Abstract
Stress-induced hormones can alter the inflammatory response to tissue injury, however, the precise mechanism by which epinephrine influences inflammatory response and wound healing is not well defined. Here we demonstrate that epinephrine alters the neutrophil (PMN)-dependent inflammatory response to a cutaneous wound. Using non-invasive real-time imaging of genetically-tagged PMNs in a murine skin wound, chronic, epinephrine-mediated stress was modeled by sustained delivery of epinephrine. Prolonged systemic exposure of epinephrine resulted in persistent PMN trafficking to the wound site via an IL-6 mediated mechanism, and this in turn impaired wound repair. Further, we demonstrate that β2 adrenergic receptor-dependent activation of pro-inflammatory macrophages is critical for epinephrine-mediated IL-6 production. This study expands our current understanding of stress hormone-mediated impairment of wound healing and provides an important mechanistic link to explain how epinephrine stress exacerbates inflammation via increased number and lifetime of PMNs.
Introduction
Impairment of skin wound healing results in chronic skin ulceration, a burgeoning clinical problem. Common etiologies include venous leg ulcers, diabetic foot ulcers, and pressure ulcers. While there are diverse pathologic processes at work in these different entities, one common effector pathway is believed to be persistent wound inflammation (Diegelmann and Evans, 2004; Liu et al., 2011) that is associated with prolongation of healing in venous, diabetic, and pressure ulcers (Gohel et al., 2008; Pradhan et al., 2009).
One recognized modulator of the inflammatory response is stress, either acute or chronic (Gouin et al., 2008; Viswanathan et al., 2005). Various types of stressors can activate either the hypothalamic-pituitary-adrenal axis or the sympathetic nerve system, resulting in the systemic release of stress hormones including glucocorticoids (GC) and catecholamines (Glaser and Kiecolt-Glaser, 2005; Webster et al., 2002). The systemic elevation of GC can attenuate the normal inflammatory and cellular immune responses to tissue injury and infection, and thereby impair wound healing (Hubner et al., 1996; Padgett et al., 1998). In contrast to the immune suppressive effects of GC, a large body of evidence supports the opposing action of stress-released catecholamines in augmenting the acute inflammatory response, especially when the response is in conjunction with tissue injury and infection (Bergmann and Sautner, 2002; Flierl et al., 2007). Catecholamines such as epinephrine can interact with all the cellular components of the immune system, since lymphocytes, macrophages, and neutrophils (PMNs) express α and β-adrenergic receptors (ARs) (Kavelaars, 2002; Kohm and Sanders, 2001). Systemic elevation of catecholamine is emblematic of most physiological stressors, including trauma, burn injury, and sepsis as well as chronic psychological stress (Engelman et al., 1983; Sivamani et al., 2009; Vanitallie, 2002). Levels of circulating epinephrine can increase up to 10-fold in patients exposed to high stress during burn injury (Jeschke et al., 2011; Sedowofia et al., 1998). These elevated levels of epinephrine can result in enhanced release of pro-inflammatory cytokines including tumor necrosis factor-α (TNFα), interleukin (IL)-1β, and IL-6 (Frost et al., 2004; Johnson et al., 2005; Wong et al., 2012). Since these cytokines can recruit PMNs to the site of inflammation, their generation as a result of catecholamine stress could provide a link to the observed increase in PMN trafficking to the lung and liver of mice that have been challenged with high doses of epinephrine (Morken et al., 2002; von Montfort et al., 2008). Additionally, extraneuronal generation of epinephrine within the wound by PMNs themselves provides a potential for amplification of the catecholamine-mediated inflammatory response (Flierl et al., 2007). Despite recent advances in understanding of the role of epinephrine on inflammatory response and emerging evidence that stress impairs healing, the precise mechanism by which stress-induced epinephrine impacts upon the PMN-dependent innate immune response and contributes to wound chronicity is unclear.
Using an animal model to mimic chronic epinephrine-dependent stress, we examined how β2 AR activation by epinephrine alters the kinetics of PMN trafficking to the site of a skin wound and its effect on the wound healing. These studies demonstrate that epinephrine-stress promotes sustained PMN trafficking within the wound site. Further we provide evidence that IL-6 is the key proinflammatory cytokine that is critical for this epinephrine-induced PMN wound trafficking and the resultant impairment of wound healing.
