Abstract
Objective
To compare the effects of 4 months of isolated lumbar resistance exercise and total body resistance exercise on walking performance in obese, older adults with chronic low back pain. A secondary analysis examined whether responsiveness to training modulated walking improvement.
Design
Randomized, controlled trial.
Setting
Research laboratory affiliated with tertiary care facility.
Methods and Intervention
Participants (N = 49; 60–85 years) were randomized into a 4-month resistance exercise intervention (TOTRX), lumbar extensor exercise intervention (LEXT), or a control group (CON).
Main Outcome Measurements
Walking performance, maximal low back strength and leg strength, and average resting and low back pain severity score (from an 11-point numerical pain rating scale; NRSpain) were collected at baseline and month 4.
Results
The TOTRX and LEXT improved lumbar extensor strength relative to CON and the TOTRX (P < .05). NRSpain scores at month 4 were lowest in the TOTRX group compared with the LEXT and CON groups, respectively (2.0 ± 1.7 points vs 3.7 ± 2.6 points and 4.6 ± 2.4 points; P < .006). A total of 53% and 67% of participants in the TOTRX and LEXT groups were responders who made lumbar extensor strength gains that achieved ≥20% greater than baseline values. Although the TOTRX demonstrated the greatest improvement in walking endurance among the intervention groups, this did not reach significance (10.1 ± 12.2% improvement in TOTRX vs 7.4 ± 30.0% LEXT and −1.7 ± 17.4% CON; P = .11). Gait speed increased most in the TOTRX (9.0 ± 13.5%) compared with the LEXT and CON groups (P < .05). The change in lumbar extensor strength explained 10.6% of the variance of the regression model for the change in walking endurance (P = .024).
Conclusions
The use of LEXT and TOTRX produced similar modest improvements in patients’ walking endurance. Lumbar extensor strength gain compared with leg strength gain is a moderate but important contributor to walking endurance in obese older adults with chronic low back pain. Responders to resistance exercise programs (event those with only lumbar extension exercise) who make at least a 20% improvement in strength can expect better improvement in walking endurance than those who do not achieve this strength improvement.
INTRODUCTION
Muscle strength is important for the maintenance of walking ability as a person ages. Weakness in the leg muscles compromises walking endurance, gait speed, crouch, stair climbing, and rising from a chair [1,2]. Emerging evidence suggests that strength deficits in persons with no current mobility disability can predict a high risk of developing future mobility impairment [3]. As degenerative joint diseases develop in the lower extremity and spine, physical activity and muscle strength may decrease. Most authors have investigated the effects of knee extensor, knee flexor, hip extensor, and ankle plantar flexor strength on patients’ walking and mobility tasks [1,3,4]. The maintenance of lumbar strength is also important for physical aspects of quality of life [5] in aging, and lumbar strength deficits exist in persons with low back pain (LBP) [6]. However, lumbar muscle strength is not commonly measured in aging-related mobility research. This is unfortunate because lumbar muscles are involved in key mobility tasks, and these muscles are activated at approximately 30% of maximal voluntary strength values during walking [7].
Lumbar strengthening might be a key therapeutic component in treating chronic LBP and walking impairment. The region encompassing the low thoracic and lumbar spine and associated muscles is among the most prevalent sites for pain in older adults [8], and chronic LBP is related to a greater prevalence of physical disability and difficulties performing activities of daily living and self-care [9]. Excess body weight exacerbates mobility limitations in older adults with LBP compared with healthy-weight counterparts [10]. Walking impairment diminishes the quality of life and might contribute to additional weight gain. Obesity-related lumbar muscle strength deficits [11] worsen pain severity and mobility impairment. Older patients with unrelenting LBP often resort to costly medications or medical procedures [12,13]. The physical and economic burdens of excess weight coupled with chronic LBP will likely worsen as our older population grows. Cost-effective strategies that preserve mobility are vital in preventing this escalating health care burden in the obese, older population [14].
A possible approach to improve walking performance (endurance and speed) in the obese older adult with chronic LBP is to correct strength deficits such as isolated lumbar extension exercise or a total body program that incorporates lumbar extension. We previously reported that 6 months of resistance exercise (including lumbar extension) increases lumbar strength across the range of lumbar flexion motion in healthy, overweight older adults [15]. The relative contributions of exercise-induced lumbar extension strength or leg strength to improvements in walking mobility in obese, older adults with LBP are not yet clear. The purpose of this study was to compare the effects of 4 months of isolated lumbar resistance exercise and total body resistance exercise on walking performance (endurance, gait speed) in obese, older adults with chronic LBP. A secondary analysis was performed to examine whether responsiveness to training (low back strength gain of ≥20% from baseline) modulated walking endurance and speed in each training group.
We hypothesized that the total body exercise group would make superior improvement in walking performance than the other groups. We also hypothesized that improvements in low back and leg strength would similarly contribute to walking endurance and speed. Finally, we hypothesized that the participants who responded better to the training (by demonstrating a ≥20% increase in low back strength) would achieve greater improvements in walking endurance and speed compared with participants who did not respond well (did not achieve at least a 20% increase in low back extensor strength). This improvement value was chosen because it exceeds previously published improvements in leg and lower back strength after resistance training exercise in diabetic, overweight, and obese older persons [15,16].
METHODS
Participants
Individuals with chronic LBP were recruited from the Gainesville, Florida, area and surrounding regions using the UF Orthopaedics Clinics, the Clinical Trials Register, study flyers and newspaper advertisements, and a list of older adults provided by the UF Claude Pepper Aging Center.
