Abstract
Context
Lung cancer patients experience multiple, simultaneous symptoms related to their disease and treatment that impair functioning and health-related quality of life (HRQL). Computer technology can reduce barriers to nonsystematic, infrequent symptom assessment and potentially contribute to improved patient care.
Objectives
To evaluate the efficacy of technology-based symptom monitoring and reporting in reducing symptom burden in patients with advanced lung cancer.
Methods
This was a prospective, multisite, randomized controlled trial (RCT). Two hundred fifty-three patients were enrolled at three sites and randomized to monitoring and reporting (MR) or monitoring alone (MA). Patients completed questionnaires at baseline, 3, 6, 9 and 12 weeks and symptom surveys via interactive voice response (IVR) weekly for 12 weeks. MR patients’ clinically significant symptom scores generated an e-mail alert to the site nurse for management. The primary endpoint was overall symptom burden; secondary endpoints included HRQL, treatment satisfaction, symptom management barriers, and self-efficacy.
Results
This RCT failed to demonstrate efficacy of symptom monitoring and reporting in reducing symptom burden compared with monitoring alone in lung cancer. HRQL declined over 12 weeks in both groups (P<0.006 to P<0.025); at week 12, treatment satisfaction was higher in MA than MR patients (P<0.012, P<0.027). Adherence to weekly calls was good (82%) and patient satisfaction was high.
Conclusion
Feasibility of using a technology-based system for systematic symptom monitoring in advanced lung cancer patients was demonstrated. Future research should focus on identifying patients most likely to benefit and other patient, provider and health system factors likely to contribute to the system’s success.
Keywords: lung cancer, symptoms, randomized controlled trial, health information technology, telemonitoring
Introduction
Patients with advanced lung cancer face a shortened life expectancy and typically experience multiple, simultaneous, debilitating symptoms related to their disease and its treatment. In addition to impairing patients’ daily functioning and health-related quality of life (HRQL) (1), unrecognized or poorly controlled symptoms can cause emergency department (ED) visits and hospitalizations for management as well as decreased treatment efficacy (2, 3). Outpatient chemotherapy is usually administered over several months, with office visits scheduled two to four weeks apart. As a result, many symptoms emerge between scheduled clinic appointments (4, 5), which creates challenges for the effective and timely monitoring and management of symptoms.
There are many patient, provider and health system barriers to adequate symptom management (1, 6). Two widely reported barriers are inadequate or nonsystematic symptom assessment (6–10) and limited patient-provider communication about symptoms (1, 11, 12). Clinicians vary in their ability to elicit information about patient symptoms (13–16) and systematically underestimate them (17–20). Patients often forget to report important medical information (21, 22) and fail to accurately report symptom levels (23). Systematic symptom assessment and reporting to the provider is associated with reduced symptom distress (24, 25), better pain control (26, 27) and improved, more focused symptom communication (6, 28, 29).
Advances in health information technology (HIT) enable routine, systematic assessment of patient-reported outcomes (PROs; e.g., symptoms and HRQL) that can be conducted from home, between office visits, with minimal burden (5, 6, 30). Technology-based monitoring is feasible and well-accepted by patients (31–36), improves patient-provider communication (37– 39), and focuses attention on priority symptoms (39–45).
We elected to use telephone-based interactive voice response (IVR) technology for our Symptom Monitoring and Reporting System for Lung Cancer (SyMon-L) because of the telephone’s widespread adoption and familiarity. In our observational, single institution pilot study (46), we found IVR monitoring to be feasible and acceptable to patients, which also has been demonstrated by others (47–56). This paper describes a multisite, prospective, randomized controlled trial (RCT) evaluating whether technology-based weekly symptom monitoring and automated reporting of problematic symptoms to the clinical team reduces on-treatment symptom burden of people with advanced lung cancer compared with monitoring alone (i.e., with no reporting to the clinical team). We hypothesized that SyMon-L monitoring and reporting, a more active intervention, would reduce symptom burden to a greater extent than a more passive monitoring intervention by facilitating timely care management realized because of early problem identification and intervention, with secondary benefit to HRQL, treatment satisfaction, perceived barriers to symptom management, and self-efficacy.
Methods
Study Design
Following approval by the research site institutional review boards, we enrolled ambulatory patients with advanced (Stage III or IV) non-small cell lung cancer (NSCLC) or small cell lung cancer (SCLC) in a non-blinded, randomized, controlled trial of technology-based symptom monitoring with reporting (MR group) to the clinical team compared with symptom monitoring alone (MA group). Both groups monitored their symptoms weekly; they differed in two respects: (1) automated delivery of reports of clinically significant symptoms of the MR group to their clinical team for further assessment and/or management; and (2) availability of paper copies of longitudinal, graphical displays of symptom scores to MR patients and their clinical teams at scheduled clinic visits.
We hypothesized that the mechanism by which symptom burden would be reduced is earlier reporting of clinically significant symptoms to the clinical team. Thus, we originally proposed also to evaluate the time-to-spontaneous reporting of any clinically significant symptom by MA patients to the clinical team, which required that we assess their weekly symptom burden, as we did with MR patients. However, discussions with nursing staff prior to RCT implementation indicated that this would not be feasible because of the demands of and delays in documenting patients’ phone calls, so this endpoint was dropped.
The primary endpoint was symptom burden over 12 weeks, as defined by a measure of symptom distress. We also hypothesized that the MR intervention would result in reduced patient-perceived barriers to symptom management and increased patient self-efficacy, HRQL, and satisfaction with treatment relative to the MA condition, which were conceptualized as secondary endpoints. If SyMon-L was effective in reducing symptom burden, reductions in some types of health care utilization also might be expected, although some types of utilization also may increase. Thus, health care utilization was included as an exploratory endpoint. The clinical interventions undertaken in response to alerts (MR only) and scheduled clinic visits (MR and MA) were documented as an additional exploratory endpoint.
After providing informed consent, participants completed baseline measures and were randomly assigned by computer in a 1:1 ratio to the MR or the MA group. Randomization was blocked, stratified by institution, with a goal of enrolling 100 participants from each of the three sites (total N=300), 150 in each group.
All participants selected a day for their weekly IVR calls. After entering their study PIN, they completed a 13-item symptom survey (Functional Assessment of Cancer Therapy [FACT] – Lung Symptom Index [FLSI] (57) by entering a response from 1 to 5 (“not at all” to “very much”) using the telephone keypad. They received reminder calls on two consecutive days after their selected day if they failed to call. This survey was completed weekly for 12 weeks.
For MR participants, any responses meeting a pre-defined threshold for a symptom “alert” generated an e-mail to the site nurse. Based on results from a previous standard setting exercise (unpublished), we set a conservative threshold of “quite a bit” or “very much” or a two-point worsening from the previous week. A site nurse contacted the participants within one business day to assess the symptom and provide clinical care as warranted. Paper copies of longitudinal graphs of symptom and HRQL scores were provided to physicians and MR participants at scheduled visits, approximately every three weeks, with the intent of facilitating discussion between physicians and patients, but their use was not further prescribed or monitored. All participants completed additional assessments at 3, 6, 9 and 12 weeks post-baseline (see Measures).
Provider Participants
Clinics at the three sites participating in this study reflected differences in organization and staffing, but all physicians who treated lung cancer at each of the sites were enrolled along with one or more designated clinical staff. Provider participants at one academic medical center included the sole thoracic oncologist, one registered nurse (RN) and physician assistant; at the second academic medical center, provider participants included all three thoracic oncologists and one RN. At the county public hospital, five medical oncologists, nine oncology fellows and one RN participated in the study.
During initial face-to-face meetings with participating providers at all sites, the study team provided training on all aspects of the study protocol, including providers’ roles and responsibilities. The training included interpretation of the symptom graphs (e.g., color-coded symptom “alerts”; interpreting high versus low scores) but did not include a prescription for how symptoms should be managed.
Patient Participants
Patient participants were enrolled at the three sites described above. Eligibility criteria included: being at least 18 years old, English-speaking, having advanced NSCLC or SCLC, receiving active treatment with traditional chemotherapy no later than Day 1 of Cycle 2 or receiving oral therapy, having access to a telephone and life expectancy of at least six months.
Measures
Sociodemographic and Clinical Information
Participant sociodemographic and clinical information was collected at baseline. Eastern Cooperative Oncology Group Performance Status Rating (ECOG PSR) (58) was obtained from participants and providers at baseline and each assessment.
Primary Endpoint
Different measures were used for the primary endpoint and the weekly symptom measure to avoid potential training effects. Overall symptom burden was measured by the Symptom Distress Scale (SDS) (59, 60). Higher scores reflect greater distress, with scores ranging from 13 to 65. In addition to established reliability and validity (59, 60), brevity, and symptom congruence with the FLSI, the SDS was selected because its scoring yields a total symptom distress score.
Weekly Symptom Assessment
The FLSI was developed by surveying oncologists about priority advanced lung cancer symptoms and concerns(57) and includes general cancer and pulmonary symptoms, side effect bother, emotional distress, and contentment with HRQL.
