Abstract
In the last decade, we have gained significant understanding of the mechanism by which vesicular stomatitis virus (VSV) specifically kills cancer cells. Dysregulation of translation and defective innate immunity are both thought to contribute to VSV oncolysis. Safety and efficacy are important objectives to consider in evaluating VSV as a therapy for malignant disease. Ongoing efforts may enable VSV virotherapy to be considered in the near future to treat drug-resistant ovarian cancer when other options have been exhausted. In this article, we review the development of VSV as a potential therapeutic approach for recurrent or drug-resistant ovarian cancer.
Keywords: Ovarian cancer, therapy, VSV, oncolytic virus, innate immunity, STING, interferons
Current State of Ovarian Cancer Treatment
Epithelial ovarian cancer is a disease with poor prognosis, few useful early diagnostic markers, and limited treatment options[1]–[3]. Chemotherapeutic agents based on platinum derivatives have been widely used to treat a broad range of cancers including epithelial ovarian cancer with some success[4]–[7]. Generally, platinum compounds are DNA damaging agents, and proliferating cancer cells are more prone to being killed by these DNA damaging compounds[8]. Currently, a platinum- and taxane-based combination regimen remains the standard front-line chemotherapy for ovarian cancer[9]–[14]. In advanced disease, the standard treatment following diagnosis (in the United States) is maximal surgical debulking followed by paclitaxel/carboplatin chemotherapy to remove residual cancer. The widely adapted protocol is administration of paclitaxel (135 mg/m2, 24 h infusion) plus cisplatin (75 mg/m2) or paclitaxel (175 mg/m2, 3 h infusion) plus carboplatin[11]. These basic regimens can be tailored to many clinical studies by adjusting the dose and duration, sequence and route of administration, and combination of additional agents to maximize the effectiveness of the therapy.
Unfortunately, intrinsic and acquired resistance to cisplatin/taxane has greatly limited the efficacy of this therapy[8],[9]. New second-line agents under development and testing, such as gemcitabine, doxorubicin, and topotecan that convey anti-cancer activities via different mechanisms, are being evaluated in clinical trials for treatment of cisplatin-resistant cancer, and some have been adopted for clinical application[8]–[11]. These new agents are often found to have incremental effects on improving survival of ovarian cancer patients. The mechanism of drug resistance has been an intense subject of investigation in ovarian cancer. Progressive understanding on this topic has been obtained, and strategies to reverse drug-resistance are being tested. Nevertheless, current treatment options are still very limited. Resistance to cytotoxic chemotherapy remains a key problem, and most women ultimately die of ovarian cancer. Development of additional chemotherapeutic regimens, biological therapeutic agents, and other unique approaches for treatment of ovarian cancer is a high priority. Certainly, the therapeutic approaches are expected to be progressively fine-tuned and improved, but no dramatic advance or alternative approaches that are projected to significantly impact the survival rate of ovarian cancer patients are anticipated on the immediate horizon.
A Brief History of Oncolytic Virus
The idea to use viruses to kill cancer cells may have been around for many years, and the notion of using a “harmful agent” to treat a “dreadful illness” was rooted in the thinking of traditional oriental medicine. The first well-known, truly scientific attempt in modern times was in 1996, when Frank McCormick and colleagues conceived the idea of using a mutant adenovirus that lacks the E1B gene, which encodes a protein that inactivates p53, to treat cancer[15]–[17]. They hypothesized that the Onyx-15 mutant adenovirus would only replicate in and kill cancer cells, which generally lack the p53 tumor suppressor, and would be attenuated in p53-containing non-cancer cells[15]–[17]. The idea was brilliant, but proven incorrect because the mutant virus killed tumor cells preferentially regardless of p53 mutation status[18]. The work was rapidly advanced to clinical application for cancer therapy in subsequent years [19]. However, for financial and regulatory reasons, human trials of the Onyx-15 mutant adenovirus (Onyx Pharmaceuticals) for cancer therapy were aborted before going into phase III trial in 2000[18]. Nevertheless, an almost identical virus, H101, was shown to have efficacy in the first cancer viral therapy in humans and achieved some degree of success in November 2005 in China[20],[21]. Further studies and trials showed that there are substantial limitations of the mutant adenovirus as an oncolytic agent, and continuing development and improvement of mutant adenovirus for cancer treatment persists[18].
Following the studies of mutant adenovirus as potential cancer therapy, the idea to use particular types of viruses as agents to selectively kill cancer cells firmly took root[18],[22],[23]. These viruses, referred to as oncolytic viruses, are capable of replicating in cancer cells but not in normal cells[18],[24]. A number of viruses that exhibit oncolytic activity, including retrovirus, measles, Newcastle disease virus, mumps, influenza, and vesicular stomatitis virus, are under investigation. Various labs and small companies are currently studying biology and testing strategies with genetic engineering to optimize the recombinant viruses for cancer therapy. Some of these oncolytic viruses are currently in phase I trials or late preclinical development[22],[23]. Certainly, there are many obstacles, both technical and logistical, in the development of oncolytic viral therapy. The established concept and further understanding of biology, along with the ongoing research effort will likely bring us one or more effective cancer oncolytic therapies.
As discussed above, the oncolytic virus that is most considered and invested in development as a cancer therapy in the past decades is adenovirus[18],[22],[23]. Therapy with engineered conditional, replication-competent, oncolytic adenovirus was regarded with much enthusiasm in preclinical studies[19]. However, clinical trials of oncolytic adenovirus encountered several difficult barriers including the lack of tumor-specific viral targeting/infection and clearance of the virus by the immune system[18],[25],[26]. In humans, weak tumor cell targeting by adenovirus is a serious shortcoming. Although adenovirus can efficiently infect most mammalian cells through specific receptors, the infectivity seems to be reduced in neoplastic cells, and the majority of adenovirus delivered is sequestered in the liver. While oncolytic adenovirus still has promise, strategies to modify the basic viral structure will be needed to overcome the existing shortcomings.
Currently, several other viral vectors that appear to lack the intrinsic faults associated with adenoviral vectors are being studied[22],[23]. One such virus, the vesicular stomatitis virus (VSV), is emerging as a promising new oncolytic vector. With its specificity for transformed cells and its ability to target tumors without accumulating in other organs, VSV may have great promise as an oncolytic agent for treating drug-resistant ovarian cancer. We discuss here the potential of VSV as an anti-cancer therapeutic agent[27]–[31].
VSV Biology
VSV is a negative-stranded RNA virus of the Rhabdoviridae family, which has more than one hundred members with hosts that include plants, invertebrates, vertebrates, and mammals[25]. VSV can infect a wide spectrum of cell types through an as-yet-unidentified but likely ubiquitous cell surface receptor(s). VSV replicates in the cytoplasm of infected cells, but the viral genome does not integrate into the host genome, nor does it have transforming activity[32],[33]. Additionally, the VSV genome can be manipulated to modify properties and to insert and express transgenes, making it suitable to be studied and engineered in the laboratory setting[29],[34],[35].
