Abstract
Aurora kinases play a key role in mitosis and are frequently overexpressed in a variety of tumor cells. Inhibition of aurora kinases results in mitotic arrest and death of cancer cells, and has been explored as an anticancer strategy. However, how aurora inhibition kills cancer cells is poorly understood. In this study, we found that inhibition of aurora kinases by siRNA or small-molecule inhibitors led to induction of PUMA, a BH3-only Bcl-2 family protein, in colorectal cancer cells irrespective of p53 status. Deficiency in PUMA increased polyploidy, improved cell survival, and abrogated mitochondria-mediated apoptosis induced by aurora kinase inhibitors. In response to aurora kinase inhibition, PUMA was directly activated by p65 through the canonical NF-κB pathway following AKT inhibition. Furthermore, PUMA was necessary for the chemosensitization and in vivo antitumor effects of aurora kinase inhibitors in colon cancer cells. These results suggest that PUMA induction mediates the apoptotic response to mitotic arrest imposed by aurora kinase inhibition, and may be a useful indicator for the anticancer activity of aurora kinase inhibitors.
Keywords: aurora kinase, PUMA, apoptosis, NF-κB, colon cancer
Introduction
Aurora kinases are a group of highly conserved serine/threonine kinases that play a key role in mitosis. They are frequently overexpressed in a variety of tumors and promote cell cycle progression (1). There are three aurora kinases in mammalian cells, including aurora A, B and C, among which aurora A and B are most abundantly expressed in cancer cells (2). Small-molecule inhibitors of aurora kinases have been developed and shown promises in recent preclinical studies (3). In response to aurora kinase inhibition, cancer cells undergo mitotic arrest and eventually, cell death (4). Accumulating evidence suggests that aurora kinase inhibitors kill cancer cells by inducing apoptosis via mitochondrial dysfunction (5). However, the mechanisms by which mitotic arrest imposed by aurora kinase inhibitors triggers apoptotic cell death are poorly understood (6).
PUMA (p53 upregulated modulator of apoptosis) is a BH3-only Bcl-2 family member which functions as a critical initiator of apoptosis in cancer cells (7). It is transcriptionally activated by p53 in response to DNA damage, and is indispensable for p53-dependent apoptosis induced by radiation and cytotoxic chemotherapeutic drugs (8). PUMA can also be induced in a p53-independent manner by a variety of non-genotoxic stimuli, such as kinase inhibitors, growth factor deprivation and inflammatory cytokines (9–14). p53-independent PUMA induction can be mediated by different transcription factors, including the p53 homologue p73, FoxO3a (Forkhead Box O3a), and NF-κB (nuclear factor κB) (9–14). Upon its induction, PUMA potently induces apoptosis in cancer cells by acting upon other Bcl-2 family members such as Bax, Bcl-2 and Bcl-XL, resulting in mitochondrial outer membrane permeabilization and activation of the caspase cascade (15–17).
PUMA was previously shown to be induced by antimitotic drugs such as microtubule-targeting agents (18), and upregulated in response to polyploidy (19, 20), suggesting it may play a role in initiating the apoptotic response to mitotic arrest. In this study, we found that PUMA is transcriptionally activated through the canonical NF-κB pathway following aurora kinase inhibition, which contributes to the in vitro and in vivo anticancer activities of aurora kinase inhibitors. Our results suggest that PUMA induction may be a useful indicator for the therapeutic effects of aurora kinase inhibitors.
Materials and Methods
Cell culture and drug treatment
The human colorectal cancer cell lines, including HCT116, DLD1, RKO, HT29, SW480, and SW48 were obtained from the American Type Culture Collection. Cell lines were last tested and authenticated for genotypes, drug response, morphology, and absence of mycoplasma in October, 2012. p53-knockout (KO) and PUMA-KO colon cancer cell lines and wide-type (WT) and PUMA-KO mouse embryonic fibroblasts (MEF) cells were previously described (13). All cell lines were maintained at 37°C in 5% CO2 and cultured in McCoy’s 5A modified media (Invitrogen) supplemented with 10% defined FBS (HyClone), 100 units/ml penicillin, and 100 µg/ml streptomycin (Invitrogen). For drug treatment, cells were plated in 12-well plates at 20% to 30% density 24 hours before treatment. The DMSO (Sigma) stocks of agents used, including ZM-447439 (Selleck Chemicals), VX-680, Gefinitib (LC Laboratories), 5-fluorouracil (5-FU; Sigma), BAY 11-7082 (Merck Chemicals), GX15-070 (Cayman Chemical), were diluted to appropriate concentrations with the cell culture medium. Human TNFα (R&D system) was diluted with phosphate-buffered saline. For NF-κB inhibition, cells were pre-treated with BAY 11–7082 for 1 hour before ZM-447439 treatment. Transfection of expression constructs of WT and constitutive AKT was performed as described (12).
Real-time reverse transcription-PCR
Total RNA was isolated from cells using the Mini RNA Isolation II kit (Zymo Research) according to the manufacturer's protocol. Total RNA (1 µg) was used to generate cDNA using SuperScript II reverse transcriptase (Invitrogen). Real-time PCR was carried out for PUMA and GAPDH as described (13).
Western blotting
Antibodies used for Western blotting included those against active caspase 3, p-ERK(T202/Y204), IκB, p-IκB (S22/23), p-GSK3β, p65 (total), p-p65 (Ser 536), FoxO3a (total), AKT (total), p-AKT (S473) (Cell Signaling Technology), cytochrome c, α-tubulin, Bcl-XL (BD Biosciences), caspase 9 (Stressgen Bioreagents), cytochrome oxidase subunit IV (Cox IV; Invitrogen), Bcl-2 (Dako), Flag (Sigma), PUMA (17), p53, Bim, Bid, Noxa, and β-actin (EMD Biosciences). Western blotting analysis was performed as previously described (13). The release of cytochrome c was detected in the cytosol following subcelluar fractionations as described (13).
Transfection and siRNA knockdown
Cells were transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Knockdown experiments were performed 24 hours before ZM-447439 or VX-680 treatment using 400 pmoles of siRNA. All siRNA have been previously described and were from Dharmacon (Lafayette), including those for aurora A (21), aurora B (22), GSK3β (sc-35527; Santa Cruz) (13), p65 (11), p73 (9), FoxO3a (10), and the control scrambled siRNA. A non-degradable IκBα super repressor mutant (S32/36A; IκBαM) was previously described (11).
Analysis of NF-κB nuclear translocation
HCT 116 cells pre-treated with BAY 11–7082 were subjected to ZM-447439 or TNF-α for 3 hours. NF-κB nuclear translocation was analyzed by nuclear fractionation. Briefly, nuclear extracts were isolated from cells plated and treated in 75-cm2 flasks using the NE-PER nuclear/cytoplasmic extraction kit (Thermo Fisher) according to the manufacturer’s instructions, and probed by Western blotting for p65.
Luciferase assays
PUMA luciferase reporter constructs have been previous described (9). Mutations were introduced into the p65 binding sites of Fragment A using QuickChange XL site-directed mutagenesis kit (Agilent Technologies) as previous described (13). Cells were transfected with PUMA reporters containing either WT or mutant p65 binding sites (13), with the transfection control β-galactosidase reporter pCMVβ (Promega), and treated with 15 µM ZM-447439 for 24 hours. Cell lysates were collected and luciferase activities were measured as previously described (13). All reporter experiments were done in triplicate and repeated three times.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was done using the Chromatin Immunoprecipitation Assay kit (Millipore) with p65 (Santa Cruz) antibody for chromatin precipitation as described (13). The precipitates were analyzed by PCR using primers 5’-GTCGGTCTGTGTACGCATCG-3’ and 5’-CCCGCGTGACGCTACGGCCC -3’ as previously described (13).
