Summary
Objective
ADAMTS13 cleaves von Willebrand factor (VWF), thereby inhibiting thrombus formation. Proteolytic cleavage relies on the amino-terminal (MDTCS) domains, but the role of the more distal carboxyl-terminal domains of ADAMTS13 is not fully understood. A previous study demonstrated the presence of multiple surface- exposed free sulfhydryls on ADAMTS13 that appeared to interact with those on VWF under shear. Here, we determined the physiological relevance of such an interaction in antithrombotic responses under flow.
Approaches and Results
A microfluidic assay demonstrated that a carboxyl-terminal fragment of ADAMTS13, comprising either 2-8 thrombospondin type 1 (TSP1) repeats and CUB domains (T2C) or 5-8 TSP1 repeats and CUB domains (T5C), directly inhibited platelet adhesion/aggregation on a collagen surface under arterial shear. In addition, an intravital microscopic imaging analysis showed that the carboxyl-terminal fragment of ADAMTS13 (T2C or T5C) was capable of inhibiting the formation and elongation of platelet-decorated ultra large (UL) VWF strings and the adhesion of platelets/leukocytes on endothelium in mesenteric venules after oxidative injury. The inhibitory activity of T2C and T5C on platelet aggregation and ULVWF string formation was dependent on the presence of their surface free thiols; pretreatment of T2C and T5C or full-length ADAMTS13 with N-ethylmaleimide that reacts with free sulfhydryls abolished or significantly reduced its antithrombotic activity.
Conclusion
Our results demonstrate for the first time that the carboxyl-terminus of ADAMTS13 has direct antithrombotic activity in a free-thiol dependent manner. The free thiols in the carboxyl-terminal domains of ADAMTS13 may also contribute to the overall antithrombotic function of ADAMTS13 under pathophysiological conditions.
Introduction
von Willebrand factor (VWF), an ultra large (UL) or large multimeric adhesion glycoprotein in blood, is primarily synthesized in endothelial cells, megakaryocytes, and platelets 1. The newly synthesized VWF is stored in the Weibel-Palade bodies of endothelial cells or α-granules of platelets. ULVWF is released from these storage organelles upon stimulation by epinephrine, histamine, thrombin, and inflammatory cytokines or toxins 2-4. The newly released ULVWF forms “string-like” structures anchored on the cell surface 2-4, which are hyperactive and recruit flowing platelets from circulation to the site of endothelial activation or injury. Cell-bound ULVWF strings are highly susceptible to proteolysis by plasma metalloprotease ADAMTS13 2, 3. This proteolytic cleavage results in a VWF-free endothelial surface, preventing unwanted and excessive platelet adhesion/aggregation and thrombus formation after injury. However, VWF released into circulation remains quite large and therefore requires further processing by plasma ADAMTS13, 5 other leukocyte proteases 6, and complement factor H 7. An inability to cleave or process cell-bound ULVWF or circulating large VWF multimers into smaller ones results in a potentially fatal syndrome, thrombotic thrombocytopenic purpura (TTP)8, 9, which is characterized by severe thrombocytopenia and microangiopathic hemolytic anemia with various degrees of organ failure 8, 9.
Previous studies have demonstrated that the proteolytic cleavage of VWF by ADAMTS13 depends on the amino-terminal portion of ADAMTS13 (i.e. MDTCS domains) 10-16. An extensive exosite interaction between the ADAMTS13-DTCS domains and the VWF-A2 domain 11, 17 appears to be necessary for productive VWF cleavage. A mutation or deletion in the DTCS domains 18-20 or an autoantibody that targets the spacer domain or others 19, 21-24 dramatically reduces or inhibits the ability of ADAMTS13 to cleave its VWF substrate. However, the role of more distal C-terminal domains of ADAMTS13 including the 2-8 TSP1 repeats and CUB domains is little known.
Recently, Yeh et al have reported that the C-terminal TSP1 repeats and CUB domains of ADAMTS13 contain a cluster of surface-exposed free thiols (-SH) 25. Using biochemical assays, these investigators demonstrated that the free thiols on recombinant ADAMTS13 interact with those on cell-bound ULVWF or soluble VWF under flow 25. However, the physiological relevance of such an interaction has not been fully established. We hypothesize that by interacting with the free thiols on VWF, the C-terminal domains of ADAMTS13 may have direct antithrombotic activity under pathophysiological conditions.
To test this hypothesis, we have developed a microfluidic flow assay and an intravital microscopic imaging technique to assess the role of ADAMTS13C-terminal domains and their surface free thiols. We demonstrate that a C-terminal ADAMTS13 fragment comprising either 2-8 TSP1 repeats and CUB domains (T2C) or 5-8 TSP1 repeats and CUB domains (T5C) inhibits platelet aggregation on a collagen-coated surface under arterial flow. Moreover, the C-terminal fragment of ADAMTS13 (T2C or T5C) reduces the formation and elongation of platelet-decorated ULVWF strings and the aggregation of platelets/leukocytes on the endothelial cell surface in mesenteric venules after oxidative injury. These inhibitory activities are nearly completely abolished after T2C or T5C is pretreated with the alkylating agent N-ethylmaleimide (NEM) that blocks its surface free thiols. Moreover, pretreatment of a full-length ADAMTS13 with NEM also reduces its ability by ~50% to inhibit the formation of platelet-decorated ULVWF strings on endothelial cells. Together, these results indicate that the C-terminal domains of ADAMTS13 are of direct antithrombotic activity in a free thiol-dependent manner, and these free thiols on the C-terminal domains of ADAMTS13 may contribute to overall antithrombotic activity of ADAMTS13 under (patho) physiologically relevant conditions.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
Characterization of recombinant ADAMTS13 and its C-terminal fragments
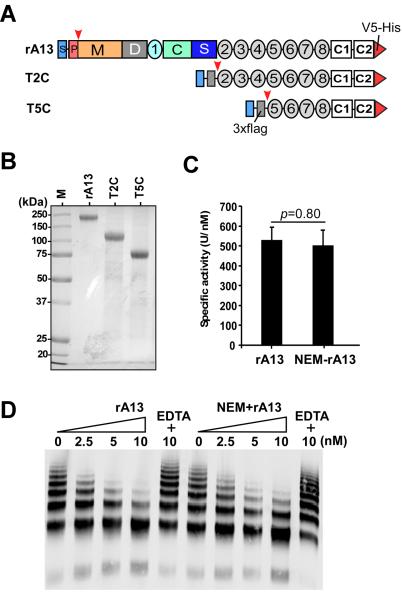
Recombinant human full-length ADAMTS13 (rA13) and its C-terminal fragments (T2C and T5C) (Fig. 1A) were expressed in stably transfected CHO-s cell lines and purified to homogeneity with a combination of Q-fast flow anion exchange and Ni-chelating affinity chromatography 15, 18. As shown in Fig. 1B, rA13, T2C, and T5C were purified to greater than 95% purity, revealed by a SDS-polyacrylamide gel with coomassie blue staining. The C-terminal domains of ADAMTS13 were shown in a previous study to contain multiple surface-exposed free thiols 25. To confirm the presence of free thiols in our recombinant products, we performed labeling with N-ethylmaleimide (NEM). In these experiments, purified rA13, T2C, and T5C were incubated at 4 °C with NEM (5 mM) at pH 7.5. Under these conditions, NEM specifically reacts with the sulfhydryl (-SH) groups on free cysteine residues without affecting preformed disulfide bonds and protein structure and functionality. In our control experiments, we found that the multimer distribution of NEM-treated VWF was not different from that of untreated VWF (data not shown). In addition, the NEM-labeled rA13 exhibited normal proteolytic activity in cleaving the FRETS-VWF73 peptide (Fig. 1C) and multimeric VWF (Fig. 1D) as compared with the untreated rA13.