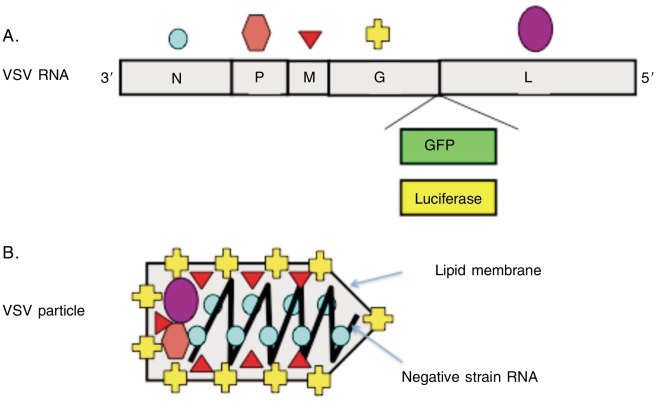
The 11-kb VSV genome encodes 5 proteins: nucleocapsid (N), phosphoprotein (P), large polymerase (L), matrix (M), and surface glycoprotein (G)[34],[35] (Figure 1). The negative sense, single-stranded VSV RNA is bound by multiple copies of N protein, forming a bead-on-a-string helical structure, and is encapsulated in a bullet-shaped viral particle approximately 100–400 nm long and 45–100 nm in diameter. VSV is an enveloped virus coated with multiple copies of trimeric surface glycoprotein G, which is embedded in a lipid membrane. Within the lipid capsule, trace amounts of P, L, and M proteins are packaged along for the initiation of viral replication following entry into cells.
Figure 1. Structure and genes of vesicular stomatitis virus (VSV).
A, the single-strained RNA encodes five viral proteins. The green fluorescence protein (GFP) or luciferase transgene can be inserted to monitor viral infection and proliferation. B, the five viral proteins function as follows: the glycoprotein (G) catalyzes fusion of viral and cell membranes; the nucleoprotein (N) binds the RNA and forms an RNA-dependent RNA-polymerase complex with the phosphoprotein (P) and large polymerase protein (L); and the matrix protein (M) has multiple critical roles in viral replication and pathogenesis.
VSV particles enter cells through surface glycoprotein G–mediated binding to the cell surface, endocytosis, and membrane fusion[36],[37]. Upon entry into the cytoplasm, packaged P and L proteins form an RNA-dependent RNA polymerase complex with the N protein-bound VSV RNA genome and initiate transcription to produce capped and polyadenylated sub-genomic transcripts. The VSV mRNA transcripts depend on the host translation machinery, Golgi apparatus, and endoplasmic reticulum to synthesize and process the viral proteins[38]. At some point after sufficient viral proteins are produced, the RNA-dependent RNA polymerase complex starts to generate full-length viral RNAs, which are then used as templates to copy the negative strain viral RNA genome. The full-length viral RNA genome is then bound by multiple copies of the N protein, and the ribonucleic acid complexes are shuttled to plasma membranes. With additional assembly and packaging, functional VSV virions are budded off and released from the cell surface[39]–[43]. The entire process of viral replication takes place in the cytoplasm.
The M protein plays multiple regulatory roles in viral assembly and pathogenesis[39]–[45]. M protein connects the VSV nucleoprotein capsule and cellular plasma membrane in the assembly of viral particles [40],[42],[45] and plays a role in the release of the viral particles by budding from the host cells[45]. Mutations in the M gene can also produce spherical extracellular viral particles instead of the bullet shape of native VSV[46],[47]. M protein is also important for modulation of host gene expression, interaction with host cellular signaling pathways, and altering immunosurveillance [44],[48],[49]. By itself, M protein can induce death in mammalian cells[41],[44] and can also modulate cellular signaling pathways to affect the immune response[49]. M protein is not essential for viral replication because M-mutant VSV can still infect and replicate in mammalian cells, albeit with reduced efficiency[50]. M-mutant VSV can also cause prolonged infection of neuronal cells, instead of inducing rapid cell death[51].
VSV Clearance in Immune-Competent Hosts
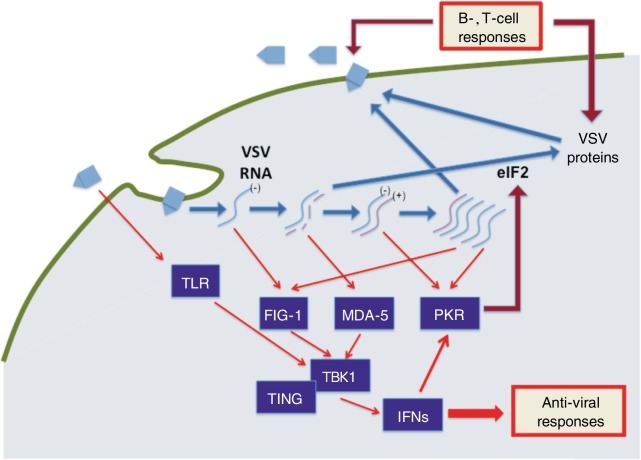
VSV can infect essentially all human cells in culture and undergo robust replication in certain (often cancerous) cells; however, VSV is relatively non-pathologic for humans, likely because of the induction of strong immune response to suppress viral replication and amplification[32],[33]. While VSV is largely asymptomatic for humans, domestic and farm animals can become non-lethally infected, with symptoms such as lesions in the mucous membranes of the mouth and nose[52],[53]. VSV has also been reported to be neuropathic in mice, following intranasal inoculation and subsequent infection and replication in the central nervous system[54]–[56]. VSV infection can be cleared through activation of both the innate and adaptive immune responses[53]–[56] (Figure 2). The interferons (IFN) are critically important in antiviral innate immunity and are a family of cytokines produced in response to VSV infection[57]–[60]. Upon VSV infection, the initial immune response against the virus is the activation of the innate immune system and production of interferon beta (IFN-β). The released IFN-β protein acts in an autocrine or paracrine manner and leads to the activation of IFN-stimulated genes, up-regulation of antigen processing machinery, and activation/maturation of antigen presenting cells (i.e., dendritic cells, NK cells, macrophages)[61]–[63]. Signaling via IFN results in suppression of viral gene expression and clearance of infected cells by leukocytes[64]–[67]. Extensive studies in mutant mice have identified several key pathways and mechanisms involved in clearing VSV. For example, mouse embryonic fibroblasts (MEFs) and mice deficient in double-stranded RNA–dependent RNA kinase (PKR), signal transducer and activator of transcritpion 1 (STAT1), nuclear factor associated with soluble stranded-RNA (NFAR), or interferon alpha receptor (IFNAR) are highly susceptible to infection by VSV[68]–[77].
Figure 2. VSV replication and host immune response.
