Abstract
Serglycin belongs to a family of small proteoglycans with Ser-Gly dipeptide repeats, and it is modified with different types of glycosaminoglycan side chains. Intracellular serglycin affects the retention and secretion of proteases, chemokines, or other cytokines by physically binding to these factors in secretory granules. Extracellular serglycin has been found to be released by several types of human cancer cells, and it is able to promote the metastasis of nasopharyngeal carcinoma cells. Serglycin can bind to CD44, which is another glycoprotein located in cellular membrane. Serglycin's function of promoting cancer cell metastasis depends on glycosylation of its core protein, which can be achieved by autocrine as well as paracrine secretion mechanisms. Further investigations are warranted to elucidate serglycin signaling mechanisms with the goal of targeting them to prevent cancer cell metastasis.
Keywords: Serglycin, cancer, metastasis, proteoglycan
Serglycin is a proteoglycan that has its core peptide coded by the gene SRGN in humans. It belongs to a family of small proteoglycans with serine-glycine dipeptide repeats and is modified with various glycosaminoglycan side chains. Serglycin is also known as a secretory granule proteoglycan core protein or hematopoietic proteoglycan core protein[1]. Rat Srgn gene was originally cloned and sequenced from the rat yolk sac carcinoma cell line L2 in 1985; human serglycin was isolated from platelets in 1986[2],[3]. The amino acid sequence of human serglycin is closely homologous to those of the mouse and rat[4].
Human serglycin consists of a core protein decorated with glycosaminoglycan chains, for example, chondroitin-4-sulfate (CS-4), CS-6, CS-E, CS-B, or heparin[5]. The core protein of human serglycin has 158 amino acid residues that form three functional domains. A signal peptide domain, encoded by exon 1, is composed of amino acid residues 1–27; the N-terminal domain, encoded by exon 2, is composed of amino acid residues 28–76; and the glycosylation domain, encoded by exon 3, is composed of amino acid residues 77–158[6], as shown in Figure 1.
Figure 1. The protein structure of serglycin.
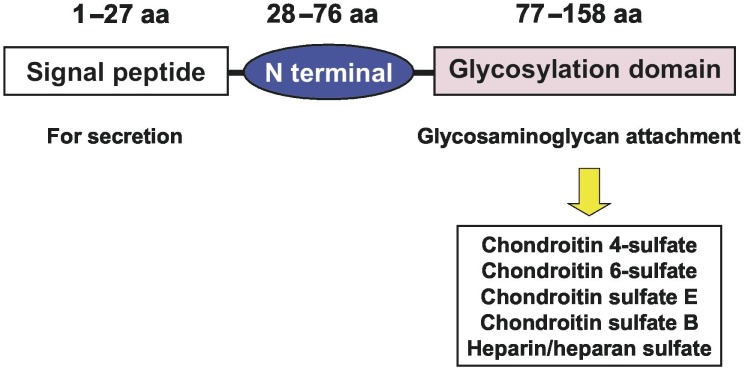
Previous studies have shown that there are two forms of serglycin in human cells, intracellular and extracellular[7]. Intracellular serglycin is a key mediator of granulopoiesis in mast cells, cytolytic T lymphocytes (CTLs), and neutrophils[8]–[11], and it is involved in the retention or secretion of proteases, histamine, cytokines, and chemokines in the storage granules of mast cells or canine kidney cells[12]–[15]. Extracellular serglycin released from the storage granules of mast cells and CTLs (or from monocytes, macrophages, and endothelial cells that constitutively secrete serglycin) has been shown to interact with CD44 in hematopoietic cells, suggesting that serglycin may take on an important role in cell-cell interactions[16]–[18]. Secretion of serglycin is involved in the release of tissue-type plasminogen activator from endothelial cells, tumor necrosis factor-α from macrophages, and matrix metalloproteinase-9 (MMP-9) from monocytes[19]–[21]. Although serglycin-knockout mice have been generated by Abrink et al.[22] the biological functions of serglycin have not been studied in detail.
In this article, we focus on the biological roles of serglycin in tumor metastasis as well as some related physiological characteristics of this interesting molecule.
