Abstract
Oncolytic herpes simplex virus (HSV) can replicate in and kill cancer cells without harming normal tissue. G47Δ is a third-generation HSV vector. In this study, the therapeutic effects of G47Δ on human nasopharyngeal carcinoma (NPC) were determined in vitro and in vivo. The human NPC cell lines CNE-2 and SUNE-1, primary normal nasopharyngeal epithelial cells (NPECs), and immortalized nasopharyngeal cells NP-69 and NPEC2/Bmi1 were infected with G47Δ at different multiplicities of infection (MOIs). The survival of infected cells was observed daily. Two subcutaneous models of NPC were established with CNE-2 and SUNE-1 in Balb/c nude mice. G47Δ or virus buffer as control was injected into the subcutaneous tumors. Tumor size was measured twice a week, and animals were euthanized when the diameter of their tumors exceeded 18 mm or when the animals appeared moribund. For the NPC cell lines CNE-2 and SUNE-1, more than 85% and 95% of cells were killed on day 5 after G47Δ infection at MOI = 0.01 and MOI = 0.1, respectively. Similar results were observed for an immortalized cell line NPEC2/Bmi-1. A moderate effect of G47Δ was also found on another immortalized cell line NP-69, of which only 27.7% and 75.9% of cells were killed at MOI = 0.01 and MOI = 0.1, respectively. On the contrary, there was almost no effect observed on NPECs. The in vivo experiments showed that tumors in mice in the G47Δ-treated group regressed completely, and the mice exhibited much longer survival time than those in the control groups. Our results suggest that the potential therapeutic effects of G47Δ would be applicable for treatment of NPC patients in the future.
Keywords: Nasopharyngeal carcinoma, primary culture, oncolytic herpes simplex virus, cytotoxicity, subcutaneous models
Nasopharyngeal carcinoma (NPC) is a unique malignant head and neck cancer that has been recognized for over 100 years. It arises from the mucosal epithelium lining the upper part of the pharynx behind the nasal cavities. NPC is one of the most poorly understood and commonly misdiagnosed malignancies[1]. Rarely reported in the West, NPC occurs at high frequency in southern China and Southeast Asia, where the annual incidence is more than 20 cases per 100 000 individuals[1],[2]. Its frequency is very high in the Guangzhou area, where the annual incidence reaches 25 cases per 100 000[3]. The reason for this geographic distribution remains unclear. Possible reasons are dietary factors (consumption of traditional preserved food), strong association with the Epstein-Barr virus (EBV), and genetic predisposition[3],[4]. The 5-year overall survival rates of NPC were 95% to 70%, 83% to 65%, 76% to 54%, and 56% to 29% for patients with stages I, II, III, and IV tumor, respectively, according to the data of the American Joint Committee on Cancer (AJCC) staging system[5]–[7].
Because the nasopharynx is surrounded by critical organs, it is difficult to surgically excise nasopharyngeal tumors. Surgery is usually offered only when there is evidence of local recurrence or persistent disease. NPC is highly radiosensitive. Currently available therapeutic approaches for NPC are either radiotherapy alone or chemoradiotherapy, which combines the effectiveness of chemotherapy on distant metastases and radiotherapy on local disease[8]. Patients with an early stage disease have a high cure rate after radiotherapy[8]. Chemoradiotherapy has been shown to improve local control, disease-free survival, and overall survival rates for patients with primary stage NPC[9]–[13]. However, the prognosis remains poor in a significant number of patients with late stage diseases[14]–[17].
Although NPC is more radiosensitive compared with other head and neck carcinomas, the high frequency of local recurrences and distant metastasis resulted in the 5-year overall survival rate from 32% to 52%[13]. A high rate of treatment failure is observed in patients with advanced NPC. Most NPC patients have advanced disease at the time of diagnosis because of the insidious clinical symptoms, difficulty of examination, and relatively poorly accessible anatomic location of the nasopharynx. In addition, long-term side effects of radiotherapy and chemotherapy are obvious and include secondary malignancies[18]. Thus, there is an intense need for specific therapies that effectively target NPC itself rather than those associated with the damage to normal tissue.
When evaluating novel therapeutic strategies for NPC, the two most highly desirable features are (1) the ability to destroy the tumor without harming normal tissue and (2) the ability to activate the antitumor immune responses to seek and kill covert tumors. Oncolytic HSV, which is designed to be inherently cytotoxic to tumor cells, seems to meet these criteria. The spread of the vector is confined to tumor cells and spares normal cells, leading to minimal toxicity to normal tissue[19]. Spread of the vector can also trigger a tumor-specific immune response. The self-perpetuating nature of oncolytic HSV allows a small amount of input virus to amplify after several cycles of replication in situ until the target cells are destroyed. Therapeutic transgenes can also be transferred to the tumor. As these viruses kill tumor cells mainly by oncolysis, they do not have cross-resistance with other therapeutic strategies such as radiotherapy and chemotherapy. Therefore, it is practical to combine viral therapy with other therapeutic approaches for enhanced effectiveness.