Inclusion Criteria
Men and women who were 60–85 years, who had a waist circumference greater than 88 cm (women) and 102 cm (men), a body mass index value of ≥30 kg/m2, who were suffering from LBP for ≥6 months [17], who had abdominal obesity [18], and who were free of abnormal cardiovascular responses during the graded maximal walk test were eligible for the study. Exclusion criteria included being wheelchair bound, having a specific LBP or acute back injury [17], having spinal stenosis with neurogenic claudication, having back surgery within the previous 2 years [17], or currently using any pharmacologic or lifestyle weight-loss interventions. This study was approved by the University of Florida Institutional Review Board. All study processes and procedures on human subjects were conducted following the Helsinki Declaration of 1975 guidelines, as revised in 1983. All participants completed an informed consent process with the study team and signed an informed consent form. The study was registered as a clinical trial (NCT01250262).
Symptoms of LBP
The severity of back pain while the patient stood at rest was self-reported with the use of an 11-point numerical pain rating scale (NRSpain) with anchors of 0, indicating no pain, and 10, indicating the worst possible pain. The NRSpain measure is an accepted outcome for chronic pain conditions, as described in the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials [19]. This measurement is reliable and valid [20] for assessing pain intensity.
Walking Tests and Strength
Each participant performed walking tests (endurance time, gait speed at 2 velocities) and maximal strength testing of the lumbar muscles and the lower extremity. The primary outcomes were walking endurance time and gait speed.
Graded Treadmill Walking Exercise Test
The participant’s peak aerobic capacity, or rate of oxygen consumption, was measured by the use of a graded exercise test (modified incremental treadmill Naughton) [21]. All tests followed the guidelines of the American College of Sports Medicine [22], with electrocardiogram heart monitoring and periodic blood pressure measures recorded. Open-circuit spirometry was used to determine the peak rate of oxygen use and carbon dioxide production. LBP symptoms and severity were collected with the NRSpain rating scale pre-exercise, at every workload change, and every 2 minutes during recovery until stopping the treadmill. Peak NRSpain ratings are reported, as peak ratings consistently occurred at the greatest treadmill workload. During the treadmill test, NRSpain severity values were collected every 2 minutes in parallel with changes in workload. Walking time until voluntary exhaustion was recorded.
Gait Speed
After practice trials of walking on a 26-foot long gait mat (GaitRite; CIRSystems, Inc.; Havertown, PA), 6 trials were collected at a self-selected, comfortable pace and at the fastest possible pace. Measurement of gait characteristics at the fastest pace represents the physical ability to perform greater-intensity exercise compared with the self-selected comfortable pace. Short distance walking speed of distances less than 10 m strongly predicts adverse health events and early mortality in older adults [23]. Fast walking speed also has prognostic value to predict lower extremity physical limitations [24] and loss of functional independence [25].
Lower Extremity and Lumbar Muscle Strength
Assessments of maximal muscle strength (1 repetition maximum, or 1RM) were determined for all major muscle groups. After a 5-minute period of treadmill walking for warm-up, the participant performed 5 light-intensity repetitions, 3 moderate-intensity repetitions, and progressively heavier repetitions separated by a 1-minute rest period on the 3 exercises: the leg press, leg extension, and lumbar extension. The maximum weight that was lifted once with appropriate form was identified as the 1RM for the exercise. Heart rates and blood pressures were monitored immediately after each maximal lift.
Resistance Exercise Interventions
All training was performed in the UF Department of Orthopaedics and Rehabilitation in the Human Dynamics Laboratory. Participants were randomly assigned to 1 of 3 study groups by one study coordinator: a total body resistance exercise group (TOTRX; includes lumbar extension), an isolated lumbar extension resistance exercise group (LEXT) or a nonexercise control group (CON). A computer-generated list was used to randomly assign the group allocation; the assignments per participant number were placed in numbered sealed envelopes, and each new enrolled participant opened an envelope to receive the group assignment. The Principal Investigator (H.K.V.) was blinded to the allocation sequence. The overall random allocations sequence was not revealed until all participants had been assigned. Single-blinding is an accepted procedure for exercise-based studies as indicated by the Consolidated Standards of Reporting Trials Group [26].
For the TOTRX group, 3 resistance exercise sessions were performed per week, and one set of each bilateral exercise was completed with MedX (Ocala, FL) resistance machines: leg press, leg curl, leg extension, chest press, seated row, overhead press, triceps dip, lumbar extension, biceps curl, calf press, and abdominal curl. Each set contained 15 repetitions performed at a resistance load of 60% of the 1RM for that exercise to reduce the risk of injury. Participants subjectively rated the effort of the exercise set using the 6- to 20-point Borg scale [27]. An average rest period between each set was 90 seconds. The resistance load was increased by ~2% for the set per week to maintain the same relative level of muscle effort at ~16–18 for the exercise over time [27]. For the LEXT group, during the first 2 weeks, participants performed 2 sets of lumbar extensions as they acclimate to the exercise (15 repetitions until volitional fatigue) once a week. From 2 weeks until month 4, participants performed one set of lumbar extensions (15 repetitions) 3 times a week. Although this prescription of once a week has been shown to elicit similar strength gains as programs that use 2–3 sessions a week for long term functional improvements [28], we controlled for the study team contact similar to that of the TOTRX group.
