Abstract
Introduction
The impact of a postoperative troponin elevation on long-term survival after vascular surgery is not well-defined. We hypothesize that a postoperative troponin elevation is associated with significantly reduced long-term survival.
Methods
The Vascular Study Group of New England registry identified all patients who underwent carotid revascularization, open abdominal aortic aneurysm repair (AAA), endovascular AAA repair, or infrainguinal lower extremity bypass (2003–2011). The association of postoperative troponin elevation and myocardial infarction (MI) with 5-year survival was evaluated. Multivariable models identified predictors of survival and of postoperative myocardial ischemia.
Results
In the entire cohort (n = 16,363), the incidence of postoperative troponin elevation was 1.3% (n = 211) and for MI was 1.6% (n = 264). Incidences differed across procedures (P < .0001) with the highest incidences after open AAA: troponin elevation, 3.9% (n = 74); MI, 5.1% (n = 96). On Kaplan-Meier analysis, any postoperative myocardial ischemia predicted reduced survival over 5 years postoperatively: no ischemia, 73% (standard error [SE], 0.5%); troponin elevation, 54% (SE, 4%); MI, 33% (SE, 4%) (P < .0001). This pattern was observed for each procedure subgroup analysis (P < .0001). Troponin elevation (hazard ratio, 1.45; 95% confidence interval, 1.1–2.0; P = .02) and MI (hazard ratio, 2.9; 95% confidence interval, 2.3–3.8; P < .0001) were independent predictors of reduced survival at 5 years.
Conclusions
Postoperative troponin elevation and MI predict a 26% or a 55% relatively lower survival in the 5 years following a vascular surgical procedure, respectively, compared with patients who do not experience myocardial ischemia. This highlights the need to better characterize factors leading to postoperative myocardial ischemia. Postoperative troponin elevation, either alone, or in combination with an MI, may be a useful marker for identifying high-risk patients who might benefit from more aggressive optimization in hopes of reducing adverse long-term outcomes.
Cardiovascular death remains a major cause of late mortality following vascular surgical procedures.1–4 The prevalence of coronary artery disease (CAD) in this patient population has been well-documented, beginning with a large review from the Cleveland Clinic nearly 30 years ago5 demonstrating that 92% of patients undergoing major vascular surgical procedures have significant CAD when screening cardiac catheterization is performed. Similarly, postoperative cardiac events remain a significant source of morbidity and mortality associated with vascular operations. Evidence suggests that postoperative myocardial infarction (MI) negatively impacts both short- and long-term survival.4,6–9 In a recent series, utilizing Vascular Study Group of New England (VSGNE) data, postoperative MI was correlated with worse survival following carotid revascularization.10 Troponin elevations have also been shown to correlate with negative outcomes for a wide range of diagnoses, from acute coronary syndromes,11,12 to critically ill medical patients,13 to all types of noncardiac surgical procedures,14 and to vascular surgical procedures in particular.6,15,16 Because it does not, in isolation, constitute an MI, many clinicians, both surgeons and consulting nonsurgeons alike, express less concern for troponin elevations; however, the long-term survival implications of these events remain uncertain. Troponin is a regulatory protein associated with contraction of myocardial muscle cells; serum elevations of troponin are believed to correlate with myocyte injury from myocardial ischemia.17 The universal definition of MI classifies myocardial ischemia into five types based on the associated pathophysiology; the majority of patients who sustain myocardial ischemia following noncardiac surgery are believed to have type 2 ischemia, which relates to an imbalance in supply and demand of myocardial oxygen, which can result from many perioperative factors including anemia or hypotension, with or without underlying cardiac disease.18 We sought to determine the association of postoperative troponin elevation with long-term survival in patients undergoing vascular surgical procedures.
METHODS
Study design
Prospectively collected data from the VSGNE database was retrospectively reviewed to identify all patients who underwent carotid angioplasty and stent placement, carotid endarterectomy (CEA), open infrarenal abdominal aortic aneurysm (AAA) repair, endovascular AAA repair, and infrainguinal lower extremity bypass between January 1, 2003 and December 31, 2011. The VSGNE is a regional cooperative quality improvement initiative, developed in 2002, to prospectively evaluate outcomes in patients undergoing vascular surgical procedures. It is comprised of 140 physicians from 25 academic and community medical centers across New England. Details of this registry have been published previously19 and are available online (www.vsgne.org). Data are physician-reported at the time of operation and include preoperative, intraoperative, and in-hospital postoperative details. Follow-up data are entered at approximately 1 year postoperatively. All information is sent to a central data repository where it is aggregated and audited. Research analysts are blinded to patient, surgeon, and hospital identity.
Primary end point and exposure variable
The primary end point was long-term survival. Survival was determined by matching patients in the registry with the Social Security Death Index. An adjusted analysis of survival over the first 5 years postoperatively was also constructed, using the mean of all nonexposure variables. An additional exploratory analysis of survival was done that excluded patients who died within 30 days of operation in order to test whether an effect is driven by fatal MI only.
The primary exposure variable was postoperative myocardial ischemia. Postoperative myocardial ischemia was categorized using variables that are collected postoperatively in the VSGNE dataset in the following manner: (1) a troponin elevation beyond the normal upper limit, as defined by the testing laboratory, without electrocardiographic (ECG) changes or (2) clinical symptoms (severe chest pain and/or radiation to the left arm or jaw) and/or ECG changes consistent with MI, as defined at each participating institution. The precise nature of the ECG changes (ST elevations, Q waves, etc) was not specified. The primary exposure variable, “postoperative ischemia,” is nominal with three possible levels: no ischemia, troponin elevation only, or MI. The hierarchy of the variable ensures that patients were not dually classified as both “troponin only” and “clinical/ECG MI”; the “clinical/ECG MI” supersedes the other designation. Patients were not routinely screened for troponin elevations or postoperative ECG changes; they were obtained at the discretion of the provider postoperatively, based on clinical suspicion or risk factor prevalence. Therefore, it is not possible to know the total number of patients for whom troponin assays were sent, but resulted within normal limits. In addition, a literal interpretation of the second part leaves open the possibility that patients who experienced clinical symptoms alone would be categorized as having sustained an MI. Since most practitioners would likely not consider this sufficient evidence to make that diagnosis, we have assumed that there would be very few instances of this misclassification when reporting outcomes also. We instead suspect that most often, this variable is understood to mean clinical symptoms in addition to troponin elevation, since the other levels of this nominal variable are “no MI,” or troponin elevation only. Troponin levels were categorized as elevated or normal but not quantified as a continuous variable.
