Abstract
ON123300 is a low molecular weight multi-kinase inhibitor identified through a series of screens that supported further analyses for brain tumor chemotherapy. Biochemical assays indicated ON123300 was a strong inhibitor of Ark5 and CDK4 as well as growth factor receptor tyrosine kinases such as Beta-type platelet-derived growth factor receptor [PDGFRβ]. ON123300 inhibited U87 glioma cell proliferation with an IC50 = 3.4 ± 0.1 μM and reduced phosphorylation of Akt, yet it also unexpectedly induced Erk activation; both in a dose- and time-dependent manner that subsequently was attributed to relieving Akt-mediated C-Raf S259 inactivation and activating a p70S6K initiated PI3K negative feedback loop. Co-treatment with the EGFR inhibitor gefitinib [GFN] produced synergistic cytotoxic effects. Pursuant to the in vitro studies, in vivo pharmacokinetic [PK] and pharmacodynamic [PD] studies of ON123300 were completed in mice bearing intracerebral U87 tumors following IV doses of 5 mg/kg and 25 mg/kg alone, and also at the higher dose concurrently with GFN. ON123300 showed high brain and brain tumor accumulation based on brain partition coefficient values of at least 2.5. Consistent with the in vitro studies, single agent ON123300 caused a dose-dependent suppression of phosphorylation of Akt as well as activation of Erk in brain tumors, whereas addition of GFN to the ON123300 regimen significantly enhanced p-Akt inhibition and prevented Erk activation. In summary, ON123300 demonstrated favorable PK characteristics and future development for brain tumor therapy would require use of combinations, such as GFN, that mitigated its Erk activation and enhanced its activity.
Keywords: Pharmacokinetics, Pharmacodynamics, ON1233300, GBM, RTK signaling pathway, kinase inhibitor, gefitinib, preclinical
Introduction
Glioblastoma multiforme [GBM] represents the most common primary brain tumor in adults and is amongst the most lethal of all cancers. Despite multimodality treatment consisting of surgical resection followed by concurrent or sequential treatment with radiation and chemotherapy, the prognosis for patients with GBM is poor, with a median survival time of approximately 14 months (1). Standard chemotherapy is based on DNA alkylating agents, most often temozolomide; however due to the modest long-term benefits efforts there is substantial interest to identify molecularly targeted agents that can be used in combination with temozolomide.
Receptor tyrosine kinase[RTK]/RAS/PI(3)K signaling cascades are amongst the most frequently altered signaling pathways in GBM that are likely key requirements for disease progression in a majority of GBM (2-5). RTK signaling hyperactivation are most commonly caused by EGFR mutation/amplification or PDGFR amplification/overexpression, largely mediated through the PI3K/Akt/mTOR and Ras/mitogen-activated protein kinase [MAPK] downstream signaling pathways. Pathological fibroblast growth factor receptor 1 [FGFR1] signaling also occurs in GBMs which exhibit FGFR1 kinase domain gain-of-function mutations (6). These pathway aberrations have stimulated an effort to discover novel modulators, albeit not necessarily directed at GBM, due in part to its orphan disease status and the more restrictive requirements to identify novel compounds with adequate BBB transport (7, 8).
Our own efforts in anticancer drug discovery has led to a number of agents in clinical trials (9-13), and more recently we have employed a pharmacokinetic/pharmacodynamic [PK/PD]-driven drug development paradigm to identify agents suitable for brain tumor chemotherapy (14-16). Application of a PK/PD-driven approach to the ON123 series - 154 low molecular weight moieties - produced ON123300 as the lead compound [Fig.1A] that possessed favorable PK properties including the ability to penetrate the BBB. A biochemical kinase screen indicated ON123300 was a multi-targeted kinase inhibitor with primary targets of Ark5 and CDK4, PDGFRβ, FGFR1, proto-oncogene Ret receptor tyrosine kinase and proto-oncogene Fyn tyrosine-protein kinase. Ark5 is a member of the AMPK family and found to be directly phosphorylated and activated by Akt to prevent cell death. It was reported that Ark5-mediated mTOR phosphorylation induced by IGF-1 plays a key role in tumor malignancy and transient RNAi-mediated ARK5 knockdown caused significant reductions in cell proliferation and brain invasion in a glioma xenograft mouse model (17-20). CDK4 has been shown to be responsible for hyperphosphorylation of tumor suppressor protein Rb releasing its inhibition on G1/S cell cycle progression through activation of the transcription factor E2F (21). CDK4-silenced cells undergo apoptosis and displayed decreased colony formation capacity (22). Small molecule inhibitors of CDK4 and CDK6 have shown preclinical efficacy in GBM models (23, 24). Given the interesting and potential importance of ON123300’s targets and its positive PK profile we undertook a detailed PK/PD analysis in a common preclinical brain tumor model [U87MG].
Figure 1.
ON123300 regulated MAPK and Akt pathways. [A] Chemical structure of ON123300. [B] U87 cells cultured on 10 cm plate were treated with 6.3 μM ON123300 for 1 h and cell lysates were analyzed by phospho-MAPK Array Kit (see Fig S1); Selected proteins shown above. ON123300 treatment decreased phosphorylated Akt, CREB and JNK pan expression levels. In contrast, p-Erk and p38γ were increased in cells treated with ON123300. [C] U87 cells cultured on 10 cm plate were treated with 6.3 μM ON123300, 70 μM ON1231120 or 10 μM ON1231320 for 1 h, and then cell homogenates were separated by SDS-PAGE gel and expression of p-Akt, phosphorylation levels of its downstream proteins and p-Erk were detected by western blotting. Total proteins and β-actin expression were used as control. ON123300 inhibited phosphorylation of Akt and its downstream signaling components, P70S6K, 40S ribosomal protein S6 [rpS6] and Rb S780 [decreased to 40.1% ± 5.7; 31.8 %± 2.1; 60.5% ± 1.0; 54.5% ± 6.3 relatively to control], yet increased p-Erk[increased to 120 % ± 6.9 relative to control]. The data shown are the measured mean values and S.D. of at least three experiments. *: p<0.05; **: p<0.01.
Methods and Materials
Materials
ON123300 [Fig. 1A], ON1231120 and ON1231320 compounds were supplied by Dr. M.V. Reddy [Department of Oncological Sciences, Icahn School of Medicine at Mount Sinai](16). Gefitinib [GFN] was purchased from LC Laboratories [Woburn, MA]. Temozolomide [TMZ] and β-actin antibody was purchased from Sigma-Aldrich [St. Louis, MO], and IRDye 800CW-conjugated and alexa fluor 680-conjugated secondary antibodies were from Rockland [Gilbertsville, PA] and Invitrogen [Grand Island, NY], respectively. All other antibodies were purchased from Cell Signaling Technology [Danvers, MA]. Ninety-six-well p-Akt and p-Erk whole cell lysate kits were purchased from Meso Scale Discovery [Gaithersburg, MD]. Human phospho-MAPK array kit was purchased from R&D System [Minneapolis, MN]. All other chemicals and solvents were obtained from commercial sources.
