Abstract
Neoadjuvant chemotherapy (NACT), which can reduce the size and therefore increase the resectability of tumors, has recently evolved as a treatment for locally advanced cervical cancer. NACT has been reported to decrease the risk of pathologic factors related to prognosis of cervical cancer. To further assess the effects of NACT on surgery and the pathologic characteristics of cervical cancer, we reviewed 110 cases of locally advanced cervical cancer treated with radical hysterectomy with or without NACT at the People's Hospital of Peking University between January 2006 and December 2010. Of 110 patients, 68 underwent platinum-based NACT prior to surgery (NACT group) and 42 underwent primary surgery treatment (PST group). Our results showed 48 of 68 (70.6%) patients achieved a complete response or partial response to NACT. Estimated blood loss, operation time, and number of removed lymph nodes during surgery, as well as complication rates during and after surgery were not significantly different between the NACT group and the PST group. The rates of deep stromal invasion, positive parametria, positive surgical vaginal margins, and lymph node metastasis were not significantly different between the two groups. However, the rate of lymph-vascular space involvement (LVSI) was significantly lower in the NACT group than in the PST group (P = 0.021). In addition, the response rate of NACT was significantly higher in the patients with chemotherapeutic drugs administrated via artery than via vein. Our results suggest that NACT is a safe and effective treatment for locally advanced cervical cancer and significantly decreases the rate of LVSI.
Keywords: Neoadjuvant chemotherapy, cervical cancer, radical hysterectomy, lymph-vascular space involvement
Worldwide, cervical cancer is the seventh most common malignancy and the second most common female cancer. According to World Health Statistics 2008, there are 53 million new cases of cervical cancer and 27.5 million cervical cancer-related deaths annually[1]. After radical hysterectomy, the 5-year survival rate of patients with early stage (FIGO stage Ia–Ib1) cervical cancer may be as high as 80%–90%[2],[3]. However, radical surgery is limited to patients with FIGO stage IIa or earlier disease. Patients with advanced (FIGO stage Ib2 and above) disease usually do not undergo primary surgery due to bulky lesion foci, local hemorrhage, inflammatory necrosis, and parametrial invasion, but do undergo traditional treatment such as radiation therapy. Although radiation therapy can have the same efficacy as surgery, damage to surrounding tissues caused by radiation may lead to loss of ovarian function and sexual capacity, which significantly impacts patients' quality of life.
Friedlander et al.[4] first reported cervical cancer sensitivity to chemotherapy in 1983. A meta-analysis of randomized controlled clinical trials showed that the combination of radiotherapy and chemotherapy can significantly improve the prognosis of cervical cancer patients and lower the relative risk of local and distant relapse compared to radiotherapy alone[5].Since 1999, the United States National Cancer Institute has recommended that a combination of radiotherapy and chemotherapy should be used to treat all patients with locally advanced cervical cancer. Now chemotherapy, especially neoadjuvant chemotherapy (NACT), is widely used in clinical therapy for cervical cancer[6],[7].
NACT, also known as early or pre-chemotherapy, is used to reduce tumor volume prior to surgery or radiotherapy. For patients with advanced cervical cancer, 2 to 3 cycles of NACT can improve the success rate of surgical resection. Previous studies show that NACT can inhibit tumor micrometastases[8] and enhance resection rate by shrinking tumor volume[9]. Recent ASCO phase II clinical trials also indicate that cervical cancer has a high response rate to NACT and that the toxicity of chemotherapy is acceptable[10]. In addition, NACT has also been shown to reduce lymph node metastasis and parametrial invasion in patients with cervical cancer[11]. However, as some investigators have reached opposite conclusions[11],[12], there is still no agreement on whether NACT can significantly improve the prognosis of cervical cancer. NACT regimens, cycles, and drug dosages vary among different studies, which led to difficulties in drawing certain conclusions in previous meta-analyses[11]. Although the optimal treatment regimen has been explored in some studies by comparing different NACT regimens as well as various clinicopathologic response rates[13],[14], no significant difference has been observed among distinct NACT regimens to date. Phase III randomized clinical trials on NACT for cervical cancer (EORTC55994, NCT00193739) are still ongoing, and NACT efficacy for locally advanced cervical cancer still needs further exploration.
Since 2006, 68 patients with FIGO Ib2–IIb cervical cancer have been successfully treated with NACT at the People's Hospital, Peking University. In this study, we analyzed the clinicopathologic response rate of these patients compared to patients who underwent direct surgical treatment to determine the significance of NACT in treatment of locally advanced cervical cancer.
Patients and Methods
Patient data
The clinical data of 110 patients with FIGO Ib2–IIb primary cervical cancer were analyzed retrospectively. All patients, diagnosed with the guidance of colposcopy or biopsy and staged by two senior gynecologic oncologists based on the revised 2009 FIGO staging system, were enrolled and treated at the People's Hospital, Peking University between January 2006 and December 2010. For stage IIa cases diagnosed before the introduction of the 2009 FIGO staging system, patient data were reviewed to determine the sub-stage of stage II, stage IIa1 or stage IIa2. Pathology and degree of differentiation were determined by examining postoperative specimens. The pathologic diagnosis included muscle invasion, parametrium and vaginal cuff involvement, lymph-vascular space involvement (LVSI), and lymph node metastasis. Of 110 patients, 68 underwent NACT before surgery and 42 underwent only primary surgery treatment (PST).
Treatment regimens
In the PST group, patients with no surgical contraindications were treated with surgery. The surgical approach included radical hysterectomy plus pelvic lymph node dissection. Some patients with suspicious pelvic lymph node metastasis underwent para-aortic lymph node dissection or lymph node biopsy, and some patients, based on their ages and individual willingness, underwent bilateral or unilateral salpingo-oophorectomy, as well as bilateral or unilateral ovarian transposition to the abdominal cavity.
