Abstract
The evolutionarily conserved Notch signaling pathway affects tissue-specific cell differentiation, proliferation, and apoptosis. In the immune system, Notch has been implicated in the development and function of both adoptive and innate immune cells. Notch signaling is initiated by Notch receptor binding to cognate ligands, which results in the enzymatic cleavage and intranuclear translocation of the intracellular domain of Notch receptor (ICN). Recent murine models of chronic inflammation highlighted a critical role for a Notch ligand, Delta-like ligand 4 (Dll4), in granuloma formation. In this study we aimed to assess Notch-1 receptor activation and Dll4 expression in human cutaneous granulomas and in cultured human macrophages and multinucleated giant cells. ICN1 and Dll4 expression was evaluated by immunohistochemistry of cutaneous foreign body (n=15) and sarcoidal (n=19) granulomas. The results showed consistent intranuclear staining for ICN1 in foreign body but not in sarcoidal granulomas and strong cytoplasmic staining for Dll4 in mononuclear histiocytes and multinucleate giant cells in both types of granulomas. Additionally, immunofluorescence confocal microscopy showed ICN1 and Dll4 expression by cultured human macrophages undergoing fusion in the presence of granulocyte-macrophage colony-stimulating factor (GMCSF) and interleukin (IL)-4. These findings indicate a potential role for the Notch1-Dll4 signaling pathway in foreign body-induced granulomatous reactions and possibly distinct Notch pathway utilization in sarcoidal granulomas.
Keywords: Notch, Delta-like ligand, granuloma, multinucleated giant cells, sarcoidosis, foreign body
INTRODUCTION
Granulomas are compact nodular collections of epithelioid histiocytes with variable numbers of admixed multinucleated giant cells (MNGC), which are thought to limit tissue damage and dissemination of infectious organisms such as mycobacteria, fungi or helminths. Granulomatous inflammation can also be evoked by foreign, non-infectious particles such as splinters, suture and keratin fragments released from ruptured epithelial structures. Sarcoidosis is a multisystem granulomatous disease of unknown etiology, which is likely a manifestation of a genetic predisposition to granuloma formation(1).
Cytokines play a crucial role in orchestrating the granulomatous immune response. Tumor necrosis factor (TNF)-α, interleukin (IL)-1, and interferon (IFN)-γ are instrumental for the differentiation and tight aggregation of epithelioid histiocytes during granuloma assembly. Other cytokines, such as granulocyte-macrophage colony stimulating factor (GM-CSF), IL-4 or IL-13 are critical for foreign-body-type giant cell formation by promoting the fusion of alternatively activated macrophages. Furthermore, IFN-γ and IL-17-dependent fusion pathways could potentially underlie giant cell formation in infectious or sarcoidal granulomas. Despite these significant advancements, however, the precise molecular mechanisms underpinning the granulomatous immune response are unknown(2–5).
Notch is an evolutionarily conserved morphogenic signaling pathway that critically determines cell fate decisions in lineage development, differentiation and survival(6). Notch receptors (Notch1-4) are transmembrane proteins which upon binding of a ligand (Jagged1-2, Delta-like (Dll)-1, -3 and -4) triggers two proteolytic cleavage events by A Disintegrin and Metalloproteinase domain (ADAM)-type proteinase and γ-secretase in the Notch receptor, which results in release and nuclear translocation of the intracellular domain of Notch (ICN). In the nucleus, ICN forms a tri-molecular complex with the DNA-binding protein RBP-J, also known as CSL, and a member of the Mastermind (MAML) family and activates transcription of Notch target genes including hes-1 and deltex-1.
Notch signaling shapes the adoptive immune system, by regulating the development and activation of T and B lymphocytes. Recent data suggests a critical role for Notch in the development and function of innate immune cells such as selected dendritic cells and macrophages(7–10). While a recent murine model of mycobacterium antigen-elicited granulomatous inflammation revealed an important role for the Notch ligand Dll4 in granulomatous inflammation, a role for the Notch pathway in human cutaneous granulomatous conditions is not known. Here we assessed whether activated Notch-1 receptor and Dll4 are expressed by tissue histiocytes in foreign body and sarcoidal granulomas as well as by fusing human macrophages and multinucleated giant cells in vitro.
