importantly, the hospital mortality in the Medicare study was twice as high in hospitals in the lowest decile of surgical volume compared to hospitals in the highest decile of surgical volume (13.0% versus 6.0%). As these data were risk adjusted, they suggest that higher volume centers place greater attention to quality of care and thus provide better outcomes. In carefully selected patients undergoing surgery in experienced centers, the operative morbidity and mortality are low even in the elderly.86,87
The indications for AVR are clear in symptomatic patients17,88 but are much less clear and remain the subject of debate in asymptomatic patients.89-91 Although the current evidence-based guidelines recommend a watchful waiting approach for most asymptomatic patients, numerous studies have shown that patients with severe AS have a high likelihood of developing symptoms and requiring surgery within 3 to 5 years,6,15,17,18 and other series have reported that asymptomatic patients with severe AS are also at risk of death when managed without surgery.38,86 As the operative risk of AVR is low in experienced centers,86,87 there is ongoing interest in identifying high risk asymptomatic patients who might benefit from early, preemptive AVR rather than a watchful waiting approach, as noted previously.
On the other hand, there is also evidence that many symptomatic patients who fulfill clear Class I indications for AVR are not referred for surgery appropriately in Europe and the United States,17,92-94 with as many as 30% of symptomatic patients not undergoing AVR despite definite indications. Although this treatment gap might be explained by the reluctance of internists and cardiologists to recommend surgery in elderly patients with many comorbidities, even low risk symptomatic patients are often not referred for surgery. Bach et al. reported that 22% of symptomatic patients with severe AS and an operative mortality risk less than 10% as estimated by the EuroSCORE were not referred for surgery.94 It is widely understood that the EuroSCORE overestimates actual observed operative mortality, so these were indeed relatively low risk patients for surgery. The mortality of the symptomatic patients in that series who did not undergo AVR was 53% at 36 months, in keeping Ross and Braunwald's concept of 40 years ago that severe symptomatic AS has a dismal prognosis.71
Transcatheter aortic valve replacement
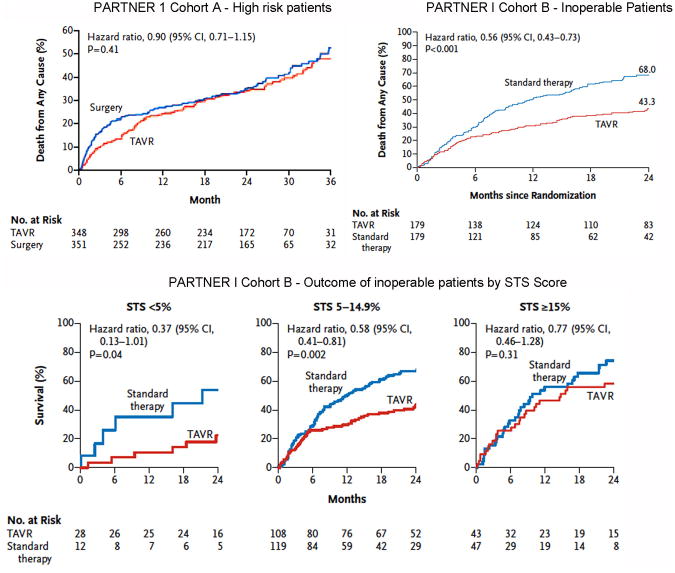
In elderly, higher-risk individuals, age and comorbidities do conspire to increase the risk of surgical AVR, and it is this target population in whom transcatheter AVR (TAVR) has become an exciting treatment option.95 Developed over 10 years ago and now performed in over 40,000 patients worldwide, TAVR has been the subject of numerous reports in the medical literature in recent years, with large national and international registries and the landmark Placement of Transcatheter Aortic Valves (PARTNER) trial96,97 – a prospective randomized trial investigating the role of TAVR in high risk patients with severe AS. PARTNER B investigated the impact of TAVR on the outcome of symptomatic patients who were considered to have a prohibitive operative risk when assessed by a team of both surgeons and cardiologists, randomizing 358 patients to TAVR versus standard care.96 In this very high risk population, TAVR resulted in a 39% reduction in mortality at 1 year (30.7% versus 50.7%) compared to the results of standard therapy, which included percutaneous balloon valvuloplasty in many patients. The mortality benefit with TAVR persisted at 2 years (43.3% with TAVR and 68.0% with standard therapy) (Figure 6).98 On the basis of these results, TAVR was approved by the Food and Drug Administration (FDA) in late 2011 for inoperable patients. It is noteworthy that the risk of stroke was higher in the TAVR group compared to standard therapy, with overall rates of 13.8% versus 5.5% at 2 years, and it is important to have this discussion with patients as part of the decision making process.
Figure 6. Outcomes in the PARTNER I Trial.
From Kodali et al.99 and Makkar et al.98 Reprinted with permission.
PARTNER A investigated the impact of TAVR on outcome of symptomatic patients who were considered high risk candidates for surgical AVR with an STS score estimated 30-day mortality of 10% or higher.97 In this arm of the trial, in which 699 patients were randomized to TAVR or surgical AVR, TAVR was found to be noninferior to surgery in terms of late mortality at one year (24.2% versus 26.8%) and 2 years (33.9% versus 35.0%) (Figure 6). As a result of these findings, TAVR was approved by the FDA for high risk surgical candidates in June 2012.
In making the decision for TAVR or surgical AVR in high risk surgical candidates, one has to balance the increased risk of bleeding and AF with surgery against the increased risk of stroke and vascular damage with TAVR.97 The risk of stroke continues to increase with TAVR compared to surgical AVR over the course of time,97,99 which is presumably the result of vascular damage during device implantation or thromboembolic material from the native calcified valve tissue that remains exposed to the circulation. Both stroke and vascular damage have been linked to higher mortality rates after TAVR.97,99,100 In addition, other complications that occur at higher frequency with TAVR than with surgical AVR, including complete heart block, left bundle branch block, and paravalvular regurgitation, have also been linked to higher long term mortality after TAVR.99-101 Although one would anticipate that the sudden volume load associated with moderate to severe paravalvular regurgitation would be poorly tolerated in a hypertrophied left ventricle that had previously adapted to chronic pressure overload, even mild regurgitation has been associated with a significant increase in long term mortality.119 Whereas moderate to severe regurgitation after TAVR is relatively uncommon (occurring in 8%-12% of patients), mild regurgitation developed in 43% of patients in PARTNER A.99 Possible mechanisms for paravalvular regurgitation include malposition, undersizing, underexpansion, and malapposition of the prosthesis. In addition, aggressive dilatation of the valve during deployment in order to prevent paravalvular regurgitation increases the risk of stroke and can produce central regurgitation through the valve leaflets themselves, contributing to the total regurgitant volume.