VSV particles enter host cells by binding to a ubiquitous surface receptor and undergoing endocytosis. Upon reaching the cytoplasm, the viral negative-strain RNA genome undergoes transcription and replication, and viral proteins are produced and packaged with newly replicated viral genomic RNA to form new VSV particles that are released outside cells. The pattern recognition receptors retinoic acid inducible gene I (RIG-1), toll-like receptor (TLR), and melanoma differentiation antigen 5 (MDA-5) recognize viral RNA and activate interferon (IFN) response through stimulator of interferon genes (STING) and tank binding kinase 1 (TBKI)–mediated signaling pathways. Double-stranded RNA-dependent RNA kinase (PKR) is an IFN-induced gene and is also activated by double-stranded RNA, forming an amplifying circuit. In a second phase of immune response, B and T cells are activated to clear viral infection.
Mice that with components of the innate immune system knocked out, such as IFNAR-, PKR-, or STAT1-null animals, show lethality around 4–5 days after infection because of the virus's ability to replicate efficiently throughout the mouse[68]–[77]. For example, PKR plays an important role in the initial immune response to VSV infection by restricting translation of viral mRNA[70],[72],[73]. PKR is an IFN-inducible serine/threonine protein kinase that becomes autophosphorylated in response to double-stranded RNA species. Activated PKR phosphorylates eukaryotic translation initiating factor 2α (eIF2α) and thus inhibits translation[70],[77]. This is the initial control of infection following induction of IFN and IFN-stimulated genes including PKR. However, activation of IFN and PKR alone is not sufficient to completely clear the VSV infection; the adapted secondary immune responses also play critical roles in VSV clearance[78].
B cell–deficient mice that maintain an otherwise intact innate response often show toxicity around 9 or 10 days post infection because of the lack of endogenous circulating antibodies and the production of neutralizing antibodies primarily against the N and G proteins by plasma cells. Further, T cell–deficient mice died 30 days after infection due to their inability to provide B cells help and generate memory against the virus[78]. These studies indicate that the innate response is a short-term acute maneuver for the initial suppression of VSV replication, and this allows time for subsequent activation of the adaptive immune response for complete viral clearance.
The innate immune system relies on several pattern recognition receptors that detect specific pathogen-associated molecular patterns, including non-self nucleic acid such as positive and negative sense single-stranded RNA, double-stranded RNA and DNA, and CpG DNA, and subsequently activates an antiviral response characterized by induction of IFNs[79]–[82] (Figure 2).
Three specific cellular DNA sensing pathways, namely the retinoic acid inducible gene I (RIG-I) and RIG-I like helicase (RLH), Toll-like receptor (TLR), and absent in melanoma 2 (AIM2) and stimulator of interferon genes (STING) pathways, are responsible for indentifying viral infection and activating an antiviral response[80]–[83]. Activation of the RLH cascade to produce IFN-α and -β relies on two DExD/H helicases, RIG-I and melanoma differentiation antigen 5 (MDA-5)[84],[85]. RIG-I recognizes 5′-triphosphates on viral RNA, such as double-stranded RNA intermediates produced during viral replication and those encoded by negative-stranded viruses like VSV[84],[85]. MDA-5 recognizes longer RNAs (>1 kb) that are encoded by positive-stranded viruses such as the picornavirus encephalomyocarditis virus[85].
The recently identified STING and AIM2 (absent in melanoma 2) pathway is particularly interesting in that the STING pathway is activated by cellular DNA but not RNA species[86]–[88]. Nevertheless, STING is important in response to the single-stranded RNA VSV, likely because it mediates the translocation of tank binding kinase 1 (TBK1) and interacts with the RIG-I pathways[83],[88],[89]. The NF-κB pathway is also important in activating the anti-viral interferon response[90].
Mechanistic Basis of VSV Oncolytic Selectivity
VSV infection occurs through a ubiquitous, undetermined receptor, and thus, VSV has the unique ability to target many different cell types and malignancies and is a promising vector for oncolytic therapy[91],[92]. In humans or experimental animals, VSV may initially enter and infect cells it encounters, but the activation of both innate and adapted immunity will suppress viral replication and ultimately clear infected cells. Thus, the sensitivity of malignant cells to VSV may be caused by their increased sensitivity to apoptosis or, more likely, their intrinsic defects in innate immune signaling pathways that allow the unchecked viral replication.
Significant progress has been made in understanding the mechanisms of VSV oncolytic activity and selectivity[90]–[92]. Cancer cells generally have defective immune signaling[93],[94], which may be crucial for proliferation and escape from the tumor suppression mechanism of host immunosurveillance. In addition, the defective immune regulatory system, which includes dysregulation of interferon induction and response, is an important factor in regulating VSV replication in cancer cells[30],[69]. Cancer cells have the ability to proliferate continuously because of translation dysregulation, which allows high levels of viral protein production and replication within malignant cells and leads to cell deathi[70],[95],[96]. A defective PKR-mediated innate immune signaling pathway, which results in the inability to suppress viral protein translational in cancer cells, is another key factor for VSV replication and oncolytic activity[95],[96]. Other defects in innate immune signaling pathways in cancer cells include alternative splicing of IRF3 or MyD88, CpG methylation of IRF7, mutations of CYLD, reduced phosphorylation of STAT1, suppressed transcription of IFN-stimulated genes, and mutations in JAKs and TRAF-3[97]–[100]. Thus, oncolytic viruses can likely kill transformed cells preferentially over normal cells because of defects in both the innate signaling pathways and the translational control systems.
The robust production of VSV proteins in cancer cells likely leads to the killing of the malignant cells. M protein produced in cancer cells can bind the nuclear pore, suppress cell cycle progression[101], and induce apoptosis[42],[102]. Moreover, the released VSV particles can then infect and kill adjacent cancer cells in the tumor, leading to additional activation of host immune response for tumor elimination.
The Potential of Recombinant VSV in Ovarian Cancer Treatment
VSV's ability to selectively replicate in and kill malignant but not normal cells has been well established in cultured cells and mouse xenografts of human cancer cells[95],[103]–[113]. VSV has oncolytic activity in a large range of cancer types[95],[103]–[113], including ovarian cancer cells[114]. Mechanistic studies indicate that tumor cells transformed by oncogenic Ras, c-Myc, or p53 inactivation are susceptible to killing by VSV[103]. The differential susceptibility of normal and ovarian cancer cells to VSV oncolytic activity is unequivocal. When VSV was added to culture cells, only a small fraction of primary ovarian surface epithelial cells were observed to express a low level of VSV-encoded proteins, indicating VSV infection and proliferation. Normal ovarian surface epithelial cells maintained a normal growth pattern up to 3 weeks following exposure to VSV. In contrast, all of the ovarian cancer cell lines tested were sensitive to VSV, and the cells died within 3 days of adding VSV to the cultures[114].
