Abstract
Objective
African American women present an understudied population in menopause research, yet face greater post-menopausal challenges associated with mortality than their white peers. We investigated the effect of a mild-intensity aerobic exercise training program on markers of mortality risk in both pre- and post-menopausal African American women.
Methods
16 pre- and 19 post-menopausal women underwent 6 months of mild-intensity aerobic exercise training. Measurements included markers of blood lipid and glucose profile, inflammation, kidney function, vascular health and aerobic fitness before and after the exercise intervention.
Results
Before the exercise intervention the pre- and post-menopausal groups only differed in terms of age, LDL, and total cholesterol levels, with the latter two being higher in the post-menopausal group. Both triglycerides and markers of early stage endothelial dysfunction (CD62E+ endothelial microparticles) improved in both groups with aerobic exercise training. Aerobic fitness, glomerular filtration rate, body mass index, plasma glucose levels, and markers of late stage endothelial dysfunction (CD31+/CD42b− endothelial microparticles) only improved in the pre-menopausal group.
Conclusions
Mild-intensity aerobic exercise training was successful in improving some markers of cardiovascular disease and mortality in post-menopausal women. Higher levels of exercise intensity or perhaps additional interventions may need to be considered in this population to further decrease mortality risk.
Keywords: menopause, aerobic exercise, endothelial microparticles, African American, kidney function
Introduction
With the onset of menopause (MP) women face greater metabolic challenges when compared to their pre-menopausal (pre-MP) years that pre-dispose them to a greater mortality risk. Most notably, post-menopausal (post-MP) women have an increased likelihood to develop metabolic syndrome (MS) and cardiovascular disease (CVD) ultimately resulting in increased mortality1-7. A powerful tool in the prevention and treatment of CVD and MS is aerobic exercise training (AEXT)8-16. However, only few studies have investigated the effects of AEXT on CVD risk factors in post-MP women in vivo. Findings include beneficial adaptations to AEXT in terms of arterial stiffness, body weight, triglycerides, total cholesterol and LDL cholesterol levels, as well as intima media thickness17-19. Actual comparisons of adaptations to AEXT between pre- and post-MP women are scarce in the literature. Measures in these studies were usually limited to lipid profile markers. Similar improvements between groups were found in terms of triglycerides, LDL and total cholesterol levels with HDL levels remaining unchanged in both pre- and post-MP women20,21. African American women (AAW) present an understudied population in terms of MP-associated CVD risk factors and their modification. Yet, AAW inherently have a greater risk for CVD, MS and associated mortality22-26. Additionally, AAW reach MP at a younger age than their white peers which in itself increases the risk for CVD and mortality27-29. Among the few studies that have investigated MP in AAW, an increased severity of vasomotor symptoms and presence of CVD risk factors when compared to other racial groups were found30,31.
To the best of our knowledge, no in vivo studies have investigated the effect of AEXT on CVD risk factors in this population, let alone compared AEXT adaptations to their pre-MP peers. Additionally, this is the first study to investigate the effect of AEXT on several markers of kidney and vascular function including endothelial microparticles, a novel marker in vascular health and disease in post-MP women32,33.
The primary aim of this pilot study was to determine whether a standardized AEXT program similarly benefits a small sample of high risk post-MP women and pre-MP women. We hypothesized that post-MP AAW would have a blunted exercise response in terms of CVD risk factors, warranting further investigation.
Methods
Participants
Thirty-five middle- to older-aged (40-75 years old) AAW selected from the Fit4Life study were included in the study and stratified by menopausal status. All participants were sedentary (aerobic exercise ≤ two times per week), non-diabetic (fasting blood glucose ≤ 125 mg/dL), non-smoking (≥ 2 years), had a clinical blood pressure (BP) < 160/100 mmHg, were non-hyperlipidemic (total cholesterol ≤ 240mg/dL), had no signs of cardiovascular/renal/pulmonary disease and were not on any lipid lowering medication or medications affecting cardiovascular or renal hemodynamics. Participants who were post-MP (N=19) were not on any hormone therapy. Each participant gave written informed consent. The protocol was approved by the Temple University Institutional Review Board.
Screening
After initial phone interviews, participants were invited to three consecutive screening visits to determine eligibility according to the study criteria. The first visit followed a twelve hour fast and included collection of blood and urine samples for analysis of blood chemistry and urinalysis by Quest Diagnostics®. Glomerular Filtration Rate (GFR) was estimated using the fourvariable CKD-EPI equation34. The second visit was a cardiologist administered physical examination and review of health history. The third visit required the participants to undergo a cycle ergometer echocardiogram stress test to further rule out cardio-pulmonary abnormalities and discrepancies among the study sample. Additionally, participants’ BPs were assessed by measuring seated BPs using an aneroid sphygmomanometer (Omron, Model 11-675D, Vernon Hills, IL) and averaging three measures each from three separate visits. During each visit, participants had to rest for five minutes prior to the first measurement. Each consecutive measure was five minutes apart. Measures of internal consistency and reliability for were 0.9 for Cronbach’s alpha and a mean of 0.75 for inter-items correlation for both systolic and diastolic blood pressure measurements.