Figure 1.
Constructs and purified recombinant ADAMTS13 and its C-terminal fragments. A. Full-length human ADAMTS13 (rA13) comprises a signal peptide (S), a propeptide (P), a metalloprotease (M), a disintegrin domain (D), a cysteine rich (C), the first thrombospondin type 1 repeats (TSP1), and a spacer domain (S). The more distal C-terminus contains 7 more TSP1 repeats (2-8) and two CUB domains (C1-C2). The signal peptide and propeptide are cleaved ( ) upon secretion and a V5-His epitope is attached to the C-terminal end in each construct. In addition, a 3xFlag tag was added to the C-terminal fragments of ADAMTS13 after the leader sequence (Igk) was removed ( ) during secretion. T2C or T5C refers the construct consisting of 2-8 TSP1 repeats plus CUB domains or 5-8 TSP1 repeats plus CUB domains. B. SDS-polyacrylamide (10%) gel electrophoresis and coomassie blue staining determined the purity of recombinant ADAMTS13 (rA13), T2C, and T5C (~5-10 μg of total protein/lane). M in lane 1 is a pre-stained and broad range protein marker. C and D are the relative activity of recombinant ADAMTS13 (rA13) with or without NEM treatment in the proteolytic cleavage of FRETS-VWF73 peptide and multimeric VWF, respectively.
The NEM-labeled rA13 and the C-terminal fragments were resolved on a 10% SDS-polyacrylamide gel and stained with coomassie blue. Specific bands were cut out from the gel and digested overnight with trypsin using a standard protocol established at the Penn Proteomic Core. The resulting peptides were identified by a Nano LC-tandem mass spectrometer. The mass to charge ratio (m/z) was determined for each peptide derived from the proteins with or without NEM modifications. Table 1 shows the identification of cysteine residues that were modified by NEM in the C-terminal domains of ADAMTS13. These included Cys710, Cys769, Cys799, Cys919, Cys933, Cys977, Cys1213, and Cys1275 in the C-terminal TSP1 repeats and CUB-1 domain of ADAMTS13. Occasionally, additional NEM-labeled cysteine residues were detected in the N-terminal MDTCS domains (not shown) if a full-length rA13 protein instead of C-terminal fragments of ADAMTS13 was used in the experiments. However, due to the low recovery of peptides in the N-terminal MDTCS domains, the exact number of free thiols in this region remains to be determined. Four other cysteine residues including Cys710, Cys799, Cys919, and Cys977 found to be modified by NEM in the current study were not identified in the previous study 25. One cysteine residue (Cys1192) that was identified to be a free thiol in the previous study was not determined in this study due to failure of recovering the peptide (Table I). Nevertheless, our results are largely in agreement with those reported previously 25, independently confirming the presence of multiple free cysteine residues that are clustered on the C-terminal TSP1 repeats and CUB domains of recombinant ADAMTS13 protein.
Table I.
Identification of the free thiols by Nano LC-tandem mass spectrometry in the C-terminal domains of ADAMTS 13
| Peptide | Cys residue | Location | This study | Yeh et al |
|---|---|---|---|---|
| WVNYSCLDQAR | 710 | TSP1-2 | + | − |
| CVEAQGSLLK | 769 | TSP1-3 | + | + |
| AGAQQPAVALETCNPQPCPAR | 799 | TSP1-3 | + | − |
| FLCMDSALR | 919 | TSP1-5 | + | − |
| VPVQEELCGLASKPGSR | 933 | TSP1-5 | + | + |
| RILYCAR | 977 | TSP1-6 | + | − |
| LLPGPQENSVQSSACGR | 1192 | CUB1 | ND | + |
| GPGQADCAVAIGRPLGEVVTLR | 1213 | CUB1 | + | + |
| RCGRPGGGVLLR | 1275 | CUB1 | + | + |
Notes: TSP1, thrombospondin type 1 repeats; CUB, complement C1r/C1s, Uegf, bone morphogenic protein 1; the underlined cysteine residue is potentially reactive (+) or not reactive (−) with N-ethylmaleimide; ND, not determined because the peptide was not recovered.
