Abstract
A 96-well plate sandwich assay based on Invader capture/signalling probes is used to recognize 28-mer mixed-sequence dsDNA targets specific to Salmonella, Campylobacter and Escherichia coli. Targets are detected at 20-55 pM concentration with excellent binding specificity.
Development of assays and platforms that enable rapid, sensitive and specific DNA detection is a burgeoning field with important implications in food industry, agriculture, clinical diagnostics, forensics and homeland security.1-6 For example, there is increased demand to replace culture-growing and PCR-based methods used for detection of microbial pathogens, with faster and less resource-intensive approaches.4,7-9 Methods based on nucleic acid hybridization are of particular interest due to the predictability and specificity of Watson-Crick base pairing. With the exception of a few approaches based on classic probes targeting double-stranded DNA (dsDNA),10-12 most approaches utilize single-stranded DNA as the analyte. However, it is attractive to develop methods that detect dsDNA instead as this eliminates the need for denaturation prior to analysis and potentially allows for amplification-free detection of genomic DNA.6 Unfortunately, conventional dsDNA targeting probe strategies, such as triplex forming oligonucleotides,13 peptide nucleic acids (PNAs),14 and minor groove binding polyamides,15 require polypurine targets and/or display other limitations, which reduces the number of suitable dsDNA targets.
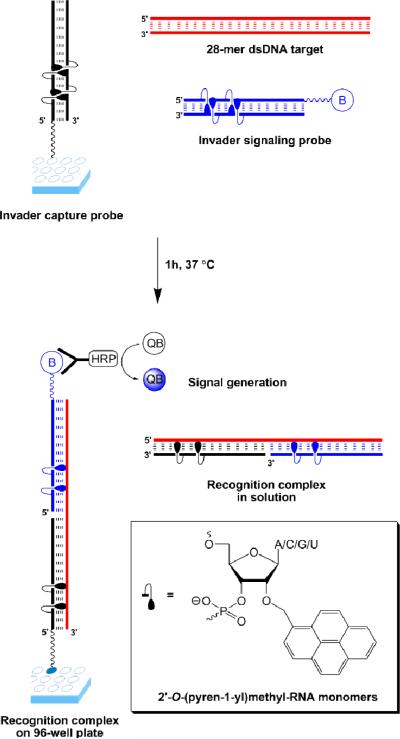
Our laboratory is pursuing Invaders as an alternative strategy for mixed-sequence recognition of dsDNA.16-19 Briefly explained, Invaders are short double-stranded oligonucleotide probes, which are activated for dsDNA-recognition through modification with one or more ‘+1 interstrand zippers’ of intercalator-functionalized nucleotides such as 2'-O-(pyren-1-yl)methyl-RNA monomers (Fig. 1; for a formal definition of the zipper terminology, see ESI†).16-19 This monomer arrangement presumably forces the intercalating pyrene moieties into the same region of the probe duplex, which results in localized duplex perturbation and destabilization (Fig. 1).19 Conversely, the two strands comprising an Invader display very strong affinity toward complementary single-stranded DNA (ssDNA) as duplex formation results in pyrene intercalation and stabilizing π-π-stacking interactions with flanking nucleobases (Fig. 1 bottom).16-22 We have previously used the stability difference between Invaders and probe-target duplexes to realize mixed-sequence recognition of linear dsDNA targets,16,17 DNA hairpins18,19 and chromosomal DNA.18
Figure 1.
Invader-based sandwich assay for detection of dsDNA. Invader capture probe (black); Invader signaling probe (blue); dsDNA target (red); biotin (B); streptavidin-horseradish peroxide conjugate (HRP); QuantaBlu (QB); adenin-9-yl/cytosin-1-yl/guanin-9-yl/uracil-1-yl (A/C/G/U).
Here, we report the development of a 96-well plate based sandwich assay in which Invader capture/signalling probes are used to recognize 28-mer mixed-sequence dsDNA targets specific to three important food pathogens, i.e., Salmonella, Campylobacter and Escherichia coli, which account for approximately half of the ~230,000 annual hospitalizations in the United States caused by foodborne pathogens.23 The principle of this assay is shown in Figure 1. Amine-terminated Invader capture probes are attached to the surface of 96-well plates and co-incubated with biotinylated Invader signalling probes and dsDNA targets. Successful Invader-mediated recognition of the corresponding dsDNA target results in the formation of two ternary complexes, i.e., one in solution and one tethered to the well. Following incubation with streptavidin-horseradish peroxidase (SA-HRP) conjugate and subsequent removal of unbound species, a HRP substrate is added, which results in the formation of a fluorescent product that reports on the original recognition event.