Covariates examined
Patient information for >100 clinical and demographic variables (available at www.vsgne.org) was collected. Demographic information included age at the time of the procedure, gender, and race. Comorbidities examined included CAD (history of MI without current symptoms, stable angina, or unstable angina or MI within the past 6 months), chronic obstructive pulmonary disease (COPD; medication-dependent or home oxygen-dependent), congestive heart failure (CHF; by history), diabetes mellitus (DM; insulin-dependent and/or controlled by oral medication or diet), hypertension (HTN; history of hypertension or blood pressure ≥140/90 mm Hg on the preoperative evaluation), and history of tobacco use (never, <1 year prior, or current). Renal disease was categorized in three strata: normal (serum creatinine ≤1.8mg/dL), renal insufficiency (serum creatinine >1.8 mg/dL), and dialysis-dependent. Results of preoperative stress testing was defined as not performed, negative for ischemia, or positive for ischemia, MI, or both. History of previous coronary revascularization included both coronary artery bypass graft or percutaneous coronary intervention. While statin use is recorded in the database, there is no information on specific lipid levels; the use of a statin was not considered a proxy for hyperlipidemia.
Medication use was recorded, including beta-blockers, antiplatelet agents (aspirin, clopidogrel, or both), and statins. Information was available both on the use of these medications preoperatively, as well as at the time of discharge. Data regarding preoperative medication use were included as covariates on multivariable modeling of postoperative myocardial ischemia, while medication use at the time of discharge was used in multivariable modeling of long-term survival; this was based on our clinical judgment of the relevance of the timing of medication use relative to the end point of interest.
Subgroup analyses were performed by procedure type, which included carotid revascularization, open AAA repair, endovascular AAA repair, and lower extremity bypass. Carotid revascularization was defined as either CEA or carotid stenting (CAS); the two procedures were grouped together because exploratory analyses revealed that the two groups were not statistically different and that the primary outcome event incidences were very low (<1%) in the carotid stenting group. Lower extremity bypass was limited to infrainguinal bypass. Elective, urgent, and emergent cases were included.
Statistical analysis
Descriptive statistics were used to assess the incidence of postoperative myocardial ischemia. Univariate analyses were conducted with t-test for the continuous variable, age, and χ2 analysis for all other variables, which were categorical variables; this included all demographic information (other than age), comorbid conditions, procedure type, and medication use. Survival analyses were performed according to the Kaplan-Meier method, with intergroup comparisons made using log-rank testing. Analyses were performed on the entire cohort, with additional subgroup analyses based on procedure type.
Cox proportional hazards modeling was used to determine the magnitude of effect of postoperative myocardial ischemia on survival. An interaction term was tested in the model to assess for interaction between procedure type and postoperative ischemia. Multivariable logistic regression was used to assess independent predictors of any postoperative myocardial ischemia (both isolated troponin elevation and MI). Clinically relevant covariates were chosen a priori to ensure face validity of the multivariable models. Significance was accepted at the P < .05 level. All analyses were conducted using SAS version 9.2 software (SAS Institute, Cary, NC).
RESULTS
Cohort characteristics
Between January 2003 and December 2011, a total of 16,421 patients underwent vascular surgical procedures recorded in the VSGNE database (Table I). Fifty-eight patients were excluded because the exposure variable, postoperative myocardial ischemia, was missing. The majority were men (n = 10,908; 67%), with a history of tobacco use (n = 13,527; 83%). Carotid revascularization comprised the majority of the cohort (n = 8317; 51%), with 7836 (94%) CEA and 481 (6%) CAS procedures. There were 3991 lower extremity bypasses (24%), 1883 open AAAs (12%), and 2172 endovascular AAA (13%) procedures.
Table I.
Demographics for patients in the VSGNE cohort (2003–2011), stratified according to postoperative isolated troponin elevation, MI, or no ischemia
| Covariate | Total (n = 16,363) |
No ischemia (n = 15,888) |
Troponin elevation (n = 211) |
MI (n = 264) |
P value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years | 69.8 ± 10 | 69.7 ± 10 | 73.1 ± 8.7 | 72.8 ± 9.2 | <.0001 |
| Gender (male) | 10908 (66.7) | 10593 (66.7) | 148 (70.1) | 167 (63.3) | .28 |
| Preoperative factors | |||||
| Smoking status | .55 | ||||
| Never | 2797 (17.1) | 2726 (17.2) | 33 (15.8) | 38 (14.3) | |
| Former/current | 13527 (82.9) | 13134 (82.8) | 176 (84.2) | 217 (85.1) | |
| COPD | <.0001 | ||||
| None/not treated | 13764 (84.2) | 13409 (84.4) | 152 (72.4) | 203 (77.8) | |
| Medications | 2190 (13.4) | 2098 (13.2) | 46 (21.9) | 46 (17.6) | |
| Home oxygen | 396 (2.4) | 372 (2.3) | 12 (5.7) | 12 (4.6) | |
| CAD | <.0001 | ||||
| None | 10850 (66.4) | 10634 (67) | 94 (44.8) | 122 (47.5) | |
| History of MI, asymptomatic | 3415 (20.9) | 3276 (20.6) | 7 (34.3) | 67 (26.1) | |
| Stable angina | 1794 (11) | 1697 (10.7) | 42 (20) | 55 (21.4) | |
| Unstable angina or MI within 6 months | 278 (1.7) | 277 (1.7) | 4 (1.8) | 13 (5.1) | |
| History of coronary revascularization | 5136 (31.4) | 4973 (31.3) | 74 (35.1) | 89 (34.5) | .29 |
| Stress testing | .01 | ||||
| Not performed | 9783 (60.1) | 9510 (60.2) | 120 (57.1) | 153 (58.4) | |
| Normal | 4550 (28) | 4437 (28.1) | 53 (25.2) | 60 (22.9) | |
| Ischemia/MI/both | 1944 (11.9) | 1858 (11.8) | 37 (17.6) | 49 (18.7) | |
| CHF | 1716 (10.5) | 1598 (10.1) | 57 (27.1) | 61 (23.6) | <.0001 |
| HTN | 14091 (86.2) | 13663 (86) | 188 (89.1) | 240 (92.3) | .01 |
| DM | 5243 (32.1) | 5044 (31.8) | 89 (42.4) | 110 (42.3) | <.0001 |
| Renal function | <.0001 | ||||
| Cr ≤1.8 mg/dL | 14622 (92.5) | 14244 (91.8) | 160 (78.1) | 218 (86.9) | |
| Cr >1.8 mg/dL | 1037 (6.5) | 972 (6.3) | 38 (18.5) | 27 (10.8) | |
| Dialysis-dependent | 318 (2) | 305 (2) | 7 (3.4) | 6 (2.4) | |
| Preoperative medications | |||||
| Antiplatelet agent | 13291 (81.3) | 12929 (81.4) | 157 (74.8) | 205 (80.1) | .04 |
| Beta blocker | 12725 (77.9) | 12337 (77.7) | 170 (81) | 218 (83.5) | .05 |
| Statin | 11507 (70.4) | 11188 (70.5) | 141 (67.1) | 178 (69.3) | .25 |
| Postoperative medications | |||||
| Antiplatelet agent | 13491 (87.9) | 13177 (87.9) | 147 (85) | 167 (88.8) | .46 |
| Beta blocker | 9592 (76.2) | 9336 (76) | 122 (80.8) | 134 (85.4) | .01 |
| Statin | 9841 (78.1) | 9603 (78.1) | 114 (75.5) | 124 (79) | .72 |
| Procedure type | <.0001 | ||||
| Carotid revascularization | 8317 (50.8) | 8229 (51.8) | 38 (18) | 50 (18.9) | |
| AAA (open) | 1883 (11.5) | 1713 (10.8) | 74 (35.1) | 96 (36.4) | |
| AAA (endovascular) | 2172 (13.3) | 2118 (13.3) | 27 (12.8) | 27 (10.2) | |
| Lower extremity bypass | 3991 (24.4) | 3828 (24.1) | 72 (34.1) | 91 (34.5) |
AAA, Abdominal aortic aneurysm; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HTN, hypertension; MI, myocardial infarction; VSGNE, Vascular Study Group of New England.
