Some health plans have implemented coverage restrictions to stem the increased use of lumbar fusion operations in patients with back pain associated with degenerative changes.[1–3] States have adopted a variety of coverage and reimbursement strategies for workers’ compensation (WC) patients, whose outcomes are generally worse compared to non-workers compensation patients.[4, 5] However, there is little information about whether these policies modify the use, costs, or surgical safety of lumbar fusion.
Guidelines suggest that lumbar fusion may be an option for patients with severe back pain who have not improved with conservative treatment.[6, 7] Restricting motion and providing structural support with instrumented fusion may be effective for some diagnoses, including degenerative spondylolisthesis, fractures, and scoliosis.[8, 9] In randomized trials, although lumbar fusion is more effective than routine non-operative care, fusion surgery is only equivalent to structured rehabilitation, but less safe and more costly.[10–12] For patients with disc herniation or spinal stenosis, decompression alone is effective.[13, 14] The use of more complex lumbar procedures is associated with higher complication rates without evidence of improved functional outcomes. [15–17]
One insurance policy strategy has been to limit complex lumbar procedures, including those involving adding fusion to a decompression procedure for unilateral herniated disc with radiculopathy, multiple vertebral levels, certain implanted devices, and circumferential surgical approaches. This strategy was adopted by Washington State’s Department of Labor and Industries in 1996 and revised in 2006 (Table 1), based on its analyses that lumbar fusion innovations did not improve worker disability or quality of life, but increased reoperations.[3, 5, 18] Washington uses a prospective utilization review of lumbar fusion requests, requires x-ray imaging confirmation of spinal instability, and limits initial fusions to a single disc level.[19]
Table 1.
Key components of Workers’ Compensation Programs for lumbar fusion in Washington and California.
| Policy Component | Washington State | California |
|---|---|---|
| Review Process | Prospective review | Prospective review |
| Claims processing | Through state Labor & Industries fund, unless employer is certified as self-insured. | Through employer-purchased private policy, unless employer is certified as self-insured. |
| Procedure type | Limited to single level | Not limited |
| Repeat spine surgery approval | Subject to utilization review & approval unless emergent | Not limited |
| Second opinion | No requirement | Binding |
| Payment | Based on DRG | Based on DRG + additional reimbursement for surgical implants |
DRG, Diagnosis Related Group.
In contrast, California’s workers compensation system uses a legislated binding second opinion.[20] This policy requires an employer to authorize the procedure if the patient receives a second surgical opinion that concurs with the initial recommendation.[21] California allows additional payment for surgical instrumentation to stabilize adjacent vertebrae (screws, rods, plates, cages) and bone growth enhancers (bone morphogenetic protein, BMP).[22]
Hospital discharge registries allow for population-based comparisons of utilization, safety indicators, and costs between states. This information would help guide policy debate in the emerging area of cost and quality control related to spinal surgery.[23, 24] Since complex fusion surgery for back pain alone has little justification on the basis of patient-reported randomized trial data, differences in safety profiles may influence patients’ opinions on acceptable risk for uncertain benefit. Therefore, we compared Washington and California’s WC population data for rates of lumbar fusion surgery, complexity of surgery (use of instrumentation, fusion adjuncts, surgical approach), costs, readmissions, revision surgery, and other complications.
Methods
Data source
We examined the State Inpatient Database (SID) for California and Washington. The Agency for Healthcare Research and Quality (AHRQ) maintains SID, which is a component of the Healthcare Cost and Utilization Project (HCUP).[25] Data from HCUP has previously been used to study spinal procedures.[1, 26–29] SID is an all-payer inpatient discharge registry that provides International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) diagnoses and procedure codes, patient demographics, and hospital charges for approximately 90% of hospitals in participating states. AHRQ translates discharge information into uniform definitions to facilitate multi-state comparisons. Several states, including Washington and California, include encrypted patient identifiers that allow us to identify readmissions of individual patients even if care is provided by multiple hospitals.
Sex- and age-stratified (by 5-year age increments) population data within each state was obtained from the U.S. Census Bureau, along with estimates of the proportion of employed populations within each stratum.
Study population
We identified adults (age 20 – 65) undergoing thoracolumbar, lumbar, or lumbosacral fusion for degenerative spinal conditions in 2008 or 2009 whose primary payer was workers’ compensation. Patients were identified using relevant diagnosis and procedure codes from the October 2010 ICD-9-CM update.[30] A detailed coding algorithm for classifying spine-related medical encounters into clinically meaningful diagnosis groups, procedure categories, and surgical safety measures is available from the lead author.
Each hospitalization in SID contained up to 25 diagnosis codes and 21 procedure codes. We searched all codes to classify respondents into a hierarchy of indications for fusion based on existing literature. This hierarchy classifies fusions from the least to the most controversial indications as: scoliosis, spondylolisthesis, stenosis, disc herniation (with and without myelopathy), and disc degeneration (e.g. spondylosis).
We excluded patients with non-degenerative spinal pathology such as vertebral fractures, spinal cord injury, intraspinal abcess, or inflammatory spondylopathy. We also excluded patients for accidents, neoplasm, immune deficiency, osteomyelitis, and cervical diagnoses or procedures (Table 2).
Table 2.
