Abstract
Background and aims
The time to first cigarette (TTFC) of the day is an indicator of nicotine intake in adults and adolescents. However, the relation between TTFC and biological markers of nicotine addiction and health risk in youths has not been well described. The current study examined whether an earlier TTFC predicts higher levels of a tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridal)-1 (NNAL), in regular and intermittent adolescent smokers and if this relation is mediated by nicotine intake (measured by cotinine) or cigarettes per day (CPD).
Design
A cross-sectional analysis of a nationally representative subsample of adolescents.
Setting
A general community sample from the 2007–2008 and 2009–2010 National Health and Nutrition and Examination Survey.
Participants
215 adolescents in the United States between the ages of 12 and 19 who reported smoking at least once in the 5 days prior to data collection.
Measurements
The primary outcome measure was urinary levels of NNAL.
Findings
In both regular and intermittent smokers, earlier TTFC was dose-dependently associated with higher levels of NNAL (p < 0.03 in both cases). TTFC had an indirect effect on NNAL, mediated by nicotine intake (cotinine) in both regular (β = −.08, SE = .03, 95% CI [−.15, −.04]), and intermittent (β = −.02, SE = .01, 95% CI [−.05, −.002]) smokers. CPD was not found to be an important mediator of the relation between TTFC and NNAL.
Conclusions
Time between waking and the first cigarette of the day is correlated in daily and non-daily adolescent smokers with overall nicotine and therefore carcinogen intake.
Keywords: Time to First Cigarette, NNAL, Carcinogen, Adolescent, Regular Smoker, Intermittent Smoker, NHANES
Introduction
Nearly all adult smokers begin smoking in their adolescent years [1]. Accordingly, adolescence is a critical period for the study of smoking behaviors. Despite the known hazards, teen smokers tend to dismiss the dangers of smoking– often because they rarely demonstrate the negative effects associated with regular cigarette use (e.g., coughs, limited functioning, tobacco-related diseases), and do not consider themselves to be very addicted [2]. Overall, adolescent smokers have a great deal of variability in their smoking patterns and smoking trajectories into adulthood[3]. For example, adolescents tend to smoke fewer cigarettes per day than adults, are less likely to inhale, are less likely to smoke when they are ill, and tend to smoke more on weekends than on weekdays [4–6]. Despite these irregular patterns, the onset of dependence after initiation in adolescent smokers is thought to occur relatively quickly and dependence symptoms are often experienced even among light and non-daily smokers [7,8]. Due to constraints such as social restrictions at home, in the community and at school, and limitations on access to cigarettes, adolescents who wish to smoke more regularly may not be able to do so; indeed, these restrictions may play a role in the relatively high prevalence of intermittent smoking among adolescents [9,10]. Accordingly, it may be particularly difficult to adequately assess which teens smoke intermittently because they choose to do so and which teens smoke intermittently due to environmental circumstances. These irregular patterns of smoking contrasted by relatively high levels of dependence and withdrawal result in difficulties characterizing adolescent nicotine addiction and fully assessing potential risk, especially among those adolescents who do not smoke daily.
The time to first cigarette (TTFC),an item included in several commonly used nicotine dependence questionnaires[11–15], was originally designed to assess smokers' degree of physical dependence resulting from overnight nicotine deprivation. The original Fagerstrom Tolerance Questionnaire (FTQ; [14]), among the first widespread measures of nicotine dependence, assessed TTFC with a two-level item: 1) smoking the first cigarette of the day 30 minutes or fewer after waking, or 2) smoking more than 30 minutes after waking. Studies classifying smokers as “early smokers” and “late smokers” conducted by Kozlowski and colleagues were the first to find that TTFC was a better predictor of dependence, and better linked to cessation outcomes, than cigarettes per day (CPD;[16]). Looking to standardize the scoring of both TTFC and CPD, the Heavy Smoking Index (HSI; [15]) lead to the 4-level scoring system for TTFC (i.e., 1=within 5 minutes; 2=6 to 30 minutes; 3=from more than 30 minutes to one hour; and 4=more than one hour) that has been adopted by later nicotine dependence questionnaires, including the Fagerstrom Test for Nicotine Dependence (FTND; [11]). Since Kozlowski and colleagues' initial work on TTFC, a number of studies have confirmed that TTFC may be the single best predictor of nicotine dependence; an earlier time to first cigarette is closely associated with poor smoking out comes such as increased levels of tobacco exposure biomarkers, increased tolerance, and decreased likelihood of quitting during and an inability to maintain abstinence following a quit attempt[16–23].
