Abstract
Combination active antiretroviral therapy prevents HIV from replicating and ravaging the immune system, thus allowing people to age with this disease. Unfortunately, the synergistic effects of HIV and aging can predispose many to become more at-risk of developing cognitive deficits which can interfere with medical management, everyday functioning, and quality of life. The purpose of this article is to describe the role of cognitive reserve and neuroplasticity on cognitive functioning in those aging with this disease. Specifically, the role of environment and the health of these individuals can compromise cognitive functioning. Fortunately, some cognitive interventions such as prevention and management of co-morbidities, cognitive remediation therapy, and neurotropic medications may be of value in preventing and rehabilitating the cognitive consequences of aging with HIV. Novel approaches such as cognitive prescriptions, transcranial direct stimulation, and binaural beat therapy may also be considered as possible techniques for cognitive rehabilitation.
Keywords: HIV/AIDS, Aging, Cognitive Remediation Therapy, cART, Neuroplasticity, Cognitive Reserve, Microbial Translocation
Fifty-two percent of adults with HIV experience some measurable cognitive deficit according to Heaton and colleagues.1 In deriving this percentage, they conducted neuropsychological evaluations with 1,555 adults with HIV from 6 sites across the United States. They categorized 33% with asymptomatic impairment, 12% with mild impairment, 5% with confounded impairment, and 2% with dementia. These cognitive deficits are observed across multiple cognitive domains including attention, memory, speed of processing, executive functioning, and psychomotor ability.2–10
These cognitive deficits can create a poor person-environment fit, thus creating problems with everyday functioning such as with employment, financial management,11 driving,12–15 remembering medical appointments, and medication adherence.16,17 For example, in a sample of adults with (n = 42) and without HIV (n = 21), Marcotte and colleagues found that those with HIV performed more poorly on a cognitive measure of visual attention and visual speed of processing (i.e., the Useful Field of View test) than those without HIV. Furthermore, they found that those classified with poor visual attention and speed of processing experienced a higher rate of automobile crashes compared to those classified with normal visual attention and speed of processing. As people age normally, cognitive loss in visual attention and speed of processing commonly occur which also affects driving safety;18 thus, there is concern that those aging with HIV may experience even more problems with driving.
As people age with HIV, the synergistic effects of aging and HIV may translate into more vulnerability of developing such cognitive deficits that impact everyday functioning.9,19–24 This phenomenon is of concern given that by 2015 half of those living with HIV in the United States will be 50 and older.25 Therefore, this percentage of 52% with cognitive deficits is likely to rise, thus representing a public health problem. Understanding this problem and developing appropriate and affordable solutions for preventing and rehabilitating for such cognitive deficits is imperative.
The purpose of this article is to review the cognitive consequences of aging with HIV within the framework of cognitive reserve and neuroplasticity. From this framework, the role of environment and health on cognition is reviewed as it pertains to HIV. Following this, novel cognitive interventions are presented that may theoretically prevent cognitive loss or rehabilitate such cognitive functioning. Finally, although not exhaustive, novel cognitive interventions are examined as potential interventions worthy of future study.
Principles of Cognitive Reserve and Neuroplasticity
As mentioned, as people age with HIV, they will be more vulnerable of developing cognitive deficits. In a cross-sectional study with 161 older (50+) and younger (<50) adults with and without HIV, Vance and colleagues administered seven neuropsychological and two laboratory-based everyday functioning tests to these participants.26 They found that, as a group, older adults with HIV performed the worst on all of these tests; this was followed by younger adults with HIV and older adults without HIV, and then with younger adults without HIV performing the best. This and other studies support similar findings.9,19–22,27
One reason for these cognitive deficits may be due to the depletion of cognitive reserve. Cognitive reserve refers to the amount of damage the brain can absorb and yet maintain functioning. Cognitive reserve has become a theoretical explanation for describing the differences observed in brain pathology and the clinical or observed manifestation of the physiological damage on individuals who are diagnosed with Alzheimer’s disease.28,29 In fact, cognitive reserve may explain why individuals experience differential rates of change in cognitive function, and that such cognitive reserve capacity may provide a buffer to cognitive decline.30 Recent evidence demonstrates that cognitive reserve offers protection from neuronal damage.31 Unfortunately, once the damage accumulates to a certain threshold, cognitive deficits begin to emerge.32–34
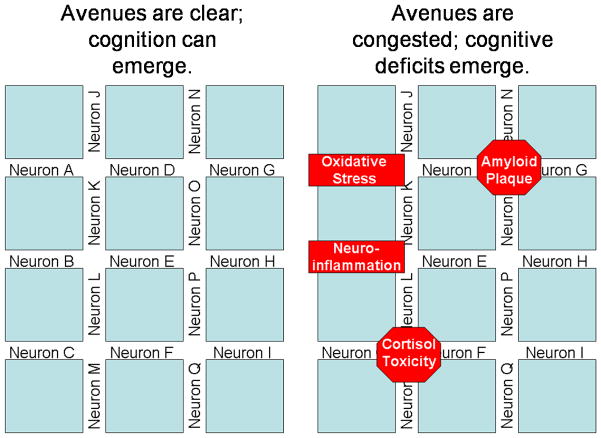
This process of cognitive reserve can be exemplified in the following manner. Imagine neurons are avenues (left panel of Figure 1), which is an apt analogy since electrical impulses travel from synapse to cell body to dendrites to the terminal buttons where neurotransmitters are released into the synaptic cleft. In this analogy, the synaptic clefts can be likened to the intersections. As electrical impulses (i.e., traffic) travel from neuron to neuron, cognition emerges. Cognition can continue to emerge as long as the avenues are open and the intersections are clear. But as seen in the right panel of Figure 1, as the intersections (i.e., synaptic clefts) are clogged by amyloid plaques or the avenues (i.e., neurons) are less viable because of oxidative stress, neurofibrillary tangles, or cortisol toxicity, the electrical impulses are no longer as free to travel. These hindrances result in traffic being slower even though it may be bypassed onto other avenues. But eventually, if the intersections and avenues become too clogged, traffic comes to a standstill; likewise, in the brain, if the neurons and their connections become too damaged, electrical impulses cannot travel and cognition becomes slowed or does not even emerge.
Figure 1.
Neuron-Avenue Analogy of Cognitive Reserve. Left panel demonstrates healthy cognitive reserve. Right panel demonstrates a depleted cognitive reserve.
For those aging, those with HIV, and especially those aging with HIV, there are several mechanisms that can deplete cognitive reserve. Some of these mechanisms can be encapsulated under two processes: oxidative stress and neuroinflammation. Oxidative stress refers to the process whereby tissues of the body react to oxygen molecules. Although oxygen is needed by the body to convert food into energy, particular oxygen molecules, referred to as reactive oxygen species, is actually corrosive and damages tissues. Fortunately, the body possesses a complex system of enzymes that absorbs these damaging molecules and makes them inert; in fact, certain foods such as green tea and red wine contain polyphenols (an antioxidant) that render these reactive oxygen species inert. But with age, the body becomes less able to defend itself against these damaging molecules. Likewise, when tissues are damaged, the body produces an inflammatory response.35 Inflammation is a complex biochemical protective response of the endocrine system that aids in repairing tissues. Specialized proteins called cytokines and leukocytes (white blood cells) eradicate pathogens and molecularly rebuild tissue. But inflammation over prolonged periods of time can actually damage tissue as well. Brain tissue is also vulnerable to these effects, thus giving rise to neuroinflammation. Several studies show that prolonged neuroinflammation is associated with increased risk of cognitive impairment, stroke, and dementia.35–37
Neuroinflammation is of particular concern for those aging with HIV. HIV is considered a slow burner “inflammation” disease; some may even remark that AIDS stands for Acquired Inflammation Disease Syndrome instead of Acquired Immunodeficiency Syndrome. For many reasons (and several have been considered), HIV produces neuroinflammation. In fact, cognitive deficits and changes in brain metabolism have been detected as early as one year post HIV diagnosis.38,39
As HIV crosses the blood brain barrier, although it generally does not infect neurons per se, it is able to enter glial cells (i.e., microglia, macrophages) and replicate, thus killing these cells.40,41 Given that glial cells facilitate neuronal health and contribute to neuronal modulation, this is of concern. Continuing with the neuron-avenue cognitive reserve analogy (Figure 1), glial cells help keep the avenues clear so that electrical impulses can continue to travel. But as glial cells become damaged or die (i.e., astrogliosis), neurotoxic byproducts are produced such as cytokines and quinolic acid. These byproducts: 1) compromise the functioning of other glial cells, 2) elevate oxidative stress and neuroinflammation, and 3) compromise or kill neurons.41,42 As a result, this reduces cognitive reserve, rendering people more susceptible of developing cognitive deficits.