In its current state of the art, TAVR represents a transformative technology with the potential to improve symptoms and prolong life in patients who previously had no surgical options. There are a number of ethical and cost effectiveness issues that will need to be addressed, including methods to identify patients who have such severe comorbid illness that even TAVR will not result in improved outcome. Clinical safety and efficacy must temper consumer enthusiasm for TAVR,102 as surgical AVR represents the standard with proven safety and durability for the majority of patients. One of the remarkable findings of the PARTNER A trial is that the surgical mortality was much lower than anticipated (8.0% actual mortality in those who received surgical AVR compared to average estimated surgical mortality of 11.8%), which is the result of the development of highly skilled heart valve teams in the trial. Whether this can be achieved in expanding the access of TAVR to the community is uncertain. Thus, the broad application of TAVR will present challenges in patient selection, cost effectiveness and the need for dedicated expert heart valve centers.
Hybrid approaches to aortic stenosis and coronary artery disease
Several heart valve centers have developed programs to address management of higher risk patients with AS who have concomitant CAD. To minimize cardiopulmonary bypass times during open heart surgery, such approaches include percutaneous coronary intervention immediately prior to surgical AVR or TAVR. Hybrid operating rooms permitting the full range of interventional and surgical options are being implemented in a growing number of institutions.
Risk Stratification of the Symptomatic Patient
While AVR is indicated for symptomatic severe AS, some patients present late in the disease course with severely advanced disease or have such extensive co-morbidities that AVR is unlikely to improve survival and/or quality of life. In these patients, additional risk stratification may be needed to clarify the risk-benefit ratio of AVR. With the recent introduction of TAVR, it will become increasingly important to recognize when there is so little potential benefit to the patients that valve replacement would be futile.
Dobutamine echocardiogram
In patients with low flow, low gradient AS and reduced EF, dobutamine echocardiography both confirms the presence of truly severe AS and provides an evaluation of LV contractile reserve. Although operative mortality is substantially increased in AS patients without contractile reserve, long-term survival may be improved by AVR.21,22 However, the subset of patients with a very low resting transvalvular mean gradient (<20mmHg) have a particularly poor prognosis, suggesting futility of AVR.21,22 TAVR may provide better outcomes than surgical AVR in patients with severe LV dysfunction,103 but further studies are needed.
BNP
While most attention has been focused on the utility of BNP as a risk stratification tool in the asymptomatic patient, in symptomatic patients with low flow, low gradient AS and a reduced EF a very elevated BNP is associated with a markedly decreased 1-year survival after AVR 104 Even after adjusting for EuroScore, an elevated BNP predicts mortality in patients referred for AVR.33 Further studies are needed regarding the relationship between a very high BNP and adverse clinical outcomes to determine whether there are cut-offs above which a favorable clinical result is unlikely.
Myocardial fibrosis
Weidemann et al. showed that patients with severe AS and severe ventricular fibrosis (without CAD and with a preserved mean EF) are more likely to have worse pre-operative symptoms and less improvement in symptoms after AVR, whereas those with none or minimal fibrosis generally improved.46 In patients with concomitant severe LV dysfunction and CAD, it is possible that assessment of fibrosis (due to prior infarction and/or pressure overload from AS) may indicate whether ventricular recovery and symptomatic improvement are likely after AVR.
Severe pulmonary disease
Patients with severe AS who have O2-dependent chronic obstructive pulmonary disease (COPD) have a poor prognosis. Among patients considered inoperable with O2-dependent COPD, 1-year mortality was not significantly better with TAVR than standard therapy,96 and at 2 years O2-dependent COPD was an independent risk factor for mortality in those who received TAVR.98 This deleterious impact on survival has been demonstrated in other TAVR cohorts.53,105 Nonetheless, in these patients, there is a quality of life benefit of TAVR at 12 months compared to standard therapy, even though that benefit may not be observed early after treatment.106 While not a contraindication to AVR, severe COPD is an important co-morbidity to consider when assessing the likelihood of clinical improvement.
Frailty
Frailty likely influences both procedural risk and likelihood of clinical improvement after AVR. Qualitative assessment of frailty has long been incorporated into the clinical evaluation of patients considered for AVR, commonly referred to as the “eyeball test.” Only more recently have attempts been made to measure frailty quantitatively.107 Afilalo et al. recently demonstrated that a 5 meter walk time of ≥6 seconds was an independent predictor of death or major morbidity after cardiac surgery after adjusting for STS risk score.108 Further studies validating objective measures of frailty and determining the impact of frailty on clinical outcomes are needed.
Renal dysfunction
Although not a contraindication to AVR, more severe renal impairment is consistently an independent risk factor for mortality in surgical AVR and TAVR patients.53,99,109,110 Whether the risk of TAVR will be lower than surgical AVR in patients with significant renal impairment requires further study.
Very High STS Scores
The STS score provides an integrated, global snapshot of patient risk as it estimates the risk of mortality and/or serious morbidity for various cardiac surgeries based upon numerous individual cardiac and non-cardiac clinical characteristics. While not perfect, the STS score is perhaps that most accurate risk algorithm for predicting mortality in patients undergoing AVR.111 The 2-year results of the PARTNER B Trial of inoperable patients demonstrated that TAVR provides no mortality benefit compared to standard therapy in those with the highest estimated operative risk (STS score ≥15%) (Figure 6).98 However, in these same very high-risk patients TAVR does appear to improve quality of life compared to standard therapy at 6 and 12 months.106 Further studies are needed to develop risk algorithms specifically designed for patients with AS (which also integrate important features, such as frailty, currently missing from risk algorithms) and to clarify which cut-points may identify individuals who are too sick to benefit from valve replacement.