EMP Analysis
EMPs were analyzed as previously reported35. Briefly, plasma samples were centrifuged twice for 1500g for 20 minutes at 24°C to obtain cell free plasma. 100 μL of sample was then incubated with fluorochrome-labeled antibodies and fixed with 93 μL of 10% formaldehyde followed by gentle agitation (500 RPM) for 20 minutes at 24°C. Samples were then diluted in 500 mL of 0.22 μm double-filtered PBS and analyzed using a BDLSRII flow cytometer and BD FACSDIVA software (BD Biosciences, San Jose, CA). Labeled events <1.0 μm were defined as EMPs and indentified through the use of a logarithmic scale for forward scatter signal, side scatter signal and each fluorescent channel. The flow rate was set to medium and all samples were run for 180 seconds yielding a mean sample volume of 101 μL per 180 seconds. EMPs were expressed as events per μL plasma. EMPs labeled with CD31+/CD42b− were used to identify EMPs released as a result of endothelial cell apoptosis, whereas EMPs labeled with CD62E+ were used to identify EMPs released as a result of endothelial cell activation33. Inter-Assay and Intra-Assay CVs for CD62E+ were 15% and 8% and 6% and 8% for CD31+/CD42b−.
Exercise Training Intervention
Each participant performed a sub-maximal graded exercise test using the modified Bruce Protocol to determine aerobic fitness levels through continuous measurement of oxygen consumption (VO2) via a metabolic cart (Vmax Encore, SensorMedics, Yorba Linda, CA) for estimation of maximal aerobic fitness capacity (VO2max). Each test was preceded by flow volume and gas mixture calibration to ensure validity and reproducibility. Participants then underwent six months of closely-supervised AEXT. Initially, participants exercised for 20 minutes at an intensity of 50% of VO2max three times per week. Frequency was maintained and duration of exercise was increased weekly in five minute increments until a total exercise time of 40 minutes was achieved. At this point exercise intensity was increased from 50% of VO2max to 65% VO2max in weekly increments of 5% of VO2max. For the remainder of the intervention participants exercised at this moderate intensity for 40 minutes three times per week. Exercise modes included treadmill walking/jogging, stair stepping, stationary cycling, rowing ergometry, arm ergometry and elliptical cross-training. Exercise duration and intensity were supervised by study personnel through the use of heart rate monitors and recorded every ten minutes for the duration of exercise.
Validity and Reliability of Measurements
Statistical Analysis
Data are expressed as mean ± standard error of the mean. The distribution of each variable was examined using the Shapiro-Wilk test of normality. Non-parametric tests were used when appropriate. For before and after AEXT comparisons within groups paired-sample tests were used (T-test or Wilcoxin signed-rank test). For between group comparisons at the same time points (before or after AEXT) independent-samples tests were performed (T-test or Mann- Whitney U test). For correlation analysis Pearson product-moment correlation coefficient or Spearman’s rank correlation coefficient were determined. Statistical significance was defined as a p-value ≤ 0.05. All statistical analyses were performed using PSAW version 17.0 (SPSS Inc., Chicago, IL). Differences in the number of participants between variables are a result of issues with participant scheduling, acquisition of blood samples, or assay procedure.