Increasing Cognitive Reserve
Fortunately, cognitive reserve can be increased; this is a useful fact should one be interested in preventing cognitive loss in later life.33 Continuing with the neuron-avenue cognitive reserve analogy (Figure 1), new avenues (i.e., neuronal pathways) can be created; this process is referred to as positive neuroplasticity. Simply stated, positive neuroplasticity is a complex biochemical process whereby new connections (i.e., avenues) are created between neurons. In contrast, negative neuroplasticity is a complex biochemical process whereby connections are pruned, atrophied, or destroyed. These processes are affected by inflammation, disease and pathogens, insults to the body and brain, and the environment. In fact, the enriched environment paradigm (below) poignantly epitomizes these processes of neuroplasticity.
In this classic enriched environment paradigm, animals (usually rats or mice) are randomly selected to live in one of three environments – an enriched, a standard, or an impoverished environment.33 Animals in the enriched environment live with other animals of their kind and have toys with which they can interact. Animals in the standard environment are grouped in threes and placed in a cage with no toys. Animals in the impoverished environment are placed in isolation with no toys. Otherwise, the environmental conditions (e.g., lighting, temperature, food) are kept constant across the three environments. Researchers have found that those animals placed in the enriched environment have more brain-derived neurotropic factors that favor positive neuroplasticity along with larger brains and more dense dendritic connections compared to animals placed in the other two conditions. Similarly, animals placed in the impoverished environment have less brain-derived neurotropic factors indicative of negative neuroplasticity along with smaller brains and less dense dendritic connections compared to animals placed in the other two conditions. Likewise, these physiological differences translate into maze performance tests in that animals placed in the enriched environment negotiate such mazes faster and more efficiently than animals placed in the other two conditions; the same comparison can be observed between animals in the standard environment compared to those in the impoverished environment.33,34 These effects are also observed in human studies whereby exposure to leisurely, intellectual, physical, and occupational activities has been shown to change the morphology of the brain and delay the onset of cognitive aging and dementia.33,43
In the classic London Taxi Driver Study, Maguire and colleagues used MRI scans to examine neuroplasticity in taxi drivers and bus drivers.44 They exploited a naturalistic opportunity whereby taxi drivers and bus drivers learned and practiced their profession. Typically, London taxi drivers have to train for 2 to 4 years to learn to effectively traversee the many streets of London and learn various points of interest along the way. In contrast, London bus drivers do not have to undergo such a vigorous training program; instead, they drive in set routes while the taxi drivers do not have any set route. Similar to the enriched environmental paradigm above, the taxi drivers represent the enriched environment and the bus drivers represent the standard environment. These researchers found that the taxi drivers had larger mid-posterior hippocampi than the bus drivers; furthermore, the size of the hippocampi was associated with the years of driving. Clearly environment plays a cruicial role in neuroplasticity. Likewise, learning new information has been shown in other studies to develop brain regions and even perhaps slow the progression of dementia.33,45,46
Decreasing Cognitive Reserve
Just as there are factors that increase cognitive reserve, there are also factors that decrease cognitive reserve, thus creating cognitive deficits. As already mentioned, oxidative stress and neuroinflammation are contributing factors. However, the medications used to treat HIV can also produce metabolic syndromes that can result in diabetes, hypercholesterolemia, hypertension, and heart disease.47 As people age with HIV, they will be more vulnerable of developing such co-morbidities. Thus, there is concern that these co-morbidities will indirectly deplete cognitive reserve in these adults. Numerous studies show that such co-morbidities can increase oxidative stress, cortisol levels, and neuroinflammation, all of which decrease cognitive reserve.35,48 Additionally, combined antiretroviral therapy (cART)-related mitochondrial damage also contributes to this process of decreasing cognitive reserve.49
In addition to the physical factors that can contribute to a decrease in cognitive reserve in those aging with HIV, psychological and social factors are also involved. First of all, across age groups, ~50% and ~20% of adults with HIV experience depression and anxiety, respectively.47 Likewise, a significant number of adults with HIV suffer from concomitant psychiatric co-morbidities such as bipolar disorder (~2%) and substance use/abuse (~25%).47 Such psychiatric conditions create a stress response within the hypothalamus-adrenal-pituitary axis resulting in the release of cortisol; sustained release of cortisol causes neuroinflammation.35
Second, HIV is a stigmatizing disease which can result in social withdrawal and isolation.50 In a study of 160 older (50+) adults with HIV in New York City, Shippy and Karpiak found that 71% lived alone, two-thirds were not in a significant intimate relationship, and 57% reported that their emotional needs were unmet.51 Thus, from this and other studies,52–54 it was shown these older adults have a fragile social network, and as a group, may not have the social and personal resources to help mitigate the physical and psychological stressors common with HIV. In fact, studies show that social isolation and social rejection in adolescents results in increased inflammatory biomarkers (i.e, interleukin-6, C-reactive protein).55 Thus, it is likely that those aging with HIV who experience such social isolation and social rejection may also experience an increased stress response that may fuel inflammation and neuroinflammation.56,57
Third, as observed in several studies, social interaction is helpful in promoting brain health and positive neuroplasticity; likewise, no or little social interaction can result in negative neuroplasticity and thus decrease cognitive reserve.43,56,58 For example, Wilson and colleagues followed 823 older adults without Alzheimer’s disease for 4 years and examined their perceived loneliness.59 When they compared the most lonely (above the 90th percentile) to the least lonely (below the 10th percentile), they found that those who were lonely experienced a two-fold increase in developing Alzheimer’s disease compared to the non-lonely group. Other studies also suggest that social interaction is important for brain health and positive neuroplasticity.58 This point is very important when one considers that many older adults with HIV may not work, and thus do not have that opportunity to interact socially with coworkers, an activity hypothesized to stimulate cognition.43,60
Cognitive Interventions
Although there are several interventions for preventing and rehabilitating cognitive deficits in adults with HIV, a few of the more well-known and practical interventions are cART, co-morbidity prevention and management, neurotropic medications, and cognitive remediation therapy.
cART
Al-khindi, Zakzaic, and van Gorp reviewed 23 studies on the effects of cART on neurological functioning in those with HIV; they found that such antiretrovirals are effective in improving virological outcomes, functioning in a variety of cognitive domains including psychomotor, executive, and attention also occur.61 Most likely, cART-induced reduction of viral load also affects the brain, with consequent reductions in neuronal injury. Although there are probably many other mechanisms in which cART improve brain health (e.g., reducing neuroinflammation caused by HIV; improved immunological response and fewer opportunistic infections), there are still some concerns about a few cART medications (e.g., efavirenz) that may actually create mitochondrial toxicity, neurotoxic effects, and co-morbidities.47 In fact, Giunta and colleagues studied the use of cART and the incidence of HIV-associated neurocognitive disorders (HAND).62 They found that cART inhibited microglial phagocytosis (i.e., devouring foreign particles) of amyloid beta protein known to accumulate in the brains of those with Alzheimer’s disease, thus leading to neurotoxicity. Despite this finding, cART is by far the first line of defense against preventing cognitive deficits by restoring and/or protecting overall health and preventing AIDS and opportunistic infections.
Physical and Psychiatric Co-morbidity Prevention and Management
As stated, physical and psychiatric co-morbidities have been shown to increase cortisol levels which can produce cytokines and neuroinflammation, resulting in poorer cognitive reserve and thus cognitive functioning.3,63–65 For those with HIV experiencing common physical co-morbidities such as diabetes, hypertension, hypercholesterolemia, microbial translocation (i.e., leaky gut syndrome), heart disease, liver disease, and renal disease, this represents added stress on the body and thus the brain.47,66–68 In fact, there are several mechanisms in which each of these co-morbidities can create problems in brain function. For example, in a study of 145 adults with HIV, Valcour and colleagues observed that greater levels of cognitive impairment were associated with greater levels of insulin resistance (the hallmark of diabetes).48 In fact, in 22 older adults (50+) with HIV, irregular glucose metabolism is associated with abnormal microstructural changes in the hippocampus and caudate nucleus, brain structures responsible for memory formation and consolidation.69 Fortunately, some studies show that diabetes management can reverse or prevent such cognitive deficits.70 This prevention and reversal of cognitive deficits through effective medical management has also been observed in other co-morbid conditions.71,72
Likewise, psychiatric co-morbidities can negatively impact cognition through: 1) competing for cognitive resources through rumination (i.e., depressive or suicidal thoughts that prevent other thoughts from emerging),73 2) displaying different cognitive profile (e.g., executive dysfunction is common in schizophrenia), and 3) producing cytokines and neuroinflammation.65 As with physical co-morbidities, some studies show that treating such psychiatric co-morbidities may help reverse or prevent some cognitive deficits from developing.65,74,75 For example, although hypertension can cause white matter lesions and result in poorer cognitive functioning, taking antihypertensive medications can prevent or even improve cognitive functioning in those with hypertension.76
Furthermore, since HIV is considered an inflammation and neuroinflammation disease, proactively reducing such inflammation should be considered as a way to protect or improve cognition.77 Becker and colleagues examined MRIs of 84 adults with HIV and 71 adults without HIV.78 Compared to the HIV-negative sample, those with HIV had more cortical thinning, especially in the right and left putamen and caudate nucleus. Cleary, such thinning may be a result of such neuroinflammation. Therefore, in addition to treating both physical and psychiatric co-morbidities, addressing the underlying neuroinflammation caused by these co-morbidities, aging, and HIV represents a potential therapeutic approach.2 One way to reduce such systemic inflammation as well as neuroinflammation may be to incorporate polyphenols and other antioxidants into the diet of those aging with HIV. For example, using HIV-Tat transgenic mice, Rrapo and colleagues found that by introducing green tea, a powerful polyphenol, into the diet of these mice actually reduced the death of glial cells (i.e., astrogliosis), cells that support neuronal functioning.79 In fact, it has also been suggested that non-steroidal anti-inflammatory medicines such as ketorolac, aspirin, and ibuprofen may reduce inflammation and protect the brain by decreasing such neuroinflammation;80–82 however, further research is recommended to determine the safety and efficacy of this approach in adults with HIV.