U87MG [U87] and U251 human glioma cells were purchased from ATCC [Manassas, VA] in August 2011. U87/EGFRvIII and U87/PTEN cell lines were a generous gift from Dr. Webster Cavenee (University of California-San Diego) and Dr. Paul Mischel [University of California Los Angeles, Los Angeles, CA] obtained in December 2009, respectively. Cells were authenticated using western blotting assays in June 2013. GBM10 and GBM39 cells were obtained from Dr. Jann Sarkaria at the Mayo Clinic [Rochester, MN] in December 2011. Cells were cultured in Dulbecco’s modified Eagle’s medium [DMEM] supplemented with 10% standard fetal bovine serum, 100 units/ml penicillin, and 100 units/ml streptomycin and maintained in a humidified atmosphere of 5% CO2 in air at 37°C.
NIH SWISS nude mouse were purchased from Taconic [Petersburgh, NY] and maintained in the American Association for the Accreditation of Laboratory Animal Care accredited Laboratory Animal Resources of Icahn School of Medicine at Mount Sinai. All study procedures were approved by the Institutional Animal Care and Use Committee.
In Vitro Cytotoxicity and Combination Drug Studies
The cytotoxicity of ON123300 was determined using a colorimetric SRB-based assay (25). Suspensions of glioma cells [100 μL containing 2×103 cells] were seeded in 96-well plates and allowed to attach to the surface by overnight incubation. The cells were then treated with increasing concentrations of ON123300 for 72 hour [h]. At the end of the treatment, cells were fixed with 10% [v/v] TCA and stained with 0.4% SRB. The optical densities were measured with a SpectraMax M2 microplate reader [Molecular Devices, Downingtown, PA] at a wavelength of 570 nm. A Sigmoid Emax model [WinNonlin, Pharsight Corporation, Mountain View, CA] was used to calculate IC50 values [mean of three independent studies], which were defined as the drug concentration that was required to reduce the number of viable cells to 50% compared with control treatment [vehicle alone]. For combination studies, cells were treated with ON123300 [from 0.03-16 μM] and GFN [from 0.16-80 μM] alone or together at a fixed concentration ratio of 0.2, or treated with ON123300 [from 0.1-25 μM], temozolomide [from 10-2500 μM], alone or together at a fixed concentration ratio of 0.01 for 72 h and then processed using the SRB assay. The resultant cell proliferation data were used to calculate the combination indexes at designated degrees-50% [ED50], 75% [ED75] and 90% [ED90] -of cell toxicity, respectively, using the CompuSyn program [ComboSyn, Inc.] (26). Combination indices of < 1 indicate synergy and those > 1 antagonisms.
Flow Cytometric Analysis
U87 cells seeded on 6-well plates were cultured overnight and treated with ON123300 and GFN alone or concurrently for 24 h. The cell medium was aspirated and the remaining cells washed and then collected by scraping in 1 ml PBS. Cells were centrifuged at 500 × g for 5 min at 4 °C and then resuspended in 0.5 ml PBS at a density of 2 × 106 cells/ml. After adding 4.5 ml 70% ice cold ethanol the cells were kept on ice for 2 h, and then washed and incubated in PBS containing 20 μg/ml propidium iodide, 0.2 mg/ml DNase-free RNase and 0.1% [v/v] Triton X-100 for 30 minutes [min] at room temperature. The cell cycle of intact/attached cells was analyzed on a flow cytometer [FACSCalibur; BD Biosciences, San Jose, CA] and only live cells were gated and quantitated by FlowJo software [Tree Star, Inc, Ashland, OR, USA]. For Annexin V studies, U87 cells seeded on 12-well plates were cultured overnight and treated with ON123300 and GFN alone or concurrently for 24 h. Both floating and attached cells were harvested, stained with AnnexinV-FITC 633 (10 μg/ml) in binding buffer [10 mM HEPES pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2], and analyzed by flow cytometry [FACScan, BD Biosciences; 5000 events/sample].
Live Cell Microscopy
U87 Cells were grown in 6-well plates overnight and treated with ON123300, GFN or the combination for 24 h. treated cells were then directly visualized with an Olympus IX70 fluorescence live cell microscope [Olympus Imaging, Center Valley, PA] controlled by InVivo software [Media Cybernetics, Bethesda, MD].
Protein Sample Preparation
Cell lysates from treated cells or brain tumors were prepared in a cell lysis buffer (27) containing 1 mM PMSF, phosphatase inhibitor I, phosphatase inhibitor II and protease inhibitor cocktail at a ratio of 600 μl of buffer to 80-90% confluent cells in 10 cm plate or 20 μl of buffer to 1 mg of brain tumor tissue. After adding cell lysis buffer the cells and tissues were brief sonicated, incubated on ice for 30 min and then centrifuged at 21,000 × g for 10 min at 4°C. The supernatants were collected and stored at −80°C. Protein concentrations of both supernatants were measured by a BSA assay according to the manufacturer’s instructions.
Phospho-MAPK array
Human phospho-MAPK arrays were processed according to the manufacturer’s protocol. Briefly, U87 cells treated with 6.3 μM ON123300 for 1 h were rinsed with ice-cold PBS and solubilized with lysis buffer to reach a cell concentration of 1 × 107/ml. Cell supernatants were collected after cell lysates were rocked at 4°C for 30 min and then centrifuged for 5 min. Subsequently, 200 μg of the cell supernatants were adjusted to final volume of 1.5 ml with Array Buffer 1 followed by the addition of 20 μL of a detection antibody cocktail and incubated at room temperature for 1 h. The prepared sample-Ab mixtures were added to membranes that had been pre-incubated with Array Buffer 5 and then incubated overnight at 4°C. Next day, the membranes were washed 3 times with washing buffer and incubated with diluted Streptavidin-HRP Ab for 30 min at room temperature. The membranes were then developed by Chemi Reagent Mix and exposed to X-ray film.
Western Blot Assay
Equal amounts of protein samples [20 μg] were electrophoresed on SDS-polyacrylamide gels and transferred to polyvinylidene fluoride membranes that were blocked with 5% nonfat milk at room temperature for 1 h, and subsequently probed with various primary antibodies for 1 h at room temperature or overnight at 4°C. The blots were washed and then incubated with IRDye 800CW-conjugated or alexa fluor 680-conjugated secondary antibodies at room temperature for 1 h and then quantitated for protein using an Odyssey 2.1 [LI-COR Biotechnology, Lincoln, Nebraska, USA] system and the ImageJ software. All western blots were repeated at least three times.
Meso Scale Discovery [MSD] assay
MSD 96-well multispot p-Akt/total Akt and p-Erk/total Erk assays were carried out as per the manufacturer’s protocol with minor modifications. Briefly, plates were blocked with the MSD blocking solution for 1 h at room temperature while shaking and then washed 4 times with Tris wash buffer. For each well, 25 μl of protein [20 μg for Akt and 5 μg for Erk] was added to the plate in duplicate and incubated overnight at 4°C. The plates were washed four times with Tris wash buffer and incubated with 25 μl of detection antibody in each well at room temperature for 2 h with shaking. The plates were analyzed on a SECTOR™ 6000 instrument [Meso Scale Discovery, Gaithersburg, MD] after washing with Tris wash buffer and addition of 150 μl of read buffer. Both the phosphor- and total signals were corrected for background [BSA spots] and for any effects of the lysis buffer. The percentage of phosphorylation against total protein was calculated and the expression of phosphorylation in each treated group was expressed as percentage relative to the control group.
