Abstract
Background
Mild cognitive impairment in Parkinson’s disease (PD-MCI) is common and increases the risk for dementia. Establishing distinct PD-MCI cognitive subtypes could be valuable for eventually predicting those most likely to convert to dementia. To date, however, the study of PD-MCI subtypes has not yielded consistent results among cohorts.
Objective
To determine whether there are distinct cognitive subtypes among participants diagnosed with PD-MCI in the Pacific Northwest Udall Center Clinical Consortium.
Methods
We cognitively subtyped 95 patients with PD-MCI using the Movement Disorders Society Task Force diagnostic guidelines. Psychometric test scores were then subjected to principle components factor analysis to determine whether similar cognitive subgroups could be identified using statistical methodology.
Results
Multiple-domain PD-MCI was diagnosed in 95% of the sample, and a range of cognitive impairments were noted. Factor analysis yielded seven factors, and demonstrated overlap of phonemic verbal fluency on two factors, as well as the loading of verbal fluency on the same factor as a visuospatial measure; however, these factors did not partition the sample into distinct cognitive subtypes.
Conclusions
Separation of cognitive subtypes based on the current PD-MCI criteria, or via statistical methods, may not provide sufficient information to describe distinct PD groups. Future efforts to validate the PD-MCI criteria and identify combinations of genetic or other risk factors for cognitive impairment are warranted.
Keywords: Parkinson disease, cognition, mild cognitive impairment, neuropsychological assessment
INTRODUCTION
Cognitive impairment in Parkinson’s disease (PD) is common and can substantially impact the quality of life for patients with PD.1, 2 Early detection of dementia may permit patients, caregivers, and physicians to plan adequately for the future, monitor symptoms more closely, and to adjust treatment decisions as necessary.3 However, efforts to identify the prevalence and characteristics of mild cognitive impairment in PD (PD-MCI) have been limited by the same issues that have challenged the study of MCI in Alzheimer’s disease; namely, variations across studies in terms of diagnostic criteria and methodology.4
The heterogeneity of cognitive impairment in PD presents additional challenges. Given the near ubiquitous nature of cognitive disability in PD, the identification of cognitive subtypes, with the ultimate goal of determining those that may predict subsequent cognitive decline and dementia, has been a recent focus of PD-related research.5-7 Different trajectories have been suggested for those with mild executive impairments, which are fairly pervasive in PD and which may remain stable for years, and those with cognitive impairments that indicate possible underlying posterior cortical deficits (e.g., semantic fluency, visuospatial deficits), which may herald the impending onset of more significant cognitive decline, including dementia.8-10 Attempts to describe discrete groups may be impeded by the heterogeneity of cognitive impairments and progression in PD patients. Further, there is disagreement in the literature as to the ideal methods for assessing cognitive impairment in PD.4
Given the methodological difficulties associated with identifying PD-MCI and its subtypes, substantial efforts toward standardizing diagnostic criteria for PD-MCI and its subtypes have been recently undertaken,11 and are now being implemented in several cohorts. Despite the benefits of having a standard set of criteria with which to diagnose PD-MCI across studies, these criteria nonetheless have yet to be validated. Recent studies have demonstrated the prevalence of PD-MCI and initial longitudinal outcomes using these criteria;12-15 however, although larger validation studies are currently underway,16 the extent to which the subtype criteria adequately represent the underlying cognitive processes in these patients is not currently well-understood.
The current study aims to determine whether there are distinct cognitive subtypes among participants diagnosed with PD-MCI in the Pacific Northwest Udall Center (PANUC) Clinical Consortium, a large observational cohort with comprehensive clinical and cognitive assessments.17 The cohort was classified using the Movement Disorders Society (MDS) Task Force PD-MCI diagnostic guidelines for cognitive subtypes; unique to this study, we examined whether statistical methodology could be used to independently identify similar cognitive subtypes in the cohort.
METHODS
Participants
Participants from the PANUC Clinical Consortium were enrolled using methods previously described.17 Briefly, the PANUC Clinical Consortium is comprised of prevalent cohorts of participants with idiopathic PD assembled at the University of Washington/VA Puget Sound, Oregon Health Sciences University/VA Portland, and the University of Cincinnati. The “Main” sample consists of participants who volunteer to undergo detailed clinical and neuropsychological evaluation. The “Genetics” sample is a larger cohort designed to generate the greater number of blood samples required for genetics projects and other biomarker efforts. In the current study, only participants from the Main sample were included because they underwent the more extensive battery of neuropsychological measures required to apply the MDS Task Force guidelines for PD-MCI subtypes. In addition, participants underwent comprehensive clinical examination that incorporated assessment of motor symptoms, focused past medical history, environmental exposures, and family history. Information concerning activities of daily living was gathered from both the patient and caregiver; a structured formal Clinical Dementia Rating score was assigned. Motor and cognitive diagnoses were adjudicated at regular diagnostic conferences. All participants met the United Kingdom Parkinson’s Disease Society Brain Bank (UKBB) clinical diagnostic criteria for idiopathic PD.18 Exclusion criteria included failure to meet UKBB criteria for PD or history of other neurologic disorders that would significantly impact cognition, e.g., large-vessel stroke or severe traumatic brain injury.
For the current analyses, cross-sectional baseline data from those participants diagnosed with PD-MCI at clinical consensus conference were included (n=142). Of these, 20 participants did not complete at least two measures across five cognitive domains due to sensory impairments, motor difficulties, fatigue, or other intermediary factors, and 27 did not meet the PD-MCI criteria specified by the MDS Task Force as described below due to slight differences between the PANUC diagnostic criteria for MCI17 and the MDS criteria (final n=95). Table 1 summarizes the demographic features, clinical scores, and neuropsychological performances for included and excluded participants.