Cognitive Remediation Therapy
Cognitive remediation therapy refers to a variety of techniques that are designed to enhance existing cognitive functioning. Typically, the delivery mechanism for such therapies include computer programs via videogames, workshops and theatre groups, workbooks, and other modalities that exercise the mind.83,84 In a recent pre-post experimental study, Vance and colleagues randomized 46 middle-aged and older adults (40+ years) with HIV into two groups; one group played approximately 10 hours of a computerized game designed to improve their visual attention and speed of processing and the other group served as a no-contact control group.85 Compared to the control group, those in the training group improved significantly on a cognitive measure of visual attention and speed of processing (i.e., Useful Field of View), which also translated into significant improvement in a measure of everyday functioning (i.e., Timed Instrumental Activities of Daily Living Test). These results are encouraging because this training is quite similar to another training program designed to improve visual speed of processing, which has been found to be related to lower depression and improve locus of control (i.e., sense of control over one’s life) and health-related quality of life in normal community-dwelling older adults;86–89 obviously these are areas that must also be addressed in those aging with HIV.47
Neurotropic Medications
Methylphenidate (Ritalin) and modafinil are neurotropic medications that have been shown to improve cognitive functioning in adults with HIV. Methylphenidate has been found to improve cognition in healthy adults and those with attention deficit hyperactivity disorder.90 In a single-blind crossover study involving 16 adults with HIV, Hinkin and colleagues found that a daily dose of 30 mg of methylphenidate was effective in improving cognition.91 In fact, these researchers also found that those who displayed more depressive symptomatology and slower speed of processing improved the most. Other studies also show that methylphenidate is effective in reducing low density lipoprotein cholesterol, triglycerides, and total cholesterol as well as ameliorating the effects of fatigue.92,93 These added effects are particularly advantageous given that of those with HIV, 38% experience hypercholesterolemia and nearly 50% experience a range of depressive symptomatology.47,94
Similar to methylphenidate, modafinil has also been shown to ameliorate the symptoms of fatigue as well as improve cognitive functioning in those with attention deficit hyperactivity disorder.95 As such, this has also been used effectively in adults with HIV. In a sample of 103 adults with HIV, McElhiney and colleagues randomized participants into two groups, modafinil or placebo, and followed them over a 4-week period.96 Those who were prescribed modafinil improved on global cognition and experienced fewer cognitive complaints compared to those who received the placebo. Although no large randomized clinical studies have been conducted on the effects of methylphenidate and modafinil on cognition in adults with HIV, these results are encouraging, especially since these are medications that have been studied well in other populations and have been shown to be well tolerated and generally safe.91
Unfortunately, other neurotropic medications such as memantine and donepezil, which improve cognition in those with Alzheimer’s disease, are not recommended at this time. Memantine has been shown to be tolerated well in adults with HIV; but improvement on cognitive functioning has not been found at this time.97 Furthermore, based on a recent search in PubMed (April 4, 2013), there are no studies that have examined the use of donepezil in adults with HIV.
Future Consideration
Several novel approaches may be considered for preventing or rehabilitating cognitive deficits in adults aging with HIV. These include cognitive prescriptions, addressing microbial translocation, lithium, transcranial direct current stimulation, vagus nerve stimulation, and binaural beat therapy. Although innovative, these approaches require additional research before being used as a part of standard of care.
Cognitive prescriptions are individually written behavioral programs designed to improve several areas that support overall cognitive functioning.98 This holistic approach targets behavioral improvements in sleep hygiene, physical exercise, intellectual activity, mood, social interaction, and nutrition; all of which have been shown to be related to positive neuroplasticity, cognitive reserve, and cognitive functioning.99–110 For example, in generating a behavioral goal for physical activity, the patient may enjoy swimming but has not done so for years; therefore the goal may be to “Go to the pool and swim 10 laps twice a week”; as a result, this may improve cardiovascular functioning and mood, both of which support brain health. In the area of nutrition, the behavioral goal may be to “Eat salmon once a week” given that this is a rich source of omega-3-fatty acid which is also linked to brain health. All of the behavioral goals are either: 1) geared to improve body health and thus brain health, or 2) facilitate positive neuroplasticity (i.e., intellectual activity, social interaction) and increase cognitive reserve. Practical implementation of this has not been tested yet; however, several intervention studies that have targeted individual areas have been shown to support cognitive functioning. For example, targeting nutritional needs in adult 50 to 70 years, Durga and colleagues conducted a 3-year randomized controlled study and found that those assigned to take folic acid supplementation experienced lower homocysteine levels and better cognitive functioning compared to those assigned to the control group.111 Therefore, this holistic approach with cognitive prescriptions may provide even more cognitive support and assist with successful cognitive aging.
Addressing microbial translocation (i.e., leaky gut syndrome) is another novel area that needs to be addressed in those aging with HIV.112,113 The prevalence of cognitive deficits is present despite virologic suppression from cART suggesting independence of this decline from viral replication.114,115 Instead, cognitive deficits have been linked to inflammatory responses to microbial products (i.e., lipopolysaccharide) leaked into and circulating within the bloodstream.116 As a result of inflammatory cytokines and depletion of key immune cells in the gut associated lymph tissue, the effects of microbial translocation are increased which amplify the immune response to these products and result in chronic inflammation.112,113,117 Even despite higher CD4 levels, the amplification of immune activation leads to early aging in people living with HIV and has been linked with HIV-associated dementia in which higher levels of microbial products were associated with higher levels of inflammatory immune response.113 Studies show that even in those with well controlled HIV, microbial translocation is common and may contribute to neuroinflammation. In a study with 97 adults with HIV, Lyons and colleagues found that soluble CD14 (an indicator of microbial translocation), was associated with poorer cognitive functioning; this could increase neuroinflammation.118 Studies have suggested that a healthy intestinal flora reduces microbial translocation, thereby subsequent immune activation evidenced by reduced levels of soluble CD14. One way to cultivate healthy intestinal flora is through regular consumption of yogurt and/or supplementation of over the counter probiotics at recommended doses. Therefore, in line with the cognitive prescription focus on nutrition, a dietary intervention with probiotic supplementation may produce cognitive benefits as well.119
Lithium has been used since the 1940s to treat mood disorders but recent evidence also suggests that lithium may be used to protect against decreases in cognitive reserve and improve cognitive functioning.120 Lithium inhibits the glycogen synthase kinase-3β enzyme and other enzymes that downstream reduce amyloid-β42 and tau protein phosphorylation, proteins associated with Mild Cognitive Impairment and Alzheimer’s disease. Also, lithium has been found to stimulate the production of vascular endothelial growth factor and brain-derived neurotropic factor. These particular factors protect against the neurotoxic effects of amyloid-β42 and promote hippocampal neurogenesis. Furthermore, lithium helps decrease oxidative stress and inflammation. For these reasons, this may be why neuroprotective effects are observed in adults with bipolar disorder who have been prescribed lithium for years. In fact, case registry studies indicate that such adults with bipolar disorder taking lithium long-term experience a lower incidence for dementia.18,121 Furthermore, structural imaging studies have found that both short-term and long-term lithium use are associated with increased cortical thickening as well as increased amygdala and hippocampal volume.120
In a 12-month double-blind study of 45 adults with amnestic Mild Cognitive Impairment, Forlenza and colleagues found that those assigned to lithium had lower conversion rates to Alzheimer’s disease compared to those assigned to a placebo.122 Based on these and other studies, subtherpeutic doses of lithium (0.2 – 0.4 mmol/L) may be used to protect cognitive reserve and cognitive functioning in normal adults120 – and perhaps adults aging with HIV. Albeit, lithium use is not without risks (e.g., gastrointestinal disturbances, tremor, hypothyroidism, renal dysfunction) and can adversely interact with other medications (e.g., nonsteroidal anti-inflammatories, thiazide diuretics).120 Yet, more research is needed to examine whether using lithium would be an effective neuroprotective agent in adults aging with HIV.