Secondary Endpoints
HRQL
The FACT-General (FACT-G) (61) is a 27-item questionnaire measuring Physical Well-Being (PWB), Social/Family Well-Being (SWB), Emotional Well-Being (EWB), and Functional Well-Being (FWB). It is a well-validated and widely used measure of HRQL (61, 62).
Treatment Satisfaction
Satisfaction with treatment was measured with the Functional Assessment of Chronic Illness Therapy-Treatment Satisfaction-Patient Satisfaction (FACIT-TS-PS) (63, 64), with a focus on the Explanations, Interpersonal, Comprehensive Care, and Decision-Making subscales.
Patient-Perceived Barriers to Symptom Management
The Symptom Management Barriers Questionnaire (SMBQ) is a modification of previous barriers questionnaires (11, 15), and assesses patient attitudinal barriers to symptom management.
Self-Efficacy
A 27-item measure was developed by study investigators to optimize its fit with the particular domains of interest in this study (65), specifically, self-efficacy related to patient-physician communication, health behaviors and knowledge about accessing care.
Clinical Activity
Using primarily the medical record supplemented by provider query, the research assistant documented any of 11 clinical management activities (e.g., diagnostic test, referral) by symptom that were undertaken at each scheduled oncologist visit (MR and MA) and in response to alerts (MR only).
Medical Care Utilization
Participants completed a checklist of medical care utilization episodes, including unscheduled clinic visits, ED visits, hospitalizations, and calls to physicians and nurses at each oncologist visit.
Participant and Provider Perceptions of SyMon-L
End-of-study surveys of participants and providers captured perceptions of usability and usefulness of the SyMon-L system.
Data Collection
SDS, ECOG PSR, medical utilization, and clinical activity assessments were completed at weeks 3, 6, 9 and 12 post-baseline; FACT-G, FACIT-TS-PS and self-efficacy assessments were completed at weeks 6 and 12; and SMBQ and end-of study evaluations were completed at week 12. Most data were collected from participants using a touchscreen tablet PC; some data were obtained via interview by the research assistant and entered into the tablet PC.
Participants were provided with nominal monetary compensation for their participation.
Statistical Analysis
Interim Analyses
To ensure that the intervention had not harmed patients, a blinded interim analysis of symptom severity and study burden data was planned after half of the randomized patients (N=150) had reached the Week 12 assessment, and this analysis was reviewed by the institutional cancer center data and safety monitoring board (DSMB).
Main Analyses
The study was powered to detect a difference between the two study groups in SDS total score. For this endpoint, a standardized effect size (mean group difference/common standard deviation) of 0.33 has been suggested to be meaningful in the measurement of PROs in several different cancer populations (61, 66–71).
Secondary endpoints included the FACT-G, FACIT-TS-PS, SMBQ, self-efficacy, and medical utilization counts, assessed at multiple time points. Effect sizes considered meaningful for the secondary PRO endpoints included 0.40 for the FACT-G (72), 0.45 for the FACIT-TS-PS (73, 74), and 0.33–0.45 for the barriers and self-efficacy endpoints (75).
To achieve 80% power to detect these effects, using a two-sided significance level, α=0.05, a sample size of at least 146 per group was required (76, 77). Based on our pilot study with over 100 participants, we had a 0% refusal to participate and less than a 15% rate of withdrawal during the study. Thus, we projected a 17% withdrawal rate in the 12-week RCT and increased the accrual goal to 360 (180 in each group), 120 at each site.
Analyses were based on intention-to-treat in all randomized participants and were not adjusted for multiple comparisons. All analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC).
The primary endpoint was estimated by calculating the area under the curve (AUC) of the total SDS score plotted over time for each participant. Interpolation between non-missing observations was used if intermediate assessments were missing. Study drop-outs were either assigned a value of the worst possible score in the case of death, or worst or last observed score, depending on reason for dropping out. The AUC was then divided by the total time to rescale back to the original units. A general linear model was used to compare study groups on the primary endpoint adjusted for baseline SDS.
For secondary endpoints, t-tests were used to compare PRO scores between groups at baseline and subsequent time points.
Results
Interim Analyses
Interim analyses (N=150) showed no difference between groups in the rate of drop-out, sociodemographic or clinical information, FACT-G, FLSI, or SDS. Questions reflecting participant burden revealed no differences in satisfaction with symptom management, perceived benefit of participation, willingness to use SyMon-L, or frequency of preferred use.
Because the rate of enrollment was slower than anticipated, we conducted futility analyses as part of the planned interim analyses. The conditional power was calculated under the original assumptions and under the observed data (78, 79). The unconditional power to detect a hypothesized effect size of 0.33 as statistically significant, given the sample size (N=150), was 51%, and the conditional power was estimated to be 3.8%. Computing the conditional power under the empirical treatment effect yielded an estimate of 0.3%. That is, if the observed trend in the data was true, we had less than a 1% chance of a statistically significant difference at the end of the trial (78, 79). Enrollment was continued to the extent permitted by funding to a final total sample size of 253.
Participant Enrollment and Baseline Characteristics
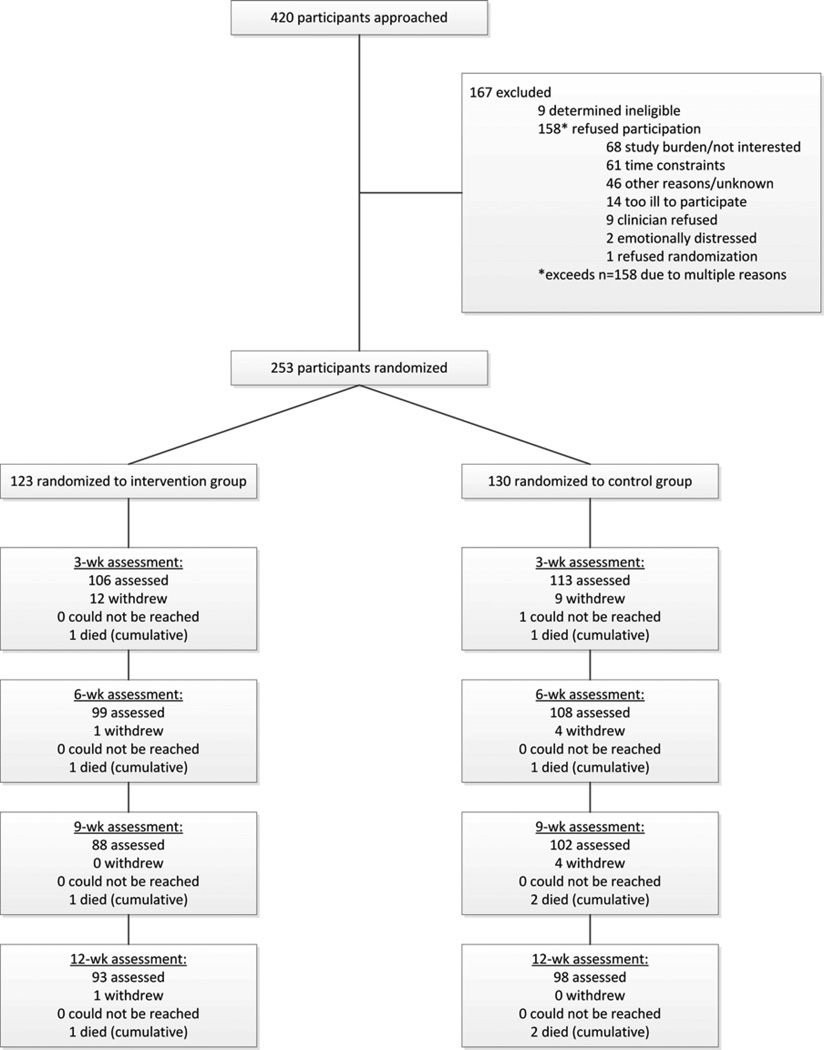
Fig. 1 summarizes the participant flow. Of 411 eligible participants approached, 253 were enrolled and randomized. The overall refusal rate was 40%, ranging from 28% at the public hospital to 44–45% at the other two sites. There were no significant differences in sociodemographic characteristics between those who agreed and those who refused to participate.
Fig. 1.
Flowchart of participants in the SyMon-L trial.
The study groups were equivalent in baseline characteristics (Table 1) and PROs (Table 2). However, participants treated at the public hospital reported worse ECOG PSR (P<0.001) and were more likely to be receiving first-line chemotherapy (P=0.006) than those at the other two sites.
Table 1.