While the efficacy of VSV for cancer therapy has been established in preclinical studies, further studies in immune-competent model organisms, rigorous evaluation of safety, and careful documentation of potential toxic side effects may move VSV oncolytic therapy to the next step, a clinical trial in human cancer patients. To that end, targeting of ovarian tumors by VSV was evaluated in an immune-competent Wv (white spotting variant) mouse model that develops ovarian epithelial tumors spontaneously [114]–[116]. Wv mice are deficient in ovarian germ cells and initially develop benign ovarian epithelial tumors known as tubular adenomas, which can acquire increasingly neoplastic features in older mice[116]. The in situ ovarian tumor-bearing mice are anatomically correct and more accurately reflect the accessibility of VSV to tumor cells and the potential toxic side effects of oncolytic therapy. This study demonstrated that VSV delivered through various routes in immune-competent mice explicitly targeted ovarian tumors without significantly affecting any other organs or showing observable toxicity. VSV treatment was very effective in eliminating the epithelial component of tumors, leaving only tumor-free ovarian stroma after treatment[114].
Likely, expression and replication of VSV proteins induces apoptosis in the infected tumor cells, and the progeny VSV particles released will infect and kill surrounding tumor cells in a manner of amplification. Additionally, tumor antigens that are released from cell lysis may be recognized and processed by immune cells. Ultimately, the tumor cell antigens are cross-presented to naïve T cells, triggering a tumor-specific T cell response that potentially induces immunologic memory against the tumor[117]–[119]. Indeed, replication-incompetent VSV (VSV-Δ G) prompted both antiviral and anti-tumor immune responses [120], thereby demonstrating the contribution of anti-tumor immune response after VSV exposure.
Recurrent and drug-resistant ovarian epithelial cancer appears to be a very suitable setting for treatment with oncolytic virus administered into the peritoneal cavity. These ovarian tumors present as numerous small nodules seeded throughout the peritoneum and on the surface of organs. VSV particles infused into the abdomen will likely be able to access, infect, and kill all tumor cells in each small nodule.
Naturally, VSV is not a human pathogen, hence deliberate inoculation of high dose viral particles to humans in therapy will be a safety concern. One advantage of using VSV for cancer therapy is the widespread tropism to readily infect cells. Unlike adenovirus, which is largely sequestered in the liver, VSV can infect most organ and cell types and only proliferate in the susceptible cancer cells upon injection into experimental animals. Also, VSV has a low incidence in the human population and is mostly naïve for the human adaptive immune system, which is another advantage over adenovirus, a flu-like virus that humans have often encountered and developed immune recognition. Thus, injection of VSV for cancer treatment will not cause an immediate immune response to clear the virus, a major limitation of using adenovirus.
Barriers to VSV as A Cancer Therapy
The oncolytic activity and efficacy of VSV as a cancer therapy are well demonstrated in mouse models, and the important remaining issues are the safety and selectivity[121]. One risk factor associated with VSV is its ability to infect the central nervous system and thereby cause potential neuropathology. VSV infection in neurons may lead to immediate morbidity or even mortality in humans, or it may cause a persistent non-lethal infection as seen in other mammals, presenting as sores on the feet or mucus membranes of the mouth and nose[52],[53]. One idea for improving the safety of VSV oncolytic therapy is to design recombinant VSV that has reduced virulence in the neuronal system and/or has altered tropism to be selective for tumor cells[122]–[127]. Strategies include engineering VSV surface protein to bind tumor antigens and including transgenes or manipulating the viral genome to modulate virulence in neuronal cells. Many of these strategies are currently being actively investigated.
Another obstacle is the human adaptive immune response. Upon challenge by high-dose VSV in therapy, the patient may experience a possibly massive immune response and inflammatory reaction that may be very harmful or lethal. Thus, host immune and inflammatory responses need to be carefully monitored and modulated during treatment to prevent inflammatory injury. The immune response may also clear VSV before tumor killing is completed. If the immune system kills the virus faster than viral replication and tumor cell killing, one possible solution is to administer drugs to suppress B cells during VSV oncolytic therapy.
Remarks
Preclinical studies have established the possibility of using VSV to treat ovarian cancer. While safety is a concern, continuing studies to create and test better VSV strains for safety, efficacy, and selectivity are ongoing[128]. Thus, VSV oncolytic therapy may be a very promising approach, especially for recurrent and drug-resistant ovarian cancer for which treatment options have been exhausted.
Additional studies of the biology of VSV, its oncolytic activity, and its regulation in benign and malignant cells, as well as rigorous testing in additional ovarian cancer animal models for safety and efficacy may bring VSV oncolytic therapy closer for treatment of drug-resistant ovarian cancer patients.
Acknowledgments
We acknowledge the works of many authors we have cited in this article, and apologize for the many other important papers we have not cited due to the limited length of the manuscript.
The studies were supported by grants R01 CA099471, R01 CA79716, and R01 CA75389 from NCI, NIH, and W81XWH-06-1-0095 from DOD to Xiang-Xi Xu; and by grants R01 CA095924 and P01CA128115 from NIH, and grant W81XWH-11-1-0266 from DOD to Glen N. Barber. We thank Dr. Elizabeth Smith for reading, commenting, and editing during the process of preparing the manuscript. We also appreciate Mr. and Mrs. Kamal T. and Leila S. Farah of Miami, Florida for their encouragement and philosophical discussion for our research and this paper.