In addition to its ability to predict nicotine dependence, TTFC is also an emerging predictor of cancer risk. In adults, an earlier TTFC is associated with an increased susceptibility to tobacco-related cancers [24–27]. This increased susceptibility appears to reflect that an earlier TTFC is associated with higher levels of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), the metabolite of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) among adult smokers[28]. Whereas the conventional measure of nicotine exposure and risk has often been the total number of cigarettes per day, this measure may not fully reflect biological exposure levels, especially among adolescent smokers. Not only does TTFC predict cotinine levels, a general marker of nicotine intake, better than the number of cigarettes per day among adolescents [29], there is a non-linear relation between cigarettes per day and levels of cotinine with a plateau effect occurring at as few as four cigarettes per day [30]. Importantly, NNAL has a terminal half-life significantly longer than other commonly used biomarkers of nicotine exposure (10 to 18 days vs. cotinine's half-life of 16 hours), and can be used to detect tobacco smoke among both daily and non-daily users [31–33]. Because NNAL is slowly cleared from the body, and is not as reactive to acute changes following smoking behavior as cotinine or other biomarkers, NNAL may be an excellent biomarker to confirm longer-term cessation outcomes and to determine exposure among non-daily youth smokers where cotinine, carbon monoxide or other measures may fail to detect use.
Despite a range of studies demonstrating associations between TTFC and nicotine intake, cessation failure and disease risk, the mechanisms by which TTFC affects these outcomes is not well understood. It has been theorized that TTFC reflects greater dependence, which in turn results in smoking cigarettes more intensively, thus resulting in greater exposure to tobacco carcinogens. However, this theory has not yet been empirically tested. Among regular youth smokers, TTFC is highly related to cotinine in dependent of cigarettes per day[29]. This suggests that TTFC could also be a marker for increased cancer susceptibility if it is associated with increased intake of carcinogens as well. However, there have been no previous studies examining the relation between TTFC and biomarkers of carcinogen exposure in adolescent smokers. The current study sought to examine the hypothesis that TTFC is an indicator of increased carcinogen exposure, as measured by NNAL levels, in both regular and intermittent adolescent smokers. Furthermore, given previous evidence that TTFC is a stronger indictor of nicotine intake than even cigarettes per day in adolescent smokers, we hypothesize that the relation between TTFC and NNAL is mediated by increased intensity of smoking (or nicotine intake), which can be measured by cotinine levels and not by the number of cigarettes smoked per day in both regular and intermittent smokers.
Methods
Participants
The current analysis was conducted by pooling the 2007–2008 and 2009–2010 National Health and Nutrition and Examination Survey (NHANES) data. NHANES uses a multistage probability sampling design to obtain a representative sample of the non-institutionalized United States population, with over sampling in minority and older age groups. A full description of the NHANES data collection methodology is available from the Centers for Disease Control and Prevention [34]. We selected all participants between the ages of 12 and 19 who reported smoking at least one cigarette in the last five days (to best capture the window to quantify cotinine levels among both daily and non-daily smokers) and those who had provided urine samples for NNAL analyses. The final sample consisted of 215 adolescent smokers (65% male) with an average age of 15.47 years (SD=2.27).
Measures
Completion of interviews, self-report questionnaires and laboratory examinations with adolescents took place following parental consent at an in-home interview. Participants between the ages of 12 and 19 years answered questionnaires and provided consent and blood and urine samples during a single visit to the NHANES Mobile Examination Center (MEC). For adolescent participants, smoking and related questions were answered using the Audio Computer – Assisted Self Interview (ACASI) technique which allows participants to respond to sensitive questions confidentially via computerized administration. Questionnaires included demographic measures, smoking behaviors and smoking history. Smoking behavior questions included the number of days smoked in the last 30 days and the last 5 days, average number of cigarettes smoked per day in the last 30 days and the last 5 days, time to first cigarette of the day, age smoked first whole cigarette, and whether there are other smokers in their home to assess exposure to second hand smoke. The item “how soon after you wake do you smoke” was classified into four categories: 1=within 5 minutes; 2=6 to 30 minutes; 3=from more than 30 minutes to one hour; and 4=more than one hour.
Urine samples were collected and processed according to the defined NHANES protocol. For a full description of collection and procession of biological specimens, see the NHANES Laboratory/Medical Technologists Procedures Manual [35]. We analyzed data on total urinary NNAL measurements (e.g. NNAL and its glucuronides) using urinary creatinine as a correction factor. All procedures were approved by the National Center for Health Statistics Research Ethics Review Board.