Characteristics of the Study Participants at Baseline (N=253)
|
N (%) |
||||
|---|---|---|---|---|
| Control (n=130) |
Intervention (n=123) |
Total (n=253) |
P- value |
|
| Age, yrs, mean (SD) | 60.2 (10.1) | 61.0 (10.3) | 60.6 (10.2) | 0.488 |
| Gender (male) | 68 (52.3) | 57 (46.3) | 125 (49.4) | 0.343 |
| Race (missing=1) | ||||
| White | 76 (58.5) | 71 (58.2) | 147 (58.3) | 0.307 |
| Black or African American | 50 (38.5) | 41 (33.6) | 91 (36.1) | |
| Other | 4 (3.1) | 10 (8.2) | 14 (5.6) | |
| Ethnicity (Hispanic/Latino; missing=4) | 4 (3.1) | 7 (5.8) | 11 (4.4) | 0.593 |
| Occupational status (missing=2) | ||||
| Homemaker | 7 (5.5) | 13 (10.6) | 20 (8.0) | 0.313 |
| Unemployed | 24 (18.8) | 20 (16.3) | 44 (17.5) | |
| Retired | 45 (35.2) | 38 (30.9) | 83 (33.1) | |
| On disability/leave of absence | 31 (24.2) | 22 (17.9) | 53 (21.2) | |
| Full- or part-time employed | 20 (15.5) | 30 (24.3) | 50 (19.9) | |
| Full-time student only | 1 (0.8) | 0 | 1 (0.3) | |
| Education completed (missing=3) | ||||
| 8th grade or less | 3 (2.4) | 6 (4.8) | 9 (3.6) | 0.721 |
| Some High School | 24 (18.9) | 12 (9.8) | 36 (14.4) | |
| High School Grad/GED | 34 (26.8) | 39 (31.7) | 73 (29.2) | |
| Some college/Technical/AA | 27 (21.3) | 32 (26.0) | 59 (23.6) | |
| College degree (BA/BS) | 22 (17.3) | 19 (15.4) | 41 (16.4) | |
| Advanced degree (MA, PhD, MD) | 17 (13.4) | 15 (12.2) | 32 (12.8) | |
| Health Insurance status (missing=14) | ||||
| Medicare | 42 (27.1) | 44 (28.4) | 86 (27.7) | 0.680 |
| Medicaid | 10 (6.4) | 10 (6.4) | 20 (6.4) | 0.954 |
| Private insurance | 72 (46.5) | 69 (44.5) | 141 (45.5) | 0.871 |
| No insurance | 31 (20.0) | 32 (20.7) | 63 (20.4) | 0.794 |
| Diagnosis (missing=17) | ||||
| NSCLC | 102 (85.7) | 102 (87.2) | 204 (86.4) | 0.742 |
| SCLC | 17 (14.3) | 15 (12.8) | 32 (13.6) | |
| Current stage of illness (missing=11) | ||||
| Stage IIIa | 15 (12.1) | 16 (13.6) | 31 (12.8) | 0.717 |
| Stage IIIb | 25 (20.2) | 28 (23.7) | 53 (21.9) | |
| Stage IV | 70 (56.4) | 64 (54.2) | 134 (55.4) | |
| Small Cell | 14 (11.3) | 10 (8.4) | 24(9.9) | |
| Planned Chemotherapy (missing=5) | ||||
| Yes, single | 15 (11.9) | 8 (6.6) | 23 (9.3) | 0.073 |
| Yes, combination | 111 (88.1) | 112 (91.8) | 223 (89.9) | |
| Yes, both single + combination | 0 | 2 (1.6) | 2 (0.8) | |
| Line of Chemotherapy (missing=5) | ||||
| First | 96 (76.2) | 97 (79.5) | 193 (77.8) | 0.969 |
| Second | 20 (15.9) | 14 (11.5) | 34 (13.7) | |
| Third or more | 10 (7.9) | 11 (9.0) | 21 (8.5) | |
| Clinician-rated ECOG PSR (missing=7) | ||||
| Normal activity, no symptoms | 40 (32.3) | 32 (26.2) | 72 929.3) | 0.635 |
| Some symptoms, no bed rest | 71 (57.3) | 81 (66.4) | 152 (61.8) | |
| Require bed rest for < 50% day | 12 (9.7) | 7 (5.7) | 19 (7.7) | |
| Require bed rest for > 50% day | 1 (0.8) | 2 (1.6) | 3 (1.2) | |
| Patient-rated ECOG PSR (missing=1) | ||||
| Normal activity, no symptoms | 39 (30.2) | 39 (31.7) | 78 (31.0) | 0.794 |
| Some symptoms, no bed rest | 57 (44.2) | 54 (43.9) | 111 (44.0) | |
| Require bed rest < 50% day | 21 (16.3) | 20 (16.3) | 41 (16.3) | |
| Require bed rest > 50% day | 12 (9.3) | 9 (7.3) | 21 (8.3) | |
| Unable to get out of bed | 0 | 1 (0.8) | 1 (0.4) | |
Table 2.
Patient-Reported Outcome Scores at Baseline and Week 12
| Baseline | Week 12 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n=130) |
Intervention (n=123) |
Total (n=253) |
P- value |
Control (n=87–96) |
Intervention (n=77–83) |
Total n=(164–179) |
P-value | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| SDS | 24.8 | 8.6 | 25.3 | 9.2 | 20.6 | 5.3 | 0.662 | 23.3 | 8.0 | 22.0 | 6.5 | 22.7 | 7.3 | 0.235 |
| FACT-G PWB | 21.1 | 5.9 | 20.6 | 6.5 | 22.0 | 6.0 | 0.553 | 20.6 | 5.3 | 20.3 | 6.2 | 20.5 | 5.8 | 0.731 |
| FACT-G SWB | 22.1 | 5.8 | 23.2 | 5.0 | 18.5 | 4.6 | 0.130 | 22.0 | 6.0 | 23.4 | 4.7 | 22.7 | 5.5 | 0.088 |
| FACT-G EWB | 17.6 | 4.3 | 17.7 | 4.7 | 16.0 | 7.1 | 0.783 | 18.5 | 4.6 | 18.3 | 4.8 | 18.4 | 4.7 | 0.804 |
| FACT-G FWB | 16.4 | 6.6 | 16.1 | 6.9 | 77.1 | 18.0 | 0.728 | 16.0 | 7.1 | 15.8 | 7.7 | 15.9 | 7.4 | 0.887 |
| FACT-G Total | 77.2 | 16.9 | 77.6 | 17.6 | 77.4 | 17.2 | 0.838 | 77.1 | 18.0 | 77.9 | 19.8 | 77.5 | 18.8 | 0.787 |
| FLSI | 36.2 | 9.2 | 35.1 | 9.3 | 30.3 | 8.1 | 0.368 | 36.1 | 9.0 | 38.4 | 8.2 | 37.2 | 8.7 | 0.084 |
| Self-Efficacy Communication | 30.6 | 6.6 | 29.0 | 8.1 | 40.6 | 9.0 | 0.109 | 30.3 | 8.1 | 29.0 | 7.9 | 29.7 | 8.0 | 0.270 |
| Self-Efficacy Health Behaviors | 40.6 | 7.1 | 40.4 | 7.2 | 19.6 | 4,9 | 0.841 | 40.6 | 9.0 | 39.3 | 8.0 | 40.0 | 8.6 | 0.322 |
| Self-Efficacy Knowledge | 20.3 | 3.4 | 20.0 | 4.0 | 90.5 | 19.9 | 0.642 | 19.6 | 4.9 | 19.7 | 4.5 | 19.7 | 4.7 | 0.840 |
| Self-Efficacy Total | 91.4 | 14.7 | 89.7 | 16.8 | 52.7 | 16.9 | 0.389 | 90.5 | 19.9 | 88.2 | 18.9 | 89.4 | 19.4 | 0.448 |
| SMBQ | 53.4 | 17.8 | 53.4 | 14.9 | 53.4 | 16.4 | 0.976 | 52.7 | 16.9 | 56.7 | 14.4 | 54.5 | 15.8 | 0.094 |
| FACIT-TS-PS Explanationsa | - | - | - | - | - | - | - | 2.70 | 0.53 | 2.60 | 0.57 | 2.65 | 0.55 | 0.230 |
| FACIT-TS-PS Interpersonala | - | - | - | - | - | - | - | 2.79 | 0.46 | 2.70 | 0.58 | 2.75 | 0.52 | 0.266 |
| FACIT-TS-PS Comprehensive Carea | - | - | - | - | - | - | - | 2.48 | 0.67 | 2.20 | 0.78 | 2.34 | 0.74 | 0.012 |
| FACIT-TS-PS Decision Makinga | - | - | - | - | - | - | - | 2.52 | 0.61 | 2.27 | 0.86 | 2.41 | 0.75 | 0.027 |
SDS = Symptom Distress Scale; FACT-G = Functional Assessment of Cancer Therapy-General; FLSI = FACT Lung Symptom Index; SMBQ = Symptom Management Barriers Questionnaire; FACIT-TS-PS = Functional Assessment of Chronic Illness Therapy-Treatment Satisfaction-Patient Satisfaction.
Only administered at Week 12.
Over the course of the study, 35 participants withdrew (13.8%; 20 MA, 15 MR, P=0.463). Compliance with completion of weekly symptom monitoring phone calls was 82.1% (83.4% MA, 80.8% MR).
Primary Endpoint
There was no difference between groups in mean SDS AUC, adjusted for baseline (MA mean=25.5, SD=8.3; MR mean=25.3, SD=8.5; P=0.505).