Apoptosis assays
Adherent and floating cells were harvested, stained with Hoechst 33258 (Invitrogen), and analyzed for apoptosis by nuclear staining assay. A minimum of 300 cells were analyzed for each treatment. For colony formation assays, equal numbers of cells were subjected to various treatments and plated into 12-well plates at different dilutions. Colonies were visualized by crystal violet (Sigma) staining 14 days after plating as previously described (13). Each experiment was performed in triplicate and repeated at least twice.
Xenograft tumors
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pittsburgh. WT and PUMA-KO HCT116 xenografts were established and measured as described (13). In brief, 5–6 week old female athymic nude mice (Harlan) were inoculated with 5×106 cells per site on both flanks. Tumors were allowed to establish for 7 days. The mice were treated by i.p. injection for 14 consecutive days with 80 mg/kg/d ZM-447439 diluted in 10% DMSO, or vehicle control. The tumor volumes were measured in two dimensions using a vernier caliper. Mice were randomized into groups such that the average tumor volume across the groups was the same prior to treatment. For all in vivo experiments, tumor volumes were measured every other day in 2 dimensions and volumes were determined in mm3 using the formula l × b2 ×0.5 (where l is the larger diameter and b is the smaller diameter of the tumor). Mice were euthanized 5 (for Western analysis) or 21 days after the treatment. Tumors were dissected and fixed in 10% formalin and embedded in paraffin. Active caspase 3 immunostaining was performed on 5 µm paraffin-embedded tumor sections as previously described (23), with an AlexaFluor 594-conjugated secondary antibody (Invitrogen) for signal detection.
Statistical Analysis
Statistical analyses were carried out using GraphPad Prism IV software. p values were calculated by the student’s t-test and were considered significant if p <0.05. The means ± one standard deviation (s.d.) are displayed in the figures.
Results
p53-independent PUMA induction in response to aurora kinase inhibition
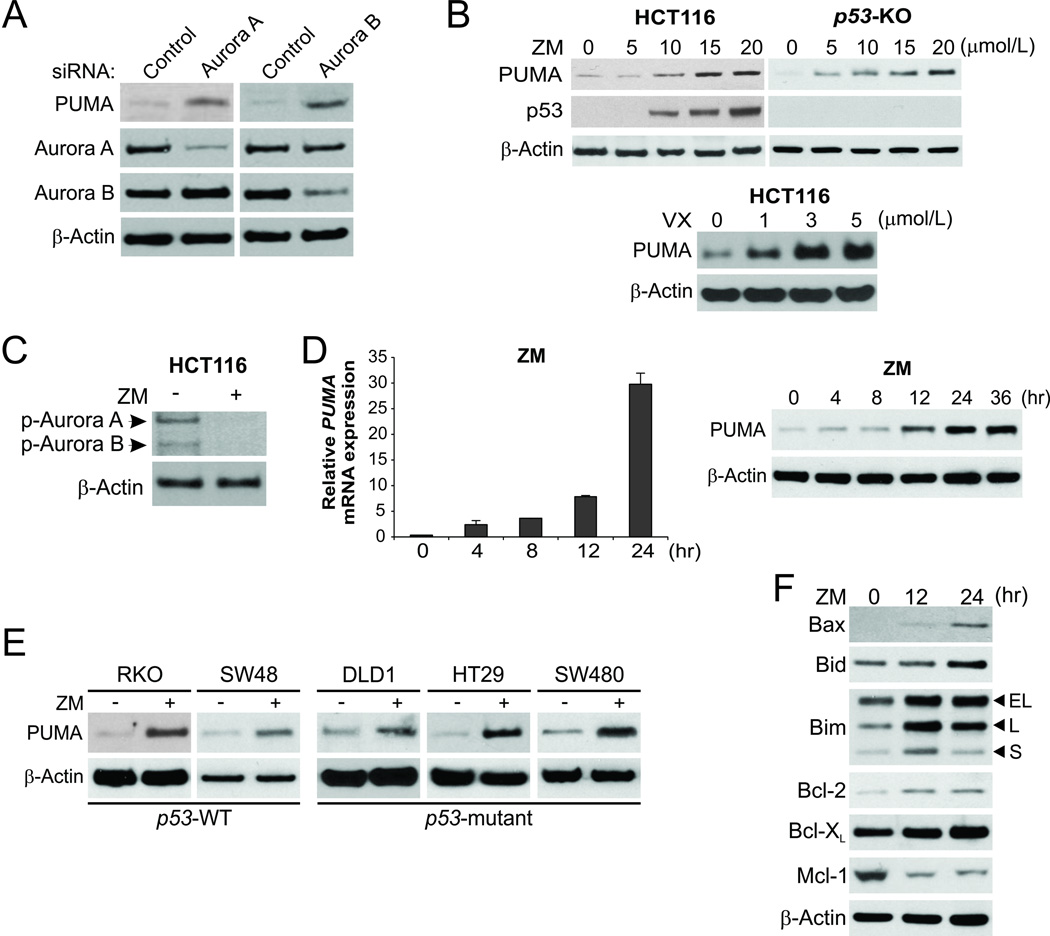
Aurora kinases, in particular aurora A and B, are frequently overexpressed in colon cancer cells (2). To determine how aurora kinases are involved in cell survival, we transfected p53-wildtype (WT) HCT116 colon cancer cells with siRNA for aurora A or aurora B. Knockdown of either aurora A or B led to the induction of PUMA (Fig. 1A). Treatment with ZM-447439 (ZM), a selective inhibitor of aurora A and B, at 5–20 µmol/L induced PUMA expression in a dose-dependent manner (Fig. 1B, upper), and suppressed the activating phosphorylation of T288 of aurora A and T232 of aurora B (Fig. 1C) (1). Treatment with the pan-aurora inhibitor VX-680 (VX; Tozasertib) at 1–40 µmol/L also strongly induced PUMA (Fig. 1B, lower and Fig. S1A). Following ZM or VX treatment, PUMA mRNA was induced as early as 4 hours, while PUMA protein started to accumulate between 8–12 hours (Fig. 1D and S1B). Both ZM and VX induced p53 in HCT116 cells (Fig. 1B and data not shown). However, the induction of PUMA by these agents was intact in p53-knockout (KO) HCT116 cells (Fig. 1B and S1A), and was observed in colon cancer cells with different p53 status, including p53-WT RKO and SW48 cells, and p53-mutant DLD1, HT29 and SW480 cells (Fig. 1E and S1C). Furthermore, the Bcl-2 family members, including Bax, Bid, Bim and Bcl-2, were upregulated, while Mcl-1 was depleted following ZM treatment (Fig. 1F). Therefore, the expression of multiple Bcl-2 family proteins including PUMA is modulated in response to aurora kinase inhibition, which may collectively initiate an apoptotic response in colon cancer cells.
Figure 1. p53-independent PUMA induction by aurora kinase inhibitors in colon cancer cells.
(A) HCT116 colon cancer cells were transfected with a control scrambled siRNA or siRNA against aurora A or aurora B. Expression of indicated proteins at 48 hours after siRNA transfection was analyzed by western blotting. (B) Upper, WT and p53-knockout (p53-KO) HCT 116 cells were treated with the aurora kinase inhibitor ZM-447439 (ZM) at indicated concentrations for 24 hours. Indicated proteins were analyzed by Western blotting; lower, HCT 116 cells were treated with the aurora kinase inhibitor VX-680 (VX) at the indicated concentrations for 24 hours. PUMA expression was analyzed by Western blotting. (C) Western blotting of p-aurora A (T288) and p-aurora B (T232) in HCT116 cells treated with 15 µmol/L ZM-447439 for 24 hours. (D) HCT116 cells were treated with 15 µmol/L ZM-447439. Left, real-time reverse transcriptase (RT) PCR analysis of PUMA mRNA induction time course; right, western blot analysis of PUMA protein induction time course. (E) Indicated colon cancer cell lines with different p53 statuses were treated with 15 µmol/L ZM-447439 for 24 hours. PUMA expression was analyzed by western blotting. (F) Western blot analysis of the expression of Bcl-2 family members at indicated time points in HCT 116 cells treated with 15 µmol/L ZM-447439.