Since the first report of the successful treatment of a brain tumor model by an oncolytic HSV vector in 1991[20], researchers have focused on the development of increasingly efficacious and safe oncolytic HSV vectors. A variety of oncolytic HSV vectors have been developed, and several among these (e.g., G207, 1716, NV1020, and OncoVex GM-CSF) have safely completed phase I clinical trials involving patients with various solid tumors[21]–[26].
G47Δ, the vector used in this study, is a third-generation oncolytic HSV vector. It is a multigene mutant of HSV-1 that contains three main mutations (γ34.5, ICP6, and α47), which results in its selective cytotoxicity to tumor cells. The major determinant of neurovirulence is γ34.5[27]. G47Δ also precludes the shut-off of host protein synthesis in host cells[28], which guarantees the confined replication of the virus only in tumor cells. The ICP6(UL39) gene encodes a subunit of viral ribonucleotide reductase, which is the key enzyme for DNA synthesis in nondividing cells[29],[30]. Without this enzyme, DNA replication is blocked in normal cells[31]. Insertion of the Escherichia coli lacZ gene inactivates the ICP6 gene. Notably, mutation of ICP6 makes the virus more sensitive to the drugs acyclovir and ganciclovir[32], which augments the safety of the virus as a clinical therapy. Although the mutations of γ34.5 and ICP6 confer significant safety attributes[30], they attenuate viral growth. Mutation of α47 can enhance antitumor activities because it places the late US11 gene under control of the immediate-early α47 promoter, which induces the amplification of γ34.5-mutant growth. Furthermore, the α47 gene binds to the antigen presentation transporter (TAP) protein and blocks peptide loading of major histocompatibility complex (MHC) class I molecules[33]. This deletion of α47 increases MHC class I presentation, thus enhancing stimulation of lymphocytes and decreasing NK cytolysis of host cells[34],[35]. These important features of oncolytic HSV G47Δ enhanced its antitumor immune responses when treating advanced tumors, especially those with few treatment options.
In the present study, we evaluated the cytotoxic effects of G47Δ on two human NPC cell lines, two immortalized human nasopharyngeal cell lines, and one primary cultured normal nasopharyngeal cell line. Furthermore, we evaluated the therapeutic effects of G47Δ in xenograft models of two human NPC cell lines.
Materials and Methods
Cells and viruses
Two poorly differentiated NPC cell lines, CNE-2 and SUNE-1, were cultured in RPMI-1640 with 4.5 g/L glucose (Mediatech, Inc., Herndon, VA) supplemented with 10% fetal calf serum (Hyclone Laboratories, Logan, UT) at 37°C in an atmosphere of 5% CO2.
Immortalized nasopharyngeal cell lines NP-69 (obtained from Dr. George SW Tsao, Cancer Center, Hong Kong University, Hong Kong) and NPEC2/Bmi1, as well as primary NPECs were cultured in keratinocyte/serum-free medium (Invitrogen, Grand Island, NY) at 37°C in an atmosphere of 5% CO2[36]. African green monkey kidney Vero cells (purchased from the Committee on Type Culture Collection of Chinese Academy of Sciences, Shanghai, China) were cultured in Dulbecco's modified Eagle medium (DMEM) with 4.5 g/L glucose (Mediatech, Inc.) supplemented with 10% fetal calf serum (Hyclone Laboratories, Logan, UT) at 37°C in an atmosphere of 5% CO2.
Oncolytic HSV G47Δ was provided by MediGene, Inc. (San Diego, CA). Viruses were multiplied in monolayer cultures of Vero cells in DMEM containing 3% inactivated fetal calf serum at 34.5°C. First, G47Δ was diluted in phosphate-buffered saline (PBS) containing 1 % inactivated fetal calf serum. Vero cells were then infected at multiplicities of infection (MOIs) of 0.02 to 0.03 and incubated at 37°C in an atmosphere of 5% CO2 for 90 min. Viral inoculums were removed and DMEM containing 3% inactivated fetal calf serum was added. The cells were placed in a 34.5°C incubator with an atmosphere of 5% CO2 for 2 to 3 days until most cells became rounded and refractile. The infected cells were harvested and resuspended in a 1:1 mix of DMEM (no serum) and virus buffer (150 mmol/L NaCl/20 mmol/L Tris at pH 7.5). The cell suspension was rapid-frozen in ethanol/dry ice and thawed 3 times to lyse cells and release viruses. The cell debris was removed by low-speed centrifugation (2000 × g for 10 min at 4°C). G47Δ was titrated into Vero cells by plaque assay.