Secondary Endpoints
There were no differences between groups in FACT-G subscales or total score at any time point, although in the combined sample (MR and MA), PWB, FWB and FACT-G total scores declined significantly over the 12 weeks (P<0.008, P<0.006, P<0.025, respectively). There was no difference between groups on the total FACIT-TS-PS score at any time point, but the MR group had lower scores (i.e., lower satisfaction) than the MA group on the Comprehensive Care (perceptions of having concerns understood; P=0.012) and Decision Making (receiving adequate information, time and support; P=0.027) subscales at week 12. There were no differences over time or between groups in self-efficacy or perceived barriers.
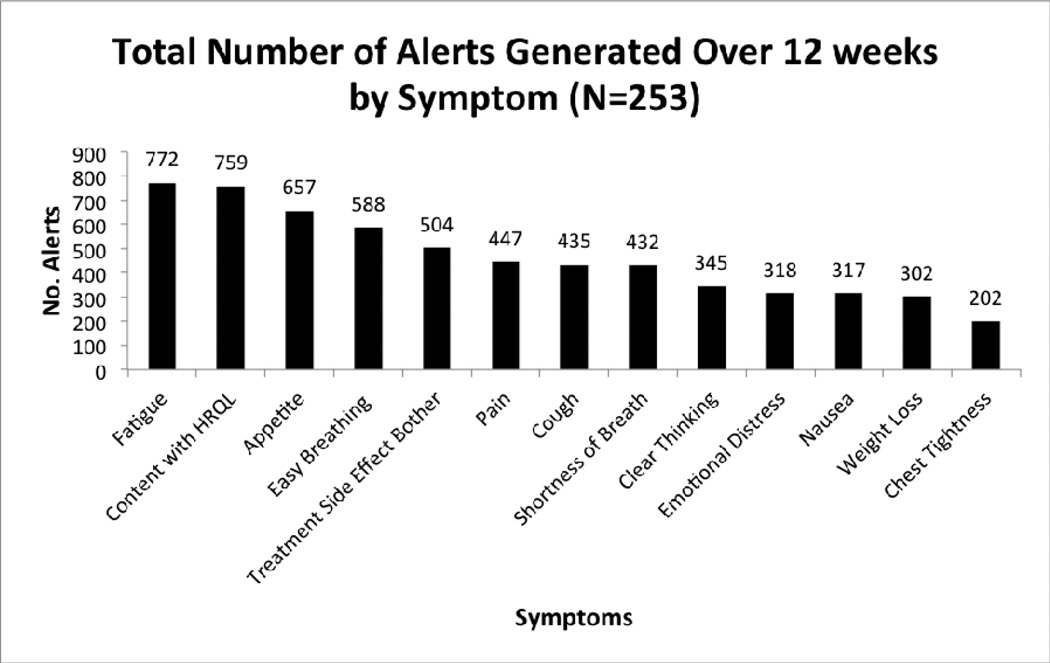
The symptoms reported most frequently at an alert level for the combined group were fatigue, poor appetite, difficulty breathing, and treatment side effect bother, as displayed in Fig. 2. There were 6078 symptom responses meeting the alert threshold during the study.
Fig. 2.
Total number of alerts by symptom over 12 weeks (n=253).
Exploratory Endpoints
Medical Utilization
There were no significant differences between groups in any of the medical utilization variables except that MR participants reported making more phone calls to nurses than MA participants (P=0.022), as displayed in Table 3.
Table 3.
Patient-Reported Medical Utilization Over the 12-Week Study Period
| Medical Utilization |
Monitoring Alone | Monitoring & Reporting | P-value | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Number of hospital admissions | ||||||
| 0 | 80 | 67.8 | 75 | 67.0 | ||
| 1 | 22 | 18.6 | 19 | 17.0 | ||
| 2 | 8 | 6.8 | 14 | 12.5 | ||
| 3 | 2 | 1.7 | 0 | .0 | ||
| 4 | 2 | 1.7 | 1 | .9 | ||
| 5–9 | 4 | 3.4 | 3 | 2.6 | ||
| Mean (SD) | .67 (1.43) | .63 (1.27) | 0.877 | |||
| Number of ER visits | ||||||
| 0 | 81 | 68.6 | 77 | 68.8 | ||
| 1 | 20 | 16.9 | 16 | 14.3 | ||
| 2 | 11 | 9.3 | 8 | 7.1 | ||
| 3 | 3 | 2.6 | 7 | 6.3 | ||
| 4–9 | 3 | 2.6 | 4 | 3.5 | ||
| Mean (SD) | .58 (1.16) | .69 (1.40) | 0.848 | |||
| Number of unscheduled clinic visits | ||||||
| 0 | 100 | 84.7 | 86 | 76.8 | ||
| 1 | 11 | 9.3 | 16 | 14.3 | ||
| 2 | 3 | 2.6 | 5 | 4.5 | ||
| 3–6 | 4 | 3.4 | 5 | 4.4 | ||
| Mean (SD) | .25 (.71) | .41 (.97) | 0.129 | |||
| Number of phone calls to physician | ||||||
| 0 | 80 | 67.8 | 66 | 58.9 | ||
| 1 | 15 | 12.7 | 27 | 24.1 | ||
| 2 | 10 | 8.5 | 7 | 6.3 | ||
| 3 | 4 | 3.4 | 3 | 2.7 | ||
| 4 | 3 | 2.5 | 4 | 3.6 | ||
| 5 | 2 | 1.7 | 3 | 2.7 | ||
| 6–8 | 4 | 3.4 | 2 | 1.7 | ||
| Mean (SD) | .81 (1.57) | .85 (1.47) | 0.323 | |||
| Number of phone calls to nurse | ||||||
| 0 | 65 | 55.1 | 46 | 41.1 | ||
| 1 | 20 | 16.9 | 25 | 22.3 | ||
| 2 | 15 | 12.7 | 10 | 8.9 | ||
| 3 | 8 | 6.9 | 10 | 8.9 | ||
| 4–6 | 6 | 5.0 | 18 | 16.2 | ||
| 7–15 | 4 | 3.4 | 3 | 2.6 | ||
| Mean (SD) | 1.14 (1.85) | 1.79 (2.52) | 0.022 | |||
Clinical Activity
Clinical interventions were documented for MA participants for scheduled clinic visits only, whereas for MR participants, interventions were documented in response to system-generated alerts and scheduled clinic visits. In addition, some MR alerts were generated the day before or day of a clinic visit and addressed at the time of the clinic visit, so those interventions were documented as “both.” Table 4 displays the counts of interventions provided for the two groups in response to visits and/or alerts. MR group participants received a total of 1323 interventions in response to alerts (Table 5). Over the course of the 12-week study, a given MR participant may have received an intervention for dyspnea or emotional distress up to 15 times; similarly, a given MR participant may have received an education/counseling or additional follow-up visit up to 32 or 48 times, respectively.
Table 4.
Number of Clinical Interventions Provided to Monitoring Alone (MA) and Monitoring and Reporting (MR) Participants
| Number of Clinical Interventions | ||||
|---|---|---|---|---|
| MA Participants | MR Participants | Total | ||
| Alert | N/A | 1323 | 1323 | |
| Scheduled Visit | 1316 | 728 | 2044 | |
| Botha | N/A | 880 | 880 | |
| Total | 1316 | 2931 | 4247 | |
Alerts occurring in close proximity to scheduled visits and managed at time of visit.
Table 5.
Number of Interventions for Monitoring and Reporting (MR) Participants in Response to Alerts (N=108)
| Number | Mediana | Minimuma | Maximuma | ||
|---|---|---|---|---|---|
| By symptom: | |||||
| Pain Interventions | 183 | 0 | 0 | 11 | |
| Fatigue Interventions | 180 | 1 | 0 | 9 | |
| Coughing Interventions | 138 | 0 | 0 | 11 | |
| Dyspnea Interventions | 167 | 0 | 0 | 15 | |
| Appetite Interventions | 145 | 0 | 0 | 12 | |
| Nausea / Vomiting Interventions | 104 | 0 | 0 | 6 | |
| Bowel Changes Interventions | 17 | 0 | 0 | 5 | |
| Weight Loss Interventions | 58 | 0 | 0 | 11 | |
| Cognitive Interventions | 53 | 0 | 0 | 8 | |
| Neurologic Interventions | 10 | 0 | 0 | 3 | |
| Side-Effect Bother Interventions | 161 | 1 | 0 | 9 | |
| Emotional Distress Interventions | 97 | 0 | 0 | 15 | |
| Disease Status Change Interventions | 10 | 0 | 0 | 3 | |
| Total | 1323 | ||||
| By type of intervention: | |||||
| New symptom supportive prescription | 70 | 0 | 0 | 4 | |
| Changed dose/schedule of symptom supportive prescription | 50 | 0 | 0 | 8 | |
| New anti-cancer/chemo treatment | 0 | 0 | 0 | 0 | |
| Changed anti-cancer/chemo dose or schedule | 6 | 0 | 0 | 2 | |
| Diagnostic test ordered | 21 | 0 | 0 | 2 | |
| Procedure ordered | 12 | 0 | 0 | 3 | |
| Referral | 40 | 0 | 0 | 15 | |
| Education/counseling | 746 | 4.5 | 0 | 48 | |
| Additional follow-up visit | 345 | 1 | 0 | 32 | |
| Emergency Department referral | 10 | 0 | 0 | 7 | |
| Hospital admission | 23 | 0 | 0 | 7 | |
| Total | 1323 | ||||
Calculated per patient.