Increased polyploidy, improved survival, and reduced apoptosis in PUMA-deficient cells upon aurora kinase inhibition
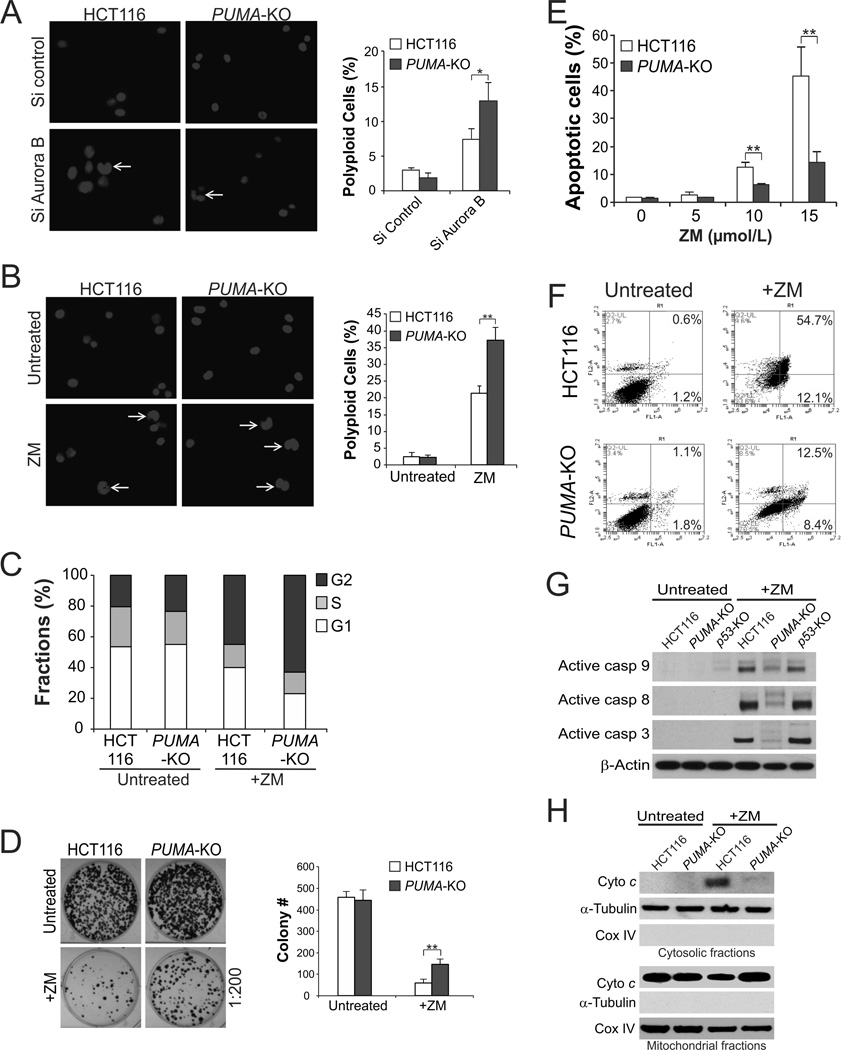
To determine whether PUMA induction plays a functional role in response to aurora kinase inhibition, we analyzed the DNA content and cell cycle progression of WT and PUMA-knockout (PUMA-KO) HCT116 cells following aurora kinase inhibition. Knockdown of aurora B, but not aurora A, by siRNA resulted in a higher fraction of polyploid PUMA-KO cells compared to WT HCT116 cells (Fig. 2A and data not shown). ZM treatment also induced a higher level of polyploidy (Fig. 2B), and enhanced accumulation of the G2 population in PUMA-KO cells relative to WT cells (Fig. 2C). Consistent with these findings, PUMA-KO cells had improved survival than WT HCT116 cells after ZM or VX treatment in a long-term colony formation assay (Fig. 2D and S2A).
Figure 2. PUMA mediates apoptosis induced by aurora kinase inhibitors through the mitochondrial pathway.
(A) WT and PUMA-KO HCT116 cells were transfected with control scrambled siRNA or aurora B siRNA. Cell nuclei were stained with Hoechst 33258 48 hours after siRNA transfection. Left, representative pictures (400×) are shown with arrows indicating polyploid nuclei; right, quantification of polyploid cells. (B) WT and PUMA-KO HCT116 cells were treated with 15 µmol/L ZM-447439 for 48 hours. Cell nuclei were stained with Hoechst 33258 after treatment. Left, representative pictures (400×) with arrows indicating polyploid nuclei; right, quantification of polyploid cells. (C) Cell cycle profiles in WT and PUMA-KO HCT116 cells treated with ZM as in (B) were determined by flow cytometry. Sub-G1 cells were excluded from the analysis. (D) Colony formation assay was done by seeding an equal number for ZM-447439-treated (10 µmol/L) WT and PUMA-KO HCT116 cells in 12-well plates, and then staining attached cells with crystal violet 14 days later. Left, representative pictures of colonies; right, quantification of colony numbers. (E) WT and PUMA-KO HCT116 cells were treated with ZM-447439 at the indicated concentrations for 48 hours. Apoptosis was analyzed by counting condensed and fragmented nuclei following nuclear staining with Hoechst 33258. (F) WT and PUMA-KO HCT116 cells were treated with 15 µmol/L ZM-447439 for 48 hours. Cells were stained with annexin V/propidium iodide and analyzed by flow cytometry. The percentages of annexin V-positive cells are indicated in the two right quadrants. (G) Western blot analysis of active caspases 3, 8 and 9 in WT, p53-KO, and PUMA-KO HCT116 cells with or without 15 µmol/L ZM-447439 treatment for 24 hours. (H) Cytosolic and mitochondrial fractions isolated from WT and PUMA-KO HCT116 cells treated with 15 µmol/L ZM-447439 for 36 hours were probed for cytochrome c by Western blotting. α -Tubulin and cytochrome oxidase subunit IV (Cox IV), which are expressed in cytoplasm and mitochondria, respectively, were analyzed as the control for loading and fractionation. Results in (A), (B), (D) and (E) were expressed as means ± SD of 3 independent experiments. **, P <0.01; *, P <0.05.
PUMA-KO cells were found to be defective in apoptosis induced by aurora kinase inhibitors. Nuclear staining revealed that apoptosis induced by ZM or VX at different concentrations was markedly reduced in PUMA-KO cells than WT cells (Fig. 2E and S2B). Annexin V/PI staining confirmed the reduction of ZM- and VX-induced apoptosis in the absence of PUMA (Fig. 2F and S2C). ZM-induced and PUMA-dependent apoptosis is not cell line specific, and was observed in p53-mutant DLD1 colon cancer cells (Fig. S2D), and in mouse embryonic fibroblasts (MEFs) (Fig. S2E). Furthermore, PUMA deficiency abrogated ZM-induced mitochondrial events including activation of caspases 3, 8, and 9 (Fig. 2G) and cytochrome c release (Fig. 2H). Together, these results suggest that cells undergoing mitotic arrest following aurora kinase inhibition are eliminated in part through PUMA-dependent apoptosis.