Results
Epinephrine stress mediates persistent PMN trafficking into skin wounds and delays their healing
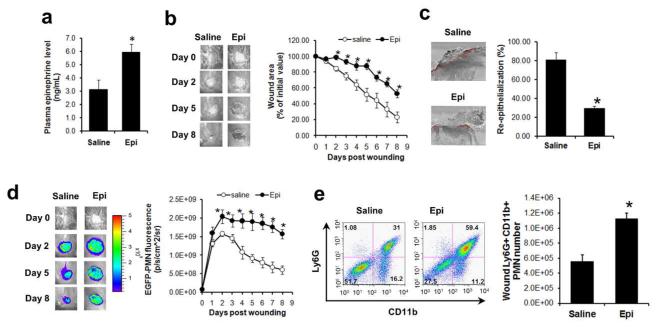
Chronic epinephrine-stress was emulated by the implantation of an osmotic pump that provided sustained delivery of epinephrine (5 mg/kg/day) or saline (control) immediately after skin wounding. The continuous delivery of epinephrine resulted in a 2-fold increase in plasma epinephrine levels compared to saline control at day 5 post-wounding (Fig. 1a), which is comparable to the value measured in a murine model of chronic isolation stress (Itoh et al., 2006). Mice that were exposed to this sustained levels of epinephrine exhibited delayed wound healing compared to control mice (saline-infused) (Figs.1b and 1c), or mice treated with a lower dose of epinephrine infusion (2.5 mg/kg/day) (Fig. S1). To investigate how increased levels of circulating epinephrine influence dynamics of PMN infiltration within the wound bed, whole animal fluorescence imaging of transgenic mice expressing the lysozyme-EGFP gene (EGFP-lys-mice) was employed. This model provides a linear EGFP signal with increased influx over time, in which >95% of the EGFPhigh cells were previously confirmed as mature PMNs (Kim et al., 2008). At day 2 post wounding, the extent of PMN recruitment was significantly augmented in the wounds of epinephrine-stressed mice and their increased trafficking to the wound persisted up to day 8 (Fig. 1d). This is in contrast to the gradual decline in wound PMNs observed in the control mice (Fig. 1d) or in the low dose epinephrine-stressed mice (Fig. S1), which paralleled the normal wound closure trajectory. The increased PMN trafficking to wounds of epinephrine-stressed mice was also confirmed by flow cytometric analysis of cells harvested from wounds at day 7. The number of Ly6G+ CD11b+ PMNs within the wounds of epinephrine-stressed mice was double that of control mice (Fig. 1e). Taken together, these data show that the extent and persistence of PMN recruitment is increased in mice that have elevated levels of plasma epinephrine, correlating with the observed delay in wound closure.
Figure 1. Effects of epinephrine on wound PMN trafficking and wound healing.
EGFP-lys-mice (a-d) and C57BL6 wild-type mice (e) were systemically delivered with sterile saline or epinephrine (5 mg/kg/day). (a) Plasma epinephrine levels were measured at day 5 post-wounding. (b) Kinetics of wound closure and representative wound images. (c) Wound re-epithelialization measured from histological sections collected at day 8 post-wounding. Red-line indicates a re-epithelialized region. (d) Kinetics of wound EGFP-PMN fluorescence and representative images. (e) Wound PMN (CD11b+Ly6G+ cell) numbers were quantified by flow cytometric analysis of cells harvested from wounded skin of mice treated with either saline or epinephrine at day 7 post-wounding. Representative FACS plot (left panel) and quantification (right panel). n=6-8 mice in each group. *, p<0.05 vs Saline group.
The persistence of wound PMN trafficking is responsible for epinephrine stress-mediated delayed healing
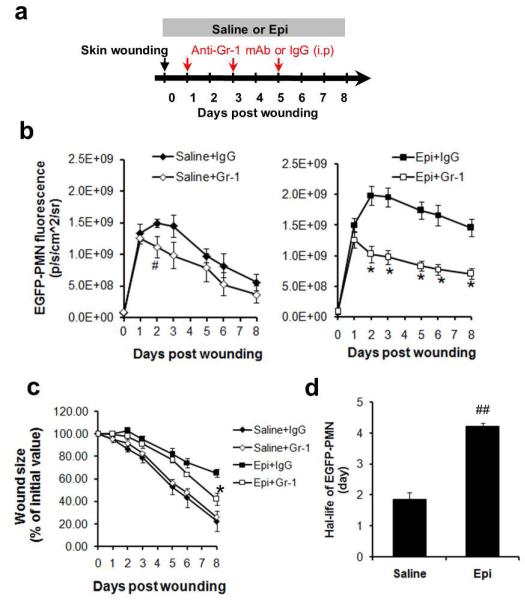
Since epinephrine-stressed mice exhibited both a marked increase in PMN trafficking to the wound and a delay in healing, we set out to determine whether these two observations were causally related. For this, mice were systemically depleted of PMNs with an anti-neutrophil monoclonal antibody (Gr-1) that was administered daily, beginning at day 1 post-wounding- the time point that PMN recruitment increased in response to epinephrine compared to saline infusion (Fig. 2a). When stressed with systemic elevation of epinephrine, the PMN-depleted mice had significantly attenuated numbers of EGFP-PMNs recruited to the wound at days 2 through 8 as compared with mice treated with an IgG isotype control (Fig. 2b). Additionally, the antibody inhibition of PMN trafficking into the wound significantly increased the rate of wound closure for epinephrine-stressed mice (by 57%, p<0.05 vs epinephrine only), but the effect was negligible on saline-treated mice (Fig. 2c). These findings support the notion that persistent PMN trafficking contributes to the impaired wound healing observed in epinephrine-stressed mice.
Figure 2. The persistence of wound PMN trafficking is responsible for epinephrine stress-mediated delayed healing.
(a) Experimental design for delayed depletion of systemic EGFP-PMNs. Systemic PMNs were depleted with anti-neutrophil antibody (Gr-1 mAb) treatment from saline-treated (Saline+Gr-1) and epinephrine-treated EGFP-lys-mice (5 mg/kg/day, Epi+Gr-1). Kinetics of wound EGFP-PMNs (b) and wound closure (c) were determined and results were compared with mice that received with isotype control (Saline+IgG or Epi+IgG). n=5-6 mice in each group. #, p<0.05 between Saline+IgG vs Saline+Gr-1 and *, p<0.05 between Epi+IgG vs Epi+Gr-1. (d) Calculation of the half-life of PMNs infiltrating wound based on the rate of EGFP-fluorescence signal decay (see Methods and Fig. S4). n=3-4 mice in each group. ##, p<0.05 vs Saline control.