Table 1. Demographics and neuropsychological test scores for PANUC clinical consortium participants diagnosed with PD-MCI.
| Included sample (n=95) | Omitted sample (n=47) | |||
|---|---|---|---|---|
| Mean (sd) | Range | Mean (sd) | Range | |
| Education (years) | 16.2 (2.4) | 12-23 | 16.6 (2.7) | 11-24 |
| Age at visit (years) | 63.4 (9.4) | 36.2-81.3 | 69.1 (1.2)*** | 52.2 – 85.1 |
| Disease duration (years) | 4.9 (4.8) | 0.08-19.6 | 5.7 (5.9) | 0.13 – 25.8 |
| MDS-UPDRS, motor subscale |
24.0 (10.9) | 7 - 66 | 24.2 (11.2) | 3 - 66 |
| Hoehn & Yahr | 2 (median) | 1 - 3 | 2 (median) | 1.5 - 4 |
| GDS | 5.6 (1.6) | 2 - 14 | 5.8 (1.4) | 4 - 9 |
| Gender | 67.4 (% male) | 74.5 (%male) | ||
| MoCA | 24.4 (2.4) | 17 - 29 | 25.1 (2.3) | 20 - 29 |
| MMSE | 27.8 (1.6) | 24 - 30 | 28.0 (1.7) | 24 - 30 |
| DRS-2 | 137.4 (5.9) | 103 - 143 | 137.3 (5.5) | 121 - 144 |
| Shipley-2 | 33.8 (3.6) | 21 - 40 | 35.3 (2.2)** | 30 - 39 |
| Mean raw score (sd) |
Raw score range |
Mean raw score (sd) |
Raw score range |
|
|---|---|---|---|---|
| Mean z-score (sd) |
Z-score range | Mean z-score (sd) |
Z-score range | |
| HVLT-R immediate | 21.8 (4.3) −0.99 (0.95) |
13 - 32 −3.07 - 1.3 |
22.0 (4.2) −0.70 (0.86) |
14 - 34 −2.38 – 1.64 |
| HVLT-R delayed | 6.4 (3.8) −1.49 (1.85) |
0 - 12 −5.45 - 1.38 |
7.2 (3.3) −0.81 (1.6)* |
0 - 12 −4.94 – 1.38 |
| Logical Memory I | 10.8 (3.7) −0.90 (0.93) |
1 - 20 −3.47 - 1.52 |
12.8 (3.1)** −0.23 (0.68)** |
2 - 17 −2.42 – 0.73 |
| Logical Memory II | 9.5 (3.8) −0.86 (0.93) |
0 - 17 −3.14 - 0.97 |
10.5 (3.4) −0.43 (0.73)* |
2 - 17 −2.41 – 1.0 |
| Clock Drawing | 8.4 (1.6) −0.25 (0.70) |
4 - 10 −2.15 - 0.46 |
‡
‡ |
‡
‡ |
| Phonemic verbal fluency | 40.8 (12.01) 0.14 (0.99) |
21 - 81 −1.9 - 3.6 |
40.0 (12.5) 0.34 (0.97) |
17 - 68 −2.17 – 2.51 |
| Trailmaking, Part A | 33.1 (12.74) −.32 (1.03) |
12 - 83 −3.98 - 1.22 |
34.3 (20.6) −0.14 (1.3) |
15 – 150 −6.54 – 1.4 |
| Trailmaking, Part B | 91.1 (45.6) −.42 (1.05) |
32 - 300 −4.59 - 1.12 |
88.4 (42.4) −0.21 (0.99) |
36 - 207 −3.45 – 0.88 |
| Digit Symbol | 44.1 (11.2) −0.76 (0.96) |
17 - 75 −2.96 - 1.93 |
44.3 (7.2) −0.39 (0.62)* |
30 – 59 −1.69 – 1.06 |
| Letter Number Sequencing |
9.3 (2.2) 0.06 (0.84) |
0 - 15 −3 - 2 |
9.2 (2.0) 0.21 (0.83) |
4 - 13 −2.0 – 1.33 |
| Digit Span, forward length |
6.9 (1.0) 0.04 (0.88) |
4 - 8 −2.33 - 1.27 |
6.9 (0.9) 0.21 (0.75) |
6 - 8 −0.66 – 1.27 |
| Digit Span, reverse length |
4.7 (1.1) −0.31 (0.93) |
2 - 7 −2.5 - 1.91 |
4.9 (1.2) −0.09 (0.92) |
2 – 7 −2.38 – 1.46 |
| Animal fluency | 19.0 (5.26) −0.44 (0.90) |
7 - 34 −2.61 - 2.12 |
20.4 (4.8) 0.02 (0.76)** |
8 – 31 −1.92 – 1.74 |
| Vegetable fluency | 12.9 (3.81) −0.62 (0.84) |
5 - 22 −2.46 - 1.31 |
13.6 (4.7) −0.28 (0.98)* |
3 – 25 −2.53 – 1.95 |
| Boston Naming Test | 28.2 (1.9) 0.15 (0.66) |
19 - 30 −2.41 - 0.83 |
28.4 (1.7) 0.34 (0.58) |
23 – 30 −1.71 – 1.05 |
| Judgment of Line Orientation |
11.8 (2.6) 1.06 (2.18) |
5 - 15 −2.45 - 3.99 |
12.4 (2.2) 1.30 (1.98) |
5 - 15 −2.45 – 3.99 |
| Cube Copy | 15.9 (4.7) −1.06 (2.03) |
2 - 10 −7.09 – 0.74 |
‡
‡ |
‡
‡ |
Abbreviations: sd=standard deviation, MDS-UPDRS=Unified Parkinson’s Disease Rating Scale, Movement Disorder Society revision, GDS=Geriatric Depression Scale, MoCA=Montreal Cognitive Assessment, MMSE= Mini Mental State Examination, DRS-2=Dementia Rating Scale-2, HVLT-R=Hopkins Verbal Learning Test-Revised
p < 0.05
p<0.01
p<0.001 all p values based on t-test comparisons between included and excluded groups
10 point clock scores and 20 point cube scores not available for these participants
Standard protocol approvals, registrations, and patient consents
The institutional review boards at all institutions approved the study, and all subjects (or their legal surrogates) provided written informed consent.