Direct activation of PUMA by NF-κB in response to aurora kinase inhibition
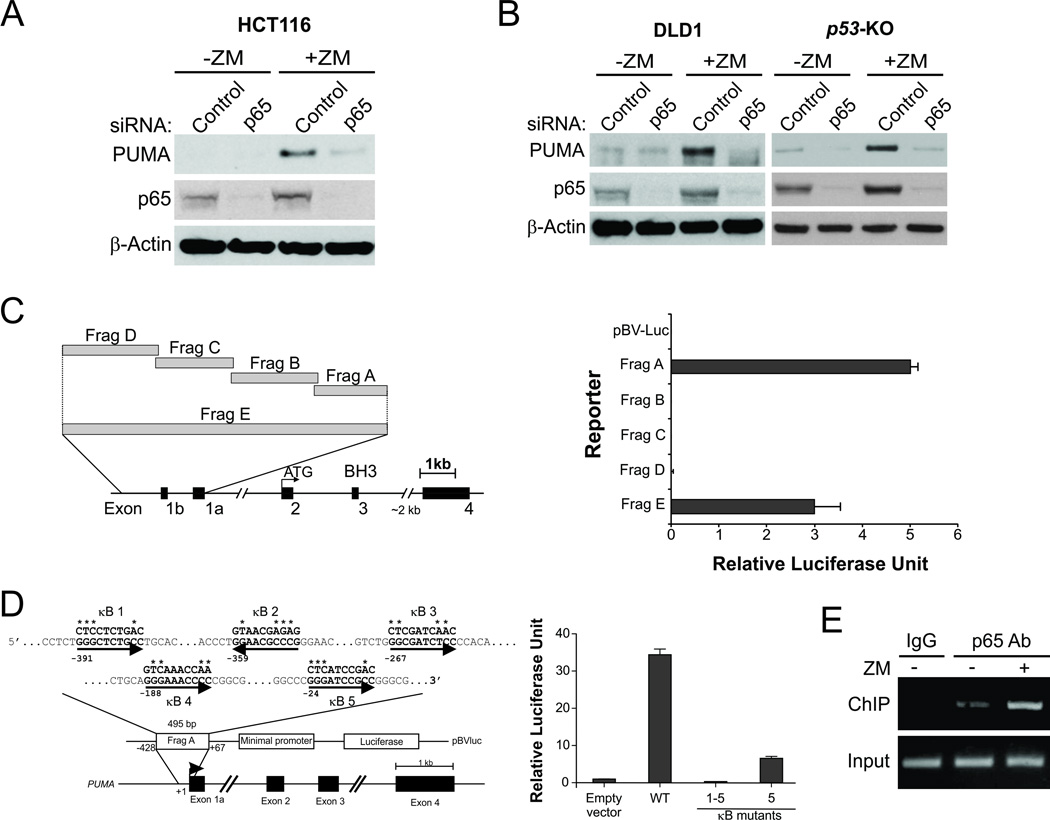
We then investigated the mechanism by which aurora kinase inhibitors induce PUMA in the absence of p53 by examining several transcription factors. The expression of FoxO3a and p73, which can induce PUMA in p53-deficient cells (9, 24), was unchanged after ZM or VX treatment (data not shown). Knockdown of FoxO3a or p73 by siRNA also did not affect PUMA induction by ZM (Fig. S3, A–C), indicating that FoxO3a and p73 are not involved in PUMA induction by aurora kinase inhibitors. The p65 subunit of NF-κB was recently identified as a transcriptional activator of PUMA in response to TNF-α or the c-Raf inhibitor sorafenib (11, 13). Suppression of p65 expression by siRNA abrogated PUMA induction following ZM treatment in HCT116 cells (Fig. 3A), and in p53-KO HCT116 and p53-deficient DLD1 cells (Fig. 3B). Knockdown of p65 also attenuated PUMA induction following VX treatment (Fig. S3D).
Figure 3. p65 directly binds to the PUMA promoter to activate its transcription following aurora kinase inhibition.
(A) HCT 116 cells were transfected with either a control scrambled siRNA or a p65 siRNA for 24 hours and then treated with 15 µmol/L ZM-447439 for 24 hours. p65 and PUMA expression was probed by Western blotting. (B) DLD1 and p53-KO HCT 116 cells were transfected with control or p65 siRNA, and then treated with 15 µmol/L ZM-447439 for 24 hours. p65 and PUMA expression was probed by Western blotting. (C) Left, schematic representation of the genomic structure of PUMA highlighting the PUMA promoter fragments (Frag) A–E used in the luciferase experiment; right, p53-KO HCT116 cells were transfected overnight with a luciferase reporter plasmid containing fragments A–E of the PUMA promoter and then treated with 10 µmol/L ZM-447439. Reporter activities were measured by a luciferase assay 16 hours later. (D) Left, schematic representation of the 5 p65 binding sites in fragment A of the PUMA promoter. Asterisks represent the mutated nucleotides; right, p53-KO HCT 116 cells were transfected overnight with a luciferase reporter plasmid containing the indicated κB site mutants and then treated and assayed as in (C). (E) Chromatin immunoprecipitation (ChIP) was performed using a p65-specific antibody on HCT116 cells following treatment with 15 µmol/L ZM-447439 for 8 hours. IgG was used as the ChIP control for antibody specificity. PCR was carried out using primers surrounding the p65 binding sites in the PUMA promoter.
To determine whether NF-κB directly activates PUMA transcription in response to aurora kinase inhibition, p53-KO HCT116 cells were transfected with luciferase reporter constructs containing different regions of the PUMA promoter (fragments A–D; Fig. 3C, left) (9). Upon ZM treatment, only the proximal 495-bp region of the PUMA promoter (fragments A and E) was found to be strongly activated (Fig. 3C, right). In contrast, the NF-κB responsive element distal within fragment D, which is required for PUMA induction by TNF-α (11), was not activated (Fig. 3C). The activated PUMA promoter region contains at least 5 putative κB sites (Fig. 3D, left) (13), among which, site 5 seems to be most critical for activation of the PUMA promoter by ZM treatment (Fig. 3D, right and Fig. S3E). But complete blockage of PUMA promoter activation was only observed when all 5 κB sites were mutated (Fig. 3D, right), suggesting that multiple, if not all, of the 5 κB sites contribute to the activation of the PUMA promoter. Furthermore, chromatin immunoprecipitation (ChIP) analysis revealed increased recruitment of p65 to the region containing the κB sites following ZM treatment (Fig. 3E). These results indicate that p65 directly binds to multiple κB sites in the PUMA promoter to drive its transcription in response to aurora kinase inhibition.