Participant and Provider Study Evaluations
Ninety-two percent of the full participant sample reported no problems using the telephone for weekly surveys, and 86% indicated it was (“very much”/“quite a bit”) convenient. SyMon-L improved communication with their doctor (46%), helped focus on important issues (49%), and revealed additional issues that may not have been discussed otherwise (38%); 93% indicated they would use the system if it was part of their care.
Because of missing data from providers because of schedule constraints, individual semistructured interviews were subsequently conducted with providers, which will be reported separately.
Exploratory Analyses
In response to the null findings, we sought to maximize our understanding of the data and conducted additional, unplanned exploratory analyses.
Given the challenge of achieving reduction in overall symptom burden in the presence of multiple simultaneous symptoms in advanced disease, we used AUC analyses of individual SDS items for which there are efficacious treatments: nausea, pain, breathing/cough. There were no differences in AUCs (adjusted for baseline) for these symptoms, with effect sizes less than one-tenth of a standard deviation (P=0.595, P=0.927, P=0.934, respectively).
Whereas the SDS was completed approximately every three weeks, the FLSI was administered weekly. Because symptom burden may have changed more frequently than could have been detected by the SDS, and imputations for missing SDS data might have resulted in an underestimation of symptom burden, we examined symptom burden using the FLSI total score and the FLSI Pulmonary Symptom Index (PSI: sum of four pulmonary items). These AUC analyses indicated no difference in symptom burden between the groups for the total score (P=0.246) or PSI (P=0.563), again with effect sizes of one-tenth of a standard deviation or less.
To examine potential responders and nonresponders to symptom management, change scores from baseline were calculated for the 12 FLSI time points and classified as improved, worsened or unchanged based on the four-point minimally important difference (80). The proportion of participants in each change group was compared between groups at each week using Mantel-Haenszel Chi-square tests for ordinal data. There were no differences between groups in any of the classification groups (P range=0.117 to 0.862).
Discussion
Our RCT of an active monitoring and reporting intervention failed to demonstrate efficacy in reducing symptom burden when compared with a more passive monitoring intervention in patients with advanced lung cancer. The hypotheses of benefits to HRQL, treatment satisfaction, self-efficacy and perceived symptom management burden also were not supported. However, the trial demonstrated that the SyMon-L intervention is feasible and well accepted by patients.
Although the two groups did not differ in terms of their total FACIT-TS-PS scores, the MR group reported lower satisfaction with two elements of treatment (e.g., having concerns understood, receiving decision making support) than MA participants. An unexpected finding, one possible explanation of this is that the MR intervention may have elevated expectations about their symptom care beyond what patients experienced.
There was no difference between the groups in number of hospitalizations, ED visits, unscheduled clinic visits or calls to physicians, but the MR group reported more calls to nurses. Based on anecdotal reports from research assistants, we suspect that, contrary to instructions, participants may have recollected calls from nurses in response to MR alerts.
One of the exploratory endpoints in this pragmatic RCT was the symptom-focused clinical interventions provided to MA and MR participants. This proved to be complex, both in terms of implementing the data collection and in its analysis and interpretation. We were not able to document interventions for MA participants in response to any interactions beyond scheduled clinic visits (e.g., patient-initiated calls), so our count of interventions provided to these participants is likely an underestimate. Furthermore, it is not possible to disentangle MR interventions categorized as “both” (i.e., alert or visit), which limits our interpretation of this data. As a result, we are not able to ascertain if one group received more clinical interventions than the other. It does appear that the information provided by MR participants’ alerts was acted upon, based on the 1323 interventions (plus some unknown percent from “both”) provided. There is some degree of overlap between the symptom-based interventions provided in response to alerts (e.g., dyspnea, emotional distress, appetite, pain, coughing) and the symptoms generating the most alerts (fatigue, appetite, breathing easily, treatment side effect bother), but the overlap is not perfect. These observations, along with considerably higher numbers of specific types of interventions (e.g., education/counseling, additional follow-up visits), suggest potential areas of focus for future research. Thus, although this endpoint is limited in aiding interpretation of current findings, it may inform hypotheses for future research
RCTs of technology-based PRO monitoring in oncology have demonstrated acceptability to patients (38, 81, 82); improved well-being (38); and reduced post-operative symptom severity (32). Our weekly call compliance rate of 82% and the findings of others (33) suggest that patients with advanced cancers are capable of using such symptom monitoring systems, contrary to the concerns of some (34, 38, 83). However, the majority of RCTs and other studies provide limited evidence of efficacy in achieving improvements in symptom or health status (36, 84). Another RCT of lung cancer patients, randomized to standard care or paper HRQL diaries completed over 16 weeks with encouragement to share information with providers, showed small but non-significant negative effects on HRQL for patients in the diary group and no effect on satisfaction with care or communication (85).
A recent editorial commenting on the null findings of an RCT of telemonitoring in older adults at high risk for hospitalization (86), cautioned against discounting the study’s findings, as the effectiveness of “telehealth” programs may be context- and outcome-dependent, mediated by factors specific to the intervention implementation and its evaluation metrics (87). It is in the spirit of attempting to understand null findings from the perspective of context and implementation that we offer potential explanations for our study as a guide for future research.
We failed to meet our accrual goal of enrolling 300 participants. Although there were early delays in technology development, we believe the primary reason was a much higher than anticipated refusal rate for the RCT (28–45%) compared with our pilot study (0%). Other symptom/HRQL monitoring RCTs also have found high rates of refusal and attrition (85). We anticipated distinct differences in patient populations between the pilot and RCT sites but clearly underestimated the magnitude of potential refusals, especially at the two medical center sites. One possibility is that a study requiring active patient participation over a prolonged period of time (12 weeks) represents an overwhelming burden for some who are struggling to cope with advanced disease. We have anecdotal reports that clinic wait-times at the public hospital are considerably longer than at the academic medical center sites, possibly accounting for the lower refusal rate at that site. No other explanation for the discrepancy is immediately apparent, but requirements for successfully implementing such studies, such as an expanded data collection period, warrant further evaluation and consideration in future studies.
Our intervention failed to impact symptom burden over the 12-week study period. Sensitivity and frequency of administration of the outcome measure are two possible explanations. However, evaluation of the endpoint using a different, weekly symptom measure also found no differences. Some have suggested that patients with advanced lung cancer experience symptom variability warranting daily assessment (88), but we have no data to support this as an explanation for our findings. It may be unrealistic to achieve reduction in symptom burden in advanced lung cancer, where some disease or treatment symptoms are controlled as others emerge with disease progression (89). Whereas some symptoms lack effective treatment (e.g., fatigue), we also did not find evidence of reduced burden when we examined symptoms for which there is efficacious treatment. Finally, the threshold for generating an alert was standardized across symptoms, whereas some have suggested that each symptom threshold should be set and validated independently (88). As designed, the system generated a significant number of alerts (>1323) for MR participants, which could have resulted in alert “fatigue,” distraction or being overwhelmed with information without prescriptive guidelines. Further research that obtains clinicians’ input on preferences for clinically actionable symptom alert thresholds may inform the design of future symptom monitoring studies. This also may argue for simple, straightforward, targeted interventions augmented with practice-changing recommendations (37, 38, 90, 91).
For weekly symptom data collection, we used telephone-based technology because of widespread accessibility (92). An auditory-based assessment also offers advantages for those with limited reading skills. Nevertheless, measurement error could have been introduced through patients’ mishearing or misremembering response choices or misentering responses. It also is possible that some patients were not comfortable communicating about their symptoms using technology, as opposed to a live conversation.
The review of interim analyses suggested no harm to study participants, but we cannot exclude this possibility, given that the MA group reported more favorable treatment satisfaction. We did not adjust for multiple comparisons, so there is no way to know if these results are artifacts of multiple comparisons or real differences, and they should be interpreted cautiously until replicated in other studies.