Results
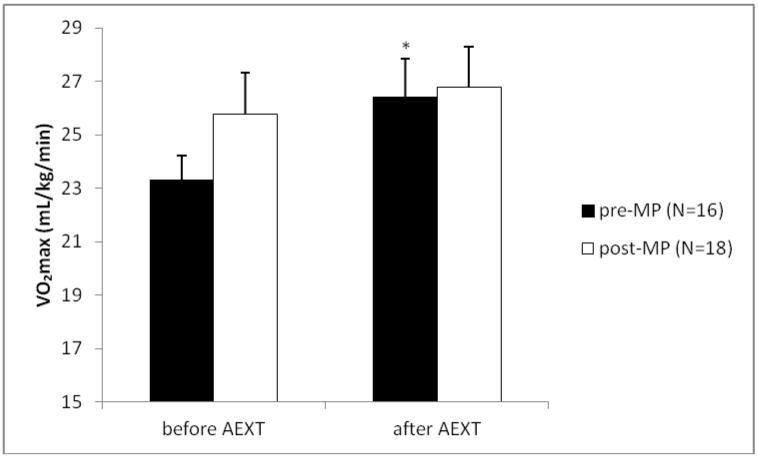
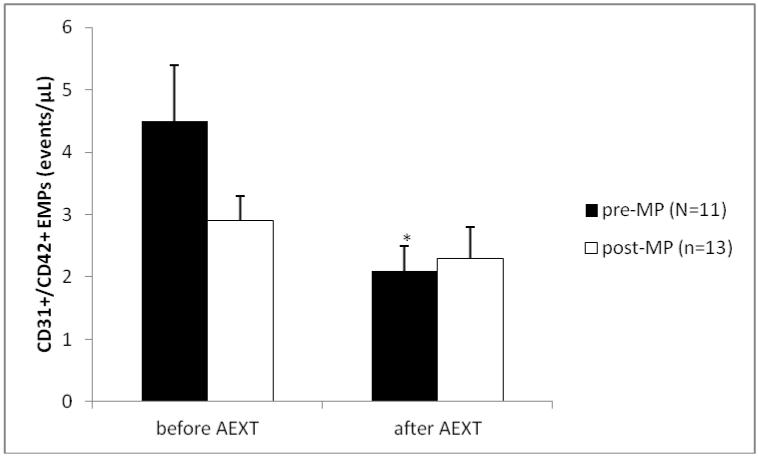
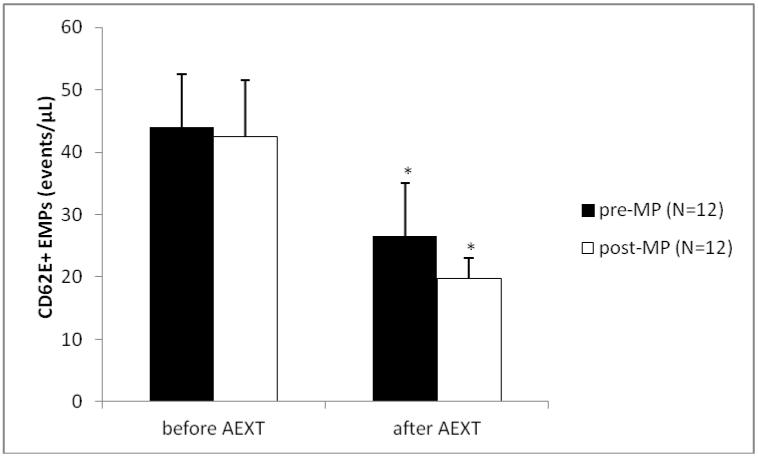
The study group consisted of 16 pre- and 19 post-MP AAW. Summary characteristics for both groups are listed in Table 1. As expected, age was significantly lower in the pre-MP group when compared to the post-MP group (51±1.2 vs. 56±1.4 yrs., p≤0.012). When looking at the entire sample, age was positively correlated with cholesterol (r=0.539, p≤0.0001), LDL (r=0.389, p≤0.011) and interestingly HDL (r=0.314, p≤0.043). No other variables were correlated with age. Before AEXT BMI was not different between pre- and post-MP women (30.3±1.0 vs. 32.4±1.7 kg/m2), but only women in the pre-MP group had lowered (p≤0.049) their BMI after AEXT (30.3±1.0 vs. 29.7±1.1 kg/m2). Total cholesterol levels were significantly lower in the pre-MP group when compared to the post-MP group, before AEXT (182.8±6.5 vs. 202.5±6.1 mg/dL, p≤0.011). Neither group had improved their cholesterol levels after AEXT. Triglyceride levels were not different between pre- and post-MP women (85.6±9.1 vs. 78.9±6.3 mg/dL), but both pre- (85.6±9.1 vs. 71.9±4.8 mg/dL, p≤0.047) and post-MP groups (78.9±6.3 vs. 68.9±5.5 mg/dL, p≤0.041) had decreased levels after AEXT. LDL levels were significantly different between pre- and post-MP women before AEXT (98.3±4.5 vs. 119.8±6.1 mg/dL, p≤0.006), although neither group had decreased LDL levels after AEXT. Glucose levels were not significantly different between pre- and post-MP women before AEXT (94.8±2.3 vs. 92.4±2.6 mg/dL), but only the pre-MP group had lowered their glucose levels after AEXT (94.8±2.3 vs. 82.9±2.2 mg/dL, p≤0.001). Systolic and diastolic blood pressures were not significantly different between groups and neither group was able to improve either variable with AEXT. Aerobic fitness, measured via VO2max was not significantly different between groups before AEXT (23.3±0.9 vs. 25.8±1.6 mL/kg/min), but only the pre-MP group had increased fitness levels after AEXT (23.3±0.9 vs. 26.4±1.4 mL/kg/min, p≤0.009) [Figure 1]. Renal function, measured by estimated GFR, was not different between groups before AEXT (90.6±4.4 vs. 94.9±4.1 mL/min/1.73m3), but had improved significantly only in the pre-MP group after AEXT (90.6±4.4 vs. 99.2±4.3 mL/min/1.73m3, p≤0.05) [Figure 2]. Markers of late phase endothelial dysfunction and endothelial cell apoptosis, CD31+/CD42b− EMPs, were not significantly different between groups before AEXT (4.5±0.9 vs. 2.9±0.4 events/μL). However, only the pre-MP group had lowered CD31+/CD42b− EMP levels after AEXT (4.5±0.9 vs. 2.1±0.4 events/μL, p≤0.015) [Figure 3]. Markers of early phase endothelial dysfunction and endothelial cell activation, CD62E+ EMPs, also were not significantly different between groups before AEXT (44.0±8.5 vs. 42.5±9 events/μL), but both pre- (44.0±8.5 vs. 26.6±8.4 events/μL, p≤0.034) and post-MP women (42.5±9 vs. 19.8±3.2 events/μL, p≤0.008) had lowered their levels after AEXT [Figure 4].