How might epinephrine stress lead to persistence of PMN numbers within the wound? A recent study has ascribed the persistent PMN trafficking observed in wounds in restraint-stressed mice to an increase in wound bacterial burden (Tymen et al., 2012). This may be a function of the increase in cortisol levels or experimental conditions that is associated with the latter model (Campbell et al., 2001; Ridder et al., 2005). In our current study using epinephrine-stressed mice, a very low bacterial colony formation was detected with no significant difference between saline and epinephrine treatment (Fig. S2). Since PMN numbers in the peripheral circulation were comparable between epinephrine and saline control treated animals despite the remarkable difference in wound PMN trafficking (Fig. S3), we hypothesized that this persistence might be due to the capacity of PMNs infiltrating the wound to evade apoptosis and extend their survival. This was examined by an in vivo quantitation of the half-life of EGFP-labeled mature PMNs adoptively transferred and infiltrating the wound using a technique that we reported recently (Kim et al., 2011). Non-proliferative mature EGFP-PMNs, defined as c-kit− EGFPhigh cells (1×107 cells), were isolated by FACS-sorting from bone marrow cells of EGFP-lys-mice and adoptively transferred by intravenous injection into C57BL/6 mice that were epinephrine-stressed or saline controls. Since the number of circulating EGFPhigh cells rapidly decrease to undetectable levels post infusion by day 2 (Fig. S4a), any observed EGFP-PMN fluorescence signal within the wound after this time point is contributed to prolonged survival of adoptively transferred PMNs that rapidly infiltrate the wound. Estimation of the half-life of EGFP-PMNs based on the rate of fluorescence signal decay (Fig.S4b) revealed that PMNs within wounds of epinephrine-stressed mice survived 2-fold longer than those in the saline treated controls (Fig. 2d; 4.23±0.05 days for epinephrine vs. 1.86±0.21 days for saline control; p<0.01). These data suggest that epinephrine promoted persistent PMN trafficking in the wound in part by prolonging the survival of the PMNs that infiltrated the wound environment.
Epinephrine stress-mediated PMN persistence and impaired wound healing is β2 AR dependent
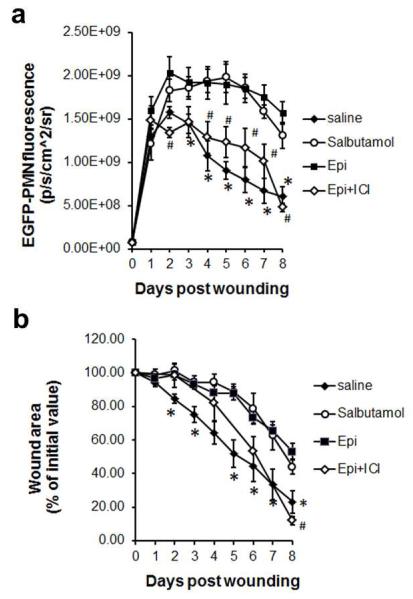
The effects of epinephrine are mediated by their binding to subtypes of ARs, α1, α2, β1, β2, and β3 receptors, and their downstream signaling post- activation (Insel, 1996; Small et al., 2003). Recent published data supports the potential role of β2 AR in wound healing, both by modulating the pro-inflammatory response (Rough et al., 2009; Tan et al., 2007) and impairing wound re-epithelialization (Sivamani et al., 2009). Hence, we examined whether epinephrine-stress induced PMN trafficking to wound sites and impaired wound healing was mediated by a β2AR-dependent mechanism. We therefore evaluated the role of β2AR-dependent signaling on PMN trafficking and wound size using a pharmacological agonist (salbutamol) and antagonist (ICI 118,551) specific for the β2AR (Baker, 2005) in wounded EGFP-lys-mice. Treatment of mice with salbutamol (3 mg/kg/day) resulted in a delay in wound healing and prolonged trafficking of PMN to the wound, equivalent to that induced by epinephrine-stress (Figs.3a and 3b). On the other hand, treatment of epinephrine-stressed mice with the antagonist ICI 118,551 (0.7 mg/kg/day) remarkably attenuated PMN trafficking to the wound. This was also confirmed by flow cytometric analysis of Ly6G+ CD11b+ PMNs number harvested from wounds at day 7, in which ICI 118,551 treatment significantly reduced Ly6G+ CD11b+ PMNs within wounds down to the level of saline control mice (Fig. S5). While the kinetics of wound closure were comparable in the epinephrine- and salbutamol-treated mice, treatment with the β2AR antagonist (ICI 118,551) significantly accelerated wound closure in epinephrine-treated mice at day 8, which is comparable to that of saline control mice (Fig. 3b). These findings suggest that that prolonged exposure to elevated levels of epinephrine results in persistent PMN presence in the wound site via β2AR-activation, which in turn delays wound repair.
Figure 3. Effects of β2AR blockade on epinephrine-mediated PMN recruitment and wound closure.
Kinetics of wound EGFP-PMN trafficking (a) and wound closure (b) from EGFP-lys-mice treated with saline, β2AR agonist (salbutamol, 3 mg/kg/day), epinephrine (5 mg/kg/day), and epinephrine with β2AR antagonist (ICI 118,551, 0.7 mg/kg/day). n=5-6 mice in each group. *, p<0.05 between Saline vs Epi group and #, p<0.05 between Epi vs Epi+ICI group.