Orthotopic Glioma Model and Dosing Studies
The U87 glioma model used in this study was described previously. Briefly, NIH Swiss nude mice were anesthetized with a cocktail [ketamine:acepromazine:xylazine:saline = 3:2:1:24 volume ratio; ketamine: 100mg/ml; acepromazine: 10mg/ml; xylazine: 20mg/ml] at an intraperitoneal dose of 5 ml/kg. After secured in a stereotactic apparatus mice were implanted with a suspension of U87 cells [106 cells in 10 μl phosphate-buffered saline] into the caudate putamen at a position 0.7 mm anterior and 2.2 mm lateral from the bregma at a depth of 2.5 mm using a 10 μl Hamilton syringe [Hamilton Co., Reno, NV]. Once the animals recovered from anesthesia [about 20 min] they were returned to the animal care facilities, and provided food and water ad libitum. Mice were monitored daily and then entered into the PK/PD studies upon the appearance of clinical symptoms [i.e. unkempt appearance, arched back, unsteady gait] or a body weight loss of 2 g over 2 consecutive days.
ON123300 was administered to groups of brain tumor-bearing mice as an IV bolus at a dose of either 5 mg/kg or 25 mg/kg via a tail vein. Blood, normal brain, and brain tumor were collected from 3-4 mice at each time point [5, 15, 30 min, and 1, 2, 4, 6, 10 h post-administration] in which the mice were briefly anesthetized with isoflurane and exsanguinated by cardiac puncture. Plasma was separated by centrifugation [14000 rpm, 2 min, 4°C] and brain tissues obtained by gross dissection. Normal brain and brain tumor samples were rinsed thoroughly with 0.9 % saline solution and together with plasma samples stored at −80°C until analyzed by LC/MS/MS. For the ON12300-GFN treatment study, ON123300 was administered at an IV dose of 25 mg/kg via a tail vein 1 h after an oral dose of 200 mg/kg GFN [set time point of GFN administration as of −1], and had blood, normal brain and brain tumor samples collected at each time point [0, 0.08, 0.25, 0.5, 1, 2, 4, 6, 10, 17 h post-ON123300 administration] and analyzed as described for ON123300 only treatment.
LC/MS/MS Analysis
All plasma and tissue samples from the PK studies were analyzed with an electrospray ionization LC/MS/MS system [HPLC, Shimadzu, Kyoto, Japan; QTrap 5500, Applied Biosystems, Foster City, CA] described previously(16). To 10 μl samples of plasma, normal brain or brain tumor homogenate [20% w/w tissue/water], 40 μl of cold acetonitrile was used to precipitate proteins followed by centrifugation at 15000 rpm for 5 min. For both plasma and brain samples, 10 μl aliquots of the resultant supernatant were injected into the LC/MS/MS system. Data collection and analysis were performed using Analyst 1.5.1 software [Applied Biosystems MDS Sciex, Ontario, Canada].
Pharmacokinetic and Pharmacodynamic Data and Statistical Analysis
Noncompartmental analyses [NCA] were performed using WinNonlin™ Phoenix, version 6.3 software [Pharsight, Cary, NC] to estimate PK and PD parameters that included for each compound the areas between the effect-time curve in brain tumor [ABEC], and the areas under the drug concentration-time curve in plasma, brain and brain tumor [AUCp, AUCb and AUCbt, respectively], half-life [t1/2], systemic clearance [CL], and the apparent volume of distribution at steady-state [Vss]. The normal brain or brain tumor partition coefficient [Pb and Pbt] was calculated as the ratio of either the AUCb or AUCbt over the corresponding AUCp. All AUC calculations were based on the area to the last observable concentration using the linear-log interpolation method. Data were statistically evaluated by a student t-test or one-way ANOVA with the level of significant chosen as P<0.05.
Results
In Vitro studies
Our previous PK/PD driven-screen of 154 ON123 compounds indicated ON123300 had potential as an anticancer drug for brain tumor chemotherapy based on in vitro cytotoxicity and an integrated panel of in vitro PK assays [i.e. metabolic stability, BBB permeability and plasma and brain tissue binding](16). Further delineation of its in vitro cytotoxicity of ON123300 to U87 cells indicated it had an IC50 equal to 3.4 [mean] ± 0.1 [S.D.] μM. In addition, ON123300 inhibition of cell proliferation in a panel of 11 glioma models including a patient-derived model [GBM10] was quite comparable to that observed to U87 cells [Fig. S1]. A previously completed biochemical screen of 288 kinases using recombinant proteins of ON123300 found the following primary targets; Ark5 [IC50= 5 nM] and CDK4 [IC50 = 3.9 nM], as well as appreciable inhibitory action against, PDGFRβ [IC50= 26 nM], FGFR1 [IC50= 26 nM], proto-oncogene Ret receptor tyrosine kinase [RET, IC50= 9.2 nM] and proto-oncogene Fyn tyrosine-protein kinase [FYN, IC50= 11 nM].
To determine how ON123300 target modulation influenced cell signaling, we measured the activities of several critical molecules in both the PI3K/Akt/mTOR and Ras/MAPK signaling cascades using a phospho-MAPK array containing 26 different capture antibodies [supplement Fig. S2]. Selected proteins that were significantly different [p< 0.05] in treated and control are shown in Fig. 1B. ON123300 decreased expression of phosphorylated Akt, CREB and JNK pan decreased whereas p-Erk and p-p38γ increased in cells. Based on these disparate findings related to Akt and Erk we further evaluated these and related proteins including Ark5, a key target, by western blot analyses. ON123300 inhibited phosphorylation of Akt and its downstream signaling components, P70S6K, 40S ribosomal protein S6 [rpS6] and Rb S780 [see Fig. 1C]. In conjunction with p-Ark5 and p-Akt inhibition there was an increase in p-Erk upon exposure to ON123300 [see Fig. 1C]. Two additional ON123 series compounds, ON1231120 and ON1231320, had negligible effects on these proteins. As measured by western blot and MSD assays the opposite effects of ON12300 on both p-Akt and p-Erk were concentration-dependent with an IC50 for p-Akt inhibition equal to 5.2 μM [Fig. 2A] - which roughly correlated to that for cytotoxicity - and 100% increase in p-Erk at a concentration of about 7 μM compared to vehicle. Using the same experimental system the time-dependent behavior of p-Akt inhibition and p-Erk activation is shown in Fig. 2B in which the effects of ON123300 on p-Akt can be seen as early as 10 min, a nadir at 30 min and sustained inhibition over 6 h at 6.3 μM of ON123300. The p-Erk time profile was oscillatory in nature with a rapid increase at 5 min followed by a decline and then a second peak at 2 h with another modest decline over 6 h yet still elevated about 2-fold relative to pre-treatment. Similar dose- and time-dependent behavior on p-Akt and p-Erk was also observed in two other glioma models; U251 glioma cells and GBM39 cells, a patient-derived brain tumor model [supplement Fig. S3]. Specifically, in the GBM39 model at 4μM ON123300 [close to that used in U87 cells], nadir p-Akt inhibition was 55% and peak p-Erk activation was 174% relative to the control.
Figure 2.