Table 1. Participant Characteristics.
| pre-menopausal | post-menopausal | |||||
|---|---|---|---|---|---|---|
| baseline | final | baseline | final | |||
| N | mean | mean | N | mean | mean | |
| Age (yrs) | 16 | 51±1.2 | 19 | 56±1.4 # | ||
| Height (cm) | 16 | 165.8±1.1 | 19 | 165.0±1.1 | ||
| Weight (kg) | 16 | 83.2±2.9 | 81.7±3.0 | 16 | 86.7±5.3 | 85.9±5.2 |
| BMI (kg/m2) | 16 | 30.3±1.0 | 29.7±1.1 * | 16 | 32.4±1.7 | 31.4±1.7 |
| Body Fat (%) | 15 | 42.0±1.3 | 41.7±1.7 | 16 | 42.7±1.6 | 42.7±2.0 |
| Cholesterol (mg/mL) | 14 | 182.8±6.5 | 181.0±7.3 | 16 | 202.5±6.1 # | 203.8±8.0 # |
| Triglycerides (mg/mL) | 14 | 85.6±9.1 | 71.9±4.8 * | 16 | 78.9±6.3 | 68.9±5.5 * |
| HDL (mg/mL) | 14 | 67.3±6.6 | 68.4±7.4 | 16 | 66.9±2.9 | 65.0±3.6 |
| LDL (mg/mL) | 14 | 98.3±4.5 | 98.3±4.4 | 16 | 119.8±6.1 # | 125.1±6.9 # |
| Glucose (mg/mL) | 14 | 94.8±2.3 | 82.9±2.2 ** | 16 | 92.4±2.6 | 91.5±2.1 |
| CRP (mg/mL) | 16 | 3.7±0.8 | 3.3±0.8 | 15 | 3.5±0.7 | 2.9±0.7 |
| SBP (mmHg) | 16 | 127.8±3.1 | 129.7±4.0 | 19 | 121.5±3.0 | 119.2±2.8 |
| DBP (mmHg) | 16 | 80.2±1.8 | 81.9±2.1 | 19 | 76.6±1.8 | 76.1±1.7 |
significantly different (p≤0.05) after AEXT vs. before AEXT within the same group (pre- or post-menopause)
significantly different (p≤0.001) after AEXT vs. before AEXT within the same group (pre- or post-menopause)
significantly different (p≤0.05) in post- vs pre-menopausal group at the same time point (before or after AEXT)
Figure 1.
Measure of VO2max before and after Aerobic Exercise Training (AEXT). The black bars represent the pre-menopausal (pre-MP) group, the white bars represent the post-menopausal (post-MP) group. Bars are expressed as mean ± SEM. *Denotes significantly different (p≤0.05) after Aerobic Exercise Training (AEXT) when compared to before AEXT within the same MP group.
Figure 2.
Measure of estimated Glomerular Filtration Rate before and after Aerobic Exercise Training (AEXT). The black bars represent the pre-menopausal (pre-MP) group, the white bars represent the post-menopausal (post-MP) group. Bars are expressed as mean ± SEM. *Denotes significantly different (p≤0.05) after AEXT when compared to before AEXT within the same MP group.
Figure 3.
Measure of CD31+/CD42b− Endothelial Microparticles before and after Aerobic Exercise Training (AEXT). The black bars represent the pre-menopausal (pre-MP) group, the white bars represent the post-menopausal (post-MP) group. Bars are expressed as mean ± SEM. *Denotes significantly different (p≤0.05) after AEXT when compared to before AEXT within the same MP group.
Figure 4.
Measure of CD62E+ Endothelial Microparticles before and after Aerobic Exercise Training (AEXT). The black bars represent the pre-menopausal (pre-MP) group, the white bars represent the post-menopausal (post-MP) group. Bars are expressed as mean ± SEM. *Denotes significantly different (p≤0.05) after AEXT when compared to before AEXT within the same MP group.
Discussion
In summary, our pilot study revealed that mild-intensity AEXT was successful in improving some markers of CVD and mortality in a small sample of post-MP women. However, AEXT was not successful in improving certain other markers of CVD and mortality that the pre-MP group showed improvements in. We believe this may be due to the effects of menopause and not merely be an effect of aging as evidenced by the lack of correlation of age with most of the other measured variables.
Interestingly, aerobic fitness did not improve in the post-MP group after six months of AEXT. It has been previously suggested that post-MP women present abnormalities in the peripheral circulation that could impair aerobic exercise capacity36. As such, the lack of improvements in aerobic fitness in the present study may, in part, be explained by abnormalities in the peripheral circulation. If confirmed in more large scale randomized controlled trials, the findings might suggest a need for different exercise prescriptions among post-MP women to elicit similar exercise adaptations as in the pre-MP group. This finding should, however, be interpreted with some caution as the small sample size may have limited our statistical power to detect improvements in aerobic fitness. Nevertheless, we suspect that a lack of statistical power likely did not confound this study finding as both groups (pre- and post-MP women) received the same exercise intervention and this intervention was sufficient to elicit significant improvements in the pre-MP women.