Acknowledgments
Sources of Funding: Dr. Lindman was supported by Washington University Institute of Clinical and Translational Sciences grants UL1 TR000448 and KL2 TR000450 from the National Center for Advancing Translational Sciences of the NIH.
Nonstandard Abbreviations and Acronyms
- 2D
two dimensional
- 3D
three dimensional
- AS
aortic stenosis
- AVA
aortic valve area
- AVR
aortic valve replacement
- BNP
brain natriuretic peptide
- CABG
coronary artery bypass grafting
- CAD
coronary artery disease
- CMR
cardiac magnetic resonance
- COPD
chronic obstructive pulmonary disease
- CT
computed tomographic imaging
- EF
ejection fraction
- LV
left ventricular
- ΔPmean
mean transaortic pressure gradient
- STS
Society of Thoracic Surgeons
- TAVR
transcatheter aortic valve replacement
- Vmax
maximum aortic velocity
- Zva
valvuloarterial impedance
Footnotes
Disclosures: None
References
- 1.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 2.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 3.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 4.Owens DS, Katz R, Takasu J, Kronmal R, Budoff MJ, O'Brien KD. Incidence and progression of aortic valve calcium in the Multi-ethnic Study of Atherosclerosis (MESA) Am J Cardiol. 2010;105:701–708. doi: 10.1016/j.amjcard.2009.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novaro GM, Katz R, Aviles RJ, Gottdiener JS, Cushman M, Psaty BM, Otto CM, Griffin BP. Clinical factors, but not C-reactive protein, predict progression of calcific aortic-valve disease: the Cardiovascular Health Study. J Am Coll Cardiol. 2007;50:1992–1998. doi: 10.1016/j.jacc.2007.07.064. [DOI] [PubMed] [Google Scholar]
- 6.Otto CM, Burwash IG, Legget ME, Munt BI, Fujioka M, Healy NL, Kraft CD, Miyake-Hull CY, Schwaegler RG. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation. 1997;95:2262–2270. doi: 10.1161/01.cir.95.9.2262. [DOI] [PubMed] [Google Scholar]
- 7.Rosenhek R, Klaar U, Schemper M, Scholten C, Heger M, Gabriel H, Binder T, Maurer G, Baumgartner H. Mild and moderate aortic stenosis. Natural history and risk stratification by echocardiography. Eur Heart J. 2004;25:199–205. doi: 10.1016/j.ehj.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Rosenhek R. Aortic stenosis: disease severity, progression, timing of intervention, and role in monitoring transcatheter valve implantation. In: Otto CM, editor. The Practice of Clinical Echocardiography. 4th. Elsevier/Saunders; 2012. pp. 425–449. [Google Scholar]
- 9.Ayad RF, Grayburn PA, Ko JM, Filardo G, Roberts WC. Accuracy of two-dimensional echocardiography in determining aortic valve structure in patients >50 years of age having aortic valve replacement for aortic stenosis. Am J Cardiol. 2011;108:1589–1599. doi: 10.1016/j.amjcard.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr. 2009;10:1–25. doi: 10.1093/ejechocard/jen303. [DOI] [PubMed] [Google Scholar]
- 11.Ozkan A, Kapadia S, Tuzcu M, Marwick TH. Assessment of left ventricular function in aortic stenosis. Nat Rev Cardiol. 2011;8:494–501. doi: 10.1038/nrcardio.2011.80. [DOI] [PubMed] [Google Scholar]
- 12.Lindman BR, Arnold SV, Madrazo JA, Zajarias A, Johnson SN, Perez JE, Mann DL. The adverse impact of diabetes mellitus on left ventricular remodeling and function in patients with severe aortic stenosis. Circ Heart Fail. 2011;4:286–292. doi: 10.1161/CIRCHEARTFAILURE.110.960039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page A, Dumesnil JG, Clavel MA, Chan KL, Teo KK, Tam JW, Mathieu P, Despres JP, Pibarot P. Metabolic syndrome is associated with more pronounced impairment of left ventricle geometry and function in patients with calcific aortic stenosis: a substudy of the ASTRONOMER (Aortic Stenosis Progression Observation Measuring Effects of Rosuvastatin) J Am Coll Cardiol. 2010;55:1867–1874. doi: 10.1016/j.jacc.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 14.Bonow RO, Carabello BA, Kanu C, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, O'Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Nishimura R, Page RL, Riegel B. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease) Circulation. 2006;114:e84–231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 15.Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–617. doi: 10.1056/NEJM200008313430903. [DOI] [PubMed] [Google Scholar]
- 16.Stewart RA, Kerr AJ, Whalley GA, Legget ME, Zeng I, Williams MJ, Lainchbury J, Hamer A, Doughty R, Richards MA, White HD. Left ventricular systolic and diastolic function assessed by tissue Doppler imaging and outcome in asymptomatic aortic stenosis. Eur Heart J. 2010;31:2216–2222. doi: 10.1093/eurheartj/ehq159. [DOI] [PubMed] [Google Scholar]
- 17.Pellikka PA, Sarano ME, Nishimura RA, Malouf JF, Bailey KR, Scott CG, Barnes ME, Tajik AJ. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation. 2005;111:3290–3295. doi: 10.1161/CIRCULATIONAHA.104.495903. [DOI] [PubMed] [Google Scholar]
- 18.Rosenhek R, Zilberszac R, Schemper M, Czerny M, Mundigler G, Graf S, Bergler-Klein J, Grimm M, Gabriel H, Maurer G. Natural history of very severe aortic stenosis. Circulation. 2010;121:151–156. doi: 10.1161/CIRCULATIONAHA.109.894170. [DOI] [PubMed] [Google Scholar]