All exercise sessions were supervised by experienced exercise physiologists. The total training duration was 4 months. The physiologists would coach the participants to recognize the sensation of working a muscle during the exercise, of experiencing the soreness 24–48 hours after the exercise (if applicable). The time, perceived effort, exercise load, and repetitions completed for each exercise per session were recorded in a personalized training log in the laboratory. If a participant showed worsening of back pain or development of new symptoms during training, the physicians and experts on the study evaluated the participant to determine whether continuation with training was appropriate. Only one participant required a physician review.
Nonexercise, Standard Care
Patients received normal medical care and follow-up during the 4-month study and no exercise intervention. Printed recommendations from the Centers for Disease Control and Prevention and the American Heart Association regarding physical activity and nutrition guidelines were provided and reviewed with the participant as part of standard care. Provision of guidelines have been used in control groups in similarly designed strengthening exercise studies [29]. These guideline materials were also provided to the participants in the 2 resistance exercise groups. Standard care participants were offered the opportunity to complete a total body resistance exercise program after the control period. Telephone contact was made every 2 weeks to encourage the participants to follow the health guidelines.
Sample Size
Sample size was calculated by the use of our previously published data regarding differences elicited in walking endurance after a 6-month resistance exercise training program in obese older adults [30]. The primary outcome measure was walking endurance time between standard care and MedX resistance exercise in obese persons [30]. The results from a randomized, controlled training study in older healthy adults revealed that mean differences in walking endurance values were 1.7 minutes (exercise group) and −1.45 minutes (control group) with pooled standard deviations of 1.1 minutes at baseline and 0.7 minutes posttraining. The power analysis revealed that a total sample size of 27 participants (n = 9 per group) would yield 90% power to detect these differences between groups at the α level of 0.05. In our exercise study, the average drop-out rate was 25% [15]. Therefore, the sample size was increased to account for these dropouts.
Statistics
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS; v.21.0, SPSS Institute, Chicago, IL). Data were managed using REDCap [31]. Descriptive statistics and frequencies were obtained to characterize the study groups. χ2 tests were used to determine whether differences existed in the proportions of participants with comorbidities. Repeated-measures analyses of variance were used to determine whether the 3 groups responded differently to the intervention over time. The between subject factor was study group (CON, LEXT, TOTRX) and the within-subject variables included walking test scores, gait speed and 1RM scores.
A secondary analysis of the main variables (walking test scores, gait speed) was performed on the basis of whether participants were responders to training (achieved ≥20% in lumbar strength) or low responders to training (achieved <20% in lumbar strength). Interactions of study group (CON, LEXT, TOTRX) by responder status (low responders, responders) were tested using univariate analyses of variance. To determine whether improvements in lumbar muscle strength or lower extremity strength predicted walking endurance or daily activity, stepwise regression analysis was performed. The change in walking endurance time from pre-post training was the dependent variable. Training-induced changes in lumbar pain were a covariate, and the changes in lumbar strength, leg press and leg extension were then added to each model. Significance was established at P < .05 for all statistical tests.
RESULTS
Participant Characteristics
The Consolidated Standards of Reporting Trials diagram is shown in Figure 1. A total of 196 people were screened by phone, and 72 signed the informed consent form. Seven failed the exercise screening test, and the remaining patients were lost to follow-up for various reasons. Participants who did not complete the training were not included in the final analyses because the reasons for drop out were major, unforeseen life issues that made participation impossible and these reasons had nothing to do with back pain or the intervention (eg, diagnosis of lung cancer, became caretakers of spouse or other family members after serious health issues, distance travel became impossible, financial). Baseline participant characteristics are shown in Table 1 [22]. There were no differences in the physiologic characteristics or prevalence of comorbidities among the 3 study groups (P value for χ2 tests ranged from .323 to .919). The compliance with study testing sessions was 100% in all groups, and the percentage of resistance exercise training sessions completed during the 4 months averaged 87% in both LEXT and TOTRX groups. Body weight did not change from baseline to month 4. Month four body weight values were 88.9 ± 13.8 kg (CON), 89.5 ± 14.6 kg (LEXT), and 89.8 ± 21.9 kg (TOTRX). Compared with the average standing resting pain scores at baseline, the pain scores at month 4 were lowest in the TOTRX group compared with the LEXT and CON groups, respectively (2.0 ± 1.7 points vs 3.7 ± 2.6 points and 4.6 ± 2.4 points; P < .012).
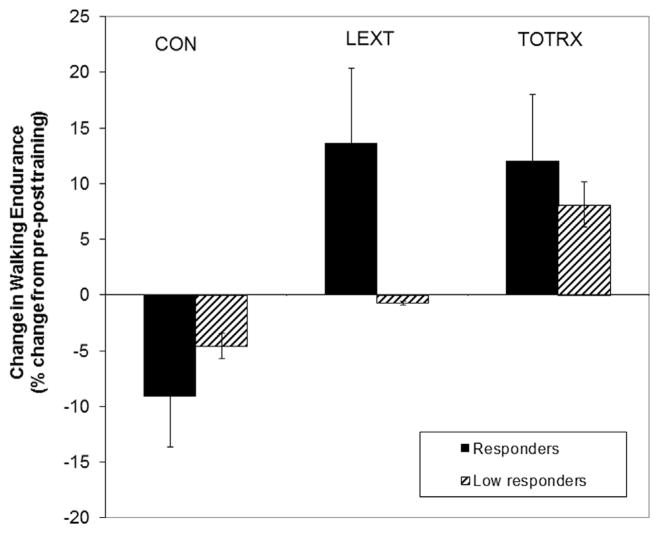
Figure 1.