Expression of Serglycin in Hematopoietic Cells
Both serglycin mRNA and protein have been detected in human hematopoietic cancer cell lines. Niemann et al.[23] found that serglycin is distinctively expressed in acute myeloid leukemia (AML) relative to lymphoblastic leukemia. Serglycin is also a selective marker distinguishing AML from Philadelphia chromosome-negative chronic myeloproliferative disorders[23]. In multiple myeloma cells, serglycin is a secreted protein decorated predominantly with CS-4, suggesting that serglycin has an effect on inhibition of bone mineralization[24]. Moreover, serglycin demonstrates a role in protecting myeloma cells from complement attacks induced by antibody immunotherapy, therefore promoting the survival of malignant myeloma cells[25]. Up-regulation of serglycin expression is found in drug-resistant tumor cell lines of hematopoietic origin, indicating that serglycin may be involved in the drug resistance of human cancer cells[26]. In a variety of hematopoietic cells, including myelomonocytes, macrophages, and lymphoma, myeloma, mastochytoma, or thymoma cells, serglycin has been shown to interact with cell surface protein CD44 if serglycin has attached CS-4 or CS-6 moieties, but not heparin or heparan sulfate[16].
Serglycin Promotes Metastasis of Nasopharyngeal Carcinoma Cells
Serglycin is highly expressed in high-metastasis nasopharyngeal carcinoma (NPC) cells[27]. In primary NPC tissues, a higher level of serglycin serves as an independent prognostic indicator for disease-free survival and distant metastasis-free survival of patients. In vitro and in vivo studies have proven that serglycin can promote motility, invasion, and metastasis of NPC cells via induction of a mesenchymal molecule vimentin [27]. This important function of serglycin depends on full glycosylation of the core protein[27]. In NPC cells, intracellular serglycin has a molecular weight of about 130 kDa, but the secreted form is about 300 kDa. Overexpression of serglycin by NPC cells dramatically increases only the amount of secreted serglycin but does not alter the intracellular serglycin level, suggesting that its metastasis-promoting function is mainly determined by the secreted form[27].
Serglycin Secretory Mechanism in Human Cells
Sequence analyses of the serglycin core protein revealed that amino acid residues 1–27 form the signal peptide[5]. In some hematopoietic cells, including monocytes or macrophages, myeloma cells, and human endothelial cells, serglycin is constitutively secreted [19],[20],[24],[25],[28],[29]. In other hematopoietic cells, including neutrophils and mast cells, serglycin is stored in secretory granules and secreted after cell activation[9],[13]. Cytokines, chemokines, and proteases interact with serglycin during secretion from secretory granules and can still be in complex with serglycin after its release from cells[12],[30]–[34]. In the extracellular matrix (ECM), serglycin is involved in the activation of MMPs, which have important roles in inflammation, wound repair, cellular invasion, and other fundamental processes[21],[35],[36]. Secretion of tumor necrosis factor from macrophages has been reported to be regulated by serglycin[20]. In addition, serglycin from CTLs or natural killer (NK) cells can form macro-complexes with granzyme B and perforin to induce the apoptosis of target cells[37]–[40]. The apoptosis-promoting effect of serglycin was found in mast cells, and this effect was associated with the release of serglycin and serglycin-dependent proteases into the cytosol[41]. Studies of serglycin released from human cancer cells should provide insight into new signaling pathways through which serglycin is involved in tumor progression.
Diversity of Glycan Chains on Serglycin
The glycosaminoglycan chains attached to serglycin vary among different cell types. In hematopoietic cells—including platelets, CTLs, NK cells, and mucosal mast cells (other than mast cells in connective tissues or the peritoneal cavity)—chondroitin sulfate is the major glycan, and CS-4 is the dominant form in most of these cell types[42]. Heparin is the glycan attachment to serglycin in peritoneal or connective tissue mast cells[15].
The localization of serglycin is determined by the types of glycan attached to the serglycin protein. In Madin-Darby canine kidney (MDCK) cells, chondroitin sulfate serglycin is generally secreted into the apical medium and heparin serglycin is mainly transported to basolateral membrane of the cells[43]–[45]. In human endothelial cells, serglycin is a major proteoglycan that carries mainly chondroitin sulfate chains and some heparin chains. A major amount of serglycin is secreted into apical medium and a small amount co-localizes with growth-related Oncogene α (GROα /CXCL1) in vesicles[46]. In acinar pancreatic cells, serglycin without attached glycans is not able to be sorted into secretory granules[47]. Glycan modification of serglycin is a key factor determining its localization and biological roles in mammalian cells. Although serglycin's function in promoting metastasis depends on full glycosylation[27], glycan modification of serglycin is poorly understood in human cancer cells.