Three 28-mer mixed-sequence dsDNA targets (GC-content 32-50%) - known from literature to be specific for Escherichia coli O157:H7,24Salmonella enterica,25 and Campylobacter jejuni26-were selected as model targets (Table 1).
Table 1.
Sequence and thermal denaturation temperatures of model dsDNA targets studied herein.a
| Species | Sequence | Tm (°C) |
|---|---|---|
| C. jejuni | 5′-TATGCCATTTGAAAAGCTATAAGAGTTC 3′-ATACGGTAAACTTTTCGATATTCTCAAG |
62.0 |
| S. enterica | 5′-CACGTTCGGGCAATTCGTTATTGGCGAT 3′-GTGCAAGCCCGTTAAGCAATAACCGCTA |
72.0 |
| E. coli | 5′-AACAGTTCTATCAGGCATGGCTCTTGAT 3′-TTGTCAAGATAGTCCGTACCGAGAACTA |
68.0 |
Species: Campylobacter jejuni open reading frame (ORF), Salmonella enterica invasin A (invA) and Escherichia coli O157:H7 hemolysin A (hlyA). For conditions of thermal denaturation experiments, please see footnote of Table 2.
14-mer Invader signalling and capture probes were designed to span these dsDNA targets. Each probe contains three +1 interstrand zippers, as this probe design has proven efficient for recognition of dsDNA in our earlier work (Table 2).18 The three letter code used to name the probes denotes the target species (e.g., E. coli), its function (signalling or capture) and whether a strand is the ‘upper’ or ‘lower’ strand of an Invader probe.
Table 2.
Thermal denaturation temperatures (Tm) and thermal advantages (TA) of 2′-O-(pyren-1-yl)methylribonucleotide modified probes.a
| ON |
Sequence |
Tm [ΔTm] (°C) |
TAc (°C) |
||
|---|---|---|---|---|---|
| upper strand vs ssDNA |
lower strand vs ssDNA |
Invaderb |
|||
|
CSU
CSL |
5′-UAUGCCATTUGAAA 3′-b-AUACGGTAAACTTT |
55.5 [+11.5] | 59.0 [+15.0] | 44.5 [+0.5] | 26.0 |
|
CCU
CCL |
5′-AGCUAUAAGAGUTC 3′-TCGAUAUTCTCAAG-C6NH2 |
60.5 [+16.0] | 62.5 [+18.0] | 47.5 [+3.0] | 31.0 |
|
ESU
ESL |
5′-AACAGTTCUATCAG 3′-b-TTGUCAAGAUAGUC |
61.0 [+16.0] | 64.0 [+19.0] | 40.0 [−5.0] | 40.0 |
|
ECU
ECL |
5′-GCAUGGCTCTUGAT 3′-CGUACCGAGAACTA-C6NH2 |
65.0 [+13.0] | 69.0 [+17.0] | 56.0 [+4.0] | 26.0 |
|
SSU
SSL |
5′-CACGTTCGGGCAAT 3′-b-GUGCAAGCCCGUTA |
72.5 [+15.0] | 66.5 [+9.0] | 60.5 [+3.0] | 21.0 |
|
SCU
SCL |
5′-TCGTUATUGGCGAT 3′-AGCAAUAACCGCUA-C6NH2 |
66.0 [+14.5] | 68.0 [+16.5] | 54.5 [+3.0] | 28.0 |
ΔTm = change in Tm relative to the corresponding unmodified reference duplexes (Tm = 44.0 °C, 44.5 °C, 45.0 °C, 52.0 °C, 57.5 °C and 51.5 °C for the unmodified reference duplexes of CSU/CSL, CCU/CCL, ESU/ESL, ECU/ECL, SSU/SCL, respectively); thermal denaturation curves were recorded in medium salt buffer ([Na+] = 110 mM, [Cl−] = 100 mM, pH 7.0 (NaH2PO4/Na2HPO4)), using 1.0 μM of each strand; see main text for definition of TA. For structures of A, C, G and U, see Fig. 1; “b” and C6NH2”, denotes 3′-biotin TEG and 5′-amino-modifier C6 units, respectively.
thermal denaturation curves of Invaders display broad transitions.
example of TA calculation: TA (ESU:ESL) = Tm (ESU:ssDNA) + Tm (ESL:ssDNA) – Tm (ESU:ESL) – Tm (reference dsDNA of ESU:ESL) = 61.0 + 64.0 – 40.0 – 45.0 = 40.0 °C
To verify the design of the Invader capture and signaling probes, we first studied their thermal denaturation properties. In agreement with our earlier studies,18,19,21 individual Invader strands form much more thermostable duplexes with complementary single-stranded DNA (ssDNA) than do reference oligodeoxyribonucleotides (ΔTm = 9-19 °C, first two ΔTm columns, Table 2). In contrast, double-stranded Invader probes are much less thermostable (ΔTm from -5 to +4 °C, ‘Invader’ column, Table 2). Thus, all of the designed Invader capture/signalling probes are activated for recognition of their respective dsDNA targets as judged by the large differences in thermostability between probe-target duplexes versus Invaders and iso-sequential dsDNA targets. The term thermal advantage, defined as TA = Tm (‘upper’ strand vs ssDNA) + Tm (‘lower’ strand vs ssDNA) - Tm (Invader) - Tm (dsDNA), quantifies this difference (TA = 21-40 °C, Table 2).