In vitro cytotoxicity
All cell types were seeded separately into 6-well plates at a density of 1 × 105 cells per well. When the 6-well plates were 50% covered, the cells were infected with G47Δ at MOIs of 0.01 and 0.1, whereas controls were mock-infected with PBS. After 24 h of incubation at 37°C, the number of surviving cells was counted with a hemocytometer daily for 5 days. Average numbers of cells from duplicate wells are plotted as percentages of the mock wells. To eliminate floating cells before counting, the plates were washed with 1 mL of PBS twice before adding trypsin. X-gal staining was performed daily to visualize the infected cells.
X-gal histochemistry
On days 1 to 5 post-infection, the medium was removed and the cells were fixed with 0.2% glutaraldehyde/2% paraformaldehyde for 5 min. They were then incubated with X-gal substrate solution (PBS at pH 7.2 containing 0.5 mg/mL 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside, 5 mmol/L potassium ferricyanide, 5 mmol/L potassium ferrocyanide, and 2 mmol/L magnesium chloride) at 37°C for 2 h. The cells showing blue staining were considered X-gal positive cells.
Animal studies
Four-week-old female Balb/c nude mice were purchased from the Shanghai Institutes for Biological Sciences (Chinese Academy of Sciences, Shanghai, China) and bred with 5 mice in each cage. All animal procedures were approved by the Animal Care and Use and Institute Research Ethics Committees of Sun Yat-sen University. The mice were monitored daily for palpable tumor formation, and tumors were measured by a Vernier caliper (length and width were represented by a and b, respectively) and the mice were weighed twice a week.
Subcutaneous tumor treatment
Poorly differentiated NPC CNE-2 cells (1 × 106) and SUNE-1 cells (5 × 105) were suspended in 100 µL of RPMI-1640 complete culture with 25% Matrigel (BD Biosciences) and implanted subcutaneously into the left flanks of 4-week-old nude mice. When the maximal diameter of the subcutaneous tumors was approximately 5 mm, the tumor were injected twice a week for 2 weeks with 2 × 107 pfu/50 µL of G47Δ or virus buffer (150 mmol/L NaCl and 20 mmol/L Tris at pH 7.5) as control. Tumor size was measured by Vernier calipers, and tumor volume was calculated with the formula V = a × b2/2, where V represents tumor volume and a and b represent length and width, respectively. Animals were euthanized when the maximal diameter of their tumors exceeded 18 mm or when the animals appeared moribund as characterized by a rough hair coat, lethargy, hunched posture, recumbent posture, or limited ambulatory movements in response to stimulation; this was recorded as the end point for survival studies. For histological hematoxylin and eosin staining, the excised subcutaneous tumors were fixed in formaldehyde and embedded in paraffin.
Virus biodistribution studies
CNE-2 and SUNE-1 subcutaneous NPC model mice were treated 4 times with 2 × 107 pfu/50 µL of G47Δ. The subcutaneous tumors were excised and embedded with opti-mum cutting temperature (OCT) compound. Cryostat sections (10 µm thick) were cut and fixed with 2% paraformaldehyde in PBS for 10 min and then washed thoroughly with PBS 3 times. Following incubation with PBS containing 2 mmol/L magnesium chloride, 0.01% sodium deoxycholate, and 0.02% NP40 for 10 min at 4°C, the sections were stained with X-gal substrate solution (PBS at pH 7.2 containing 1 mg/mL 5-bromo-4-chloro-3-indolyl-h-D-galactopyranoside, 5 mmol/L potassium ferricyanide, 5 mmol/L potassium ferrocyanide, 2 mmol/L magnesium chloride, 0.01% sodium deoxycholate, and 0.02% NP40) at 34°C for 4 h. Sections were washed with PBS containing 2 mmol/L EDTA and counterstained with eosin before mounting.
Statistical analyses
Statistical analyses were performed using SPSS 13.0. Differences among variables were assessed by the log-rank test. A P value less than 0.05 was considered significant.