Continuous data are presented as mean ± standard deviation and categoric data as number (%).
Overall, preoperative use of all three classes of cardio-protective medications was high, with 70% of patients on statins, 78% of patients on beta blockers, and 81% of patients on antiplatelet agents. Patients who suffered both types of postoperative myocardial ischemia were less likely to be on a beta blocker (P = .04) but were more likely to be on an antiplatelet agent (P = .04). Patients with a history of CAD were more likely to experience postoperative myocardial ischemia (P < .0001). Patients with positive preoperative stress testing were also more likely to experience postoperative myocardial ischemia (P = .01). Postoperative myocardial ischemia was associated with a history of CHF (P < .0001), history of COPD (P < .0001), DM (P < .0001), HTN (P = .01), and renal insufficiency (P < .0001) on univariate analysis.
Postoperative events and long-term outcomes
Postoperative myocardial ischemia occurred in 475 patients (3%), with MI slightly more common (n = 264; 2%) than troponin elevation (n = 211; 1%; Table II). On univariate analysis, incidences of myocardial ischemia were significantly different between procedure types (P < .0001), with the highest incidence after open AAA repair (troponin elevation, n = 74; 4% and MI, n = 96; 5%). Postoperative myocardial ischemia was least common after carotid revascularization, with 38 patients experiencing troponin elevation (0.5%) and 50 patients experiencing MI (0.6%).
Table II.
Event rates stratified by procedure type
| Procedure type | No ischemia, No. (%) |
Troponin elevation, No. (%) |
MI, No. (%) |
|---|---|---|---|
| Total cohort | 15,888 (97.1) | 211 (1.3) | 264 (1.6) |
| Carotid revascularization | 8229 (98.9) | 38 (0.5) | 50 (0.6) |
| AAA (open) | 1713 (91) | 74 (3.9) | 96 (5.1) |
| AAA (endovascular) | 2118 (97.5) | 27 (1.2) | 27 (1.2) |
| Lower extremity bypass | 3828 (95.9) | 72 (1.8) | 91 (2.3) |
AAA, Abdominal aortic aneurysm; MI, myocardial infarction.
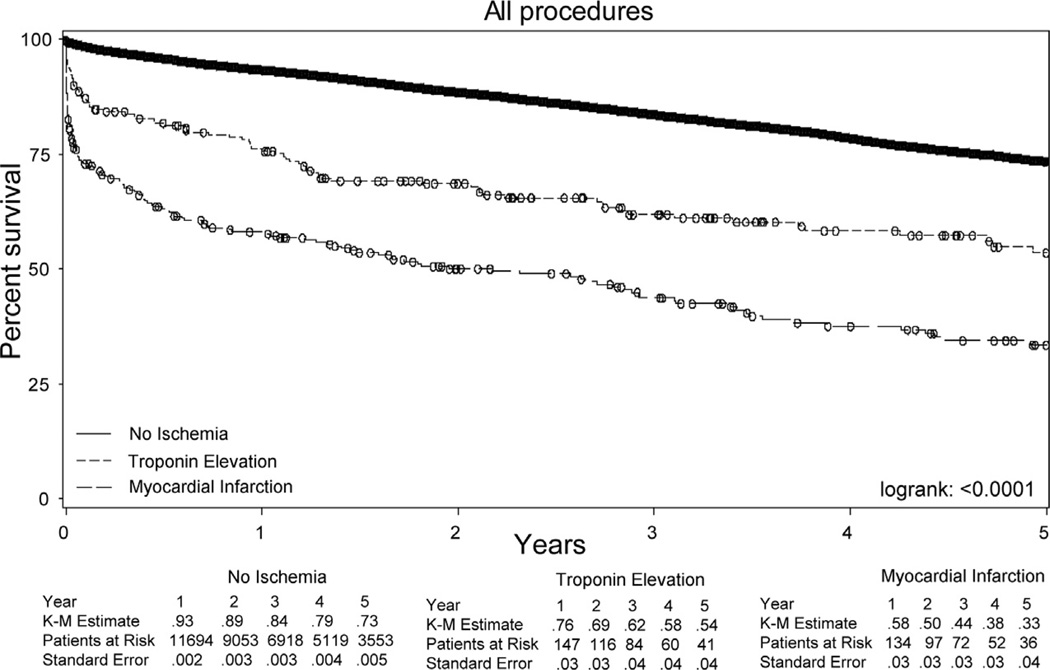
Kaplan-Meier analysis revealed a significant difference in survival over the first 5 years postoperatively when the cohort was stratified according to the specific type of postoperative myocardial ischemia (Fig 1). Among those without myocardial ischemia, survival over the first 5 years postoperatively was 73% (standard error [SE], 0.5%), whereas among those with a postoperative troponin elevation or MI, survival was 54% (SE, 4%) and 33% (SE, 4%), respectively (log-rank, P < .0001).
Fig 1.
Univariate Kaplan-Meier (K-M) analysis of 5-year survival among the total cohort, stratified by postoperative myocardial ischemia; log-rank, P < .0001.