- 19.Otto CM, Pearlman AS. Doppler echocardiography in adults with symptomatic aortic stenosis. Diagnostic utility and cost-effectiveness. Arch Intern Med. 1988;148:2553–2560. [PubMed] [Google Scholar]
- 20.Monin JL, Quere JP, Monchi M, Petit H, Baleynaud S, Chauvel C, Pop C, Ohlmann P, Lelguen C, Dehant P, Tribouilloy C, Gueret P. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation. 2003;108:319–324. doi: 10.1161/01.CIR.0000079171.43055.46. [DOI] [PubMed] [Google Scholar]
- 21.Levy F, Laurent M, Monin JL, Maillet JM, Pasquet A, Le Tourneau T, Petit-Eisenmann H, Gori M, Jobic Y, Bauer F, Chauvel C, Leguerrier A, Tribouilloy C. Aortic valve replacement for low-flow/low-gradient aortic stenosis operative risk stratification and long-term outcome: a European multicenter study. J Am Coll Cardiol. 2008;51:1466–1472. doi: 10.1016/j.jacc.2007.10.067. [DOI] [PubMed] [Google Scholar]
- 22.Tribouilloy C, Levy F, Rusinaru D, Gueret P, Petit-Eisenmann H, Baleynaud S, Jobic Y, Adams C, Lelong B, Pasquet A, Chauvel C, Metz D, Quere JP, Monin JL. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol. 2009;53:1865–1873. doi: 10.1016/j.jacc.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 23.Clavel MA, Dumesnil JG, Capoulade R, Mathieu P, Senechal M, Pibarot P. Outcome of Patients With Aortic Stenosis, Small Valve Area, and Low-Flow, Low-Gradient Despite Preserved Left Ventricular Ejection Fraction. J Am Coll Cardiol. doi: 10.1016/j.jacc2011.12.054. Published online ahead of print 5 June 2012. [DOI] [PubMed] [Google Scholar]
- 24.Lancellotti P, Magne J, Donal E, Davin L, O'Connor K, Rosca M, Szymanski C, Cosyns B, Pierard LA. Clinical outcome in asymptomatic severe aortic stenosis: insights from the new proposed aortic stenosis grading classification. J Am Coll Cardiol. 2012;59:235–243. doi: 10.1016/j.jacc.2011.08.072. [DOI] [PubMed] [Google Scholar]
- 25.Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856–2864. doi: 10.1161/CIRCULATIONAHA.106.668681. [DOI] [PubMed] [Google Scholar]
- 26.Jander N, Minners J, Holme I, Gerdts E, Boman K, Brudi P, Chambers JB, Egstrup K, Kesaniemi YA, Malbecq W, Nienaber CA, Ray S, Rossebo A, Pedersen TR, Skjaerpe T, Willenheimer R, Wachtell K, Neumann FJ, Gohlke-Barwolf C. Outcome of patients with low-gradient “severe” aortic stenosis and preserved ejection fraction. Circulation. 2011;123:887–895. doi: 10.1161/CIRCULATIONAHA.110.983510. [DOI] [PubMed] [Google Scholar]
- 27.Picano E, Pibarot P, Lancellotti P, Monin JL, Bonow RO. The emerging role of exercise testing and stress echocardiography in valvular heart disease. J Am Coll Cardiol. 2009;54:2251–2260. doi: 10.1016/j.jacc.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 28.Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J. 2005;26:1309–1313. doi: 10.1093/eurheartj/ehi250. [DOI] [PubMed] [Google Scholar]
- 29.Marechaux S, Hachicha Z, Bellouin A, Dumesnil JG, Meimoun P, Pasquet A, Bergeron S, Arsenault M, Le Tourneau T, Ennezat PV, Pibarot P. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J. 2010;31:1390–1397. doi: 10.1093/eurheartj/ehq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation. 2005;112(Suppl):I377–382. doi: 10.1161/CIRCULATIONAHA.104.523274. [DOI] [PubMed] [Google Scholar]
- 31.Lancellotti P, Magne J, Donal E, O'Connor K, Dulgheru R, Rosca M, Pierard LA. Determinants and prognostic significance of exercise pulmonary hypertension in asymptomatic severe aortic stenosis. Circulation. 2012;126:851–859. doi: 10.1161/CIRCULATIONAHA.111.088427. [DOI] [PubMed] [Google Scholar]
- 32.Bergler-Klein J, Klaar U, Heger M, Rosenhek R, Mundigler G, Gabriel H, Binder T, Pacher R, Maurer G, Baumgartner H. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation. 2004;109:2302–2308. doi: 10.1161/01.CIR.0000126825.50903.18. [DOI] [PubMed] [Google Scholar]
- 33.Pedrazzini GB, Masson S, Latini R, Klersy C, Rossi MG, Pasotti E, Faletra FF, Siclari F, Minervini F, Moccetti T, Auricchio A. Comparison of brain natriuretic peptide plasma levels versus logistic EuroSCORE in predicting in-hospital and late postoperative mortality in patients undergoing aortic valve replacement for symptomatic aortic stenosis. Am J Cardiol. 2008;102:749–754. doi: 10.1016/j.amjcard.2008.04.055. [DOI] [PubMed] [Google Scholar]
- 34.Monin JL, Lancellotti P, Monchi M, Lim P, Weiss E, Pierard L, Gueret P. Risk score for predicting outcome in patients with asymptomatic aortic stenosis. Circulation. 2009;120:69–75. doi: 10.1161/CIRCULATIONAHA.108.808857. [DOI] [PubMed] [Google Scholar]
- 35.Messika-Zeitoun D, Aubry MC, Detaint D, Bielak LF, Peyser PA, Sheedy PF, Turner ST, Breen JF, Scott C, Tajik AJ, Enriquez-Sarano M. Evaluation and clinical implications of aortic valve calcification measured by electron-beam computed tomography. Circulation. 2004;110:356–362. doi: 10.1161/01.CIR.0000135469.82545.D0. [DOI] [PubMed] [Google Scholar]
- 36.Dichtl W, Alber HF, Feuchtner GM, Hintringer F, Reinthaler M, Bartel T, Sussenbacher A, Grander W, Ulmer H, Pachinger O, Muller S. Prognosis and risk factors in patients with asymptomatic aortic stenosis and their modulation by atorvastatin (20 mg) Am J Cardiol. 2008;102:743–748. doi: 10.1016/j.amjcard.2008.04.060. [DOI] [PubMed] [Google Scholar]