Serglycin Signaling for Clinical Applications
As a secreted glycoprotein, serglycin could be an ideal serum marker for predicting and monitoring cancer progression. Further, serglycin's role in promoting metastasis depends on interaction between the secreted protein and cancer cells, making that interaction an ideal drug target. Serglycin promotes NPC cell metastasis via autocrine and paracrine signaling[27], but how secreted serglycin triggers the cascade leading to cellular motility remains unknown. It is therefore crucial to identify the membrane-binding protein(s) of serglycin that help promote migration, invasion, and metastasis.
Serglycin binds to CD44 on hematopoietic cells[16]. CD44 itself is a glycosylated protein found at cell surface, and it is involved in cell-cell interactions, cell adhesion, and cellular migration[48],[49]. CD44 is a cancer stem cell marker for a variety of cancer types, including carcinomas of the pancreas, colon, ovary, breast, and liver[50]–[54]. Some aggressive behaviors of cancer cells, e.g., apoptosis resistance, epithelial-mesenchymal transition, and metastasis, have been linked to CD44[49]. It is therefore important to clarify whether serglycin signaling depends on binding to CD44 at the cell surface.
Summary
Serglycin is widely expressed in various hematopoietic cells and highly metastatic NPC cells, and it is an important molecule in regulating NPC metastasis. Although great potential for clinical applications can be predicted, the exact molecular mechanism of serglycin signaling remains unclear. Further explorations in identifying serglycin-binding proteins and its signaling cascade in promoting cellular motility are needed.
Acknowledgments
This work was supported by a grant from the State Key Program of National Natural Science Foundation of China (No. 81030043). We thank David Nadziejka, Grand Rapids, Michigan, for critical reading of the manuscript.
References
- 1.Schick BP. Regulation of expression of megakaryocyte and platelet proteoglycans [J] Stem Cells. 1996;14(Suppl. 1):220–231. doi: 10.1002/stem.5530140729. [DOI] [PubMed] [Google Scholar]
- 2.Bourdon MA, Oldberg A, Pierschbacher M, et al. Molecular cloning and sequence analysis of a chondroitin sulfate proteoglycan cDNA [J] Proc Natl Acad Sci USA. 1985;82(5):1321–1325. doi: 10.1073/pnas.82.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okayama M, Oguri K, Fujiwara Y, et al. Purification and characterization of human platelet proteoglycan [J] Biochem J. 1986;233(1):73–81. doi: 10.1042/bj2330073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avraham S, Stevens RL, Nicodemus CF, et al. Molecular cloning of a cDNA that encodes the peptide core of a mouse mast cell secretory granule proteoglycan and comparison with the analogous rat and human cdna [J] Proc Natl Acad Sci U S A. 1989;86(10):3763–3767. doi: 10.1073/pnas.86.10.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolset SO, Tveit H. Serglycin—structure and biology [J] Cell Mol Life Sci. 2008;65(7–8):1073–1085. doi: 10.1007/s00018-007-7455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicodemus CF, Avraham S, Austen KF, et al. Characterization of the human gene that encodes the peptide core of secretory granule proteoglycans in promyelocytic leukemia HL-60 cells and analysis of the translated product [J] J Biol Chem. 1990;265(10):5889–5896. [PubMed] [Google Scholar]
- 7.Kolset SO, Prydz K, Pejler G. Intracellular proteoglycans [J] Biochem J. 2004;379(Pt 2):217–227. doi: 10.1042/BJ20031230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niemann CU, Cowland JB, Klausen P, et al. Localization of serglycin in human neutrophil granulocytes and their precursors [J] J Leukoc Biol. 2004;76(2):406–415. doi: 10.1189/jlb.1003502. [DOI] [PubMed] [Google Scholar]