PUMA induction by aurora kinase inhibitors through the canonical NF-κB pathway and AKT inhibition
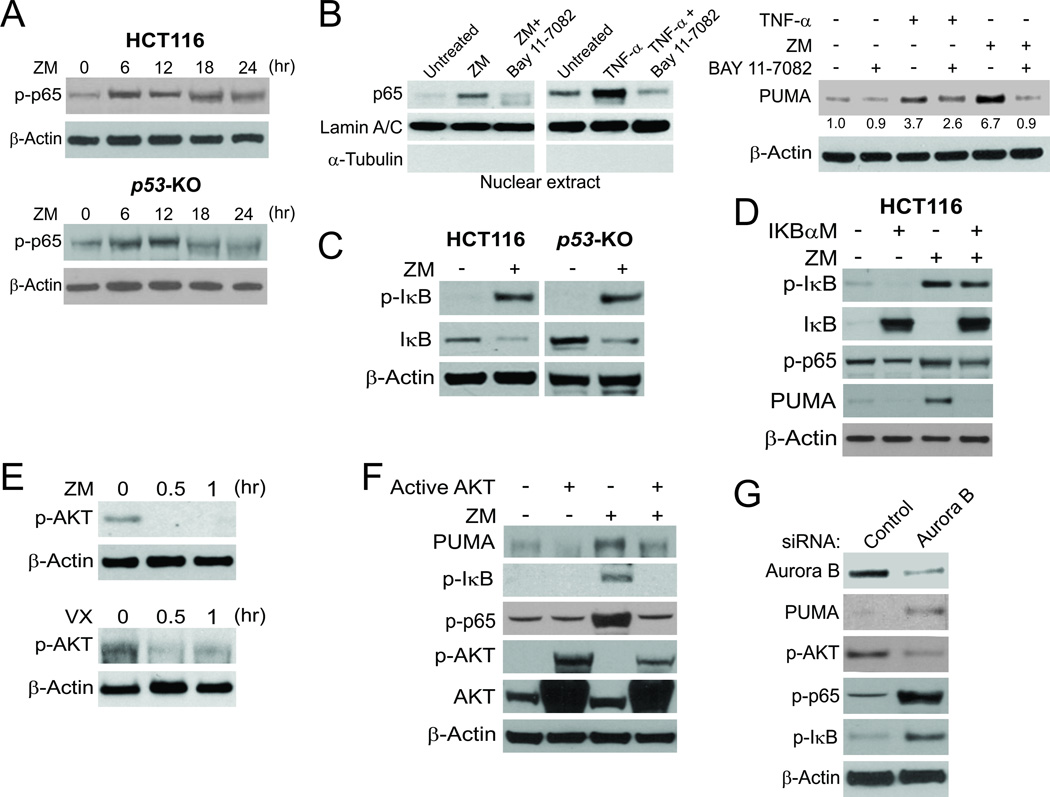
Activation of NF-κB signaling is characterized by p65 phosphorylation on several residues and its subsequent translocation to the nucleus, where it activates transcription of NF-κB target genes (25). We found that ZM treatment enhanced the phosphorylation of S536, the major regulatory site of p65 (25), in both WT and p53-KO HCT116 cells (Fig. 4A). Probing p65 in nuclear fractions detected its nuclear translocation in cells treated with ZM or the control TNF-α (Fig. 4B). Suppressing p65 nuclear translocation, by pre-treating cells with the NF-κB inhibitor BAY 11–7082 (Fig. 4B, left), impeded PUMA induction by ZM or TNF-α (Fig. 4B, right), suggesting that PUMA induction following aurora kinase inhibition is mediated by p65 nuclear translocation. The canonical NF-κB pathway activated by agents such as TNF-α is mediated by IκB S22/S23 phosphorylation and subsequent degradation (25). ZM treatment led to IκB phosphorylation and degradation (Fig. 4C). Transfecting cells with IκBαM, a non-degradable mutant of IκB (11), reduced ZM-induced PUMA expression and p65 phosphorylation in WT and p53-KO HCT116 cells (Fig. 4D and S4A), suggesting that ZM-induced p65 activation is mediated by IκB depletion through the canonical NF-κB pathway.
Figure 4. Aurora kinase inhibitors induce PUMA through AKT inhibition and the canonical NF-κB pathway.
(A) WT and p53-KO HCT 116 cells were treated with 15 µmol/L ZM-447439. Expression of p-p65 (S536) and β-actin at the indicated time points was analyzed by Western blotting. (B) HCT 116 cells were treated with 10 µmol/L BAY11–7082 for 1 hour, and then with 15 µmol/L ZM-447439 or 10 ng/mL TNF-α for 24 hours. Left, nuclear fractions were isolated from cells and analyzed for p65 expression by Western blotting. Lamin A/C and α-tubulin, which are expressed in the nucleus and cytoplasm, respectively, were used as controls for loading and fractionation; right, Western blot analysis of PUMA and β-actin expression in whole cell lysates after ZM-447439 or TNF-α treatment. Relative PUMA expression of each sample, determined by Image J program and normalized to that of the loading control β-actin, is indicated, with that of the untreated cells arbitrarily set as 1.0. (C) WT and p53-KO HCT116 cells were treated with 15 µmol/L ZM-447439 for 24 hours. Expression of p-IκB (S22/23) and IκB was analyzed by Western blotting. (D) HCT116 cells were transfected overnight with pCMV or IκBαM and then treated with 15 µmol/L ZM-447439 for 24 hours. Expression of PUMA, p-IκB, IκB and p-p65 was analyzed by Western blotting. (E) p53-KO HCT116 cells were treated with 15 µmol/L ZM-447439 or 20 µmol/L VX-680 for the indicated time and expression of p-AKT was probed by Western blotting. (F) p53-KO HCT116 cells were transfected with either control empty vector or a constitutively active AKT expression construct for 16 hours, and then treated with 15 µmol/L ZM-447439 for 24 hours. Indicated proteins were analyzed by Western blotting. (G) HCT 116 cells were transfected with control scrambled siRNA or aurora B siRNA. Expression of the indicated proteins at 48 hours after siRNA transfection was analyzed by Western blotting.
We further analyzed the signaling events leading to NF-κB activation, focusing on several kinases known to act upon the NF-κB pathway (25). ZM treatment did not significantly affect the activity of ERK kinase (Fig. S4B), but suppressed the inhibitory S9 phosphorylation of GSK3β (26) (Fig. S4C). However, knockdown of GSK3β by siRNA did not affect the induction of PUMA by ZM in both WT and p53-KO HCT116 cells (Fig. S4D). AKT phosphorylation was decreased shortly after ZM or VX treatment (Fig. 4E). Exogenous expression of WT or active AKT suppressed PUMA induction, IκB phosphorylation and p65 phosphorylation following ZM treatment (Fig. 4F), also downregulated endogenous PUMA expression in untreated cells (Fig. S4E). Furthermore, knockdown of aurora B recapitulated these effects of ZM on AKT, IκB, and p65 phosphorylation (Fig. 4G). Collectively, these observations suggest that aurora kinase inhibitors induce PUMA through a pathway involving AKT inhibition, IκB degradation and subsequent p65 activation.