Reasons for exclusion.
| Exclusion factors (not mutually exclusive) | Number with exclusion |
|---|---|
| Cervical diagnosis or procedures | 4,268 |
| Less than 3 months of surveillance | 613 |
| Trauma | 354 |
| Age > 65 | 332 |
| Artificial disc replacement | 217 |
| Open treatment of fracture | 174 |
| Not an initial observed lumbar fusion admission | 174 |
| Congenital or other anomaly | 154 |
| Fracture or dislocation | 85 |
| Neurological impairment | 57 |
| Drug abuse | 40 |
| Cancer | 34 |
| Osteomyelitis | 17 |
| Dynamic stabilizing device | 16 |
| Spinal spacer | 12 |
| HIV or immune deficiency | 7 |
| Intraspinal abscess | 4 |
| Inflammatory spondylopathy | 3 |
| Spinal cord injury | 1 |
| Pregnancy | 0 |
| ANY OF ABOVE EXCLUSIONS | 6,756 |
Lumbar fusions combined with discectomy or laminectomy were included, as were patients with codes implying previous spine surgery (e.g., “refusion”). However, because previous surgery increases the probability of yet further reoperations,[31] we included this as an adjustment variable.
Another approach to dealing with previous surgery is to exclude patients for whom we identify a previous spine operation within the database. Because the unique patient identifier for Washington changed in 2007, we were unable to “look back” in the database for previous spinal operations. In California, we were able to “look back” over a three year period. Therefore, we conducted a two sensitivity analyses: 1) excluding patients with procedure codes suggesting revision surgery during the index hospitalization in both states; and 2) additionally excluding patients with previous operations in California, but not Washington.
Patients undergoing artificial disc replacement, corpectomy, osteotomy, kyphectomy, and insertion of spinal spacers or dynamic stabilizing devices were excluded, even if performed in conjunction with a fusion.
Measuring safety
Repeat lumbar surgery, readmissions (all cause), wound problems, device and life-threatening complications within 3 months were identified for each patient. These outcomes were not mutually exclusive. With the exception of device complications, these well-accepted indicators of quality are part of the National Surgical Quality Improvement Program (NSQIP)[32] and the Healthcare Effectiveness Data and Information Set (HEDIS).[33] We used 3 month surveillance because readmissions and complications during this short interval are likely to be consequences of the index procedure, are associated with poor patient-reported outcomes, and are commonly-used as a quality indicator by the Medicare Payment Advisory Commission.[34, 35]
Device complications were defined as readmissions with diagnosis or procedure codes indicating loosening, breakage or malfunction of an internal orthopaedic device. Device complication codes used during the index operation were not counted because we could not determine whether they reflected problems at the index operation or a previous operation. Reoperations were identified as the first instance of any subsequent inpatient lumbar operation and not necessarily a repeat of the same procedure. We required device complications and reoperations to have a lumbar spine-specific ICD-9-CM diagnosis or procedure code.
Previous algorithms, which are similar to AHRQ quality indicators,[36] were used to identify life-threatening complications and wound problems during the index admission and during a 3-month post-operative period.[17] Life-threatening complications included major medical events such as respiratory failure, myocardial infarction, cardiopulmonary resuscitation, endotracheal intubation, pneumonia, stroke, and mechanical ventilation. Myocardial infarctions and strokes that were coded as being “present-on-admission” were not counted as a complication. Wound problems included hemorrhage, debridement, wound disruption, seroma, and hematoma. Complications requiring only ambulatory care were not counted.
Surgical characteristics
Operations were characterized by the use of surgical approach (anterior, posterior, or circumferential approach), fusions combined with decompression (discectomy or laminectomy), fusions of three or more disc levels (4 or more vertebrae), use of instrumentation, and bone morphogenetic protein.
Covariates
Because patient characteristics could explain differences in outcomes between states, we also adjusted for age, sex, comorbidity, previous surgery, and diagnosis. An “enhanced” version of the Charlson index was used to adjust for comorbidity.[37] This index was entered into our analysis as a categorical variable grouped as “none”, “one”, or “two or more”. The latter category was designed because only a small number of patients had two or more listed comorbidity conditions. Since this index includes myocardial infarctions and strokes, and these are among the life-threatening complications that we sought to identify, we excluded these items from the comorbidity score.
Analysis
The annual rates of lumbar fusion operations for degenerative diagnoses paid by WC programs were directly standardized by sex and age using state-specific population denominators of employed adults (ages 20–65) from the US Census Bureau. Direct standardization involves reporting the sum of the age- and sex-specific crude rates that we observed multiplied by their corresponding proportions in the denominator. The denominator for employed populations was calculated by multiplying the state-specific civilian population within each age and sex-stratum by their corresponding proportion for employed individuals.
Differences between the two state’s cohorts in patient characteristics, comorbidity, diagnoses, and surgical features were described along with chi-square or t-test comparisons (Table 3).
Table 3.