In the NACT group, patients with large lesion foci, local bleeding or obvious inflammation, loss of cervical structure, wide vaginal invasion, suspicious parametrial invasion and whose disease was confirmed by biopsy, were given chemotherapy after informed consent if no chemotherapy contraindications were present.
Chemotherapy regimens
Single- or multiple-agent chemotherapy was selected according to the size of lesion foci and the state of suspicious pelvic lymph node involvement based on magnetic resonance imaging (MRI) or computed tomography (CT) scanning. Cisplatin (DDP) was used in most single-agent chemotherapy regimens. The regimens of multiple-agent chemotherapy were determined individually by chemotherapy oncologists according to the functions of heart, liver, and kidney. Most patients in this group were treated with BIP regimen (DDP + bleomycin + ifosfamide), whereas some were treated with PF regimen (DDP + 5-fluorouracil), and a few underwent other platinum-based chemotherapy at other hospitals.
Chemotherapy dosages
Chemotherapy dosages were adjusted according to patients' height, weight, and body surface area.
Chemotherapy administration routes
For patients with large lesion foci and bleeding, DDP was administrated via arterial intervention. More specifically, the superselective descending cervical branch of the uterine artery was percutaneously catheterized via the femoral artery, and each side was given a half dose of drug. For patients with heavy vaginal bleeding, the drug was initially administered via arterial intervention supplemented with bilateral uterine artery embolization by liquid gelatin sponge to control local bleeding, and was later switched to intravenous chemotherapy. For patients whose economic conditions were poor, intravenous chemotherapy was applied.
Evaluation of chemotherapy efficacy and cycle
The chemotherapy efficacy was evaluated 2 weeks after each chemotherapy cycle by gynecological examination. In the NACT group, tumor sizes were measured by gynecological examination and imaging instruments before chemotherapy and by pathologic gross specimen examination after surgery. Therapeutic effect was graded according to WHO criteria: complete remission (CR), tumor completely disappeared; partial remission (PR), more than 50% tumor shrinkage; stable disease (SD), tumor grows or shrinks no more than 25% and no new lesion foci appear; and progressive disease (PD), tumor grows more than 25% or new lesion foci appear. As it is very difficult to directly measure cervical lesion volume, maximum lesion diameter was used in this study. Response was defined to include CR and PR. Moreover, restoration of cervical prototype and improvement of parametrial conditions were also considered as good responses. If response was apparent, surgical treatment was applied, but if not, chemotherapy cycles were increased or chemotherapy regimen was changed.
Statistical methods
Statistical analysis was performed using SPSS16.0 software. The t-test and Chi-square test were used to analyze the differences between two groups. A P value of less than 0.05 was defined as significantly different.
Results
General patient information and histopathologic type, stage, and differentiation
Of 110 cases, 97 were squamous cell carcinoma, 5 were adenosquamous cell carcinoma, 3 were adenocarcinoma, 2 were endometrial carcinoma, 1 was mucinous adenocarcinoma, 1 was serous carcinoma, and 1 was small cell carcinoma. There was no significant difference in patients' age, gravidity, parity, body mass index (BMI), histological type, or cell differentiation between the NACT group and the PST group (Table 1).
Table 1. The general Information and histopathologic characteristics of patients with cervical cancer.
| Characteristic | NACT | PST | P a |
| Age (years) | 45.7 ± 8.1 | 49.1 ± 10.4 | 0.058 |
| Gravidity | 2.9 ± 1.6 | 3.5 ± 1.4 | 0.063 |
| Parity | 1.8 ± 1.0 | 2.1 ± 1.4 | 0.118 |
| Body mass index | 23.4 ± 3.7 | 23.4 ± 4.0 | 0.960 |
| Histologic type | 0.555 | ||
| Squamous | 61 | 36 | |
| Non-squamous | 7 | 6 | |
| Stage | <0.001 | ||
| Ib2 | 12 | 17 | |
| IIa1 | 9 | 17 | |
| IIa2 | 8 | 7 | |
| IIb | 39 | 1 | |
| Differentiationb | 0.098 | ||
| G1 | 6 | 6 | |
| G2 | 38 | 14 | |
| G3 | 23 | 22 |
aThe comparison of age, gravidity, parity, and body mass index between two groups was tested by ANOVA, whereas the comparison of histologic type, stage, and differentiation was tested by the Chi-square test. bOne patient was diagnosed with small cell cancer. Therefore, the statistical analyses were performed with data from 109 patients.
The clinical stage was significantly different between the two groups. Of 40 patients with stage IIb cervical cancer, 39 underwent NACT and 1 refused preoperative chemotherapy but underwent direct surgery.
Comparison of surgical characteristics between the NACT and PST groups
In this study, 108 patients underwent radical hysterectomy and 2 patients, 1 in each group, underwent wide laparotomic excision of the cervical stump. In the NACT group, 52 patients underwent laparotomy and 16 underwent laparoscopic surgery, with 1 switching from laparoscopic surgery to laparotomy due to intraoperative iliac vein injury. In the PST group, 36 patients underwent laparotomy and 6 underwent laparoscopic surgery. The ratio of laparoscopic surgery to laparotomy was not significantly different between the two groups (P = 0.424).
Of 110 patients, 72 underwent double oophorectomy and 38 underwent unilateral or bilateral peritoneal ovarian transposition to evade the field of external beam radiation therapy. Ninety patients underwent pelvic lymph node dissection, and 20 underwent para-aortic lymph node dissection or lymph node biopsy. One patient in the NACT group retained hypogastric nerves, and 1 with cancer in the left kidney in the PST group underwent laparoscopic extraperitoneal resection of the left kidney. Two patients, 1 in each group, were given retroperitoneal transvaginal surgery as required.