ON1233300 regulated p-Akt and p-Erk activities through PDGFRB and FGFR1 signaling pathways, P70S6K/PI3K/Akt negative feedback loops and crosstalk between PI3K/Akt and Ras/Erk pathways. U87 cells were incubated with increasing concentration of ON123300 for 1 h [A] or with 6.3 μM of ON123300 for indicated times [B]. The p-Akt and p-Erk expression levels against total Akt and Erk expressions were visualized by western blot or quantified by MSD assays. [C] U87 cells were serum-starved for 24 h and then treated for 2 h with 3 μM ON123300 before stimulation with bFGF (20 ng/ml) and PDGF-BB (50 ng/ml) for 10 min. The activation status of the receptor, downstream signal molecules, Akt and Erk, were determined by western blot analysis. [D] U87 cells or U87 PTEN cells cultured on 6-well plate were treated with 6.3 μM ON123300 for 1 h after pretreating with vehicle, 50 μM LY294002 or 1 μM wortmannin for 1 h. The p-Akt, p-Erk, p-C-Raf S259 and p-PTEN expression levels were visualized by western blot. Total proteins and β-actin expression were used as control. [E] Overview of the biochemical pathways involved in ON123300 targeting. The negative feedback loop and Akt induced Raf inactivation are shown in red lines. The data shown are percentages of the mean value and are presented as the mean ± S.D. of three experiments.
ON123300 did not directly inhibit Akt based on the biochemical kinase screen but did show appreciable inhibitory action against upstream tyrosine kinase receptors [PDGFRB, FGFR1 and VEGFR3] that we inferred was the cause for reduced Akt activity. To elucidate the importance of FGFR1 and PDGFRB signaling in Akt inhibition, we examined their response upon stimulation by their cognate ligands, bFGF [20ng/ml] and PDGF-BB [50ng/ml], respectively (28, 29). The activation status of the receptor and Akt and Erk were determined by western blot analysis [Fig. 2C] in U87 cells that were serum-starved for 18 h and then treated for 2 h with 3 μM ON123300 before stimulation with each ligand for 10 min. Ligand stimulation with bFGF and PDGF-BB induced high levels of p-Akt and p-Erk in control cells that was abolished completely for p-Akt and partially for p-Erk by ON123300.
Previous studies reported that mTOR inhibition activates Erk by release of a concomitant negative feedback loop in which activation of p70S6K suppresses its upstream signaling component PI3K and causes inactivation of Erk (30, 31). To test if ON123300-induced Erk activation was mediated through this PI3K/Ras/Raf/Erk negative feedback loop, PI3K activity was inhibited either by pretreating cells with 50 μM LY294002 or 1 μM wortmannin for 1 h or augmented by using U87/PTEN cells that possess normal PTEN activity. As shown in Fig. 2D, abrogation of the PI3K feedback by pharmacological inhibition of PI3K or by overexpression of PI3K suppressor PTEN reduced Erk activation in cells treated with either vehicle or ON123300. These data suggested that Erk activation may be partially modulated by release of feedback inhibition via PI3K activity but may also involve other signaling pathways. Under certain conditions - ligand concentration-dependent and time-dependent - Akt directly phosphorylates C-Raf at a highly conserved serine residue S259 and this phosphorylation results in recruiting 14-3-3 protein binding to C-Raf causing inactivation leading to Erk inactivation (32, 33). Thus, inhibition of Akt by ON123300 could prevent phosphorylation of C-Raf S259 via this cross-talk mechanism and promote Raf and then Erk activation. ON123300 treatment alone, in either U87 or U87PTEN cells, partially decreased p-C-Raf S259 expression [Fig. 2D] and when combined with LY294002 or wortmanin even greater inhibition of p-C-Raf S259 was achieved supporting this pathway connection in Erk activation. In summary, our results demonstrated that ON123300-induced Erk activation is caused by the release of the p70S6K/PI3K/Ras/Raf/Erk negative feedback loop and by suppression of Akt-induced Raf inhibition. Fig. 2E illustrates the proposed mechanisms involved in ON123300 signaling.
In Vitro Combination Studies
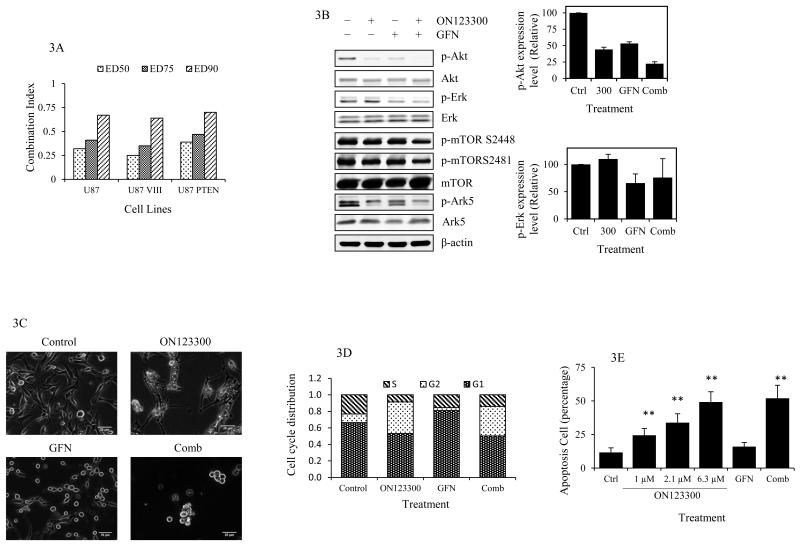
Based on the fact that ON123300 activated Erk through PI3K and Raf mechanisms we considered that the addition of the EGFR inhibitor GFN could prevent this negative effect and enhance ON123300 activity. First, the in vitro cytotoxicity assays and associated combination index values being less than 1 [range 0.25 – 0.7] for the ON123300-GFN combination indicated synergy for all designated degrees of cell toxicity in all glioma cell lines [Fig 3A] (26). We completed a combination study with ON123300 and TMZ and found an antagonistic effect based on the combination indices being > 1 [Table S1]. Although initially perplexing this antagonism could be explained by the ability of TMZ to induce Erk1/2 in U87 cells (34, 35), yet further investigations would be indicated to further characterize this combination. To investigate the molecular mechanisms underlying the synergistic cytotoxic effect of the combination of ON123300 and GFN western blot analyses of U87 cells were completed that demonstrated GFN not only enhanced ON123300 blockade of p-Akt, p-Ark5 and p-mTOR [S2481 and 2448], but also decreased p-Erk levels relative to control [Fig. 3B]. The favorable responses to GFN are attributed to EGFR inhibition that is upstream of both the PI3K/Ras/Raf/Erk negative feedback loop and Akt-Raf activation that led to Erk activation.
Figure 3.
Mechanisms of action of concurrent treatment with ON123300 and GFN. [A] Combination indices for ON123300 and gefitinib combination therapy. U87 parental, U87 VIII, U87/ PTEN cells were treated with different concentration of ON123300, GFN or with a fixed concentration ratio of 0.2 of both for 72 h. Combination indices were computed using the CompuSyn program for designated degrees of toxicity; ED50, ED75 and ED90. [B] Immunoblot analysis was performed on Akt, Erk, mTOR and Ark5 expression after treatment of U87 cells with 6.3 μM ON123300, 13.3 μM GFN or both for 1 h. p-Akt and p-Erk expression levels were quantitated by Image J software. The expression of p-mTOR S2448 and p-mTOR S2481 were 86% ± 3.1 and 102% ± 5.2 when treated with ON123300 alone and reduced to 64% ± 2.8 and 84% ± 4.5 when treated in combination. [C-E] After treatment of U87 cells with 6.3 μM ON123300, 13.3 μM GFN or both for 24 h, Cells were analyzed by live cell imaging for morphology [C], and by flow cytometry for cell cycle [D] and apoptosis quantification[E]. Scale bars = 20 μm. The data shown are percentages of the mean value and are presented as the mean ± S.D. of three experiments. **: p<0.01.