Patient characteristics, diagnosis and operative features of workers compensation patient undergoing inpatient lumbar fusion.
| California (n = 4,082) | Washington (n = 546) | Overall (n = 4628) | P-value for difference between states | ||
|---|---|---|---|---|---|
| Rate per 100,000 (95%CI) employed adults aged 20–65 [1] | 19.0 (18.6 – 19.5) | 12.9 (12.0 – 13.8) | 18.0 (17.6 – 18.4) | <0.001 | |
| Age, mean (sd) | 47.1 (9.5) | 46.6 (9.4) | 47.0 (9.5) | 0.253 | |
| Age group | 20–24 | 29 (1%) | 6 (1%) | 35 (1%) | 0.691 |
| 25–29 | 149 (4%) | 18 (3%) | 167 (4%) | ||
| 30–34 | 288 (7%) | 44 (8%) | 332 (7%) | ||
| 35–39 | 443 (11%) | 64 (12%) | 507 (11%) | ||
| 40–44 | 619 (15%) | 70 (13%) | 689 (15%) | ||
| 45–49 | 789 (19%) | 111 (20%) | 900 (19%) | ||
| 50–54 | 755 (18%) | 108 (20%) | 863 (19%) | ||
| 55–59 | 618 (15%) | 80 (15%) | 698 (15%) | ||
| 60–64 | 392 (10%) | 45 (8%) | 437 (9%) | ||
| Sex, % | Male | 2614 (65%) | 390 (71%) | 3004 (66%) | 0.003 |
| Female | 1405 (35%) | 156 (29%) | 1561 (34%) | ||
| Charlson comorbidity [2], % | None | 3157 (77%) | 434 (79%) | 3591 (78%) | 0.384 |
| 1 | 795 (19%) | 93 (17%) | 888 (19%) | ||
| 2+ | 130 (3%) | 19 (3%) | 149 (3%) | ||
| Length of stay, days (SD) | 4.39 (2.8) | 3.06 (1.8) | 4.2 (2.8) | <0.001 | |
| Diagnosis | Disc Degeneration | 1161 (28%) | 115 (21%) | 1276 (28%) | <0.001 |
| Herniated | 1390 (34%) | 102 (19%) | 1492 (32%) | ||
| Herniated + myelopathy | 111 (3%) | 10 (2%) | 121 (3%) | ||
| Stenosis | 257 (6%) | 79 (15%) | 336 (7%) | ||
| Spondylolisthesis | 999 (25%) | 220 (41%) | 1219 (26%) | ||
| Scoliosis | 159 (4%) | 17 (3%) | 176 (4%) | ||
| Codes that imply previous surgery | No | 3203 (79%) | 407 (75%) | 3610 (78%) | 0.056 |
| Yes | 874 (21%) | 136 (25%) | 1010 (22%) | ||
| Procedure | Fusion only | 1183 (29%) | 154 (28%) | 1337(29%) | 0.707 |
| Fusion + decompression | 2899 (71%) | 392 (72%) | 3291(71%) | ||
| Instrumentation | No | 896 (22%) | 112 (21%) | 1008 (22%) | 0.445 |
| Yes | 3186 (78%) | 434 (79%) | 3620 (78%) | ||
| 3+ disc levels fused | No | 3654 (90%) | 521 (95%) | 4175 (90%) | <0.001 |
| Yes | 428 (10%) | 25 (5%) | 453 (10%) | ||
| BMP [3] | No | 2,037 (50%) | 378 (69%) | 2415 (52%) | <0.001 |
| Yes | 2,045 (50%) | 168 (31%) | 2213 (48%) | ||
| Approach | Posterior | 2428 (60%) | 475 (87%) | 2903 (63%) | <0.001 |
| Anterior | 586 (14%) | 41 (8%) | 627 (14%) | ||
| Circum. | 1054 (26%) | 28 (5%) | 1082 (23%) | ||
Age- and sex-adjusted rate of fusion for degenerative disease reimbursed through WC systems per 100,000 employed adults aged 20–65 based on U.S. Census denominator.
Charlson index modified to remove acute myocardial infarction and stroke
BMP, Bone Morphogenetic Proteins
We then examined differences in the rates of reoperations, readmissions, and complications, including only the patients who had a minimum of 3 months of surveillance available to assess each outcome. We performed a log-binomial regression of each outcome, adjusting for patient age, sex, diagnosis, previous surgery and comorbidity. All variables except age were included as categorical variables. Age and age-squared (continuous polynomial) were only weakly important in some models, but retained in all models for precision and consistency. State-specific robust standard errors improved the precision of our estimates and our ability to test the difference between states.[38] We did not adjust for difference in operative features because their discretionary use is the target of the coverage and reimbursement policies that we examine.
Adjusted rates for each outcome were estimated from the regression models by setting all covariates to their mean distributions in the sample. Specifically, we used the results from the regression model to assess the risk of complication for an “average” patient. This was accomplished by setting the covariates for age, sex, previous surgery, and comorbidity to the mean sample distributions (including proportionate distributions for each level of the categorical covariates) as displayed in table 2. Each observation was then weighted using the beta-coefficient associated with the corresponding variables from our regression models. This produces a normative risk for each patient based on the experience of a sample with similar characteristics. To examine variation in outcomes across hospitals we added hospital-specific intercepts to the adjusted model.[39, 40]
California has a substantially higher proportion of non-white and Hispanic residents compared to Washington State. However, race and ethnicity was not included in our models because it was largely missing from Washington. To help understand the association of race and ethnicity on outcomes we separately examined models using only California.
Inpatient charges, excluding professional fees and non-covered services, are included with SID. HCUP hospital cost-to-charge ratios were used to estimate costs. A small number of cases (n=21) with missing charges were imputed by setting them to the mean values of the sample. To account for inflation, we referenced the medical component of the Consumer Price Index to adjust charges in 2007 to their 2008 equivalents.[41] We estimated average costs (charges) adjusting for age (age and age-squared), sex, comorbidity, previous surgery, and diagnosis using generalized linear regressions that accounted for skewed distributions (inverse Gaussian family with log link function).