MATERIALS AND METHODS
Patient Selection
Following Institutional Review Board approval, a retrospective query of archived tissue specimens was performed to identify cases of human FBGR with non-polarizable material and cutaneous sarcoidosis. All sarcoidosis cases were evaluated under polarized microscopy and no foreign material was observed. Cases were reviewed by two board certified dermatopathologists.
Human Macrophage Culture and Fusion Assay
This study was reviewed and approved by human subjects review boards at the University of Pennsylvania (Philadelphia, PA, USA). Human macrophages were generated by culturing freshly purified monocytes from healthy donors (Human Immunology Core of the University of Pennsylvania) at density of 1×106/ml in the presence of 10 ng/ml of macrophage colony stimulating factor (M-CSF) for 8 days. MCSF was refreshed at day 3 and day 6. For macrophage fusion assays, macrophages (250,000/well) were cultured in 8-well chamber slides (Nunc® Lab-Tek® Chamber Slide™ system) in the absence or presence of fusogenic cytokines IL-4 (1ng/ml) and GM-CSF (1ng/ml) for 6 days. Culture medium and cytokines were refreshed on day 3.
Immunohistochemical Staining
Immunohistochemistry of formalin-fixed paraffin-embedded tissue sections (4 µm) were performed following derparaffinization, rehydration [xylene (3 times), 95% ethanol, and 75% ethanol] and antigen retrieval [citrate buffer (pH 6.0) pressure cooker (10 min)]. To detect Notch-1 receptor signaling, we used a primary antibody specific to activated Notch1 (ICN1) receptor, which is not cross reactive with full-length Notch1 (cleaved Notch1, Val 1744, Ab #2421; Cell Signaling Technology) at a 1:100 dilution. Anti-Dll4 antibody (Novus Biologicals NBP1-59702) was used at 1:225 dilution. The slides were visualized by DAB immunostaining using the REAL EnVision Detection System(DAKO) and Mayer’s hematoxylin counterstain. For negative controls, the primary antibody was omitted from the staining protocol.
Immunofluorescence Staining
Cultured macrophages and multinucleated giant cells were washed in phosphate-buffered saline solution (3×5 min), fixed in 4% paraformaldehyde (15 min) and permeabilized in 0.1% Triton-X in PBS for 10 minutes. After permeabilization, cells were blocked in 1% bovine serum albumin (BSA) for 30 min. After incubation with primary antibody (against ICN1 or Dll4) in PBS containing 3% BSA for 1 h, cells were washed with PBS and incubated with secondary antibodies (Alexa-Fluor™568-anti-rabbit) and Phalloidin-Alexa-Fluor™488 for 45 min. Slides were washed and treated with VECTASHIELD Mounting Medium with DAPI for nuclear visualization. Slides were imaged using an LSM-510 laser scanning confocal microscope (Carl Zeiss, Germany; 20× Plan APOCHROMAT, NA 0.8; 63× EC Plan-NEOFLUAR; NA 1.3).
RESULTS
Activated Notch1 receptor expression in foreign body but not in sarcoidal granulomas
Low power magnification of immunohistochemically stained slides demonstrated significant staining with ICN1 in both foreign body granulomatous reactions (FBGR) and sarcoidal granulomas (Fig. 1A, and C, respectively). At high power magnification, ICN1 staining demonstrated strong nuclear staining within epithelioid histiocytes and MNGCs in all cutaneous FBGR samples studied. (15/15=100%) (Fig. 1B). In contrast, ICN1 staining was mostly cytoplasmic and showed only weak, focal nuclear staining in sarcoidal granulomas (19/19=100%) (Fig. 1D). Strong nuclear staining of ICN1 in epidermal keratinocytes served as an internal control (Fig. 1E).
Figure 1.