Cognitive diagnosis
Participants were assigned to one of the following cognitive diagnostic categories based on clinical and neuropsychological information: Parkinson’s disease dementia,19 PD-MCI, or no cognitive impairment by a consensus panel of movement disorder and cognitive specialists.
PD-MCI was diagnosed according to the MDS Task Force Level 2 criteria:11 1) observed objective cognitive decline defined by performance that is at least one standard deviation below the published normative mean on two or more tests across five specified cognitive domains (described below); 2) reported/observed cognitive decline by the clinician, participant, or collateral; and 3) cognitive impairments that are not sufficient to substantially interfere with activities of daily living. The Task Force criteria permit individual centers to set their own method for determining level of cognitive impairment; one such method includes those who fall between 1-2 standard deviations below the normative mean. A less restrictive level of impairment (one standard deviation) was used in the PANUC cohort in order to allow diagnosis of PD-MCI in highly educated individuals who have notable cognitive complaints but milder impairments on testing. Participants were further divided into cognitive subtypes according to the Task Force criteria: 1) single-domain subtype, which requires impairment on two tests within only one of the five cognitive domains; and 2) multiple domain subtype, which requires impairment on at least one test across more than one cognitive domain. The domains were further described according to which of the five cognitive domains specified by the MDS Task Force (described below) were impaired.
The excluded participants in the PD-MCI sample met MDS PD-MCI Level 1 criteria.11 According to the Task Force criteria, those diagnosed according to Level 1 criteria do not have sufficient data to determine cognitive subtype (single or multiple), and thus were not included in the current analyses.
Neuropsychological assessment
Cognitive domains assessed were chosen according to the criteria specified by the MDS Task Force, and included learning and memory (Hopkins Verbal Learning Test-Revised [HVLT-R],20 Logical Memory subtest from the Wechsler Memory Scale-Revised),21 executive (phonemic verbal fluency,22 clock drawing test23), attention/working memory (Digit Symbol subtest from the Wechsler Adult Intelligence Scale-Revised,24 Letter-Number Sequencing subtest from the Wechsler Adult Intelligence Scale-III,25 Digit Span subtest from the Wechsler Adult Intelligence Scale-Revised,24 Trailmaking Test26), language (animal and vegetable category [semantic] fluency,27 Boston Naming Test28), and visuospatial (Judgment of Line Orientation,29 cube copy30,31). Global cognitive function was assessed by the Mini Mental State Examination (MMSE),32 Montreal Cognitive Assessment (MoCA),33 and Dementia Rating Scale-2 (DRS-2).34
Analyses
Descriptive statistics (e.g., means, standard deviations, and ranges) were calculated for demographic, clinical, and neuropsychological test scores. The prevalence of MDS Task Force cognitive subtypes (single domain, multiple domain) and the prevalence of impairment across individual cognitive domains (learning/memory, attention/working memory, executive, language, and visuospatial) were further described by frequency estimates. T-tests and Fisher’s exact test were used to determine differences in mean performance between included and excluded groups. A principal components factor analysis (PCFA) was performed independently from the pre-specified cognitive domains delineated by the MDS Task Force criteria in order to collapse the 17 domain-specific psychometric test scores (Table 2) into a smaller number of independent factors that account for most of the variation and the underlying correlation pattern. Raw psychometric test scores were adjusted for age at visit, duration since disease diagnosis, years of education, and sex using linear regression, and the standardized residuals were included in the PCFA for those subjects with complete psychometric testing and demographic data. The initial PCFA components as linear combinations of the psychometric tests were extracted with an eigen-value threshold of 1 or greater. These components or factors were then rotated using a varimax orthogonal rotation and interpreted by considering only those variables with a factor loading magnitude ≥|0.40|. Factor scores for each of the rotated components were calculated as weighted sums of the individual psychometric tests and represent the underlying cognitive domains characterized by the strong factor loadings of particular cognitive tests. We used the factors to determine whether there were cognitive subgroups represented by the clustering of data between cognitive domain factors using scatterplots. All analyses were conducted using Stata 12 (Stata Corp., College Station, TX).
Table 2. Factors and factor loadingsa from principal components factor analysis using 17 raw scoresb.