Preliminary Analyses
To date, there has been no agreed upon definition of the terms “regular” and “intermittent” cigarette use, particularly among adolescent smokers. Indeed, the terminology used to define nondaily cigarette use is often not consistent between studies of adolescents and adults [36]. Previous adolescent smoking studies have used terms such as “light” or “intermittent” to create distinctions among types of adolescent nondaily smokers. Additional studies have differentiated between daily smokers and “low” and “high” intermittent smokers, as determined by a midpoint in a sample distribution [2]. Given the difficulties of determining what constitutes “regular” and “intermittent” smoking, Discriminant Function Analyses (DFA) were used in the present study to predict classification based on four predictor variables associated with smoking frequency: 1) number of cigarettes per day in the last 30 days, 2) age smoked first whole cigarette, 3) current age, and 4) number of days smoked in the last five day. We conducted two DFA tests with the first predicting regular smokers defined as having smoked on at least 27 of the last 30 days, and intermittent smokers defined as having smoked on less than 27 days. We selected 27 days as the criterion of the first analyses to reflect definitions used by Shiffman and colleagues for adult intermittent smokers [37]. The second DFA defined regular smokers as having smoked on 20 or more days in the last 30, and intermittent smokers as having smoked on fewer than 20 days. This definition was based on the National Youth Tobacco Survey which defined “frequent users” as those using 20 or more days in the last 30 days [38]. We evaluated overall classification, specificity and sensitivity and effect size (canonical correlation squared).
The results of the DFA tests suggested that the four variables were significant predictors of regular and intermittent smokers with the cutoff defined as 27 days, Λ= .48, χ2 (4, 215)= 155.75, p < .001, and defined as 20 days, Λ= .52, χ2 (4, 215)= 140, p < .001. Overall, regular and intermittent smokers were correctly classified 85.6% of the time in both models. For the model with the cutoff for regular smoker defined as 27 days, specificity was 76% and sensitivity was 95%, and the overall effect size was canonical R2 = .52. For the model with the cutoff for regular smoker defined as 20 days, specificity was 77% and sensitivity was 93%, and the overall effect size was canonical R2 = .50.
Given that adolescent smoking is characterized, in part, by irregular patterns [4], and that the DFA results were nearly identical, and to be comparable with the National Youth Tobacco Survey definition of “frequent” youth smokers, the present study defined regular smokers as smoking 20 or more days in the last 30, and intermittent smokers as smoking on fewer than 20 days in the last 30.
Main Analyses
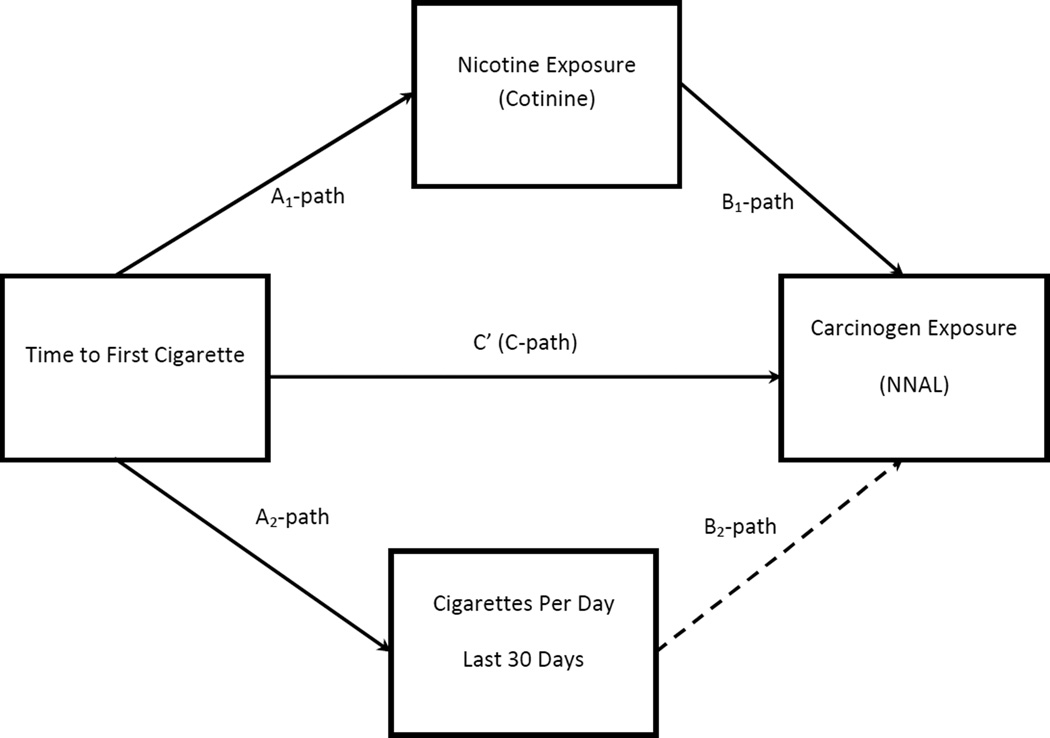
A priori power analyses suggest that our sample size of 215 was sufficient to detect a small effect size (f2 = .07) with power of .80 with alpha = .05. To address the hypotheses that the relation between TTFC and NNAL is mediated by the level of smoke intake (e.g., intensity/volume of puffing), as measured by cotinine and not necessarily by the number of cigarettes smoked, we utilized multiple mediation analyses using bootstrapping methods with bias-corrected confidence intervals [39,40]. The multiple mediator regression models estimated path coefficients and confidence intervals for overall direct and indirect (mediated) effects. Similar to mediation analyses using the causal step method proposed by Baron and Kenny [41], the bootstrapping method of Preacher and Hayes [42] establishes relations between an independent variable and a dependent variable (path “C” in Figure 1), between an independent variable and a proposed mediator (path “A” in Figure 1), and between a proposed mediator and a dependent variable (path “B” in Figure 1). Unlike the causal step approach, however, the bootstrapping method allows for the simultaneous modeling of multiple mediators (cotinine and cigarettes per day in the present study) and calculates total indirect effects and bias corrected confidence intervals for all proposed mediators together, as well as measures of indirect effects for each mediator independently. Additionally, unlike the causal steps method, the interpretation of the indirect (mediated) effects in the bootstrapping method places little emphasis on the statistical significance of the individual A and B paths; rather, it focuses on the direction and size of the indirect effects, which are calculated using thousands of bootstrapping resamples to determine a 95% confidence interval of the effect size [42].