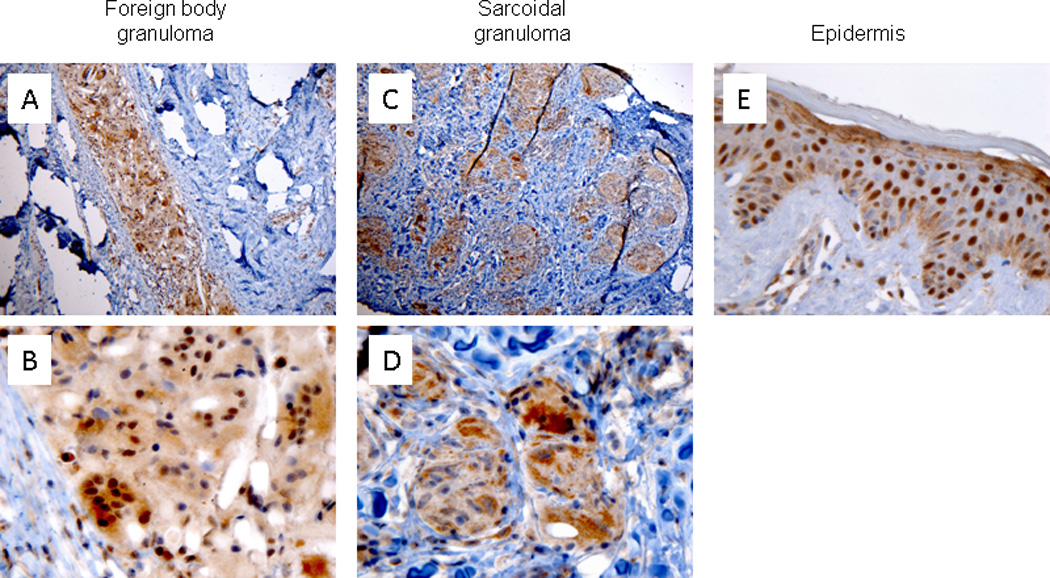
Cleaved Notch-1 (ICN1) immunohistochemical staining of human foreign body granulomas and cutaneous sarcoidosis. Prominent nuclear ICN1 staining within mono- and multinucleated histiocytes in FBGR (A, 100× magnification and B, 600× magnification). ICN1 demonstrated cytoplasmic staining within epithelioid histiocytes and MNGCs in cutaneous sarcoidosis (C, 100× magnification and D, 600× magnification). ICN1 expression by epidermal keratinocytes (E, 600× magnification).
Dll4 expression in human foreign body and sarcoidal granulomas
Immunohistochemical staining for Dll4 showed strong cytoplasmic staining within histiocytes and MNGCs in both FBGR (Figs. 2A and B) and sarcoidosis (Fig. 2C and D) in all cases examined. Dll4 decorated vascular endothelial cells served as an internal control (Fig. 2E).
Figure 2.
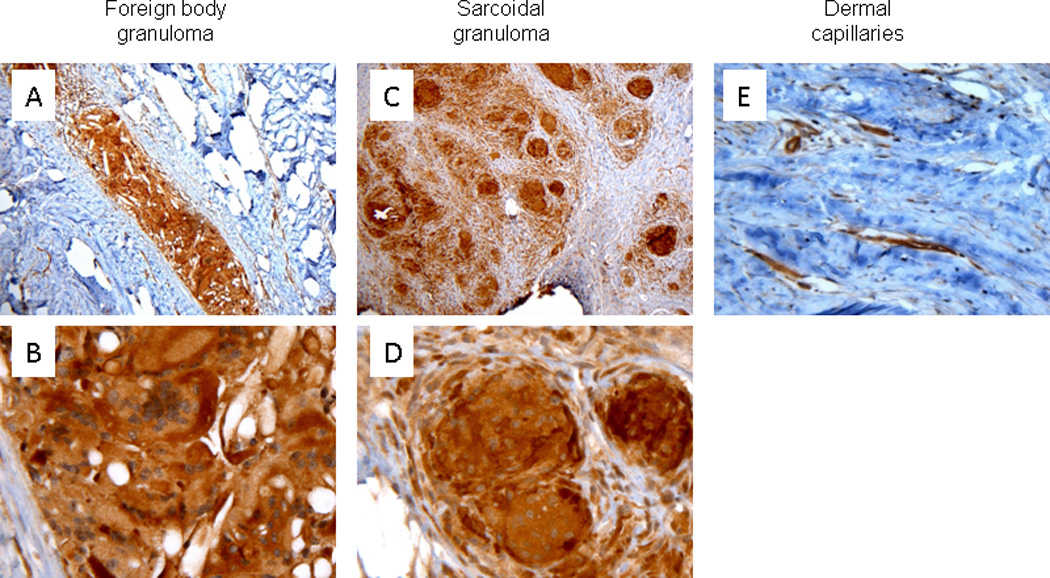
Delta-like ligand 4 (Dll4) immunohistochemical staining of human cutaneous foreign body granulomatous reactions and sarcoidosis. Dll4 demonstrated specific cytoplasmic and variable nuclear staining within mono- and multinucleated histiocytes in both FBGR (A, 100× magnification and B, 600× magnification) and epithelioid histiocytes in cutaneous sarcoidosis (C, 100× magnification and D, 600× magnification). Dll4 staining of dermal endothelial cells (E, 400× magnification).