| Factor Variable | Factor1: Attention/ Processing Speed |
Factor2: Fluency/ Visuospatial |
Factor3: Contextual Declarative Memory |
Factor4: Working Memory/ Executive |
Factor5: List-Learning Declarative Memory |
Factor6: Construction |
Factor7: Language |
|---|---|---|---|---|---|---|---|
| HVLT-R (total recall) | 0.19 | 0.19 | 0.25 | 0.13 | 0.73 | −0.14 | −0.07 |
| HVLT-R (delayed) | −0.05 | 0.13 | 0.08 | −0.07 | 0.77 | −0.09 | 0.32 |
| Logical Memory I | −0.06 | 0.00 | 0.94 | −0.01 | 0.06 | 0.08 | 0.05 |
| Logical Memory II | 0.04 | 0.02 | 0.95 | −0.06 | 0.10 | −0.06 | −0.03 |
| Digit Span forward | 0.11 | −0.08 | −0.16 | 0.78 | 0.19 | 0.00 | −0.14 |
| Digit Span backward | −0.11 | −0.06 | 0.03 | 0.78 | −0.26 | −0.08 | 0.23 |
| Digit Symbol | 0.77 | 0.12 | −0.13 | −0.04 | −0.32 | −0.03 | 0.16 |
| Letter Number Sequencing |
0.15 | 0.11 | 0.05 | 0.56 | 0.16 | 0.11 | 0.34 |
| Trailmaking Part A | − 0.78 | 0.00 | −0.05 | 0.01 | −0.17 | −0.12 | −0.32 |
| Trailmaking Part B | − 0.80 | −0.08 | −0.04 | −0.15 | −0.19 | −0.07 | 0.16 |
| Phonemic verbal fluency |
0.24 | 0.45 | −0.10 | 0.47 | 0.17 | 0.02 | −0.16 |
| Clock Drawing Test | 0.01 | −0.06 | 0.05 | −0.02 | −0.16 | 0.84 | 0.00 |
| Semantic verbal fluency (animals) |
0.06 | 0.83 | 0.06 | −0.04 | 0.19 | −0.02 | 0.12 |
| Semantic verbal fluency (vegetables) |
0.04 | 0.81 | −0.02 | −0.11 | 0.10 | −0.05 | −0.14 |
| Boston Naming Test | 0.15 | 0.01 | 0.00 | 0.08 | 0.13 | 0.06 | 0.85 |
| Judgment of Line Orientation |
0.11 | 0.56 | 0.00 | 0.31 | −0.31 | 0.13 | 0.34 |
| Cube copy | 0.13 | 0.04 | −0.03 | 0.00 | 0.01 | 0.79 | 0.09 |
| % Total variance | 12.02 | 11.51 | 11.27 | 11.22 | 9.72 | 8.39 | 7.97 |
| % Cumulative variance | 12.02 | 23.53 | 34.80 | 46.02 | 55.74 | 64.13 | 72.10 |
Factor loadings represent the correlation between the individual variable and each factor (after rotation); bold font and gray highlights indicate magnitude of factor loadings ≥ 0.40
PCFA was implemented on the standardized residuals from the linear regression of the raw score adjusted for age at visit, education, disease duration, and gender.
RESULTS
Cognitive subtypes according to MDS Task Force criteria
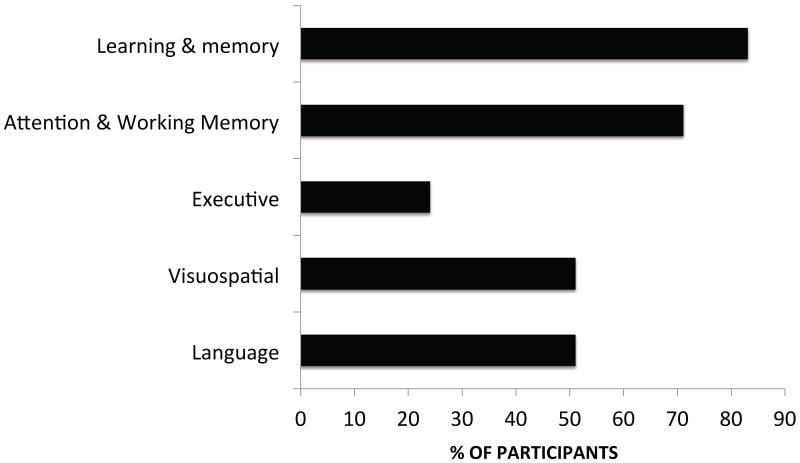
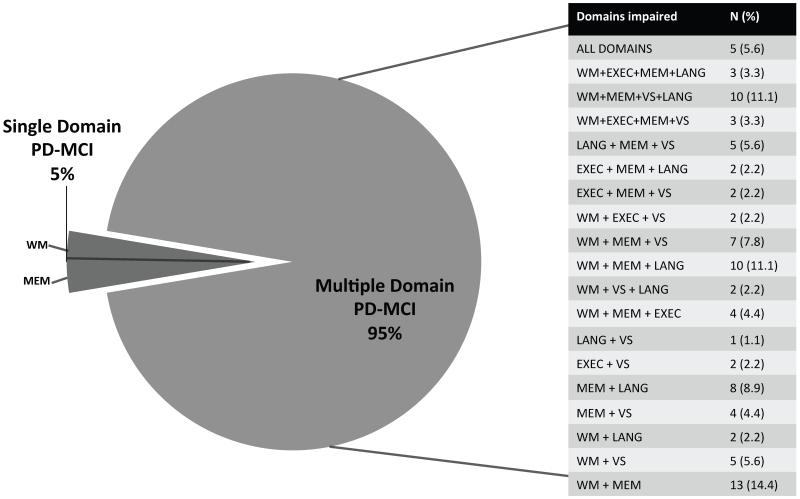
The majority of cognitive impairments identified in the sample were in the learning/memory and attention/working memory domains (Figure 1). When subtypes were defined using the MDS PD-MCI criteria (single vs. multiple domain), 95% of participants were identified as having multiple-domain PD-MCI. Those with the multiple domain subtype demonstrated a diverse collection of impairments across cognitive domains, although working memory and learning/memory were features common to many of the subgroups (Figure 2).
Figure 1. Percentage of PD-MCI participants in the PANUC Clinical Consortium with impairment by cognitive domain.
Figure 2. Number of participants diagnosed with single- and multiple-domain PD-MCI subtypes in the PANUC Clinical Consortium.
Abbreviations: MEM: Learning/Memory WM: Attention/Working Memory EXEC: Executive VS: Visuospatial LANG: Language. Percentages in the table are based on the total number of participants with Multiple Domain PD-MCI. Total percentage does not equal 100 due to rounding.
Principle components factor analyses
An independent principle components factor analysis performed on the sample extracted from 17 standardized residual test scores the following seven factors which were characterized by the cognitive tests with strongest factor loadings: 1) attention/processing speed, 2) fluency/visuospatial 3) contextual declarative memory, 4) working memory/executive, 5) list-learning declarative memory, 6) construction and 7) language. These seven factors accounted for 72% of the total variance. Phonemic verbal fluency loaded on more than one factor (Factors 2 and 4). See Table 2 for PCFA results.