Analyses were performed using StataMP, version 11 (College Station, TX), and a two-sided alpha level of 0.05. A waiver of human subjects review for publicly available data was obtained from the Committee for the Protection of Human Subjects at Dartmouth College.
Results
Study population
A total of 11,384 patients were identified as having an inpatient spinal fusion paid through WC programs in Washington (n=1,624; 14%) or California (n= 9,760; 86%). We excluded 6,756 patients (59%; Table 2), leaving 4,628 eligible patients with a diagnosis of lumbar degenerative disease. The age- and sex-adjusted rate of lumbar fusions for degenerative conditions paid by WC programs was 19.0 per 100,000 employed adults (aged 20–65) in California, compared to 12.9 in Washington State (p<0.001; table 3).
Of the 4,628 eligible patients who received an initial lumbar fusion, 546 (11.8%) were from Washington. A larger percentage of patients in California were female (35% versus 29%; p=0.004). Mean age (47.0 years; sd 9.5) and comorbidity (22% with any) did not differ between the two states (Table 3).
Workers undergoing fusion surgery in California were significantly more likely than those in Washington to have a diagnosis of disc degeneration (28% versus 21%; p<0.001) or disc herniation (37% versus 21%; p<0.001), and less likely to have stenosis (6% versus 15%; p<0.001) or spondylolisthesis (25% versus 41%; p<0.001). The proportion of patients with scoliosis was small (4%), and similar between the two states (p=0.38). A significantly higher proportion of patients in California received anterior (14% versus 8%; p<0.001) or circumferential approaches (26% versus 5%; p<0.001), had 3+ disc levels fused (10% versus 5%; p<0.001), and received bone morphogenetic protein (50% versus 31%; p<0.001). The two states had similar rates of instrumented fusion (78%; p=0.45) and simultaneous decompression procedures (71%; p=0.71).
Safety outcomes
Workers in California had significantly higher rates of reoperation (5.0% versus 2.2%, p=0.002) and readmission (14.4% versus 10.3%, p=0.007) within 3 months, compared to those in Washington (Table 4). Adjusting for age, sex, comorbidity, previous surgery, and diagnosis, the rate of reoperation in California was 4.8%, compared to 1.9% in Washington (RR 2.28; 95%CI 2.27 – 2.29; p<0.001); and the adjusted rate for any readmission in California was 14.0%, compared to 9.1% in Washington (RR 1.45; 95%CI 1.44 – 1.47; p<0.001).
Table 4.
Multivariate analysis of complications, repeat surgery, and re-hospitalization within 3 months of an inpatient lumbar fusion, as well as hospital costs and charges.
| Unadjusted analysis [1] | Adjusted analysis [2] | Adjusted analysis excluding those with implied previous surgery codes [2] | Adjusted analysis excluding those with implied surgery and, for CA only, spine surgery observed in previous three years [2] | |||||
|---|---|---|---|---|---|---|---|---|
| Rate | RR (95% CI) | Rate | RR (95%CI) | Rate | RR (95%CI) | Rate | ||
| Repeat lumbar surgery | Washington | 12/546 (2.2%) | 1.00 (ref) | 1.9% | 1.00 (ref) | 1.4% | 1.00 (ref) | 1.2% |
| California | 210/4,082 (5.1%) | 2.28 (2.27 – 2.29) | 4.8% | 2.91 (2.91 – 2.92) | 4.7% | 1.84 (1.83 – 1.84) | 2.4% | |
| p-value | 0.001 | <0.001 | <0.001 | <0.001 | ||||
| Readmission (all cause) | Washington | 56/546 (10.3%) | 1.00 (ref) | 9.1% | 1.00 (ref) | 9.2% | 1.00 (ref) | 9.0% |
| California | 607/4,082 (14.9%) | 1.45 (1.44 – 1.47) | 14.0% | 1.30 (1.27 – 1.33) | 13.3% | 1.11 (1.08 – 1.13) | 11.0% | |
| p-value | 0.003 | <0.001 | <0.001 | <0.001 | ||||
| Device complication | Washington | 3/546 (0.6%) | 1.00 (ref) | 0.3% | 1.00 (ref) | <0.1 | 1.00 (ref) | <0.01% |
| California | 41/4,082 (1.0%) | 2.49 (2.38 – 2.61) | 0.7% | 2.09 (1.80 – 2.43) | 0.2 | 1.47 (1.19 – 1.82) | 0.2% | |
| p-value | 0.478 | <0.001 | <0.001 | <0.001 | ||||
| Wound problems | Washington | 11/546 (2.0%) | 1.00 (ref) | 1.5% | 1.00 (ref) | 1.1% | 1.00 (ref) | 1.1% |
| California | 207/4,082 (5.1%) | 2.64 (2.62 – 2.65) | 4.2% | 2.67 (2.62 – 2.71) | 3.3% | 2.49 (2.42 – 2.57) | 3.2% | |
| p-value | 0.001 | <0.001 | <0.001 | <0.001 | ||||
| Life-threatening problems | Washington | 18/546 (3.3%) | 1.00 (ref) | 2.4% | 1.00 (ref) | 2.4% | 1.00 (ref) | 2.5% |
| California | 154/4,082 (3.7%) | 1.31 (1.31 – 1.31) | 3.3% | 1.11 (1.10 – 1.12) | 2.7% | 1.09 (1.07 – 1.11) | 2.8% | |
| p-value | 0.717 | <0.001 | <0.001 | <0.001 | ||||
| Unadjusted [3] | Adjusted [4] | Adjusted [4] | Adjusted [4] | |||||
| USD | USD (95% CI) | USD (95% CI) | USD (95% CI) | |||||
| Charges, mean | Washington | 104,170 | 103,221 (102,512 – 103,931) | 98,868 (98,417 – 99,318) | 99,293 (98,724 – 99,861) | |||
| California | 161,015 | 160,988 (160,529 – 161,447) | 158,813 (158,678 – 158,948) | 158,565 (158,433 – 158,697) | ||||
| p-value | <0.001 | < 0.001 | <0.001 | <0.001 | ||||
| Costs, mean | Washington | 40,693 | 40,327 (40,114 – 40,542) | 38,660 (38,518 – 38,801) | 38,858 (38,681 – 39,036) | |||
| California | 49,565 | 49,430 (49,347 – 49,512) | 48,525 (48,507 – 48,542) | 48,425 (48,407 – 48,444) | ||||
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | ||||
P-value between states based on 2-sided Fisher exact chi-square
P-values and estimates based on log-binomial regression with state specific robust standard errors, controlling for age, age-squared, sex, comorbidity, previous surgery (except where excluded as specified), and diagnosis.