Activated Notch1 and Dll4 are expressed by fusogenic macrophages in vitro
Our findings of specific nuclear ICN1 staining in FBGR-associated macrophages and MNGCs prompted us to examine the expression of ICN1 and Dll4 in an in vitro model of MNGC formation. Human monocyte-derived macrophages cultured in the presence of GMCSF and IL-4 fuse to form foreign body-type MNGCs(11). We used this model with immunofluorescence confocal imaging to assess the protein expression of activated Notch-1 receptor and Dll4 ligand in resting human macrophages and in macrophages undergoing cytokine induced fusion. Macrophages cultured in the absence of cytokines showed no significant intranuclear ICN1 expression and only rare cells expressed Dll4 (Figs. 3A, and D). In contrast, two days after exposure of macrophages to fusogenic cytokines GMCSF and IL-4 resulted in enhanced intranuclear ICN1 (Fig. 3B) and cytoplasmic Dll4 expression (Fig. 3E) in mononuclear macrophages. At day 4 of cytokine supplemented cultures, strong nuclear ICN1 expression was seen in MNGCs (Fig. 3C) and Dll4 expression was present at the distal tips of macrophage dendrites that were in close proximity to MNGCs (Fig. 3F).
Figure 3.
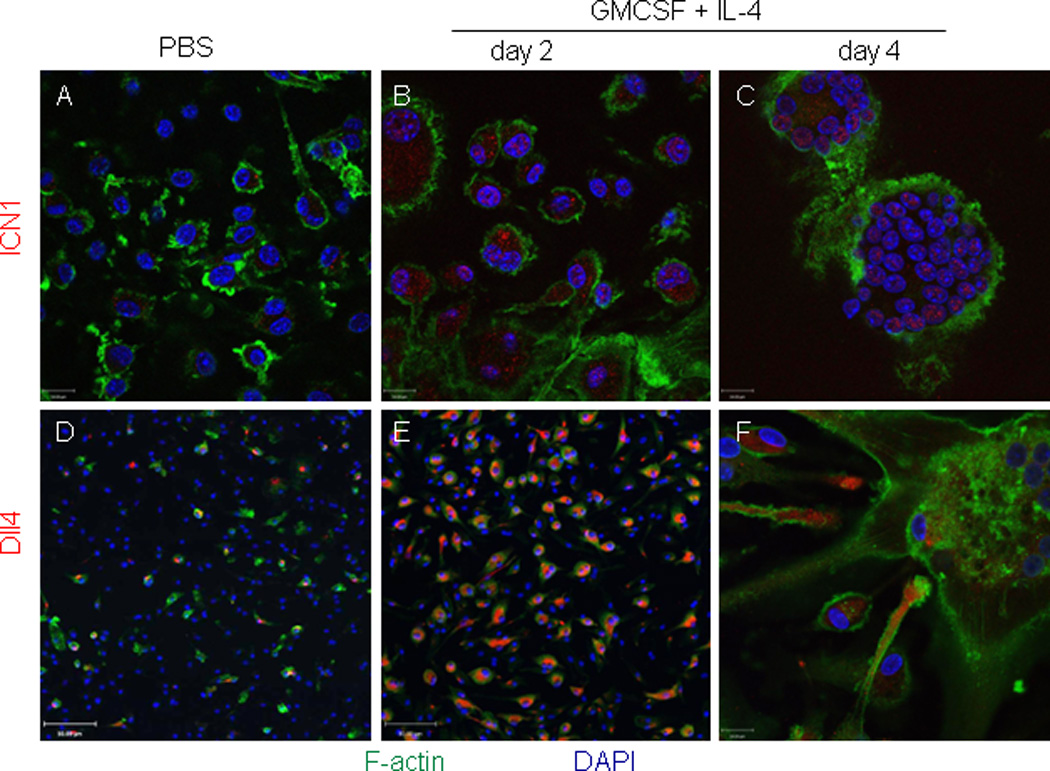
Confocal immunofluorescence imaging of ICN1 and Delta-like ligand 4 (Dll4) expression by cultured human mononuclear macrophages and multinucleated giant cells. Human monocyte-derived macrophages were cultured in the presence of vehicle (PBS) or fusogenic cytokines GMCSF and IL-4 for 2 and 4 days. Panels A–C: ICN1 (red), F-actin (green); magnification 630×. Panels D–F: Dll4 (red) and F-actin (green) at 200× (D and E) and 630× (F) magnifications. Nuclei were visualized by DAPI staining (blue).
DISCUSSION
Notch signaling is essential in embryogenesis, adult tissue homeostasis and neoplastic transformation in various organ systems. In the immune system, distinct Notch-receptor utilization underpins the steady-state differentiation and activation of adoptive and innate immune cells. Notch-1 receptor signals are instrumental for intrathymic T-cell and dendritic cell differentiation(12) as well as macrophage activation(13). Notch-2 