It has been previously reported18 that higher levels of physical activity were associated with lower BMI in post-MP women when compared to non-exercisers. Our findings suggest that AEXT was not sufficient to decrease BMI in the post-MP group. Whether this phenomenon is population specific or is due to the relatively mild exercise intensity of our intervention remains unanswered, but again may suggest the need for a higher exercise intensity in the post-MP to achieve similar results as the pre-MP group.
Total cholesterol and LDL levels were elevated in the post-MP group as previously reported6,7. We also found that LDL and total cholesterol were correlated with age in the total sample. Increases in the these two lipid markers may therefore not be a result of reaching MP, but simply be a function of age in this population, unlike previously reported3,37. Both LDL and total cholesterol remained unchanged with AEXT in both pre- and post-MP women, contrary to previous studies20,21. Also, HDL cholesterol did not differ between pre- and post-MP women and did not improve with AEXT. Controversy on the matter exists, as some studies indicate no improvement with AEXT in terms of HDL in both pre- and post-MP women20,21, others report improvements38,39. The lack of change in HDL, LDL and total cholesterol in our group could be explained by the relatively mild exercise intensity used in our study. Triglycerides were the only lipid profile marker that changed in response AEXT, although there were no differences between pre- and post-MP women.
Glucose levels, although not different between groups prior to the exercise intervention, only improved in the pre-MP group. This may indicate differences in glucose metabolism unique to post-MP women who coincidentally also present a higher risk for diabetes mellitus when compared to their pre-MP peers40.
Kidney function measured by estimated GFR did not differ between groups, but only the pre- MP group had improved estimated GFR post-exercise. This could suggest that AEXT may not be the best means in protecting against progressive declines in kidney function among post-MP women. This is important, because African Americans in particular have much higher rates of Chronic Kidney Disease (CKD) than their white peers and have been shown to progress more rapidly from the early stages of CKD to more advanced stages41,42. Additionally, studies have illustrated the beneficial effects of AEXT in the modification of several CKD risk factors and in the prevention of CKD43,44. However, our data indicate that AEXT may not be sufficient to improve kidney function among post-MP women as evidenced by the lack of improvement in estimated GFR in this high risk population.
CD62+ EMPs which are a measure of endothelial health status improved in both the pre- and post-MP group and there were no differences between groups at any time point. Increases in CD62+ EMPs are usually associated with activation of endothelial cells which is a sign of early stage endothelial dysfunction, a predecessor for atherosclerosis and hypertension32,33. Conversely, CD31+/CD42b− EMP levels, a measure of late stage endothelial dysfunction and apoptosis, only improved among the pre-MP group. Although purely speculative, these findings may suggest that AEXT has the potential to ameliorate early stage endothelial dysfunction, regardless of MP status, but may be ineffective to ameliorate later stage endothelial dysfunction among post-MP women.
Conclusion
Before AEXT, in our study sample post-MP AAW had a higher CVD risk than their pre-MP peers only in terms of lipid profile. Although, mild-intensity AEXT does not seem to improve cardio- respiratory fitness, glucose, LDL and total cholesterol levels, nor kidney function in post-MP AAW, improvements in vascular and endothelial function may warrant adoptions of a physically active lifestyle to decrease CVD risk post-MP in this population. Further exploration of these findings may be necessary to confirm whether similar exercise effects can be observed in a larger sample and as such this investigation should only be regarded as a pilot study. Additional lifestyle modifications or perhaps an increased exercise stimulus to address the other CVD risk factors unchanged by mild-intensity AEXT should also be explored to potentially further decrease mortality risks post-MP.