Figure 1.
Multiple Mediator Model
Using this approach, we created two separate mediation models, each entering four covariates associated with NNAL in smokers: 1) age, 2) age smoked first whole cigarette, 3) gender, and 4) creatinine, to account for differences in urinary dilution[43]. One model was run for regular smokers and one model was run for intermittent smokers. For all mediation models, the outcome (NNAL) was log-transformed to normalize skewness in values.
Results
Table 1 shows descriptive demographic information on the participants and TTFC distribution between regular and intermittent smokers. Of the 215 adolescent smokers, 120 were classified as regular smokers (56%). As expected, there were significant differences between regular and intermittent smokers on background characteristics and smoking behaviors, see Table 1.
Table 1.
Descriptive and comparative statistics for study variables
| Regular Smokers n=120 MEAN/(SD) |
Intermittent Smokers n=95 MEAN/(SD) |
t | df | p | 95% CI Lower |
95% CI Upper |
|
|---|---|---|---|---|---|---|---|
| Age | 17.64 (1.41) | 16.88 (1.66) | −3.62 | 213 | .000 | −1.17 | −.35 |
| Age smoked 1st whole cigarette | 13.81 (2.70) | 14.43 (2.30) | 1.80 | 213 | .07 | −.06 | 1.31 |
| Cigarettes per day / 30 days | 10 (10.05) | 2.76 (2.74) | −6.82 | 213 | .000 | −9.33 | −5.14 |
| Cigarette per day / 5 days | 8.87 (8.60) | 2.56 (2.40) | −13.16 | 213 | .000 | −2.45 | −1.81 |
| Number of days smoked / 30 days | 28.36 (3.10) | 7.28 (5.23) | −36.73 | 213 | .000 | −22.20 | −19.94 |
| χ2 | df | p | |||||
| Male Gender | 65.3% | 65% | .002 | 1 | .96 | ||
| Other smokers in the home | 46% | 22% | 12.70 | 1 | .000 | ||
| Time to first cigarette | |||||||
| Within 5 minutes | 24% | 7% | 10.73 | 1 | .001 | ||
| 6 – 30 minutes | 31% | 6% | 19.21 | 1 | .000 | ||
| 31–60 minutes | 18% | 12% | 1.45 | 1 | .22 | ||
| > 60 minutes | 27% | 73% | 47.63 | 1 | .000 | ||
The overall multiple mediator regression model for intermittent smokers was significant, F (7,73) = 9.88, p < .001, adjusted R2 = .44. The analyses simultaneously evaluated two moderators of the relation between TTFC and NNAL: 1) cotinine and 2) cigarettes per day. Results demonstrated significant relations between TTFC and NNAL (labeled “C” path in Figure 1), between TTFC and cotinine (labeled “A1” path in Figure 1) and between cotinine and NNAL (labeled “B1” path in Figure 1). Additionally, there was a significant relation between TTFC and cigarettes per day (labeled “A2” path in Figure 1). The relation between cigarettes per day and NNAL was found to be non-significant (labeled “B2” path in Figure 1). See Table 2 for full results. Tests of total and mediated indirect effects demonstrate that there was an indirect effect of cotinine, but no indirect effect of both mediators combined and no indirect effect of cigarettes per day alone. See Table 3 for full results of indirect mediated effects.
Table 2.