U.S. dollars. P-values and estimates based on t-test.
U.S. dollars. P-values and estimates based on generalize linear regression with robust standard errors, controlling for age, age-squared, sex, comorbidity, previous surgery (except where excluded as specified) and diagnosis. Wald distributional family & log link function.
After adjusting for age, sex, comorbidity, previous surgery, and diagnosis, California also had higher rates of device complications (0.7% versus 0.3%; RR 2.49; 95%CI 2.39 – 2.61; p<0.001), wound problems (4.2% versus 1.5%; RR 2.64; 95%CI 2.62 – 2.65; p<0.001) and life threatening complications (3.3% versus 2.4%; RR 1.31; 95%CI 1.31 – 1.31; p<0.001).
Hospital outcomes
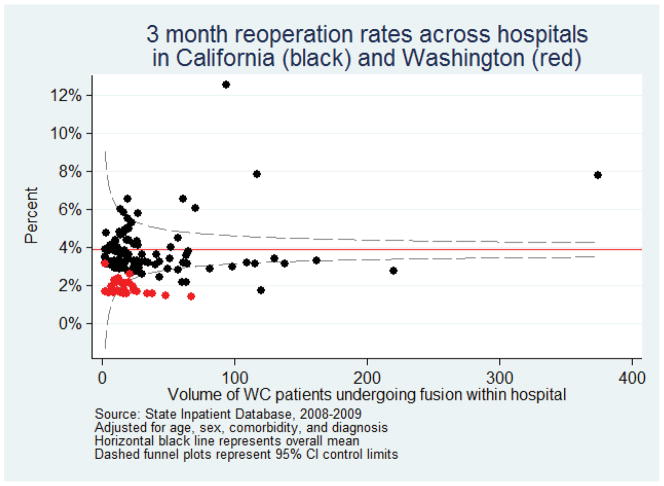
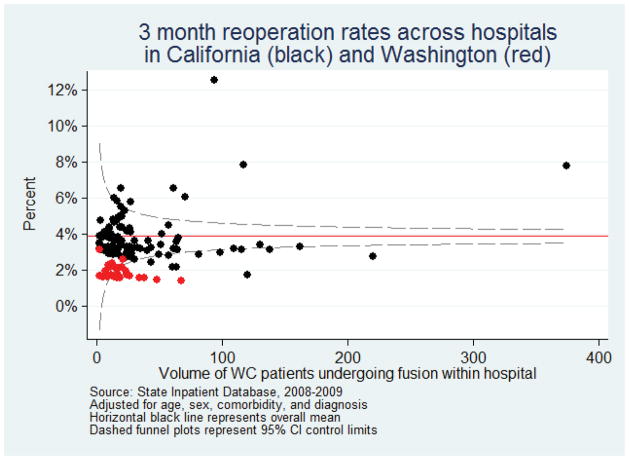
To examine whether these differences were due to hospitals with outlying surgical rates or concentrated in hospitals with low or high surgical volume, we examined variation in adjusted reoperation rates aggregated across hospitals (Figure 1). Low-volume hospitals had a greater variance around the mean, but our findings were not driven by the few hospitals with unusually high rates.
Figure 1.
Rates of repeat lumbar surgery within 3 month among hospitals performing lumbar fusion operations among worker compensation patients, State Inpatient Database 2008–2009 combined. Each point represents a single hospital from California (black) or Washington (red). The horizontal solid line represents the overall mean for all hospitals.
Costs
Mean hospitalization costs were higher in California than in Washington ($49,430 versus $40,327; p< 0.001), after adjusting for age, sex, comorbidity, and diagnosis.
Sensitivity analysis
Codes implying previous spine operations were associated with higher rates of complications and readmission, and these effects were greater in California than in Washington. However, the low frequency of these outcomes in Washington (the referent) prohibited us from examining an interaction term. The risk ratio for readmissions in California did not substantially change after excluding patients with previous surgery codes, and complications were only slightly attenuated. When we further excluded patients from California (but not Washington) who had a spine operation in the previous three years (n=724), the risks for repeat surgery (RR 1.84; 95%CI 1.83 – 1.84; p<0.001) or readmission (RR 1.11 95%CI 1.08 – 1.13; p<0.001) in California were reduced, but still greater than in Washington. Wound and life threatening complication risks in California did not substantially change.