References
- 1.Ozols RF, Bookman MA, Connolly DC, et al. Focus on epithelial ovarian cancer [J] Cancer Cell. 2004;5(1):19–24. doi: 10.1016/s1535-6108(04)00002-9. [DOI] [PubMed] [Google Scholar]
- 2.Cho KR, Shih IeM. Ovarian cancer [J] Annu Rev Pathol. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bast RC, Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation [J] Nat Rev Cancer. 2009;9(6):415–428. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozols RF, Bookman MA, Young RC. Intraperitoneal chemotherapy for ovarian cancer [J] N Engl J Med. 2006;354(15):1641–1643. doi: 10.1056/NEJMc060264. [DOI] [PubMed] [Google Scholar]
- 5.Ozols RF, Young RC. Chemotherapy of ovarian cancer [J] Semin Oncol. 1984;11(3):251–263. [PubMed] [Google Scholar]
- 6.McGuire WP, 3rd, Markman M. Primary ovarian cancer chemotherapy: current standards of care [J] Br J Cancer. 2003;89(Suppl 3):S3–S8. doi: 10.1038/sj.bjc.6601494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGuire WP, Ozols RF. Chemotherapy of advanced ovarian cancer [J] Semin Oncol. 1998;25(3):340–348. [PubMed] [Google Scholar]
- 8.Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways [J] Clin Cancer Res. 2008;14(5):1291–1295. doi: 10.1158/1078-0432.CCR-07-2238. [DOI] [PubMed] [Google Scholar]
- 9.Christian MC, Trimble EL. Salvage chemotherapy for epithelial ovarian carcinoma [J] Gynecol Oncol . 1994;55(3 Pt 2):S143–S150. doi: 10.1006/gyno.1994.1354. [DOI] [PubMed] [Google Scholar]
- 10.Ozols RF. Advanced ovarian cancer: a clinical update on first-line treatment, recurrent disease, and new agents [J] J Natl Compr Canc Netw. 2004;2(Suppl 2):S60–S73. [PubMed] [Google Scholar]
- 11.Martin LP, Schilder RJ. Management of recurrent ovarian carcinoma: current status and future directions [J] Semin Oncol. 2009;36(2):112–125. doi: 10.1053/j.seminoncol.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 12.McGuire WP, Brady MF, Ozols RF. The Gynecologic Oncology Group experience in ovarian cancer [J] Ann Oncol. 1999;10(Suppl 1):29–34. doi: 10.1023/a:1008303300675. [DOI] [PubMed] [Google Scholar]
- 13.Ozols RF, Bookman MA, du Bois A, et al. Intraperitoneal cisplatin therapy in ovarian cancer: comparison with standard intravenous carboplatin and paclitaxel [J] Gynecol Oncol. 2006;103(1):1–6. doi: 10.1016/j.ygyno.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 14.Ozols RF. Paclitaxel (Taxol)/carboplatin combination chemotherapy in the treatment of advanced ovarian cancer [J] Semin Oncol. 2000;27(3 Suppl 7):3–7. [PubMed] [Google Scholar]
- 15.Kirn DH, McCormick F. Replicating viruses as selective cancer therapeutics [J] Mol Med Today. 1996;2(12):519–527. doi: 10.1016/s1357-4310(97)81456-6. [DOI] [PubMed] [Google Scholar]
- 16.Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells [J] Science. 1996;274(5286):373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 17.Heise C, Sampson-Johannes A, Williams A, et al. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents [J] Nat Med. 1997;3(6):639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 18.McCormick F. Cancer-specific viruses and the development of ONYX-015 [J] Cancer Biol Ther. 2003;2(4 Suppl 1):S157–S160. [PubMed] [Google Scholar]
- 19.Kirn D, Hermiston T, McCormick F. ONYX-015: clinical data are encouraging [J] Nat Med. 1998;4(12):1341–1342. doi: 10.1038/3902. [DOI] [PubMed] [Google Scholar]
- 20.Garber K. China approves world's first oncolytic virus therapy for cancer treatment [J] J Natl Cancer Inst. 2006;98(5):298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- 21.Yu W, Fang H. Clinical trials with oncolytic adenovirus in China [J] Curr Cancer Drug Targets. 2007;7(2):141–148. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]
- 22.Rowan K. Oncolytic viruses move forward in clinical trials [J] J Natl Cancer Inst. 2010;102(9):590–595. doi: 10.1093/jnci/djq165. [DOI] [PubMed] [Google Scholar]
- 23.Eager RM, Nemunaitis J. Clinical development directions in oncolytic viral therapy [J] Cancer Gene Ther. 2011;18(5):305–317. doi: 10.1038/cgt.2011.7. [DOI] [PubMed] [Google Scholar]
- 24.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering [J] Mol Ther. 2007;15(4):651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 25.Hermiston T. A demand for next-generation oncolytic adenoviruses [J] Curr Opin Mol Ther. 2006;8(4):322–330. [PubMed] [Google Scholar]
- 26.Nettelbeck DM. Cellular genetic tools to control oncolytic adenoviruses for virotherapy of cancer [J] J Mol Med. 2008;86(4):363–377. doi: 10.1007/s00109-007-0291-1. [DOI] [PubMed] [Google Scholar]
- 27.Balachandran S, Barber GN. Vesicular stomatitis virus (VSV) therapy of tumors [J] IUBMB Life. 2000;50(2):135–138. doi: 10.1080/713803696. [DOI] [PubMed] [Google Scholar]
- 28.Barber GN. Vesicular stomatitis virus as an oncolytic vector [J] Viral Immunol. 2004;17(4):516–527. doi: 10.1089/vim.2004.17.516. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez M, Porosnicu M, Markovic D, et al. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease [J] J Virol. 2002;76(2):895–904. doi: 10.1128/JVI.76.2.895-904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stojdl DF, Lichty B, Knowles S, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus [J] Nat Med. 2000;6(7):821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 31.Stojdl DF, Lichty BD, tenOever BR, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents [J] Cancer Cell. 2003;4(4):263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 32.Wagner RR, Rose JK. In: Rhabdoviridae: the viruses and their replication [M] Fields BN, Knipe DM, editors. New York: Lippincott-Raven; 1996. pp. 1121–1136. [Google Scholar]
- 33.Rickinson A, Kieff E. Epstein-Barr virus [M] In: Knipe DaH P., editor. Fields Viroloy. 4th edition. Philadelphia: Lippincott Williams & Wilkins; 2001. p. 1579. [Google Scholar]
- 34.Whelan SP, Ball LA, Barr JN, et al. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones [J] Proc Natl Acad Sci USA. 1995;92(18):8388–8892. doi: 10.1073/pnas.92.18.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawson ND, Stillman EA, Whitt MA, et al. Recombinant vesicular stomatitis viruses from DNA [J] Proc Natl Acad Sci USA. 1995;92(10):4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cureton DK, Massol RH, Saffarian S, et al. Vesicular stomatitis virus enters cells through vesicles incompletely coated with clathrin that depend upon actin for internalization [J] PLoS Pathog. 2009;5(4):e1000394. doi: 10.1371/journal.ppat.1000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johannsdottir HK, Mancini R, Kartenbeck J, et al. Host cell factors and functions involved in vesicular stomatitis virus entry [J] J Virol. 2009;83(1):440–453. doi: 10.1128/JVI.01864-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed M, Lyles DS. Effect of vesicular stomatitis virus matrix protein on transcription directed by host RNA polymerases I, II, and III [J] J Virol. 1998;72(10):8413–8419. doi: 10.1128/jvi.72.10.8413-8419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raux H, Obiang L, Richard N, et al. The matrix protein of vesicular stomatitis virus binds dynamin for efficient viral assembly [J] J Virol. 2010;84(24):12609–12618. doi: 10.1128/JVI.01400-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Connor JH, McKenzie MO, Lyles DS. Role of residues 121 to 124 of vesicular stomatitis virus matrix protein in virus assembly and virus-host interaction [J] J Virol. 2006;80(8):3701–3711. doi: 10.1128/JVI.80.8.3701-3711.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kopecky SA, Willingham MC, Lyles DS. Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus [J] J Virol. 2001;75(24):12169–12181. doi: 10.1128/JVI.75.24.12169-12181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flood EA, Lyles DS. Assembly of nucleocapsids with cytosolic and membrane-derived matrix proteins of vesicular stomatitis virus [J] Virology. 1999;261(2):295–308. doi: 10.1006/viro.1999.9856. [DOI] [PubMed] [Google Scholar]