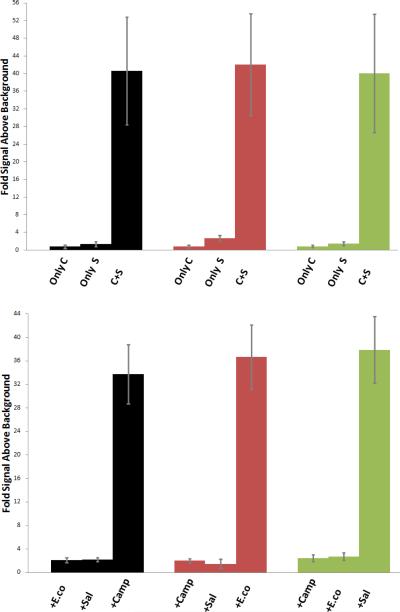
Indeed, incubation of Invader capture and signalling probes with their corresponding 28-mer dsDNA targets (1h at 37 °C) results in dose-dependent signal formation (Fig. S1).† As expected, both the capture and signalling probes are required for signal formation (Fig. 2 – upper panel). Moreover, only very weak signals are observed when signalling/capture probes designed for a particular species (e.g., E. coli) are incubated with dsDNA targets from a different species (e.g., Salmonella) (Fig. 2 – lower panel), which demonstrates that Invader-mediated dsDNA-recognition is specific. The sensitivity of this unoptimized assay (signal above background = 3 at ~20/30/55 pM target concentration, Fig. S1†) is noteworthy considering i) the high Tm (62-72 °C), high GC-content (32-50%), and double-stranded nature of the DNA targets, and ii) the fact that we did not use highly polymeric versions of SA-HRP conjugates for signal generation.§
Figure 2.
Upper panel: detection of dsDNA targets (1 nM concentration) specific to Campylobacter jejuni (black), E. coli O157:H7 (red) and Salmonella enterica (green), using only species-specific capture probe (only C), using only species-specific signaling probe (only S), or using both species-specific capture and signaling probes (C+S) (upper panel) – or using capture and signaling probes designed against E. coli (+E.co), Salmonella (+Sal) or Campylobacter (+Camp) (lower panel). Lines represent standard deviation.
In conclusion, this proof-of-concept study describes a simple Invader-based sandwich assay, which enables mixed-sequence recognition of double-stranded DNA using a 96-well plate platform. In the present study, the assay is used to detect dsDNA targets specific to food pathogens, but we envision that a much broader range of targets can be detected via this approach, especially since Invader probes do not exhibit the inherent sequence limitations that many conventional dsDNA-targeting probes exhibit. Importantly, this assay potentially eliminates the need for DNA denaturation prior to analysis. This, along with the possibility of coupling the assay with existing sample preparation and enrichment protocols, and integrating it with microarrays4 and/or single molecule detection techniques,6 suggest that exciting applications of Invader-based sandwich assays in nucleic acid diagnostics and biotechnology are possible.
Supplementary Material
Acknowledgments
This study was supported by Award Number R01 GM088697 from the National Institute of General Medical Sciences, USDA (No. 2009-34479-19833 and 2010-34479-20715) and The Office of Naval Research (N00014-10-1-0282). We appreciate input from Dr. Shiva Rastogi, Prof. D. Eric Aston (both Univ. Idaho), Dr. Josh Branen and Prof. A. Larry Branen (both Biotracking LLC, Moscow, Idaho). We thank Dr. Lee Deobald (EBI Murdock Mass Spectrometry Center, Univ. Idaho) for assistance with mass spectrometric analysis.
Footnotes
Electronic supplementary information (ESI) available: Definition of zipper terminology, experimental protocols, MS-table of synthesized probes (Table S1) and dose-response curve (Fig. S1). See DOI: XXX
Highly polymeric SA-HRP conjugates have been used to increase the sensitivity of sandwich assays (see, ref. 11).