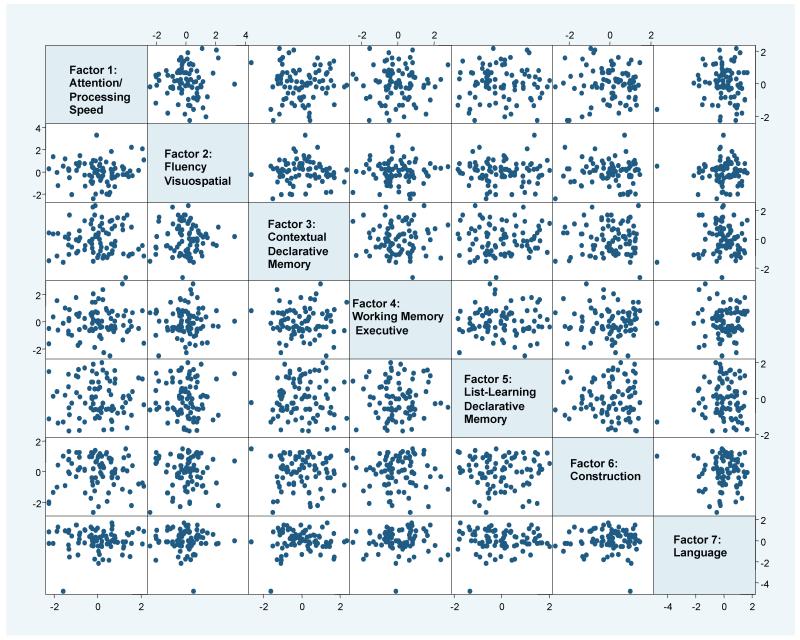
Figure 3 demonstrates that, while seven factors were identified, the factors did not characterize distinctly separate cognitive groups.
Figure 3. Pairsplot of 7 factors based on PCFA of 17 psychometric scoresa in the PANUC cohort.
A matrix of all possible two-dimensional scatterplots for the seven factor scores obtained from PCFA. Each data point represents an individual subject’s pair of factor scores (based on the horizontal and vertical axes). Given all possible configurations of pairwise factor scatterplots, there is a lack of any distinct clustering of subjects.
aPCFA was implemented on the standardized residuals from the linear regression of the raw scores adjusted for age at visit, education, disease duration, and gender
DISCUSSION
The current study demonstrates an apparent lack of distinct cognitive subtypes among patients diagnosed with PD-MCI using both methods described by the MDS Task Force and independent statistical methodology. Subtype categorization using the diagnostic methods specified by the MDS Task Force for PD-MCI yielded a sample that was largely identified as having multiple-domain PD-MCI, consistent with other recent investigations that reported 92-93% of PD-MCI participants were diagnosed with the multiple-domain subtype when using the MDS Task Force criteria.12, 13 These findings are in part due to the constraints imposed by the specific Task Force criteria: if only one test within a domain is required to produce a multiple-domain diagnosis and two tests within a domain are required for a single-domain diagnosis, then by definition most participants will be diagnosed with multiple-domain subtype. Alternatively, other diagnostic methods have identified single-domain MCI as being more prevalent in the PD population.6, 7, 35 Thus, results from the current study and others using the MDS Task Force criteria suggest that these criteria may lead to a high prevalence of multiple-domain MCI in PD patients. Indeed, in our sample and others,13, 14 when impairment on two tests were required within each domain for the multiple domain subtype, a much higher prevalence of the single domain subtype was found (Table e-1).
Interestingly, however, when statistical methods were employed to evaluate cognitive test performance in PD-MCI participants, the resulting seven factors did not partition the sample into distinctly identified cognitive subgroups. These findings are consistent with results reported to date using the MDS Task Force recommendations, and support the higher prevalence of multiple-domain MCI found when implementing the Task Force criteria. Thus, our independent statistical methods help to validate our clinical findings. The lack of distinct cognitive subgroups may be a function of impairments in frontally-mediated abilities (which range from simple attention to higher order executive functions) that are frequently seen in patients with PD.36, 37 As demonstrated in the current study, a majority of PD patients have some type of impairment on tests that measure attention/working memory, skills generally considered to be mediated by anterior/subcortical regions which in turn may influence many other more posterior-mediated functions.
The factor analytic methods used in this study produced interesting results, including the finding that visuospatial skills and verbal fluency (particularly semantic fluency) loaded on the same factor. This is not an intuitive outcome; different brain regions are thought to underlie performance on these measures. Interestingly, however, both semantic fluency and visuospatial impairment have been identified as possible precursors to more rapid cognitive decline and dementia.10, 38-40 It has been suggested that, while executive cognitive impairments may arise as a result of dopamine depletion, vascular insults, and other subcortical pathology in PD, more cortically-based deficits are required for the onset of dementia.9 Thus, although subjects are likely to exhibit multiple impairments that do not partition into distinct cognitive subtypes per se, it is possible that impairments on these specific tasks may identify those most likely to progress. Longitudinal measurement in the cohort will provide additional information concerning performance on specific cognitive tests and progression to dementia.
Importantly, our PCFA results do not completely support the division of tests into the five cognitive domains identified by the MDS Task Force. For example, some cognitive tests measure multiple cognitive functions beyond their purported primary assessment domain (e.g., phonemic fluency is both a measure of language and executive ability), while others do not fit neatly into the cognitive domains to which they are typically assigned (e.g., the clock drawing test loaded on a construction factor but did not load with the other executive measures). In addition, frontally-mediated functions include a wide range of abilities that encompass simple attention, concentration, working memory, and higher-order executive abilities, such as reasoning, abstraction, and task-switching. It is not always possible to disentangle these functions from each other: higher order functions require lower-level abilities. Thus, the division of cognitive domains into “executive” and “attention/working memory” may be arbitrary. Indeed, our “executive” measures tended to load on factors not always associated with executive abilities (and thus may account for the lower than expected prevalence of impairment on this domain as defined by the Task Force criteria), while “attention/working memory” measures loaded on separate factors (“attention/processing speed” and “working memory/executive”). Future endeavors may seek either to redefine the cognitive domains or to redistribute the measures among the domains.