Development of a microfluidic flow assay for assessing proteolytic and non-proteolytic activities of ADAMTS13 under flow
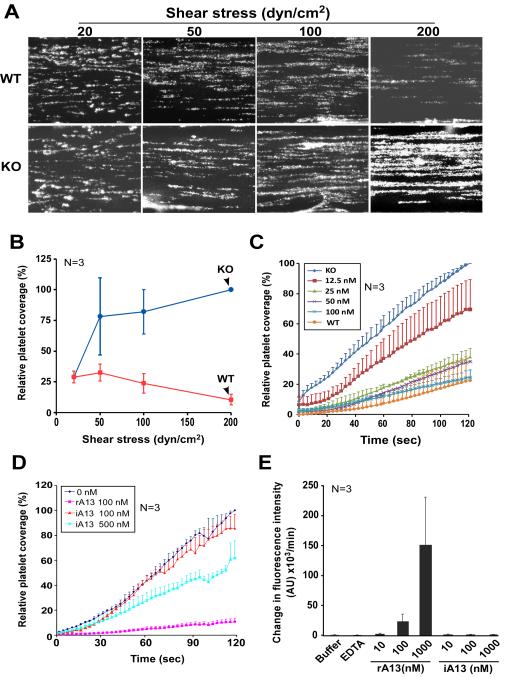
To assess the biological functions of ADAMTS13 under more physiologically relevant conditions, we established a microfluidic flow assay. In this assay, the microfluidic flow channels were coated with fibrillar collagen and perfused over with PPACK-anticoagulated whole blood from wild type (WT) or Adamts13−/− (KO) mice under various shear stresses. The percentage coverage of fluorescein-labeled platelets on the collagen surface over time was determined. We showed that in the absence of plasma ADAMTS13 (such as in KO mice), the percentage of surface coverage by fluorescent platelets increased dramatically as a function of increasing shear stresses (Figs. 2A & 2B). In contrast, in the presence of physiological concentrations of ADAMTS13 (such as in WT mice), the percentage of platelet coverage on the collagen surface did not significantly increase (or even slightly decreased) as a function of increasing shear stresses (Figs. 2A & 2B). For instance, at the highest shear stress (~200 dyne/cm2), the percentage of KO mouse platelets that adhered and aggregated on the collagen surface was ~8-fold higher than that of WT mouse platelets (p<0.01) (Fig. 2B). However, such a difference was not observed when blood was perfused at a relatively low arterial shear stress (20 dyne/cm2) (Fig. 2B). These results are highly consistent with those reported previously26-28, suggesting that mice lacking plasma ADAMTS13 activity are prothrombotic, particularly under pathological shear stresses.
Figure 2.
A microfluidic flow system determines antithrombotic activity of rA13 and its C-terminal fragments. A. Representative images depicting the surface coverage of platelet aggregates on a fibrillar collagen-coated surface under various arterial shears (20, 50, 100, and 200 dyne/cm2) after perfusion of PPACK-anticoagulated whole blood from wild-type mice (WT) and Adamts13−/− (KO) mice. B. Quantitation of relative surface platelet coverage (or relative fluorescent intensity) from images obtained in panel A as a function of fluid shear stress from three individual experiments (n=3). The maximal fluorescent intensity in the KO mice was defined as 100% at the end of 120 seconds. The data shown are the means ± one standard deviation. C. The percentage of surface coverage of WT and KO murine platelets on fibrillar collagen under high shear (100 dyne/cm2) in the presence of rA13 (0, 12.5, 25, 50, and 100 nM) (mean ± standard deviation, N=3). D. The percentage of murine (KO) platelet coverage on a fibrillar collagen surface in the presence of active human rA13 (100 nM) and heat-inactivated rA13 (iA13) (100 and 500 nM) under 100 dyne/cm2. The data shown are the mean+ standard error) (n=3). E. The relative proteolytic activity of rA13 and heat-inactivated rA13 (iA13) at various concentrations (10, 100, and 1,000 nM) in cleaving the FRETS-VWF73 peptide.
The prothrombotic phenotypes of KO mice may be attributed by increased plasma levels of ULVWF and lack of anti-thrombotic ADAMTS13 protease. To further determine the role of plasma ADAMTS13 in the surface coverage of murine platelets on the collagen surface, we reconstituted PPACK-anticoagulated whole blood from KO mice with human rA13 at various concentrations (0, 12.5, 25, 50, and 100 nM). After perfusion through collagen-coated microfluidic flow channels under ~100 dyne/cm2, we found that the percentage of platelet coverage was inhibited by supplemented rA13 in a concentration-dependent manner (Fig. 2C). For instance, in the presence of 12.5 nM and 100 nM of rA13, the percentage of platelet coverage at the end of 2-min perfusion reduced by 30% and 80%, respectively, similar to that in WT mouse platelets (Fig. 2C). Surprisingly, even in the presence of heat-inactivated rA13 (500 nM), which exhibited no residual proteolytic activity toward FRETS-VWF73 (Fig. 2E), the percentage of platelet coverage on the collagen surface was also significantly reduced (Fig. 2D). There results suggest that the microfluidic flow assay is highly sensitive to assess both proteolytic and non-proteolytic activities of ADAMTS13.
C-terminal fragments of ADAMTS13 are capable of inhibiting platelet adhesion and aggregation on a collagen surface under flow
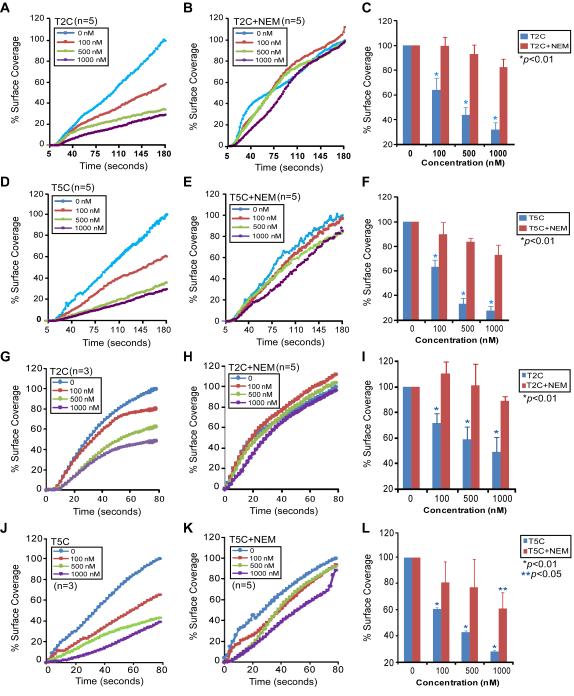
With the establishment of the microfluidic flow assay, we asked the question if the C-terminal domains of ADAMTS13, in the absence of proteolytic activity, inhibit platelet adhesion/aggregation on a collagen surface under flow. PPACK-anticoagulated human or murine (KO) whole blood was incubated with increasing concentrations of a C-terminal fragment of ADAMTS13 (T2C or T5C) and then flown over soluble (Figs. 3A-F) or fibrillar (Figs. 3G-L) collagen-coated surface under arterial shear stresses. In either case, T2C or T5C inhibited the percentage of platelet coverage on the collagen surface in a concentration-dependent manner. For instance, in the presence of ~500-1000 nM of T2C (Figs. 3A & 3C) or T5C (Figs. 3D & 3F), the percentages of human platelets adhered and aggregated on the soluble collagen surface at the end of 3-min perfusion were reduced by ~70% or ~80% (Figs. 3C & 3F), similar to the inhibitory effect of 10 nM rA13 (not shown). Similar but slightly less inhibitory effects of T2C (Figs. 3G & 3I) and T5C (Figs. 3J & 3L) on the percentage of KO mouse platelet coverage on the fibrillar collagen surface under high shear (~200 dyne/cm2) were observed. These results demonstrate for the first time that the C-terminal domains of ADAMTS13 without a catalytic subunit are capable of inhibiting platelet adhesion/aggregation and thrombus formation under arterial shear stresses.