Four patients had surgical complications: 1 in the NACT group had intraoperative iliac vein injury and then switched to laparotomy from laparoscopic surgery; 1 in the NACT group had bladder injury and then underwent bladder repair during laparotomy; 1 in the NACT group had suspicious right ureter injury and then underwent right ureter cystoscope lumen tube placement during laparotomy; and 1 in the PST group had rectal injury and then underwent rectal repair surgery during laparotomy. Two patients had postoperative complications: 1 in the NACT group developed vein thrombosis in the low limbs after laparoscopic surgery and recovered after anticoagulant therapy, and 1 in the PST group had urinary retention after laparotomy and recovered a month after catheter insertion and long-term retention. There was no significant difference in the incidences of Intraoperative and postoperative complications, the amount of bleeding, the operation time, or the number of removed lymph nodes between the two groups (Table 2).
Table 2. The effect of NACT on surgery and histopathologic characteristics in patients with cervical cancer.
| Item | NACT (n = 68) | PST (n = 42) | P a | |
| Surgery characteristic | Estimated blood loss (mL) | 1061 ± 728 | 932 ± 537 | 0.324 |
| The number of lymph nodes removed | 29.5 ± 11.6 | 31.6 ± 10.5 | 0.342 | |
| Operation time (min) | 247 ± 68 | 240 ± 89 | 0.634 | |
| Complications | 4 | 2 | 1.000 | |
| Pathologic characteristic | Deep stromal invasion | 40 | 26 | 0.842 |
| Parametrial involvement | 3 | 1 | 1.000 | |
| Vaginal margin involvement | 5 | 3 | 1.000 | |
| LVSI | 21 | 22 | 0.021 | |
| Lymph node metastasis | 21 | 11 | 0.669 |
NACT, neoadjuvant chemotherapy; PST, primary surgery treatment; LVSI, lymph-vascular space involvement. aThe estimated blood loss, number of lymph nodes removed, and operation time were all tested by ANOVA. The Chi-square test was used to test the deep stromal invasion, parametrial involvement, vaginal margin involvement, LVSI, and lymph node metastasis rate.
Pathologic characteristics of the NACT and PST groups
Pathologic data showed that there was no statistical difference in deep myometrial invasion, parametrial involvement, vaginal stump, or lymph node metastasis between the two groups. However, the NACT group had a significantly lower LVSI rate than did the PST group (P = 0.021, Table 2).
NACT regimens
Of 68 patients in the NACT group, 64 underwent NACT in our hospital and 4 in other hospital (s). Among these 4 patients, 1 underwent 1 cycle of chemotherapy but the detailed regimen, dose, and administration route was unknown; 1 underwent 1 cycle of DDP arterial embolization with unknown dose followed by 1 cycle of intravenous BIP chemotherapy in our hospital; 1 underwent TP (paclitaxel and cisplatin) chemotherapy with unknown dose followed by 1 cycle of DDP arterial embolization in our hospital; and 1 underwent 1 cycle of intravenous BIP chemotherapy, though detailed records of chemotherapy were unavailable, and then underwent direct surgery without further chemotherapy in our hospital (Table 3).
Table 3. The effect of various NACT regimens on chemotherapy response rate and pathologic characteristics.
| Item | Chemotherapy response rate |
LVSI |
Lymph node metastasis |
||||
| Response rate | P a | Positive rate | P a | Metastasis rate | P a | ||
| Regimen | 0.534 | 0.534 | 0.354 | ||||
| (n = 67)a | Single agent (DDP) | 10/16 | 6/16 | 3/16 | |||
| Combined chemotherapy | 37/51 | 14/51 | 18/51 | ||||
| BIP | 30/41 | 1.000 | 11/41 | 1.000 | 13/41 | 0.296 | |
| Non-BIP | 7/10 | 3/10 | 5/10 | ||||
| Cycle | 0.429 | 0.416 | 0.282 | ||||
| (n = 68) | 1 | 26/40 | 14/40 | 11/40 | |||
| 2 | 20/25 | 7/25 | 10/25 | ||||
| ≥=3 | 2/3 | 0/3 | 0/3 | ||||
| Intervention | 0.005 | 0.179 | 0.283 | ||||
| (n = 67)a | In vein | 12/25 | 10/25 | 10/25 | |||
| Artery intervention | 35/42 | 10/42 | 11/42 | ||||
| Artery embolism | 15/18 | 0.230 | 3/18 | 0.230 | 3/18 | 0.146 | |
| No embolism | 32/49 | 17/49 | 18/49 | ||||
All variables were analyzed by Chi-square test.
aOne patient who underwent a cycle of NACT in a local hospital was excluded due to unknown regimen and intervention approach.
Of the 67 patients with definite chemotherapy regimens, 51 underwent combination chemotherapy, of which 10 underwent non-BIP regimen, including 6 who underwent PF regimen, 2 who underwent TP regimen (1 in our hospital and 1 in another hospital), 1 who underwent TC regimen (taxol + carboplatin), and 1 who underwent CF regimen (carboplatin + fluorouracil).
Analysis of NACT efficacy
Patients with good economic condition underwent MRI and other imaging examinations before and after chemotherapy (Figure 1). Most cases were evaluated according to changes of tumor sizes before initial chemotherapy and before surgery. Of the 68 patients, 48 (70.6%) responded to chemotherapy, including 4 with CR, 9 with PR, 23 with recovery of cervical morphology, and 12 with significant improvement in parametrial invasion. Twenty patients did not respond to chemotherapy, all of whom showed no significant improvement of parametrial invasion or cervical morphology.