- 43.Irie T, Licata JM, Jayakar HR, et al. Functional analysis of late-budding domain activity associated with the PSAP motif within the vesicular stomatitis virus M protein [J] J Virol. 2004;78(14):7823–7827. doi: 10.1128/JVI.78.14.7823-7827.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blondel D, Harmison GG, Schubert M. Role of matrix protein in cytopathogenesis of vesicular stomatitis virus [J] J Virol. 1990;64(4):1716–1725. doi: 10.1128/jvi.64.4.1716-1725.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jayakar HR, Murti KG, Whitt MA. Mutations in the PPPY motif of vesicular stomatitis virus matrix protein reduce virus budding by inhibiting a late step in virion release [J] J Virol. 2000;74(21):9818–9827. doi: 10.1128/jvi.74.21.9818-9827.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ge P, Tsao J, Schein S, et al. Cryo-EM model of the bullet-shaped vesicular stomatitis virus [J] Science. 2010;327(5966):689–693. doi: 10.1126/science.1181766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lyles DS, McKenzie MO, Kaptur PE, et al. Complementation of M gene mutants of vesicular stomatitis virus by plasmid- derived M protein converts spherical extracellular particles into native bullet shapes [J] Virology. 1996;217(1):76–87. doi: 10.1006/viro.1996.0095. [DOI] [PubMed] [Google Scholar]
- 48.Ahmed M, Marino TR, Puckett S, et al. Immune response in the absence of neurovirulence in mice infected with m protein mutant vesicular stomatitis virus [J] J Virol. 2008;82(18):9273–9277. doi: 10.1128/JVI.00915-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Renukaradhya GJ, Khan MA, Shaji D, Brutkiewicz RR. Vesicular stomatitis virus matrix protein impairs CD1d-mediated antigen presentation through activation of the p38 MAPK pathway [J] J Virol. 2008;82(24):12535–12542. doi: 10.1128/JVI.00881-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmed M, Puckett S, Lyles DS. Susceptibility of breast cancer cells to an oncolytic matrix (M) protein mutant of vesicular stomatitis virus [J] Cancer Gene Ther. 2010;17(12):883–892. doi: 10.1038/cgt.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Desforges M, Despars G, Bérard S, et al. Matrix protein mutations contribute to inefficient induction of apoptosis leading to persistent infection of human neural cells by vesicular stomatitis virus [J] Virology. 2002;295(1):63–73. doi: 10.1006/viro.2001.1329. [DOI] [PubMed] [Google Scholar]
- 52.Martinez I, Rodriguez LL, Jimenez C, et al. Vesicular stomatitis virus glycoprotein is a determinant of pathogenesis in swine, a natural host [J] J Virol. 2003;77(14):8039–8047. doi: 10.1128/JVI.77.14.8039-8047.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bi Z, Barna M, Komatsu T, et al. Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity [J] J Virol. 1995;69(10):6466–6672. doi: 10.1128/jvi.69.10.6466-6472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huneycutt BS, Plakhov IV, Shusterman Z, et al. Distribution of vesicular stomatitis virus proteins in the brains of BALB/c mice following intranasal inoculation: an immunohistochemical analysis [J] Brain Res. 1994;635(1–2):81–95. doi: 10.1016/0006-8993(94)91426-5. [DOI] [PubMed] [Google Scholar]
- 55.Huneycutt BS, Bi Z, Aoki CJ, et al. Central neuropathogenesis of vesicular stomatitis virus infection of immunodeficient mice [M] J Virol. 1993;67(11):6698–6706. doi: 10.1128/jvi.67.11.6698-6706.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Christian AY, Barna M, Bi Z, et al. Host immune response to vesicular stomatitis virus infection of the central nervous system in C57BL/6 mice [J] Viral Immunol. 1996;9(3):195–205. doi: 10.1089/vim.1996.9.195. [DOI] [PubMed] [Google Scholar]
- 57.Platanias LC, Fish EN. Signaling pathways activated by interferons [J] Exp Hematol. 1999;27(11):1583–1592. doi: 10.1016/s0301-472x(99)00109-5. [DOI] [PubMed] [Google Scholar]
- 58.Honda K, Yanai H, Takaoka A, et al. Regulation of the type I IFN induction: a current view [J] Int Immunol. 2005;17(11):1367–1178. doi: 10.1093/intimm/dxh318. [DOI] [PubMed] [Google Scholar]
- 59.Balachandran S, Roberts PC, Kipperman T, et al. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/Caspase-8 death signaling pathway [J] J Virol. 2000;74(3):1513–1523. doi: 10.1128/jvi.74.3.1513-1523.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balachandran S, Venkataraman T, Fisher PB, et al. Fas-associated death domain-containing protein-mediated antiviral innate immune signaling involves the regulation of Irf7 [J] J Immunol. 2007;178(4):2429–2439. doi: 10.4049/jimmunol.178.4.2429. [DOI] [PubMed] [Google Scholar]
- 61.Zou W, Kim JH, Handidu A, et al. Microarray analysis reveals that Type I interferon strongly increases the expression of immune-response related genes in Ubp43 (Usp18) deficient macrophages [J] Biochem Biophys Res Commun. 2007;356(1):193–199. doi: 10.1016/j.bbrc.2007.02.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Der SD, Zhou A, Williams BR, et al. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays [J] Proc Natl Acad Sci USA. 1998;95(26):15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith PL, Lombardi G, Foster GR. Type I interferons and the innate immune response—more than just antiviral cytokines [J] Mol Immunol. 2005;42(8):869–877. doi: 10.1016/j.molimm.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 64.Katze MG, He Y, Gale M., Jr Viruses and interferon: a fight for supremacy [J] Nat Rev Immunol. 2002;2(9):675–687. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- 65.Hecht TT, Paul WE. Limitation of VSV infection by the host's response to VSV-associated cellular antigens [J] J Immunol. 1982;129(4):1736–1741. [PubMed] [Google Scholar]
- 66.Lande R, Gilliet M. Plasmacytoid dendritic cells: key players in the initiation and regulation of immune responses [J] Ann N Y Acad Sci. 2010;1183:89–103. doi: 10.1111/j.1749-6632.2009.05152.x. [DOI] [PubMed] [Google Scholar]
- 67.Takaoka A, Yanai H. Interferon signalling network in innate defence [J] Cell Microbiol. 2006;8(6):907–922. doi: 10.1111/j.1462-5822.2006.00716.x. [DOI] [PubMed] [Google Scholar]