To examine the effects of the GFN and ON123300 combination on cell dynamics, live cell microscopy and flow cytometry were completed. Consistent with cytotoxicity assays, the live cell microscopy showed a dramatic decrease of total cell number of U87 cells following single or combination treatments for 24 h [Fig. 3C]. Flow cytometry of live cells demonstrated significant effects on cell cycle progression following 24 h drug exposures [Fig. 3D] with ON123300 alone and in combination with GFN causing an approximate 2.5-fold increase in the percentage of cells in the G2/M phase [38.2 ± 01.4% (alone) 35.8±0.4% (combined) vs 10.4 ± 0.6% control], whilst GFN caused cell cycle redistribution mainly at the G1 phase [80.9 ± 2.5% vs 66 ± 0.9%]. ON123300 induced G2/M phase arrest may be through Akt inhibition as it has been reported that Akt promotes G2/M cell cycle progression by inducing phosphorylation-dependent 14-3-3 theta binding and cytoplasmic localization of WEE1(36, 37) that switches the balance of regulator activities and causes the initial activation of cyclin B-Cdc2 at the meiotic G2/M-phase transition. ON123300 could interfere with this action by inhibition of Akt. In addition, based on Annexin V staining, ON123300 induced cell death by apoptosis in a dose-dependent manner. GFN treatment alone did not cause apoptotic cell death and in combination with ON123300 had no effect on ON123300 induced apoptosis [Fig. 3E].
In Vivo PK/PD Studies
From our prior PK screening analyses of the ON123 series, ON123300 was the lead compound that possessed favorable PK characteristics suggestive of distribution into brain (16). To determine if these findings translated to an in vivo setting, a series of PK/PD studies of ON123300 alone and combined with GFN were conducted in mice bearing intracerebral U87 tumors. These efforts were based on the measurement of two biomarkers, p-Akt and p-Erk in brain tumor samples and plasma, normal brain and brain tumor concentrations of ON123300 and GFN following doses.
In Vivo PD Profiles
In agreement with in vitro PD profiles, in vivo PD results showed decreased p-Akt expression and increased p-Erk activity in brain tumors upon ON123300 administration at both IV doses of 5 mg/kg and 25 mg/kg [Fig. 4A and 4B]. p-Akt rapidly declined and reached nadir values of 73% and 60% of control within 30 min after 5 mg/kg and 25 mg/kg dose levels, respectively. This was followed by a rapid and pronounced rebound within the first 2 h of dosing and then a more sustained period of p-Akt inhibition with levels at the higher dose reaching near nadir values of 60% at 10 h. To ascertain the cumulative effect of ON123300 on p-Akt levels the ABEC parameters were 63.2 and 304.9 %-h at IV doses of 5 mg/kg and 25 mg/kg, respectively, which is a proportional dose-dependent inhibition [5-fold change, see Table 1]. Unlike the p-Akt profiles that shared similar patterns of inhibition at both dose levels, the p-Erk profiles were somewhat dissimilar with the 25 mg/kg dose group exhibiting appreciable activation that was biphasic reaching a maximum of 161% relative to baseline, whereas at the lower dose of ON123300 the biphasic pattern was muted with a singular spike of p-Erk at 5 min of 159%. As shown in Table 1, The ABEC values of p-Erk were 50 %-h below the baseline and 144 %-h above the baseline following administration of ON123300 at 5 mg/kg and 25 mg/kg IV, respectively. This suggests that at the lower 5 mg/kg dose of ON123300 the Akt-dependent feedback inhibition mechanisms were not completely blocked.
Figure 4.
PD profiles of brain tumor in U87 brain tumor-bearing mouse administered at an IV dose of 5 mg/kg or 25 mg/kg alone or combinational after 1 h of oral dosing of 200 mg/kg GFN. p-Akt [A, C] and p-Erk [B, C] expression levels against total Akt and Erk expression in brain tumor homogenates at various time points quantified by MSD analysis. The data shown are percentages of the mean value and are presented as the mean ± S.D. of at least three mice in each time point.
Table 1.
Pharmacokinetic and pharmacodynamic parameters after the administration of 5 mg/kg and 25 mg/kg ON123300 [5mg/kg and 25mg/kg] alone or with oral dosing of 200 mg/kg GFN [25mg/kg+GFN] on mice bearing U87 tumors. Noncompartmental analyses [NCA] were performed using WinNonlin™ Phoenix, version 6.3 software [Pharsight, Cary, NC] to estimate PK and PD parameters that included for each compound the areas under the drug concentration-time curve in plasma, brain and brain tumor [AUCp, AUCb and AUCbt, respectively], the areas between the effect-time curve in brain tumor [ABEC], half-life [t1/2], systemic clearance [CL], and the apparent volume of distribution at steady-state [Vss]. The normal brain or brain tumor partition coefficient [Pb and Pbt] was calculated as the ratio of either the AUCb or AUCbt over the corresponding AUCp. All AUC calculations were based on the area to the last observable concentration using the linear-log interpolation method. It is assumed that the density of tissues equals 1 g/ml. #: ABEC above the baseline.
| Tissue | Parameter | Unit | Dose | |||
|---|---|---|---|---|---|---|
| 5mg/kg | 25mg/kg | 25mg/kg+GFN | ||||
| Plasma | AUCp | h*ng/ml | 1206 | 4224 | 6351 | |
| t½ | h | 1.5 | 1.5 | 2.3 | ||
| CL | L/h/kg | 4146 | 5919 | 3937 | ||
| Vss | L/kg | 3597 | 10193 | 7078 | ||
| Brain | AUCb | h*ng/ml | 3123 | 11679 | 19794 | |
| Cmax | ng/ml | 5753 | 12432.5 | 16300 | ||
| Tmax | h | 0.083 | 0.083 | 0.25 | ||
| Pb | 2.6 | 2.8 | 3.1 | |||
| Brain tumor | AUCbt | h*ng/ml | 6886 | 63221 | 49440 | |
| Cmax | ng/ml | 6493 | 27250 | 20900 | ||
| Tmax | h | 0.083 | 1 | 0.5 | ||
| Pbt | 5.7 | 15 | 7.8 | |||
| ABEC(%_h) | p-Akt | (%_h) | 63.2 | 304.9 | 765.0 | |
| p-Erk | (%_h) | 50.0 | 144.1# | 273.9 | ||
The addition of GFN to the ON123300 25 mg/kg dose regimen produced prominent effects on both the p-Akt and p-Erk profiles. As with single agent ON123300, the p-Akt profile was biphasic yet the signal was further reduced [ABEC: 765 %-h, Table 1] and achieved a nadir of about 30% at 4 h after the ON123300 dose [Fig. 4C]. The p-Erk response, although biphasic, was attenuated to a large degree with only a single peak activation of 120% at 5 min that was followed by a more durable decrease [ABEC: 273.9 %-h below the baseline, Table 1] that was at 80% of control at 17 h following the ON123300 dose [Fig. 4C]. Overall, the addition of GFN to ON123300 produced similar effects on p-Akt and p-Erk responses in brain tumor-bearing mice as that observed in U87 cells in vitro.