Multiple mediation regression model results
| B | SE (B) | t | p | ||
|---|---|---|---|---|---|
| A1 Path (TTFC → COT) | |||||
| Regular | −.22 | .04 | −5.46 | .000 | |
| Intermittent | −.31 | .12 | −2.50 | .01 | |
| A2 Path (TTFC → CPD) | |||||
| Regular | −2.94 | .66 | −4.49 | .000 | |
| Intermittent | −2.02 | .27 | −7.35 | .000 | |
| B1 Path (COT → NNAL) | |||||
| Regular | .35 | .11 | 3.15 | .00 | |
| Intermittent | .08 | .01 | 5.57 | .000 | |
| B2 Path (CPD → NNAL) | |||||
| Regular | .01 | .01 | .71 | .48 | |
| Intermittent | −.00 | .01 | −.53 | .60 | |
| C Path (TTFC → NNAL) No mediators | |||||
| Regular | −.10 | .03 | −2.14 | .03 | |
| Intermittent | −.06 | .02 | −3.67 | .001 | |
| C’ Path (TTFC → NNAL) With mediators | |||||
| Regular | .00 | .05 | .08 | .93 | |
| Intermittent | −.04 | .02 | −2.41 | .02 | |
Table 3.
Indirect (mediated) effects
| B | SE (B) | 95% CI Lower |
95% CI Upper |
|
|---|---|---|---|---|
| Regular smokers | ||||
| Total indirect effect (both mediators) | −.10 | .03 | −.16 | −.04 |
| Indirect effect mediated through COT | −.08 | .03 | −.15 | −.03 |
| Indirect effect mediated through CPD | −.02 | .02 | −.06 | .01* |
| Intermittent smokers | ||||
| Total indirect effect (both mediators) | −.02 | .01 | −.05 | .008* |
| Indirect effect mediated through COT | −.02 | .01 | −.05 | −.002 |
| Indirect effect mediated through CPD | .01 | .01 | −.02 | .03* |
No indirect effects as the 95% CI includes a zero value.
Note: Results calculated with 5000 bootstrap resamples
Identical models conducted for regular smokers demonstrated a significant overall model, F (7,103) = 5.07, p < .001, adjusted R2 = .21. Analyses revealed significant associations between TTFC and NNAL (C path),between TTFC and cotinine (A1 path), between cotinine and NNAL (B1 path), and between TTFC and cigarettes per day (A2 path). The relation between cigarettes per day and NNAL (B2 path) was non-significant. Tests of total and mediated indirect effects demonstrated that there was a total indirect effect of cotinine and cigarettes per day combined. However, whereas there was an independent indirect effect of cotinine, there was no independent indirect effect of cigarettes per day. This suggests that the relation of TTFC on NNAL is mediated by cotinine, but not cigarettes per day. See Table 3 for results of indirect effects.
Discussion
Research has shown that 91% of all lung cancers in men and 69% in women are related directly to cigarette use[44]. Among the numerous carcinogens found in tobacco smoke, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is thought to play a significant role in the development of lung tumors [45,46]. The main metabolite of NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), can be readily detected in urine and has been independently related to lung cancer among adult smokers [44]. Although it might be expected that the total number of cigarettes per day (CPD) would be the best measure of NNAL among smokers, there is a non-linear relation between CPD and NNAL[47], and other factors may be equally – or better – able to predict NNAL levels among smokers. Given there are meaningful differences in NNAL levels between individuals, understanding factors that may contribute to an increase in NNAL – and therefore to an increased risk of lung cancer risk – is essential. Whereas indicators of exposure risk such as NNAL in adolescent smokers have not been well documented, it has been suggested that factors such as lack of stabilization of smoking behaviors (inexperienced smokers may go about smoking each cigarette differently than experienced smokers) and differences in the types of cigarettes smoked may be important contributors to total NNAL levels in adolescent smokers [48]. Unfortunately, however, there are few studies that help us understand predictors of NNAL among adolescent smokers. Understanding the relation between NNAL and adolescent smoking may be an important step in helping researchers, clinicians and even youth smokers better understand nicotine dependence and risk factors resulting from smoking.
This study confirms previous observations that shorter TTFC is strongly correlated with higher intensity of puffing, as shown by higher urinary nicotine, which results in increased levels of NNK. Specifically, this study demonstrated that the time to the first cigarette of the day (TTFC) has a significant association with NNAL in both regular and intermittent adolescent smokers, such that NNAL levels increased with earlier TTFC in a dose-dependent fashion. This finding is consistent with studies of adult smokers demonstrating an association between earlier TTFC and increased levels of NNAL [28]. Because increased NNAL levels have been shown to indicate subsequent cancer risk among adults, this finding suggests that TTFC may be able to identify a high-risk subgroup of young smokers despite differences in smoking frequency. A major reason teenagers smoke is their sense of invulnerability. Smoking is considered dangerous in the abstract and teens have little sense of their individual risk[49]. Thus, assessing an adolescent’s TTFC independent of their CPD may provide researchers with the opportunity to identify such individuals and develop appropriate intervention strategies to help these high-risk young smokers to quit.