Transcranial direct current stimulation represents another novel area whereby low voltage current is applied to the scalp via electrodes. In doing so, this procedure produces changes in the cerebral cortex and influences cognition.123,124 For example, in a studying involving 96 healthy adults, Clark and colleagues placed 10 cm2 sponge electrodes directly over the right inferior frontal cortex above the sphenoid bone (an area referred to as F10) as participants played a virtual reality game whereby they received feedback as to where deadly objects (i.e., bombs) were concealed.125 Electrical current was applied 5 minutes before playing the game and continued during the first 30 minutes of playing the game. These researchers found that those who received 2 milliamps compared to those who received 0.1 milliamps learned the game much better. Although it is not clear how this technology stimulates the brain or why different amperages produce varying effects, direct current stimulation has the potential to induce positive neuroplasticity. Unfortunately, no large randomized clinical trials have been conducted; however, smaller studies have shown that it can improve cognitive functioning in normal healthy adults as well as those with stroke and Parkinson’s disease.126 Thus, this technology may also be effective in those aging with HIV who suffer from cognitive deficits; albeit, studies must be conducted to examine its effectiveness in this clinical population.
The vagus cranial nerve is mixed in nature, capable of afferent (sensory) and efferent (motor) responses. Traditionally, this nerve has been associated with efferent parasympathetic responses in visceral organs, whereas vagus afferent fibers and their many projections in the forebrain and brain stem (thalamus, cerebellum, orbitofrontal cortex, limbic system, hypothalamus, and medulla) modulate cortical synchrony of electrical impulses, as well as cognition and memory-related functions.127–129 Neurostimulation of the vagus nerve can be achieved through implanted devices connected to electrodes near the left cervical branch of the vagus nerve, or transcutaneously at the level of the left auricular portion of the vagus nerve.130 Indeed, vagus nerve stimulation by electric currents has been demonstrated to decrease the frequency and duration of seizures in epileptic individuals,131–134 being now an approved adjunct method of treatment of refractory epilepsies and depression. Incidentally, it was also noted that a significant portion of treated cohorts had improvements in cognition, particularly with respect to attention and memory recall.135–141 These observations lead to the establishment of several studies investigating the potential of vagus nerve stimulation as treatment for various mood and cognitive disorders, including anxiety, depression, and Alzheimer disease.141 To date, vagus nerve stimulation has proved effective in enhancing memory recall of conditioned behaviors in animal models.100 Even though improved attention and memory recall have been described in some studies involving human participants,95, 97, 98 other studies suggested no cognitive improvements with vagus nerve stimulation.103–105 Still, the physiological associations of afferent fibers of the vagus nerve with various cognitive-related aspects of the brain, coupled with evidence suggesting that vagus nerve stimulation might increase cognitive reserve in humans, warrant further exploration of this therapy for persons experiencing chronic cognitive deficits, including older adults with HIV who often sustain cognitive deficits as an intrinsic aspect of their disease course.
Binaural beat therapy is designed to change brain waves in people to a more meditative state. It does this by separately presenting sounds with similar frequencies to each ear (e.g., 345 hertz to the left ear, 355 hertz to the right ear). The brain registers these differences and processes them through the superior olivary nucleus and the reticular activating system which stimulates the cerebral cortex and the thalamus which then produces theta waves.142 Theta waves, which correspond to being in a relaxed meditative state, have been shown to facilitate executive functioning, promote reasoning, and help reduce anxiety.143 For example, in a group of 15 people with anxiety, LaScouarnec and colleagues gave these participants music tapes with binaural beats embedded in the background sounds.144 Participants were instructed to listen to the tapes five times a week for a month. These researchers found that anxiety was reduced after a month. Others studies have found similar effects of binaural beats on anxiety.145 Given the high levels of anxiety and mood problems experienced by many with HIV, and given that such mood problems can interfere with cognitive functioning, binaural beat technology represents a potential solution for augmenting cognition in those aging with HIV.
Conclusion
As the graying of the HIV epidemic continues, healthcare providers will struggle with ways to improve the quality of life of their patients as they age. To facilitate such successful aging, Rowe and Kahn suggested that three things are needed: 1) prevention of disease and/or disability, 2) meaningful social engagement, and 3) maximization of physical and cognitive functioning.146 Clearly, as adults age with HIV, they are vulnerable to developing other co-morbidities such as heart disease and diabetes which can be debilitating,47 may encounter HIV-related stigma that can result in fragile and unsupportive social networks,51 and may experience cognitive deficits. As presented in this article, such cognitive deficits can negatively impact the other two areas needed for successful aging. Poor cognitive functioning can lead to poor medication adherence which can result in even more disease and disability. Reduced cognitive functioning can also lead to poor driving and perhaps reduced driving which in turn may result in less social contact.147 Therefore, addressing the cognitive consequences of aging with HIV cannot be underscored enough given that it is so important for other components of life and successful aging. Unfortunately, sometimes other health concerns seem to take priority over addressing cognitive health, at least until one’s cognitive abilities begin to deteriorate. Thus, it is important to proactively address cognitive health as well as physical health in routine clinic visits. This point is important because cognitive deficits may be avoided or mitigated.
Acknowledgments
We have no financial acknowledgements.
Footnotes
There are no conflicts of interest.
Contributor Information
David E. Vance, Email: devance@uab.edu, Associate Director of the Center for Nursing Research, PhD Coordinator, NB Building Room 2M026, School of Nursing, 1701 University Boulevard, University of Alabama at Birmingham (UAB), Birmingham, AL 35294-1210, Office: 205-934-7589, Fax: 205-996-7183.
Graham J. McDougall, Jr, Email: gjmcdougall@ua.edu, Martha Lucinda Luker Saxon Endowed Chair in Rural Health Nursing, The University of Alabama, Capstone College of Nursing, Box 870358, Tuscaloosa, AL 35487-0358, Office: 205-348-0650.
Natalie Wilson, Email: nataliewilson@uab.edu, University of Alabama at Birmingham. School of Nursing, 1701 University Blvd. Birmingham, AL 35294-1210. Phone: 980-355-1064.
Marcus Otavio Debiasi, Email: made@uab.edu, School of Nursing, NB Building Room 352, University Boulevard, University of Alabama at Birmingham (UAB), Birmingham, AL 35294-1210, Office: 205-996-9825.
Shameka L. Cody, Email: slcody@uab.edu, School of Nursing, NB Building Room 2M026, 1701 University Boulevard, University of Alabama at Birmingham (UAB), Birmingham, AL 35294-1210, Office: 205-934-7589, Fax: 205-996-7183.