IL-6 is a critical regulator of persistent PMN trafficking in local wounds of epinephrine-treated mice
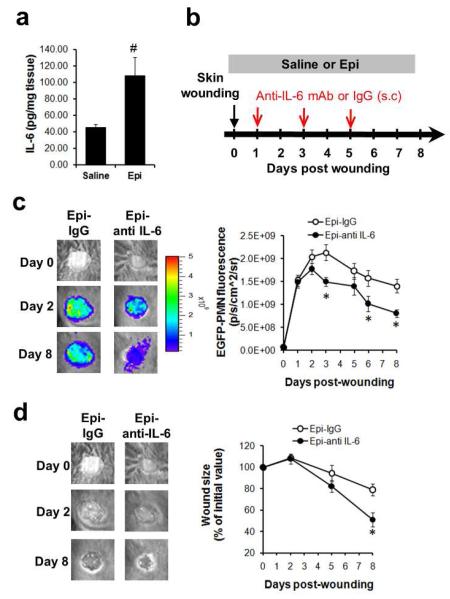
We next examined whether the epinephrine-stress mediated alterations in wound PMN trafficking correlated with altered expression of pro-inflammatory cytokines during the late phase of wound repair. Protein levels of IL-1β, TNF-α, IL-1α, IL-6 and GM-CSF were measured from tissue harvested from the wounded skin on day 5 post wounding using a multiplex cytokine assay. Wound tissue levels of IL-6 were substantially increased by 140% (Fig. 4a, p<0.05 vs saline), in the epinephrine treated animals, but protein levels of other proinflammatory cytokines (IL-1β, TNF-α, IL-1α, and GM-CSF) were not significantly altered as compared to the saline controls, despite their trend towards increase in tissue levels (Fig. S6).
Fig. 4. IL-6 is a critical regulator of persistent PMN trafficking in local wounds of epinephrine-treated mice.
(a) IL-6 protein level in the wound. Wounded skin samples were harvested from EGFP-lys-mice treated with either saline or epinephrine (5 mg/kg/day) at day 5 post wounding and protein levels of IL-6 were measured. (b) Experimental design for anti-IL-6 antibody treatment into the wounds of mice. Either anti-IL-6 antibody or isotype control was subcutaneously injected into the periphery of wound bed of epinephrine-treated EGFP-lys-mice (5 mg/kg/day) every other day, starting 24hr after wounding up to day 5. Kinetics of wound EGFP-PMNs (c) and wound closure (d) were determined. n=4-5 mice in each group. #, p<0.05 vs Saline group and *, p<0.05 vs Epi+IgG group.
This finding prompted us to investigate whether an increased tissue level of IL-6 mediated the persistent EGFP-PMN trafficking seen in the epinephrine-stressed wounds. To accomplish this, the local activity of IL-6 was blocked by the subcutaneous injection of an anti-IL-6 antibody (0.1 mg) into the wound bed, beginning on day 1 post-wounding, and continued every other day up to day 5 post-wounding (Fig. 4b). Indeed, the anti-IL-6 antibody treatment significantly attenuated wound EGFP-PMN trafficking by 40% at day 8 post-wounding in epinephrine-stressed mice (Epi-anti-IL-6) compared to animals treated with the isotype control (Epi-IgG) (Fig. 4c). Furthermore, the rate of wound closure in the epinephrine-stressed and anti-IL-6 treated mice was accelerated 2-fold compared to mice that received isotype control (Fig. 4d). Taken together, these data indicate that epinephrine upregulates IL-6 levels at the site of local skin wounding and this provides a critical signal to promote PMN persistence in the wound that results in delayed wound healing.
Epinephrine-stress mediates increased IL-6 expression in macrophages via β2 AR dependent mechanism
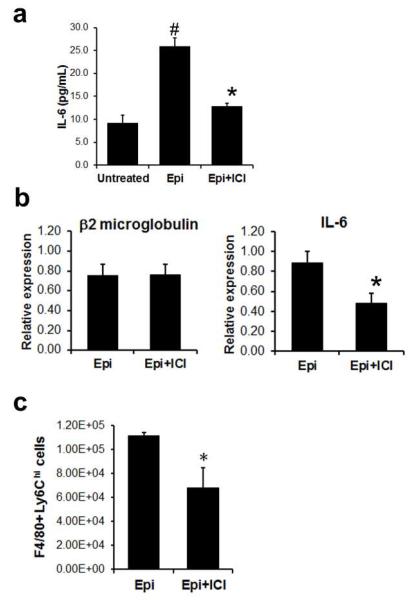
Macrophages within inflammatory wounds play an important role in generating a number of pro-inflammatory cytokines, including IL-6 (Stow et al., 2009). Given the crucial role of IL-6 in mediating epinephrine-stress in PMN wound trafficking, along with the observation that blockade of β2AR activation can reduce epinephrine-mediated PMN trafficking, we examined if epinephrine can stimulate enhanced IL-6 from naïve macrophage via β2AR activation. Treatment of macrophages with epinephrine (1 μM) resulted in ~3-fold increase in IL-6 production compared to untreated control (Fig. 5a). The epinephrine-induced production of IL-6 was blocked by the antagonist ICI 118,551 (1μM). We next sought in vivo evidence of whether wound macrophages were responsible for β2AR-dependent IL-6 production in response to epinephrine. For this, F4/80+ macrophages were harvested from day 8 skin wounds in epinephrine-stressed mice (5 mg/kg/day) or epinephrine-stressed mice treated with ICI 118,551 (0.7 mg/kg/day), and IL-6 gene expression was then determined using real-time qPCR analysis. Consistent with in vitro data of IL-6 ELISA (Fig. 5a), treatment of the epinephrine-stressed animals with ICI 118,551 (Epi+ICI) significantly attenuated IL-6 gene expression of F4/80+ macrophages by ~50% (p<0.05) (Fig. 5b). These data support a crucial role for wound-resident macrophages in producing IL-6 via β2AR activation in response to systemic elevations of epinephrine.