Results
In vitro cytotoxicity
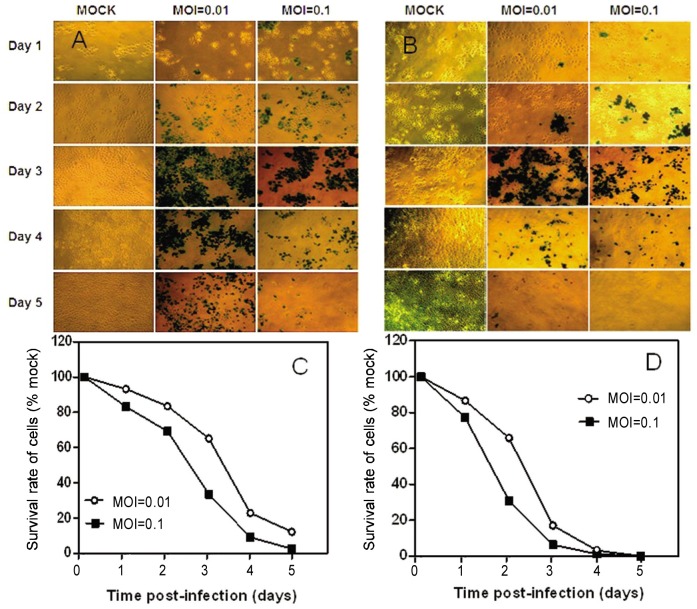
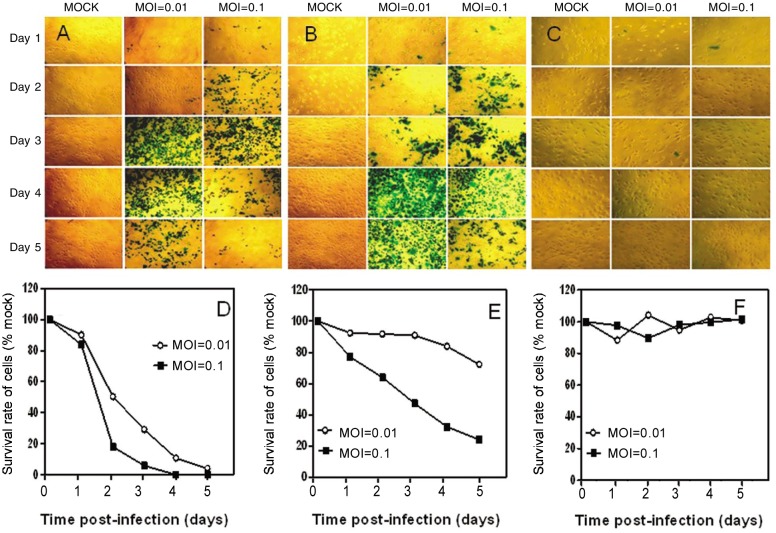
To assess the cytotoxicity of oncolytic HSV vector G47Δ in vitro, monolayers of human NPC cell lines CNE-2 and SUNE-1, immortalized nasopharyngeal cell lines NP-69 and NPEC2/Bmi1, and NPECs were infected with G47Δ at low MOIs (MOI = 0.01 and MOI = 0.1, Figure 1). For the NPC cell line CNE-2, more than 85% and 95% of cells were killed by G47Δ at MOI = 0.01 and MOI = 0.1, respectively, on day 5 after infection (Figures 1A and 1C), and 100% of SUNE-1 cells in the MOI = 0.01 and MOI =0.1 groups were killed on day 5 (Figures 1B and 1D). For the immortalized nasopharyngeal cell line NPEC2/Bmi1, an effect similar to that in the NPC cell lines was observed; more than 95% and 100% of cells were killed by G47Δ at MOI = 0.01 and MOI = 0.1, respectively, on day 5 after infection (Figure 2). G47Δ had a moderate effect in NP-69 cells, killing only 27.7% and 75.9% of cells at MOI = 0.01 and MOI = 0.1, respectively, on day 5 (Figure 2E). On the contrary, NPECs were viable and intact even 5 days after infection (Figure 2F). Because G47Δ contains the lacZ transgene in the ICP6 region, infected cells could be histochemically stained by X-gal. The infected cells and the spread of G47Δ were illustrated by blue staining (Figures 1A and 2B, and 2A-C).
Figure 1. Cytotoxicity of G47Δ in two human NPC cell lines in vitro.
A, X-gal staining of CNE-2 cells infected with G47Δ. Monolayers of CNE-2 cells in 6-well dishes were infected with G47Δ or mock infected and incubated with DMEM containing 1% heat-inactivated FBS at 37°C. On the following 5 days after infection, the cells were stained with X-gal solution. Infected cells (expressing lacZ) were stained with X-gal (blue). B, X-gal staining of SUNE-1 cells infected with G47Δ. Monolayers of CNE-2 cells (C) and SUNE-1 cells (D) in 6-well plates were infected with G47Δ (MOI = 0.01 and MOI = 0.1), incubated in DMEM containing 1% heat-inactivated FBS at 37°C, and counted on a hemocytometer on the days indicated. Average numbers of cells from duplicate wells are plotted as percentages of the mock-infected cells.
Figure 2. Cytotoxicity of G47Δ in two human immortalized nasopharyngeal cell lines and primary cultured NPEC in vitro.