References
- 1.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010 Dec 7;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malaspina L, Woods SP, Moore DJ, et al. Successful cognitive aging in persons living with HIV infection. J Neurovirol. 2011 Feb;17(1):110–119. doi: 10.1007/s13365-010-0008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thames AD, Becker BW, Marcotte TD, et al. Depression, cognition, and self-appraisal of functional abilities in HIV: an examination of subjective appraisal versus objective performance. The Clinical neuropsychologist. 2011 Feb;25(2):224–243. doi: 10.1080/13854046.2010.539577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011 Feb;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vance DE, McGuinness T, Musgrove K, Orel NA, Fazeli PL. Successful aging and the epidemiology of HIV. Clin Interv Aging. 2011;6:181–192. doi: 10.2147/CIA.S14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ettenhofer ML, Foley J, Behdin N, Levine AJ, Castellon SA, Hinkin CH. Reaction time variability in HIV-positive individuals. Archives of clinical neuropsychology: the official journal of the National Academy of Neuropsychologists. 2010 Dec;25(8):791–798. doi: 10.1093/arclin/acq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis RJ, Deutsch R, Heaton RK, et al. Neurocognitive impairment is an independent risk factor for death in HIV infection. San Diego HIV Neurobehavioral Research Center Group. Arch Neurol. 1997 Apr;54(4):416–424. doi: 10.1001/archneur.1997.00550160054016. [DOI] [PubMed] [Google Scholar]
- 8.Hardy DJ, Castellon SA, Hinkin CH. Perceptual span deficits in adults with HIV. J Int Neuropsychol Soc. 2004 Jan;10(1):135–140. doi: 10.1017/S1355617704101148. [DOI] [PubMed] [Google Scholar]
- 9.Hardy DJ, Vance DE. The neuropsychology of HIV/AIDS in older adults. Neuropsychol Rev. 2009 Jun;19(2):263–272. doi: 10.1007/s11065-009-9087-0. [DOI] [PubMed] [Google Scholar]
- 10.Marcotte TD. Cognitive disorders in the era of combination HIV antiviral treatment. Focus. 2008 Sep;23(3):1–5. [PubMed] [Google Scholar]
- 11.Thames AD, Kim MS, Becker BW, et al. Medication and finance management among HIV-infected adults: the impact of age and cognition. J Clin Exp Neuropsychol. 2011 Feb;33(2):200–209. doi: 10.1080/13803395.2010.499357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foley JM, Gooding AL, Thames AD, et al. Visuospatial and Attentional Abilities Predict Driving Simulator Performance Among Older HIV-infected Adults. American journal of Alzheimer’s disease and other dementias. 2013 Jan 11; doi: 10.1177/1533317512473192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcotte TD, Wolfson T, Rosenthal TJ, et al. A multimodal assessment of driving performance in HIV infection. Neurology. 2004 Oct 26;63(8):1417–1422. doi: 10.1212/01.wnl.0000141920.33580.5d. [DOI] [PubMed] [Google Scholar]
- 14.Marcotte TD, Lazzaretto D, Scott JC, et al. Visual attention deficits are associated with driving accidents in cognitively-impaired HIV-infected individuals. J Clin Exp Neuropsychol. 2006 Jan;28(1):13–28. doi: 10.1080/13803390490918048. [DOI] [PubMed] [Google Scholar]
- 15.Marcotte TD, Heaton RK, Wolfson T, et al. The impact of HIV-related neuropsychological dysfunction on driving behavior. The HNRC Group. J Int Neuropsychol Soc. 1999 Nov;5(7):579–592. doi: 10.1017/s1355617799577011. [DOI] [PubMed] [Google Scholar]
- 16.Ettenhofer ML, Foley J, Castellon SA, Hinkin CH. Reciprocal prediction of medication adherence and neurocognition in HIV/AIDS. Neurology. 2010 Apr 13;74(15):1217–1222. doi: 10.1212/WNL.0b013e3181d8c1ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinkin CH, Hardy DJ, Mason KI, et al. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004 Jan 1;18( Suppl 1):S19–25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessing LV, Forman JL, Andersen PK. Does lithium protect against dementia? Bipolar disorders. 2010 Feb;12(1):87–94. doi: 10.1111/j.1399-5618.2009.00788.x. [DOI] [PubMed] [Google Scholar]
- 19.Vance DE. A review of metacognition in aging with HIV. Percept Mot Skills. 2006 Dec;103(3):693–696. doi: 10.2466/pms.103.3.693-696. [DOI] [PubMed] [Google Scholar]
- 20.Valcour VG, Shikuma CM, Watters MR, Sacktor NC. Cognitive impairment in older HIV-1-seropositive individuals: prevalence and potential mechanisms. AIDS. 2004 Jan 1;18( Suppl 1):S79–86. doi: 10.1097/00002030-200401001-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valcour V, Shikuma C, Shiramizu B, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004 Sep 14;63(5):822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valcour V, Shikuma C, Shiramizu B, et al. Age, apolipoprotein E4, and the risk of HIV dementia: the Hawaii Aging with HIV Cohort. J Neuroimmunol. 2004 Dec;157(1–2):197–202. doi: 10.1016/j.jneuroim.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 23.Hinkin CH, Castellon SA, Atkinson JH, Goodkin K. Neuropsychiatric aspects of HIV infection among older adults. J Clin Epidemiol. 2001 Dec;54( Suppl 1):S44–52. doi: 10.1016/s0895-4356(01)00446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodkin K, Wilkie FL, Concha M, et al. Aging and neuro-AIDS conditions and the changing spectrum of HIV-1-associated morbidity and mortality. J Clin Epidemiol. 2001 Dec;54( Suppl 1):S35–43. doi: 10.1016/s0895-4356(01)00445-0. [DOI] [PubMed] [Google Scholar]
- 25.Kirk JB, Goetz MB. Human immunodeficiency virus in an aging population, a complication of success. J Am Geriatr Soc. 2009 Nov;57(11):2129–2138. doi: 10.1111/j.1532-5415.2009.02494.x. [DOI] [PubMed] [Google Scholar]
- 26.Vance DE, Fazeli PL, Gakumo CA. The Impact of Neuropsychological Performance on Everyday Functioning Between Older and Younger Adults With and Without HIV. J Assoc Nurses AIDS Care. 2013 Mar;24(2):112–125. doi: 10.1016/j.jana.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fazeli PL, Marceaux JC, Vance DE, Slater L, Long CA. Predictors of cognition in adults with HIV: implications for nursing practice and research. J Neurosci Nurs. 2011 Feb;43(1):36–50. doi: 10.1097/jnn.0b013e3182029790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamat A, Misra V, Cassol E, et al. A plasma biomarker signature of immune activation in HIV patients on antiretroviral therapy. PloS one. 2012;7(2):e30881. doi: 10.1371/journal.pone.0030881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steffener J, Stern Y. Exploring the neural basis of cognitive reserve in aging. Biochim Biophys Acta. 2012 Mar;1822(3):467–473. doi: 10.1016/j.bbadis.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vance DE, Wright MA. Positive and negative neuroplasticity: implications for age-related cognitive declines. J Gerontol Nurs. 2009 Jun;35(6):11–17. doi: 10.3928/00989134-20090428-02. quiz 18–19. [DOI] [PubMed] [Google Scholar]
- 31.Foley JM, Ettenhofer ML, Kim MS, Behdin N, Castellon SA, Hinkin CH. Cognitive reserve as a protective factor in older HIV-positive patients at risk for cognitive decline. Applied neuropsychology. 2012 Jan;19(1):16–25. doi: 10.1080/09084282.2011.595601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vance DE, Webb NM, Marceaux JC, Viamonte SM, Foote AW, Ball KK. Mental stimulation, neural plasticity, and aging: directions for nursing research and practice. J Neurosci Nurs. 2008 Aug;40(4):241–249. doi: 10.1097/01376517-200808000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Vance DE, Roberson AJ, McGuinness TM, Fazeli PL. How neuroplasticity and cognitive reserve protect cognitive functioning. J Psychosoc Nurs Ment Health Serv. 2010 Apr;48(4):23–30. doi: 10.3928/02793695-20100302-01. [DOI] [PubMed] [Google Scholar]
- 34.Vance DE, Crowe M. A Proposed Model of Neuroplasticity and Cognitive Reserve in Older Adults. Activities, Adaptation & Aging. 2006;30(3):61–79. [Google Scholar]
- 35.Satori AC, Vance DE, Slater LZ, Crowe M. The impact of inflammation on cognitive function in older adults: Implications for healthcare practice and research. J Neurosci Nurs. 2012;44(4):206–217. doi: 10.1097/JNN.0b013e3182527690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitehead SN, Cheng G, Hachinski VC, Cechetto DF. Progressive increase in infarct size, neuroinflammation, and cognitive deficits in the presence of high levels of amyloid. Stroke. 2007 Dec;38(12):3245–3250. doi: 10.1161/STROKEAHA.107.492660. [DOI] [PubMed] [Google Scholar]
- 37.Cechetto DF, Hachinski V, Whitehead SN. Vascular risk factors and Alzheimer’s disease. Expert review of neurotherapeutics. 2008 May;8(5):743–750. doi: 10.1586/14737175.8.5.743. [DOI] [PubMed] [Google Scholar]