Figure 3.
The C-terminal ADAMTS13 fragments inhibit platelet aggregation on a collagen surface under flow. A to F represent dynamic changes in the mean percentage of coverage or aggregation of human platelets from a PPACK anti-coagulated whole blood on type 1 soluble collagen surface under shear (~20 dyne/cm2) over time (180 seconds) in the absence (0 nM) and presence of various concentrations (100, 500, and 1000 nM) of untreated (A, D) or NEM-treated (B, E) T2C or T5C as indicated in each panel. G to L indicate the dynamic changes in the mean percentage of coverage of murine (Adamts13−/−) platelets on type 1 fibrillar collagen surface over time under high shear (~100 dyne/cm2) in the absence (0 nM) and presence of various concentrations (100, 500, and 1000 nM) of untreated (G, J) or NEM-treated (H, K) T2C or T5C as indicated in each panel. C, F, I, and L are the means and standard errors (SEM) of the mean platelet coverage or aggregation on collagen surfaces at the end of perfusion. Each experiment was repeated for 3-5 times as indicated in each panel. Statistical analysis was performed by ANOVA analysis. The p values<0.05 and 0.01 were considered statistically significant and highly significant, respectively.
To determine whether the inhibitory activity of T2C or T5C on platelet adhesion and aggregation on the collagen surfaces depends on its surface free thiols, a purified T2C or T5C was pretreated with NEM which blocks surface free thiols. As shown, reconstitution of PPACK-anticoagulated KO mouse whole blood with NEM-treated T2C (Figs. 3B & E) or T5C (Figs. 3H & 3K) at various concentrations (0, 100, 500, and 1000 nM) had no significant inhibitory effect on the percentage of platelet coverage on the fibrillar collagen surface under the same conditions (Figs. 3C, F, I, & L-red bars). Only at the highest concentration (1,000 nM) was the inhibitory activity of NEM-treated T5C on platelet adhesion/aggregation detected (Figs. 3L-red bar). These results indicate that antithrombotic activity of the C-terminal domains of ADAMTS13 is mediated by their surface thiols.
Of interest, binding of rA13 and C-terminal fragments to immobilized type 1 collagen and VWF (as positive controls) was performed. As shown in the suppl. Fig. I, rA13, T2C, and T5C bound to collagen with dissociation constants (KD) (S) of 257±87 nM, 79±29 nM, and 106±30 nM, respectively. The KD (S) was 35, 9, and 18 fold higher than those of binding of rA13 (KD =8.2±1.6 nM), T2C (KD=8.4±2.5 nM), and T5C (KD=5.3±1.2 nM) to immobilized VWF under the same conditions (Suppl. Fig. I). These results suggest that full-length ADAMTS13 and its C-terminal fragments can directly interact with collagen, but this low affinity binding is not expected to affect high affinity interaction between VWF and collagen.
To determine if rA13 and C-terminal fragments affects platelet-collagen binding, we determined the platelet aggregation induced by type 1 collagen in the absence and in the presence of purified rA13, T2C, and T5C. Suppl. Fig. II shows that in the presence of 10 nM rA13, 100 nM T2C, and 100 nM T5C, no inhibition of murine platelet aggregation induced by type 1 collagen. These results indicate that the antithrombotic effect of rA13 and its C-terminal fragments is less likely to be mediated by direct interference with VWF-collagen or platelet-collagen interaction.