A, monolayers of NPEC2/Bmi1 cells in 6-well dishes were infected with G47Δ or mock infected and incubated with DMEM containing 1% heat-inactivated FBS at 37°C. On the following 5 days after infection, the cells were stained with X-gal solution. Infected cells (expressing lacZ) were stained with X-gal (blue). B, X-gal staining of NP-69 cells infected with G47Δ. C, X-gal staining of NPECs infected with G47Δ. Monolayers of NPEC2/Bmi1 (D), NP-69 (E), and NPECs (F) in 6-well plates were infected with G47Δ (MOI = 0.01 and MOI = 0.1), incubated in DMEM containing 1% heat-inactivated FBS at 37°C, and counted on a Coulter counter on the days indicated. The average numbers of cells from duplicate wells are plotted as percentage of the mock-infected cells.
Anti-tumor effects of G47Δ in NPC xenografts
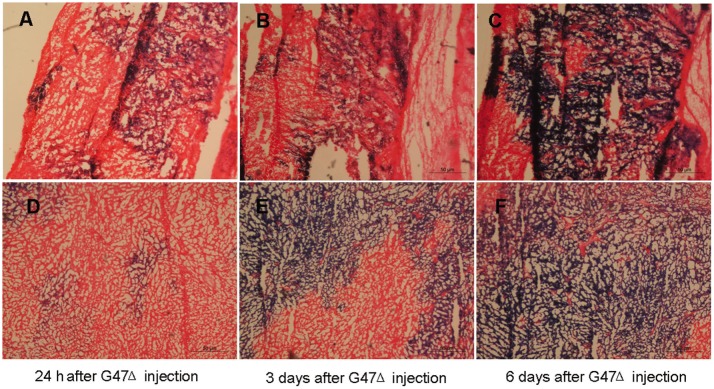
For the in vivo studies, CNE-2 and SUNE-1 cells were implanted in the left flanks of female Balb/c nude mice. The tumors were then injected with G47Δ (2 × 107 pfu, 4 times) when they became palpable (approximately 5 mm in diameter). To evaluate G47Δ replication in vivo, G47Δ-treated tumor-bearing mice were euthanized, and X-gal histochemistry were performed on sectioned subcutaneous tumors to detect G47Δ-infected cells. The blue staining represented the replication of G47Δ. Coronal sections through a subcutaneous tumor of a nude mouse 24 h (Figures 3A and 3D), 3 days (Figures 3B and 3E), and 6 days (Figures 3C and 3F) after G47Δ injection illustrate G47Δ replication in the subcutaneous tumors.
Figure 3. Virus biodistribution in the subcutaneous tumors.
A-C, coronal sections of CNE-2 subcutaneous tumor in a nude mouse 24 h, 3 days, and 6 days after G47Δ (2 × 107 pfu) injection illustrate the G47Δ replication, respectively. Sections were stained with X-gal solution to identify cells containing replicating G47Δ (blue) and counterstained with eosin. D–F, coronal section of SUNE-1 subcutaneous tumor in a nude mouse 24 h, 3 days, and 6 days after G47Δ (2 × 107 pfu) injection, respectively.
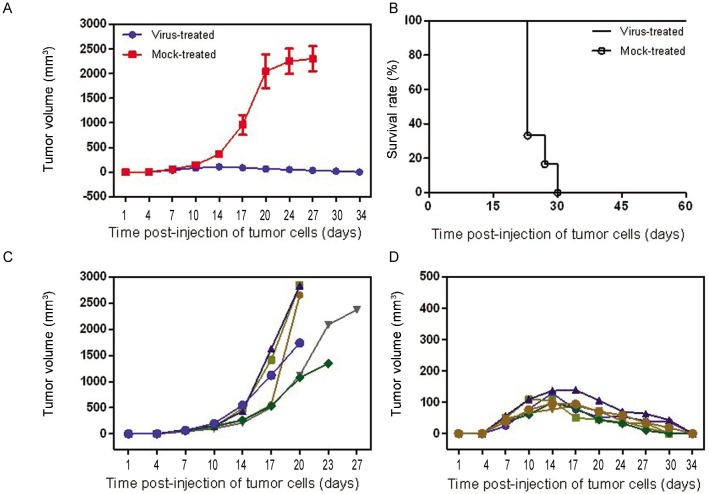
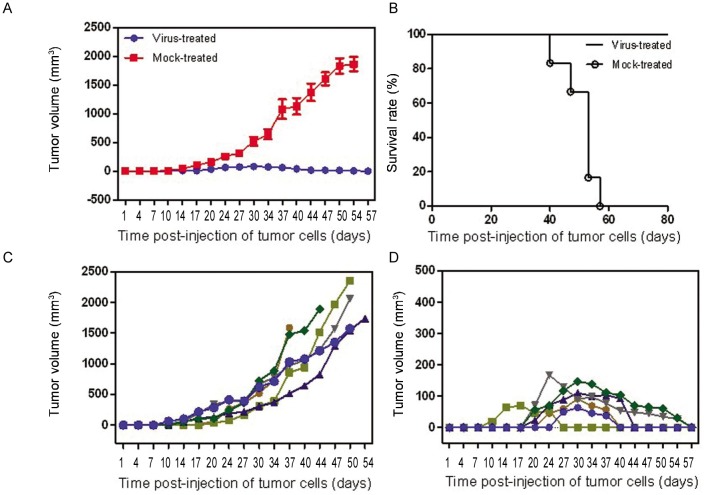
The subcutaneous tumor volume increased obviously within a few days in the mock-treated group, whereas in the G47Δ -treated group, the tumor regressed gradually or even disappeared in both the CNE-2 (Figures 4A, 4C, 4E, and 4G ) and SUNE-1 (Figures 4B, 4D, 4F, and 4H) groups. The mice in the G47Δ -treated CNE-2 or SUNE-1 groups did not appear moribund, so they were euthanized on days 60 and 80, respectively; however, the median survival times of mock-treated mice were 20 (χ2 = 11.74, P < 0.001, log-rank test) and 50 days (χ2 = 10.74, P < 0.001, log-rank test), respectively (Figures 5 and 6). The difference of CNE-2 tumor volume between the mock-treated groups and the virus-treated groups was obvious with the elapse of time (Figure 5). The difference of SUNE-1 tumor volume between the mock-treated groups and the virus-treated groups was obvious with the elapse of time (Figure 6).