- 37.Cueff C, Serfaty JM, Cimadevilla C, Laissy JP, Himbert D, Tubach F, Duval X, Iung B, Enriquez-Sarano M, Vahanian A, Messika-Zeitoun D. Measurement of aortic valve calcification using multislice computed tomography: correlation with haemodynamic severity of aortic stenosis and clinical implication for patients with low ejection fraction. Heart. 2011;97:721–726. doi: 10.1136/hrt.2010.198853. [DOI] [PubMed] [Google Scholar]
- 38.Nistri S, Faggiano P, Olivotto I, Papesso B, Bordonali T, Vescovo G, Dei Cas L, Cecchi F, Bonow RO. Hemodynamic progression and outcome of asymptomatic aortic stenosis in primary care. Am J Cardiol. 2012;109:718–723. doi: 10.1016/j.amjcard.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 39.Dumesnil JG, Pibarot P, Carabello B. Paradoxical low flow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J. 2010;31:281–289. doi: 10.1093/eurheartj/ehp361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cramariuc D, Cioffi G, Rieck AE, Devereux RB, Staal EM, Ray S, Wachtell K, Gerdts E. Low-flow aortic stenosis in asymptomatic patients: valvular-arterial impedance and systolic function from the SEAS Substudy. JACC Cardiovasc Imaging. 2009;2:390–399. doi: 10.1016/j.jcmg.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 41.Hachicha Z, Dumesnil JG, Pibarot P. Usefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic stenosis. J Am Coll Cardiol. 2009;54:1003–1011. doi: 10.1016/j.jacc.2009.04.079. [DOI] [PubMed] [Google Scholar]
- 42.Lancellotti P, Donal E, Magne J, Moonen M, O'Connor K, Daubert JC, Pierard LA. Risk stratification in asymptomatic moderate to severe aortic stenosis: the importance of the valvular, arterial and ventricular interplay. Heart. 2010;96:1364–1371. doi: 10.1136/hrt.2009.190942. [DOI] [PubMed] [Google Scholar]
- 43.Cioffi G, Faggiano P, Vizzardi E, Tarantini L, Cramariuc D, Gerdts E, de Simone G. Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart. 2011;97:301–307. doi: 10.1136/hrt.2010.192997. [DOI] [PubMed] [Google Scholar]
- 44.Duncan AI, Lowe BS, Garcia MJ, Xu M, Gillinov AM, Mihaljevic T, Koch CG. Influence of concentric left ventricular remodeling on early mortality after aortic valve replacement. Ann Thorac Surg. 2008;85:2030–2039. doi: 10.1016/j.athoracsur.2008.02.075. [DOI] [PubMed] [Google Scholar]
- 45.Mihaljevic T, Nowicki ER, Rajeswaran J, Blackstone EH, Lagazzi L, Thomas J, Lytle BW, Cosgrove DM. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg. 2008;135:1270–1278. doi: 10.1016/j.jtcvs.2007.12.042. [DOI] [PubMed] [Google Scholar]
- 46.Weidemann F, Herrmann S, Stork S, Niemann M, Frantz S, Lange V, Beer M, Gattenlohner S, Voelker W, Ertl G, Strotmann JM. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577–584. doi: 10.1161/CIRCULATIONAHA.108.847772. [DOI] [PubMed] [Google Scholar]
- 47.Lafitte S, Perlant M, Reant P, Serri K, Douard H, DeMaria A, Roudaut R. Impact of impaired myocardial deformations on exercise tolerance and prognosis in patients with asymptomatic aortic stenosis. Eur J Echocardiogr. 2009;10:414–419. doi: 10.1093/ejechocard/jen299. [DOI] [PubMed] [Google Scholar]
- 48.Azevedo CF, Nigri M, Higuchi ML, Pomerantzeff PM, Spina GS, Sampaio RO, Tarasoutchi F, Grinberg M, Rochitte CE. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol. 2010;56:278–287. doi: 10.1016/j.jacc.2009.12.074. [DOI] [PubMed] [Google Scholar]
- 49.Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, Banya W, Gulati A, Roussin I, Raza S, Prasad NA, Wage R, Quarto C, Angeloni E, Refice S, Sheppard M, Cook SA, Kilner PJ, Pennell DJ, Newby DE, Mohiaddin RH, Pepper J, Prasad SK. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271–1279. doi: 10.1016/j.jacc.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 50.Herrmann S, Stork S, Niemann M, Lange V, Strotmann JM, Frantz S, Beer M, Gattenlohner S, Voelker W, Ertl G, Weidemann F. Low-gradient aortic valve stenosis myocardial fibrosis and its influence on function and outcome. J Am Coll Cardiol. 2011;58:402–412. doi: 10.1016/j.jacc.2011.02.059. [DOI] [PubMed] [Google Scholar]
- 51.Faggiano P, Antonini-Canterin F, Ribichini F, D'Aloia A, Ferrero V, Cervesato E, Pavan D, Burelli C, Nicolosi G. Pulmonary artery hypertension in adult patients with symptomatic valvular aortic stenosis. Am J Cardiol. 2000;85:204–208. doi: 10.1016/s0002-9149(99)00643-8. [DOI] [PubMed] [Google Scholar]
- 52.Melby SJ, Moon MR, Lindman BR, Bailey MS, Hill LL, Damiano RJ., Jr Impact of pulmonary hypertension on outcomes after aortic valve replacement for aortic valve stenosis. J Thorac Cardiovasc Surg. 2011;141:1424–1430. doi: 10.1016/j.jtcvs.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodes-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, Osten M, Natarajan MK, Velianou JL, Martucci G, DeVarennes B, Chisholm R, Peterson MD, Lichtenstein SV, Nietlispach F, Doyle D, DeLarochelliere R, Teoh K, Chu V, Dancea A, Lachapelle K, Cheema A, Latter D, Horlick E. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55:1080–1090. doi: 10.1016/j.jacc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 54.Mutlak D, Aronson D, Carasso S, Lessick J, Reisner SA, Agmon Y. Frequency, determinants and outcome of pulmonary hypertension in patients with aortic valve stenosis. Am J Med Sci. 2012;343:397–401. doi: 10.1097/MAJ.0b013e3182309431. [DOI] [PubMed] [Google Scholar]