Whereas a strength of this study was randomization to intervention groups, a limitation was the lack of a placebo control group, which has been criticized as a shortcoming of most studies in HRQL/symptom management (89). This trial was originally designed to allow us to test whether using technology to initiate and accelerate the reporting of problematic symptoms could reduce symptom burden. Unfortunately, we were not able to capture data on the time of first report by MA participants of any clinically significant symptom to the team. Our experience from a previous RCT of mixed cancer patients (including lung), demonstrated that assessment alone had no impact on HRQL or treatment satisfaction (91). Other similar findings (37, 38) have suggested that information obtained by assessment without direct, immediate feedback to clinicians may not be acted upon by patients or utilized by the medical team, although some studies of computer-based monitoring in oncology have demonstrated symptom benefit in the absence of providing feedback to providers (38, 84, 93, 94). In the current study, even though we augmented passive monitoring with more active feedback to clinicians, one strong possibility is that the MA group experienced an enhancement of symptom awareness that may have increased the likelihood they would report those symptoms to the clinical team, thereby reducing the magnitude of a detectable benefit associated with using the full SyMon-L intervention. Our findings suggest that further research is required to determine if an even more active version of the intervention is required to effect changes in clinical endpoints, such as symptom management guidelines for providers and/or patients, as others have suggested is important for effective use of PRO data (37, 38, 90, 91).
In addition to the limitations discussed above, we randomized at the patient level, rather than physician or site, because of the scope and logistical constraints, including the small number of physician participants. We recognize that exposure to the intervention may have affected physicians’ discussion/management with patients in the MA group, although they did not have access to the symptom reports or study data and previous research has documented the difficulty in changing physician behavior (7, 95–97). A cross-over design was not deemed practical because, with advanced disease, it seemed likely that outcomes in the first period would differentially affect outcomes in the second period.
Especially as cancer treatments are increasingly delivered in outpatient and primary care settings, it is important to continue to develop and evaluate efficient and acceptable ways of monitoring patients’ symptoms and well-being while they are away from the clinic. Some have suggested that standard clinical cancer care should now include electronic patient interfaces that allow symptom reporting (98). Our data and experience support the conclusion that a technology-based symptom monitoring and reporting system can be easy to use and well-received by patients. However, this field is profoundly in need of research focused on identifying which patients are most likely to benefit from such a system, and the other patient, provider and health system contextual factors that are likely to contribute to successful adoption, use and improvements in clinical care (98).
Acknowledgments
This research was funded by the National Institutes of Health, National Cancer Institute (R01-CA115361). The funder had no role in study design or in the collection, analysis, interpretation or the development of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no conflicts of interest.
References
- 1.Cleeland CS. Cancer-related symptoms. Semin Radiat Oncol. 2000;10:175–190. doi: 10.1053/srao.2000.6590. [DOI] [PubMed] [Google Scholar]
- 2.Borden EC, Parkinson DA. perspective on the clinical effectiveness and tolerance of interferon-alpha. Semin Oncol. 1998;25(1) Suppl 1:3–8. [PubMed] [Google Scholar]
- 3.Meyers CA, Seabrooke LF, Albitar M, Estey EH. Association of cancer-related symptoms with physiological parameters. J Pain Symptom Manage. 2002;24:359–361. doi: 10.1016/s0885-3924(02)00501-8. [DOI] [PubMed] [Google Scholar]
- 4.Whelan TJ, Mohide EA, Willan AR, et al. The supportive care needs of newly diagnosed cancer patients attending a regional cancer center. Cancer. 1997;80:1518–1524. doi: 10.1002/(sici)1097-0142(19971015)80:8<1518::aid-cncr21>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Mooney KH, Beck SL, Friedman RH, Farzanfar R. Telephone-linked care for cancer symptom monitoring: a pilot study. Cancer Pract. 2002;10:147–154. doi: 10.1046/j.1523-5394.2002.103006.x. [DOI] [PubMed] [Google Scholar]
- 6.Naughton M, Homsi J. Symptom assessment in cancer patients. Curr Oncol Rep. 2002;4:256–263. doi: 10.1007/s11912-002-0024-0. [DOI] [PubMed] [Google Scholar]
- 7.VonRoenn J, Cleeland CS, Gonin R, Hatfield AK, Pandya KJ. Physician attitudes practice in cancer pain management. A survey from the Eastern Cooperative Oncology Group. Ann Intern Med. 1993;119:121–126. doi: 10.7326/0003-4819-119-2-199307150-00005. [DOI] [PubMed] [Google Scholar]
- 8.Cleeland CS, Janjan NA, Scott CB, Seiferheld WF, Curran WJ. Cancer pain management by radiotherapists: a survey of radiation therapy oncology group physicians. Int J Radiat Oncol Biol Phys. 2000;47:203–208. doi: 10.1016/s0360-3016(99)00276-x. [DOI] [PubMed] [Google Scholar]
- 9.Dalton JA, Blau W, Carlson J, et al. Changing the relationship among nurses' knowledge, self-reported behavior, and documented behavior in pain management: does education make a difference? J Pain Symptom Manage. 1996;12:308–319. doi: 10.1016/s0885-3924(96)00183-2. [DOI] [PubMed] [Google Scholar]
- 10.Schuit KW, Sleijfer DT, Meijler WJ, et al. Symptoms and functional status of patients with disseminated cancer visiting outpatient departments. J Pain Symptom Manage. 1998;16:290–297. doi: 10.1016/s0885-3924(98)00091-8. [DOI] [PubMed] [Google Scholar]
- 11.Ward SE, Goldberg N, Miller-McCauley V, et al. Patient-related barriers to management of cancer pain. Pain. 1993;52:319–324. doi: 10.1016/0304-3959(93)90165-L. [DOI] [PubMed] [Google Scholar]
- 12.Wilkie DJ, Huang HY, Berry DL, et al. Cancer symptom control: feasibility of a tailored, interactive computerized program for patients. Fam Community Health. 2001;24:48–62. [PubMed] [Google Scholar]
- 13.Beckman HB, Frankel RM. The effect of physician behavior on the collection of data. Ann Intern Med. 1984;101:692–696. doi: 10.7326/0003-4819-101-5-692. [DOI] [PubMed] [Google Scholar]
- 14.Fallowfield L, Ratcliffe D, Jenkins V, Saul J. Psychiatric morbidity and its recognition by doctors in patients with cancer. Br J Cancer. 2001;84:1011–1015. doi: 10.1054/bjoc.2001.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passik SD, Kirsh KL, Donaghy K, et al. Patient-related barriers to fatigue communication: Initial validation of the fatigue management barriers questionnaire. J Pain Symptom Manage. 2002;24:481–493. doi: 10.1016/s0885-3924(02)00518-3. [DOI] [PubMed] [Google Scholar]
- 16.Roter D, Hall J. Physicians’ interviewing styles and medical information obtained from patients. J Gen Intern Med. 1987;2:325–329. doi: 10.1007/BF02596168. [DOI] [PubMed] [Google Scholar]
- 17.Newell S, Sanson-Fisher RW, Girgis A, Bonaventura A. How well do medical oncologists' perceptions reflect their patients' reported physical and psychosocial problems? Data from a survey of five oncologists. Cancer. 1998;83:1640–1651. [PubMed] [Google Scholar]
- 18.Stromgren AS, Groenvold M, Sorensen A, Andersen L. Symptom recognition in advanced cancer. A comparison of nursing records against patient self-rating. Acta Anaesthesiol Scand. 2001;45:1080–1085. doi: 10.1034/j.1399-6576.2001.450905.x. [DOI] [PubMed] [Google Scholar]
- 19.Nekolaichuk CL, Maguire TO, Suarez-Almazor M, Rogers WT, Bruera E. Assessing the reliability of patient, nurse, and family caregiver symptom ratings in hospitalized advanced cancer patients. J Clin Oncol. 1999;17:3621–3630. doi: 10.1200/JCO.1999.17.11.3621. [DOI] [PubMed] [Google Scholar]
- 20.Stephens RJ, Hopwood P, Girling DJ, Machin D. Randomized trials with quality of life endpoints: are doctors' ratings of patients' physical symptoms interchangeable with patients' self- ratings? Qual Life Res. 1997;6:225–236. doi: 10.1023/a:1026458604826. [DOI] [PubMed] [Google Scholar]
- 21.Thomason TE, McCune JS, Bernard SA, et al. Cancer pain survey: patient-centered issues in control. J Pain Symptom Manage. 1998;15:275–284. doi: 10.1016/s0885-3924(98)00016-5. [DOI] [PubMed] [Google Scholar]