Figure 4. Anti-tumor effect of G47Δ in NPC xenografts in mice.
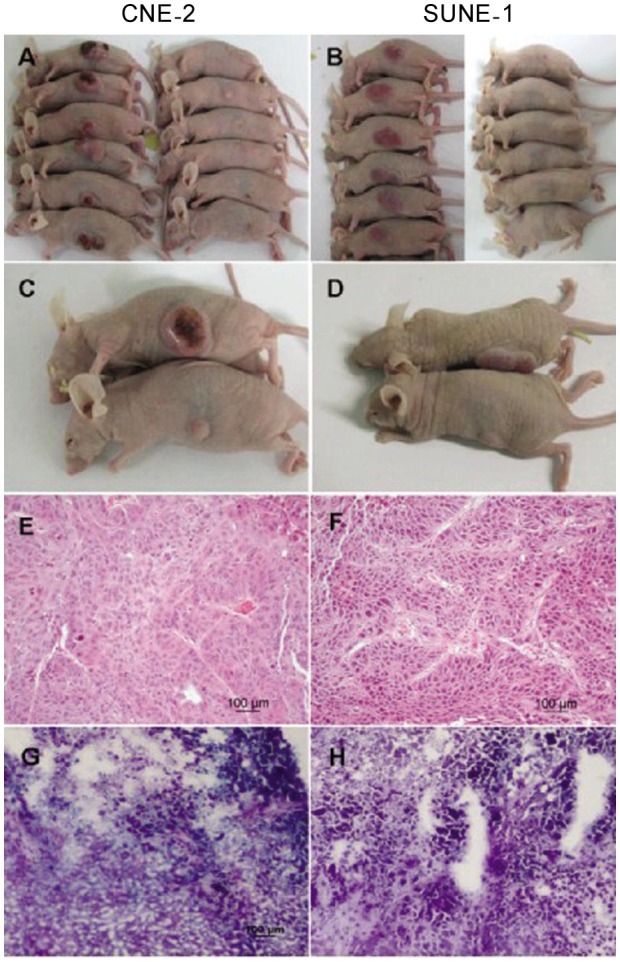
CNE-2 cells (1 × 106) and SUNE-1 cells (5 × 105) were suspended in 100 mL RPMI-1640 complete medium with 25% Matrigel (BD Biosciences) and implanted subcutaneous into the left flanks of 4-week-old nude mice. When the maximal diameter of the subcutaneous tumors was approximately 5 mm, G47Δ (2 × 107 pfu /50 µL) or virus buffer (150 mmol/L NaCl and 20 mmol/L Tris at pH7.5) was injected into the subcutaneous tumors twice a week for 2 weeks. A, the subcutaneous CNE-2 tumor regressed by treatment with G47Δ on day 34 or appeared as large tumors after the mock treatment on day 20 post-injection of tumor cells. B, the subcutaneous SUNE-1 tumor regressed by treatment with G47Δ or appeared as large tumors after the mock treatment on day 37 post-injection of tumor cells. Comparison of mice with subcutaneous CNE-2 tumors (C) or subcutaneous SUNE-1 tumors (D) in the mock-treated group (upper) and the virus-treated group (lower) on day 20 or day 37 post-injection of tumor cells. Hematoxylin and eosin (HE) staining of CNE-2 (E), SUNE-1 (F), G47Δ-treated CNE-2 (G), and G47Δ-treated SUNE-1 (H) subcutaneous tumors (×200).
Figure 5. Features of subcutaneous CNE-2 tumors treated with G47Δ.