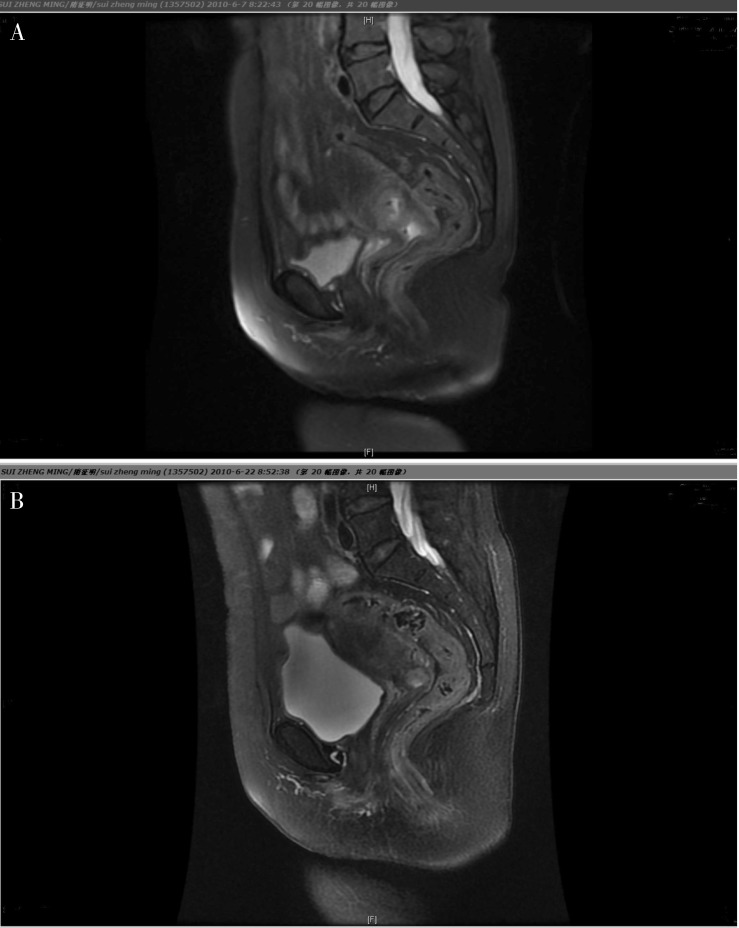
Figure 1. MRI was used to assess the response of cervical cancer to neoadjurant chemotherapy in one patient.
A, before chemotherapy, the size of the tumor is big and the border between the tumor and surrounding tissues is not very clear. B, after chemotherapy, the tumor shrank significantly and the original shape of the cervix was restored.
Effects of NACT regimens on chemotherapy outcomes, pathologic characteristics, and surgical outcomes
The efficacy of NACT was similar between single-and multiple-agent chemotherapy, BIP and non-BIP regimen chemotherapy, or various chemotherapy cycles. The pathologic features of the tumor, including the rates of LVSI and lymph node metastasis, were not significantly related with chemotherapy regimens or cycles. However, among various administration routes, the efficacy of arterial infusion chemotherapy was significantly higher than the efficacy of chemotherapy administered intravenously. Nevertheless, the efficacy was not associated with uterine arterial embolization or tumor pathologic features (Table 3).
The amount of bleeding and the number of removed lymph node in the PST group showed no relationship with chemotherapy regimens, administration routes, or dose. Arterial infusion chemotherapy and arterial embolization in single-agent DDP chemotherapy did significantly shorten operation time but did not significantly affect the number of chemotherapy cycles or the application of BIP (Table 4).
Table 4. The effect of various NACT regimens on surgery.
| Item | Estimated blood loss |
The number of removed lymph nodes |
Operation time |
|||
| Average(mL) | P | The number | P | Average(min) | P | |
| Regime (n = 67)a | 0.683 | 0.791 | 0.025 | |||
| Single agent (DDP) | 1116 ± 834 | 29.9 ± 11.4 | 219 ± 28 | |||
| Combine chemotherapy | 1029 ± 699 | 0.675 | 29.1 ± 11.6 | 0.919 | 255 ± 60 | 0.595 |
| BIP regimen | 1050 ± 740 | 29.0 ± 11.2 | 257 ± 57 | |||
| Non-BIP regimens | 945 ± 521 | 29.4 ± 13.7 | 246 ± 74 | |||
| Cycle (n = 68) | 0.830 | 0.127 | 0.853 | |||
| 1 | 1078 ± 871 | 29.3 ± 12.5 | 249 ± 56 | |||
| 2 | 1010 ± 489 | 28.3 ± 8.6 | 246 ± 58 | |||
| ≥3 | 1267 ± 115 | 42.7 ± 16.9 | 230 ± 66 | |||
| Intervention (n = 67)a | 1028 ± 796 | 0.850 | 29.7 ± 12.7 | 0.823 | 267 ± 62 | 0.024 |
| Vein | 1063 ± 694 | 29.0 ± 10.8 | 235 ± 49 | |||
| Artery | 864 ± 492 | 0.207 | 28.4 ± 12.5 | 0.724 | 219 ± 44 | 0.014 |
| Embolism | 1118 ± 791 | 29.6 ± 11.2 | 257 ± 57 | |||
| No embolism | ||||||
BIP regimen, DDP + bleomycin + ifosfamide. Other footnotes as provided in Table 3.
In addition, the efficacy of chemotherapy and the decrease of pathologic lesion foci were not significantly related with total dose or single dose of cisplatin or the interval between chemotherapy and surgery (Table 5).