The change in walking endurance from pre-post training (4 months) in participants who were high responders (demonstrated ≥20% increase in lumbar extensor 1RM from baseline to month 4) or low responders (demonstrated less than <20% increase in lumbar extensor 1RM from baseline to month four). Values are mean ± standard deviation (SD).
Table 1.
Baseline characteristics of the study groups
| CON (n = 14) | LEXT (n = 18) | TOTRX (n = 17) | |
|---|---|---|---|
| Age, y | 67.5 ± 6.4 | 68.7 ± 7.1 | 68.6 ± 7.3 |
| Height, cm | 169 ± 10 | 167 ± 12 | 167 ± 12 |
| Mass, kg | 89.8 ± 14.3 | 89.9 ± 21.2 | 95.1 ± 15.2 |
| Women, % | 61.1 | 66.7 | 69.6 |
| BMI, kg/m2 | 31.2 ± 4.2 | 32.0 ± 4.8 | 33.9 ± 5.1 |
| Waist circumference, cm | 103 ± 9 | 104 ± 11 | 107 ± 10 |
| White | 94.4 | 87.5 | 91.3 |
| Black | 5.6 | 12.5 | 4.3 |
| Average resting NRSpain in low back, points | 5.2 ± 2.4 | 5.0 ± 1.8 | 4.3 ± 1.8 |
| Comorbidities, % | |||
| Arthritis | 68.4 | 68.0 | 58.3 |
| Diabetes mellitus | 10.5 | 12.0 | 16.7 |
| Hypertension | 68.4 | 64.0 | 58.3 |
| Heart disease | 5.3 | 8.0 | 8.3 |
| Hypothyroid | 15.8 | 20.0 | 8.3 |
| High cholesterol | 42.1 | 44.0 | 58.3 |
| Neuropathy | 5.3 | 12.0 | 20.8 |
| Depression | 26.3 | 16.0 | 33.3 |
CON = control group; LEXT = lumbar extensor exercise intervention; TOTRX = resistance exercise intervention; BMI = body mass index; NRSpain = numerical pain rating scale, where 0 indicates no pain and 10 indicates the worst imaginable pain.
Values are means ± SD or percent of the group.
Waist circumference = narrowest part of torso [22].
Muscle Strength and Responsiveness to Training
The 1RM values for 4 of the major muscle groups at baseline and month 4 are shown in Table 2. The TOTRX and LEXT improved lumbar extensor strength relative to CON, and the TOTRX demonstrated a significant improvement in leg press strength relative to the CON group (both P < .05).
Table 2.
Maximal muscle strength values (1RM) from baseline to month 4
| CON
|
LEXT
|
TOTRX
|
||||
|---|---|---|---|---|---|---|
| Baseline | Month 4 | Baseline | Month 4 | Baseline | Month 4 | |
| Leg extension | 165 ± 52 | 169 ± 59 | 160 ± 78 | 168 ± 65 | 172 ± 78 | 196 ± 95 |
| Leg press | 359 ± 140 | 351 ± 99 | 323 ± 116 | 369 ± 94 | 314 ± 113 | 364 ± 109* |
| Abdominal curl | 45 ± 19 | 48 ± 17 | 43 ± 27 | 64 ± 61 | 44 ± 25 | 75 ± 78 |
| Lumbar extension | 151 ± 62 | 161 ± 63 | 147 ± 71 | 164 ± 70 | 140 ± 66 | 182 ± 94* |
1RM = 1 repetition maximum; CON = control group; LEXT = lumbar extensor exercise intervention; TOTRX = resistance exercise intervention. Strength values were measured by the use of bilaterally performed motions.
Values are mean ± SD. Strength values are expressed in Nm.
Different between LEXT and TOTRX compared with CON, P < .05.
Participants were categorized into responders (who achieved 20% improvement in lumbar extensor strength from baseline to month 4) and low responders (who achieved <20% improvement in lumbar strength during the 4-month period). A total of 53% and 67% of participants in the TOTRX and LEXT groups were high responders who made lumbar extensor strength gains that achieved at least 20% greater than baseline values (χ2 distribution not different between groups, P = .325).
Walking Endurance and Gait Speed
Walking endurance and speed before and after 4 months of training are shown in Table 3. Although the TOTRX demonstrated the greatest improvement in walking endurance among the intervention groups, this did not reach significance (P = .11). The secondary analysis of walking endurance change on the basis of responder status to training is shown in Figure 2. The high responders demonstrated a greater improvement in walking endurance compared with those with less lumbar strength improvement. The responders in the LEXT group achieved the greatest change in the walking endurance compared with CON and TOTRX, although this did not achieve statistical significance. Lumbar pain severity during the walking test decreased by 42%–58% in the LEXT and TOTRX groups compared with the 7% decrease in the CON group by month 4 (P = .046). The self-selected walking speed significantly increased in the TOTRX group compared to the remaining groups by month 4 (P = .045). There was not a significant interaction of study group by responder status.
Table 3.