Acknowledgements
Funding: This research was supported by NIH/NHLBI Grant RO1 [HL085497] to Michael D. Brown
Footnotes
Conflicts of interest: none
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.KANNEL WB, HJORTLAND MC, McNAMARA PM, GORDON T. Menopause and Risk of Cardiovascular DiseaseThe Framingham Study. Annals of Internal Medicine. 1976;85(4):447–452. doi: 10.7326/0003-4819-85-4-447. Available at: http://dx.doi.org/10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 2.Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease. The Framingham Study. Annals of Internal Medicine. 1978;89(2):157–161. doi: 10.7326/0003-4819-89-2-157. Available at: http://www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?db=m&form=6&dopt=r&uid=677576. [DOI] [PubMed] [Google Scholar]
- 3.Matthews KA, Crawford SL, Chae CU, et al. Are Changes in Cardiovascular Disease Risk Factors in Midlife Women Due to Chronological Aging or to the Menopausal Transition? Journal of the American College of Cardiology. 2009;54(25):2366–2373. doi: 10.1016/j.jacc.2009.10.009. Available at: http://dx.doi.org/10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rappelli A. Hypertension and obesity after the menopause. Journal of hypertension Supplement official journal of the International Society of Hypertension. 2002;20(2):S26–S28. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12183846. [PubMed] [Google Scholar]
- 5.Rosano GMC, Vitale C, Marazzi G, Volterrani M. Menopause and cardiovascular disease: the evidence. Climacteric. 2007;10(s1):19–24. doi: 10.1080/13697130601114917. Available at: http://dx.doi.org/10.1080/13697130601114917. [DOI] [PubMed] [Google Scholar]
- 6.Janssen I, Crawford S, Lasley B, Sutton-Tyrrell KPLH. Menopause and the metabolic syndrome: The study of women's health across the nation. Archives of Internal Medicine. 2008;168(14):1568–1575. doi: 10.1001/archinte.168.14.1568. Available at: http://dx.doi.org/10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawford SL, Johannes CB. The Epidemiology of Cardiovascular Disease in Postmenopausal Women. Journal of Clinical Endocrinology & Metabolism. 1999;84(6):1803–1806. Available at: http://jcem.endojournals.org/content/84/6/1803.short. [Google Scholar]
- 8.Thompson PD, Buchner D, Piña IL, et al. Exercise and Physical Activity in the Prevention and Treatment of Atherosclerotic Cardiovascular Disease: A Statement From the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23(8):e42–e49. doi: 10.1161/01.ATV.0000087143.33998.F2. Available at: http://atvb.ahajournals.org/content/23/8/e42.short. [DOI] [PubMed] [Google Scholar]
- 9.Hansen D, Dendale P, Loon LC, Meeusen R. The Impact of Training Modalities on the Clinical Benefits of Exercise Intervention in Patients with Cardiovascular Disease Risk or Type 2 Diabetes Mellitus. Sports Medicine. 2010;40(11):921–940. doi: 10.2165/11535930-000000000-00000. LA - English. Available at: http://dx.doi.org/10.2165/11535930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Whelton SP, Chin A, Xin X, He J. Effect of Aerobic Exercise on Blood PressureA Meta-Analysis of Randomized, Controlled Trials. Annals of Internal Medicine. 2002;136(7):493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. Available at: http://dx.doi.org/10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 11.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. The American Journal of Clinical Nutrition. 1999;69(3):373–380. doi: 10.1093/ajcn/69.3.373. Available at: http://ajcn.nutrition.org/content/69/3/373.abstract. [DOI] [PubMed] [Google Scholar]
- 12.Miller T, Balady G, Fletcher G. Exercise and its role in the prevention and rehabilitation of cardiovascular disease. Annals of Behavioral Medicine. 1997;19(3):220–229. doi: 10.1007/BF02892287. LA - English. Available at: http://dx.doi.org/10.1007/BF02892287. [DOI] [PubMed] [Google Scholar]
- 13.Hellénius M-L, de Faire U, Berglund B, Hamsten A, Krakau I. Diet and exercise are equally effective in reducing risk for cardiovascular disease. Results of a randomized controlled study in men with slightly to moderately raised cardiovascular risk factors. Atherosclerosis. 1993;103(1):81–91. doi: 10.1016/0021-9150(93)90042-s. Available at: http://www.sciencedirect.com/science/article/pii/002191509390042S. [DOI] [PubMed] [Google Scholar]
- 14.Pattyn N, Cornelissen V, Eshghi ST, Vanhees L. The Effect of Exercise on the Cardiovascular Risk Factors Constituting the Metabolic Syndrome. Sports Medicine. 2013;43(2):121–133. doi: 10.1007/s40279-012-0003-z. LA - English. Available at: http://dx.doi.org/10.1007/s40279-012-0003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bateman LA, Slentz CA, Willis LH, et al. Comparison of Aerobic Versus Resistance Exercise Training Effects on Metabolic Syndrome (from the Studies of a Targeted Risk Reduction Intervention Through Defined Exercise - STRRIDE-AT/RT) The American Journal of Cardiology. 2011;108(6):838–844. doi: 10.1016/j.amjcard.2011.04.037. Available at: http://www.sciencedirect.com/science/article/pii/S0002914911017838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finucane FM, Sharp SJ, Purslow LR, et al. The effects of aerobic exercise on metabolic risk, insulin sensitivity and intrahepatic lipid in healthy older people from the Hertfordshire Cohort Study: a randomised controlled trial. Diabetologia. 2010;53(4):624–631. doi: 10.1007/s00125-009-1641-z. LA - English. Available at: http://dx.doi.org/10.1007/s00125-009-1641-z. [DOI] [PubMed] [Google Scholar]