- 38.Basso MR, Bornstein RA. Estimated premorbid intelligence mediates neurobehavioral change in individuals infected with HIV across 12 months. J Clin Exp Neuropsychol. 2000 Apr;22(2):208–218. doi: 10.1076/1380-3395(200004)22:2;1-1;FT208. [DOI] [PubMed] [Google Scholar]
- 39.Lentz MR, Kim WK, Kim H, et al. Alterations in brain metabolism during the first year of HIV infection. J Neurovirol. 2011 Jun;17(3):220–229. doi: 10.1007/s13365-011-0030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuang X, Scofield VL, Yan M, Stoica G, Liu N, Wong PK. Attenuation of oxidative stress, inflammation and apoptosis by minocycline prevents retrovirus-induced neurodegeneration in mice. Brain Res. 2009 Aug 25;1286:174–184. doi: 10.1016/j.brainres.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams R, Yao H, Peng F, Yang Y, Bethel-Brown C, Buch S. Cooperative induction of CXCL10 involves NADPH oxidase: Implications for HIV dementia. Glia. 2010 Apr;58(5):611–621. doi: 10.1002/glia.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fields RD. The other brain. New York, NY: Simon & Schuster; 2009. [Google Scholar]
- 43.Adam S, Bonsang E, Grotz C, Perelman S. Occupational activity and cognitive reserve: Implications in terms of prevention of cognitive aging and Alzheimer’s disease. Clinical Interventions in Aging. 2013;8:377–390. doi: 10.2147/CIA.S39921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maguire EA, Woollett K, Spiers HJ. London taxi drivers and bus drivers: a structural MRI and neuropsychological analysis. Hippocampus. 2006;16(12):1091–1101. doi: 10.1002/hipo.20233. [DOI] [PubMed] [Google Scholar]
- 45.Boyke J, Driemeyer J, Gaser C, Buchel C, May A. Training-induced brain structure changes in the elderly. J Neurosci. 2008 Jul 9;28(28):7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007 Nov 13;69(20):1911–1920. doi: 10.1212/01.wnl.0000271087.67782.cb. [DOI] [PubMed] [Google Scholar]
- 47.Vance DE, Mugavero M, Willig J, Raper JL, Saag MS. Aging with HIV: a cross-sectional study of comorbidity prevalence and clinical characteristics across decades of life. J Assoc Nurses AIDS Care. 2011 Jan-Feb;22(1):17–25. doi: 10.1016/j.jana.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Valcour VG, Sacktor NC, Paul RH, et al. Insulin resistance is associated with cognition among HIV-1-infected patients: the Hawaii Aging With HIV cohort. J Acquir Immune Defic Syndr. 2006 Dec 1;43(4):405–410. doi: 10.1097/01.qai.0000243119.67529.f5. [DOI] [PubMed] [Google Scholar]
- 49.Valcour V, Shiramizu B. HIV-associated dementia, mitochondrial dysfunction, and oxidative stress. Mitochondrion. 2004 Jul;4(2–3):119–129. doi: 10.1016/j.mito.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Vance DE. The Cognitive Consequences of Stigma, Social Withdrawal, and Depression in Adults Aging with HIV. J Psychosoc Nurs Ment Health Serv. 2013 Mar 22;:1–3. doi: 10.3928/02793695-20130315-01. [DOI] [PubMed] [Google Scholar]
- 51.Shippy RA, Karpiak SE. The aging HIV/AIDS population: fragile social networks. Aging & mental health. 2005 May;9(3):246–254. doi: 10.1080/13607860412331336850. [DOI] [PubMed] [Google Scholar]
- 52.Grant JS, Vance DE, Keltner NL, White W, Raper JL. Reasons why persons living with HIV include individuals in their chosen families. J Assoc Nurses AIDS Care. 2013 Jan-Feb;24(1):50–60. doi: 10.1016/j.jana.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 53.Poindexter C, Shippy RA. Networks of older New Yorkers with HIV: fragility, resilience, and transformation. Aids Patient Care STDS. 2008 Sep;22(9):723–733. doi: 10.1089/apc.2007.0260. [DOI] [PubMed] [Google Scholar]
- 54.Slater LZ, Moneyham L, Vance DE, Raper JL, Mugavero MJ, Childs G. Support, stigma, health, coping, and quality of life in older gay men with HIV. J Assoc Nurses AIDS Care. 2013 Jan-Feb;24(1):38–49. doi: 10.1016/j.jana.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 55.Murphy MLM, Slavich GM, Rohleder N, Miller GE. Targeted rejection triggers differential pro- and anti-inflammatory gene expression in adolescents as a function of social status. Clinical Psychology Science. 2013;1(1):30–40. doi: 10.1177/2167702612455743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vance DE. The Cognitive Consequences of Stigma, Social Withdrawal, and Depression in Adults Aging with HIV. J Psychosoc Nurs Ment Health Serv. 2013 Mar 22;:1–3. doi: 10.3928/02793695-20130315-01. [DOI] [PubMed] [Google Scholar]
- 57.Karelina K, Norman GJ, Zhang N, Morris JS, Peng H, DeVries AC. Social isolation alters neuroinflammatory response to stroke. Proc Natl Acad Sci U S A. 2009 Apr 7;106(14):5895–5900. doi: 10.1073/pnas.0810737106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andres MK, Rockwood KK. Social vulnerability predicts cognitive decline in a prospective cohort of older Canadians. Alzheimer Dis Assoc Disord. 2010;6(4):319–325. doi: 10.1016/j.jalz.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 59.Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007 Feb;64(2):234–240. doi: 10.1001/archpsyc.64.2.234. [DOI] [PubMed] [Google Scholar]
- 60.Vance DE. Implications of positive and negative neuroplasticity on cognition in HIV. Med Sci Mon. 2010 Apr;16(4):HY3–5. [PubMed] [Google Scholar]
- 61.Al-Khindi T, Zakzanis KK, van Gorp WG. Does antiretroviral therapy improve HIV-associated cognitive impairment? A quantitative review of the literature. J Int Neuropsychol Soc. 2011 Nov;17(6):956–969. doi: 10.1017/S1355617711000968. [DOI] [PubMed] [Google Scholar]
- 62.Giunta B, Ehrhart J, Obregon DF, et al. Antiretroviral medications disrupt microglial phagocytosis of beta-amyloid and increase its production by neurons: implications for HIV-associated neurocognitive disorders. Molecular brain. 2011;4(1):23. doi: 10.1186/1756-6606-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vance D, Larsen KI, Eagerton G, Wright MA. Comorbidities and cognitive functioning: implications for nursing research and practice. J Neurosci Nurs. 2011 Aug;43(4):215–224. doi: 10.1097/JNN.0b013e3182212a04. [DOI] [PubMed] [Google Scholar]
- 64.Katon WJ, Lin EH, Williams LH, et al. Comorbid depression is associated with an increased risk of dementia diagnosis in patients with diabetes: a prospective cohort study. J Gen Intern Med. 2010 May;25(5):423–429. doi: 10.1007/s11606-009-1248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vance DE, Dodson JE, Watkins J, Kennedy BH, Keltner NL. Neurological and Psychiatric Diseases and Their Unique Cognitive Profiles: Implications for Nursing Practice and Research. J Neurosci Nurs. 2013 Apr;45(2):77–87. doi: 10.1097/JNN.0b013e3182829038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hinkin CH, Castellon SA, Levine AJ, Barclay TR, Singer EJ. Neurocognition in individuals co-infected with HIV and hepatitis C. J Addict Dis. 2008;27(2):11–17. doi: 10.1300/j069v27n02_02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hilsabeck RC, Castellon SA, Hinkin CH. Neuropsychological aspects of coinfection with HIV and hepatitis C virus. Clin Infect Dis. 2005 Jul 1;41( Suppl 1):S38–44. doi: 10.1086/429494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Foley J, Ettenhofer M, Wright MJ, et al. Neurocognitive functioning in HIV-1 infection: effects of cerebrovascular risk factors and age. The Clinical neuropsychologist. 2010 Feb;24(2):265–285. doi: 10.1080/13854040903482830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakamoto BK, Jahanshad N, McMurtray A, et al. Cerebrovascular risk factors and brain microstructural abnormalities on diffusion tensor images in HIV-infected individuals. J Neurovirol. 2012 Aug;18(4):303–312. doi: 10.1007/s13365-012-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Logroscino G, Kang JH, Grodstein F. Prospective study of type 2 diabetes and cognitive decline in women aged 70–81 years. BMJ. 2004 Mar 6;328(7439):548. doi: 10.1136/bmj.37977.495729.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vance DE, Larsen KI, Eagerton G, Wright MA. Comorbidities and cognitive functioning: Implications for nursing practice and research. J Neurosci Nurs. 2011;43(4):215–224. doi: 10.1097/JNN.0b013e3182212a04. [DOI] [PubMed] [Google Scholar]
- 72.Beeri MS, Ravona-Springer R, Silverman JM, Haroutunian V. The effects of cardiovascular risk factors on cognitive compromise. Dialogues in clinical neuroscience. 2009;11(2):201–212. doi: 10.31887/DCNS.2009.11.2/msbeeri. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vance DE, Moneyham L, Farr KF. Suicidal ideation in adults aging with HIV: neurological and cognitive considerations. J Psychosoc Nurs Ment Health Serv. 2008 Nov;46(11):33–38. doi: 10.3928/02793695-20081101-11. [DOI] [PubMed] [Google Scholar]