- 55.Briand M, Dumesnil JG, Kadem L, Tongue AG, Rieu R, Garcia D, Pibarot P. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol. 2005;46:291–298. doi: 10.1016/j.jacc.2004.10.081. [DOI] [PubMed] [Google Scholar]
- 56.Kupari M, Turto H, Lommi J. Left ventricular hypertrophy in aortic valve stenosis: preventive or promotive of systolic dysfunction and heart failure? Eur Heart J. 2005;26:1790–1796. doi: 10.1093/eurheartj/ehi290. [DOI] [PubMed] [Google Scholar]
- 57.Miller JD, Weiss RM, Heistad DD. Calcific aortic valve stenosis: methods, models, and mechanisms. Circ Res. 2011;108:1392–1412. doi: 10.1161/CIRCRESAHA.110.234138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Barwolf C, Holme I, Kesaniemi YA, Malbecq W, Nienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 59.Chan KL, Teo K, Dumesnil JG, Ni A, Tam J. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121:306–314. doi: 10.1161/CIRCULATIONAHA.109.900027. [DOI] [PubMed] [Google Scholar]
- 60.O'Brien KD, Shavelle DM, Caulfield MT, McDonald TO, Olin-Lewis K, Otto CM, Probstfield JL. Association of angiotensin-converting enzyme with low-density lipoprotein in aortic valvular lesions and in human plasma. Circulation. 2002;106:2224–2230. doi: 10.1161/01.cir.0000035655.45453.d2. [DOI] [PubMed] [Google Scholar]
- 61.O'Brien KD, Probstfield JL, Caulfield MT, Nasir K, Takasu J, Shavelle DM, Wu AH, Zhao XQ, Budoff MJ. Angiotensin-converting enzyme inhibitors and change in aortic valve calcium. Arch Intern Med. 2005;165:858–862. doi: 10.1001/archinte.165.8.858. [DOI] [PubMed] [Google Scholar]
- 62.Rosenhek R, Rader F, Loho N, Gabriel H, Heger M, Klaar U, Schemper M, Binder T, Maurer G, Baumgartner H. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation. 2004;110:1291–1295. doi: 10.1161/01.CIR.0000140723.15274.53. [DOI] [PubMed] [Google Scholar]
- 63.Cote N, Couture C, Pibarot P, Despres JP, Mathieu P. Angiotensin receptor blockers are associated with a lower remodelling score of stenotic aortic valves. Eur J Clin Invest. 2011;41:1172–1179. doi: 10.1111/j.1365-2362.2011.02522.x. [DOI] [PubMed] [Google Scholar]
- 64.Litwin SE, Katz SE, Weinberg EO, Lorell BH, Aurigemma GP, Douglas PS. Serial echocardiographic-Doppler assessment of left ventricular geometry and function in rats with pressure-overload hypertrophy. Chronic angiotensin-converting enzyme inhibition attenuates the transition to heart failure. Circulation. 1995;91:2642–2654. doi: 10.1161/01.cir.91.10.2642. [DOI] [PubMed] [Google Scholar]
- 65.Chockalingam A, Venkatesan S, Subramaniam T, Jagannathan V, Elangovan S, Alagesan R, Gnanavelu G, Dorairajan S, Krishna BP, Chockalingam V. Safety and efficacy of angiotensin-converting enzyme inhibitors in symptomatic severe aortic stenosis: Symptomatic Cardiac Obstruction-Pilot Study of Enalapril in Aortic Stenosis (SCOPE-AS) Am Heart J. 2004;147:E19. doi: 10.1016/j.ahj.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 66.Nadir MA, Wei L, Elder DH, Libianto R, Lim TK, Pauriah M, Pringle SD, Doney AD, Choy AM, Struthers AD, Lang CC. Impact of renin-angiotensin system blockade therapy on outcome in aortic stenosis. J Am Coll Cardiol. 2011;58:570–576. doi: 10.1016/j.jacc.2011.01.063. [DOI] [PubMed] [Google Scholar]
- 67.Di Eusanio M, Fortuna D, De Palma R, Dell'Amore A, Lamarra M, Contini GA, Gherli T, Gabbieri D, Ghidoni I, Cristell D, Zussa C, Pigini F, Pugliese P, Pacini D, Di Bartolomeo R. Aortic valve replacement: results and predictors of mortality from a contemporary series of 2256 patients. J Thorac Cardiovasc Surg. 2011;141:940–947. doi: 10.1016/j.jtcvs.2010.05.044. [DOI] [PubMed] [Google Scholar]
- 68.Kapadia SR, Goel SS, Yuksel U, Agarwal S, Pettersson G, Svensson LG, Smedira NG, Whitlow PL, Lytle BW, Tuzcu EM. Lessons learned from balloon aortic valvuloplasty experience from the pre-transcatheter aortic valve implantation era. J Interv Cardiol. 2010;23:499–508. doi: 10.1111/j.1540-8183.2010.00577.x. [DOI] [PubMed] [Google Scholar]
- 69.Khot UN, Novaro GM, Popovic ZB, Mills RM, Thomas JD, Tuzcu EM, Hammer D, Nissen SE, Francis GS. Nitroprusside in critically ill patients with left ventricular dysfunction and aortic stenosis. N Engl J Med. 2003;348:1756–1763. doi: 10.1056/NEJMoa022021. [DOI] [PubMed] [Google Scholar]
- 70.Lindman BR, Zajarias A, Madrazo JA, Shah J, Gage BF, Novak E, Johnson SN, Chakinala MM, Hohn TA, Saghir M, Mann DL. Effects of phosphodiesterase type 5 inhibition on systemic and pulmonary hemodynamics and ventricular function in patients with severe symptomatic aortic stenosis. Circulation. 2012;125:2353–2362. doi: 10.1161/CIRCULATIONAHA.111.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ross J, Jr, Braunwald E. Aortic stenosis. Circulation. 1968;38(1 Suppl):61–67. doi: 10.1161/01.cir.38.1s5.v-61. [DOI] [PubMed] [Google Scholar]
- 72.Bach DS, Cimino N, Deeb GM. Unoperated patients with severe aortic stenosis. J Am Coll Cardiol. 2007;50(20):2018–2019. doi: 10.1016/j.jacc.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 73.Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G, Flachskampf F, Hall R, Iung B, Kasprzak J, Nataf P, Tornos P, Torracca L, Wenink A. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J. 2007;28:230–268. doi: 10.1093/eurheartj/ehl428. [DOI] [PubMed] [Google Scholar]