receptor signals are implicated in marginal zone B-cell and selected splenic DC differentiation and macrophage polarization(12). The contribution of Notch signaling in the pathogenesis of inflammatory diseases is poorly understood. In humans, aberrations in Notch signaling have been associated with autoimmune conditions such as multiple sclerosis, systemic sclerosis, and systemic lupus erythematosus(14–16). Furthermore, Notch-Dll4 signaling had been observed in macrophages within atherosclerotic plaques(10). Despite the critical role of Notch in both adoptive and innate immunity, the pathogenic role of Notch in human granulomatous reactions is largely unknown. Here we demonstrate that the Notch receptor ligand Dll4 is expressed in both foreign body and sarcoidal granulomas whereas the molecular signature of Notch-1 receptor activation is specifically seen in mono- and multinucleated histiocytes in FBGR but not in sarcoidosis.
Previous in vitro studies demonstrated that toll-like receptor (TLR) stimulation of human macrophages leads to the initiation of Notch signaling, which in turn activates a transcriptional program that augments the expression of granuloma-associated proinflammatory cytokines, such as IFN-γ, IL-6 and IL-17(13). Recent murine models confirmed a mechanistic connection between Notch signaling and granulomatous inflammation. Mycobacterial antigens efficiently induce Dll4-expression through Toll-like receptor (TLR)-9 signaling with concomitant Th17 expression and modulation of granuloma formation (8). Similar requirements for the Dll4/Notch axis for Th1 and Th17 immune responses were observed in experimental encephalitis models(17). Thus, our findings of Dll4 expression on sarcoidal granulomas could indicate a pathogenic role of Notch signaling in the acquisition of the Th1/Th17 immune signature characteristic of sarcoidal granulomas(18). Besides maintaining the TH-cell milieu during chronic inflammation, it is also conceivable that Notch promotes granuloma-associated innate immune functions as Notch-1 signaling in macrophages had been associated with enhanced antigen presentation and cytotoxic activity(19).