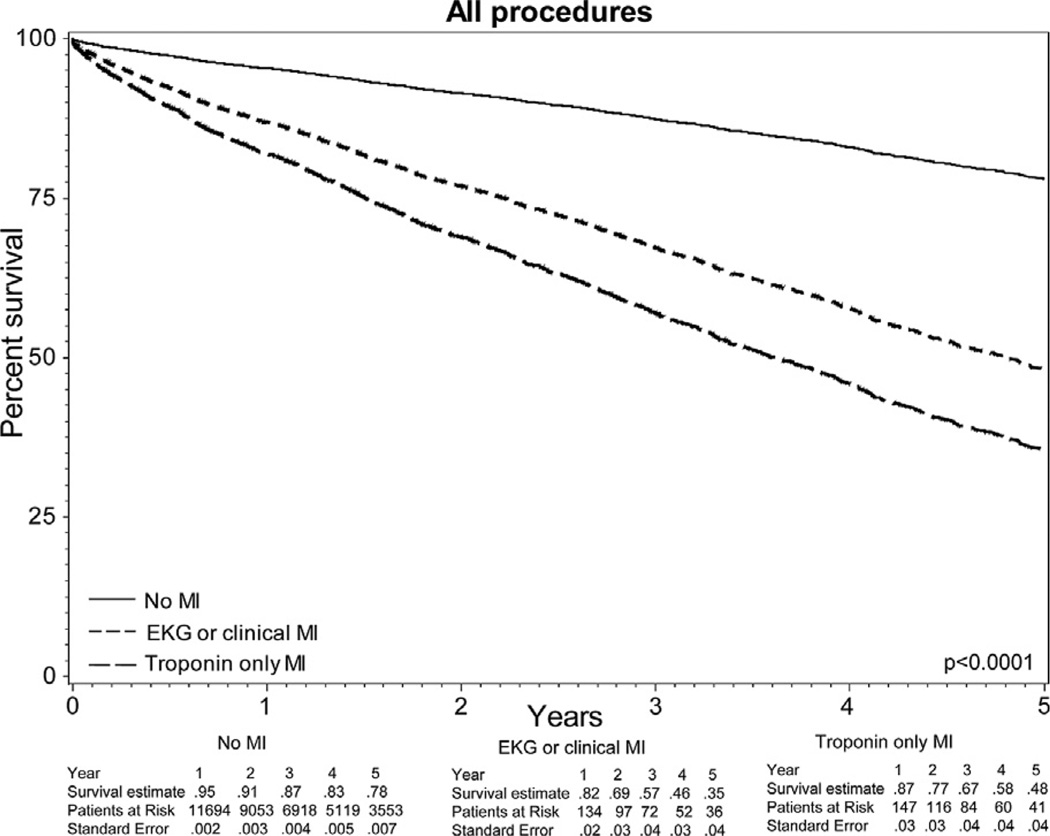
Adjusted Kaplan-Meier analysis, using the mean values for nonexposure variables, demonstrated a significant difference in survival over the first 5 years postoperatively (Fig 2). Among those without myocardial ischemia, survival over the first 5 years postoperatively was 78% (SE, 0.7%), whereas among those with a postoperative troponin elevation or MI, survival was 48% (SE, 4%) and 35% (SE, 4%), respectively (log-rank, P < .0001).
Fig 2.
Kaplan-Meier analysis of 5-year survival among the total cohort, stratified by postoperative myocardial ischemia and adjusted for the mean values of all nonexposure variables; log-rank, P < .0001. EKG, Electrocardiograph; MI, myocardial infarction.
An exploratory analysis that excluded patients who died in the perioperative period continued to demonstrate a significant difference in survival over the first 5 years postoperatively, although the point estimates were different. Among those without myocardial ischemia, survival over the first 5 years postoperatively was 74% (SE, 0.5%), whereas among those with a postoperative troponin elevation or MI, survival was 61% (SE, 5%) and 46% (SE, 5%), respectively (log-rank, P < .0001).
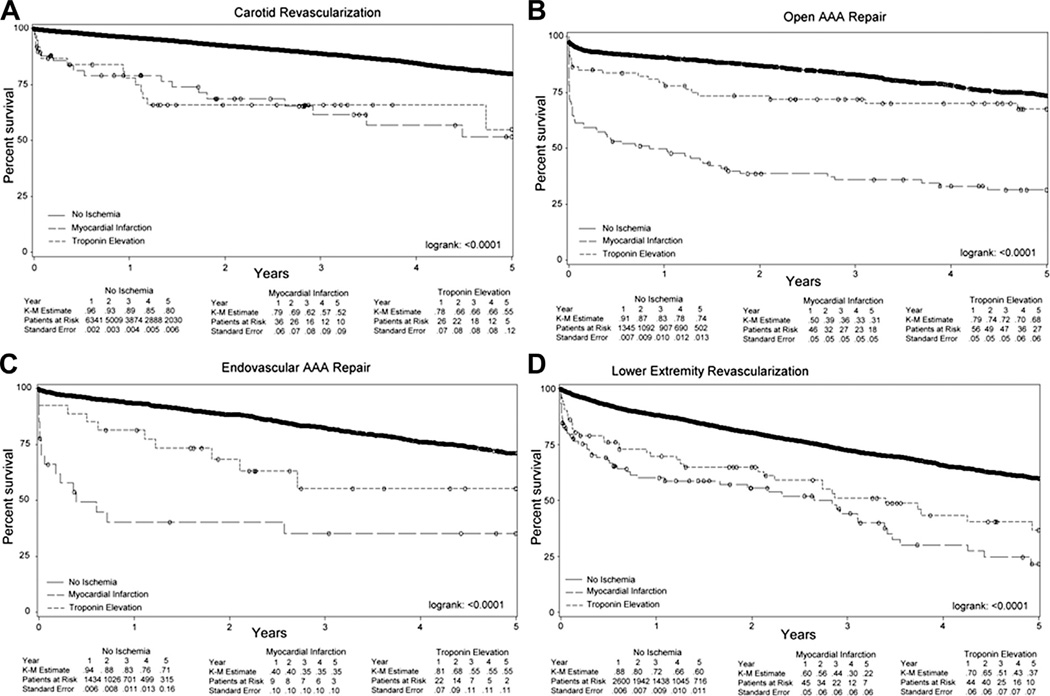
Subgroup analysis, using the entire cohort, by procedure type demonstrated a similar trend for all procedures (log-rank, P < .0001 for all; Fig 3).
Fig 3.
Univariate Kaplan-Meier (K-M) curves of survival at 5 years, stratified according to postoperative myocardial ischemia, among (A) carotid revascularization; P < .0001, (B) open abdominal aortic aneurysm (AAA) repair; P < .0001, (C) endovascular AAA repair; P < .0001, and (D) lower extremity bypass; P < .0001.