- 68.Baltzis D, Qu LK, Papadopoulou S, et al. Resistance to vesicular stomatitis virus infection requires a functional cross talk between the eukaryotic translation initiation factor 2alpha kinases PERK and PKR [J] J Virol. 2004;78(23):12747–12761. doi: 10.1128/JVI.78.23.12747-12761.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Obuchi M, Fernandez M, Barber GN. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity [J] J Virol. 2003;77(16):8843–8856. doi: 10.1128/JVI.77.16.8843-8856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Balachandran S, Barber GN. Defective translational control facilitates vesicular stomatitis virus oncolysis [J] Cancer Cell. 2004;5(1):51–65. doi: 10.1016/s1535-6108(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 71.Pfeifer I, Elsby R, Fernandez M, et al. NFAR-1 and -2 modulate translation and are required for efficient host defense [J] Proc Natl Acad Sci USA. 2008;105(11):4173–4178. doi: 10.1073/pnas.0711222105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harashima A, Guettouche T, Barber GN. Phosphorylation of the NFAR proteins by the dsRNA-dependent protein kinase PKR constitutes a novel mechanism of translational regulation and cellular defense [J] Genes Dev. 2010;24(23):2640–2653. doi: 10.1101/gad.1965010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stojdl DF, Abraham N, Knowles S, et al. The murine double-stranded RNA-dependent protein kinase PKR is required for resistance to vesicular stomatitis virus [J] J Virol. 2000;74(20):9580–9585. doi: 10.1128/jvi.74.20.9580-9585.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Detje CN, Meyer T, Schmidt H, et al. Local type I IFN receptor signaling protects against virus spread within the central nervous system [J] J Immunol. 2009;182(4):2297–2304. doi: 10.4049/jimmunol.0800596. [DOI] [PubMed] [Google Scholar]
- 75.Balachandran S, Thomas E, Barber GN. A FADD-dependent innate immune mechanism in mammalian cells [J] Nature. 2004;432(7015):401–405. doi: 10.1038/nature03124. [DOI] [PubMed] [Google Scholar]
- 76.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines [J] J Biol Chem. 2007;282(28):20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 77.Perkins DJ, Barber GN. Defects in translational regulation mediated by the alpha subunit of eukaryotic initiation factor 2 inhibit antiviral activity and facilitate the malignant transformation of human fibroblasts [J] Mol Cell Biol. 2004;24(5):2025–2040. doi: 10.1128/MCB.24.5.2025-2040.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thomsen AR, Nansen A, Andersen C, et al. Cooperation of B cells and T cells is required for survival of mice infected with vesicular stomatitis virus [J] Int Immunol. 1997;9(11):1757–1766. doi: 10.1093/intimm/9.11.1757. [DOI] [PubMed] [Google Scholar]
- 79.Kawai T, Akira S. TLR signaling [J] Semin Immunol. 2007;19(1):24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 80.Kawai T, Akira S. Toll-like receptor and RIG-l-like receptor signaling [J] Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 81.Kawai T, Akira S. Antiviral signaling through pattern recognition receptors [J] J Biochem. 2007;141(2):137–145. doi: 10.1093/jb/mvm032. [DOI] [PubMed] [Google Scholar]
- 82.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response [J] Nature. 2006;442(7098):39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 83.Barber GN, et al. Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses [J] Curr Opin Immunol. 2011;23(1):10–20. doi: 10.1016/j.coi.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hornung V, Ellegast J, Kim S, et al. 5′-Triphosphate RNA is the ligand for RIG-I [J] Science. 2006;314(5801):994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 85.Kato H, Takeuchi O, Sato S, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses [J] Nature. 2006;441(7089):101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 86.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity [J] Nature. 2009;461(7265):788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signaling [J] Nature. 2008;455(7213):674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ishikawa H, Barber GN. The STING pathway and regulation of innate immune signaling in response to DNA pathogens [J] Cell Mol Life Sci. 2011;68(7):1157–1165. doi: 10.1007/s00018-010-0605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ishii KJ, Kawagoe T, Koyama S, et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines [J] Nature. 2008;451(7179):725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- 90.Sharma S, tenOever BR, Grandvaux N, et al. Triggering the interferon antiviral response through an IKK-related pathway [J] Science. 2003;300(5622):1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 91.Lichty BD, Power AT, Stojdl DF, et al. Vesicular stomatitis virus: re-inventing the bullet [J] Trends Mol Med. 2004;10(5):210–216. doi: 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 92.Parato KA, Senger D, Forsyth PA, et al. Recent progress in the battle between oncolytic viruses and tumours [J] Nat Rev Cancer. 2005;5(12):965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 93.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells [J] Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Critchley-Thorne RJ, Simons DL, Yan N, et al. Impaired interferon signaling is a common immune defect in human cancer [J] Proc Natl Acad Sci USA. 2009;106(22):9010–9015. doi: 10.1073/pnas.0901329106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Balachandran S, Barber GN. Defective translational control facilitates vesicular stomatitis virus oncolysis [J] Cancer Cell. 2004;5(1):51–65. doi: 10.1016/s1535-6108(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 96.Barber GN. VSV-tumor selective replication and protein translation [J] Oncogene. 2005;24(52):7710–7719. doi: 10.1038/sj.onc.1209042. [DOI] [PubMed] [Google Scholar]
- 97.Mullighan CG, Zhang J, Harvey RC, et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia [J] Proc Natl Acad Sci USA. 2009;106(23):9414–9418. doi: 10.1073/pnas.0811761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Almeida S, Maillard C, Itin P, et al. Five new CYLD mutations in skin appendage tumors and evidence that aspartic acid 681 in CYLD is essential for deubiquitinase activity [J] J Invest Dermatol. 2008;128(3):587–593. doi: 10.1038/sj.jid.5701045. [DOI] [PubMed] [Google Scholar]
- 99.Critchley-Thorne RJ, Yan N, Nacu S, et al. Down-regulation of the interferon signaling pathway in T lymphocytes from patients with metastatic melanoma [J] PLoS Med. 2007;4:e176. doi: 10.1371/journal.pmed.0040176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nagel I, Bug S, Tönnies H, et al. Biallelic inactivation of TRAF3 in a subset of B-cell lymphomas with interstitial del (14) (q24.1q32.33) [J] Leukemia. 2009;23(11):2153–2155. doi: 10.1038/leu.2009.149. [DOI] [PubMed] [Google Scholar]