PUMA-mediated chemosensitization by aurora kinase inhibitors
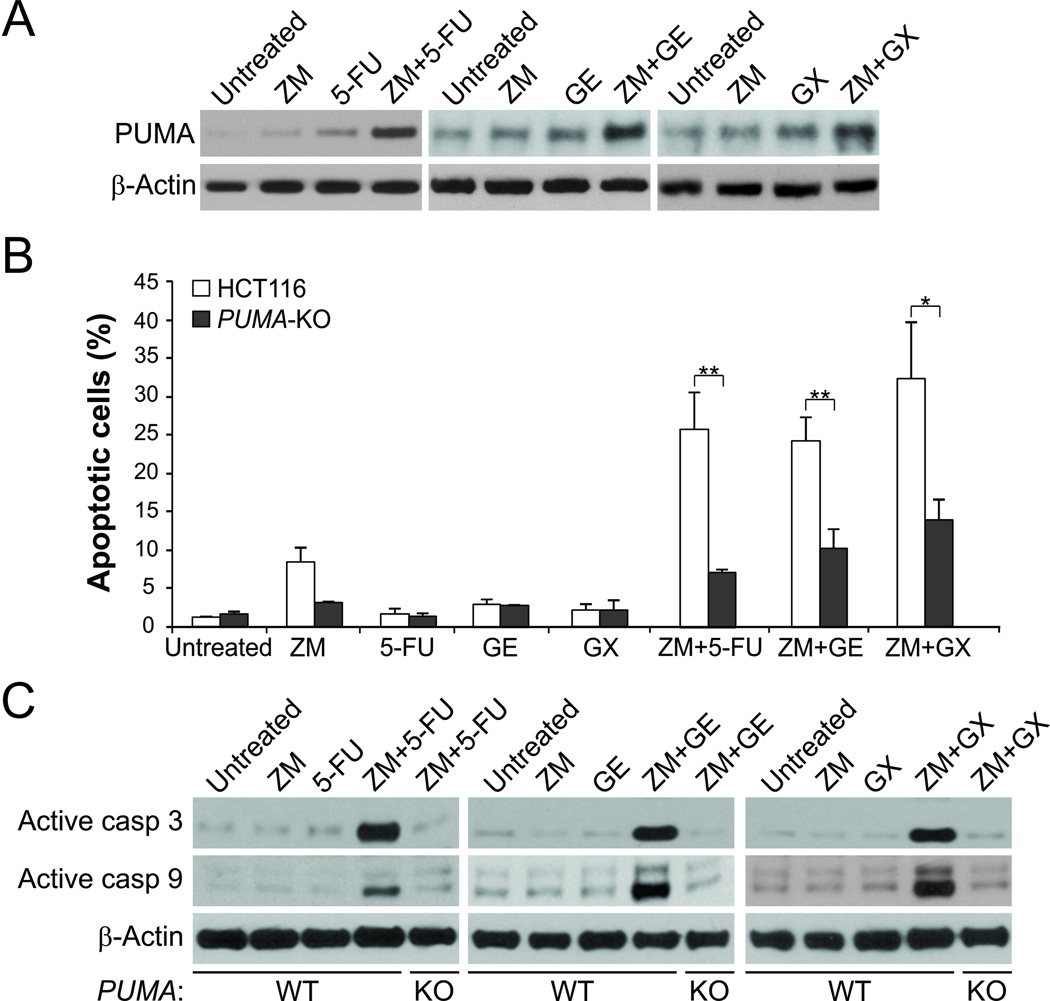
Aurora kinase inhibitors have been used in combination with conventional cytotoxic drugs in clinical studies (3). We reasoned that PUMA induction may mediate the chemosensitization effects of aurora kinase inhibitors, because of concurrent PUMA induction by aurora kinase inhibitors and other agents through different mechanisms. Indeed, we found that the combination of ZM with other anticancer agents, at a concentration that did not robustly induce PUMA or apoptosis, such as the DNA damaging drug 5-fluorouracil (5-FU), the EGFR inhibitor gefitinib (GE), or the BH3-mimetic GX15-070 (Obatoclax mesylate) (27), induced PUMA at a much higher level compared to a single agent alone (Fig. 5A). Accordingly, the levels of apoptosis and caspase 3 activation were also significantly higher in HCT116 cells following the combination treatments (Fig. 5B and 5C). However, the enhanced induction of apoptosis and caspase 3 activation was largely suppressed in PUMA-KO cells (Fig. 5B and 5C). These data suggest that PUMA mediates the chemosensitization effects of aurora kinase inhibitors, and robust induction of PUMA is indicative of effective drug combinations.
Figure 5. PUMA mediates the chemosensitization effects of aurora kinase inhibitors through apoptosis induction.
(A) Western blot analysis of PUMA expression in HCT 116 cells treated with 5 µmol/L ZM-447439, 20 µg/ml 5-fluorouracil (5-FU), 25 µmol/L Gefitinib (GE), or 100 µmol/L GX5-070 (GX) alone, or their combinations for 48 hours. (B) WT and PUMA-KO HCT 116 cells were treated with ZM, 5-FU, GE, or GX alone or their combinations as in (A). Apoptosis was determined by nuclear staining with Hoechst 33258. Results were expressed as means ± SD of 3 independent experiments. **, P <0.01; *, P <0.05. (C) Western blotting of processed active caspase 3 and caspase 9 in WT and PUMA-KO HCT 116 cells treated as in (A).
PUMA-dependent in vivo therapeutic activity of aurora kinase inhibitors
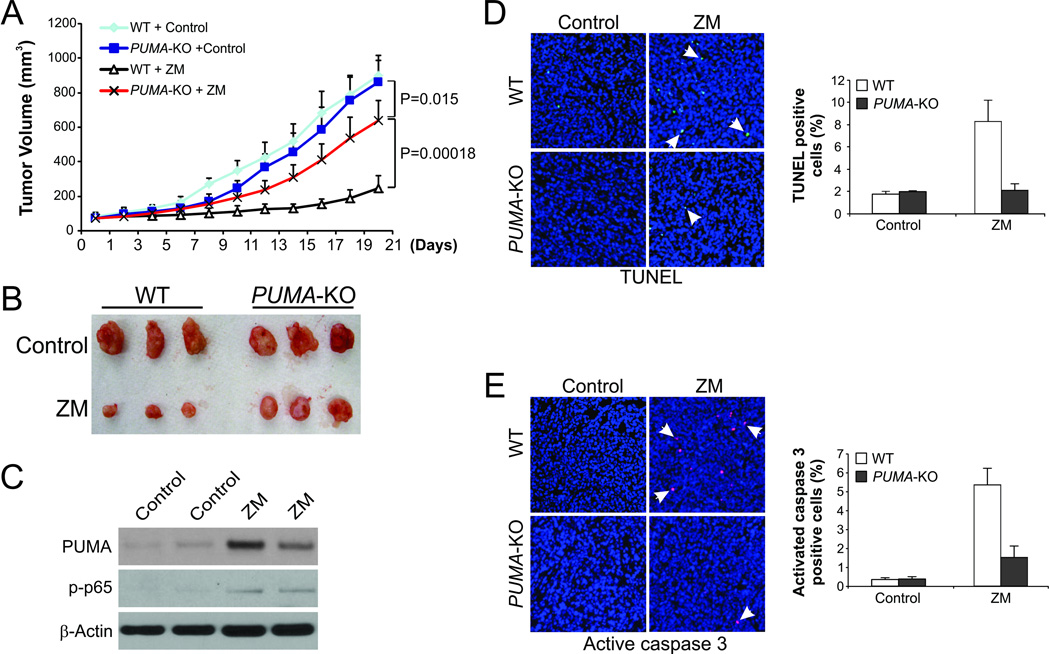
We then used a xenograft tumor model to determine whether PUMA is necessary for the antitumor effects of aurora kinase inhibitors. WT and PUMA-KO HCT116 cells were injected subcutaneously into nude mice to establish xenograft tumors. Mice were then treated with 80 mg/kg/d ZM or the control vehicle by i.p. injection for 14 consecutive days. WT and PUMA-KO tumors without drug treatment were not different in their growth (Fig. 6A and 6B). While ZM treatment resulted in 72.8% growth inhibition relative to the vehicle control in WT tumors, it only led to 25.7% growth inhibition in PUMA-KO tumors (Fig. 6A and 6B). The differences between WT and PUMA-KO groups were statistically significant in the ZM arm (P<0.001), but not in the control vehicle arm (Fig. 6A), indicating that PUMA accounts for a substantial portion of the antitumor activity of ZM. Increased p65 phosphorylation and PUMA expression were detected in the ZM-treated WT tumors (Fig. 6C). Substantial apoptosis induction was revealed by TUNEL staining in the ZM-treated WT tumors, but not in the control tumors. In contrast, apoptosis was barely detectable in the PUMA-KO tumors after ZM treatment (Fig. 6D). Staining for active caspase 3 verified defective apoptosis in ZM-treated PUMA-KO tumors (Fig. 6E). Therefore, the in vivo antitumor activity of ZM is PUMA-dependent, and also involves NF-κB activation.
Figure 6. PUMA mediates the antitumor effect of ZM-447439 in a xenograft model.
(A) Nude mice were injected s.c. with 5×106 WT or PUMA-KO HCT116 cells. After 1 week, mice were treated with 80 mg/kg/d ZM-447439 (i.p. injection) or the control 10% DMSO for 14 consecutive days. Tumor volume at the indicated time points after treatment was calculated and plotted (n=5 in each group). (B) Representative tumors at the end of the experiment in (A). (C) WT and PUMA-KO HCT 116 xenograft tumors were treated with 80 mg/kg/d ZM-447439 or control DMSO by i.p. injection as in (A) for 5 consecutive days. p-p65(S536) and PUMA expression in representative tumors was analyzed by Western blotting. (D) Paraffin-embedded sections from tumors in (C) were analyzed by TUNEL staining. Left, representative TUNEL staining pictures with arrows indicating example TUNEL-positive cells (400×); right, TUNEL-positive cells were counted and plotted. (E) Tumor sections were analyzed by active caspase 3 staining. Left, representative staining pictures arrows indicating example active caspase 3-positive cells (400×); right, active caspase 3-positive cells were counted and plotted. Results of (D) and (E) were expressed as means ±SD.