Tumor size was measured by Vernier calipers, and tumor volume was calculated with the formula V = a ×b2/2, where a and b are tumor length and width, respectively. Animals were euthanized when the maximal diameter of their tumors exceeded 18 mm or when the animals appeared moribund; this was recorded as the end point for survival studies. G47Δ effectively inhibited tumor growth, and the tumors in the treated group completely regressed. A, the mean tumor volumes in the virus-treated group and mock-treated group at different time points. B, median survival was increased from 20 days in the mock-treated group to 60 days in the G47Δ-treated group on different days post-injection of tumor cells. C, growth of the subcutaneous tumors (tumor volume) of each mouse in the mock-treated group. D, growth of the subcutaneous tumors (tumor volume) of each mouse in the virus-treated group on different days post-injection of tumor cells.
Figure 6. Features of subcutaneous. SLJNE-1 tumors treated with G47Δ.
Tumor size was measured by Vernier calipers, and tumor volume was calculated with the formula V = a × b2/2, where a and b are tumor length and width, respectively. Animals were euthanized when the maximal diameter of their tumors exceeded 18 mm or when the animals appeared moribund; this was recorded as the end point for survival studies. G47Δ effectively inhibited tumor growth, and the tumors in the treated group completely regressed. A, the mean tumor volumes in the virus-treated group and mock-treated group at different time points. B, median survival was increased from 50 days in the mock-treated group to 80 days in the G47Δ-treated group. C, growth of the subcutaneous tumors (tumor volume) of each mouse in the mock-treated group on different days post-injection of tumor cells. D, growth of the subcutaneous tumors (tumor volume) of each mouse in the virus-treated group on different days post-injection of tumor cells.
Discussion
In this study, we investigated the cytotoxic effects of the third-generation oncolytic HSV G47Δ on normal NPECs, immortalized nasopharyngeal cells, and NPC cells. G47Δ effectively killed human NPC cells and immortalized nasopharyngeal cells but not normal nasopharyngeal cells in vitro. We then demonstrated its therapeutic value in the treatment of NPC through animal experimentation. Intratumor injection of G47Δ induced an obvious therapeutic effect and effectively prolonged the survival of treated mice bearing NPC tumors.
NPC is one of the most highly prevalent and harmful malignant head and neck tumors. It occurs sporadically in most Western countries but has a high incidence in southern China, Hong Kong, Taiwan, Singapore, and Malaysia[37]. The worldwide incidence of NPC exceeds 80 000 cases per year[38].
The available therapeutic options for NPC include surgery, brachytherapy, radiosurgery, stereotactic RT, and IMRT, each with or without cisplatin-based chemotherapy. NPC is relatively radiosensitive and occurs in an anatomic location that is poorly accessible to surgeons. Therefore, high-dose radiotherapy combined with chemotherapy has long been the mainstay of therapy modalities and is a primary component of curative-intent treatment for nondisseminated NPC[9],[39]. The standard medicine used in chemotherapy-radiotherapy is cisplatin, which, when used in the combined treatment approach, procides a benefit for overall survival and improves both local and distant control. However, the survival advantages of cisplatin-based chemotherapy alone has not been verified for NPC[39]. Patients presenting an early stage NPC have a high cure rate after radiotherapy. Unfortunately, NPC patients tend to present advanced stage diseases with highly metastatic potential because the anatomic site of the tumor is cryptic and the disease is usually asymptomatic at early stages.
Despite advances in radiotherapy technology, patients with NPC have not experienced any fundamental improvements in survival rate or prognosis, thus prompting the development of novel therapeutic strategies. An ideal therapeutic modality should have both good tumor specificity and high killing efficacy with well-proven safety. Recent studies indicate that engineered oncolytic viruses represent a new modality for malignant tumor treatment. Indeed, these oncolytic viruses have shown highly promising results in clinical trials[40]. Therefore, progress in this field is gaining momentum.
Since the 1990s, oncolytic viruses have been utilized to treat patients with malignant carcinoma from phase I to phase III trials. Several kinds of oncolytic viruses were used for the treatment of NPC. These include VSVΔ 51 (vesicular stomatitis virus), which can be further augmented when combined with ionizing radiation[41], and H101 (adenovirus), which has already finished clinical trials and been formally approved by the State Food and Drug Administration (SFDA) [42]–[45]. However, there are few reports about the utilization of oncolytic HSV in the treatment of human NPC.
HSV-1 is an enveloped, double-stranded DNA virus with a genome size of approximately 152 kb. HSV-1 vectors target actively dividing tumor cells whereas sparing adjacent normal cells, making them a possible therapy for selective tumor destruction. The infected tumor cells are destroyed by the replication of oncolytic HSV-1, which releases many progeny virions. Viral amplification and lateral transmission result in further destruction of surrounding tumor cells until tumor cells are totally destroyed. Because this kind of virus kills tumor cells by oncolysis, it does not have cross-resistance with other therapeutic options such as radiotherapy and chemotherapy and thus can serve as a complementary approach to these therapeutic modalities.