Table 5. The associations of the DDP dosage and the interval between last NACT and surgery with the rate of LVSI, lymph node metastasis, and response rate to NACT.
| Variable | Total dosage of DDP (mg)a | P | DDP dosage in one cycle (mg/m2)a | P | The interval between the last NACT and surgery (days)b | P |
| Response | 0.836 | 0.959 | 0.559 | |||
| Yes | 151.9 (n = 46) | 62.6 (n = 18) | 23.9 (n = 18) | |||
| No | 147.5 (n = 18) | 62.7 (n = 46) | 27.1 (n = 48) | |||
| LVSI | 0.359 | 0.587 | 0.271 | |||
| Positive | 137.4 (n = 19) | 63.8 (n = 19) | 22.1 (n = 19) | |||
| Negative | 156.2 (n = 45) | 62.2 (n = 45) | 27.9 (n = 47) | |||
| Lymph node metastasis | 0.933 | 0.737 | 0.320 | |||
| Positive | 151.8 (n = 19) | 62.0 (n = 19) | 22.8 (n = 21) | |||
| Negative | 150.1 (n = 45) | 63.0 (n = 45) | 27.8 (n = 45) |
The differences between the two groups of each variable were analyzed by the ANOVA test.
aOf 68 patients, 67 underwent cisplatinum-based NACT and 1 underwent combination chemotherapy regimens including carboplatin. Among the 67 patients, 3 underwent NACT with unknown dosage of cisplatinum in other hospitals. Therefore, the analysis was carried out with data from 64 patients. bAmong the 68 patients treated with NACT, 4 were treated in other hospitals, including 2 patients with unclear periods or intervals of chemotherapy. Therefore, the analysis was carried out only with data from 66 patients in the NACT group.
Discussion
NACT was originally used to treat solid cancers, such as lung cancer, breast cancer, and head and neck tumors. Since the 1980s, oncologists have tried to use NACT for cervical cancer before radiotherapy to shrink tumor foci and increase radiosensitivity by reducing proportion of hypoxic cells in cancer tissue. Preoperative NACT for cervical cancer was first reported in 1988 by Benedetti et al.[15], who used combination chemotherapy (cisplatin + bleomycin + methotrexate) to treat locally advanced cervical cancer. In that study, patients achieved a response rate of 75.7%, and all patients treated with chemotherapy underwent radical hysterectomy. Since then, preoperative NACT has gradually become a common treatment of cervical cancer. NACT was even tried on patients with advanced (stage III) cervical cancer to increase the potential for successful surgical resection, and achieved certain success[16]. Moreover, in recent years quite a few studies showed that patients with early cervical cancer retained fertility after preoperative NACT[17]. Nevertheless, large scale studies by multiple institutes are absent, and the efficacy of NACT remains unclear. To date, most oncologists recommend NACT only for locally advanced cervical cancer.
Although medical imaging (MRI and CT) can provide objective evidence to determine tumor size, it has limitations in determining parametrial invasion and vaginal involvement. Due to the limited affordability of some patients, NACT efficacy was evaluated mainly by traditional pelvic examination in this study. We found that the NACT response rate in cervical cancer patients was 70.6%, which was consistent with previously reported rates (70%–88%)[18]–[22]. In 1996, Eddy et al.[23] summarized 18 phase II clinical trials on NACT for cervical cancer and found that the NACT efficacy was related with tumor stage, but a meta-analysis up to March 2009[11] did not reach a similar conclusion. As only patients with stage Ib1–IIb cervical cancer were enrolled in this study, statistical analysis revealed no correlation between NACT efficacy and cancer stage.
NACT efficacy was reported to be related with the prognosis of cervical cancer patients, and sensitivity to chemotherapy was the key factor in choosing NACT regimens[24]. A multi-institute study in France showed that sensitivity to chemotherapy was reduced in cervical cancer patients with menopause, parametrial invasion, LVSI, or cervical mucous cancer[25] Molecular factors found to be associated with NACT resistance include clusterin[26], an anti-apoptotic molecule, and excision repair cross-complementation group 1 (ERCC1)[27]. In addition, Battaglia et al.[28] suggested that NACT supplemented by small dose of radiotherapy could change the immune status of the lymph nodes and then increase the efficacy of chemotherapy. In this study, all but 1 patient with stage IIb cervical cancer underwent NACT. We failed to find any relevant factors associated with NACT sensitivity. Therefore, NACT-related basic research and application of NACT may be future promising research directions.
Unlike ovarian cancer, there is currently no standard chemotherapy for cervical cancer, but many studies have demonstrated the efficacy of platinum-based agents. To date, all NACT regimens used have contained cisplatin. In this study, cisplatin was used either alone or as a major constituent of combination chemotherapy. Our results showed that NACT efficacy was not related with chemotherapy regimens or the number of cycles. Because of the limited number of cases, the diversity of chemotherapy regimens, and various cycles, we only included patients with arterial infusion chemotherapy or simple intravenous chemotherapy in statistical analysis. We found that the efficacy of arterial infusion chemotherapy was significantly higher than that of simple intravenous chemotherapy, which is inconsistent with a previous study reporting no significant difference in their efficacy on locally advanced cervical cancer[29]. We also found no relationship between NACT efficacy and arterial embolization. Our results provide a valuable, practical basis for selection of the administration route in the future.
In 2003, Tierney et al.[30] conducted a meta-analysis of 18 clinical trials on NACT prior to radiotherapy for cervical cancer and compared the efficacy of NACT plus radical hysterectomy with that of radiotherapy alone. They found that NACT could significantly improve prognosis when the period of NACT was shorter than 14 days or the dose of cisplatin was greater than 25 mg/m2 per week. Therefore, we analyzed the dose of cisplatin in our study, including the dose per square meter and the cumulative dose, but failed to find the association between cisplatin dose and NACT efficacy. Moreover, surgical outcomes and postoperative pathologic features were not affected by cisplatin dose or the interval between NACT and surgery.