C-terminal fragments of ADAMTS13 also inhibit the formation of ULVWF strings and platelet-leukocyte aggregates on endothelial cells in vivo
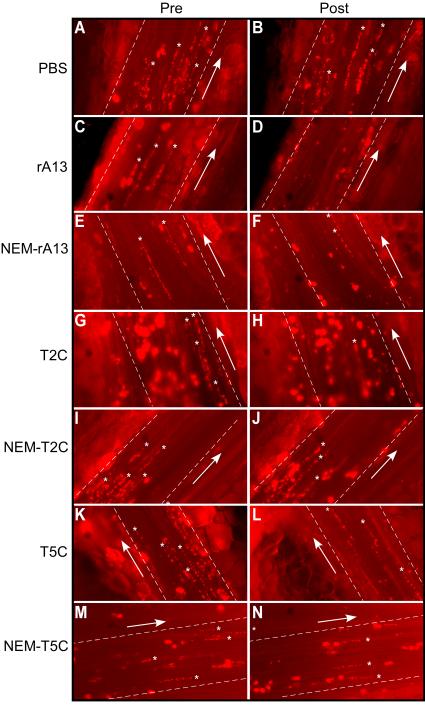
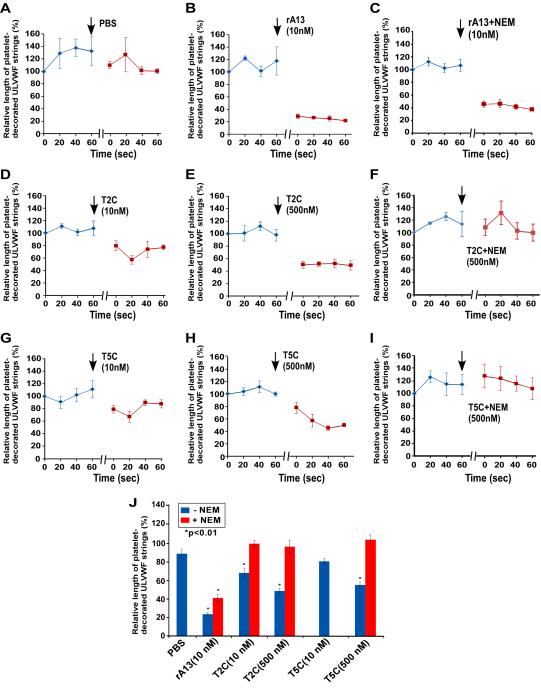
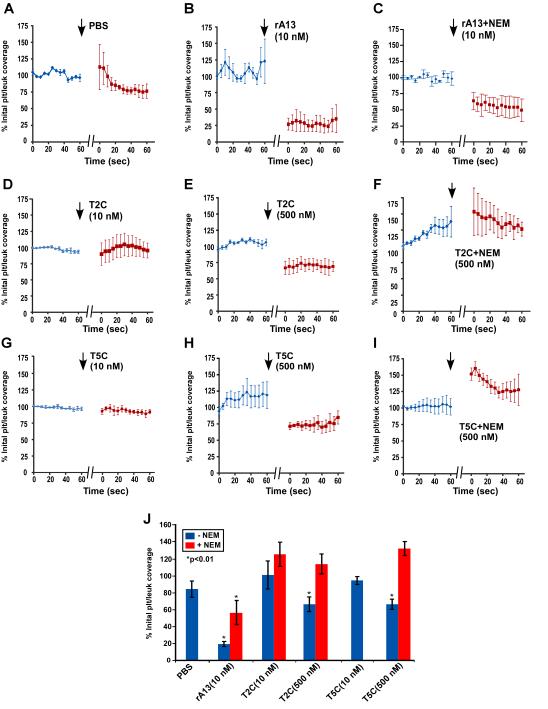
To determine the specificity of the anti-thrombotic function of the C-terminal domains of ADAMTS13, the effects of T2C or T5C on the formation of platelet-decorated ULVWF strings and adhesion/aggregation of platelets/leukocytes on endothelial cells were determined in a murine model. In these experiments, a mesenteric venule from KO mice was injured by 7.5% FeCl3, and the area immediately downstream from the injury site was located and imaged under a fluorescent microscope. Platelet-decorated ULVWF strings and/or platelet/leukocyte accumulation were clearly visible in this location. In the absence of plasma ADAMTS13, such as in the KO mice, the formation of ULVWF strings on the endothelial cell surface is highly dynamic, with some strings formed and others disappeared over time. However, the average length of ULVWF strings was fairly constant during the recording time (~180 seconds) (Figs. 4A, 4B, 5A, and Suppl. Video I). An infusion of rA13 at its physiological concentration (10 nM) resulted in a rapid and significant reduction of the average length of platelet-decorated ULVWF strings (Fig. 4D, 5B, and 5J) and the percentage of surface coverage by platelet/leukocyte aggregates on the endothelial cell surface (Figs. 4D, 6B, 6J, and Suppl. Video II). Interestingly, an infusion of T2C or T5C also significantly reduced the average length of platelets-decorated ULVWF strings (Figs. 4 & 5) and the formation of platelet/leukocyte aggregates (Figs. 4 & 6) in a concentration-dependent manner. For instance, after an infusion of 500 nM of T2C and T5C, the average length of the ULVWF strings on endothelial cells was reduced by ~50% and ~40%, respectively (Figs, 4, 5 & Suppl. Video III) whereas the percentage of surface coverage of platelet/leukocytes was reduced by ~40% (Figs. 4, 6 & Suppl. Video III). This was less than the efficacy of 10 nM rA13 under the same conditions (Fig. 6J). Nevertheless, our results demonstrate for the first time that the C-terminal non-catalytic domains of ADAMTS13 are capable of inhibiting ULVWF string formation and platelet/leukocyte accumulation on endothelium after injury.
Figure 4.
Representative snapshots of platelet/leukocyte-decorated ULVWF strings on the endothelial cells in mesenteric venules. A, C, E, G, I, K, and M represent the ULVWF string formation prior to infusion of rA13 or a C-terminal fragment; B, D, F, H, J, L, and N show the ULVWF strings one minute after infusion of PBS, rA13 (10 nM), NEM-treated rA13 (10 nM), T2C (500 nM), NEM-treated T2C (500 nM), T5C (500 nM), and NEM-treated T5C (500 nM), respectively. Asterisks indicate the presence of long and short platelet-decorated ULVWF strings; arrows indicate the blood flow direction.
Figure 5.
Dynamic changes in the mean length of platelet-decorated ULVWF strings on endothelial cells before and after infusion of rA13 and its C-terminal fragments. A to I show the dynamic changes in the mean length of platelet-decorated ULVWF strings on the endothelial cells in mesenteric venules after oxidative injury prior to and after infusion (↓) of PBS, rA13 (10 nM), T2C (10 and 500 nM), and T5C (10 and 500 nM) which were treated with or without NEM as indicated in each panel. The data represent the mean ± standard errors (SEM) from three mice (N=3) for each treatment. J summarizes the percentage changes (mean ± SEM, n=3) in the average length of platelet-decorated ULVWF strings on endothelial cells after infusion of PBS, rA13 (10 nM), T2C (10 and 500 nM), and T5C (10 and 500 nM) with (red bars) or without (blue bars) NEM treatment. Statistical analysis was performed using ANOVA analysis. Stars in panel J indicate the differences were statistically highly significant compared with PBS-control.
Figure 6.