- 17.Figueroa A, Park SY, Seo DY, Sanchez-Gonzalez MA, Baek YH. Combined resistance and endurance exercise training improves arterial stiffness, blood pressure, and muscle strength in postmenopausal women. Menopause. 2011;18(9) doi: 10.1097/gme.0b013e3182135442. Available at: http://journals.lww.com/menopausejournal/Fulltext/2011/09000/Combined_resistance_and_endurance_exercise.11.aspx. [DOI] [PubMed] [Google Scholar]
- 18.Gudmundsdottir SL, Flanders WD, Augestad LB. Physical activity and cardiovascular risk factors at menopause: The Nord-Trøndelag health study. Climacteric. 2013:1–9. doi: 10.3109/13697137.2013.768231. Available at: http://dx.doi.org/10.3109/13697137.2013.768231. [DOI] [PubMed] [Google Scholar]
- 19.Wildman RP, Schott LL, Brockwell S, Kuller LH, Sutton-Tyrrell K. A dietary and exercise intervention slows menopause-associated progression of subclinical atherosclerosis as measured by intima-media thickness of the carotid arteries. Journal of the American College of Cardiology. 2004;44(3):579–585. doi: 10.1016/j.jacc.2004.03.078. Available at: http://dx.doi.org/10.1016/j.jacc.2004.03.078. [DOI] [PubMed] [Google Scholar]
- 20.Behall KM, Howe JC, Martel G, Scott WH, Dooly CR. Comparison of resistive to aerobic exercise training on cardiovascular risk factors of sedentary, overweight premenopausal and postmenopausal women. Nutrition Research. 2003;23(5):607–619. Available at: http://www.sciencedirect.com/science/article/pii/S0271531703000150. [Google Scholar]
- 21.Blumenthal JA, Matthews K, Fredrikson M, et al. Effects of exercise training on cardiovascular function and plasma lipid, lipoprotein, and apolipoprotein concentrations in premenopausal and postmenopausal women. Arteriosclerosis, Thrombosis, and Vascular Biology. 1991;11(4):912–917. doi: 10.1161/01.atv.11.4.912. Available at: http://atvb.ahajournals.org/content/11/4/912.abstract. [DOI] [PubMed] [Google Scholar]
- 22.Matthews KA, Sowers MF, Derby CA, et al. Ethnic differences in cardiovascular risk factor burden among middle-aged women: Study of Women’s Health Across the Nation (SWAN) American Heart Journal. 2005;149(6):1066–1073. doi: 10.1016/j.ahj.2004.08.027. Available at: http://www.sciencedirect.com/science/article/pii/S0002870304005411. [DOI] [PubMed] [Google Scholar]
- 23.Group W, Mosca L, Manson JE, et al. Cardiovascular Disease in Women: A Statement for Healthcare Professionals From the American Heart Association. Circulation. 1997;96(7):2468–2482. doi: 10.1161/01.cir.96.7.2468. Available at: http://circ.ahajournals.org/content/96/7/2468.short. [DOI] [PubMed] [Google Scholar]
- 24.Ford ES, Dietz WHGWH. Prevalence of the metabolic syndrome among us adults: Findings from the third national health and nutrition examination survey. JAMA. 2002;287(3):356–359. doi: 10.1001/jama.287.3.356. Available at: http://dx.doi.org/10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 25.Park Y, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SBZS. The metabolic syndrome: Prevalence and associated risk factor findings in the us population from the third national health and nutrition examination survey, 1988-1994. Archives of Internal Medicine. 2003;163(4):427–436. doi: 10.1001/archinte.163.4.427. Available at: http://dx.doi.org/10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall WD, Clark LT, Wenger NK, et al. The Metabolic Syndrome in African Americans: a review. Ethnicity & disease. 2003;13(4):414–428. Available at: http://europepmc.org/abstract/MED/14632261. [PubMed] [Google Scholar]
- 27.Snowdon DAFAU, Kane RL, Kane RLFAU, Beeson WL, Beeson WLFAU, Burke GL, et al. Is early natural menopause a biologic marker of health and aging? :709–14. doi: 10.2105/ajph.79.6.709. (0090-0036 (Print)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper GSFAU, Sandler DP, Sandler DPLA, eng PT. Journal Article PL - UNITED STATES TA - Ann Epidemiol JT - Annals of epidemiology JID - 9100013 SB - IM SB - J MH - Adult MH - Age Factors MH - Aged MH - Aged Nonparametric MH - United States OID - IND: RH8B046 OID - 80 and over MH-*Aging MH-CDMH-CDMH-FMH-HMH-*Menopause MH-MAMH-*Mortality MH- NMH-PDMH-S. Age at natural menopause and mortality. :229–35. (1047-2797 (Print)) [Google Scholar]
- 29.Bromberger JT, Matthews KA, Kuller LH, et al. [Accessed April 22, 2013];Prospective Study of the Determinants of Age at Menopause. American Journal of Epidemiology. 1997 145(2):124–133. doi: 10.1093/oxfordjournals.aje.a009083. Available at: http://aje.oxfordjournals.org/cgi/content/long/145/2/124. [DOI] [PubMed] [Google Scholar]