In Vivo PK Profiles
The concentration-time profiles of ON123300 in plasma, brain and brain tumor following single IV doses of 5 mg/kg and 25 mg/kg are shown in Fig. 5A and 5B and the corresponding noncompartmental analysis results are summarized in Table 1. The PK profiles of plasma ON123300 concentration were multiexponential and overall declined fairly rapidly with terminal elimination half-lives of 1.5 h. The AUCp values did not increase in a dose-proportional manner, being 1206 ng-hr/ml at 5 mg/kg and 4224 ng-hr/ml at 25 mg/kg or a 3.5 fold increase that translated into an increase in total clearance [CL] at the higher dose that could reflect saturable plasma protein binding for a low clearance drug. Previously we showed that ON123300 is highly bound (99.4%) to plasma proteins in mice (16). The apparent volume of distribution of ON123300 was elevated at the higher dose also consistent with saturable plasma protein binding [Table 1]. ON123300 rapidly penetrated into brain with peak concentrations at 5 min of 5735 ng/g and 12432 ng/g at doses of 5 mg/kg and 25 mg/kg, respectively. Distribution of ON123300 into normal brain as measured by the brain partition coefficient [Pb] was equal to 2.6 and 2.8 at the low and high doses, respectively. As expected in brain tumors where the BBB is compromised distribution of ON123300 was greater and more variable with brain tumor partition coefficients [PBT] equal to 5.7 and 15 at the 5 mg/kg and 25 mg/kg dose levels, respectively. Whether the elevated partitioning into brain tumor at the 25 mg/kg dose could partially be attributed to saturation of BBB efflux transporters is unknown at this time; however based on previous studies it is unlikely to be a substrate for the ABC transporters(16). The concentration-time profiles of ON1233300 in mice pretreated with a single 200 mg/kg oral dose of GFN were similar in nature to those in mice treated with single agent ON1233300 [see Fig. 5C and Table 1]. There was a marked reduction [33%] in the total clearance of ON123300 in the presence of GFN. Normal brain and brain tumor partitioning of ON123300 was analogous to that when ON123300 was given alone, and although distribution into brain tumor was less in the presence of GFN it is more likely a reflection of interanimal variability in BBB integrity since normal brain distribution was unaltered.
Figure 5.
PK profiles of ON123300 in U87 brain tumor-bearing mouse concurrently treated with ON123300 alone or with GFN. ON123300 was administered into U87 brain tumor-bearing mouse at an IV dose of 5 mg/kg or 25 mg/kg via a tail vein alone or after 1 h of oral dosing of 200 mg/kg GFN and plasma (P), normal brain (B) and brain tumor (BT) samples were collected at various time points and analyzed by LC/MS/MS. ON123300 concentration [A, B and C] in plasma and tissue samples at an IV dose of 5 mg/kg [A], 25 mg/kg alone [B] or pretreated with GFN [C]. Data shown are presented as the mean ± S.D. of at least three mice in each time point.
Discussion
Despite the plethora of new anticancer drugs under study, appreciable improvements in patient survival have not yet followed. Specifically, with respect to brain tumors successful chemotherapy must not only inhibit key PD targets, but overcome the BBB that can greatly limit drug access to the desired target. By application of a PK/PD-driven drug development strategy to ON123300 surfaced as the lead candidate in the ON123 series based on toxicity to glioma cells and proficient BBB penetration and accumulation in normal brain (16). The current investigation was designed to characterize the PK/PD of ON123300 and assess its potential in brain tumor chemotherapy by focusing on the components in the Ras/MAPK and PI3K/Akt/mTOR pathways that are often dysregulated in brain tumor patients (7, 8, 38).
In U87 glioma cells, ON123300 did inhibit p-Akt in a dose- and time-dependent manner, yet unexpectedly also stimulated p-Erk that would be detrimental to its anticancer activity. The observation that inhibitors along the RTK/PI3K/Akt/mTOR pathway produce undesirable anti-complimentary effects has been noted previously (29-33, 39-41). Specifically, the PDGFR inhibitor imatinib elicited Erk activation that was ligand dependent in which imatinib inhibited signaling mediated by PDGF-BB, but not by PDGF-AA or stem cell factor (29, 40). The mTOR1 inhibitor rapamycin and its analogues can activate Ras and its downstream counterparts by relief of the brake supplied by PI3K and triggered by p70S6K (30, 31). This p70S6K/PI3K/Ras/Raf/MEK feedback loop exists in both cancer patients and in preclinical animal models that appeared drug schedule-dependent as we observed (30, 31). In addition, Akt antagonizes Raf activity by direct phosphorylation of S259, and thus, treatment with a PI3K or Akt inhibitor, such as LY294002, results in Erk hyperactivation by decreasing inhibitory S259 phosphorylation of c-Raf (32, 33). Our data demonstrate that ON123300 treatment appears to cause MAPK activation not only by relief of PI3K feedback inhibition triggered by p70S6K, but also by downregulation of c-Raf S259 phosphorylation. The overall action of ON123300 – decreased Akt activity and activated Erk – was observed in three different GBM models including a patient-derived model that supported the need to find another drug that could synergize with ON123300 and prevent Erk activation.
Our previous studies of single agent gefitinib suggested it would be effective in mitigating the rapid Erk activation caused by ON123300 due to its rapid and appreciable penetration of the blood-brain barrier and its inhibitory action upstream of PI3K and Akt and their points of interaction with the MAPK pathway (42-44). Other studies employing gefitinib or an EGFR inhibitor also suggested tumor growth and Erk suppression when used in combination with either an mTOR inhibitor [rapamycin] or Akt inhibitor (45). In the latter study(45), the Akt inhibitor activated Erk yet when combined with gefitinib [150 mg/kg three times/week] growth of NCI-H292 tumors in mice were significantly retarded compared to the Akt inhibitor alone. These positive preclinical findings of EGFR inhibitors and Akt/mTOR pathway inhibitors have not yet translated to the clinic as noted for the combinations of gefitinib and everolimus or sirolimus (46-48). Pharmacodynamic analyses of intratumoral MAPK, PI3K and mTOR signaling were not completed leaving the cause of the lack of efficacy unresolved (46-48). Other agents, possibly those directly inhibiting Raf[mgn]MEK[mgn]Erk signal cascades, such as the MEK inhibitor AZD6244, may also curtail ON123300-induced Erk activation (49, 50); however with the gefitinib-ON123300 combination not only was Erk activation suppressed Akt inhibition was enhanced.
In summary, through a series of in vitro and in vivo PK/PD investigations of ON123300 we confirmed it possessed suitable PK properties for brain tumor chemotherapy accentuated by brain:plasma partitioning of greater than 1. Of particular interest and significance was this multikinase inhibitor produced opposing effects on the PI3K/Akt/mTOR and Ras/MAPK pathways that were attributed to release of feedback inhibition mechanisms that were dose-dependent, and would likely curtail future development as a single agent. Since this feature may be characteristic of inhibitors of the PI3K/Akt/mTOR pathway, it is worthwhile to consider confounding pathway activations early in the drug development scheme and whether rational combination therapy can overcome, and in the process, improve disruption of oncogenic pathways.
Supplementary Material
Acknowledgments
Financial support: This work was supported by the National Institute of Health Grants CA127963 (J.M. Gallo); P01CA-130821 (E.P. Reddy); and CA157740 (J.E. Chipuk).