Dynamic changes in the surface coverage of platelet/leukocyte aggregates on endothelium before and after infusion of rA13 and its C-terminal fragments. A to I show the dynamic changes in the percentage of surface coverage of platelet/leukocyte aggregates on endothelial cells after oxidative injury prior to and after infusion (↓) of PBS, rA13 (10 nM), T2C (10 and 500 nM), and T5C (10 and 500 nM) that were untreated or treated with NEM as indicated in each panel. The data are the mean ± standard errors (SEM) from three mice (N=3) for each treatment. J summarizes the percent changes (mean ± SEM, n=3) in the surface coverage of platelet/leukocyte aggregates on endothelium after infusion of PBS, rA13 (10 nM), T2C (10 and 500 nM), and T5C (10 and 500 nM) untreated (blue bars) or treated (red bars) with NEM. Statistical analysis was performed using ANOVA analysis. Stars in panel J indicate that the differences were statistically highly significant compared with PBS-control.
To determine if the inhibitory effect of T2C and T5C on ULVWF string formation and platelet/leukocyte accumulation is mediated by their surface free thiols, similar experiments were performed using the NEM-treated proteins. As shown, pretreatment of T2C or T5C with NEM completely abolished its ability to inhibit the formation of ULVWF strings (Figs. 4 & 5) and platelet/leukocyte aggregates (Figs. 4 & 6) on activated endothelium. Furthermore, pretreatment of rA13 with NEM resulted in a ~40% reduction in inhibiting the ULVWF string formation (Figs. 4 & 5) and a ~60% reduction in attenuating the platelet and leukocyte aggregation (Figs. 4 & 6) on endothelium. Together, these results suggest that the surface free thiols on the C-terminal domains of ADAMTS13 may contribute to the protease-independent antithrombotic activity of ADAMTS13 in vivo.
Discussion
Our present study demonstrates that the C-terminal domains of ADAMTS13 are capable of inhibiting thrombus formation under flow, independent of proteolytic activity. This novel function of the C-terminus of ADAMTS13 has been demonstrated with two independent assay systems. First, a microfluidic assay demonstrates that C-terminal ADAMTS13 fragments (T2C and T5C) lacking the protease domain or heat-inactivated rA13 lacking proteolytic activity inhibits platelet adhesion/aggregation on a collagen surface under flow in a concentration-dependent manner (Figs. 2 & 3). Second, an intravital microscopic imaging analysis further show that constructs T2C and T5C inhibit the in vivo formation of elongated platelet-decorated ULVWF strings and platelet/leukocyte aggregates on endothelium after oxidative injury in a dose-dependent manner (Figs. 4-6). The inhibitory activity of T2C or T5C on the platelet aggregation on the collagen surface or ULVWF string formation on the endothelial surface requires the presence of its surface free thiols as the treatment of T2C or T5C with NEM abolishes its antithrombotic activity in vitro (Fig. 3) and in vivo (Figs. 4-6).
However, the concentration of T2C or T5C required to achieve significant antithrombotic activity appears to be 20-40 times higher than that of plasma ADAMTS13 (2.5-5 nM) in human and mice. This raises two interesting questions: is the thiol-dependent reductase activity important physiologically and can the species differences between human and murine VWF (or ADAMTS13) account for the high concentration required for an observable antithrombotic effect? NEM-treated rA13 exhibits a ~50% reduction of its inhibitory activity on the formation of ULVWF strings (Fig. 5) and platelet/leukocyte aggregates (Fig. 6) on activated endothelium despite having normal proteolytic activity in vitro (Fig. 1C & 1D), suggesting the importance of surface free thiols on ADAMTS13 in its overall antithrombotic function in vivo. Previous studies have demonstrated the low cleavage efficiency of mouse VWF by human ADAMTS13 or vice versa15, 29, supporting the notion that species differences between human and mouse VWF (or ADAMTS13) may account for the high concentrations of human recombinant ADAMTS13 and variants required for the inhibitory effects observed under our experimental conditions.
It remains to be determined, however, how the free thiols in the C-terminal domains of ADAMTS13 exert their antithrombotic function under flow. Yeh et al 25 have shown that the free thiols clustered on the C-terminal TSP1 repeats of ADAMTS13 interact with the free thiols on VWF under shear. These investigators hypothesize that such interactions may prevent VWF-VWF lateral association, thereby reducing VWF adhesiveness. NEM-labeling coupled with mass spectrometry has allowed us to identify multiple free cysteine residues in the TSP1 repeats and CUB domains of ADAMTS13 (Table I). Four free cysteine residues (Cys710, Cys799, Cys919, and Cys977) identified in this study were not reported in the previous study 25. This may be related to the repeated experiments and prolonged incubation of rA13 and C-terminal fragments with NEM in this study. A prolonged incubation may result in protein conformational changes that expose additional free cysteine residues. More free-cysteine residues have been detected if a protein such as VWF is unfolded by a denaturing agent or under fluid shear stress 25.
There was a concern that prolonged incubation of rA13 or its C-terminal fragments with NEM may result in modification of additional residues (such as lysine), reduce the preformed disulfide bonds, and alter the protein structure and function. To rule out these possibilities, we examined all NEM-labeled peptides and did not find NEM modification on other residues besides the cysteine residues. Moreover, a control multimeric VWF treated with NEM under the same conditions did not result in alteration of its multimer pattern by agarose gel electrophoresis (data not shown). Finally, the proteolytic activity of NEM-treated rA13 was compared with that of untreated rA13 in cleaving FRETS-VWF73 and multimeric VWF, and it was found to be identical (Figs. 1C and 1D). Together, these results indicate that NEM treatment under the conditions described did not significantly alter the recombinant ADAMTS13 proteins in such a way that would affect their overall functionality non-specifically.
Another intriguing question was whether rA13 and its C-terminal domains bind collagen and block VWF-collagen or platelet-collagen interaction. To address this, we have performed microtiter plate binding assays and collagen-induced platelet aggregation. As shown in suppl. Fig. I, rA13, T2C, and T5C bind to immobilized type 1 collagen, but such a low affinity binding does not appear to affect high affinity biding between VWF and collagen. Also, binding of rA13 and C-terminal domains does not appear to interfere with the interaction between collagen and platelet (Suppl. Fig. II). Together, these results indicate that antithrombotic activity of C-terminal fragments of ADAMTS13 is less likely to be mediated through its blockage of VWF-collagen or platelet-collagen interaction. In support of this, a previous study has demonstrated that under arterial shear (1,700 s−1), platelets do not adhere and aggregate onto collagen surface in the absence of VWF 30. Therefore, the VWF-ADAMTS13 axis plays a key role in platelet adhesion/aggregation on a collagen surface under arterial flow.