- 22.Brody EM, Kleban MH. Physical and mental health symptoms of older people: who do they tell? J Am Geriatr Soc. 1981;29:442–449. doi: 10.1111/j.1532-5415.1981.tb01746.x. [DOI] [PubMed] [Google Scholar]
- 23.Broderick JE, Schwartz JE, Vikingstad G, et al. The accuracy of pain and fatigue items across different reporting periods. Pain. 2008;139:146–157. doi: 10.1016/j.pain.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarna L. Effectiveness of structured nursing assessment of symptom distress in advanced lung cancer. Oncol Nurs Forum. 1998;25:1041–1048. [PubMed] [Google Scholar]
- 25.Trowbridge R, Dugan W, Jay SJ, et al. Determining the effectiveness of a clinical-practice intervention in improving the control of pain in outpatients with cancer. Acad Med. 1997;72:798–800. doi: 10.1097/00001888-199709000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Au E, Loprinzi CL, Dhodapkar M, et al. Regular use of a verbal pain scale improves the understanding of oncology inpatient pain intensity. J Clin Oncol. 1994;12:2751–2755. doi: 10.1200/JCO.1994.12.12.2751. [DOI] [PubMed] [Google Scholar]
- 27.DuPen PS, DuPen PA, Polissar N, et al. Implementing guidelines for cancer pain management: results of a randomized controlled clinical trial. J Clin Oncol. 1999;17:361–370. doi: 10.1200/JCO.1999.17.1.361. [DOI] [PubMed] [Google Scholar]
- 28.Soni MK, Cella D. Quality of life and symptom measures in oncology: an overview. Am J Manag Care. 2002;8(18 Suppl):S560–S573. [PubMed] [Google Scholar]
- 29.Cooley ME. Symptoms in adults with lung cancer. A systematic research review. J Pain Symptom Manage. 2000;19:137–153. doi: 10.1016/s0885-3924(99)00150-5. [DOI] [PubMed] [Google Scholar]
- 30.Wright EP, Selby PJ, Crawford M, et al. Feasibility and compliance of automated measurement of quality of life in oncology practice. J Clin Oncol. 2003;21:374–382. doi: 10.1200/JCO.2003.11.044. [DOI] [PubMed] [Google Scholar]
- 31.Kleiboer A, Gowing K, Holm Hansen C, et al. Monitoring symptoms at home: what methods would cancer patients be comfortable using? Qual Life Res. 2010;19:965–968. doi: 10.1007/s11136-010-9662-0. [DOI] [PubMed] [Google Scholar]
- 32.Cleeland CS, Wang XS, Shi Q, et al. Automated symptom alerts reduce postoperative symptom severity after cancer surgery: a randomized controlled clinical trial. J Clin Oncol. 2011;29:994–1000. doi: 10.1200/JCO.2010.29.8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hachizuka M, Yoshiuchi K, Yamamoto Y, et al. Development of a personal digital assistant (pda) system to collect symptom information from home hospice patients. J Palliat Med. 2010;13:647–651. doi: 10.1089/jpm.2009.0350. [DOI] [PubMed] [Google Scholar]
- 34.Basch E, Artz D, Iasonos A, et al. Evaluation of an online platform for cancer patient self-reporting of chemotherapy toxicities. J Am Med Inform Assoc. 2007;14:264–268. doi: 10.1197/jamia.M2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basch E, Iasonos A, Barz A, et al. Long-term toxicity monitoring via electronic patient-reported outcomes in patients receiving chemotherapy. J Clin Oncol. 2007;25:5374–5380. doi: 10.1200/JCO.2007.11.2243. [DOI] [PubMed] [Google Scholar]
- 36.Kearney N, McCann L, Norrie J, et al. Evaluation of a mobile phone-based, advanced symptom management system (ASyMS) in the management of chemotherapy-related toxicity. Support Care Cancer. 2009;17:437–444. doi: 10.1007/s00520-008-0515-0. [DOI] [PubMed] [Google Scholar]
- 37.Detmar SB, Muller MJ, Schornagel JH, Wever LD, Aaronson NK. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA. 2002;288:3027–3034. doi: 10.1001/jama.288.23.3027. [DOI] [PubMed] [Google Scholar]
- 38.Velikova G, Booth L, Smith AB, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol. 2004;22:714–724. doi: 10.1200/JCO.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 39.McCann L, Maguire R, Miller M, Kearney N. Patients' perceptions and experiences of using a mobile phone-based advanced symptom management system (ASyMS©) to monitor and manage chemotherapy related toxicity. Eur J Cancer Care. 2009;18:156–164. doi: 10.1111/j.1365-2354.2008.00938.x. [DOI] [PubMed] [Google Scholar]
- 40.Dy SM, Roy J, Ott GE, et al. Tell Us™: A web-based tool for improving communication among patients, families, and providers in hospice and palliative care through systematic data specification, collection, and use. J Pain Symptom Manage. 2011;42:526–534. doi: 10.1016/j.jpainsymman.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibson F, Aldiss S, Taylor RM, Maguire R, Kearney N. Involving health professionals in the development of an advanced symptom management system for young people: the ASyMS©-YG study. Eur J Oncol Nurs. 2009;13:187–192. doi: 10.1016/j.ejon.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Maguire R, McCann L, Miller M, Kearney N. Nurse's perceptions and experiences of using of a mobile-phone-based Advanced Symptom Management System (ASyMS©) to monitor and manage chemotherapy-related toxicity. Eur J Oncol Nurs. 2008;12:380–386. doi: 10.1016/j.ejon.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 43.McCall K, Keen J, Farrer K, et al. Perceptions of the use of a remote monitoring system in patients receiving palliative care at home. Int J Palliat Nurs. 2008;14:426–431. doi: 10.12968/ijpn.2008.14.9.31121. [DOI] [PubMed] [Google Scholar]
- 44.Snyder CF, Jensen R, Courtin SO, Wu AW. PatientViewpoint: a website for patient-reported outcomes assessment. Qual Life Res. 2009;18:793–800. doi: 10.1007/s11136-009-9497-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennett AV, Jensen RE, Basch E. Electronic patient-reported outcome systems in oncology clinical practice. CA Cancer J Clin. 2012;62:336–347. doi: 10.3322/caac.21150. [DOI] [PubMed] [Google Scholar]
- 46.Davis K, Yount S, Del Ciello K, et al. An innovative symptom monitoring tool for people with advanced lung cancer: a pilot demonstration. J Support Oncol. 2007;5:381–387. [PubMed] [Google Scholar]
- 47.Kornblith AB, Dowell JM, Herndon JE, et al. Telephone monitoring of distress in patients aged 65 years or older with advanced stage cancer. Cancer. 2006;107:2706–2714. doi: 10.1002/cncr.22296. [DOI] [PubMed] [Google Scholar]
- 48.Lee H, Friedman ME, Cukor P, Ahern D. Interactive voice response system (IVRS) in health care services. Nurs Outlook. 2003;51:277–283. doi: 10.1016/s0029-6554(03)00161-1. [DOI] [PubMed] [Google Scholar]
- 49.Mahoney D, Tennstedt S, Friedman R, Heeren T. An automated telephone system for monitoring the functional status of community-residing elders. Gerontologist. 1999;39:229–234. doi: 10.1093/geront/39.2.229. [DOI] [PubMed] [Google Scholar]
- 50.Piette JD. Interactive voice response systems in the diagnosis and management of chronic disease. Am J Manag Care. 2000;6:817–827. [PubMed] [Google Scholar]
- 51.Naylor MR, Keefe FJ, Brigidi B, Naud S, Helzer JE. Therapeutic interactive voice response for chronic pain reduction and relapse prevention. Pain. 2008;134:335–345. doi: 10.1016/j.pain.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baer L, Jacobs DG, Cukor P, et al. Automated telephone screening survey for depression. JAMA. 1995;273:1943–1944. [PubMed] [Google Scholar]
- 53.Mundt JCP, Moore HKP, Greist JHMD. A novel interactive voice response (IVR) system for dementia screening, education, and referral: one-year summary. Alzheimer Dis Assoc Disord. 2005;19:143–147. doi: 10.1097/01.wad.0000174992.68332.0d. [DOI] [PubMed] [Google Scholar]
- 54.Moore HK, Hughes CW, Mundt JC, et al. A pilot study of an electronic, adolescent version of the quick inventory of depressive symptomatology. J Clin Psychiatry. 2007;68:1436–1440. doi: 10.4088/jcp.v68n0917. [DOI] [PubMed] [Google Scholar]
- 55.Friedman RH, Kazis LE, Jette A, et al. A telecommunications system for monitoring and counseling patients with hypertension: impact on medication adherence and blood pressure control. Am J Hypertens. 1996;9:285–292. doi: 10.1016/0895-7061(95)00353-3. [DOI] [PubMed] [Google Scholar]
- 56.Friedman RH. Automated telephone conversations to assess health behavior and deliver behavioral interventions. J Med Syst. 1998;22:95–102. doi: 10.1023/a:1022695119046. [DOI] [PubMed] [Google Scholar]
- 57.Cella D, Paul D, Yount S, et al. What are the most important symptom targets when treating advanced cancer? A survey of providers in the National Comprehensive Cancer Network (NCCN) Cancer Invest. 2003;21:526–535. doi: 10.1081/cnv-120022366. [DOI] [PubMed] [Google Scholar]
- 58.Zubrod C, Schneiderman M, Frei E, III, et al. Appraisal of methods for the study of chemotherapy of cancer in man: comparative therapeutic trial of nitrogen mustard and triethylene. J Chronic Dis. 1960;11:7–33. [Google Scholar]
- 59.McCorkle R, Young K. Development of a symptom distress scale. Cancer Nurs. 1978;1:373–378. [PubMed] [Google Scholar]