Discussion
Upregulation of mitotic kinases such as aurora and polo kinases promotes cell division and contributes to sustained cell proliferative signaling, a hallmark of cancer cells (28). Several lines of evidence suggest that aurora B is the prominent aurora kinase in colorectal cancer cells (29–31). Aurora B is frequently overexpressed in colorectal tumors (1), and its overexpression correlates with advanced tumor stages (29). Inhibition of aurora B seems to have more profound effects in colon cancer cells than that of other aurora kinases. For example, inhibition of aurora B, but not aurora A, sensitized colon cancer cells to the Bcl-2 inhibitor ABT-263 (30). Aurora B mutations were found to be enriched in cells with acquired resistance to ZM (31). In our study, knockdown of aurora B recapitulated the effects of ZM on PUMA induction, AKT inhibition, and I-κB and p65 phosphorylation (Fig. 1A and 4G). Therefore, targeting aurora kinases in colorectal cancer may benefit from development of more specific inhibitors for aurora B.
Our results demonstrate that PUMA is induced following mitotic arrest and AKT inhibition in response to aurora kinase inhibition, and contributes to apoptosis initiation in colon cancer cells via the mitochondrial pathway. Consistent with this notion, PUMA and PUMA-dependent apoptosis can be induced by polyploidy (19, 20), or by AKT inhibition (9, 10, 12, 32). PUMA seems to function as a nodule of multiple killing pathways, and can initiate the apoptotic response to a variety of kinase inhibitors, including the pan-CDK inhibitor UCN-01 (10), the EGFR inhibitors gefitinib and erlotinib (12), the c-MET/ALK inhibitor crizotinib (33), and the multi-kinase inhibitor drugs sorafenib and sunitinib (13, 14). In addition to PUMA, other Bcl-2 family members are also modulated following aurora kinase inhibition and contribute to apoptosis induction. Multiple proapoptotic BH3-only proteins are induced by ZM and VX in colon cancer cells (Fig. 1F and S1D) (30), and ZM could induce Bax-mediated apoptosis in HCT116 cells (34). A recent study suggests that downregulation of Mcl-1 following aurora kinase inhibition has a functional role in colon cancer cells by increasing the reliance on Bcl-XL for cell survival (30).
PUMA mediates apoptosis induced by aurora kinase inhibitors in a p53-independent manner, though p53 can be stabilized and promotes apoptosis in response to aurora kinase inhibitors (34, 35). The residual apoptosis in ZM-treated PUMA-deficient cells may be attributable to p53 induction and its effects on other Bcl-2 family members (Fig. 2E), as well as other cell death mechanisms. It is unexpected that aurora kinase inhibitors activate NF-κB, better known as a pro-survival factor, to induce PUMA; however, NF-κB clearly can promote apoptosis under certain conditions (36). Unlike the induction of PUMA by sorafenib, which does not involve IκB degradation and results from GSK3β and ERK inhibition (13), the induction of PUMA by aurora kinase inhibitors is mediated by the canonical NF-κB pathway via IκB degradation and subsequent p65 nuclear translocation following AKT inhibition. These results reinforce the multi-faceted nature of NF-κB signaling, and suggest a broader functional role of NF-κB signaling in mediating therapeutic response to targeted drugs, which has not been sufficiently appreciated. In addition to p65, other aurora kinase inhibitors may function through different transcription factors, such as p73 (35), to induce PUMA and other Bcl-2 family members to induce apoptosis.
For robust induction of apoptosis, most of the experiments in this study were performed by using ZM-447439 or VX-680 in a dose range of 10–20 µmol/L, which are substantially higher than those known to be effective for aurora kinase inhibition (3). The results cannot be solely explained by inhibition of aurora kinase activity. A recent Phase I dose escalation study showed that the plasma concentration of VX-680 (MK-0457) can reach 1–5 µM in patients 24 hr after drug infusion (37). We found that VX-680 in this dose range was sufficient to induce PUMA expression (Fig. 1B, lower). PUMA is also necessary for the chemosensitization effects of ZM-447439 at 5 µmol/L (Fig. 5). These observations suggest that PUMA might be involved in the effects of aurora kinase inhibitors at clinically relevant doses.
There is great enthusiasm for developing new antimitotic agents including aurora kinase inhibitors, largely due to the success of microtubule-targeting drugs such as taxanes and vinca alkaloids. To date, at least 30 aurora kinase inhibitors have been developed, some of which have advanced into clinical trials for treating a variety of tumors (2, 4). However, these new antimitotic agents have not met the initial expectation with regard to their clinical efficacy and toxicities, raising doubt on the rationale of targeting mitosis for anticancer therapy (38). Understanding the killing mechanisms of aurora kinase inhibitors may help to improve their clinical applications (39). For example, it might be possible to use aurora kinase inhibitors at reduced doses in combination with agents with different PUMA induction mechanisms and non-overlapping toxicities. It also seems to be plausible to use apoptosis-targeting agents such as BH3 and SMAC mimetics to potentiate aurora kinase inhibitors. PUMA induction might be a useful biomarker for clinical trials testing aurora kinase inhibitors. Furthermore, delineating a critical mediator of their killing activity may help to develop more specific and more effective aurora kinase inhibitors with less toxicity.
Supplementary Material
Acknowledgments
Grant Support: This work is supported by NIH grants CA106348, CA121105, CA172136 (L. Zhang), CA129829 (J. Yu), American Cancer Society grants RSG-07-156-01-CNE (L. Zhang) and RGS-10-124-01-CCE (J. Yu). This project used the UPCI shared glassware and cell imaging facilities that were supported in part by award P30CA047904.
Abbreviations
- Ad
adenovirus
- ChIP
chromatin immunoprecipitation
- Cox IV
cytochrome oxidase subunit IV
- FoxO3a
Forkhead Box O3a
- KO
knockout
- MEFs
mouse embryo fibroblasts
- NF-κB
nuclear factor κB
- PUMA
p53 upregulated modulator of apoptosis
- RT-PCR
reverse transcriptase-PCR
- shRNA
small hairpin RNA
- siRNA
small interfering RNA
- TNF-α
tumor necrosis factor-α
- WT
wild-type
Footnotes
Disclosures: The authors declare no conflict of interest and all authors have agreed on the submission.