- 9.Niemann CU, Abrink M, Pejler G, et al. Neutrophil elastase depends on serglycin proteoglycan for localization in granules [J] Blood. 2007;109(10):4478–4486. doi: 10.1182/blood-2006-02-001719. [DOI] [PubMed] [Google Scholar]
- 10.Whitaker-Menezes D, Schechter NM, Murphy GF. Serine proteinases are regionally segregated within mast cell granules [J] Lab Invest. 1995;72(1):34–41. [PubMed] [Google Scholar]
- 11.Grujic M, Braga T, Lukinius A, et al. Serglycin-deficient cytotoxic T lymphocytes display defective secretory granule maturation and granzyme B storage [J] J Biol Chem. 2005;280(39):33411–33418. doi: 10.1074/jbc.M501708200. [DOI] [PubMed] [Google Scholar]
- 12.Henningsson F, Hergeth S, Cortelius R, et al. A role for serglycin proteoglycan in granular retention and processing of mast cell secretory granule components [J] Febs J. 2006;273(21):4901–4912. doi: 10.1111/j.1742-4658.2006.05489.x. [DOI] [PubMed] [Google Scholar]
- 13.Braga T, Grujic M, Lukinius A, et al. Serglycin proteoglycan is required for secretory granule integrity in mucosal mast cells [J] Biochem J. 2007;403(1):49–57. doi: 10.1042/BJ20061257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zernichow L, Dalen KT, Prydz K, et al. Secretion of proteases in serglycin transfected Madin-Darby canine kidney cells [J] Febs J. 2006;273(3):536–547. doi: 10.1111/j.1742-4658.2005.05085.x. [DOI] [PubMed] [Google Scholar]
- 15.Humphries DE, Wong GW, Friend DS, et al. Heparin is essential for the storage of specific granule proteases in mast cells [J] Nature. 1999;400(6746):769–772. doi: 10.1038/23481. [DOI] [PubMed] [Google Scholar]
- 16.Toyama-Sorimachi N, Kitamura F, Habuchi H, et al. Widespread expression of chondroitin sulfate-type serglycins with CD44 binding ability in hematopoietic cells [J] J Biol Chem. 1997;272(42):26714–26719. doi: 10.1074/jbc.272.42.26714. [DOI] [PubMed] [Google Scholar]
- 17.Toyama-Sorimachi N, Miyasaka M. A sulfated proteoglycan as a novel ligand for CD44 [J] J Dermatol. 1994;21(11):795–801. doi: 10.1111/j.1346-8138.1994.tb03293.x. [DOI] [PubMed] [Google Scholar]
- 18.Toyama-Sorimachi N, Sorimachi H, Tobita Y, et al. A novel ligand for CD44 is serglycin, a hematopoietic cell lineage-specific proteoglycan. Possible involvement in lymphoid cell adherence and activation [J] J Biol Chem. 1995;270(13):7437–7444. doi: 10.1074/jbc.270.13.7437. [DOI] [PubMed] [Google Scholar]
- 19.Schick BP, Gradowski JF, San Antonio JD. Synthesis, secretion, and Subcellular localization of serglycin proteoglycan in human endothelial cells [J] Blood. 2001;97(2):449–458. doi: 10.1182/blood.v97.2.449. [DOI] [PubMed] [Google Scholar]
- 20.Zernichow L, Abrink M, Hallgren J, et al. Serglycin is the major secreted proteoglycan in macrophages and has a role in the regulation of macrophage tumor necrosis factor-alpha secretion in response to lipopolysaccharide [J] J Biol Chem. 2006;281(37):26792–26801. doi: 10.1074/jbc.M512889200. [DOI] [PubMed] [Google Scholar]
- 21.Winberg JO, Kolset SO, Berg E, et al. Macrophages secrete matrix metalloproteinase 9 covalently linked to the core protein of chondroitin sulphate proteoglycans [J] J Mol Biol. 2000;304(4):669–680. doi: 10.1006/jmbi.2000.4235. [DOI] [PubMed] [Google Scholar]
- 22.Abrink M, Grujic M, Pejler G. Serglycin is essential for maturation of mast cell secretory granule [J] J Biol Chem. 2004;279(39):40897–40905. doi: 10.1074/jbc.M405856200. [DOI] [PubMed] [Google Scholar]
- 23.Niemann CU, Kjeldsen L, Ralfkiaer E, et al. Serglycin proteoglycan in hematologic malignancies: a marker of acute myeloid leukemia [J] Leukemia. 2007;21(12):2406–2410. doi: 10.1038/sj.leu.2404975. [DOI] [PubMed] [Google Scholar]
- 24.Theocharis AD, Seidel C, Borset M, et al. Serglycin constitutively secreted by myeloma plasma cells is a potent inhibitor of bone mineralization in vitro [J] J Biol Chem. 2006;281(46):35116–35128. doi: 10.1074/jbc.M601061200. [DOI] [PubMed] [Google Scholar]