Walking performance in obese older adults with low back pain before and after a 4-month resistance exercise training program
| CON
|
LEXT
|
TOTRX
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Month 4 | Change (%) | Baseline | Month 4 | Change (%) | Baseline | Month 4 | Change (%) | |
| Walking Endurance, min | 13.6 ± 3.2 | 12.8 ± 3.2 | −1.7 ± 17.4 | 12.5 ± 4.6 | 12.9 ± 4.2 | 7.4 ± 30.0 | 13.5 ± 4.8 | 14.6 ± 4.9 | 10.1 ± 12.2 |
| VO2 peak, mL/kg* min | 19.3 ± 4.0 | 17.4 ± 4.7 | −6.6 ± 30.2 | 16.7 ± 4.6 | 17.7 ± 6.0 | 7.0 ± 34.8 | 19.5 ± 4.4 | 20.4 ± 3.9 | 7.7 ± 21.1 |
| Speed, self-selected pace, cm/s | 123 ± 26 | 116 ± 27 | −1.1 ± 19.3 | 110 ± 18 | 112 ± 19 | 2.1 ± 15.2 | 112 ± 19 | 123 ± 29* | 9.0 ± 13.5 |
| Speed, maximal pace, cm/s | 159 ± 22 | 159 ± 29 | 0.5 ± 18.8 | 159 ± 33 | 158 ± 33 | 0.7 ± 12.7 | 157 ± 27 | 162 ± 26 | 5.5 ± 22.7 |
CON = control group; LEXT = lumbar extensor exercise intervention; TOTRX = resistance exercise intervention; VO2 peak = peak rate of oxygen use.
Values are mean ± SD, and % change represents the mean change for each group.
P < .05 compared with the remaining groups.
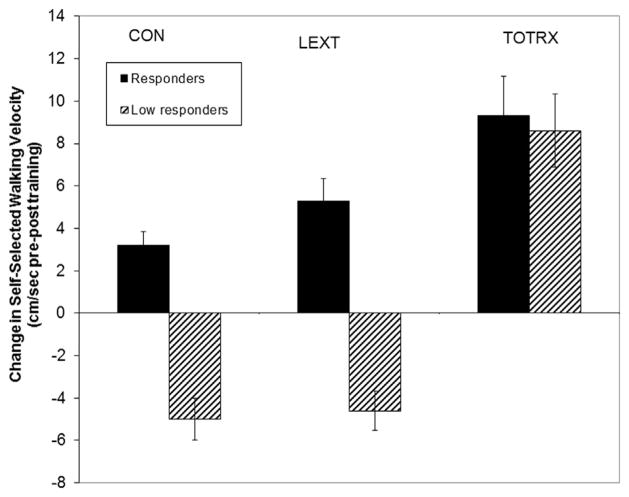
Figure 2.
The change of self-selected walking speed in participants who were high responders (demonstrated ≥20% increase in lumbar extensor 1RM from baseline to month 4) or low responders (demonstrated less than <20% increase in lumbar extensor 1RM) from baseline to month 4). Values are mean ± SD.
The secondary analyses comparing self-selected and maximal walking speed change based on training group and responder status are shown in Figures 3 and 4. The group*time interaction was not significant for the change in self-selected walking speed (Figure 2). A significant interaction between study group by responder status was found for the 4-month maximal walking speed change (Figure 3; P = .035), where the responders in the LEXT and TOTRX groups improved more than the CON group.
Figure 3.
The change of maximal walking speed in participants who were high responders (demonstrated ≥ 20% increase in lumbar extensor 1RM from baseline to month 4) or low responders (demonstrated less than <20% increase in lumbar extensor 1RM from baseline to month 4). Values are mean ± SD.
Figure 4.
The change in maximal walking speed in participants who were high responders (demonstrated 20% increase in lumbar extensor 1RM from baseline to month 4) or low responders (demonstrated less than 20% increase in lumbar extensor 1RM from baseline to month 4).
Regression Analyses
The results of the regression analyses are shown in Table 4. After covarying for the change in LBP severity after 4 months, we found that the change in lumbar extensor strength was a modest but significant contributor to the variance of the model for the change in walking endurance (explained 10.6% of the variance; P = .024). The changes in leg extension and leg press 1RM strength were not found to be significant contributors to the change in walking endurance (explained 1.0% and 5.4% of the variance the model for the change in walking endurance, respectively).
Table 4.
Stepwise regression analyses for change in the walking endurance time (from baseline to month 4)
| Walking Endurance Time | R | R2 | R2 Change | Sign of F Change (p) | B (CI) |
|---|---|---|---|---|---|
| Model 1 | |||||
| Pain change | .002 | .000 | .000 | .000 | 0.021 (−.055 to −0.09) |
| Change in back extensor strength | .325 | .106 | .106 | .024* | 0.208 (.029 to .388) |
| Model 2 | |||||
| Pain change | .002 | .000 | .000 | .991 | −0.002 (−0.078 to 0.073) |
| Change in leg press strength | .232 | .054 | .054 | .113 | 0.177 (−0.043 to 0.397) |
| Model 3 | |||||
| Pain change | .020 | .000 | .000 | .890 | −0.003 (−0.081 to 0.075) |
| Change in leg extension strength | .102 | .011 | .010 | .502 | 0.056 (−.110 to .221) |
B (CI) = unstandardized B coefficient and confidence interval; Pain change = NRSpain score change from baseline to month 4.
The change in pain was added in the first step followed by the change in muscle strength for each of the models below.
Denotes significant contribution to the model of walking endurance time, P < .05.