Given the results of the current study, it is unlikely that cognitive subtypes alone will provide substantial information concerning those participants most at risk for cognitive decline. It is possible, however, that a combination of test performance profile and some other disease marker will eventually permit identification of those likely to develop dementia. For example, motor subtype (tremor-dominant vs. postural instability/gait disturbance) has been implicated in the progression of cognitive symptoms, such that those with postural instability/gait disturbance may be more likely to progress rapidly to dementia.7, 41, 42,43 Additionally, genetic factors have been implicated in cognitive performance,9, 44, 45 as have biomarkers for neurodegeneration, vascular impairment, and beta-amyloid.8, 40, 46, 47 Importantly, the study of cognitive impairment may require analysis of test performance on a continuum rather than trying to place individual patients/subjects into distinct categories. Thus, future PD-related research should focus on developing algorithms that take multiple disease factors and longitudinal test performance into consideration when determining those most at risk for dementia.
A primary limitation of the study is its cross-sectional design; longitudinal study will permit a better understanding of which tests and impairment domains are suggestive of more rapid cognitive progression. In addition, despite the MDS Task Force’s development of operational methods for the identification of MCI in PD cohorts, the criteria allow for variability across sites in terms of setting an appropriate level of impairment. Because the PANUC cohort has higher than average estimated premorbid abilities, we have found that setting a less restrictive level of impairment permits diagnosis of highly educated individuals who have notable cognitive complaints but seemingly milder impairments on testing. Differences in level of impairment can result in prevalence rates that vary between 9-93%,48 and thus, our results may differ from others due to such variations in methodology. However, we believe that including patients who meet these less restrictive requirements will ultimately provide a greater breadth of information concerning the onset, course, and variability of PD-MCI.
The PANUC battery also incorporates a greater number of measures in the attention/working memory domain. This domain is comprised of several functions (visual attention, auditory attention, task switching, working memory, etc.) subserved by discrete areas in the frontal lobes which may be affected differentially in PD. Inclusion of measures that assess these multiple functions increases the likelihood that early changes in frontally-mediated abilities will be detected on formal testing; however, it may also artificially increase the prevalence of impairments in this domain. In addition, we did not incorporate our measure of premorbid ability into the current analyses (resulting in a reduced number of included participants); recent research suggests this creates even greater variability and is difficult to apply across populations.13 Finally, the clinical diagnostic guidelines remain to be validated, and the requirement for the inclusion of extensive testing may increase the likelihood of spurious findings. Indeed, the proportion of participants who were excluded from the current study due to inability to complete the entire battery suggests that some reduction in total number of tests required may be necessary in this population. As these subjects had been identified as having PD-MCI by an expert diagnostic panel using fewer tests, it is thus unclear to date whether the large number of tests required by the Task Force are necessary for a valid diagnosis of PD-MCI in research populations.
These results provide evidence that separation of groups based purely on cognitive subtyping may not provide sufficient information for determining distinct PD groups that may be useful for longitudinal study. However, combining cognitive test performance with other factors, such as genetic profile, imaging, biomarkers, and/or motor subtype may eventually prove to be the best method for identifying those most likely to rapidly progress to dementia. Future endeavors to validate the PD-MCI criteria and to identify combinations of disease features that most strongly determine risk for cognitive progression should be undertaken.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by NS062684 and NS065070, the Nancy and Buster Alvord Endowment, and the Department of Veterans Affairs. The funding sources did not provide scientific input for the study.
Alberto J. Espay is supported by the K23 career development award (NIMH, 1K23MH092735); has received grant support from CleveMed/Great Lakes Neurotechnologies, and Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Solvay (now Abbvie), Chelsea Therapeutics, TEVA, Impax, Merz, Pfizer, Solstice Neurosciences, Eli Lilly, and USWorldMeds; royalties from Lippincott Williams & Wilkins and Cambridge; and honoraria from Novartis, UCB, TEVA, the American Academy of Neurology, and the Movement Disorders Society. He serves as Associate Editor of Movement Disorders and Frontiers in Movement Disorders and on the editorial board of The European Neurological Journal.
Brenna A. Cholerton: Supported by NIH
Cyrus P. Zabetian: Funded by grants from the American Parkinson Disease Association, Department of Veterans Affairs, NIH, Northwest Collaborative Care, and Parkinson’s Disease Foundation.
Jia Y. Wan: Supported by NIH.
Karen L. Edwards: Supported by NIH.
Thomas J. Montine: Supported by NIH and Nancy and Buster Alvord Endowment
Joseph F. Quinn: Funded by the Department of Veterans Affairs and the NIH (UH2TR000903-02, U10NS077350-01, P30 AG008017, P50 NS062684). He serves as an Associate Editor for the Journal of Alzheimer’s Disease.
Ignacio F. Mata: Dr. Mata is funded by grants from the Department of Veterans Affairs, NIH, and Parkinson’s Disease Foundation.
Amie Peterson: VA Career Development Award
Fredy J. Revilla: Consultant and speaker for Lundbeck Speaker’s Bureau and UCB Speaker’s Bureau; supported by NIH.
Johnna Devoto: Supported by the Michael J Fox Foundation.
Shu-Ching Hu: Supported by NIH.
James B. Leverenz: Supported by the NIH, Jane and Lee Seidman Fund, and is a consultant for Boehringer-Ingelheim, Navidea Biopharmaceuticals, and Piramal Healthcare.
Footnotes
Relevant Financial Disclosures/Conflicts of Interest:
Brenna A. Cholerton: No relevant disclosures/conflicts of interest to report.
Cyrus P. Zabetian: Funded by grants from the American Parkinson Disease Association, Department of Veterans Affairs, NIH, Northwest Collaborative Care, and Parkinson’s Disease Foundation.