- 101.Petersen JM, Her LS, Varvel V, et al. The matrix protein of vesicular stomatitis virus inhibits nucleocytoplasmic transport when it is in the nucleus and associated with nuclear pore complexes [J] Mol Cell Biol. 2000;20(22):8590–8601. doi: 10.1128/mcb.20.22.8590-8601.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chakraborty P, Seemann J, Mishra RK, et al. Vesicular stomatitis virus inhibits mitotic progression and triggers cell death [J] EMBO Rep. 2009;10(10):1154–1160. doi: 10.1038/embor.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Balachandran S, Porosnicu M, Barber GN. Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or myc function and involves the induction of apoptosis [J] J Virol. 2001;75(7):3474–3479. doi: 10.1128/JVI.75.7.3474-3479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Diaz RM, Galivo F, Kottke T, et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus [J] Cancer Res. 2007;67(6):2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- 105.Ebert O, Shinozaki K, Huang TG, et al. Oncolytic vesicular stomatitis virus for treatment of orthotopic hepatocellular carcinoma in immune-competent rats [J] Cancer Res. 2003;63(13):3605–3611. [PubMed] [Google Scholar]
- 106.Ebert O, Harbaran S, Shinozaki K, et al. Systemic therapy of experimental breast cancer metastases by mutant vesicular stomatitis virus in immune-competent mice [J] Cancer Gene Ther. 2005;12(4):350–358. doi: 10.1038/sj.cgt.7700794. [DOI] [PubMed] [Google Scholar]
- 107.Shinozaki K, Ebert O, Woo SL. Eradication of advanced hepatocellular carcinoma in rats via repeated hepatic arterial infusions of recombinant VSV [J] Hepatology. 2005;41(1):196–203. doi: 10.1002/hep.20536. [DOI] [PubMed] [Google Scholar]
- 108.Hadaschik BA, Zhang K, So Al, et al. Oncolytic vesicular stomatitis viruses are potent agents for intravesical treatment of high-risk bladder cancer [J] Cancer Res. 2008;68(12):4506–4510. doi: 10.1158/0008-5472.CAN-08-0238. [DOI] [PubMed] [Google Scholar]
- 109.Lun X, Senger DL, Alain T, et al. Effects of intravenously administered recombinant vesicular stomatitis virus (VSV (deltaM51)) on multifocal and invasive gliomas [J] J Natl Cancer Inst. 2006;98(21):1546–1557. doi: 10.1093/jnci/djj413. [DOI] [PubMed] [Google Scholar]
- 110.Césaire R, Olière S, Sharif-Askari E, et al. Oncolytic activity of vesicular stomatitis virus in primary adult T-cell leukemia [J] Oncogene. 2006;25(3):349–358. doi: 10.1038/sj.onc.1209055. [DOI] [PubMed] [Google Scholar]
- 111.Lichty BD, Stojdl DF, Taylor RA, et al. Vesicular stomatitis virus: a potential therapeutic virus for the treatment of hematologic malignancy [J] Hum Gene Ther. 2004;15(9):821–831. doi: 10.1089/hum.2004.15.821. [DOI] [PubMed] [Google Scholar]
- 112.Huang TG, Ebert O, Shinozaki K, et al. Oncolysis of hepatic metastasis of colorectal cancer by recombinant vesicular stomatitis virus in immune-competent mice [J] Mol Ther. 2003;8(3):434–440. doi: 10.1016/s1525-0016(03)00204-1. [DOI] [PubMed] [Google Scholar]
- 113.Wongthida P, Diaz RM, Galivo F, et al. Type III IFN interleukin-28 mediates the antitumor efficacy of oncolytic virus VSV in immune-competent mouse models of cancer [J] Cancer Res. 2010;70(11):4539–4549. doi: 10.1158/0008-5472.CAN-09-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Capo-chichi CD, Yeasky TM, Heiber JF, et al. Explicit targeting of transformed cells by VSV in ovarian epithelial tumor-bearing Wv mouse models [J] Gynecol Oncol. 2010;116(2):269–275. doi: 10.1016/j.ygyno.2009.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang WL, Cai KQ, Smedberg JL, et al. A reduction of Cox-2 gene dosage counters the menopausal ovarian morphological aging and tumor phenotype in Wv mice [J] Am J Pathol. 2007;170(4):1325–1336. doi: 10.2353/ajpath.2007.060769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Murphy ED, et al. Hyperplastic and early neoplastic changes in the ovaries of mice after genic deletion of germ cells [J] J Natl Cancer Inst. 1972;48(5):1283–1295. [PubMed] [Google Scholar]
- 117.Breitbach CJ, De Silva NS, Falls TJ, et al. Targeting tumor vasculature with an oncolytic virus [J] Mol ther. 2011;19(5):886–894. doi: 10.1038/mt.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lun X, Chan J, Zhou H, et al. Efficacy and safety/toxicity study of recombinant vaccinia virus JX-594 in two immunocompetent animal models of glioma [J] Mol Ther. 2010;18(11):1927–1936. doi: 10.1038/mt.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wongthida P, Diaz RM, Galivo F, et al. Type III IFN interleukin-28 mediates the antitumor efficacy of oncolytic virus VSV in immune-competent mouse models of cancer [J] Cancer Res. 2010;70(11):4539–4549. doi: 10.1158/0008-5472.CAN-09-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wongthida P, Diaz RM, Galivo F, et al. VSV oncolytic virotherapy in the B16 model depends upon intact MyD88 signaling [J] Mol Ther. 2011;19(1):150–158. doi: 10.1038/mt.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bell JC, Lichty B, Stojdl D. Getting oncolytic virus therapies off the ground [J] Cancer Cell. 2003;4(1):7–11. doi: 10.1016/s1535-6108(03)00170-3. [DOI] [PubMed] [Google Scholar]
- 122.Porosnicu M, Mian A, Barber GN. The oncolytic effect of recombinant vesicular stomatitis virus is enhanced by expression of the fusion cytosine deaminase/uracil phosphoribosyltransferase suicide gene [J] Cancer Res. 2003;63(23):8366–8376. [PubMed] [Google Scholar]
- 123.Willmon CL, Saloura V, Fridlender ZG, et al. Expression of IFN-beta enhances both efficacy and safety of oncolytic vesicular stomatitis virus for therapy of mesothelioma [J] Cancer Res. 2009;69(9):7713–7720. doi: 10.1158/0008-5472.CAN-09-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Saloura V, Wang LC, Fridlender ZG, et al. Evaluation of an attenuated vesicular stomatitis virus vector expressing interferon-beta for use in malignant pleural mesothelioma: heterogeneity in interferon responsiveness defines potential efficacy [J] Hum Gene Ther. 2010;21(1):51–64. doi: 10.1089/hum.2009.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu L, Huang TG, Meseck M, et al. rVSV(M Delta 51)-M3 is an effective and safe oncolytic virus for cancer therapy [J] Hum Gene Ther. 2008;19(6):635–647. doi: 10.1089/hum.2007.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Edge RE, Falls TJ, Brown CW, et al. A let-7 MicroRNA-sensitive vesicular stomatitis virus demonstrates tumor-specific replication [J] Mol Ther. 2008;16(8):1437–1443. doi: 10.1038/mt.2008.130. [DOI] [PubMed] [Google Scholar]
- 127.Kelly EJ, Nace R, Barber GN, et al. Attenuation of vesicular stomatitis virus encephalitis through microRNA targeting [J] J Virol. 2010;84(3):1550–1562. doi: 10.1128/JVI.01788-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jenks N, Myers R, Greiner SM, et al. Safety studies on intrahepatic or intratumoral injection of oncolytic vesicular stomatitis virus expressing interferon-beta in rodents and nonhuman primates [J] Hum Gene Ther. 2010;21(4):451–462. doi: 10.1089/hum.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]