Abbreviations
- [rpS6]
40S ribosomal protein S6
- [AUC]
area under the drug concentration-time curve
- [PDGFRβ]
Beta-type platelet-derived growth factor receptor
- [ABEC]
effect-time curve
- [FGFR1]
fibroblast growth factor receptor 1
- [FYN]
Fyn tyrosine-protein kinase
- [GFN]
gefitinib
- [GBM]
Glioblastoma multiforme
- [MSD]
Meso Scale Discovery
- [MAPK]
assay mitogen-activated protein kinase
- [NCA]
Noncompartmental analyses
- [Pb and Pbt]
normal brain or brain tumor partition coefficient
- [PD]
pharmacodynamic
- [PK]
pharmacokinetic
- [RTK]
Receptor tyrosine kinase
- [RET]
Ret receptor tyrosine kinase
- [CL]
systemic clearance
- [TMZ]
temozolomide
Footnotes
Disclosure of Potential Conflicts of Interest: Dr. Premkumar Reddy and Dr. Ramana Reddy own equity and receive financial compensation for providing consulting services of Onconova Therapeutics, Inc., a privately held biopharmaceutical company that develops targeted anti-cancer therapeutics. Dr. Premkumar Reddy also receive financial compensation for serving on the Board of Directors. In addition, Dr. Premkumar Reddy and Dr. Ramana Reddy are named inventors on pending and issued patents filed by Temple University and licensed to Onconova Therapeutics that are related to novel cancer treatments. The outcome of this research project could affect the value of these patents and of Onconova Therapeutics.
Reference
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Squatrito M, Holland EC. DNA damage response and growth factor signaling pathways in gliomagenesis and therapeutic resistance. Cancer Res. 2011;71:5945–9. doi: 10.1158/0008-5472.CAN-11-1245. [DOI] [PubMed] [Google Scholar]
- 3.Network CGAR Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nazarenko I, Hede SM, He X, Hedrén A, Thompson J, Lindström MS, et al. PDGF and PDGF receptors in glioma. Ups J Med Sci. 2012;117:99–112. doi: 10.3109/03009734.2012.665097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uhrbom L, Hesselager G, Nistér M, Westermark B. Induction of brain tumors in mice using a recombinant platelet-derived growth factor B-chain retrovirus. Cancer Res. 1998;58:5275–9. [PubMed] [Google Scholar]
- 6.Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–53. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai RY, Staedtke V, Riggins GJ. Molecular targeting of glioblastoma: Drug discovery and therapies. Trends Mol Med. 2011;17:301–12. doi: 10.1016/j.molmed.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wick W, Weller M, Weiler M, Batchelor T, Yung AW, Platten M. Pathway inhibition: emerging molecular targets for treating glioblastoma. Neuro Oncol. 2011;13:566–79. doi: 10.1093/neuonc/nor039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jimeno A, Li J, Messersmith WA, Laheru D, Rudek MA, Maniar M, et al. Phase I study of ON 01910.Na, a novel modulator of the Polo-like kinase 1 pathway, in adult patients with solid tumors. J Clin Oncol. 2008;26:5504–10. doi: 10.1200/JCO.2008.17.9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olnes MJ, Shenoy A, Weinstein B, Pfannes L, Loeliger K, Tucker Z, et al. Directed therapy for patients with myelodysplastic syndromes (MDS) by suppression of cyclin D1 with ON 01910.Na. Leuk Res. 2012;36:982–9. doi: 10.1016/j.leukres.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roschewski M, Farooqui M, Aue G, Wilhelm F, Wiestner A. Phase I study of ON 01910.Na (Rigosertib), a multikinase PI3K inhibitor in relapsed/refractory B-cell malignancies. Leukemia. 2013;27:1920–3. doi: 10.1038/leu.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seetharam M, Fan AC, Tran M, Xu L, Renschler JP, Felsher DW, et al. Treatment of higher risk myelodysplastic syndrome patients unresponsive to hypomethylating agents with ON 01910.Na. Leuk Res. 2012;36:98–103. doi: 10.1016/j.leukres.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma WW, Messersmith WA, Dy GK, Weekes CD, Whitworth A, Ren C, et al. Phase I study of Rigosertib, an inhibitor of the phosphatidylinositol 3-kinase and Polo-like kinase 1 pathways, combined with gemcitabine in patients with solid tumors and pancreatic cancer. Clin Cancer Res. 2012;18:2048–55. doi: 10.1158/1078-0432.CCR-11-2813. [DOI] [PubMed] [Google Scholar]
- 14.Nuthalapati S, Zhou Q, Guo P, Lv H, Cosenza S, Reddy MV, et al. Preclinical Pharmacokinetic and Pharmacodynamic Evaluation of Novel Anticancer Agents, ON01910.Na (Rigosertib, Estybon) and ON013105, for Brain Tumor Chemotherapy. Pharm Res. 2012 doi: 10.1007/s11095-012-0780-y. [DOI] [PubMed] [Google Scholar]
- 15.Lv H, Wang F, Ramana Reddy MV, Zhou Q, Zhang X, Reddy EP, et al. Screening candidate anticancer drugs for brain tumor chemotherapy: Pharmacokinetic-driven approach for a series of (E)-N-(substituted aryl)-3-(substituted phenyl)propenamide analogues. Invest New Drugs. 2012;30:2263–73. doi: 10.1007/s10637-012-9806-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lv H, Zhang X, Sharma J, Reddy MV, Reddy EP, Gallo JM. Integrated pharmacokinetic-driven approach to screen candidate anticancer drugs for brain tumor chemotherapy. AAPS J. 2013;15:250–7. doi: 10.1208/s12248-012-9428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu S, Niu N, Guo H, Tang J, Guo W, Liu Z, et al. ARK5 promotes glioma cell invasion, and its elevated expression is correlated with poor clinical outcome. Eur J Cancer. 2013;49:752–63. doi: 10.1016/j.ejca.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Kusakai G, Suzuki A, Ogura T, Kaminishi M, Esumi H. Strong association of ARK5 with tumor invasion and metastasis. J Exp Clin Cancer Res. 2004;23:263–8. [PubMed] [Google Scholar]
- 19.Suzuki A, Lu J, Kusakai G-i, Kishimoto A, Ogura T, Esumi H. ARK5 Is a Tumor Invasion-Associated Factor Downstream of Akt Signaling. Mol Cell Biol. 2004;24:3526–35. doi: 10.1128/MCB.24.8.3526-3535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki A, Kusakai G-i, Kishimoto A, Lu J, Ogura T, Lavin MF, et al. Identification of a Novel Protein Kinase Mediating Akt Survival Signaling to the ATM Protein. J Biol Chem. 2003;278:48–53. doi: 10.1074/jbc.M206025200. [DOI] [PubMed] [Google Scholar]
- 21.Kato J, Matsushime H, Hiebert SW, Ewen ME, Sherr CJ. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7:331–42. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 22.Deng X, Ma L, Wu M, Zhang G, Jin C, Guo Y, et al. miR-124 radiosensitizes human glioma cells by targeting CDK4. J Neurooncol. 2013;114:263–74. doi: 10.1007/s11060-013-1179-2. [DOI] [PubMed] [Google Scholar]