Jia Y. Wan: No relevant disclosures/conflicts of interest to report.
Karen L. Edwards: No relevant disclosures/conflicts of interest to report.
Thomas J. Montine: No relevant disclosures/conflicts of interest to report.
Joseph F. Quinn: No relevant disclosures/conflicts of interest to report.
Ignacio F. Mata: No relevant disclosures/conflicts of interest to report.
Kathryn A. Chung: No relevant disclosures/conflicts of interest to report.
Amie Peterson: No relevant disclosures/conflicts of interest to report.
G. Stennis Watson: No relevant disclosures/conflicts of interest to report.
Shu-Ching Hu: No relevant disclosures/conflicts of interest to report.
DOCUMENTATION OF AUTHOR ROLES
Research Project: A. Conception- KLE, JYW, CPZ, IFM, BC, JBL, GSW, TJM B. Organization- KLE, JYW, CPZ, IFM, BC, JBL, GSW; C. Execution- KLE, JYW, BC, CPZ, JBL, GSW, FJR, AJE, JD, SCH
Statistical Analysis: A. Design- KLE, JYW; B. Execution- KLE, JYW; C. Review and Critique BC, CPZ, JYW, KLE, TJM, JFQ, IFM, KAC, AP, AJE, FJR, JD, GSW, SCH, JBL
Manuscript Preparation: A. Writing of the first draft-BC; B. Review and Critique- BC, CPZ, JYW, KLE, TJM, JFQ, IFM, KAC, AP, AJE, FJR, JD, GSW, SCH, JBL
REFERENCES
- 1.Reginold W, Duff-Canning S, Meaney C, et al. Impact of mild cognitive impairment on health-related quality of life in Parkinson’s disease. Dement Geriatr Cogn Disord. 2013;36(1-2):67–75. doi: 10.1159/000350032. [DOI] [PubMed] [Google Scholar]
- 2.Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G, Norwegian ParkWest Study G Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurol. 2009;72(13):1121–1126. doi: 10.1212/01.wnl.0000338632.00552.cb. [DOI] [PubMed] [Google Scholar]
- 3.Borson S, Frank L, Bayley PJ, et al. Improving dementia care: the role of screening and detection of cognitive impairment. Alzheimers Dement. 2013;9(2):151–159. doi: 10.1016/j.jalz.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldman JG, Litvan I. Mild cognitive impairment in Parkinson’s disease. Minerva Med. 2011;102(6):441–459. [PMC free article] [PubMed] [Google Scholar]
- 5.Kim KR, Lee KS, Cheong HK, Eom JS, Oh BH, Hong CH. Characteristic profiles of instrumental activities of daily living in different subtypes of mild cognitive impairment. Dement Geriatr Cogn Disord. 2009;27(3):278–285. doi: 10.1159/000204765. [DOI] [PubMed] [Google Scholar]
- 6.Sollinger AB, Goldstein FC, Lah JJ, Levey AI, Factor SA. Mild cognitive impairment in Parkinson’s disease: subtypes and motor characteristics. Parkinsonism Relat Disord. 2010;16(3):177–180. doi: 10.1016/j.parkreldis.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldman JG, Weis H, Stebbins G, Bernard B, Goetz CG. Clinical differences among mild cognitive impairment subtypes in Parkinson’s disease. Mov Disord. 2012;27(9):1129–1136. doi: 10.1002/mds.25062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Garcia D, Clavero P, Gasca Salas C, et al. Posterior parietooccipital hypometabolism may differentiate mild cognitive impairment from dementia in Parkinson’s disease. Eur J Nucl Med Mol Imaging. 2012;39(11):1767–1777. doi: 10.1007/s00259-012-2198-5. [DOI] [PubMed] [Google Scholar]
- 9.Pagonabarraga J, Kulisevsky J. Cognitive impairment and dementia in Parkinson’s disease. Neurobiol Dis. 2012;46(3):590–596. doi: 10.1016/j.nbd.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Williams-Gray CH, Mason SL, Evans JR, et al. The CamPaIGN study of Parkinson’s disease: 10-year outlook in an incident population-based cohort. J Neurol Neurosurg Psychiatry. 2013;84(11):1258–1264. doi: 10.1136/jnnp-2013-305277. [DOI] [PubMed] [Google Scholar]
- 11.Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27(3):349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldman JG, Holden S, Bernard B, Ouyang B, Goetz CG, Stebbins GT. Defining optimal cutoff scores for cognitive impairment using Movement Disorder Society Task Force criteria for mild cognitive impairment in Parkinson’s disease. Mov Disord. 2013;28(14):1972–9. doi: 10.1002/mds.25655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marras C, Armstrong MJ, Meaney CA, et al. Measuring mild cognitive impairment in patients with Parkinson’s disease. Mov Disord. 2013;28(5):626–633. doi: 10.1002/mds.25426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broeders M, de Bie RM, Velseboer DC, Speelman JD, Muslimovic D, Schmand B. Evolution of mild cognitive impairment in Parkinson disease. Neurol. 2013;81(4):346–352. doi: 10.1212/WNL.0b013e31829c5c86. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen KF, Larsen JP, Tysnes OB, Alves G. Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol. 2013;70(5):580–586. doi: 10.1001/jamaneurol.2013.2110. [DOI] [PubMed] [Google Scholar]
- 16.Geurtsen GJ, Hoogland J, Goldman JG, et al. Parkinson’s Disease Mild Cognitive Impairment: Application and Validation of the Criteria. J Parkinsons Dis. 2013 doi: 10.3233/JPD-130304. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cholerton BA, Zabetian CP, Quinn JF, et al. Pacific northwest udall center of excellence clinical consortium: study design and baseline cohort characteristics. J Parkinsons Dis. 2013;3(2):205–214. doi: 10.3233/JPD-130189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51(6):745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 20.Benedict RHB, Schretlen D, Groninger L, Brandt J. The Hopkins Verbal Learning Test-Revised: Normative data and analysis of inter-form and inter-rater reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- 21.Wechsler D. Wechsler Memory Scale-Revised Manual. The Psychological Corporation; San Antonio: 1987. [Google Scholar]