Our findings of activated intranuclear Notch-1 expression in FBGR in tissues and in IL-4 induced macrophages and foreign body type MNGCs in vitro raise the notion that Notch-1 signals lie downstream of the IL-4-induced macrophage fusion pathway(11). The lack of intranuclear ICN1 staining in sarcoidosis could be a reflection of distinct immune pathogenesis between these two granuloma types. It is conceivable that in sarcoidosis, Dll-4 activates a Notch receptor other than Notch-1. Supportive of this notion are recent findings from genome-wide association studies that identified NOTCH4 as a novel sarcoidosis-associated locus among a large cohort of African-American patients(20). Additionally, signaling through the Notch-2 receptor had been seen in macrophage activation(13).
Finally, our in vitro data showing the expression of ICN1 in fusing and multinucleated macrophages as well as the localization of Dll4 protein at the tips of dendrites of fusing macrophages suggest that Dll4-dependent Notch signals are critical for macrophage fusion. This notion had been supported by findings of Notch-Delta ligand dependent cell-cell fusion mechanisms in drosophila myoblasts and C. elegans epithelial fusion (21,22).
Overall, our data suggest that Notch-Delta signaling in histiocytes may play an essential role in cutaneous granulomatous tissue reactions. As such, a better understanding of the function of Notch receptors and their ligands in various cutaneous granulomatous inflammatory conditions may further our understanding of disease pathogenesis and could lead to new therapeutic interventions.
Acknowledgments
Funding Sources: This work was supported in part by grants received from the Penn Skin Disease Research Center (NIAMS P30-AR057217) (to A.S. and M.R.), Career Development Awards from the National Institute of Health (NIAID, K08AI080138 to A.S.) and Dermatology Foundation (to M.R.).
Abbreviations
- FBGR
foreign body granulomatous reaction
- MNGC
multinucleated giant cells
- DC
dendritic cells
- ICN1
intracellular domain of Notch-1
- Dll4
delta-like ligand
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Tchernev G, Patterson J, Nenoff P, et al. Sarcoidosis of the skin – A dermatological puzzle: important differential diagnostic aspects and guidelines for clinical and histopathological recognition. Journal of the European Academy of Dermatology and Venereology. 2010;24(2):125–137. doi: 10.1111/j.1468-3083.2009.03396.x. [DOI] [PubMed] [Google Scholar]
- 2.Hirsh BC, Johnson WC. Concepts of Granulomatous Inflammation. International Journal of Dermatology. 1984;23(2):90–100. doi: 10.1111/j.1365-4362.1984.tb05679.x. [DOI] [PubMed] [Google Scholar]
- 3.Iizasa H, Yoneyama H, Mukaida N, et al. Exacerbation of granuloma formation in IL-1 receptor antagonist-deficient mice with impaired dendritic cell maturation associated with Th2 cytokine production. J Immunol. 2005 Mar 15;174(6):3273–3280. doi: 10.4049/jimmunol.174.6.3273. [DOI] [PubMed] [Google Scholar]
- 4.Yoneyama H, Matsuno K, Zhang Y, et al. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract-associated lymphoid tissue, in a granulomatous liver disease. J Exp Med. 2001 Jan 1;193(1):35–49. doi: 10.1084/jem.193.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helming L, Gordon S. The molecular basis of macrophage fusion. Immunobiology. 2007;212(9–10):785–793. doi: 10.1016/j.imbio.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Kopan R, Ilagan MXG. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009 Apr 17;137(2):216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray SJ. Notch signalling: a simple pathway becomes complex. Nature Reviews Molecular Cell Biology. 2006 Sep 1;7(9):678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 8.Ito T, Schaller M, Hogaboam CM, et al. TLR9 regulates the mycobacteria-elicited pulmonary granulomatous immune response in mice through DC-derived Notch ligand delta-like 4. Journal of Clinical Investigation [Internet] 2008 Dec 15; doi: 10.1172/JCI35647. [cited 2012 Sep 21]; Available from: http://www.jci.org/articles/view/35647/cite. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan JS, Kousis PC, Suliman S, et al. Functions of Notch Signaling in the Immune System: Consensus and Controversies. Annual Review of Immunology. 2010;28(1):343–365. doi: 10.1146/annurev.immunol.021908.132719. [DOI] [PubMed] [Google Scholar]