- 74.Claypoole KH, Elliott AJ, Uldall KK, et al. Cognitive functions and complaints in HIV-1 individuals treated for depression. Applied neuropsychology. 1998;5(2):74–84. doi: 10.1207/s15324826an0502_3. [DOI] [PubMed] [Google Scholar]
- 75.Bremmer JD. The relationship between cognition and brain changes in posttraumatic stress disorder. Ann N Y Acad Sci. 2006;1266:1–7. doi: 10.1196/annals.1364.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Viamonte S, Vance D, Wadley V, Roenker D, Ball K. Driving-Related Cognitive Performance in Older Adults with Pharmacologically Treated Cardiovascular Disease. Clin Gerontol. 2010 Apr 1;33(2):109–123. doi: 10.1080/07317110903552180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hearps AC, Angelovich TA, Jaworowski A, Mills J, Landay AL, Crowe SM. HIV infection and aging of the innate immune system. Sexual health. 2011 Dec;8(4):453–464. doi: 10.1071/SH11028. [DOI] [PubMed] [Google Scholar]
- 78.Becker JT, Sanders J, Madsen SK, et al. Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain imaging and behavior. 2011 Jun;5(2):77–85. doi: 10.1007/s11682-011-9113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rrapo E, Zhu Y, Tian J, et al. Green Tea-EGCG reduces GFAP associated neuronal loss in HIV-1 Tat transgenic mice. American journal of translational research. 2009;1(1):72–79. [PMC free article] [PubMed] [Google Scholar]
- 80.Eggert D, Dash PK, Gorantla S, et al. Neuroprotective activities of CEP-1347 in models of neuro AIDS. J Immunol. 2010 Jan 15;184(2):746–756. doi: 10.4049/jimmunol.0902962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.High KP, Brennan-Ing M, Clifford DB, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. 2012 Jul 1;60( Suppl 1):S1–18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vance DE, Fazeli PL, Moneyham L, Keltner NL, Raper JL. Assessing and treating forgetfulness and cognitive problems in adults with HIV. J Assoc Nurses AIDS Care. 2013 Jan-Feb;24(1 Suppl):S40–60. doi: 10.1016/j.jana.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vance DE, Graham MA, Fazeli PL, Heaton K, Moneyham L. An overview of nonpathological geroneuropsychology: implications for nursing practice and research. J Neurosci Nurs. 2012 Feb;44(1):43–53. doi: 10.1097/JNN.0b013e31823ae48b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vance DE, McNees P, Meneses K. Technology, cognitive remediation, and nursing: directions for successful cognitive aging. J Gerontol Nurs. 2009 Feb;35(2):50–56. doi: 10.3928/00989134-20090201-09. [DOI] [PubMed] [Google Scholar]
- 85.Vance DE, Fazeli PL, Ross LA, Wadley VG, Ball KK. Speed of Processing Training With Middle-Age and Older Adults With HIV: A Pilot Study. J Assoc Nurses AIDS Care. 2012 May 11; doi: 10.1016/j.jana.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vance DE. Speed of processing in older adults: a cognitive overview for nursing. J Neurosci Nurs. 2009 Dec;41(6):290–297. doi: 10.1097/jnn.0b013e3181b6beda. [DOI] [PubMed] [Google Scholar]
- 87.Wolinsky FD, Mahncke HW, Weg MW, et al. The ACTIVE cognitive training interventions and the onset of and recovery from suspected clinical depression. J Gerontol B Psychol Sci Soc Sci. 2009 Sep;64(5):577–585. doi: 10.1093/geronb/gbp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wolinsky FD, Vander Weg MW, Martin R, et al. The effect of speed-of-processing training on depressive symptoms in ACTIVE. J Gerontol A Biol Sci Med Sci. 2009 Apr;64(4):468–472. doi: 10.1093/gerona/gln044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wolinsky FD, Vander Weg MW, Martin R, et al. Does cognitive training improve internal locus of control among older adults? J Gerontol B Psychol Sci Soc Sci. 2010 Sep;65(5):591–598. doi: 10.1093/geronb/gbp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Linssen AM, Vuurman EF, Sambeth A, Riedel WJ. Methylphenidate produces selective enhancement of declarative memory consolidation in healthy volunteers. Psychopharmacology (Berl) 2012 Jun;221(4):611–619. doi: 10.1007/s00213-011-2605-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hinkin CH, Castellon SA, Hardy DJ, Farinpour R, Newton T, Singer E. Methylphenidate improves HIV-1-associated cognitive slowing. J Neuropsychiatry Clin Neurosci. 2001 Spring;13(2):248–254. doi: 10.1176/jnp.13.2.248. [DOI] [PubMed] [Google Scholar]
- 92.Charach G, Kaysar N, Grosskopf I, Rabinovich A, Weintraub M. Methylphenidate has positive hypocholesterolemic and hypotriglyceridemic effects: new data. J Clin Pharmacol. 2009 Jul;49(7):848–851. doi: 10.1177/0091270009336736. [DOI] [PubMed] [Google Scholar]
- 93.Mar Fan HG, Clemons M, Xu W, et al. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer. 2008 Jun;16(6):577–583. doi: 10.1007/s00520-007-0341-9. [DOI] [PubMed] [Google Scholar]
- 94.Leserman J, Barroso J, Pence BW, Salahuddin N, Harmon JL. Trauma, stressful life events and depression predict HIV-related fatigue. AIDS Care. 2008 Nov;20(10):1258–1265. doi: 10.1080/09540120801919410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Turner DC, Clark L, Dowson J, Robbins TW, Sahakian BJ. Modafinil improves cognition and response inhibition in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2004 May 15;55(10):1031–1040. doi: 10.1016/j.biopsych.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 96.McElhiney M, Rabkin J, Van Gorp W, Rabkin R. Modafinil effects on cognitive function in HIV+ patients treated for fatigue: a placebo controlled study. J Clin Exp Neuropsychol. 2010 Jun;32(5):474–480. doi: 10.1080/13803390903201769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schifitto G, Navia BA, Yiannoutsos CT, et al. Memantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy study. AIDS. 2007 Sep 12;21(14):1877–1886. doi: 10.1097/QAD.0b013e32813384e8. [DOI] [PubMed] [Google Scholar]
- 98.Vance DE, Eagerton G, Harnish B, McKie P, Fazeli PL. Cognitive prescriptions. J Gerontol Nurs. 2011 Apr;37(4):22–29. doi: 10.3928/00989134-20101202-03. quiz 30–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Restak R. Think smart: A neuroscientist’s prescription for improving your brain performance. New York, NY: Penguin; 2009. [Google Scholar]
- 100.Atkins JH, Rubenstein SL, Sota TL, et al. Impact of social support on cognitive symptom burden in HIV/AIDS. AIDS Care. 2010 Jul;22(7):793–802. doi: 10.1080/09540120903482994. [DOI] [PubMed] [Google Scholar]
- 101.Foster PP, Rosenblatt KP, Kuljis RO. Exercise-induced cognitive plasticity, implications for mild cognitive impairment and Alzheimer’s disease. Frontiers in neurology. 2011;2:28. doi: 10.3389/fneur.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Restak R. Think smart: A neuroscientist’s prescription for improving your brain’s performance. New York, NY: Riverhead Books; 2009. [Google Scholar]
- 103.Breitling LP, Perna L, Muller H, Raum E, Kliegel M, Brenner H. Vitamin D and cognitive functioning in the elderly population in Germany. Exp Gerontol. 2012 Jan;47(1):122–127. doi: 10.1016/j.exger.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 104.Dullemeijer C, Durga J, Brouwer IA, et al. n 3 fatty acid proportions in plasma and cognitive performance in older adults. Am J Clin Nutr. 2007 Nov;86(5):1479–1485. doi: 10.1093/ajcn/86.5.1479. [DOI] [PubMed] [Google Scholar]
- 105.Durga J, Verhoef P, Anteunis LJ, Schouten E, Kok FJ. Effects of folic acid supplementation on hearing in older adults: a randomized, controlled trial. Ann Intern Med. 2007 Jan 2;146(1):1–9. doi: 10.7326/0003-4819-146-1-200701020-00003. [DOI] [PubMed] [Google Scholar]
- 106.Vance DE, Heaton K, Eaves Y, Fazeli PL. Sleep and cognition on everyday functioning in older adults: implications for nursing practice and research. J Neurosci Nurs. 2011 Oct;43(5):261–271. doi: 10.1097/JNN.0b013e318227efb2. quiz 272–263. [DOI] [PubMed] [Google Scholar]
- 107.Kramer AF, Colcombe SJ, McAuley E, et al. Enhancing brain and cognitive function of older adults through fitness training. J Mol Neurosci. 2003;20(3):213–221. doi: 10.1385/JMN:20:3:213. [DOI] [PubMed] [Google Scholar]
- 108.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychological science. 2003 Mar;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 109.Colcombe SJ, Erickson KI, Raz N, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003 Feb;58(2):176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- 110.Turner J. Your brain on food: A nutrient rich diet can protect cognitive health. Generations. 2011;35(2):99–106. [Google Scholar]