- 74.Kvidal P, Bergstrom R, Horte LG, Stahle E. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol. 2000;35:747–756. doi: 10.1016/s0735-1097(99)00584-7. [DOI] [PubMed] [Google Scholar]
- 75.Bakaeen FG, Chu D, Ratcliffe M, Gopaldas RR, Blaustein AS, Venkat R, Huh J, LeMaire SA, Coselli JS, Carabello BA. Severe aortic stenosis in a veteran population: treatment considerations and survival. Ann Thorac Surg. 2010;89:453–458. doi: 10.1016/j.athoracsur.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 76.Connolly HM, Oh JK, Schaff HV, Roger VL, Osborn SL, Hodge DO, Tajik AJ. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction:result of aortic valve replacement in 52 patients. Circulation. 2000;101:1940–1946. doi: 10.1161/01.cir.101.16.1940. [DOI] [PubMed] [Google Scholar]
- 77.Pereira JJ, Lauer MS, Bashir M, Afridi I, Blackstone EH, Stewart WJ, McCarthy PM, Thomas JD, Asher CR. Survival after aortic valve replacement for severe aortic stenosis with low transvalvular gradients and severe left ventricular dysfunction. J Am Coll Cardiol. 2002;39:1356–1363. doi: 10.1016/s0735-1097(02)01759-x. [DOI] [PubMed] [Google Scholar]
- 78.Lee R, Li S, Rankin JS, O'Brien SM, Gammie JS, Peterson ED, McCarthy PM, Edwards FH. Fifteen-year outcome trends for valve surgery in North America. Ann Thorac Surg. 2011;91:677–684. doi: 10.1016/j.athoracsur.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 79.Brown JM, O'Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg. 2009;137:82–90. doi: 10.1016/j.jtcvs.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 80.El-Hamamsy I, Eryigit Z, Stevens LM, Sarang Z, George R, Clark L, Melina G, Takkenberg JJ, Yacoub MH. Long-term outcomes after autograft versus homograft aortic root replacement in adults with aortic valve disease: a randomised controlled trial. Lancet. 2010;376:524–531. doi: 10.1016/S0140-6736(10)60828-8. [DOI] [PubMed] [Google Scholar]
- 81.EuroSCORE online risk calculator. Available at: http://www.euroscore.org/calc.html.
- 82.Online STS risk calculator. Available at: http://riskcalc.sts.org/STSWebRiskCalc273/de.aspx.
- 83.Ambler G, Omar RZ, Royston P, Kinsman R, Keogh BE, Taylor KM. Generic, simple risk stratification model for heart valve surgery. Circulation. 2005;112:224–231. doi: 10.1161/CIRCULATIONAHA.104.515049. [DOI] [PubMed] [Google Scholar]
- 84.Ashikhmina EA, Schaff HV, Dearani JA, Sundt TM, 3rd, Suri RM, Park SJ, Burkhart HM, Li Z, Daly RC. Aortic valve replacement in the elderly: determinants of late outcome. Circulation. 2011;124:1070–1078. doi: 10.1161/CIRCULATIONAHA.110.987560. [DOI] [PubMed] [Google Scholar]
- 85.Goodney PP, O'Connor GT, Wennberg DE, Birkmeyer JD. Do hospitals with low mortality rates in coronary artery bypass also perform well in valve replacement? Ann Thorac Surg. 2003;76:1131–1136. doi: 10.1016/s0003-4975(03)00827-0. [DOI] [PubMed] [Google Scholar]
- 86.Kang DH, Park SJ, Rim JH, Yun SC, Kim DH, Song JM, Choo SJ, Park SW, Song JK, Lee JW, Park PW. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation. 2010;121:1502–1509. doi: 10.1161/CIRCULATIONAHA.109.909903. [DOI] [PubMed] [Google Scholar]
- 87.Malaisrie SC, McCarthy PM, McGee EC, Lee R, Rigolin VH, Davidson CJ, Beohar N, Lapin B, Subacius H, Bonow RO. Contemporary perioperative results of isolated aortic valve replacement for aortic stenosis. Ann Thorac Surg. 2010;89:751–756. doi: 10.1016/j.athoracsur.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 88.Schwarz F, Baumann P, Manthey J, Hoffmann M, Schuler G, Mehmel HC, Schmitz W, Kubler W. The effect of aortic valve replacement on survival. Circulation. 1982;66:1105–1110. doi: 10.1161/01.cir.66.5.1105. [DOI] [PubMed] [Google Scholar]
- 89.Vaishnava P, Fuster V, Goldman M, Bonow RO. Surgery for asymptomatic degenerative aortic and mitral valve disease. Nat Rev Cardiol. 2011;8:173–177. doi: 10.1038/nrcardio.2010.203. [DOI] [PubMed] [Google Scholar]
- 90.Carabello BA. Aortic valve replacement should be operated on before symptom onset. Circulation. 2012;126:112–117. doi: 10.1161/CIRCULATIONAHA.111.079350. [DOI] [PubMed] [Google Scholar]
- 91.Shah PK. Severe aortic stenosis should not be operated on before symptom onset. Circulation. 2012;126:118–125. doi: 10.1161/CIRCULATIONAHA.111.079368. [DOI] [PubMed] [Google Scholar]
- 92.Bouma BJ, van Den Brink RB, van Der Meulen JH, Verheul HA, Cheriex EC, Hamer HP, Dekker E, Lie KI, Tijssen JG. To operate or not on elderly patients with aortic stenosis: the decision and its consequences. Heart. 1999;82:143–148. doi: 10.1136/hrt.82.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iung B, Cachier A, Baron G, Messika-Zeitoun D, Delahaye F, Tornos P, Gohlke-Barwolf C, Boersma E, Ravaud P, Vahanian A. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J. 2005;26:2714–2720. doi: 10.1093/eurheartj/ehi471. [DOI] [PubMed] [Google Scholar]
- 94.Bach DS, Siao D, Girard SE, Duvernoy C, McCallister BD, Jr, Gualano SK. Evaluation of patients with severe symptomatic aortic stenosis who do not undergo aortic valve replacement: the potential role of subjectively overestimated operative risk. Circ Cardiovasc Qual Outcomes. 2009;2:533–539. doi: 10.1161/CIRCOUTCOMES.109.848259. [DOI] [PubMed] [Google Scholar]