In this study, all patients with stage IIb cervical cancer in the NACT group successfully underwent surgery, indicating that NACT made surgery an option for patients with locally advanced cervical cancer. There were no significant differences in operation time, intraoperative blood loss, numbers of removed lymph nodes, and intra operative and postoperative complication rates between the PST and NACT groups, indicating that NACT could reduce surgical risk in patients with locally advanced cervical cancer. Furthermore, NACT has been reported not to increase operation difficulty[31],[32], and our data were consistent with most previously reported data[21],[30],[33]. In clinical practice, we often found adhesion of the uterine artery and ureter surrounding tissues in patients who had undergone arterial infusion chemotherapy and embolization, which could increase the difficulty of surgery. However, this phenomenon was not universal to all patients with arterial infusion chemotherapy and embolization. Further analysis showed that combination chemotherapy significantly prolonged the operation time. One possible explanation may be that combination therapy was mainly used in patients with more severe local lesions, on whom the operation was more difficult even without NACT. Although local adhesion increased operation difficulty in patients with arterial infusion intervention and embolization, the operation time was shortened, which may be because artery embolization blocked tumor blood supply and thus controlled local bleeding, enabling clear exposure, and shortening the operation time.
Histopathologic risk factor affects prognosis and is an important reference to determining whether NACT can be used for cervical cancer[34]–[36]. A summary of the experiences of treating patients with early stage cervical cancer over a 12-year period in England showed that lymph node metastasis and positive vaginal margins were two independent poor prognostic factors [37]. In multivariate analysis, LVSI was an independent risk factor for recurrence[35],[36]. The revised 2009 FIGO staging system recommends that (1) LVSI should not be considered in staging and should be reported separately, and (2) the status of lymph nodes should not be considered in staging, but lymph node metastasis should be considered as a poor prognostic factor. Therefore, determining whether changes in histopathologic characteristics improve patient prognosis has been the recent focus of NACT research.
Many previous studies showed that NACT can reduce the risk of pathologic factors such as tumor lesions, deep myometrial invasion, parametrial and lymph-vascular space involvement, thereby reducing the rate of postoperative adjuvant therapy[11],[21],[33],[38]. Battaglia et al.[28] found that NACT, especially when supplemented with radiotherapy, can effectively improve anti-tumor immune responses of pelvic lymph nodes in cervical cancer patients and thus reduce lymph node metastasis. Our data showed no significant difference in depth of myometrial invasion, parametrial and vaginal involvement, or lymph node metastasis between the NACT and PST groups. However, the NACT group had a significantly lower LVSI rate than did the PST group. As LVSI is a cervical cancer-specific poor prognostic factor, further studies are warranted to determine whether a decrease in LVSI reduces the occurrence of micrometastases.
The 5-year survival rate of patients with early cervical cancer ranges from 88% to 97% [39]. Several investigators proposed that NACT could improve the prognosis of cervical cancer[23], but this has not been confirmed by randomized trials[40]. A meta-analysis by Tierney et al. [30] compared NACT plus surgery with radiotherapy alone and found that NACT improved 5-year survival rate by 14% only if the period of cisplatin chemotherapy was shorter than 14 days or weekly doses of cisplatin were greater than 25 mg/m2. Other reports showed that the 5-year survival rate of locally advanced cervical cancer patients treated with NACT was 80% [18],[22],[41],[42]. However, NACT did not show any advantages in improving the survival of patients with cervical cancer with 10-year follow-up[19]. A meta-analysis published in 2010 showed that, compared with PST, NACT significantly improved progression-free survival of patients with early and locally advanced cervical cancer but did not improve the overall survival[11]. Consistent with this, several recent studies showed that NACT could not improve tumor-free survival or overall survival[11],[21],[43]. Nevertheless, NACT was found in some studies to increase local and distant recurrence rate[11] and decrease overall survival rate[38]. In our study, patients who underwent NACT had decreased LVSI, though this did not necessarily lead to improvement of prognosis and, instead, interfered with postoperative adjuvant therapy. Because NACT exposure and observation periods were limited in this study and because a considerable proportion of study patients are currently undergoing adjuvant therapy after surgery, patient prognosis needs to be further assessed.
In summary, we showed here that NACT for patients with locally advanced cervical cancer has substantial beneficial effect on local disease control. NACT increased the opportunity for surgery, reduced surgical risk, and allow young patients to retain physiological functions. We also found that arterial administration of NACT could achieve better efficacy. More importantly, this study shows that NACT significantly reduced the rate of LVSI. Further research is needed to confirm whether NACT can improve the prognosis of patients with cervical cancer.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics [J] CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Benedet JL, Odicino F, Maisonneuve P, et al. Carcinoma of the cervix uteri [J] J Epidemiol Biostat. 2001;6(1):7–43. [PubMed] [Google Scholar]