Fig.5. Epinephrine mediates increased IL-6 expression in wound macrophages via β2 AR-dependent mechanism.
(a) Macrophage production of IL-6 in response to epinephrine (1 μM) or epinephrine with ICI 118,551(1 μM). IL-6 production was quantified by ELISA assay. (b) Gene expressions of IL-6 in macrophages isolated from wounds of mice treated either epinephrine or epinephrine with ICI 118,551 at day 8 post-wounding. β2 macroglobulin was used for reference gene. (c) The effect of β2AR antagonist on epinephrine-mediated F4/80+Ly6Ghi cells trafficking in wounds. Cells were harvested from wounds of mice treated with epinephrine (5 mg/kg/day) or epinephrine with ICI 118,551 (0.7 mg/kg/day) at day 8 and were analyzed using flow cytometry. n=4-5 mice in each group. *, p<0.05 between Epi vs Epi+ICI and #, p<0.05 between Epi vs Saline.
We next sought to determine whether sustained elevation of systemic levels of epinephrine alters the inflammatory phenotype of wound macrophages. We examined the surface expression of Ly6C in F4/80+ wound macrophages, as previous studies have reported that F4/80+ Ly6Chi macrophages exhibited a pro-inflammatory phenotype and cytotoxic activities, whereas F4/80+ Ly6Clo macrophages exhibit an anti-inflammatory phenotype and enhanced tissue repair activities (Arnold et al., 2007; Nahrendorf et al., 2007). The total number of F4/80+ macrophages was not different between Epi and Epi+ICI mice (Fig. S7). However, the number of pro-inflammatory F4/80+ Ly6Chi cells was significantly decreased by ~40% in the β2AR antagonist treated animals (Epi+ICI, Fig. 5c). This data suggests that the epinephrine-stress mediated activation of β2AR signaling contributes to the increased trafficking or local differentiation of the proinflammatory macrophage within wounds, which then results in persistent inflammation and delayed wound healing.
Discussion
Using a pharmacologic stress paradigm, we demonstrate that prolonged systemic exposure of epinephrine results in persistent PMN trafficking and prolonged survival within the wound site via β2AR-dependent and IL-6-mediated mechanisms and this in turn impairs wound repair. This study expands our current understanding of stress-mediated impairment of wound healing and provides a pivotal insight into the mechanism by which epinephrine stress exacerbates inflammation and impairs wound healing.
There is significant clinical evidence linking chronic stress to the exacerbation of many disease processes in the immunologic, neoplastic and cardiovascular realms (Hassan et al., 2013; Wong et al., 2012). Recent work has expanded this link to wound healing, wherein chronic stress is associated with delayed healing in both experimental and clinical scenarios (Kiecolt-Glaser et al., 1995; Walburn et al., 2009). Here, we focused on the catecholamine epinephrine, whose elevation parallels both acute and chronic stress, and explored hypothesis that stress-induced epinephrine contributes to impairment of healing via immunomodulation of the wound environment. In support of this, we demonstrated that systemically elevated epinephrine superimposed upon skin injury resulted in impaired wound healing by prolongation of wound inflammation that led to persistent PMN trafficking. Epinephrine stress may impair healing by multiple, overlapping mechanisms. Our recent study demonstrating the direct role of epinephrine in inhibiting keratinocyte motility and re-epithelialization is one way that epinephrine stress impairs healing (Sivamani et al., 2009). This study unveils another mechanism by which epinephrine contributes to impairment of wound healing by promoting a PMN-dependent inflammatory response.
Previous studies have demonstrated systemic increases in plasma IL-6 levels in response to diverse stressors including long-term caregiving (Kiecolt-Glaser et al., 2003), burn injury (Kawakami et al., 2001), psychological stress (Takaki et al., 1994), and childhood maltreatment stress (Carpenter et al., 2010). Here we demonstrate a stress-mediated increase in IL-6 localized to the wound niche. Our findings that the local blockade of IL-6 activity with anti-IL-6 antibody could attenuate wound PMN trafficking and improve wound healing strongly supports the notion that IL-6 is a critical signal linking epinephrine effects with persistent PMN trafficking. IL-6 can promote tissue PMN trafficking during the inflammatory response via selective regulation of inflammatory chemokines or mediating anti-apoptotic events. Multiple lines of evidence support a relationship between increased IL-6 level and prolonged lifetime of PMNs (Asensi et al., 2004; Daffern et al., 1999; Ottonello et al., 2002), which is consistent with our observation of extended survival of PMNs in wounds under epinephrine stress.