DISCUSSION
This study compared the effects of 4 months of LEXT and TOTRX on walking performance in obese, older adults with chronic LBP. A secondary analysis was performed to examine whether responsiveness to training modulated walking performance in each training group. After 4 months of either LEXT or TOTRX, patients exhibited similar improvements in walking endurance compared with the CON group. A total of 67% of the LEXT and 53% of the TOTRX participants improved low back extensor strength more than 20% from baseline. The responders in the LEXT demonstrated modest increases in walking endurance compared with nonresponders. Lumbar extensor strength, but not leg strength, moderately contributed to walking endurance. Therefore, it appears that walking performance may be modestly improved by the use of progressive resistance exercise that has focused lumbar extension, or includes a lumbar extension component.
Comparative data for lumbar extension training on walking performance in obese, older adults are scant. However, 4 months of resistive exercise in older adults increased lumbar extensor endurance, and this change in endurance was a predictor of standing balance [32], and leg strength change was not related with balance measures [32]. Related training studies in active, healthy community-dwelling older adults revealed that maximal walking speed increased 17% (2.0–2.3 m/s) compared to 6% in controls [33]. The change in self-selected and maximal gait speeds with TOTRX are within the ranges of what are considered small meaningful changes in older adults with mobility challenges after resistance exercise training (4–6 cm/s) [34]. The fact that some improvement in walking endurance and gait speed occurred with LEXT in responders is very noteworthy (Figures 2–4). Strengthening of the lumbar muscles appears to be important component of an exercise or rehabilitation program for LBP. Most studies in the aging adult have focused on the importance of leg strength for functional ability and mobility tasks [1,4]. These data demonstrate that lumbar strength is very important for walking performance in this older obese sample. Increases in lumbar strength exceeding 20% can improve walking endurance. When lumbar strengthening is coupled with additional large muscle group exercise (as resulted here in the TOTRX group), a progressive improvement in walking endurance and speed occurred. Potential mechanisms underlying the changes in walking mobility may be the improved activation of lumbar muscle to support the spine and the reduction in daily and ambulatory LBP severity.
Clinical Applications
The findings from this study have clinical relevance. Although there were some modest improvements in walking endurance with LEXT and TOTRX (0.5–1 minutes), pain with walking was significantly decreased over 4 months of training. Putting the evidence into a long-term view, we anticipate that as movement quality increases and pain is minimized, continued resistance exercise training may help further increase endurance time and maintain functional independence. In obesity, there is an excess weight-induced biomechanical loading that occurs on weight bearing joints [35] and the spine [36] that are related to pain [37]. Hence, weight loss is a reasonable recommendation for obese persons with degenerative musculoskeletal conditions and chronic joint pain. However, a unique finding from this study is that despite no change in body weight, the participants in the trained groups experienced clinically relevant resting LBP relief, especially in the TOTRX group (2 points from a baseline of 4 points on an 11-point NRSpain scale) [38]. From the clinical perspective, there is value for TOTRX regimens to be used as a first-line rehabilitation program to reduce low back severity. Once pain is reduced, the obese older adult may be more prepared to begin and adhere to other regular physical activities for weight management. Future work may consider the progression of exercise mode and integration of regular resistance exercise into care plans for this population. Also, long term training with variations in the resistance exercise protocols would be valuable to help determine whether or not there is continued improvement in walking and physical function over time, and whether there is an optimal prescription of resistance exercise for this population.
Limitations and Strengths
A limitation of the current study was the relatively small sample size. This analysis was powered based on effect sizes of walking endurance after resistance training from healthy older adults with no pain. The presence of LBP and varying pain severity may have prevented the consistent magnitude of improvement in walking endurance observed in our earlier work. Future studies in this population should include greater sample sizes to account for the variation in responsiveness to the training. Interestingly, a few participants in the CON group made strength improvement despite no participation in the resistance exercise protocol. The authors of other low back–strengthening studies have found improvements in control groups [39], which was attributed to the placebo effect and an expectation of change from study participation. A possibility is that the attention and educational materials provided during the study period may have assured these individuals that performing this type of exercise was not harmful for the back. Other intervention studies have shown the importance and positive effects of psychosocial education on LBP beliefs and pain coping ability [40]. Also, assurance may have permitted the participant to fully engage in the 1RM strength test. Strengths of the study included the well-controlled exercise programs, participant adherence, and the use of validated, objective measures of walking performance and gait coupled with subjective pain assessment. When generalizing these findings to the populations with LBP, it can be expected that with regular resistance exercise there will be some improvement in muscle strength and LBP severity, but with varying responsiveness among individuals.
CONCLUSION
LEXT and TOTRX made similar modest improvements in walking endurance. Lumbar extensor strength gain compared with leg strength gain is a moderate but important contributor to walking endurance in obese older adults with chronic LBP. Responders to resistance exercise programs (even those with only lumbar extension exercise) who make at least a 20% improvement in strength can expect better improvement in walking endurance than those who do not achieve this strength improvement. Resistance exercise training may be especially useful when combined with dietary modification or other weight loss strategies for further improvement in function, mobility and quality of movement in this population.
Acknowledgments
Research support: Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR057552 and the National Institutes of Health/National Center for Advancing Translational Sciences Clinical and Translational Science Award to the University of Florida UL1 TR000064. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Portions of these findings were presented in poster form at AAPMR annual assembly.
Contributor Information
Heather K. Vincent, Department of Orthopaedics and Rehabilitation, Interdisciplinary Center for Musculoskeletal Training and Research, University of Florida, Gainesville, FL; and Department of Orthopedics and Rehabilitation, Division of Research, UF Orthopaedics and Sports Medicine Institute, PO Box 112727, Gainesville, FL 32611. Disclosure: nothing to disclose.