- 10.Fung E, Tang S-MT, Canner JP, et al. Delta-like 4 induces notch signaling in macrophages: implications for inflammation. Circulation. 2007 Jun 12;115(23):2948–2956. doi: 10.1161/CIRCULATIONAHA.106.675462. [DOI] [PubMed] [Google Scholar]
- 11.McNally AK, Anderson JM. Interleukin-4 induces foreign body giant cells from human monocytes/macrophages. Differential lymphokine regulation of macrophage fusion leads to morphological variants of multinucleated giant cells. Am J Pathol. 1995 Nov;147(5):1487–1499. [PMC free article] [PubMed] [Google Scholar]
- 12.Radtke F, Fasnacht N, Macdonald HR. Notch signaling in the immune system. Immunity. 2010 Jan 29;32(1):14–27. doi: 10.1016/j.immuni.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Hu X, Chung AY, Wu I, et al. Integrated regulation of Toll-like receptor responses by Notch and interferon-gamma pathways. Immunity. 2008 Nov 14;29(5):691–703. doi: 10.1016/j.immuni.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duvefelt K, Anderson M, Fogdell-Hahn A, et al. A NOTCH4 association with multiple sclerosis is secondary to HLA-DR*1501. Tissue Antigens. 2004 Jan;63(1):13–20. doi: 10.1111/j.1399-0039.2004.00135.x. [DOI] [PubMed] [Google Scholar]
- 15.Gorlova O, Martin J-E, Rueda B, et al. Identification of novel genetic markers associated with clinical phenotypes of systemic sclerosis through a genome-wide association strategy. PLoS Genet. 2011 Jul;7(7):e1002178. doi: 10.1371/journal.pgen.1002178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sodsai P, Hirankarn N, Avihingsanon Y, et al. Defects in Notch1 upregulation upon activation of T Cells from patients with systemic lupus erythematosus are related to lupus disease activity. Lupus. 2008 Jul;17(7):645–653. doi: 10.1177/0961203308089406. [DOI] [PubMed] [Google Scholar]
- 17.Eixarch H, Mansilla MJ, Costa C, et al. Inhibition of delta-like ligand 4 decreases Th1/Th17 response in a mouse model of multiple sclerosis. Neurosci. Lett. 2013 Apr 29;541:161–166. doi: 10.1016/j.neulet.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 18.Ten Berge B, Paats MS, Bergen IM, et al. Increased IL-17A expression in granulomas and in circulating memory T cells in sarcoidosis. Rheumatology (Oxford) 2012 Jan;51(1):37–46. doi: 10.1093/rheumatology/ker316. [DOI] [PubMed] [Google Scholar]
- 19.Monsalve E, Pérez MA, Rubio A, et al. Notch-1 Up-Regulation and Signaling following Macrophage Activation Modulates Gene Expression Patterns Known to Affect Antigen-Presenting Capacity and Cytotoxic Activity. J Immunol. 2006 May 1;176(9):5362–5373. doi: 10.4049/jimmunol.176.9.5362. [DOI] [PubMed] [Google Scholar]
- 20.Adrianto I, Lin CP, Hale JJ, et al. Genome-Wide Association Study of African and European Americans Implicates Multiple Shared and Ethnic Specific Loci in Sarcoidosis Susceptibility. PLoS ONE. 2012 Aug 27;7(8):e43907. doi: 10.1371/journal.pone.0043907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gildor B, Schejter ED, Shilo B-Z. Bidirectional Notch activation represses fusion competence in swarming adult Drosophila myoblasts. Development. 2012 Nov;139(21):4040–4050. doi: 10.1242/dev.077495. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen JP, English K, Tenlen JR, et al. Notch signaling and morphogenesis of single-cell tubes in the C. elegans digestive tract. Dev. Cell. 2008 Apr;14(4):559–569. doi: 10.1016/j.devcel.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