Beyond the association between TTFC and NNAL among regular and intermittent smokers, the present study determined that the relation between TTFC and NNAL was mediated by increased cotinine exposure, reflecting increased nicotine intake via mechanisms (e.g., smoking topography, genetic differences in nicotine metabolism) independent of the number of cigarettes smoked per day. Although previous studies have shown that earlier TTFC is associated with both increased nicotine intake in adolescents[12,29] and increased levels of NNAL in adults[28], this study is the first to formally test this mediational relationship, as well as the first to test this relationship among adolescent regular and intermittent smokers. These findings suggest that independent of how often adolescents smoke (e.g., daily or non-daily), adolescents with an earlier TTFC may smoke with greater intensity (e.g., greater puff depth or duration) or may metabolize nicotine more slowly, resulting in greater nicotine intake, leading to increased intake of nicotine and toxins (as indicted by cotinine), and ultimately resulting in elevated levels of NNAL.
The results of the present study should be considered in light of its limitations. Specifically, the data included in the analyses were cross-sectional data and do not reflect longitudinal relationships between variables. Given that mediation models are designed to assess theorized, longitudinal casual pathway, the cross-sectional models can only yield plausible relations between data. However, it should be noted that alternative models were run that systematically altered the placement of predictors and mediators (e.g., cotinine as predictor, CPD as predictor, TTFC as mediator) and none of these models achieved significance or demonstrated mediation. Future studies replicating these findings with longitudinal data will be needed to increase confidence in the results.
In summary, the findings of this study demonstrate that among both intermittent and regular adolescent smokers, an early TTFC is associated with higher levels of NNAL, and that this relationship is mediated by increased nicotine intake. These findings suggest that regardless of whether an adolescent smokes intermittently or regularly, or how many cigarettes they smoke per day, those who smoke their first cigarette of the day earlier after waking have greater nicotine intake and greater levels of NNAL, a metabolite predictive of lung cancer risk. Given that many teens either are unaware of their risk or equate smoking less frequently with less risk, these findings offer the possibility of developing targeted evidence-based messages for these teens to alert them of their high-risk status and perhaps eventually encourage cessation.
Acknowledgments
This paper was supported in part by NIH grants1R01CA169070-01A1&R01DA026815
Footnotes
Declarations of interest: None declared.
References
- 1.Benjamin RM. A new surgeon general’s report: preventing tobacco use among adolescents and young adults. Public Health Rep Wash DC 1974. 2012 Aug;127(4):360–361. doi: 10.1177/003335491212700402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenk KM, Chen V, Bernat DH, Forster JL, Rode PA. Characterizing and comparing young adult intermittent and daily smokers. Subst Use Misuse. 2009;44(14):2128–2140. doi: 10.3109/10826080902864571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costello DM, Dierker LC, Jones BL, Rose JS. Trajectories of smoking from adolescence to early adulthood and their psychosocial risk factors. Health Psychol Off J Div Health Psychol Am Psychol Assoc. 2008 Nov;27(6):811–818. doi: 10.1037/0278-6133.27.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey SR, Jeffery CJ, Hammer SA, Bryson SW, Killen DT, Ammerman S, et al. Assessing teen smoking patterns: the weekend phenomenon. Drug Alcohol Depend. 2012 Jan 1;120(1–3):242–245. doi: 10.1016/j.drugalcdep.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branstetter SA, Horn K, Dino G, Zhang J. Beyond quitting: predictors of teen smoking cessation, reduction and acceleration following a school-based intervention. Drug Alcohol Depend. 2009 Jan 1;99(1–3):160–168. doi: 10.1016/j.drugalcdep.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis-Esquerre JM, Colby SM, Tevyaw TO, Eaton CA, Kahler CW, Monti PM. Validation of the timeline follow-back in the assessment of adolescent smoking. Drug Alcohol Depend. 2005 Jul;79(1):33–43. doi: 10.1016/j.drugalcdep.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Colby SM, Tiffany ST, Shiffman S, Niaura RS. Measuring nicotine dependence among youth: a review of available approaches and instruments. Drug Alcohol Depend. 2000 May 1;59(Suppl 1):S23–S39. doi: 10.1016/s0376-8716(99)00163-5. [DOI] [PubMed] [Google Scholar]