Kevin R. Vincent, Department of Orthopaedics and Rehabilitation, Interdisciplinary Center for Musculoskeletal Training and Research, University of Florida, Gainesville, FL. Disclosure: nothing to disclose.
Amanda N. Seay, Department of Orthopaedics and Rehabilitation, Interdisciplinary Center for Musculoskeletal Training and Research, University of Florida, Gainesville, FL. Disclosure: nothing to disclose.
Bryan P. Conrad, Department of Orthopaedics and Rehabilitation, Interdisciplinary Center for Musculoskeletal Training and Research, University of Florida, Gainesville, FL. Disclosure: nothing to disclose.
Robert W. Hurley, Departments of Anesthesiology, Psychiatry, Orthopaedics, and Neurology, University of Florida, Gainesville, FL. Disclosure: nothing to disclose.
Steven Z. George, Department of Physical Therapy and Center for Pain Research and Behavioral Health, University of Florida, Gainesville, FL. Disclosure: nothing to disclose.
References
- 1.IJzerman TH, Schaper NC, Melai T, Meijer K, Willems PJ, Savelberg HH. Lower extremity muscle strength is reduced in people with type 2 diabetes, with and without polyneuropathy, and is associated with impaired mobility and reduced quality of life. Diab Res Clin Pract. 2012;95:345–351. doi: 10.1016/j.diabres.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez ME, Goldberg A, Alexander NB. Decreased muscle strength relates to self-reported stooping, crouching, or kneeling difficulty in older adults. Phys Ther. 2010;90:67–74. doi: 10.2522/ptj.20090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marko M, Neville CG, Prince MA, Ploutz-Snyder LL. Lower-extremity force decrements identify early mobility decline among community-dwelling older adults. Phys Ther. 2012;92:1148–1159. doi: 10.2522/ptj.20110239. [DOI] [PubMed] [Google Scholar]
- 4.Hicks GE, Shardell M, Alley DE, et al. Absolute strength and loss of strength as predictors of mobility decline in older adults: The InCHIANTI study. J Geront A Biol Sci Med Sci. 2012;67:66–73. doi: 10.1093/gerona/glr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imagama S, Matsuyama Y, Hasegawa Y, et al. Back muscle strength and spinal mobility are predictors of quality of life in middle-aged and elderly males. Eur Spine J. 2011;20:954–961. doi: 10.1007/s00586-010-1606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes B, Leggett S, Mooney V, Nichols J, Negri S, Hoeyberghs A. Comparison of female geriatric lumbar-extension strength: Asymptotic versus chronic low back pain patients and their response to active rehabilitation. J Spinal Dis. 1996;9:17–22. [PubMed] [Google Scholar]
- 7.Watanabe S, Eguchi A, Kobara K, Ishida H, Otsuki K. Electromyographic activity of selected trunk muscles during bicycle ergometer exercise and walking. Electromyog Clin Neurophys. 2006;46:311–315. [PubMed] [Google Scholar]
- 8.Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: Results of an Internet-based survey. J Pain. 2010;11:1230–1239. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Di Iorio A, Abate M, Guralnik JM, et al. From chronic low back pain to disability, a multifactorial mediated pathway: The InCHIANTI study. Spine (Phila) 2007;32:E.809–E.815. doi: 10.1097/BRS.0b013e31815cd422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fanuele JC, Abdu WA, Hanscom B, Weinstein JN. Association between obesity and functional status in patients with spine disease. Spine. 2002;27:306–312. doi: 10.1097/00007632-200202010-00021. [DOI] [PubMed] [Google Scholar]
- 11.Vincent HK, Seay AN, Vincent KR, Montero C, Conrad BP, Hurley RW. Low back strength adversely affects exercise capacity in obese, older adults with chronic back pain. Med Sci Sports Exerc. 2012;44:S342. [Google Scholar]
- 12.Tomé-Bermejo F, Barriga-Martín A, Martín JL. Identifying patients with chronic low back pain likely to benefit from lumbar facet radio-frequency denervation: A prospective study. J Spinal Disord Tech. 2011;24:69–75. doi: 10.1097/BSD.0b013e3181dc9969. [DOI] [PubMed] [Google Scholar]
- 13.Oneal RM, Mulka JP, Shapiro P, Hing D, Cavaliere C. Wide abdominal rectus plication abdominoplasty for the treatment of chronic intractable low back pain. Plastic Reconstr Surg. 2011;127:225–231. doi: 10.1097/PRS.0b013e3181fad2f7. [DOI] [PubMed] [Google Scholar]
- 14.Mehra M, Hill K, Nicholl D, Schadrack J. The burden of chronic low back pain with and without a neuropathic component: A healthcare resource use and cost analysis. J Med Econ. 2012;15:245–252. doi: 10.3111/13696998.2011.642090. [DOI] [PubMed] [Google Scholar]
- 15.Vincent KR, Braith RW, Vincent HK. Influence of resistance exercise on lumbar strength in older, overweight adults. Arch Phys Med Rehab. 2006;87:383–389. doi: 10.1016/j.apmr.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 16.Hameed UA, Manzar D, Raza S, Shareef MY, Hussain ME. Resistance training leads to clinically meaningful improvements in control of glycemia and muscular strength in untrained middle-aged patients with type 2 diabetes mellitus. N Am J Med Sci. 2012;4:336–343. doi: 10.4103/1947-2714.99507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helmhout PH, Harts CC, Staal JB, Candel MJ, de Bie RA. Comparison of a high-intensity and a low-intensity lumbar extensor training program as minimal intervention treatment in low back pain: a randomized trial. Eur Spine J. 2004;13:537–547. doi: 10.1007/s00586-004-0671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection EaToHBCiA. J Am Med Assoc. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 19.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Von Korff M, Jensen MP, Karoly P. Assessing global pain severity by self-report in clinical and health services research. Spine. 2000;25:3140–3151. doi: 10.1097/00007632-200012150-00009. [DOI] [PubMed] [Google Scholar]