- 25.Skliris A, Happonen KE, Terpos E, et al. Serglycin inhibits the classical and lectin pathways of complement via its glycosaminoglycan chains: Implications for multiple myeloma [J] Eur J Immunol. 2011;41(2):437–449. doi: 10.1002/eji.201040429. [DOI] [PubMed] [Google Scholar]
- 26.Beyer-Sehlmeyer G, Hiddemann W, Wormann B, et al. Suppressive subtractive hybridisation reveals differential expression of serglycin, sorcin, bone marrow proteoglycan and prostate-tumour-inducing gene I (PTI-1) in drug-resistant and sensitive tumour cell lines of haematopoetic origin [J] Eur J Cancer. 1999;35(12):1735–1742. doi: 10.1016/s0959-8049(99)00202-6. [DOI] [PubMed] [Google Scholar]
- 27.Li XJ, Ong CK, Cao Y, et al. Serglycin is a theranostic target in nasopharyngeal carcinoma that promotes metastasis [J] Cancer Res. 2011;71(8):3162–3172. doi: 10.1158/0008-5472.CAN-10-3557. [DOI] [PubMed] [Google Scholar]
- 28.Kolset SO, Zernichow L. Serglycin and secretion in human monocytes [J] Glycoconj J. 2008;25(4):305–311. doi: 10.1007/s10719-007-9073-9. [DOI] [PubMed] [Google Scholar]
- 29.Uhlin-Hansen L, Wik T, Kjellen L, et al. Proteoglycan metabolism in normal and inflammatory human macrophages [J] Blood. 1993;82(9):2880–2889. [PubMed] [Google Scholar]
- 30.Ringvall M, Ronnberg E, Wernersson S, et al. Serotonin and histamine storage in mast cell secretory granules is dependent on serglycin proteoglycan [J] J Allergy Clin Immunol. 2008;121(4):1020–1026. doi: 10.1016/j.jaci.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 31.Kolset SO, Mann DM, Uhlin-Hansen L, et al. Serglycin-binding proteins in activated macrophages and platelets [J] J Leukoc Biol. 1996;59(4):545–554. doi: 10.1002/jlb.59.4.545. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto R, Sali A, Ghildyal N, et al. Packaging of proteases and proteoglycans in the granules of mast cells and other hematopoietic cells. A cluster of histidines on mouse mast cell protease 7 regulates its binding to heparin serglycin proteoglycans [J] J Biol Chem. 1995;270(33):19524–19531. doi: 10.1074/jbc.270.33.19524. [DOI] [PubMed] [Google Scholar]
- 33.Serafin WE, Katz HR, Austen KF, et al. Complexes of heparin proteoglycans, chondroitin sulfate E proteoglycans, and [3H] diisopropyl fluorophosphate-binding proteins are exocytosed from activated mouse bone marrow-derived mast cells [J] J Biol Chem. 1986;261(32):15017–15021. [PubMed] [Google Scholar]
- 34.Pejler G, Abrink M, Wernersson S. Serglycin proteoglycan: regulating the storage and activities of hematopoietic proteases [J] Biofactors. 2009;35(1):61–68. doi: 10.1002/biof.11. [DOI] [PubMed] [Google Scholar]
- 35.Lundequist A, Abrink M, Pejler G. Mast cell-dependent activation of pro matrix metalloprotease 2: a role for serglycin proteoglycan-dependent mast cell proteases [J] J Biol Chem. 2006;387(10–11):1513–1519. doi: 10.1515/BC.2006.189. [DOI] [PubMed] [Google Scholar]
- 36.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior [J] Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metkar SS, Wang B, Aguilar-Santelises M, et al. Cytotoxic cell granule-mediated apoptosis: perforin delivers granzyme B-serglycin complexes into target cells without plasma membrane pore formation [J] Immunity. 2002;16(3):417–428. doi: 10.1016/s1074-7613(02)00286-8. [DOI] [PubMed] [Google Scholar]
- 38.Galvin JP, Spaeny-Dekking LH, Wang B, et al. Apoptosis induced by granzyme B-glycosaminoglycan complexes: implications for granule-mediated apoptosis in vivo [J] J Immunol. 1999;162(9):5345–5350. [PubMed] [Google Scholar]
- 39.Raja SM, Wang B, Dantuluri M, et al. Cytotoxic cell granule-mediated apoptosis. Characterization of the macromolecular complex of granzyme B with serglycin [J] J Biol Chem. 2002;277(51):49523–49530. doi: 10.1074/jbc.M209607200. [DOI] [PubMed] [Google Scholar]