- 30.Avis NE, Stellato R, Crawford S, et al. Is there a menopausal syndrome? Menopausal status and symptoms across racial/ethnic groups. Social Science & Medicine. 2001;52(3):345–356. doi: 10.1016/s0277-9536(00)00147-7. Available at: http://www.sciencedirect.com/science/article/pii/S0277953600001477. [DOI] [PubMed] [Google Scholar]
- 31.GA W, VG M, RH M, et al. C-reactive protein is associated with aortic stiffness in a cohort of African American and white women transitioning through menopause. Menopause. 2011;18(12):1291–1297. doi: 10.1097/gme.0b013e31821f81c2. Available at: http://pubget.com/paper/21892111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boulanger CM, Amabile N, Tedgui A. [Accessed March 12, 2013];Circulating microparticles: a potential prognostic marker for atherosclerotic vascular disease. Hypertension. 2006 48(2):180–6. doi: 10.1161/01.HYP.0000231507.00962.b5. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16801490. [DOI] [PubMed] [Google Scholar]
- 33.Chironi GN, Boulanger CM, Simon A, et al. [Accessed March 23, 2013];Endothelial microparticles in diseases. Cell and tissue research. 2009 335(1):143–51. doi: 10.1007/s00441-008-0710-9. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18989704. [DOI] [PubMed] [Google Scholar]
- 34.Stevens LAFAU, Schmid CH, Schmid CHFAU, Greene T, Greene TFAU, Zhang YL, et al. Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. pp. 486–95. LID - 10.1053/j.ajkd.2010.03.026 [doi] (1523-6838 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Babbitt DM, Diaz KM, Feairheller DL, et al. Endothelial Activation Microparticles and Inflammation Status Improve with Exercise Training in African Americans. International Journal of Hypertension. 2013;2013:1–8. doi: 10.1155/2013/538017. Available at: http://www.hindawi.com/journals/ijht/2013/538017/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mercuro G, Saiu F, Deidda M, et al. Impairment of physical exercise capacity in healthy postmenopausal women. American Heart Journal. 2006;151(4):923–927. doi: 10.1016/j.ahj.2005.06.027. Available at: http://www.sciencedirect.com/science/article/pii/S0002870305006460. [DOI] [PubMed] [Google Scholar]
- 37.Carr MC. The Emergence of the Metabolic Syndrome with Menopause. Journal of Clinical Endocrinology & Metabolism. 2003;88(6):2404–2411. doi: 10.1210/jc.2003-030242. Available at: http://jcem.endojournals.org/content/88/6/2404.abstract. [DOI] [PubMed] [Google Scholar]
- 38.Kelley GA, Kelley KS. Aerobic exercise and HDL2-C: A meta-analysis of randomized controlled trials. Atherosclerosis. 2006;184(1):207–215. doi: 10.1016/j.atherosclerosis.2005.04.005. Available at: http://www.sciencedirect.com/science/article/pii/S0021915005002698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harting GH, Moore CE, Mitchell R, Kappus CM. Relationship of menopausal status and exercise level to HDL cholesterol in women. Experimental Aging Research. 1984;10(1):13–18. doi: 10.1080/03610738408258535. Available at: http://www.tandfonline.com/doi/abs/10.1080/03610738408258535. [DOI] [PubMed] [Google Scholar]
- 40.Wedisinghe LFAU, Perera M, Perera MLA, eng PT. Journal Article PT - Review DEP - 20090616 PL - Ireland TA - Maturitas JT - Maturitas JID - 7807333 SB - IM MH - Cardiovascular Diseases/*drug therapy/etiology MH - Diabetes Mellitus T 2/complications/*drug therapy MH- *Estrogen RT effects MH-FMH-HMH-MMH-NMH-*Postmenopause MH-PG as TMH-RFRF-49 E- 2009/06/19. Diabetes and the menopause. :200–3. LID - 10.1016/j.maturitas.2009.04.005 [doi] (1873-4111 (Electronic)) [Google Scholar]
- 41.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third national health and nutrition examination survey. American Journal of Kidney Diseases. 2003;41(1):1–12. doi: 10.1053/ajkd.2003.50007. Available at: http://www.sciencedirect.com/science/article/pii/S027263860350004X. [DOI] [PubMed] [Google Scholar]
- 42.Tarver-Carr ME, Powe NR, Eberhardt MS, et al. Excess Risk of Chronic Kidney Disease among African-American versus White Subjects in the United States: A Population-Based Study of Potential Explanatory Factors. Journal of the American Society of Nephrology. 2002;13(9):2363–2370. doi: 10.1097/01.asn.0000026493.18542.6a. Available at: http://jasn.asnjournals.org/content/13/9/2363.abstract. [DOI] [PubMed] [Google Scholar]
- 43.Pechter Ü , Ots M, Mesikepp S, et al. Beneficial effects of water-based exercise in patients with chronic kidney disease. International Journal of Rehabilitation Research. 2003;26(2) doi: 10.1097/00004356-200306000-00013. Available at: http://journals.lww.com/intjrehabilres/Fulltext/2003/06000/Beneficial_effects_of_water_based_exercise_in.13.aspx. [DOI] [PubMed] [Google Scholar]
- 44.Bello AK, Nwankwo E, El Nahas a M. Prevention of chronic kidney disease: a global challenge. Kidney international. Supplement. 2005;68(98):S11–7. doi: 10.1111/j.1523-1755.2005.09802.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16108964. [DOI] [PubMed] [Google Scholar]