- 3.Landoni F, Maneo A, Colombo A, et al. Randomised study of radical surgery versus radiotherapy for stage Ib–IIa cervical cancer [J] Lancet. 1997;350(9077):535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 4.Friedlander M, Kaye SB, Sullivan A, et al. Cervical carcinoma: a drug-responsive tumor—experience with combined cisplatin, vinblastine, and bleomycin therapy [J] Gynecol Oncol. 1983;16(2):275–281. doi: 10.1016/0090-8258(83)90102-6. [DOI] [PubMed] [Google Scholar]
- 5.Green JA, Kirwan JM, Tierney JF, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and metaanalysis [J] Lancet. 2001;358(9284):781–786. doi: 10.1016/S0140-6736(01)05965-7. [DOI] [PubMed] [Google Scholar]
- 6.Lilic V, Lilic G, Filipovic S, et al. Modern treatment of invasive carcinoma of the uterine cervix [J] J Buon. 2009;14(4):587–592. [PubMed] [Google Scholar]
- 7.Legge F, Fuoco G, Lorusso D, et al. Pharmacotherapy of cervical cancer [J] Expert Opin Pharmacother. 2010;11(12):2059–2075. doi: 10.1517/14656566.2010.493556. [DOI] [PubMed] [Google Scholar]
- 8.Thigpen T, Shingleton H, Homesley H, et al. Cis-platinum in treatment of advanced or recurrent squamous cell carcinoma of the cervix: a phase II study of the Gynecologic Oncology Group [J] Cancer. 1981;48(4):899–903. doi: 10.1002/1097-0142(19810815)48:4<899::aid-cncr2820480406>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Sardi J, Sananes C, Giaroli A, et al. Neoadjuvant chemotherapy in locally advanced carcinoma of the cervix uteri [J] Gynecol Oncol. 1990;38(3):486–493. doi: 10.1016/0090-8258(90)90096-4. [DOI] [PubMed] [Google Scholar]
- 10.McCormack M, Ledermann JA, Hall-Craggs MA, et al. A phase II study of weekly neoadjuvant chemotherapy followed by radical chemoradiation for locally advanced cervical cancer [C] ASCO. 2009 doi: 10.1038/bjc.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rydzewska L, Tierney J, Vale CL, et al. Neoadjuvant chemotherapy plus surgery versus surgery for cervical cancer [J] Cochrane Database Syst Rev. 2010;(1):CD007406. doi: 10.1002/14651858.CD007406.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Tierney J F, Vale C, Symonds P. Concomitant and neoadjuvant chemotherapy for cervical cancer [J] Clin Oncol (R Coll Radiol) 2008;20(6):401–416. doi: 10.1016/j.clon.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Buda A, Fossati R, Colombo N, et al. Randomized trial of neoadjuvant chemotherapy comparing paclitaxel, ifosfamide, and cisplatin with ifosfamide and cisplatin followed by radical surgery in patients with locally advanced squamous cell cervical carcinoma: the SNAP01 (Studio Neo-Adjuvante Portio) Italian Collaborative Study [J] J Clin Oncol. 2005;23(18):4137–4145. doi: 10.1200/JCO.2005.04.172. [DOI] [PubMed] [Google Scholar]
- 14.Lissoni AA, Colombo N, Pellegrino A, et al. A phase II, randomized trial of neo-adjuvant chemotherapy comparing a three-drug combination of paclitaxel, ifosfamide, and cisplatin (TIP) versus paclitaxel and cisplatin (TP) followed by radical surgery in patients with locally advanced squamous cell cervical carcinoma: the Snap-02 Italian Collaborative Study [J] Ann Oncol. 2009;20(4):660–665. doi: 10.1093/annonc/mdn690. [DOI] [PubMed] [Google Scholar]
- 15.Benedetti Panici P, Scambia G, Greggi S, et al. Neoadjuvant chemotherapy and radical surgery in locally advanced cervical carcinoma: a pilot study [J] Obstet Gynecol. 1988;71(3 Pt 1):344–348. [PubMed] [Google Scholar]
- 16.Itoh N, Sawairi M, Hanabayashi T, et al. Neoadjuvant intraarterial infusion chemotherapy with a combination of mitomycin-C, vincristine, and cisplatin for locally advanced cervical cancer: a preliminary report [J] Gynecol Oncol. 1992;47(3):391–394. doi: 10.1016/0090-8258(92)90146-a. [DOI] [PubMed] [Google Scholar]
- 17.Rob L, Pluta M, Skapa P, et al. Advances in fertility-sparing surgery for cervical cancer [J] Expert Rev Anticancer Ther. 2010;10(7):1101–1114. doi: 10.1586/era.10.61. [DOI] [PubMed] [Google Scholar]
- 18.Wan T, Huang H, Liu JH, et al. Response to neoadjuvant chemotherapy on locally advanced cervical cancer and longterm follow-up outcome [J] Zhonghua Yi Xue Za Zhi. 2010;90(43):3045–3048. [in Chinese] [PubMed] [Google Scholar]
- 19.Benedetti-Panici P, Greggi S, Scambia G, et al. Long-term survival following neoadjuvant chemotherapy and radical surgery in locally advanced cervical cancer [J] Eur J Cancer. 1998;34(3):341–346. doi: 10.1016/s0959-8049(97)10029-6. [DOI] [PubMed] [Google Scholar]
- 20.Raspagliesi F, Ditto A, Selvaggi L, et al. A phase 2 multicenter study of irinotecan and cisplatinum as neoadjuvant treatment in patients with locally advanced cervical cancer [J] Int J Gynecol Cancer. 2010;20(9):1569–1575. doi: 10.1111/IGC.0b013e3181cc71f7. [DOI] [PubMed] [Google Scholar]
- 21.Lee JY, Kim YH, Kim MJ, et al. Treatment of stage IB2, NA bulky cervical cancer: a single-institution experience of neoadjuvant chemotherapy followed by radical hysterectomy and primary radical hysterectomy [J] Arch Gynecol Obstet. 2010 Sep 28; doi: 10.1007/s00404-010-1685-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Matsumura M, Takeshima N, Ota T, et al. Neoadjuvant chemotherapy followed by radical hysterectomy plus postoperative chemotherapy but no radiotherapy for Stage IB2-IIB cervical cancer—Irinotecan and platinum chemotherapy [J] Gynecol Oncol. 2010;119(2):212–216. doi: 10.1016/j.ygyno.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 23.Eddy GL., Sr Neoadjuvant chemotherapy before surgery in cervical cancer [J] J Natl Cancer Inst Monogr. 1996;(21):93–99. [PubMed] [Google Scholar]