We found no association between race or ethnicity and outcomes within California, but had poor power to detect difference for some race and ethnicity categories.
Discussion
Rate of surgery, selection of surgical technique, and occurrence of major complications differed substantially between California and Washington WC patients undergoing lumbar fusion. These empirical differences may in part be due to differences in coverage policies. After adjusting for demographic and clinical characteristics, WC patients with degenerative conditions in Washington had a significantly lower rate of fusion operations, reoperations, readmissions, wound problems, device complications, and life threatening complications, when compared to WC patients in California. Washington had lower use of complex procedures including, combined surgical approaches procedures, multi-level fusions, and bone morphogenetic proteins. Even though a smaller proportion of California’s WC patients had the strongest evidence-based indications for fusion, such as spondylolisthesis, they were more likely to undergo complex procedures compared to WC patients in Washington. Similar patient age and comorbidity suggest that California’s WC patients were not “sicker” than those in Washington, and previous surgery does not account for the worse outcomes in California. Inpatient costs (22% higher) and length of stay (42% higher) were greater in California than in Washington.
Coverage and reimbursement policies may account for the differences in utilization, costs and safety differences that we observed between Washington and California. Limited empirical data are available to confirm the common, largely anecdotal, belief that second surgical opinion consults are often performed by surgical colleagues who are unlikely to disagree with an initial surgical recommendation. The review by Lindsey & Newhouse[21] summarize the deficiencies in the literature and call into question the costs and value of second surgical opinion programs.
Operative features were associated with differences in utilization and outcomes between Washington and California. For example, the decision to use BMP is largely discretionary and controversial. [42] The high rate of use in California relative to Washington is not supported by evidence of improved outcomes or lower rates of reoperations.[43] As in previous claims-based studies,[39] we found that BMP use was associated with higher complication rates.
We only examined adverse outcomes reliably captured in administrative data. Our study consisted of a large population, which is advantageous for comparisons of rare safety outcomes. Discharge databases are useful for understanding how health systems influence clinical practice outside the controlled conditions of a clinical trial. Although research based on ICD-9-CM codes lack some clinical detail, administrative data capture care occurring at different institutions, improve generalizability, and reduce recruitment, measurement, and investigator biases problematic in clinical trials.[42] Although SID does not include pain intensity, imaging findings, or specific vertebral levels, we were able to describe important operative characteristics, including surgical approach and use of instrumentation. Administratively derived patient safety indicators are used by NSQIP and based on HEDIS measures; they appear to be reliable for ascertaining major complications.[44] Measuring readmission or reoperations in our analysis did not depend on ICD-9-CM codes for complications. Our estimates of readmissions and complications may be conservative because we excluded non-degenerative spinal comorbidity and previous surgery. In addition, by only counting events requiring an inpatient admission, our estimates of complication rates may underestimate the actual rate (e.g. some infections may be treated in outpatient settings).
Analyses involving observational data, such as HCUP’s claims-based discharge registries, have some inherent limitations. First, unobserved differences between WC populations in California and Washington may account for the differences in the choice of procedure and safety outcomes that we observed. For such factors to influence our findings, they would have to be substantially different between California and Washington, but not be directly related to the policies that we contrast. By excluding patients with trauma, cancer, infections, and non-degenerative spinal pathology, we have reduced some potential confounding.
Second, observational data is often prone to selection bias introduced by the non-random process of placing patients into comparison groups. Obviously, we could not randomly allocate patients to different jurisdictions and surgical management strategies. Therefore, differences in the patient population who are served by the policies might be thought of as drawn from the consequences of these policies.
We adjusted our models for observed differences in patient age, sex, comorbidity, previous surgery and diagnosis, although it is not clear why California had a higher proportion of female WC patients. One possibility is that work injuries are more common in occupations with a preponderance of male workers, and that these are more common in Washington. Compared to California, a higher proportion of Washington residents are employed in agriculture, forestry, fishing and hunting, and mining (2.5% compared to 1.9%), as well as construction (7.0% versus 6.2%).[45] This also suggests that worse outcomes in California cannot be attributed to a higher proportion of manual labor.
Finally, because we rely on an observational research design, it is technically incorrect to infer that differences in coverage policies causally lead to difference in utilization, costs and outcomes. Given their limitations, the use of observational data must be viewed with caution. However, because population-based observational data is the only practical means for evaluating differences between state-wide coverage and reimbursement policies, our results might reasonably be used as part of the decision-making process for guiding treatments.
Approval and reimbursement policies among WC programs influence utilization, cost and safety of lumbar fusion surgery. Broader coverage policy was associated with more aggressive practice, higher rates of reoperation, readmission and other complications. Some insurers have recently instituted coverage policies dramatically limiting lumbar fusion coverage for degenerative disc disease and chronic low back pain.[2] Future work should examine whether these restrictive policies are associated with differences in return-to-work and patient-reported outcomes.
Acknowledgments
Funding for this project was provided by grants P60AR062799 and R01AR054912-01A (National Institute of Arthritis, Musculoskeletal, and Skin Diseases), HS018405 (Agency for Healthcare Research and Quality), and 1RC1AG036268 (National Institute of Aging). The findings and conclusions expressed are solely those of the author(s) and do not necessarily represent the views of any agency of the Federal Government. The funding agencies had no role in the design, collection, analysis, and interpretation of the data, or with the manuscript preparation. The corresponding author confirms that he had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis as well as the decision to submit or publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
B.I. Martin, Email: Brook.I.Martin@Dartmouth.edu.