- 40.Veugelers K, Motyka B, Goping IS, et al. Granule-mediated killing by granzyme B and perforin requires a mannose 6-phosphate receptor and is augmented by cell surface heparan sulfate [J] Mol Biol Cell. 2006;17(2):623–633. doi: 10.1091/mbc.E05-07-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melo FR, Waern I, Ronnberg E, et al. A role for serglycin proteoglycan in mast cell apoptosis induced by a secretory granule-mediated pathway [J] J Biol Chem. 2011;286(7):5423–5433. doi: 10.1074/jbc.M110.176461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolset SO, Gallagher JT. Proteoglycans in haemopoietic cells [J] Biochim Biophys Acta. 1990;1032(2–3):191–211. doi: 10.1016/0304-419x(90)90004-k. [DOI] [PubMed] [Google Scholar]
- 43.Kolset SO, Vuong TT, Prydz K. Apical secretion of chondroitin sulphate in polarized madin-darby canine kidney (MDCK) cells [J] J Cell Sci. 1999;112(Pt 11):1797–1801. doi: 10.1242/jcs.112.11.1797. [DOI] [PubMed] [Google Scholar]
- 44.Mertens G, Van der Schueren B, van den Berghe H, et al. Heparan sulfate expression in polarized epithelial cells: the apical sorting of glypican (GPI-anchored proteoglycan) is inversely related to its heparan sulfate content [J] J Cell Biol. 1996;132(3):487–497. doi: 10.1083/jcb.132.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hafte TT, Fagereng GL, Prydz K, et al. Protein core-dependent glycosaminoglycan modification and glycosaminoglycan-dependent polarized sorting in epithelial madin-darby canine kidney cells [J] Glycobiology. 2011;21(4):457–466. doi: 10.1093/glycob/cwq180. [DOI] [PubMed] [Google Scholar]
- 46.Meen AJ, Oynebraten I, Reine TM, et al. Serglycin is a major proteoglycan in polarized human endothelial cells and is implicated in the secretion of the chemokine GROalpha/CXCL1 [J] J Biol Chem. 2011;286(4):2636–2647. doi: 10.1074/jbc.M110.151944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biederbick A, Licht A, Kleene R. Serglycin proteoglycan is sorted into zymogen granules of rat pancreatic acinar cells [J] Eur J Cell Biol. 2003;82(1):19–29. doi: 10.1078/0171-9335-00287. [DOI] [PubMed] [Google Scholar]
- 48.Spring FA, Dalchau R, Daniels GL, et al. The Ina and Inb blood group antigens are located on a glycoprotein of 80,000 MW (the CDw44 glycoprotein) whose expression is influenced by the In(Lu) gene [J] Immunology. 1988;64(1):37–43. [PMC free article] [PubMed] [Google Scholar]
- 49.Zoller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? [J] Nat Rev Cancer. 2011;11(4):254–267. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 50.Li C, Lee CJ, Simeone DM. Identification of human pancreatic cancer stem cells [J] Methods Mol Biol. 2009;568:161–173. doi: 10.1007/978-1-59745-280-9_10. [DOI] [PubMed] [Google Scholar]
- 51.Casagrande F, Cocco E, Bellone S, et al. Eradication of chemotherapy-resistant CD44 + human ovarian cancer stem cells in mice by intraperitoneal administration of clostridium perfringens enterotoxin [J] Cancer. 2011 Jun 20; doi: 10.1002/cncr.26215. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu LL, Fu D, Ma Y, et al. The power and the promise of liver cancer stem cell markers [J] Stem Cells Dev. 2011 Jun 8; doi: 10.1089/scd.2011.0012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53.Ricardo S, Vieira AF, Gerhard R, et al. Breast cancer stem cell markers CD44, CD24 and ALDH1: expression distribution within intrinsic molecular subtype [J] J Clin Pathol. 2011 Jun 16; doi: 10.1136/jcp.2011.090456. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 54.Su YJ, Lai HM, Chang YW, et al. Direct reprogramming of stem cell properties in colon cancer cells by CD44 [J] EMBO J. 2011;30(15):3186–3199. doi: 10.1038/emboj.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]