- 8.Rose JS, Dierker LC, Donny E. Nicotine Dependence Symptoms among Recent Onset Adolescent Smokers. Drug Alcohol Depend. 2010 Jan 15;106(2–3):126. doi: 10.1016/j.drugalcdep.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson LD, O’Malley PM, Bachman JG, Schulenberg JE. Decline in teen smoking continues in 2012 [Internet] Ann Arbor, MI: University of Michigan News Service; 2012. [cited 2012 May 21]. Available from: http://www.monitoringthefuture.org. [Google Scholar]
- 10.Urbán R. Early smoking experience in adolescents. Addict Behav. 2010 Jun;35(6):612–615. doi: 10.1016/j.addbeh.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991 Sep;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 12.Prokhorov AV, De Moor C, Pallonen UE, Hudmon KS, Koehly L, Hu S. Validation of the modified Fagerström tolerance questionnaire with salivary cotinine among adolescents. Addict Behav. 2000 Jun;25(3):429–433. doi: 10.1016/s0306-4603(98)00132-4. [DOI] [PubMed] [Google Scholar]
- 13.Prokhorov AV, Koehly LM, Pallonen UE, Hudmon KS. Adolescent Nicotine Dependence Measured by the Modified Fagerstrom Tolerance Questionnaire at Two Time Points. J Child Adolesc Subst Abuse. 1998;7(4):35–47. [Google Scholar]
- 14.Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3(3):235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- 15.Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. 1989;84(7):791–800. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- 16.Kozlowski LT, Director D, Harford MA. Tobacco dependence, restraint and time to the first cigarette of the day. Addict Behav. 1981;6(4):307–312. [Google Scholar]
- 17.Transdisciplinary Tobacco Use Research Center (TTURC) Tobacco Dependence, Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, et al. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Res Off J Soc Res Nicotine Tob. 2007 Nov;9(Suppl 4):S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toll BA, Schepis TS, O’Malley SS, McKee SA, Krishnan-Sarin S. Subjective reactivity to the first cigarette of the day as a predictor of smoking relapse: a preliminary study. Drug Alcohol Depend. 2007;89(2–3):302–305. doi: 10.1016/j.drugalcdep.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kabat GC, Wynder EL. Determinants of quitting smoking. Am J Public Health. 1987;77(10):1301–1305. doi: 10.2105/ajph.77.10.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borland R, Yong H-H, O’Connor RJ, Hyland A, Thompson ME. The reliability and predictive validity of the Heaviness of Smoking Index and its two components: Findings from the International Tobacco Control Four Country study. Nicotine Tob Res. 2010 Sep 30;12(Supplement 1):S45–S50. doi: 10.1093/ntr/ntq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Feng G, Jiang Y, Yong H-H, Borland R, Fong GT. Prospective predictors of quitting behaviours among adult smokers in six cities in China: findings from the International Tobacco Control (ITC) China Survey: Predictors of smoking cessation among Chinese smokers. Addiction. 2011 Jul;106(7):1335–1345. doi: 10.1111/j.1360-0443.2011.03444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pillitteri JL, Kozlowski LT, Sweeney CT, Heatherton TF. Individual differences in the subjective effects of the first cigarette of the day: a self-report method for studying tolerance. Exp Clin Psychopharmacol. 1997;5(1):83. doi: 10.1037//1064-1297.5.1.83. [DOI] [PubMed] [Google Scholar]
- 23.Mercincavage M, Branstetter SA, Muscat JE, Horn KA. Time to first cigarette predicts cessation outcomes in adolescent smokers. Nicotine Tob Res. 2013;15(12):1996–2004. doi: 10.1093/ntr/ntt087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muscat JE, Ahn K, Richie JP, Jr, Stellman SD. Nicotine dependence phenotype, time to first cigarette, and risk of head and neck cancer. Cancer. 2011 Dec 1;117(23):5377–5382. doi: 10.1002/cncr.26235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muscat JE, Liu H-P, Livelsberger C, Richie JP, Stellman SD. The nicotine dependence phenotype, time to first cigarette, and larynx cancer risk. Cancer Causes Control. 2012 Feb 25;23(3):497–503. doi: 10.1007/s10552-012-9909-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muscat JE, Ahn K, Richie JP, Stellman SD. Nicotine dependence phenotype and lung cancer risk. Cancer. 2011 Dec 1;117(23):5370–5376. doi: 10.1002/cncr.26236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuo K, Gallus S, Negri E, Kawakita D, Oze I, Hosono S, et al. Time to first cigarette and upper aerodigestive tract cancer risk in Japan. Cancer Epidemiol Biomarkers Prev. 2012 Sep 12;21(11):1986–1992. doi: 10.1158/1055-9965.EPI-12-0662. [DOI] [PubMed] [Google Scholar]
- 28.Branstetter SA, Muscat JE. Time to First Cigarette and 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol (NNAL) Levels in Adult Smokers; National Health and Nutrition Examination Survey (NHANES, 2007–2010. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2013 Apr;22(4):615–622. doi: 10.1158/1055-9965.EPI-12-0842. [DOI] [PubMed] [Google Scholar]