References
- 1.Lens SM, Voest EE, Medema RH. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat Rev Cancer. 2010;10:825–841. doi: 10.1038/nrc2964. [DOI] [PubMed] [Google Scholar]
- 2.Katayama H, Sen S. Aurora kinase inhibitors as anticancer molecules. Biochimica et biophysica acta. 2010;1799:829–839. doi: 10.1016/j.bbagrm.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green MR, Woolery JE, Mahadevan D. Update on Aurora Kinase Targeted Therapeutics in Oncology. Expert opinion on drug discovery. 2011;6:291–307. doi: 10.1517/17460441.2011.555395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dar AA, Goff LW, Majid S, Berlin J, El-Rifai W. Aurora kinase inhibitors--rising stars in cancer therapeutics? Mol Cancer Ther. 2010;9:268–278. doi: 10.1158/1535-7163.MCT-09-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manchado E, Guillamot M, Malumbres M. Killing cells by targeting mitosis. Cell Death Differ. 2012;19:369–377. doi: 10.1038/cdd.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gascoigne KE, Taylor SS. How do anti-mitotic drugs kill cancer cells? J Cell Sci. 2009;122:2579–2585. doi: 10.1242/jcs.039719. [DOI] [PubMed] [Google Scholar]
- 7.Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene. 2008;27(Suppl 1):S71–S83. doi: 10.1038/onc.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J, Yue W, Wu B, Zhang L. PUMA sensitizes lung cancer cells to chemotherapeutic agents and irradiation. Clin Cancer Res. 2006;12:2928–2936. doi: 10.1158/1078-0432.CCR-05-2429. [DOI] [PubMed] [Google Scholar]
- 9.Ming L, Sakaida T, Yue W, Jha A, Zhang L, Yu J. Sp1 and p73 Activate PUMA Following Serum Starvation. Carcinogenesis. 2008;29:1878–1884. doi: 10.1093/carcin/bgn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudgeon C, Wang P, Sun X, Peng R, Sun Q, Yu J, et al. PUMA induction by FoxO3a mediates the anticancer activities of the broad-range kinase inhibitor UCN-01. Mol Cancer Ther. 2010;9:2893–2902. doi: 10.1158/1535-7163.MCT-10-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang P, Qiu W, Dudgeon C, Liu H, Huang C, Zambetti GP, et al. PUMA is directly activated by NF-kappaB and contributes to TNF-alpha-induced apoptosis. Cell Death Differ. 2009;16:1192–1202. doi: 10.1038/cdd.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Q, Ming L, Thomas SM, Wang Y, Chen ZG, Ferris RL, et al. PUMA mediates EGFR tyrosine kinase inhibitor-induced apoptosis in head and neck cancer cells. Oncogene. 2009;18:2348–2357. doi: 10.1038/onc.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudgeon C, Peng R, Wang P, Sebastiani A, Yu J, Zhang L. Inhibiting oncogenic signaling by sorafenib activates PUMA via GSK3beta and NF-kappaB to suppress tumor cell growth. Oncogene. 2012;31:4848–4858. doi: 10.1038/onc.2011.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun J, Sun Q, Brown MF, Dudgeon C, Chandler J, Xu X, et al. The Multi-Targeted Kinase Inhibitor Sunitinib Induces Apoptosis in Colon Cancer Cells via PUMA. PLoS One. 2012;7:e43158. doi: 10.1371/journal.pone.0043158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ming L, Wang P, Bank A, Yu J, Zhang L. PUMA dissociates Bax and BCL-XL to induce apoptosis in colon cancer cells. J Biol Chem. 2006;281:16034–16042. doi: 10.1074/jbc.M513587200. [DOI] [PubMed] [Google Scholar]
- 16.Yu J, Wang P, Ming L, Wood MA, Zhang L. SMAC/Diablo mediates the proapoptotic function of PUMA by regulating PUMA-induced mitochondrial events. Oncogene. 2007;26:4189–4198. doi: 10.1038/sj.onc.1210196. [DOI] [PubMed] [Google Scholar]
- 17.Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci U S A. 2003;100:1931–1936. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giannakakou P, Nakano M, Nicolaou KC, O'Brate A, Yu J, Blagosklonny MV, et al. Enhanced microtubule-dependent trafficking and p53 nuclear accumulation by suppression of microtubule dynamics. Proc Natl Acad Sci U S A. 2002;99:10855–10860. doi: 10.1073/pnas.132275599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le HV, Minn AJ, Massague J. Cyclin-dependent kinase inhibitors uncouple cell cycle progression from mitochondrial apoptotic functions in DNA-damaged cancer cells. J Biol Chem. 2005;280:32018–32025. doi: 10.1074/jbc.M504689200. [DOI] [PubMed] [Google Scholar]
- 20.Castedo M, Coquelle A, Vivet S, Vitale I, Kauffmann A, Dessen P, et al. Apoptosis regulation in tetraploid cancer cells. EMBO J. 2006;25:2584–2595. doi: 10.1038/sj.emboj.7601127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mason KA, Withers HR, McBride WH, Davis CA, Smathers JB. Comparison of the gastrointestinal syndrome after total-body or total-abdominal irradiation. Radiat Res. 1989;117:480–488. [PubMed] [Google Scholar]
- 22.Yu J. Intestinal stem cell injury and protection during cancer therapy. Transl Cancer Res. 2013 [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu W, Wang X, Leibowitz B, Liu H, Barker N, Okada H, et al. Chemoprevention by nonsteroidal anti-inflammatory drugs eliminates oncogenic intestinal stem cells via SMAC-dependent apoptosis. Proc Natl Acad Sci U S A. 2010;107:20027–20032. doi: 10.1073/pnas.1010430107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, et al. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203:1657–1663. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Ming L, Yu J. BH3 mimetics to improve cancer therapy; mechanisms and examples. Drug Resist Updat. 2007;10:207–217. doi: 10.1016/j.drup.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Katayama H, Ota T, Jisaki F, Ueda Y, Tanaka T, Odashima S, et al. Mitotic kinase expression and colorectal cancer progression. J Natl Cancer Inst. 1999;91:1160–1162. doi: 10.1093/jnci/91.13.1160. [DOI] [PubMed] [Google Scholar]
- 30.Shah OJ, Lin X, Li L, Huang X, Li J, Anderson MG, et al. Bcl-XL represents a druggable molecular vulnerability during aurora B inhibitor-mediated polyploidization. Proc Natl Acad Sci U S A. 2010;107:12634–12639. doi: 10.1073/pnas.0913615107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girdler F, Sessa F, Patercoli S, Villa F, Musacchio A, Taylor S. Molecular basis of drug resistance in aurora kinases. Chemistry & biology. 2008;15:552–562. doi: 10.1016/j.chembiol.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Qiu W, Leibowitz B, Zhang L, Yu J. Growth factors protect intestinal stem cells from radiation-induced apoptosis by suppressing PUMA through the PI3K/AKT/p53 axis. Oncogene. 2009;29:1622–1632. doi: 10.1038/onc.2009.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng X, He K, Zhang L, Yu J. Crizotinib induces PUMA-dependent apoptosis in colon cancer cells. Mol Cancer Ther. 2013;12:777–786. doi: 10.1158/1535-7163.MCT-12-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li M, Jung A, Ganswindt U, Marini P, Friedl A, Daniel PT, et al. Aurora kinase inhibitor ZM447439 induces apoptosis via mitochondrial pathways. Biochem Pharmacol. 2010;79:122–129. doi: 10.1016/j.bcp.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Dar AA, Belkhiri A, Ecsedy J, Zaika A, El-Rifai W. Aurora kinase A inhibition leads to p73-dependent apoptosis in p53-deficient cancer cells. Cancer Res. 2008;68:8998–9004. doi: 10.1158/0008-5472.CAN-08-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dutta J, Fan Y, Gupta N, Fan G, Gelinas C. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene. 2006;25:6800–6816. doi: 10.1038/sj.onc.1209938. [DOI] [PubMed] [Google Scholar]
- 37.Traynor AM, Hewitt M, Liu G, Flaherty KT, Clark J, Freedman SJ, et al. Phase I dose escalation study of MK-0457, a novel Aurora kinase inhibitor, in adult patients with advanced solid tumors. Cancer chemotherapy and pharmacology. 2011;67:305–314. doi: 10.1007/s00280-010-1318-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Komlodi-Pasztor E, Sackett DL, Fojo AT. Inhibitors targeting mitosis: tales of how great drugs against a promising target were brought down by a flawed rationale. Clin Cancer Res. 2012;18:51–63. doi: 10.1158/1078-0432.CCR-11-0999. [DOI] [PubMed] [Google Scholar]
- 39.Green DR, Kroemer G. Pharmacological manipulation of cell death: clinical applications in sight? J Clin Invest. 2005;115:2610–2617. doi: 10.1172/JCI26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.