Independent predictors of long-term survival
On multivariable analysis, postoperative MI was a significant independent predictor of death over the first 5 years of follow-up postoperatively (hazard ratio [HR], 2.9; 95% confidence interval [CI], 2.3–3.8; P < .0001) compared with no myocardial ischemia (Table III). Similarly, a postoperative troponin elevation was also a significant independent predictor of death over the first 5 years postoperatively (HR, 1.5; 95% CI, 1.1–2.0; P = .01) compared with no myocardial ischemia. Compared with carotid revascularization as the reference group, patients undergoing lower extremity bypass had significantly lower 5-year survival (HR, 2.0; 95% CI, 1.8–2.2; P < .0001), while patients undergoing endovascular AAA (P = .26) and open AAA (P = .14) did not. No significant interaction was found between procedure type and postoperative ischemia (P = .0952). Increasing age demonstrated an inversely proportional relationship, with decreased survival associated with each incremental increase in age strata (compared with age <60 years; age 60–69 years, HR, 1.4; 95% CI, 1.1–1.6; P = .002; age 70–79 years, HR, 2.1; 95% CI, 1.7–2.5; P < .0001; age >80 years, HR, 4.3; 95% CI, 3.6–5.2; P < .0001).
Table III.
Cox proportional hazards modeling of the effect of postoperative myocardial ischemia on survival
| Covariate | HR (95% CI) | P value |
|---|---|---|
| Postoperative myocardial ischemia | ||
| No ischemia | Referent | |
| Troponin elevation | 1.45 (1.08–1.95) | .01 |
| MI | 2.93 (2.26–3.8) | <.0001 |
| Demographics | ||
| Age <60 years | Referent | |
| Age 60–69 years | 1.35 (1.11–1.63) | .002 |
| Age 70–79 years | 2.09 (1.75–2.51) | <.0001 |
| Age ≥80 years | 4.32 (3.59–5.2) | <.0001 |
| Female gender | 1.02 (0.92–1.13) | .77 |
| Preoperative factors | ||
| Smoking status | ||
| Never | Referent | |
| Former/current | 1.14 (1.0–1.3) | .05 |
| COPD | ||
| None/not treated | Referent | |
| Medications | 1.38 (1.21–1.57) | <.0001 |
| Home oxygen | 2.78 (2.29–3.38) | <.0001 |
| CAD | 1.35 (1.2–1.51) | <.0001 |
| History of coronary revascularization | 1.0 (0.89–1.12) | .97 |
| CHF | 1.48 (1.3–1.7) | <.0001 |
| HTN | 0.98 (0.85–1.14) | .82 |
| DM | 1.2 (1.08–1.33) | .001 |
| Renal function | ||
| Creatinine ≥1.8 mg/dL | Referent | |
| Creatinine >1.8 mg/dL | 1.89 (1.63–2.19) | <.0001 |
| Dialysis-dependent | 4.38 (3.56–5.39) | <.0001 |
| Medications at discharge | ||
| Antiplatelet agent | 0.67 (0.56–0.76) | <.0001 |
| Beta blocker | 0.93 (0.82–1.05) | .22 |
| Statin | 0.69 (0.62–0.76) | <.0001 |
| Procedure | ||
| Carotid revascularization | Referent | |
| AAA (open) | 1.14 (0.96–1.35) | .14 |
| AAA (endovascular) | 1.09 (0.94–1.27) | .26 |
| Lower extremity bypass | 1.98 (1.76–2.21) | <.0001 |
AAA, Abdominal aortic aneurysm; CAD, coronary artery disease; CHF, congestive heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HTN, hypertension; HR, hazard ratio; MI, myocardial infarction.
Certain medical comorbidities were independently associated with decreased survival over the first 5 years postoperatively: history of CAD (HR, 1.4; 95% CI, 1.2–1.5; P < .0001), COPD requiring medications (HR, 1.4; 95% CI, 1.2–1.6; P < .0001), home oxygen use (HR, 2.8; 95% CI, 2.3–3.4; P < .0001), history of DM (HR, 1.2; 95% CI, 1.1–1.3; P = .001), CHF (HR, 1.5; 95% CI, 1.3–1.7; P < .0001), renal insufficiency (Cr >1.78 mg/dL, HR, 1.9; 95% CI, 1.6–2.2; P < .0001, and dialysis dependence, HR, 4.4; 95% CI, 3.6–5.4; P < .0001). Antiplatelet therapy at the time of discharge was independently associated with a survival benefit over the first 5 years postoperatively (HR, 0.7; 95% CI, 0.6–0.8; P < .0001), as was statin use (HR, 0.7; 95% CI, 0.6–0.8; P < .0001). Beta blocker use on discharge did not confer a significant survival benefit over the first 5 years postoperatively.
Predictors of postoperative myocardial ischemia
On logistic regression, CEA had lower odds of postoperative myocardial ischemia than all other procedure types (Table IV). Open AAA had the strongest association on the occurrence of myocardial ischemia (odds ratio [OR], 9.6; 95% CI, 7.2–12.9; P < .0001), followed by lower extremity bypass (OR, 3.6; 95% CI, 2.7–4.8; P < .0001). History of CAD was independently associated with postoperative myocardial ischemia, with an increasing exposure-response relationship (compared with no history of CAD, history of MI but no symptoms, OR, 1.8; 95% CI, 1.4–2.3; P < .0001; stable angina, OR, 2.5; 95% CI, 1.9–3.4; P < .0001; unstable MI or angina within 6 months, OR, 2.6; 95% CI, 1.5–4.7; P = .001). No class of cardioprotective medications, used preoperatively, had a significant effect on postoperative myocardial ischemia.
Table IV.
Logistic regression analysis of predictors of any type of postoperative myocardial ischemia
| Covariate | OR (95% CI) | P value |
|---|---|---|
| Demographics | ||
| Age <60 years | Referent | |
| Age 60–69 years | 1.53 (1.04–2.24) | .03 |
| Age 70–79 years | 1.87 (1.29–2.7) | .0009 |
| Age ≥80 years | 2.39 (1.61–3.55) | <.0001 |
| Gender (female) | 1.27 (1.03–1.57) | .03 |
| Preoperative factors | ||
| Smoking status | ||
| Never | Referent | |
| Former/current | 1.07 (0.81–1.42) | .63 |
| COPD | ||
| None/not treated | Referent | |
| Medications | 1.25 (0.97–1.61) | .09 |
| Home oxygen | 1.59 (1.0–2.52) | .05 |
| CAD | ||
| None | Referent | |
| History of MI, asymptomatic | 1.77 (1.37–2.27) | <.0001 |
| Stable angina | 2.53 (1.9–3.37) | <.0001 |
| Unstable angina or MI within 6 months | 2.65 (1.48–4.75) | .001 |
| History of coronary revascularization | 0.70 (0.56–0.89) | .003 |
| CHF | 1.9 (1.48–2.45) | <.0001 |
| HTN | 1.46 (1.02–2.08) | .04 |
| DM | 1.63 (1.31–2.03) | <.0001 |
| Renal function | ||
| Creatinine ≤1.8 mg/dL | Referent | |
| Creatinine >1.8 mg/dL | 1.58 (1.18–2.13) | .002 |
| Dialysis-dependent | 0.77 (0.43–1.4) | .39 |
| Preoperative medications | ||
| Antiplatelet agent | 1.18 (0.91–1.51) | .21 |
| Beta blocker | 1.03 (0.79–1.35) | .82 |
| Statin | 1.12 (0.89–1.4) | .34 |
| Procedure | ||
| Carotid revascularization | Referent | |
| AAA (open) | 9.62 (7.21–12.85) | <.0001 |
| AAA (endovascular) | 2.17 (1.51–3.13) | <.0001 |
| Lower extremity bypass | 3.61 (2.73–4.76) | <.0001 |
AAA, Abdominal aortic aneurysm; CAD, coronary artery disease; CHF, congestive heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HTN, hypertension; MI, myocardial infarction; OR, odds ratio.