- 29.Branstetter SA, Muscat JE. Time to first cigarette and serum cotinine levels in adolescent smokers: National Health and Nutrition Examination Survey, 2007–2010. Nicotine Tob Res Off J Soc Res Nicotine Tob. 2013 Mar;15(3):701–707. doi: 10.1093/ntr/nts189. [DOI] [PubMed] [Google Scholar]
- 30.Rubinstein ML, Thompson PJ, Benowitz NL, Shiffman S, Moscicki A-B. Cotinine levels in relation to smoking behavior and addiction in young adolescent smokers. Nicotine Tob Res Off J Soc Res Nicotine Tob. 2007 Jan;9(1):129–135. doi: 10.1080/14622200601078517. [DOI] [PubMed] [Google Scholar]
- 31.Berg C, Schauer, Ahluwalia J, Benowitz N. Correlates of NNAL levels among nondaily and daily smokers in the college student population. Curr Biomark Find. 2012 Oct;87 doi: 10.2147/CBF.S34642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goniewicz ML, Havel CM, Peng MW, Jacob P, Dempsey D, Yu L, et al. Elimination Kinetics of the Tobacco-Specific Biomarker and Lung Carcinogen 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol. Cancer Epidemiol Biomarkers Prev. 2009 Dec 3;18(12):3421–3425. doi: 10.1158/1055-9965.EPI-09-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khariwala SS, Scheuermann TS, Berg CJ, Hayes RB, Nollen NL, Thomas JL, et al. Cotinine and Tobacco-Specific Carcinogen Exposure Among Nondaily Smokers in a Multiethnic Sample. [cited 2014 Jan 9];Nicotine Tob Res [Internet] 2013 Dec 1; doi: 10.1093/ntr/ntt194. Available from: http://ntr.oxfordjournals.org/cgi/doi/10.1093/ntr/ntt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Center for Health Statistics. Analytic and Reporting Guidelines: The National Health and Nutrition Examination Survey (NHANES) [Internet] Hyattsville, MD: Centers for Disease Control and Prevention; 2006. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/analytical_guidelines.htm. [Google Scholar]
- 35.Centers for Disease Control. Laboratory Procedures Manual [Internet]. Centers for Disease Control and Prevention. 2009 Available from: www.cdc.gov/nchs/data/nhanes/nhanes_09_10/lab.pdf.
- 36.Husten CG. How should we define light or intermittent smoking? Does it matter? Nicotine Tob Res. 2009 Feb;11(2):111–121. doi: 10.1093/ntr/ntp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiffman S, Ferguson SG, Dunbar MS, Scholl SM. Tobacco dependence among intermittent smokers. Nicotine Tob Res Off J Soc Res Nicotine Tob. 2012 Nov;14(11):1372–1381. doi: 10.1093/ntr/nts097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control. Youth Risk Behavior Surveillance -- United States, 2011. MMWR. 2012;61(4):1–168. [PubMed] [Google Scholar]
- 39.MacKinnon DP, Lockwood CM, Williams J. Confidence Limits for the Indirect Effect: Distribution of the Product and Resampling Methods. Multivar Behav Res. 2004 Jan 1;39(1):99. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput J Psychon Soc Inc. 2004 Nov;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 41.Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 42.Preacher K, Hayes A. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 43.Muscat JE, Liu A, Richie JP., Jr A comparison of creatinine vs. specific gravity to correct for urinary dilution of cotinine. Biomark Biochem Indic Expo Response Susceptibility Chem. 2011 May;16(3):206–211. doi: 10.3109/1354750X.2010.538084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan J-M, Gao Y-T, Murphy SE, Carmella SG, Wang R, Zhong Y, et al. Urinary levels of cigarette smoke constituent metabolites are prospectively associated with lung cancer development in smokers. Cancer Res. 2011 Nov 1;71(21):6749–6757. doi: 10.1158/0008-5472.CAN-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Y, Shen Z, Shen J, Liu G, Li W, Tang Y. Computational insights into the different catalytic activities of CYP2A13 and CYP2A6 on NNK. J Mol Graph Model. 2011 Sep;30:1–9. doi: 10.1016/j.jmgm.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Lin R-K, Hsieh Y-S, Lin P, Hsu H-S, Chen C-Y, Tang Y-A, et al. The tobacco-specific carcinogen NNK induces DNA methyltransferase 1 accumulation and tumor suppressor gene hypermethylation in mice and lung cancer patients. J Clin Invest. 2010 Feb;120(2):521–532. doi: 10.1172/JCI40706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xia Y, Bernert J, Jain R, Ashley D, Pirkle J. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in smokers in the United States: NHANES 2007–2008. Biomarkers. 2011 Mar;16(2):112–119. doi: 10.3109/1354750X.2010.533288. [DOI] [PubMed] [Google Scholar]
- 48.Hertsgaard LA, Hanson K, Hecht SS, Lindgren BR, Luo X, Carmella SG, et al. Exposure to a tobacco-specific lung carcinogen in adolescent versus adult smokers. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2008 Dec;17(12):3337–3343. doi: 10.1158/1055-9965.EPI-08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frankenberger KD. Adolescent Egocentrism, Risk Perceptions, and Sensation Seeking among Smoking and Nonsmoking Youth. J Adolesc Res. 2004 Sep 1;19(5):576–590. [Google Scholar]