In conclusion, we demonstrate that the C-terminal ADAMTS13 fragments have direct antithrombotic activity under pathophysiological conditions. This antithrombotic activity is mediated by a disulfide bond reduction mechanism independent of proteolytic activity. These findings shed new lights on the biology of ADAMTS13 in vivo and may provide novel insights into the mechanisms of TTP and other arterial thrombosis. Whether an isolated C-terminal ADAMTS13 fragment can be used as a therapeutic agent for TTP or other arterial thrombosis remains to be determined in a disease-specific animal model.
Supplementary Material
Significance.
Present study demonstrates for the first time that the C-terminal domains of ADAMTS13 have direct antithrombotic activity in vitro and in vivo under fluidic shear. The antithrombotic activity depends on the clustered surface free thiols on the C-terminus of ADAMTS13 but is independent of its proteolytic activity. The thiol-mediated reductase activity in the ADAMTS13 C-terminus appears to contribute to the overall antithrombotic activity of ADAMTS13 in vivo. Our findings shed new light on the molecular mechanisms underlying the antithrombotic function of ADAMTS13 under pathophysiological conditions.
Acknowledgements
This study is in part supported National Institute of Health (HL074124-project 3 to XLZ) and American Heart Association (AHA 0940100N to XLZ), and National Science Foundation of China (NSFC 81200354 to JX). XLZ is an Established Investigator of AHA and a Chutian Professor of Tongji Hospital, Huazhong University of Science and Technology, Wuhan, China.
Abbreviations
- rA13
A full-length recombinant human ADAMTS13 (A Disintegrin And Metalloprotease with Thrombospondin type 1 repeats, 13)
- TSP1
Thrombospondin type 1 repeat
- T2C
ADAMTS13 fragment consisting of 2-8 thrombospondin type 1 repeats plus CUB domains
- T5C
ADAMTS13 fragment consisting of 5-8 thrombospondin type 1 repeats plus CUB domains
- CUB
Complement C1r/C1s, Uegf, bone morphogenic protein 1
- MDTCS
ADAMTS13 variant truncated after the spacer domain
- VWF
von Willebrand factor
- ULVWF
Ultra large von Willebrand factor
- TTP
Thrombotic thrombocytopenic purpura
- NEM
N-ethylmaleimide
- CHO
Chinese hamster ovarian cell line
- HEK
Human embryonic kidney cell line
- PPACK
D-phenylalanyl-L-prolyl-L-arginine chloromethylketone
Footnotes
Conflict of interest statement
Authors declare no relevant conflict of interest.
Reference List
- 1.Wagner DD, Marder VJ. Biosynthesis of von Willebrand protein by human endothelial cells: processing steps and their intracellular localization. J Cell Biol. 1984;99:2123–2130. doi: 10.1083/jcb.99.6.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong JF, Moake JL, Nolasco L, Bernardo A, Arceneaux W, Shrimpton CN, Schade AJ, McIntire LV, Fujikawa K, Lopez JA. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–4039. doi: 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- 3.Dong JF, Moake JL, Bernardo A, Fujikawa K, Ball C, Nolasco L, Lopez JA, Cruz MA. ADAMTS-13 metalloprotease interacts with the endothelial cell-derived ultra-large von Willebrand factor. J Biol Chem. 2003;278:29633–19639. doi: 10.1074/jbc.M301385200. [DOI] [PubMed] [Google Scholar]
- 4.Huang J, Motto DG, Bundle DR, Sadler JE. Shiga toxin B subunits induce VWF secretion by human endothelial cells and thrombotic microangiopathy in ADAMTS13-deficient mice. Blood. 2010;116:3653–3659. doi: 10.1182/blood-2010-02-271957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin SY, Skipwith CG, Shang D, Zheng XL. von Willebrand factor cleaved from endothelial cells by ADAMTS13 remains ultralarge in size. J Thromb Haemost. 2009;7:1749–1752. doi: 10.1111/j.1538-7836.2009.03570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raife TJ, Cao W, Atkinson BS, Bedell B, Montgomery RR, Lentz SR, Johnson GF, Zheng XL. Leukocyte proteases cleave von Willebrand factor at or near the ADAMTS13 cleavage site. Blood. 2009;114:1666–1674. doi: 10.1182/blood-2009-01-195461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nolasco L, Nolasco J, Feng S, Afshar-Kharghan V, Moake J. Human complement factor h is a reductase for large soluble von Willebrand factor multimers--brief report. Arterioscler Thromb Vasc Biol. 2013;33:2524–2528. doi: 10.1161/ATVBAHA.113.302280. [DOI] [PubMed] [Google Scholar]
- 8.Moake JL. Thrombotic thrombocytopenic purpura: the systemic clumping "plague". Annu Rev Med. 2002;53:75–88. doi: 10.1146/annurev.med.53.082901.103948. [DOI] [PubMed] [Google Scholar]
- 9.Zheng XL, Sadler JE. Pathogenesis of Thrombotic Microangiopathies. Annu Rev Path Mech Dis. 2008;3:249–277. doi: 10.1146/annurev.pathmechdis.3.121806.154311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ai J, Smith P, Wang S, Zhang P, Zheng XL. The proximal carboxyl-terminal domains of ADAMTS13 determine substrate specificity and are all required for cleavage of von Willebrand factor. J Biol Chem. 2005;280:29428–29434. doi: 10.1074/jbc.M505513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao W, Anderson PJ, Majerus EM, Tuley EA, Sadler JE. Exosite interactions contribute to tension-induced cleavage of von Willebrand factor by the antithrombotic ADAMTS13 metalloprotease. Proc Natl Acad Sci U S A. 2006;103:19099–19104. doi: 10.1073/pnas.0607264104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao W, Anderson PJ, Sadler JE. Extensive contacts between ADAMTS13 exosites and von Willebrand factor domain A2 contribute to substrate specificity. Blood. 2008;112:1713–1719. doi: 10.1182/blood-2008-04-148759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soejima K, Matsumoto M, Kokame K, Yagi H, Ishizashi H, Maeda H, Nozaki C, Miyata T, Fujimura Y, Nakagaki T. ADAMTS-13 cysteine-rich/spacer domains are functionally essential for von Willebrand factor cleavage. Blood. 2003;102:3232–3237. doi: 10.1182/blood-2003-03-0908. [DOI] [PubMed] [Google Scholar]