DISCUSSION
This study demonstrates that, in patients undergoing vascular surgical procedures, any postoperative myocardial ischemia, whether a postoperative troponin elevation or a postoperative MI, is associated with lower survival over the first 5 years postoperatively. While the magnitude of effect of a troponin elevation (HR, 1.5) is smaller than that of MI (HR, 2.9), the association with reduced 5-year survival is substantial. The exclusion of patients who did not survive the perioperative period, a surrogate for those with fatal cardiac events, did not impact this trend. Also, adjustment of the Kaplan-Meier curves for confounders changed the point estimates (by less than 10% in each exposure level), but not the overall trend, or significance testing. Other predictors of reduced long-term survival identified in this population included advanced age, COPD, CAD, CHF, DM, impaired renal function, and a lower extremity bypass procedure. Conversely, the use of antiplatelet agents and statins at the time of discharge demonstrated a protective effect.
Previous studies have shown that, compared with age-matched controls, patients undergoing major vascular operations have decreased long-term survival.2 Cardiac death has been shown to account for a substantial portion of long-term mortality4 following major vascular procedures. Similarly, postoperative cardiac complications have been identified as predictors of death in both the short and long term.6,8,20,21 This extends the findings previously reported utilizing patients in the VSGNE who underwent carotid revascularization, a subset of the present study’s cohort; in that study, both postoperative MI and stroke were associated with worse 1-year and 5-year survival.10 Our findings corroborate the importance of cardiac morbidity on survival; patients who experienced a postoperative MI had a substantially lower survival over the first 5 years postoperatively (33%; SE, 4%) than patients who did not experience postoperative ischemia (73%; SE, .5%).
The specific influence of a postoperative troponin elevation on short-term outcomes has been investigated4,7,8,15 in previous work, but the association with worse long-term outcomes, specifically in patients undergoing major vascular operations, has not been examined. A recent meta-analysis demonstrated a strong association between postoperative troponin elevation and short-term mortality after vascular procedures.22 While not the primary focus of the present investigation, Kaplan-Meier analysis of survival at 1 year in our cohort confirmed this finding, with a significant difference between the curves (log-rank, P < .0001); for those with no ischemia, survival at 1 year was 93% (SE, 0.2%); for toponin elevation, 76% (SE, 3%); and for MI, 58% (SE, 3%). Our findings are therefore in accordance with these other studies of early survival, but extend the current body of literature by demonstrating that the negative impact of a postoperative troponin elevation on survival persists out to 5 years.
Utilizing one of the largest cooperative data registries of vascular surgical patients, we were able to elucidate the significance of a postoperative troponin elevation beyond the previously established immediate postoperative period. In addition, our results include several major procedure types, and therefore represent a more diverse patient population than previous studies analyzing a single procedure type.2–4 This, in combination with the varied population obtained from both academic and community practices throughout New England, enhances the generalizability of these findings.
Limitations of this study include the potential for bias based on a lack of protocol for obtaining postoperative troponin levels on every patient. It may be that troponins were checked in the absence of clinical symptoms only in those patients considered at highest risk for cardiac complications. Attempts to mitigate this potential bias included controlling for several comorbidities with the use of Cox proportional hazards modeling, but confounding due to unmeasured factors may persist. In addition, the incidence of postoperative myocardial ischemia was somewhat lower than that reported in several other large series of vascular surgical patients. This may relate to the heterogeneous population, with 50% of the cohort having undergone carotid revascularization, a relatively low-risk procedure for postoperative myocardial ischemia. Finally, a limitation inherent to the structure of the VSGNE dataset is the definition of MI as either “clinical symptoms consistent with MI” or “ECG changes consistent with MI” without reference to troponin level. According to the American Heart Association’s (AHA) universal definition of MI,18 at least one troponin value above the 99th percentile is required in addition to either of the two aforementioned criteria. This data collection method makes it impossible to classify patients in the VSGNE dataset according to the AHA universal definition. Nonetheless, in aggregate, these limitations would tend to overestimate the incidence of myocardial ischemia and underestimate the effect of a postoperative myocardial ischemia on 5-year survival.
We have shown in a contemporary diverse cohort of more than 16,000 patients undergoing vascular surgical procedures that survival over the first 5 years postoperatively is reduced in patients experiencing a postoperative myocardial ischemic event, including an isolated troponin elevation. Moreover, we have established the relative magnitude of effect on long-term survival associated with an isolated postoperative troponin elevation (HR, 1.5) compared with a postoperative MI (HR, 2.9). While previous investigations have shown that postoperative troponin elevations correlate with worse short-term outcomes,23 this is the first study to determine its association with reduced long-term survival.
An important question that remains unanswered pertains to the nature of the relationship between postoperative troponin elevations and reduced survival: is this a cause-and-effect relationship, with myocardial damage leading to worse survival, or a correlation that identifies a subset of patients at higher risk for decreased survival? If the former is true, then efforts should focus on preoperative cardiac risk optimization and prevention of myocardial ischemia. Methods of preoperative medical optimization are not entirely straightforward. The use of antiplatelet agents and statins in patients undergoing vascular procedures is recommended in consensus statements.24 However, studies on the use of beta blockers in the perioperative period have proved difficult to interpret, with one of the largest trials showing a reduction in the incidence of postoperative MI but also an increase in the incidence of postoperative stroke and death.25 Within our dataset, no class of medications, used perioperatively, demonstrated a significant reduction in postoperative myocardial ischemia. However, use of both statins and antiplatelet agents at the time of discharge showed a survival benefit of 30%. While adherence to evidence-based therapies such as these will likely never reach 100%, some studies have suggested significant room for improvement.26 Within our dataset, utilization of these medications was generally greater than 70%, and increased utilization was seen postoperatively compared with preoperatively. This relative decrease in preoperative cardioprotective medication usage may represent a potential opportunity for system-based quality improvement initiatives.