- 111.Durga J, van Boxtel MP, Schouten EG, et al. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet. 2007 Jan 20;369(9557):208–216. doi: 10.1016/S0140-6736(07)60109-3. [DOI] [PubMed] [Google Scholar]
- 112.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006 Dec;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 113.Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PloS one. 2008;3(6):e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Spudich S, Gonzalez-Scarano F. HIV-1-related central nervous system disease: current issues in pathogenesis, diagnosis, and treatment. Cold Spring Harbor perspectives in medicine. 2012 Jun;2(6):a007120. doi: 10.1101/cshperspect.a007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Harezlak J, Buchthal S, Taylor M, et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS. 2011 Mar 13;25(5):625–633. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kamat A, Lyons JL, Misra V, et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr. 2012 Jul 1;60(3):234–243. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brenchley JM, Paiardini M, Knox KS, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008 Oct 1;112(7):2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kessing LV, Forman JL, Andersen PK. Do continued antidepressants protect against dementia in patients with severe depressive disorder? Int Clin Psychopharmacol. 2011 Nov;26(6):316–322. doi: 10.1097/YIC.0b013e32834ace0f. [DOI] [PubMed] [Google Scholar]
- 119.Wilson NL, Moneyham LD, Alexandrov AW. A systematic review of probiotics as a potential intervention to restore gut health in HIV infection. J Assoc Nurses AIDS Care. 2013 Mar-Apr;24(2):98–111. doi: 10.1016/j.jana.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 120.Diniz BS, Machado-Vieira R, Forlenza OV. Lithium and neuroprotection: translational evidence and implications for the treatment of neuropsychiatric disorders. Neuropsychiatric disease and treatment. 2013;9:493–500. doi: 10.2147/NDT.S33086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kessing LV, Sondergard L, Forman JL, Andersen PK. Lithium treatment and risk of dementia. Arch Gen Psychiatry. 2008 Nov;65(11):1331–1335. doi: 10.1001/archpsyc.65.11.1331. [DOI] [PubMed] [Google Scholar]
- 122.Forlenza OV, Diniz BS, Radanovic M, Santos FS, Talib LL, Gattaz WF. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011 May;198(5):351–356. doi: 10.1192/bjp.bp.110.080044. [DOI] [PubMed] [Google Scholar]
- 123.Cerruti C, Schlaug G. Anodal transcranial direct current stimulation of the prefrontal cortex enhances complex verbal associative thought. J Cogn Neurosci. 2009 Oct;21(10):1980–1987. doi: 10.1162/jocn.2008.21143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bullard LM, Browning ES, Clark VP, et al. Transcranial direct current stimulation’s effect on novice versus experienced learning. Exp Brain Res. 2011 Aug;213(1):9–14. doi: 10.1007/s00221-011-2764-2. [DOI] [PubMed] [Google Scholar]
- 125.Clark VP, Coffman BA, Mayer AR, et al. TDCS guided using fMRI significantly accelerates learning to identify concealed objects. Neuroimage. 2012 Jan 2;59(1):117–128. doi: 10.1016/j.neuroimage.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nerdone R, Bergmann J, Christova M, et al. Effect of transcranial brain stimulation for the treatment of Alzheimer’s disease: A review. International Journal of Alzheimer’s Disease. 2012:1–5. doi: 10.1155/2012/687909. Article ID 687909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chae JH, Nahas Z, Lomarev M, et al. A review of functional neuroimaging studies of vagus nerve stimulation (VNS) J Psychiatr Res. 2003 Nov-Dec;37(6):443–455. doi: 10.1016/s0022-3956(03)00074-8. [DOI] [PubMed] [Google Scholar]
- 128.Pardo JV, Sheikh SA, Schwindt GC, et al. Chronic vagus nerve stimulation for treatment-resistant depression decreases resting ventromedial prefrontal glucose metabolism. Neuroimage. 2008 Aug 15;42(2):879–889. doi: 10.1016/j.neuroimage.2008.04.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Peuker ET, Filler TJ. The nerve supply of the human auricle. Clin Anat. 2002 Jan;15(1):35–37. doi: 10.1002/ca.1089. [DOI] [PubMed] [Google Scholar]
- 130.Busch V, Zeman F, Heckel A, Menne F, Ellrich J, Eichhammer P. The effect of transcutaneous vagus nerve stimulation on pain perception--an experimental study. Brain stimulation. 2013 Mar;6(2):202–209. doi: 10.1016/j.brs.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 131.Ardesch JJ, Buschman HP, Wagener-Schimmel LJ, van der Aa HE, Hageman G. Vagus nerve stimulation for medically refractory epilepsy: a long-term follow-up study. Seizure. 2007 Oct;16(7):579–585. doi: 10.1016/j.seizure.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 132.Benifla M, Rutka JT, Logan W, Donner EJ. Vagal nerve stimulation for refractory epilepsy in children: indications and experience at The Hospital for Sick Children. Childs Nerv Syst. 2006 Aug;22(8):1018–1026. doi: 10.1007/s00381-006-0123-6. [DOI] [PubMed] [Google Scholar]
- 133.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and Findings From the Rush Memory and Aging Project. Current Alzheimer research. 2012 Apr 2; doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sirven JI, Sperling M, Naritoku D, et al. Vagus nerve stimulation therapy for epilepsy in older adults. Neurology. 2000 Mar 14;54(5):1179–1182. doi: 10.1212/wnl.54.5.1179. [DOI] [PubMed] [Google Scholar]
- 135.Murphy JV, Hornig G, Schallert G. Left vagal nerve stimulation in children with refractory epilepsy. Preliminary observations. Arch Neurol. 1995 Sep;52(9):886–889. doi: 10.1001/archneur.1995.00540330064016. [DOI] [PubMed] [Google Scholar]
- 136.Helmers SL, Wheless JW, Frost M, et al. Vagus nerve stimulation therapy in pediatric patients with refractory epilepsy: retrospective study. J Child Neurol. 2001 Nov;16(11):843–848. doi: 10.1177/08830738010160111101. [DOI] [PubMed] [Google Scholar]
- 137.Valencia I, Holder DL, Helmers SL, Madsen JR, Riviello JJ., Jr Vagus nerve stimulation in pediatric epilepsy: a review. Pediatr Neurol. 2001 Nov;25(5):368–376. doi: 10.1016/s0887-8994(01)00319-8. [DOI] [PubMed] [Google Scholar]
- 138.Handforth A, DeGiorgio CM, Schachter SC, et al. Vagus nerve stimulation therapy for partial-onset seizures - A randomized active-control trial. Neurology. 1998 Jul;51(1):48–55. doi: 10.1212/wnl.51.1.48. [DOI] [PubMed] [Google Scholar]
- 139.Dodrill CB, Morris GL. Effects of Vagal Nerve Stimulation on Cognition and Quality of Life in Epilepsy. Epilepsy & behavior: E&B. 2001 Feb;2(1):46–53. doi: 10.1006/ebeh.2000.0148. [DOI] [PubMed] [Google Scholar]
- 140.Elger G, Hoppe C, Falkai P, Rush AJ, Elger CE. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res. 2000 Dec;42(2–3):203–210. doi: 10.1016/s0920-1211(00)00181-9. [DOI] [PubMed] [Google Scholar]
- 141.Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci. 1999 Jan;2(1):94–98. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- 142.Carter C. Healthcare performance and the effects of the binaural beats on human blood pressure and heart rate. Journal of hospital marketing & public relations. 2008;18(2):213–219. doi: 10.1080/15390940802234263. [DOI] [PubMed] [Google Scholar]
- 143.Baijal S, Srinivasan N. Theta activity and meditative states: spectral changes during concentrative meditation. Cognitive processing. 2010 Feb;11(1):31–38. doi: 10.1007/s10339-009-0272-0. [DOI] [PubMed] [Google Scholar]
- 144.LaScouarnec R, Poirier R, Owen J, Gautheir J, Taylor A, Foresman P. Use of binaural beat tapes for treatment of anxiety: A pilot study of tape performance and outcomes. Altern Ther Health Med. 2001;7(1):58–63. [PubMed] [Google Scholar]
- 145.Lewis AK, Osborn IP, Roth R. The effect of hemispheric synchronization on intraoperative analgesia. Anesth Analg. 2004 Feb;98(2):533–536. doi: 10.1213/01.ANE.0000096181.89116.D2. table of contents. [DOI] [PubMed] [Google Scholar]
- 146.Rowe JW, Kahn RL. Successful aging. Gerontologist. 1997 Aug;37(4):433–440. doi: 10.1093/geront/37.4.433. [DOI] [PubMed] [Google Scholar]
- 147.Liddle J, Gustafsson L, Bartlett H, McKenna K. Time use, role participation and life satisfaction of older people: Impact of driving status. Australian Occupational Therapy Journal. 2012;59(5):384–392. doi: 10.1111/j.1440-1630.2011.00956.x. [DOI] [PubMed] [Google Scholar]