Another important finding from this study was that epinephrine-mediated β2AR activation primed wound-associated macrophages into a pro-inflammatory profile, and this, in turn, was responsible for amplified IL-6 production and persistent PMN trafficking. This conclusion is supported by experiments using a β2AR -specific pharmacological antagonist to inhibit epinephrine-mediated responses. We found that blockade of β2AR signaling significantly (1) attenuates epinephrine-mediated trafficking of the pro-inflammatory phenotype of macrophages (F4/80+Ly6Chi macrophages), (2) attenuates epinephrine-induced IL-6 gene expression from wound-isolated macrophages, and (3) attenuates epinephrine-mediated wound PMN trafficking. Macrophages demonstrate considerable plasticity in adjusting their phenotype in response to environmental cues (Mosser and Edwards, 2008). Interestingly, repeated psychosocial stress can increase the number of Ly6Chi macrophages that traffic to inflamed tissue in the brain, and chronic restraint stress similarly increases the trafficking of this pro-inflammatory macrophage phenotype to breast cancer tumors, with both of these responses blocked by an β-AR antagonist (Wohleb et al., 2011). Additionally, the role of β2AR signaling in modulating immunological responses has been supported by the demonstration that its activation enhances pro-inflammatory cytokines and, conversely, by the efficacy of β2AR blockade in attenuating the inflammatory responses in various pathological conditions including traumatic injury, burn injury, and sepsis (Rough et al., 2009). Our findings are consistent with previous demonstrations of induction of a pro-inflammatory profile of macrophages by β2AR activation, and extend those findings by revealing a stress/epinephrine-mediated activation of β2AR signaling pathway that can alter IL-6 cytokine expression in macrophages in the wound, which drive persistent PMN trafficking. Additionally, it is conceivable that macrophages might be pivotal regulators in the epinephrine-mediated pro-inflammatory response, not only for PMN trafficking but also for other significant aspects of wound repair, given the observation that the inhibition of PMN infiltration with either anti-Gr1 or anti-IL-6 antibody treatment resulted in partial recovery of optimal wound healing while the blockade of β2AR signaling fully recovered wound closure in the epinephrine-treated mice (Fig. 3b).
In summary, this study reveals a role of epinephrine in promoting persistent inflammation in skin wounds and demonstrates the pivotal mechanistic role played by β2AR-dependent IL-6 production in the persistent PMN trafficking to the wound (Fig. S8). Since sustained elevation of systemic epinephrine has been associated with different stress etiologies, such as trauma, burn injury, and sepsis (Engelman et al., 1983; Sivamani et al., 2009; Vanitallie, 2002), targeting the β2AR-dependent signaling pathway in these stressed patients may provide a clinically effective immunomodulatory therapeutic strategy to normalize macrophage phenotype, optimize the number of PMNs and thereby avoid prolonged inflammation in cutaneous wounds.
Materials and Methods
Mice
Female EGFP-lysozyme M (lys) knock in-mice (EGFP-lys-mice) backcrossed onto C57BL/6 at least 8 generations (Faust et al., 2000) (kind gift from Dr. Thomas Graf) and C57BL/6 mice (Jackson laboratory) were used in the experiments. Further details are provided in Supplementary Materials and Methods.
Mouse model of skin wounding and osmotic pump implantation
Mouse skin wounding and osmotic pump implantation were performed as described previously (Kim et al., 2011; Kim et al., 2008; Sivamani et al., 2009). Briefly, a circular full thickness wound (6mm in diameter) was made on the back skin of mouse using a skin biopsy punch (Robbins instruments Inc., NJ). Immediately after skin wounding, while the mouse is still anesthetized, an osmotic minipump (1002 model, Alzet, Durect Corporation, Cupertino, CA) was implanted subcutaneously in the side flank. Further details are provided in Supplementary Materials and Methods.
Non-invasive quantification of wound EGFP-PMNs trafficking
The trafficking of EGFP-PMNs at wound sites over time was determined non-invasively using the IVIS 100 imaging system (Caliper Life Sciences, Inc. MA) as described previously (Kim et al., 2008) and in the Supplementary Materials and Methods.
Flow cytometric immunophenotyping of wound PMNs and macrophages
At seleted time points post-wounding, skin wounds were harvested from C57BL/6 mice treated with saline, epinephrine (5 mg/kg/day) or epinephrine with β2AR antagonist ICI 118,551 (0.7 mg/kg/day) and the immunophenotyping of wound PMNs and macrophages were performed as described in Supplementary Materials and Methods.
Immuno-depletion of systemic PMNs
Systemic PMNs were depleted by multiple injections (i.p) of anti-Gr-1 monoclonal antibody (0.1 mg each injection, RB6-8C5 clone, eBioscience, CA) or isotype control IgG (eBioscience, CA) every other day, starting day 1 post-wounding up to day 5.
Plasma epinephrine measurement
Plasma epinephrine measurements were performed using enzyme immunoassay kit (Epinephrine ELISA kit, Rocky mountain diagnostics, Inc., CO) as described in Supplementary Materials and Methods.
The quantification of PMN half-life in the wound
The quantification of PMN half-life in the wound was performed as described previously (Kim et al., 2011) and in the Supplementary Materials and Methods.
Multiplex cytokine assay from wounded skin
The proinflammatory cytokine levels in the wounded skin were measured using MILLIPLEX MAP 13-plex Cytokine Kit (Millipore, Billerica, MA) as described in the Supplementary Materials and Methods.
Anti-IL-6 antibody treatment of murine wounds
Monoclonal mouse anti-IL-6 antibody (clone MP520F3, R&D System, MN) or isotype control, IgG1 (R&D system, MN) was subcutaneously injected into the periphery of the wound bed (0.1 mg each injection) every other day, starting 24hr after wounding up to day 5.
IL-6 ELISA assay from mouse macrophage
IL-6 ELISA from mouse macrophages was performed as described in the Supplementary Material and Methods.
Real time-qPCR for IL-6 gene expression from wound macrophages
Real-time quantitative PCR analysis for the IL-6 gene was performed using macrophages isolated from wounds of EGFP-lys-mice treated with either epinephrine (5 mg/kg/day) or epinephrine with β2AR antagonist ICI 118,551 (0.7 mg/kg/day) at day 8 post-wounding and detailed experimental procedure was described in the Supplementary Materials and Methods.