To date, there are no established guidelines for measuring troponin levels after vascular surgical procedures. While routine serial measurements of cardiac enzymes, for all patients regardless of clinical factors, have certainly been employed in the past, current standard of care does not call for this, nor is it widely practiced. The results of this study do not allow for determination of the value of routine screening of cardiac biomarkers. However, this investigation highlights that postoperative troponin elevation, either alone, or in combination with clinical or ECG evidence of ischemia, may be useful in identifying a high-risk subset of patients that potentially warrants more aggressive postprocedural treatment to avoid adverse long-term outcomes. It remains unclear whether these events cause this worsened survival, or instead, simply identify a subset of patients at higher risk for death in the long term.
DISCUSSION
Dr Julie Ann Freischlag (Baltimore, Md). Could you define how a myocardial infarction was determined, and what you required in order to make that diagnosis?
Dr Jessica P. Simons. The way that the data capture postoperative myocardial ischemia are categorized as none, troponin elevation only, or clinical or EKG evidence of MI.
Myocardial infarction is defined at the discretion of the surgeon, ultimately, when they fill out the data sheets, but it includes either clinical evidence of myocardial infarction, such as chest pain, or EKG changes consistent with an MI.
Dr Jon Matsumura (Madison, Wisc). What are the criteria to screen a patient with a troponin? Were you only testing patients who had symptoms, or was it all the patients in the database had a routine troponin? With a prophylactic operation for aneurysm repair or asymptomatic carotid, predicting the long-term survival is helpful for the patient decision making.
Dr Simons. I think that’s a really excellent point, and that it would be really helpful to know what the indication was. We don’t have that data available to us, why a patient would have had troponins checked postoperatively. It’s not a routine part of the management of all patients in the VSGNE, but rather, it’s at the discretion of the surgeon.
Dr Michael Conte (San Francisco, Calif). I think that your slide where you contrasted the implications about causality versus association is really the most important point, because I think, again, this information may be misinterpreted in light of the types of conversations that surround it; for example, the CREST end point.
And, unfortunately, your conclusion slide actually erred back in the wrong direction by suggesting causality, the way I read it, when you said that these postoperative events were themselves directly related to later mortality. I think if you took 1000 cardiovascular patients in the clinic and gave them all a stress test, it would be no shock to find out that the ones that failed the stress test would not have quite as good long-term survival as those that passed the stress test. But, the stress test doesn’t cause the death. That is entirely analogous to the issue with the majority of postoperative MIs.
I think that this becomes a critical issue with interpretation of the findings, while I concur with the importance of working to prevent these events. My major question to you is: did you look at whether or not all of the patients were treated according to guidelines with cardioprotective medications? Because I think that really is the bottom line for perioperative management. It’s not the procedure that’s causing the late mortality, it’s really whether or not they are getting appropriate medical care before, during, and after.
Dr Simons. We did take a look to some extent at that, although I think that that question is being addressed a little bit more thoroughly with another VSGNE study. But, in brief, we did find that the use of statin and antiplatelet agents at the time of discharge was protective against death in our Cox proportional hazard model, suggesting exactly as you’re saying, that if patients are treated medically more appropriately, then perhaps that would be what impacts survival over the long term.
Dr John Chang (Roslyn, NY). What would be your suggestions in terms of optimization preoperatively? Do you think that everybody who has a positive stress test should go through cardiac catheterization or revascularization?
Dr Simons. Well, I think we probably need to lean on our cardiology colleagues a little bit more in order to figure out exactly what the right things would be to better preoptimize patients preoperatively and medically manage them postoperatively as well. Certainly the data suggest a benefit to medications such as statins and antiplatelet agents for improving survival. But, beyond that, who needs a stress test and who needs preoperative cardiac catheterization is a question I would work with our cardiology colleagues to better answer.
Dr Matthew Eagleton (Cleveland, Ohio). When you’re looking at cause of death at 5 years, do you have any categorization as to cause of death, such as cardiovascular death versus cancer death?
Dr Simons. We don’t have that data available to us in this data set.
Dr Cheong Lee (Milwaukee, Wisc). Elevation in troponin can be also related to impairment in renal function, so do you think this is actually a reflection of renal impairment that you’re seeing the elevations in troponin being related with mortality?
Dr Simons. I think it’s a little hard to know for sure. I know that looking through our data, the percentage of patients who have significant renal disease–which is defined by a creatinine greater than 1.8–is very small, so I suspect that that influence would be very minor. But, that is a reasonable point.
The other confounding issue is whether troponins were checked disproportionally among those patients with renal failure, which would bias the data. So, I can’t really answer that question specifically.
Dr Amy Reed (Hershey, Pa). I’m assuming this is from the VSGNE database. And, I know on a VQI, we do put down whether there has been any preoperative evaluation. Was that not available in this data set that you aren’t aware of that?
Dr Simons. No, we did have access to that variable. And, we did include in some of our analyses whether or not a preoperative stress test was performed. And, it was not performed for the majority of patients. I think it was positive only in an extremely small percentage, as you would expect to find. Regardless, it ultimately didn’t end up being significant on univariate screening, and that’s why it didn’t make it into the final analysis.
Footnotes
Author conflict of interest: none.
Presented during plenary session at the 2013 Vascular Annual Meeting of the Society for Vascular Surgery, San Francisco, Calif, May 30-June 1, 2013.