- 14.Tao Z, Wang Y, Choi H, Bernardo A, Nishio K, Sadler JE, Lopez JA, Dong JF. Cleavage of ultralarge multimers of von Willebrand factor by C-terminal-truncated mutants of ADAMTS-13 under flow. Blood. 2005;106:141–143. doi: 10.1182/blood-2004-11-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao J, Jin SY, Xue J, Sorvillo N, Voorberg J, Zheng XL. Essential domains of a disintegrin and metalloprotease with thrombospondin type 1 repeats-13 metalloprotease required for modulation of arterial thrombosis. Arterioscler Thromb Vasc Biol. 2011;31:2261–2269. doi: 10.1161/ATVBAHA.111.229609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng XL, Nishio K, Majerus EM, Sadler JE. Cleavage of von Willebrand factor requires the spacer domain of the metalloprotease ADAMTS13. J Biol Chem. 2003;278:30136–30141. doi: 10.1074/jbc.M305331200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akiyama M, Takeda S, Kokame K, Takagi J, Miyata T. Crystal structures of the noncatalytic domains of ADAMTS13 reveal multiple discontinuous exosites for von Willebrand factor. Proc Natl Acad Sci U S A. 2009;106:19274–19279. doi: 10.1073/pnas.0909755106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin SY, Skipwith CG, Zheng XL. Amino acid residues Arg(659), Arg(660), and Tyr(661) in the spacer domain of ADAMTS13 are critical for cleavage of von Willebrand factor. Blood. 2010;115:2300–2310. doi: 10.1182/blood-2009-07-235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pos W, Crawley JT, Fijnheer R, Voorberg J, Lane DA, Luken BM. An autoantibody epitope comprising residues R660, Y661, and Y665 in the ADAMTS13 spacer domain identifies a binding site for the A2 domain of VWF. Blood. 2010;115:1640–1649. doi: 10.1182/blood-2009-06-229203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiang Y, de Groot R, Crawley JT, Lane DA. Mechanism of von Willebrand factor scissile bond cleavage by a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) Proc Natl Acad Sci U S A. 2011;108:11602–11607. doi: 10.1073/pnas.1018559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luken BM, Turenhout EA, Hulstein JJ, Van Mourik JA, Fijnheer R, Voorberg J. The spacer domain of ADAMTS13 contains a major binding site for antibodies in patients with thrombotic thrombocytopenic purpura. Thromb Haemost. 2005;93:267–274. doi: 10.1160/TH04-05-0301. [DOI] [PubMed] [Google Scholar]
- 22.Luken BM, Kaijen PH, Turenhout EA, Kremer Hovinga JA, Van Mourik JA, Fijnheer R, Voorberg J. Multiple B-cell clones producing antibodies directed to the spacer and disintegrin/thrombospondin type-1 repeat 1 (TSP1) of ADAMTS13 in a patient with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2006;4:2355–2364. doi: 10.1111/j.1538-7836.2006.02164.x. [DOI] [PubMed] [Google Scholar]
- 23.Luken BM, Turenhout EA, Kaijen PH, Greuter MJ, Pos W, Van Mourik JA, Fijnheer R, Voorberg J. Amino acid regions 572-579 and 657-666 of the spacer domain of ADAMTS13 provide a common antigenic core required for binding of antibodies in patients with acquired TTP. Thromb Haemost. 2006;96:295–301. doi: 10.1160/TH06-03-0135. [DOI] [PubMed] [Google Scholar]
- 24.Zheng XL, Wu HM, Shang D, Falls E, Skipwith CG, Cataland SR, Bennett CL, Kwaan HC. Multiple domains of ADAMTS13 are targeted by autoantibodies against ADAMTS13 in patients with acquired idiopathic thrombotic thrombocytopenic purpura. Haematologica. 2010;95:1555–1562. doi: 10.3324/haematol.2009.019299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeh HC, Zhou Z, Choi H, Tekeoglu S, May W, III, Wang C, Turner N, Scheiflinger F, Moake JL, Dong JF. Disulfide bond reduction of von Willebrand factor by ADAMTS-13. J Thromb Haemost. 2010;8:2778–2788. doi: 10.1111/j.1538-7836.2010.04094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banno F, Kokame K, Okuda T, Honda S, Miyata S, Kato H, Tomiyama Y, Miyata T. Complete deficiency in ADAMTS13 is prothrombotic, but it alone is not sufficient to cause thrombotic thrombocytopenic purpura. Blood. 2006;107:3161–3166. doi: 10.1182/blood-2005-07-2765. [DOI] [PubMed] [Google Scholar]
- 27.Chauhan AK, Motto DG, Lamb CB, Bergmeier W, Dockal M, Plaimauer B, Scheiflinger F, Ginsburg D, Wagner DD. Systemic antithrombotic effects of ADAMTS13. J Exp Med. 2006;203:767–776. doi: 10.1084/jem.20051732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niiya M, Endo M, Shang D, Zoltick PW, Muvarak NE, Cao W, Jin SY, Skipwith CG, Motto DG, Flake AW, Zheng XL. Correction of ADAMTS13 deficiency by in utero gene transfer of lentiviral vector encoding ADAMTS13 genes. Mol Ther. 2009;17:34–41. doi: 10.1038/mt.2008.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varadi K, Rottensteiner H, Vejda S, Weber A, Muchitsch EM, Turecek PL, Ehrlich HJ, Schwarz HP. Species-dependent variability of ADAMTS13-mediated proteolysis of human recombinant von Willebrand factor. J Thromb Haemost. 2009;7:1134–1142. doi: 10.1111/j.1538-7836.2009.03453.x. [DOI] [PubMed] [Google Scholar]
- 30.Fuchs B, Budde U, Schulz A, Kessler CM, Fisseau C, Kannicht C. Flow-based measurements of von Willebrand factor (VWF) function: binding to collagen and platelet adhesion under physiological shear rate. Thromb Res. 2010;125:239–245. doi: 10.1016/j.thromres.2009.08.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.