References
- 1.Rosi NL, Mirkin CA. Chem. Rev. 2005;105:1547. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- 2.Drummond TG, Hill MG, Barton JK. Nat. Biotechnol. 2003;21:1192. doi: 10.1038/nbt873. [DOI] [PubMed] [Google Scholar]
- 3.Ranasinghe RT, Brown T. Chem. Commun. 2005:5487. doi: 10.1039/b509522k. [DOI] [PubMed] [Google Scholar]
- 4.Uttamchandani M, Neo JL, Ong BNZ, Moo S. Trends Biotechnol. 2009;27:53. doi: 10.1016/j.tibtech.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lord H, Kelley SO. J. Mater. Chem. 2009;19:3127. [Google Scholar]
- 6.Ranasinghe RT, Brown T. Chem. Commun. 2011;47:3717. doi: 10.1039/c0cc04215c. [DOI] [PubMed] [Google Scholar]
- 7.de Boer E, Beumer RR. Int. J. Food Microbiol. 1999;50:119. doi: 10.1016/s0168-1605(99)00081-1. [DOI] [PubMed] [Google Scholar]
- 8.Ivnitski D, O'Neil DJ, Gattuso A, Schlicht R, Calidonna M, Fisher R. Biotechniques. 2003;35:862. doi: 10.2144/03354ss03. [DOI] [PubMed] [Google Scholar]
- 9.Lazcka O, del Campo FJ, Munoz FX. Biosens. Bioelectron. 2007;22:1205. doi: 10.1016/j.bios.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 10.McKenzie F, Faulds K, Graham D. Chem. Commun. 2008:2367. doi: 10.1039/b802163e. [DOI] [PubMed] [Google Scholar]
- 11.Zhang N, Appella DH. J. Am. Chem. Soc. 2007;129:8424. doi: 10.1021/ja072744j. [DOI] [PubMed] [Google Scholar]
- 12.Singh I, Wendeln C, Clark AW, Cooper JM, Ravoo BJ, Burley GA. J. Am. Chem. Soc. 2013;135:3449. doi: 10.1021/ja309677h. [DOI] [PubMed] [Google Scholar]
- 13.Duca M, Vekhoff P, Oussedik K, Halby L, Arimondo PB. Nucleic Acids Res. 2008;36:5123. doi: 10.1093/nar/gkn493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen PE. Chem. Biodiversity. 2010;7:786. doi: 10.1002/cbdv.201000005. [DOI] [PubMed] [Google Scholar]
- 15.Dervan PB, Edelson BS. Curr. Opin. Struct. Biol. 2003;13:284. doi: 10.1016/s0959-440x(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 16.Hrdlicka PJ, Kumar TS, Wengel J. Chem. Commun. 2005:4279. doi: 10.1039/b506986f. [DOI] [PubMed] [Google Scholar]
- 17.Sau SP, Kumar TS, Hrdlicka PJ. Org. Biomol. Chem. 2010;8:2028. doi: 10.1039/b923465a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Didion BA, Karmakar S, Guenther DC, Sau S, Verstegen JP, Hrdlicka PJ. ChemBioChem. 2013;14:1534. doi: 10.1002/cbic.201300414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sau SP, Madsen AS, Podbevsek P, Andersen NK, Kumar TS, Andersen S, Rathje RL, Anderson BA, Guenther DC, Karmakar S, Kumar P, Plavec J, Wengel J, Hrdlicka PJ. J. Org. Chem. doi: 10.1021/jo4015936. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar TS, Madsen AS, Østergaard ME, Sau SP, Wengel J, Hrdlicka PJ. J. Org. Chem. 2009;74:1070. doi: 10.1021/jo802037v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karmakar S, Anderson BA, Rathje RL, Andersen S, Jensen T, Nielsen P, Hrdlicka PJ. J. Org. Chem. 2011;76:7119. doi: 10.1021/jo201095p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura M, Fukunaga Y, Sasa K, Ohtoshi Y, Kanaori K, Hayashi H, Nakano H, Yamana K. Nucleic Acids Res. 2005;33:5887. doi: 10.1093/nar/gki889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M-A, Roy SL, Jones JL, Griffin PM. Emerg. Infect Dis. 2011 doi: 10.3201/eid1701.P11101. DOI: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farabullini F, Lucarelli F, Palchetti I, Marrazza G, Mascini M. Biosens. Bioelectron. 2007;22:1544. doi: 10.1016/j.bios.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Rahn K, Degrandis SA, Clarke RC, McEwen SA, Galan JE, Ginocchio C, Curtiss R, Gyles CL. Mol. Cell. Probes. 1992;6:271. doi: 10.1016/0890-8508(92)90002-f. [DOI] [PubMed] [Google Scholar]
- 26.Jackson CJ, Fox AJ, Jones DM. J. Appl. Bacteriol. 1996;81:467. doi: 10.1111/j.1365-2672.1996.tb03534.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.