Statistical analysis
Data analysis was performed using GraphPad Prism version 5.0 software (GraphPad Software, San Diego, CA). Statistical significance between two groups was determined by two-tailed unpaired t tests. Statistical signficance among multiple groups were analyzed using the one-way ANOVA followed by the Turkey post-test for secondary analysis for significance. P values of <0.05 were considered statistically significant. Data are expressed as mean ± SEM.
Supplementary Material
Acknowledgments
This work was supported by California Institute for Regenerative (CIRM) grant TG2-01163 (FG), NIH NIAID RO1 AI47294 (SIS), VA Merit Surg-016-06F, CIRM TR2-01787, and NIH R33 AI080604 (RRI).
Footnotes
Conflict of interests
The authors declare no conflict of interests.
References
- Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–69. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensi V, Valle E, Meana A, Fierer J, Celada A, Alvarez V, et al. In vivo interleukin-6 protects neutrophils from apoptosis in osteomyelitis. Infect Immun. 2004;72:3823–8. doi: 10.1128/IAI.72.7.3823-3828.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol. 2005;144:317–22. doi: 10.1038/sj.bjp.0706048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann M, Sautner T. Immunomodulatory effects of vasoactive catecholamines. Wien Klin Wochenschr. 2002;114:752–61. [PubMed] [Google Scholar]
- Campbell T, Meagher MW, Sieve A, Scott B, Storts R, Welsh TH, et al. The effects of restraint stress on the neuropathogenesis of Theiler’s virus infection: I. Acute disease. Brain Behav Immun. 2001;15:235–54. doi: 10.1006/brbi.2000.0598. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35:2617–23. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffern PJ, Jagels MA, Hugli TE. Multiple epithelial cell-derived factors enhance neutrophil survival. Regulation by glucocorticoids and tumor necrosis factor-alpha. Am J Respir Cell Mol Biol. 1999;21:259–67. doi: 10.1165/ajrcmb.21.2.3605. [DOI] [PubMed] [Google Scholar]
- Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–9. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- Engelman RM, Haag B, Lemeshow S, Angelo A, Rousou JH. Mechanism of plasma catecholamine increases during coronary artery bypass and valve procedures. The Journal of thoracic and cardiovascular surgery. 1983;86:608–15. [PubMed] [Google Scholar]
- Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood. 2000;96:719–26. [PubMed] [Google Scholar]
- Flierl MA, Rittirsch D, Nadeau BA, Chen AJ, Sarma JV, Zetoune FS, et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature. 2007;449:721–5. doi: 10.1038/nature06185. [DOI] [PubMed] [Google Scholar]
- Frost RA, Nystrom GJ, Lang CH. Epinephrine stimulates IL-6 expression in skeletal muscle and C2C12 myoblasts: role of c-Jun NH2-terminal kinase and histone deacetylase activity. Am J Physiol Endocrinol Metab. 2004;286:E809–17. doi: 10.1152/ajpendo.00560.2003. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–51. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Gohel MS, Windhaber RA, Tarlton JF, Whyman MR, Poskitt KR. The relationship between cytokine concentrations and wound healing in chronic venous ulceration. J Vasc Surg. 2008;48:1272–7. doi: 10.1016/j.jvs.2008.06.042. [DOI] [PubMed] [Google Scholar]
- Gouin JP, Hantsoo L, Kiecolt-Glaser JK. Immune dysregulation and chronic stress among older adults: a review. Neuroimmunomodulation. 2008;15:251–9. doi: 10.1159/000156468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan S, Karpova Y, Baiz D, Yancey D, Pullikuth A, Flores A, et al. Behavioral stress accelerates prostate cancer development in mice. The Journal of clinical investigation. 2013 doi: 10.1172/JCI63324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner G, Brauchle M, Smola H, Madlener M, Fassler R, Werner S. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine. 1996;8:548–56. doi: 10.1006/cyto.1996.0074. [DOI] [PubMed] [Google Scholar]
- Insel PA. Seminars in medicine of the Beth Israel Hospital, Boston. Adrenergic receptors--evolving concepts and clinical implications. N Engl J Med. 1996;334:580–5. doi: 10.1056/NEJM199602293340907. [DOI] [PubMed] [Google Scholar]
- Itoh S, Yamada S, Mori T, Miwa T, Tottori K, Uwahodo Y, et al. Attenuated stress-induced catecholamine release in mice lacking the vasopressin V1b receptor. Am J Physiol Endocrinol Metab. 2006;291:E147–51. doi: 10.1152/ajpendo.00005.2006. [DOI] [PubMed] [Google Scholar]
- Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6:e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, et al. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- Kavelaars A. Regulated expression of alpha-1 adrenergic receptors in the immune system. Brain Behav Immun. 2002;16:799–807. doi: 10.1016/s0889-1591(02)00033-8. [DOI] [PubMed] [Google Scholar]
- Kawakami M, He J, Sakamoto T, Okada Y. Catecholamines play a role in the production of interleukin-6 and interleukin-1alpha in unburned skin after burn injury in mice. Crit Care Med. 2001;29:796–801. doi: 10.1097/00003246-200104000-00023. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995;346:1194–6. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003;100:9090–5. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Granick JL, Kwok C, Walker NJ, Borjesson DL, Curry FR, et al. Neutrophil survival and c-kit(+)-progenitor proliferation in Staphylococcus aureus-infected skin wounds promote resolution. Blood. 2011;117:3343–52. doi: 10.1182/blood-2010-07-296970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Liu W, Borjesson DL, Curry FR, Miller LS, Cheung AL, et al. Dynamics of neutrophil infiltration during cutaneous wound healing and infection using fluorescence imaging. J Invest Dermatol. 2008;128:1812–20. doi: 10.1038/sj.jid.5701223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohm AP, Sanders VM. Norepinephrine and beta 2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacol Rev. 2001;53:487–525. [PubMed] [Google Scholar]