G.M. Franklin, Email: Meddir@u.washington.edu.
R.A. Deyo, Email: deyor@ohsu.edu.
T Wickizer, Email: twickizer@cph.osu.edu.
J.D. Lurie, Email: Jonathan.D.Lurie@Dartmouth.edu.
S.K. Mirza, Email: Sohail.K.Mirza@Dartmouth.edu.
References
- 1.Deyo RA, Gray DT, Kreuter W, et al. United States trends in lumbar fusion surgery for degenerative conditions. Spine. 2005;30(12):1441–7. doi: 10.1097/01.brs.0000166503.37969.8a. [DOI] [PubMed] [Google Scholar]
- 2.Corporate Medical Policy Lumbar Spine Fusion Surgery. [Accessed February 1, 2012];Blue Cross Blue Shield of North Carolina. 2011 at http://www.bcbsnc.com/assets/services/public/pdfs/medicalpolicy/lumber_spine_fusion_surgery.pdf.
- 3.Elam K, Taylor V, Ciol MA, et al. Impact of a worker’s compensation practice guideline on lumbar spine fusion in Washington State. Med Care. 1997;35(5):417–24. doi: 10.1097/00005650-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Harris I, Mulford J, Solomon M, et al. Association between compensation status and outcome after surgery: a meta-analysis. JAMA. 2005;293(13):1644–52. doi: 10.1001/jama.293.13.1644. [DOI] [PubMed] [Google Scholar]
- 5.Maghout-Juratli S, Franklin GM, Mirza SK, et al. Lumbar fusion outcomes in Washington State workers’ compensation. Spine (Phila Pa 1976) 2006;31(23):2715–23. doi: 10.1097/01.brs.0000244589.13674.11. [DOI] [PubMed] [Google Scholar]
- 6.Watters WC, 3rd, et al. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spondylolisthesis. Spine J. 2009;9(7):609–14. doi: 10.1016/j.spinee.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Chou R, et al. Surgery for low back pain: a review of the evidence for an American Pain Society Clinical Practice Guideline. Spine (Phila Pa 1976) 2009;34(10):1094–109. doi: 10.1097/BRS.0b013e3181a105fc. [DOI] [PubMed] [Google Scholar]
- 8.Fischgrund JS, Mackay M, Herkowitz HN, et al. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine. 1997;22(24):2807–12. doi: 10.1097/00007632-199712150-00003. [DOI] [PubMed] [Google Scholar]
- 9.Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am. 1991;73(6):802–8. [PubMed] [Google Scholar]
- 10.Mirza SK, Deyo RA. Systematic review of randomized trials comparing lumbar fusion surgery to nonoperative care for treatment of chronic back pain. Spine. 2007;32(7):816–23. doi: 10.1097/01.brs.0000259225.37454.38. [DOI] [PubMed] [Google Scholar]
- 11.Deyo RA, Mirza SK. The case for restraint in spinal surgery: does quality management have a role to play? Eur Spine J. 2009;18 (Suppl 3):331–7. doi: 10.1007/s00586-009-0908-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Findings and Coverage of Lumbar Fusion & Discography. Washington State Health Care Authority. [Accessed February, 1, 2012];Health Technology Assessment Program. 2007 at http://www.hta.hca.wa.gov/lumbar.html.
- 13.Weinstein JN, Tosteson TD, Lurie JD. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial. JAMA. 2006;296(20):2441–50. doi: 10.1001/jama.296.20.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinstein JN, Tosteson TD, Lurie JD. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358(8):794–810. doi: 10.1056/NEJMoa0707136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vamvanij V, Fredrickson BE, Thorpe JM, et al. Surgical treatment of internal disc disruption: an outcome study of four fusion techniques. J Spinal Disord. 1998;11(5):375–82. [PubMed] [Google Scholar]
- 16.Martin BI, Mirza SK, Comstock BA, et al. Reoperation rates following lumbar spine surgery and the influence of spinal fusion procedures. Spine. 2007;32(3):382–7. doi: 10.1097/01.brs.0000254104.55716.46. [DOI] [PubMed] [Google Scholar]
- 17.Deyo RA, Mirza SK, Martin BI, et al. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303(13):1259–65. doi: 10.1001/jama.2010.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franklin GM, Haug J, Heyer NJ, et al. Outcome of lumbar fusion in Washington State workers’ compensation. Spine. 1994;19(17):1897–904. doi: 10.1097/00007632-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Surgical Guidelines for Lumbar Fusion (Arthrodesis) Office of the Medical Director, Washington State Department of Labor & Industries; 2009. [Accessed February 1, 2002]. at http://www.lni.wa.gov/ClaimsIns/Providers/TreatingPatients/ByCondition/lumbarfusionarthrodesis.asp. [Google Scholar]
- 20.Title 8 California Code of Regulations, Section 9788.01. California Department of Industrial Relations, Division of Workers Compensation; 2004. [Accessed February 1, 2012]. Spinal Surgery Second Opinion Procedure. at https://www.dir.ca.gov/dwc/ [Google Scholar]
- 21.Lindsey PA, Newhouse JP. The cost and value of second surgical opinion programs: a critical review of the literature. J Health Polit Policy Law. 1990;15(3):543–70. doi: 10.1215/03616878-15-3-543. [DOI] [PubMed] [Google Scholar]
- 22.Wynn BO, Bergamo G. Working paper: Payment for Hardware Used in Complex Spinal Procedures under California’s Official Medical Fee Schedule for Injured Workers. RAND Institute for civil justice and health; 2005. [Accessed February 1, 2012]. at https://www.dir.ca.gov/dwc/ [Google Scholar]
- 23.Errico TJ, Gatchel RJ, Schofferman J, et al. A fair and balanced view of spine fusion surgery. Spine J. 2004;4(5 Suppl):S129–38. doi: 10.1016/j.spinee.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 24.Deyo RA, Nachemson A, Mirza SK. Spinal-fusion surgery - the case for restraint. N Engl J Med. 2004;350(7):722–6. doi: 10.1056/NEJMsb031771. [DOI] [PubMed] [Google Scholar]
- 25.State Inpatient Databases (SID) [Accessed February 1, 2012];Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality. 2010 at www.hcup-us.ahrq.gov/sidoverview.jsp.