- 24.Gadducci A, Fabrini MG, Perrone F, et al. Pattern of failures and clinical outcome of patients with locally advanced cervical cancer treated with a tailored integrated therapeutic approach [J] Anticancer Res. 2010;30(9):3731–3735. [PubMed] [Google Scholar]
- 25.Poujade O, Morice P, Rouzier R, et al. Pathologic response rate after concomitant neo-adjuvant radiotherapy and chemotherapy for adenocarcinoma of the uterine cervix: a retrospective multicentric study [J] Int J Gynecol Cancer. 2010;20(5):815–820. doi: 10.1111/IGC.0b013e3181df7406. [DOI] [PubMed] [Google Scholar]
- 26.Watari H, Kanuma T, Ohta Y, et al. Clusterin expression inversely correlates with chemosensitivity and predicts poor survival in patients with locally advanced cervical cancer treated with cisplatin-based neoadjuvant chemotherapy and radical hysterectomy [J] Pathol Oncol Res. 2010;16(3):345–352. doi: 10.1007/s12253-009-9235-0. [DOI] [PubMed] [Google Scholar]
- 27.Park JS, Jeon EK, Chun SH, et al. ERCC1 (excision repair cross-complementation group 1) expression as a predictor for response of neoadjuvant chemotherapy for FIGO stage 2B uterine cervix cancer [J] Gynecol Oncol. 2011;120(2):275–279. doi: 10.1016/j.ygyno.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 28.Battaglia A, Buzzonetti A, Martinelli E, et al. Selective changes in the immune profile of tumor-draining lymph nodes after different neoadjuvant chemoradiation regimens for locally advanced cervical cancer [J] Int J Radiat Oncol Biol Phys. 2010;76(5):1546–1553. doi: 10.1016/j.ijrobp.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Adachi S, Ogasawara T, Tsubamoto H, et al. Intravenous nedaplatin and intraarterial cisplatin with transcatheter arterial embolization for patients with locally advanced uterine cervical cancer [J] Int J Clin Pharmacol Res. 2001;21(3–4):105–110. [PubMed] [Google Scholar]
- 30.Neoadjuvant Chemotherapy for Locally Advanced Cervical Cancer Meta-analysis Collaboration Neoadjuvant chemotherapy for locally advanced cervical cancer: a systematic review and meta-analysis of individual patient data from 21 randomised trials [J] Eur J Cancer. 2003;39(17):2470–2486. doi: 10.1016/s0959-8049(03)00425-8. [DOI] [PubMed] [Google Scholar]
- 31.Fujiwaki R, lida K, Ohnishi Y, et al. Intra-arterial neoadjuvant chemotherapy followed by radical surgery and radiotherapy for stage IIb cervical carcinoma [J] Anticancer Res. 1997;17(5B):3751–3755. [PubMed] [Google Scholar]
- 32.Vizza E, Pellegrino A, Milani R, et al. Total laparoscopic radical hysterectomy and pelvic lymphadenectomy in locally advanced stage IB2–IIB cervical cancer patients after neoadjuvant chemotherapy [J] Eur J Surg Oncol. 2010;37(4):364–369. doi: 10.1016/j.ejso.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Cho YH, Kim DY, Kim JH, et al. Comparative study of neoadjuvant chemotherapy before radical hysterectomy and radical surgery alone in stage IB2–IIA bulky cervical cancer [J] J Gynecol Oncol. 2009;20(1):22–27. doi: 10.3802/jgo.2009.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rutledge TL, Kamelle SA, Tillmanns TD, et al. A comparison of stages IB1 and IB2 cervical cancers treated with radical hysterectomy. Is size the real difference? [J] Gynecol Oncol. 2004;95(1):70–76. doi: 10.1016/j.ygyno.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 35.Kamelle SA, Rutledge TL, Tillmanns TD, et al. Surgical-pathological predictors of disease-free survival and risk groupings for IB2 cervical cancer: do the traditional models still apply? [J] Gynecol Oncol. 2004;94(2):249–255. doi: 10.1016/j.ygyno.2004.05.038. [DOI] [PubMed] [Google Scholar]
- 36.Marchiole P, Buenerd A, Benchaib M, et al. Clinical significance of lympho vascular space involvement and lymph node micrometastases in early-stage cervical cancer: a retrospective case-control surgico-pathological study [J] Gynecol Oncol. 2005;97(3):727–732. doi: 10.1016/j.ygyno.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Suprasert P, Srisomboon J, Charoenkwan K, et al. Twelve years experience with radical hysterectomy and pelvic lymphadenectomy in early stage cervical cancer [J] J Obstet Gynaecol. 2010;30(3):294–298. doi: 10.3109/01443610903585192. [DOI] [PubMed] [Google Scholar]
- 38.Kim HS, Kim JY, Park NH, et al. Matched-case comparison for the efficacy of neoadjuvant chemotherapy before surgery in FIGO stage IB1–IIA cervical cancer [J] Gynecol Oncol. 2010;119(2):217–224. doi: 10.1016/j.ygyno.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Rob L, Halaska M, Robova H. Nerve-sparing and individually tailored surgery for cervical cancer [J] Lancet Oncol. 2010;11(3):292–301. doi: 10.1016/S1470-2045(09)70191-3. [DOI] [PubMed] [Google Scholar]
- 40.Eddy G L, Bundy B N, Creasman W T, et al. Treatment of (“bulky”) stage IB cervical cancer with or without neoadjuvant vincristine and cisplatin prior to radical hysterectomy and pelvic/para-aortic lymphadenectomy: a phase III trial of the gynecologic oncology group [J] Gynecol Oncol. 2007;106(2):362–369. doi: 10.1016/j.ygyno.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Robova H, Halaska M, Pluta M, et al. The role of neoadjuvant chemotherapy and surgery in cervical cancer [J] Int J Gynecol Cancer. 2010;20(11 Suppl 2):S42–S46. doi: 10.1111/igc.0b013e3181f60d73. [DOI] [PubMed] [Google Scholar]
- 42.Mori T, Hosokawa K, Sawada M, et al. Neoadjuvant weekly carboplatin and paclitaxel followed by radical hysterectomy for locally advanced cervical cancer: long-term results [J] Int J Gynecol Cancer. 2010;20(4):611–616. doi: 10.1111/IGC.0b013e3181d80aa9. [DOI] [PubMed] [Google Scholar]
- 43.Mossa B, Mossa S, Corosu L, et al. Follow-up in a long-term randomized trial with neoadjuvant chemotherapy for squamous cell cervical carcinoma [J] Eur J Gynaecol Oncol. 2010;31(5):497–503. [PubMed] [Google Scholar]