- 60.McCorkle R, Quint-Benoliel J. Symptom distress, current concerns and mood disturbance after diagnosis of life-threatening disease. Soc Sci Med. 1983;17:431–438. doi: 10.1016/0277-9536(83)90348-9. [DOI] [PubMed] [Google Scholar]
- 61.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 62.Victorson D, Barocas J, Song J, Cella D. Reliability across studies from the functional assessment of cancer therapy-general (FACT-G) and its subscales: a reliability generalization. Qual Life Res. 2008;17:1137–1146. doi: 10.1007/s11136-008-9398-2. [DOI] [PubMed] [Google Scholar]
- 63.Hahn EA, Cella D, Chang CH, et al. Development of the FACIT Treatment Satisfaction Scale. Proceedings of the Seventh Annual Symposium of Contributed Papers: Quality of Life Evaluation, Drug Information Association. 2000 [Google Scholar]
- 64.Cella D, Hahn E, Webster K, et al. The FACIT treatment satisfaction measurement system. Qual Life Res. 2003;12:747. [Google Scholar]
- 65.Bandura A. Guide for constructing self-efficacy scales. In: Pajares F, Urdan TC, editors. Self-efficacy beliefs of adolescents. Greenwich, CT: Information Age Publishing; 2006. pp. 307–337. [Google Scholar]
- 66.Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 67.Cella D, Bonomi A, Leslie WT, VonRoenn J, Tchekmedyian NS. Quality of life and nutritional well-being: measurement and relationship. Oncology. 1993;7(11 Suppl):S105–S111. [Google Scholar]
- 68.Cella DF, Bonomi AE, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy- Lung (FACT-L) quality of life instrument. Lung Cancer. 1995;12:199–220. doi: 10.1016/0169-5002(95)00450-f. [DOI] [PubMed] [Google Scholar]
- 69.Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24:547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 70.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 71.Hays RD, Woolley JM. The concept of clinically meaningful difference in health-related quality-of-life research. How meaningful is it? Pharmacoeconomics. 2000;18:419–423. doi: 10.2165/00019053-200018050-00001. [DOI] [PubMed] [Google Scholar]
- 72.Cella D, Hahn EA, Dineen K. Meaningful change in cancer-specific quality of life scores: differences between improvement and worsening. Qual Life Res. 2002;11:207–221. doi: 10.1023/a:1015276414526. [DOI] [PubMed] [Google Scholar]
- 73.Bruera E, Pituskin E, Calder K, Neumann CM, Hanson J. The addition of an audiocassette recording of a consultation to written recommendations for patients with advanced cancer: a randomized, controlled trial. Cancer. 1999;86:2420–2425. [PubMed] [Google Scholar]
- 74.Ong LM, Visser MR, Lammes FB, De Haes JC. Doctor-patient communication and cancer patients' quality of life and satisfaction. Patient Educ Couns. 2000;41:145–156. doi: 10.1016/s0738-3991(99)00108-1. [DOI] [PubMed] [Google Scholar]
- 75.Gunnarsdottir S, Donovan HS, Serlin RC, Voge C, Ward S. Patient-related barriers to pain management: The Barriers Questionnaire II (BQ-II) Pain. 2002;99:385–396. doi: 10.1016/S0304-3959(02)00243-9. [DOI] [PubMed] [Google Scholar]
- 76.Overall JE, Doyle SR. Estimating sample sizes for repeated measurement designs. Control Clin Trials. 1994;15:100–123. doi: 10.1016/0197-2456(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 77.Hedeker D, Gibbons RD, Waternaux C. Sample size estimation for longitudinal designs with attrition: comparing time-related contrasts bewteen two groups. J Educ Behav Stat. 1999;24:70–93. [Google Scholar]
- 78.Lachin JM. A review of methods for futility stopping based on conditional power. Stat Med. 2005;24:2747–2764. doi: 10.1002/sim.2151. [DOI] [PubMed] [Google Scholar]
- 79.Proschan MA, Lan KKG, Wittes JT. statistical monitoring of clinical trials. New York: Springer; 2006. Power: Conditional, unconditional, and predictive; pp. 43–66. [Google Scholar]
- 80.Eton DT, Cella D, Yount SE, Davis KM. Validation of the functional assessment of cancer therapy - Lung symptom index-12 (FLSI-12) Lung Cancer. 2007;57:339–347. doi: 10.1016/j.lungcan.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 81.Hilarius DL, Kloeg PH, Gundy CM, Aaronson NK. Use of health-related quality-of-life assessments in daily clinical oncology nursing practice: a community hospital-based intervention study. Cancer. 2008;113:628–637. doi: 10.1002/cncr.23623. [DOI] [PubMed] [Google Scholar]
- 82.Matthew AG, Currie KL, Irvine J, et al. Serial personal digital assistant data capture of health-related quality of life: a randomized controlled trial in a prostate cancer clinic. Health Qual Life Outcomes. 2007;5:38–11. doi: 10.1186/1477-7525-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cox A, Illsley M, Knibb W, et al. The acceptability of e-technology to monitor and assess patient symptoms following palliative radiotherapy for lung cancer. Palliat Med. 2011;25:675–681. doi: 10.1177/0269216311399489. [DOI] [PubMed] [Google Scholar]
- 84.Hoekstra J, de Vos R, van Duijn NP, Schade E, Bindels PJE. Using the symptom monitor in a randomized controlled trial: the effect on symptom prevalence and severity. J Pain Symptom Manage. 2006;31:22–30. doi: 10.1016/j.jpainsymman.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 85.Mills ME, Murray LJ, Johnston BT, Cardwell C, Donnelly M. Does a patient-held quality-of-life diary benefit patients with inoperable lung cancer? J Clin Oncol. 2009;27:70–77. doi: 10.1200/JCO.2008.17.5687. [DOI] [PubMed] [Google Scholar]
- 86.Takahashi PY, Pecina JL, Upatising B, et al. A randomized controlled trial of telemonitoring in older adults with multiple health issues to prevent hospitalizations and emergency department visits. Arch Intern Med. 2012;172:773–779. doi: 10.1001/archinternmed.2012.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilson Sr CP. Another sobering result for home telehealth—and where we might go next: comment on “a randomized controlled trial of telemonitoring in older adults with multiple health issues to prevent hospitalizations and emergency department visits.”. Arch Intern Med. 2012;172:779–780. doi: 10.1001/archinternmed.2012.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lasheen W, Walsh D, Hauser K, Gutgsell T, Karafa MT. Symptom variability during repeated measurement among hospice patients with advanced cancer. Am J Hosp Palliat Med. 2009;26:368–375. doi: 10.1177/1049909109338352. [DOI] [PubMed] [Google Scholar]
- 89.Temel J. Complexities of quality of life analysis in non-small cell lung cancer. J Support Oncol. 2007;5:30–31. [PubMed] [Google Scholar]
- 90.Taenzer P, Bultz BD, Carlson LE, et al. Impact of computerized quality of life screening on physician behavior and patient satisfaction in lung cancer outpatients. Psychooncology. 2000;9:203–213. doi: 10.1002/1099-1611(200005/06)9:3<203::aid-pon453>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 91.Rosenbloom SK, Victorson DE, Hahn EA, Peterman AH, Cella D. Assessment is not enough: a randomized controlled trial of the effects of HRQL assessment on quality of life and satisfaction in oncology clinical practice. Psychooncology. 2007;16:1069–1079. doi: 10.1002/pon.1184. [DOI] [PubMed] [Google Scholar]
- 92.United States Census Bureau. [Accessed October 25, 2012];Housing and Household Economic Statistics Division. Available from http://www.census.gov/hhes/www/housing/census/historic/phone.html.
- 93.Basch E, Artz D, Dulko D, et al. Patient online self-reporting of toxicity symptoms during chemotherapy. J Clin Oncol. 2005;23:3552–3561. doi: 10.1200/JCO.2005.04.275. [DOI] [PubMed] [Google Scholar]
- 94.Given CW, Sikorskii A, Tamkus D, et al. Managing symptoms among patients with breast cancer during chemotherapy: results of a two-arm behavioral trial. J Clin Oncol. 2008;26:5855–5862. doi: 10.1200/JCO.2008.16.8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cella D, Tulsky DS, Linn E, Stewart I, Blendowski C. Brief intervention and its impact on quality of life. Proceedings of the 25th annual convention of the Association for the Advancement of Behavior Therapy. 1991:139. [Google Scholar]
- 96.Greenhalgh J, Meadows K. The effectiveness of the use of patient-based measures of health in routine practice in improving the process and outcomes of patient care: a literature review. J Eval Clin Pract. 1999;5:401–416. doi: 10.1046/j.1365-2753.1999.00209.x. [DOI] [PubMed] [Google Scholar]
- 97.Rhodes D, Koshy R, Waterfield W, Wu AW, Grossman S. Feasibility of quantitative pain assessment in outpatient oncology practice. J Clin Oncol. 2001;19:501–508. doi: 10.1200/JCO.2001.19.2.501. [DOI] [PubMed] [Google Scholar]
- 98.Basch E, Abernethy AP. Supporting clinical practice decisions with real-time patient-reported outcomes. J Clin Oncol. 2011;29:954–956. doi: 10.1200/JCO.2010.33.2668. [DOI] [PubMed] [Google Scholar]