- 26.Cahill KS, Chi JH, Day A, et al. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302(1):58–66. doi: 10.1001/jama.2009.956. [DOI] [PubMed] [Google Scholar]
- 27.Gray DT, Deyo RA, Kreuter W, et al. Population-based trends in volumes and rates of ambulatory lumbar spine surgery. Spine. 2006;31(17):1957–64. doi: 10.1097/01.brs.0000229148.63418.c1. [DOI] [PubMed] [Google Scholar]
- 28.Cowan JA, Dimick JB, Wainess R, et al. Changes in the utilization of spinal fusion in the United States. Neurosurgery. 2006;59(1):15–20. doi: 10.1227/01.NEU.0000219836.54861.CD. [DOI] [PubMed] [Google Scholar]
- 29.Williams BJ, Smith JS, Fu KM, et al. Does Bone morphogenetic protein increase the incidence of perioperative complications in spinal fusion? A comparison of 55,862 cases of spinal fusion with and without bone morphogenetic protein. Spine. 2011;36(20):1685–91. doi: 10.1097/BRS.0b013e318216d825. [DOI] [PubMed] [Google Scholar]
- 30.International Classification of Diseases, Ninth Revision, Clinical Modification. Centers for Disease Control and Prevention; 2010. [Accessed February 1, 2012]. at http://www.cdc.gov/nchs/icd/icd9cm.htm. [Google Scholar]
- 31.Deyo RA, Martin BI, Kretuer W, et al. Revision surgery following operations for lumbar stenosis. J Bone Joint Surg Am. 2011;93(21):1979–86. doi: 10.2106/JBJS.J.01292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiloach M, Frencher SK, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6–16. doi: 10.1016/j.jamcollsurg.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 33.HEDIS 2012 Volume2: Technical Specifications. National Committee for Quality Assurance; [Accessed February 1, 2012]. at www.ncqa.org. [Google Scholar]
- 34.Report to Congress: Promoting Greater Efficiency in Medicine. Medicare Payment Advisory Commission; 2007. [Accessed February 1, 2012]. Payment Policies for inpatient readmissions. at http://www.medpac.gov/documents/jun07_entirereport.pdf. [Google Scholar]
- 35.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418–28. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 36.Remus D, Fraser I. AHRQ Pub 04–0086-EF. Department of Health and Human Services, Agency for Healthcare Research and Quality; 2004. [Accessed February 1, 2012]. Guidance for Using the AHRQ Quality Indicators for Hospital-level Public Reporting or Payment. at http://www.qualityindicators.ahrq.gov/ [Google Scholar]
- 37.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 38.Miglioretti DL, Heagerty PJ. Marginal modeling of nonnested multilevel data using standard software. Am J Epidemiol. 2007;165(4):453–63. doi: 10.1093/aje/kwk020. [DOI] [PubMed] [Google Scholar]
- 39.Martin BI, Mirza SK, Franklin GM, et al. Hospital and Surgeon Variation in Complications and Repeat Surgery Following Incident Lumbar Fusion for Common Degenerative Diagnoses. Health Serv Res. 2013;48(1):1–25. doi: 10.1111/j.1475-6773.2012.01434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones HE, Spiegelhalter DJ. The Identification of “Unusual” Health-Care Providers From a Hierarchical Model. The American Statistician. 2011;65(5):154–162. [Google Scholar]
- 41.Consumer Price Index Calculator. U.S. Bureau of Labor Statistics; [Accessed February 1, 2012]. at http://www.bls.gov/cpi/ [Google Scholar]
- 42.Mirza SK. Folly of FDA-approval studies for bone morphogenetic protein. Spine J. 2011;11(6):495–9. doi: 10.1016/j.spinee.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Deyo RA, et al. Use of bone morphogenetic proteins in spinal fusion surgery for older adults with lumbar stenosis: trends, complications, repeat surgery, and charges. Spine (Phila Pa 1976) 2012;37(3):222–30. doi: 10.1097/BRS.0b013e31821bfa3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawthers AG, McCarthy EP Davis RB, et al. Identification of in-hospital complication from claims data. Is it valid? Med Care. 2000;38(8):785–95. doi: 10.1097/00005650-200008000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Current Population Survey. U.S. Census Bureau; 2012. [Accessed February, 1, 2012]. at http://www.census.gov/ [Google Scholar]