- 95.Holmes DR, Jr, Mack MJ. Transcatheter valve therapy a professional society overview from the american college of cardiology foundation and the society of thoracic surgeons. J Am Coll Cardiol. 2011;58:445–455. doi: 10.1016/j.jacc.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 96.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 97.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 98.Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, Thourani VH, Babaliaros VC, Webb JG, Herrmann HC, Bavaria JE, Kodali S, Brown DL, Bowers B, Dewey TM, Svensson LG, Tuzcu M, Moses JW, Williams MR, Siegel RJ, Akin JJ, Anderson WN, Pocock S, Smith CR, Leon MB. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366:1696–1704. doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- 99.Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, Fontana GP, Dewey TM, Thourani VH, Pichard AD, Fischbein M, Szeto WY, Lim S, Greason KL, Teirstein PS, Malaisrie SC, Douglas PS, Hahn RT, Whisenant B, Zajarias A, Wang D, Akin JJ, Anderson WN, Leon MB. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366:1686–1695. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 100.Genereux P, Webb JG, Svensson LG, Kodali SK, Satler LF, Fearon WF, Davidson CJ, Eisenhauer AC, Makkar RR, Bergman GW, Babaliaros V, Bavaria JE, Velazquez OC, Williams MR, Hueter I, Xu K, Leon MB. Vascular Complications After Transcatheter Aortic Valve Replacement: Insights From the PARTNER (Placement of AoRTic TraNscathetER Valve) Trial. J Am Coll Cardiol. 2012;60:1043–1052. doi: 10.1016/j.jacc.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 101.Houthuizen P, Van Garsse LA, Poels TT, de Jaegere P, van der Boon RM, Swinkels BM, Ten Berg JM, van der Kley F, Schalij MJ, Baan J, Jr, Cocchieri R, Brueren GR, van Straten AH, den Heijer P, Bentala M, van Ommen V, Kluin J, Stella PR, Prins MH, Maessen JG, Prinzen FW. Left bundle-branch block induced by transcatheter aortic valve implantation increases risk of death. Circulation. 2012;126:720–728. doi: 10.1161/CIRCULATIONAHA.112.101055. [DOI] [PubMed] [Google Scholar]
- 102.Desai CS, Bonow RO. Transcatheter valve replacement for aortic stenosis: balancing benefits, risks, and expectations. JAMA. 2012;308:573–574. doi: 10.1001/jama.2012.9427. [DOI] [PubMed] [Google Scholar]
- 103.Clavel MA, Webb JG, Rodes-Cabau J, Masson JB, Dumont E, De Larochelliere R, Doyle D, Bergeron S, Baumgartner H, Burwash IG, Dumesnil JG, Mundigler G, Moss R, Kempny A, Bagur R, Bergler-Klein J, Gurvitch R, Mathieu P, Pibarot P. Comparison between transcatheter and surgical prosthetic valve implantation in patients with severe aortic stenosis and reduced left ventricular ejection fraction. Circulation. 2010;122:1928–1936. doi: 10.1161/CIRCULATIONAHA.109.929893. [DOI] [PubMed] [Google Scholar]
- 104.Bergler-Klein J, Mundigler G, Pibarot P, Burwash IG, Dumesnil JG, Blais C, Fuchs C, Mohty D, Beanlands RS, Hachicha Z, Walter-Publig N, Rader F, Baumgartner H. B-type natriuretic peptide in low-flow, low-gradient aortic stenosis: relationship to hemodynamics and clinical outcome: results from the Multicenter Truly or Pseudo-Severe Aortic Stenosis (TOPAS) study. Circulation. 2007;115:2848–2855. doi: 10.1161/CIRCULATIONAHA.106.654210. [DOI] [PubMed] [Google Scholar]
- 105.Moat NE, Ludman P, de Belder MA, Bridgewater B, Cunningham AD, Young CP, Thomas M, Kovac J, Spyt T, MacCarthy PA, Wendler O, Hildick-Smith D, Davies SW, Trivedi U, Blackman DJ, Levy RD, Brecker SJ, Baumbach A, Daniel T, Gray H, Mullen MJ. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol. 2011;58:2130–2138. doi: 10.1016/j.jacc.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 106.Reynolds MR, Magnuson EA, Lei Y, Leon MB, Smith CR, Svensson LG, Webb JG, Babaliaros VC, Bowers BS, Fearon WF, Herrmann HC, Kapadia S, Kodali SK, Makkar RR, Pichard AD, Cohen DJ. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124:1964–1972. doi: 10.1161/CIRCULATIONAHA.111.040022. [DOI] [PubMed] [Google Scholar]
- 107.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 108.Afilalo J, Eisenberg MJ, Morin JF, Bergman H, Monette J, Noiseux N, Perrault LP, Alexander KP, Langlois Y, Dendukuri N, Chamoun P, Kasparian G, Robichaud S, Gharacholou SM, Boivin JF. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668–1676. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 109.Webb JG, Altwegg L, Boone RH, Cheung A, Ye J, Lichtenstein S, Lee M, Masson JB, Thompson C, Moss R, Carere R, Munt B, Nietlispach F, Humphries K. Transcatheter aortic valve implantation: impact on clinical and valve-related outcomes. Circulation. 2009;119:3009–3016. doi: 10.1161/CIRCULATIONAHA.108.837807. [DOI] [PubMed] [Google Scholar]
- 110.Tamburino C, Capodanno D, Ramondo A, Petronio AS, Ettori F, Santoro G, Klugmann S, Bedogni F, Maisano F, Marzocchi A, Poli A, Antoniucci D, Napodano M, De Carlo M, Fiorina C, Ussia GP. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 123:299–308. doi: 10.1161/CIRCULATIONAHA.110.946533. [DOI] [PubMed] [Google Scholar]
- 111.Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg. 2008;135:180–187. doi: 10.1016/j.jtcvs.2007.09.011. [DOI] [PubMed] [Google Scholar]