- 21.Vincent KR, Braith RW, Feldman RA, Kallas HE, Lowenthal DT. Improved cardiorespiratory endurance following 6 months of resistance exercise in elderly men and women. Arch Intern Med. 2002;162:673–678. doi: 10.1001/archinte.162.6.673. [DOI] [PubMed] [Google Scholar]
- 22.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 6. Philadelphia, PA: Lippincott Williams and Wilkins; 2000. [Google Scholar]
- 23.Cesari M, Pahor M, Lauretani F, et al. Skeletal muscle and mortality results from the InCHIANTI Study. J Geront A Biol Sci Med Sci. 2009;64:377–384. doi: 10.1093/gerona/gln031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people–results from the Health, Aging and Body Composition Study. J Am Geriat Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 25.Rantanen T, Guralnik JM, Ferrucci L, et al. Coimpairments as predictors of severe walking disability in older women. J Am Geriat Soc. 2001;49:21–27. doi: 10.1046/j.1532-5415.2001.49005.x. [DOI] [PubMed] [Google Scholar]
- 26.Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P, CONSORT G. Extending the CONSORT statement to randomized trials of non-pharmacologic treatment: Explanation and elaboration. Ann Intern Med. 2008;148:295–309. doi: 10.7326/0003-4819-148-4-200802190-00008. [DOI] [PubMed] [Google Scholar]
- 27.Vincent KR, Braith RW, Feldman RA, et al. Resistance exercise and physical performance in adults aged 60 to 83. J Am Geriat Soc. 2002;50:1100–1107. doi: 10.1046/j.1532-5415.2002.50267.x. [DOI] [PubMed] [Google Scholar]
- 28.Graves JE, Pollock ML, Foster D, et al. Effect of training frequency and specificity on isometric lumbar extension strength. Spine. 1990;15:504–509. doi: 10.1097/00007632-199006000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz KH, Hannan PJ, Stovitz SD, Bryan CJ, Warren M, Jensen MD. Strength training and adiposity in premenopausal women: Strong, healthy, and empowered study. Am J Clin Nutr. 2007;86:566–572. doi: 10.1093/ajcn/86.3.566. [DOI] [PubMed] [Google Scholar]
- 30.Vincent HK, Bourguignon C, Vincent KR. Resistance training lowers exercise-induced oxidative stress and homocysteine levels in overweight and obese older adults. Obesity (Silv Spr) 2006;14:1921–1930. doi: 10.1038/oby.2006.224. [DOI] [PubMed] [Google Scholar]
- 31.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture [REDCap]–A metadriven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suri P, Kiely DK, Leveille SG, Frontera WR, Bean JF. Increased trunk extension endurance is associated with meaningful improvement in balance among older adults with mobility problems. Arch Phys Med Rehabil. 2011;92:1038–1043. doi: 10.1016/j.apmr.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlicht J, Camaione DN, Owen SV. Effect of intense strength training on standing balance, walking speed, and sit-to-stand performance in older adults. J Geront A Biol Sci Med Sci. 2001;56:M281–M286. doi: 10.1093/gerona/56.5.m281. [DOI] [PubMed] [Google Scholar]
- 34.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriat Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 35.Powell A, Teichtahl AJ, Wluka AE, Cicuttini F. Obesity: A preventable risk factor for large joint osteoarthritis which may act through biomechanical factors. Br J Sports Med. 2005;39:4–5. doi: 10.1136/bjsm.2004.011841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lidar Z, Behrbalk E, Regev GJ, et al. Intervertebral disc height changes after weight reduction in morbidly obese patients and its effect on quality of life and radicular and low back pain. Spine. 2013;37:1947–1952. doi: 10.1097/BRS.0b013e31825fab16. [DOI] [PubMed] [Google Scholar]
- 37.Hooper MM, Stellato TA, Hallowell PT, Seitz BA, Moskowitz RW. Musculoskeletal findings in obese subjects before and after weight loss following bariatric surgery. Int J Obes (Lond) 2007;31:114–120. doi: 10.1038/sj.ijo.0803349. [DOI] [PubMed] [Google Scholar]
- 38.Farrar JT, Young JPJ, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 39.Liu-Ambrose T, Khan KM, Eng JJ, Lord SR, McKay HA. Balance confidence improves with resistance or agility training. Increase is not correlated with objective changes in fall risk and physical abilities. Gerontology. 2004;50:373–382. doi: 10.1159/000080175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.George SZ, Teyhen DS, Wu SS, et al. Psychosocial education improves low back pain beliefs: Results from a cluster randomized clinical trial ( NCT00373009) in a primary prevention setting. Eur J Spine. 2009;18:1050–1058. doi: 10.1007/s00586-009-1016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]