- 23.Cen L, Carlson BL, Schroeder MA, Ostrem JL, Kitange GJ, Mladek AC, et al. p16-Cdk4-Rb axis controls sensitivity to a cyclin-dependent kinase inhibitor PD0332991 in glioblastoma xenograft cells. Neuro Oncol. 2012;14:870–81. doi: 10.1093/neuonc/nos114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michaud K, Solomon DA, Oermann E, Kim JS, Zhong WZ, Prados MD, et al. Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res. 2010;70:3228–38. doi: 10.1158/0008-5472.CAN-09-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fricker SP. The application of sulforhodamine B as a colorimetric endpoint in a cytotoxicity assay. Toxicol In Vitro. 1994;8:821–2. doi: 10.1016/0887-2333(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 26.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–81. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Q, Guo P, Wang X, Nuthalapati S, Gallo JM. Preclinical pharmacokinetic and pharmacodynamic evaluation of metronomic and conventional temozolomide dosing regimens. J Pharmacol Exp Ther. 2007;321:265–75. doi: 10.1124/jpet.106.118265. [DOI] [PubMed] [Google Scholar]
- 28.Luo JC, Lin HY, Lu CL, Wang LY, Chang FY, Lin HC, et al. Dexamethasone inhibits basic fibroblast growth factor-stimulated gastric epithelial cell proliferation. Biochem Pharmacol. 2008;76:841–9. doi: 10.1016/j.bcp.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Servidei T, Riccardi A, Sanguinetti M, Dominici C, Riccardi R. Increased sensitivity to the platelet-derived growth factor (PDGF) receptor inhibitor STI571 in chemoresistant glioma cells is associated with enhanced PDGF-BB-mediated signaling and STI571-induced Akt inactivation. J Cell Physiol. 2006;208:220–8. doi: 10.1002/jcp.20659. [DOI] [PubMed] [Google Scholar]
- 30.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–74. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carracedo A, Baselga J, Pandolfi PP. Deconstructing feedback-signaling networks to improve anticancer therapy with mTORC1 inhibitors. Cell Cycle. 2008;7:3805–9. doi: 10.4161/cc.7.24.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–4. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
- 33.Moelling K, Schad K, Bosse M, Zimmermann S, Schweneker M. Regulation of Raf-Akt Cross-talk. J Biol Chem. 2002;277:31099–106. doi: 10.1074/jbc.M111974200. [DOI] [PubMed] [Google Scholar]
- 34.Watt HL, Rachid Z, Jean-Claude BJ. Receptor activation and inhibition in cellular response to chemotherapeutic combinational mimicries: the concept of divergent targeting. J Neurooncol. 2010;100:345–61. doi: 10.1007/s11060-010-0196-7. [DOI] [PubMed] [Google Scholar]
- 35.Sun S, Lee D, Lee NP, Pu JK, Wong ST, Lui WM, et al. Hyperoxia resensitizes chemoresistant human glioblastoma cells to temozolomide. J Neurooncol. 2012;109:467–75. doi: 10.1007/s11060-012-0923-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okumura E, Fukuhara T, Yoshida H, Hanada Si S, Kozutsumi R, Mori M, et al. Akt inhibits Myt1 in the signalling pathway that leads to meiotic G2/M-phase transition. Nat Cell Biol. 2002;4:111–6. doi: 10.1038/ncb741. [DOI] [PubMed] [Google Scholar]
- 37.Katayama K, Fujita N, Tsuruo T. Akt/protein kinase B-dependent phosphorylation and inactivation of WEE1Hu promote cell cycle progression at G2/M transition. Mol Cell Biol. 2005;25:5725–37. doi: 10.1128/MCB.25.13.5725-5737.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang S, Armstrong EA, Benavente S, Chinnaiyan P, Harari PM. Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR): combining anti-EGFR antibody with tyrosine kinase inhibitor. Cancer Res. 2004;64:5355–62. doi: 10.1158/0008-5472.CAN-04-0562. [DOI] [PubMed] [Google Scholar]
- 39.George D. Targeting PDGF receptors in cancer--rationales and proof of concept clinical trials. Adv Exp Med Biol. 2003;532:141–51. doi: 10.1007/978-1-4615-0081-0_12. [DOI] [PubMed] [Google Scholar]
- 40.Dong Y, Jia L, Wang X, Tan X, Xu J, Deng Z, et al. Selective inhibition of PDGFR by imatinib elicits the sustained activation of ERK and downstream receptor signaling in malignant glioma cells. Int J Oncol. 2011;38:555–69. doi: 10.3892/ijo.2010.861. [DOI] [PubMed] [Google Scholar]
- 41.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang S, Guo P, Wang X, Zhou Q, Gallo JM. Preclinical pharmacokinetic/pharmacodynamic models of gefitinib and the design of equivalent dosing regimens in EGFR wild-type and mutant tumor models. Mol Cancer Ther. 2008;7:407–17. doi: 10.1158/1535-7163.MCT-07-2070. [DOI] [PubMed] [Google Scholar]
- 43.Wang S, Zhou Q, Gallo JM. Demonstration of the equivalent pharmacokinetic/pharmacodynamic dosing strategy in a multiple-dose study of gefitinib. Mol Cancer Ther. 2009;8:1438–47. doi: 10.1158/1535-7163.MCT-09-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma J, Lv H, Gallo JM. Intratumoral modeling of gefitinib pharmacokinetics and pharmacodynamics in an orthotopic mouse model of glioblastoma. Cancer Res. 2013;73:5242–52. doi: 10.1158/0008-5472.CAN-13-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao RD, Mladek AC, Lamont JD, Goble JM, Erlichman C, James CD, et al. Disruption of parallel and converging signaling pathways contributes to the synergistic antitumor effects of simultaneous mTOR and EGFR inhibition in GBM cells. Neoplasia. 2005;7:921–9. doi: 10.1593/neo.05361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen TDLA, Lis E, Rosen N, Shaffer DR, Scher HI, Deangelis LM, Abrey LE. A pilot study to assess the tolerability and efficacy of RAD-001 (everolimus) with gefitinib in patients with recurrent glioblastoma multiforme (GBM) Journal of Clinical Oncology ASCO Annual Meeting Proceedings. 2006:24. [Google Scholar]
- 47.Reardon DA, Quinn JA, Vredenburgh JJ, Gururangan S, Friedman AH, Desjardins A, et al. Phase 1 trial of gefitinib plus sirolimus in adults with recurrent malignant glioma. Clin Cancer Res. 2006;12:860–8. doi: 10.1158/1078-0432.CCR-05-2215. [DOI] [PubMed] [Google Scholar]
- 48.Kreisl TN, Lassman AB, Mischel PS, Rosen N, Scher HI, Teruya-Feldstein J, et al. A pilot study of everolimus and gefitinib in the treatment of recurrent glioblastoma (GBM) J Neurooncol. 2009;92:99–105. doi: 10.1007/s11060-008-9741-z. [DOI] [PubMed] [Google Scholar]
- 49.See WL, Tan IL, Mukherjee J, Nicolaides T, Pieper RO. Sensitivity of glioblastomas to clinically available MEK inhibitors is defined by neurofibromin 1 deficiency. Cancer Res. 2012;72:3350–9. doi: 10.1158/0008-5472.CAN-12-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang Y, Dai Y, Grant S, Dent P. Enhancing CHK1 inhibitor lethality in glioblastoma. Cancer Biol Ther. 2012;13:379–88. doi: 10.4161/cbt.19240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.