- Liu YC, Margolis DJ, Isseroff RR. Does inflammation have a role in the pathogenesis of venous ulcers? A critical review of the evidence. J Invest Dermatol. 2011;131:818–27. doi: 10.1038/jid.2010.428. [DOI] [PubMed] [Google Scholar]
- Morken JJ, Warren KU, Xie Y, Rodriguez JL, Lyte M. Epinephrine as a mediator of pulmonary neutrophil sequestration. Shock. 2002;18:46–50. doi: 10.1097/00024382-200207000-00009. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–47. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottonello L, Frumento G, Arduino N, Bertolotto M, Dapino P, Mancini M, et al. Differential regulation of spontaneous and immune complex-induced neutrophil apoptosis by proinflammatory cytokines. Role of oxidants, Bax and caspase-3. J Leukoc Biol. 2002;72:125–32. [PubMed] [Google Scholar]
- Padgett DA, Marucha PT, Sheridan JF. Restraint stress slows cutaneous wound healing in mice. Brain Behav Immun. 1998;12:64–73. doi: 10.1006/brbi.1997.0512. [DOI] [PubMed] [Google Scholar]
- Pradhan L, Nabzdyk C, Andersen ND, LoGerfo FW, Veves A. Inflammation and neuropeptides: the connection in diabetic wound healing. Expert Rev Mol Med. 2009;11:e2. doi: 10.1017/S1462399409000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridder S, Chourbaji S, Hellweg R, Urani A, Zacher C, Schmid W, et al. Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. J Neurosci. 2005;25:6243–50. doi: 10.1523/JNEUROSCI.0736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rough J, Engdahl R, Opperman K, Yerrum S, Monroy MA, Daly JM. beta2 Adrenoreceptor blockade attenuates the hyperinflammatory response induced by traumatic injury. Surgery. 2009;145:235–42. doi: 10.1016/j.surg.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Sedowofia K, Barclay C, Quaba A, Smith A, Stephen R, Thomson M, et al. The systemic stress response to thermal injury in children. Clinical endocrinology. 1998;49:335–41. doi: 10.1046/j.1365-2265.1998.00553.x. [DOI] [PubMed] [Google Scholar]
- Sivamani RK, Pullar CE, Manabat-Hidalgo CG, Rocke DM, Carlsen RC, Greenhalgh DG, et al. Stress-mediated increases in systemic and local epinephrine impair skin wound healing: potential new indication for beta blockers. PLoS Med. 2009;6:e12. doi: 10.1371/journal.pmed.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small KM, McGraw DW, Liggett SB. Pharmacology and physiology of human adrenergic receptor polymorphisms. Annu Rev Pharmacol Toxicol. 2003;43:381–411. doi: 10.1146/annurev.pharmtox.43.100901.135823. [DOI] [PubMed] [Google Scholar]
- Stow JL, Low PC, Offenhauser C, Sangermani D. Cytokine secretion in macrophages and other cells: pathways and mediators. Immunobiology. 2009;214:601–12. doi: 10.1016/j.imbio.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Takaki A, Huang QH, Somogyvari-Vigh A, Arimura A. Immobilization stress may increase plasma interleukin-6 via central and peripheral catecholamines. Neuroimmunomodulation. 1994;1:335–42. doi: 10.1159/000097185. [DOI] [PubMed] [Google Scholar]
- Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM. Beta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-kappaB-independent mechanisms. Cell Signal. 2007;19:251–60. doi: 10.1016/j.cellsig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Tymen SD, Rojas IG, Zhou X, Fang ZJ, Zhao Y, Marucha PT. Restraint stress alters neutrophil and macrophage phenotypes during wound healing. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.07.013. DOI: 10.1016/j.bbi.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanitallie TB. Stress: a risk factor for serious illness. Metabolism: clinical and experimental. 2002;51:40–5. doi: 10.1053/meta.2002.33191. [DOI] [PubMed] [Google Scholar]
- Viswanathan K, Daugherty C, Dhabhar FS. Stress as an endogenous adjuvant: augmentation of the immunization phase of cell-mediated immunity. Int Immunol. 2005;17:1059–69. doi: 10.1093/intimm/dxh286. [DOI] [PubMed] [Google Scholar]
- von Montfort C, Beier JI, Guo L, Kaiser JP, Arteel GE. Contribution of the sympathetic hormone epinephrine to the sensitizing effect of ethanol on LPS-induced liver damage in mice. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1227–34. doi: 10.1152/ajpgi.00050.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walburn J, Vedhara K, Hankins M, Rixon L, Weinman J. Psychological stress and wound healing in humans: a systematic review and meta-analysis. J Psychosom Res. 2009;67:253–71. doi: 10.1016/j.jpsychores.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–63. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, et al. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277–88. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DL, Tai TC, Wong-Faull DC, Claycomb R, Meloni EG, Myers KM, et al. Epinephrine: a short- and long-term regulator of stress and development of illness: a potential new role for epinephrine in stress. Cell Mol Neurobiol. 2012;32:737–48. doi: 10.1007/s10571-011-9768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.