- 22.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14(2):167–177. [PubMed] [Google Scholar]
- 23.Rouleau I, Salmon DP, Butters N, Kennedy C, McGuire K. Quantitative and qualitative analyses of clock drawings in Alzheimer’s and Huntington’s disease. Brain Cogn. 1992;18(1):70–87. doi: 10.1016/0278-2626(92)90112-y. [DOI] [PubMed] [Google Scholar]
- 24.Wechsler D. Wechsler Adult Intelligence Scale-Revised manual. The Psychological Corporation; San Antonio: 1987. [Google Scholar]
- 25.Wechsler D. WAIS-III® Administration and Scoring Manual. The Psychological Corporation Harcourt Brace & Company; San Antonio, TX: 1997. [Google Scholar]
- 26.Army Individual Test Battery: Manual of directions and scoring. War Department, Adjutant General’s Office; Washington, DC: 1944. [Google Scholar]
- 27.Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: administration, norms, and commentary. 3rd ed. Oxford University Press; Oxford; New York: 2006. [Google Scholar]
- 28.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- 29.Benton AL, Sivan AB, Hamsher K, Varney N, Spreen O. Contributions to Neuropsychological Assessment-A Clinical Manual. Psychological Assessment Resources; Lutz, FL: 1994. [Google Scholar]
- 30.Maeshima S, Itakura T, Nakagawa M, Nakai K, Komai N. Visuospatial impairment and activities of daily living in patients with Parkinson’s disease: a quantitative assessment of the cube-copying task. Am J Phys Med Rehabil. 1997;76(5):383–388. doi: 10.1097/00002060-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Buchhave P, Stomrud E, Warkentin S, Blennow K, Minthon L, Hansson O. Cube copying test in combination with rCBF or CSF A beta 42 predicts development of Alzheimer’s disease. Dement Geriatr Cogn Disord. 2008;25(6):544–552. doi: 10.1159/000137379. [DOI] [PubMed] [Google Scholar]
- 32.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 33.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 34.Jurica P, Leitten C, Mattis S. Dementia Rating Scale-2™ (DRS-2™) 2001. [Google Scholar]
- 35.Caviness JN, Driver-Dunckley E, Connor DJ, et al. Defining mild cognitive impairment in Parkinson’s disease. Mov Disord. 2007;22(9):1272–1277. doi: 10.1002/mds.21453. [DOI] [PubMed] [Google Scholar]
- 36.Owen AM. Cognitive dysfunction in Parkinson’s disease: the role of frontostriatal circuitry. Neuroscientist. 2004;10(6):525–537. doi: 10.1177/1073858404266776. [DOI] [PubMed] [Google Scholar]
- 37.Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci. 2003;23(15):6351–6356. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stepkina DA, Zakharov VV, Yakhno NN. Cognitive impairments in progression of Parkinson’s disease. Neurosci Behav Physiol. 2010;40(1):61–67. doi: 10.1007/s11055-009-9223-6. [DOI] [PubMed] [Google Scholar]
- 39.Johnson DK, Galvin JE. Longitudinal changes in cognition in Parkinson’s disease with and without dementia. Dement Geriatr Cogn Disord. 2011;31(2):98–108. doi: 10.1159/000323570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Compta Y, Pereira JB, Rios J, et al. Combined dementia-risk biomarkers in Parkinson’s disease: a prospective longitudinal study. Parkinsonism Relat Disord. 2013;19(8):717–724. doi: 10.1016/j.parkreldis.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Burn DJ, Rowan EN, Allan LM, Molloy S, O’Brien JT, McKeith IG. Motor subtype and cognitive decline in Parkinson’s disease, Parkinson’s disease with dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2006;77(5):585–589. doi: 10.1136/jnnp.2005.081711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor JP, Rowan EN, Lett D, O’Brien JT, McKeith IG, Burn DJ. Poor attentional function predicts cognitive decline in patients with non-demented Parkinson’s disease independent of motor phenotype. J Neurol Neurosurg Psychiatry. 2008;79(12):1318–1323. doi: 10.1136/jnnp.2008.147629. [DOI] [PubMed] [Google Scholar]
- 43.Poletti M, Frosini D, Pagni C, et al. Mild cognitive impairment and cognitive-motor relationships in newly diagnosed drug-naive patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2012;83(6):601–606. doi: 10.1136/jnnp-2011-301874. [DOI] [PubMed] [Google Scholar]
- 44.Alcalay RN, Mejia-Santana H, Tang MX, et al. Self-report of cognitive impairment and mini-mental state examination performance in PRKN, LRRK2, and GBA carriers with early onset Parkinson’s disease. Journal Clin and Exp Neuropsychol. 2010;32(7):775–779. doi: 10.1080/13803390903521018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuang D, Leverenz JB, Lopez OL, et al. APOE epsilon4 increases risk for dementia in pure synucleinopathies. JAMA Neurol. 2013;70(2):223–228. doi: 10.1001/jamaneurol.2013.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Filoteo JV, Reed JD, Litvan I, Harrington DL. Volumetric correlates of cognitive functioning in nondemented patients with Parkinson’s disease. Mov Disord. 2013 doi: 10.1002/mds.25633. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Theilmann RJ, Reed JD, Song DD, et al. White-matter changes correlate with cognitive functioning in Parkinson’s disease. Front Neurol. 2013;4:37. doi: 10.3389/fneur.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liepelt-Scarfone I, Graeber S, Feseker A, et al. Influence of different cut-off values on the diagnosis of mild cognitive impairment in Parkinson’s disease. Parkinson’s disease. 2011:540–843. doi: 10.4061/2011/540843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.