AUTHOR CONTRIBUTIONS
Conception and design: JS, AS
Analysis and interpretation: JS, DB, PG, LM, JC, AS
Data collection: AS
Writing the article: JS, AS
Critical revision of the article: JS, DB, PG, DB, WR, JC, LM, AS
Final approval of the article: JS, DB, PG, DB, WR, JC, LM, AS
Statistical analysis: JS, AS
Obtained funding: AS
Overall responsibility: AS
REFERENCES
- 1.Massie MT, Rohrer MJ, Leppo JA, Cutler BS. Is coronary angiography necessary for vascular surgery patients who have positive results of dipyridamole thallium scans? J Vasc Surg. 1997;25:975–982. doi: 10.1016/s0741-5214(97)70120-4. discussion: 982–3. [DOI] [PubMed] [Google Scholar]
- 2.Johnston KW. Nonruptured abdominal aortic aneurysm: six-year follow-up results from the multicenter prospective Canadian aneurysm study. Canadian Society for Vascular Surgery Aneurysm Study Group. J Vasc Surg. 1994;20:163–170. doi: 10.1016/0741-5214(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 3.Hertzer NR, Mascha EJ, Karafa MT, O’Hara PJ, Krajewski LP, Beven EG. Open infrarenal abdominal aortic aneurysm repair: the Cleveland Clinic experience from 1989 to 1998. J Vasc Surg. 2002;35:1145–1154. doi: 10.1067/mva.2002.123686. [DOI] [PubMed] [Google Scholar]
- 4.McFalls EO, Ward HB, Moritz TE, Apple FS, Goldman S, Pierpont G, et al. Predictors and outcomes of a perioperative myocardial infarction following elective vascular surgery in patients with documented coronary artery disease: results of the CARP trial. Eur Heart J. 2008;29:394–401. doi: 10.1093/eurheartj/ehm620. [DOI] [PubMed] [Google Scholar]
- 5.Hertzer NR, Beven EG, Young JR, O’Hara PJ, Ruschhaupt WF, 3rd, Graor RA, et al. Coronary artery disease in peripheral vascular patients. A classification of 1000 coronary angiograms and results of surgical management. Ann Surg. 1984;199:223–233. doi: 10.1097/00000658-198402000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mangano DT, Browner WS, Hollenberg M, Li J, Tateo IM. Long-term cardiac prognosis following noncardiac surgery. The Study of Perioperative Ischemia Research Group. JAMA. 1992;268:233–239. doi: 10.1001/jama.268.2.233. [DOI] [PubMed] [Google Scholar]
- 7.Kim LJ, Martinez EA, Faraday N, Dorman T, Fleisher LA, Perler BA, et al. Cardiac troponin I predicts short-term mortality in vascular surgery patients. Circulation. 2002;106:2366–2371. doi: 10.1161/01.cir.0000036016.52396.bb. [DOI] [PubMed] [Google Scholar]
- 8.Landesberg G, Shatz V, Akopnik I, Wolf YG, Mayer M, Berlatzky Y, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol. 2003;42:1547–1554. doi: 10.1016/j.jacc.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Raby KE, Barry J, Creager MA, Cook EF, Weisberg MC, Goldman L. Detection and significance of intraoperative and postoperative myocardial ischemia in peripheral vascular surgery. JAMA. 1992;268:222–227. [PubMed] [Google Scholar]
- 10.Simons JP, Goodney PP, Baril DT, Nolan BW, Hevelone ND, Cronenwett JL, et al. The effect of postoperative stroke and myocardial infarction on long-term survival after carotid revascularization. J Vasc Surg. 2013;57:1581–1588. doi: 10.1016/j.jvs.2012.11.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antman EM, Tanasijevic MJ, Thompson B, Schactman M, McCabe CH, Cannon CP, et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med. 1996;335:1342–1349. doi: 10.1056/NEJM199610313351802. [DOI] [PubMed] [Google Scholar]
- 12.Galvani M, Ottani F, Ferrini D, Ladenson JH, Destro A, Baccos D, et al. Prognostic influence of elevated values of cardiac troponin I in patients with unstable angina. Circulation. 1997;95:2053–2059. doi: 10.1161/01.cir.95.8.2053. [DOI] [PubMed] [Google Scholar]
- 13.Landesberg G, Vesselov Y, Einav S, Goodman S, Sprung CL, Weissman C. Myocardial ischemia, cardiac troponin, and long-term survival of high-cardiac risk critically ill intensive care unit patients. Crit Care Med. 2005;33:1281–1287. doi: 10.1097/01.ccm.0000166607.22550.87. [DOI] [PubMed] [Google Scholar]
- 14.Levy M, Heels-Ansdell D, Hiralal R, Bhandari M, Guyatt G, Yusuf S, et al. Prognostic value of troponin and creatine kinase muscle and brain isoenzyme measurement after noncardiac surgery: a systematic review and meta-analysis. Anesthesiology. 2011;114:796–806. doi: 10.1097/ALN.0b013e31820ad503. [DOI] [PubMed] [Google Scholar]
- 15.Marston N, Brenes J, Garcia S, Kuskowski M, Adabag S, Santilli S, et al. Peak postoperative troponin levels outperform preoperative cardiac risk indices as predictors of long-term mortality after vascular surgery Troponins and postoperative outcomes. J Crit Care. 2012;27:66–72. doi: 10.1016/j.jcrc.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 16.McFalls EO, Ward HB, Santilli S, Scheftel M, Chesler E, Doliszny KM. The influence of perioperative myocardial infarction on long-term prognosis following elective vascular surgery. Chest. 1998;113:681–686. doi: 10.1378/chest.113.3.681. [DOI] [PubMed] [Google Scholar]
- 17.Hannibal GB. Interpretation of serum troponin elevation. AACN Adv Crit Care. 2013;24:224–228. doi: 10.1097/NCI.0b013e31828b41f3. [DOI] [PubMed] [Google Scholar]
- 18.Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 19.Cronenwett JL, Likosky DS, Russell MT, Eldrup-Jorgensen J, Stanley AC, Nolan BW. A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VSGNNE) J Vasc Surg. 2007;46:1093–1101. doi: 10.1016/j.jvs.2007.08.012. discussion: 1101–2. [DOI] [PubMed] [Google Scholar]
- 20.Le Manach Y, Perel A, Coriat P, Godet G, Bertrand M, Riou B. Early and delayed myocardial infarction after abdominal aortic surgery. Anesthesiology. 2005;102:885–891. doi: 10.1097/00000542-200505000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Back MR, Leo F, Cuthbertson D, Johnson BL, Shamesmd ML, Bandyk DF. Long-term survival after vascular surgery: specific influence of cardiac factors and implications for preoperative evaluation. J Vasc Surg. 2004;40:752–760. doi: 10.1016/j.jvs.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 22.Redfern G, Rodseth RN, Biccard BM. Outcomes in vascular surgical patients with isolated postoperative troponin leak: a meta-analysis. Anaesthesia. 2011;66:604–610. doi: 10.1111/j.1365-2044.2011.06763.x. [DOI] [PubMed] [Google Scholar]
- 23.Devereaux PJ, Chan MT, Alonso-Coello P, Walsh M, Berwanger O, Villar JC, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307:2295–2304. doi: 10.1001/jama.2012.5502. [DOI] [PubMed] [Google Scholar]
- 24.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof EL, Fleischmann KE, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:e169–e276. doi: 10.1161/CIRCULATIONAHA.109.192690. [DOI] [PubMed] [Google Scholar]
- 25.Devereaux PJ, Yang H, Yusuf S, Guyatt G, Leslie K, Villar JC, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371:1839–1847. doi: 10.1016/S0140-6736(08)60601-7. [DOI] [PubMed] [Google Scholar]
- 26.Hoeks SE, Scholte op Reimer WJ, Lenzen MJ, van Urk H, Jorning PJ, Boersma E, et al. Guidelines for cardiac management in noncardiac surgery are poorly implemented in clinical practice: results from a peripheral vascular survey in the Netherlands. Anesthesiology. 2007;107:537–544. doi: 10.1097/01.anes.0000281892.15637.fb. [DOI] [PubMed] [Google Scholar]