NPC is typically heterogenous, surgically inaccessible, and resistant to some single agent chemotherapies. Although radiotherapy is effective to some extent, there is evidence of radiotherapy treatment failure, and radiotherapy-related injury and oncogenicity are common. Therefore, no single therapy is completely effective for NPC treatment. Nevertheless, oncolytic HSV-1 vectors are different from routine treatment modalities like chemotherapy and radiotherapy and thus provide an additional approach for treatment.
Unlike chemotherapy and radiotherapy, oncolytic HSV-1 is not limited by tumor stage or patient health status. Furthermore, the viral genes affecting neuropathogenicity are knocked down in HSV-1, reducing the potential for severe illness. In addition, other antiviral agents, such as acyclovir and ganciclovir, can effectively shut off viral replication, so this kind of therapy approach will not lead to high-dose toxicities. These features suggest wide application and bright prospects of oncolytic HSV in the treatment of NPC. The antitumor effect of G47Δ has been verified in several kinds of malignant tumors, including glioblastoma[46], schwannoma tumor [47], prostate cancer[48], and breast cancer[50]. Recently, G47Δ has entered phase I clinical trial for progressive glioblastoma.
Because of their anatomic location and proximity to crucial adjacent organs, NPCs require a highly selective antitumor therapy. Thus, it was very important to show that G47Δ confines replication to dividing cells without damaging normal cells. Therefore, we established primary cultured NPECs to test their affinity to G47Δ. Our results showed that the normal NPECs were intact even 5 days post-G47Δ infection, which indicated the safety of G47Δ. However, the cytotoxic effects in the NPC cell lines CNE-2 and SUNE-1 were obviously observed. This data suggest that G47Δ is a safe and valid as a novel biological therapy.
Immortalization and bypass of senescence are considered early steps in tumor development. In this study, we also observed that G47Δ had cytotoxicity effects at a very low MOI in two different immortalized NPEC lines, indicating that G47Δ may function effectively in the early stages of tumorigenesis. Because the injection of G47Δ does not pose any potential health hazard, G47Δ could probably be used as a preventive agent against pre-malignant diseases in patients with a high risk of NPC.
To investigate the in vivo antitumor effect of G47Δ on NPC, we established a subcutaneous model of NPC using two different cell lines, CNE-2 and SUNE-1. G47Δ was administered twice a week without any observable toxicity in the animals. In the virus-treated group, a higher survival rate and significant reduction in tumor growth were noted after therapy. The subcutaneous tumors of all treated mice gradually regressed in both the CNE-2 and SUNE-1 groups, suggesting that G47Δ has potential as a treatment to effectively inhibit different types of NPC tumors.
Although we observed cytotoxicity effects of G47Δ on immortalized NPEC lines, we have yet to explore the underlying mechanism of these effects. In addition, the limited types of NPC cell lines used in this study may have encumbered the stringency of the effectivity for G47Δ in the treatment of NPC. Thus, to fully test the antitumor effects of G47Δ, another clinical study with a larger cohort of samples should be performed.
In conclusion, oncolytic HSV vector G47Δ was effective in both immortalized nasopharyngeal cells and NPC cells but spared normal NPECs. This feature of restricted replication gives it potential as both a therapeutic and preventive agent for human NPC. Direct intratumoral inoculation of G47Δ induced an obvious therapeutic effect on NPC, suggesting G47Δ may be useful in future clinical applications in cancer therapy.
Acknowledgments
This study was supported by grants from the National Science Foundation of China (30672410) and the Guangdong Natural Science Foundation (06104599).
References
- 1.Cho WC. Nasopharyngeal carcinoma: molecular biomarker discovery and progress [J] Mol Cancer. 2007;6:1. doi: 10.1186/1476-4598-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marks JE, Phillips JL, Menck HR. The national cancer data base report on the relationship of race and national origin to the histology of nasopharyngeal carcinoma [J] Cancer. 1998;83(3):582–588. doi: 10.1002/(sici)1097-0142(19980801)83:3<582::aid-cncr29>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 3.Busson P, Keryer C, Ooka T, et al. EBV-associated nasopharyngeal carcinomas: from epidemiology to virus-targeting strategies [J] Trends Microbiol. 2004;12(8):356–360. doi: 10.1016/j.tim.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Lo KW, To KF, Huang DP. Focus on nasopharyngeal carcinoma [J] Cancer Cell. 2004;5(5):423–428. doi: 10.1016/s1535-6108(04)00119-9. [DOI] [PubMed] [Google Scholar]
- 5.Chua DT, Sham JS, Kwong DL, et al. Prognostic value of paranasopharyngeal extension of nasopharyngeal carcinoma. A significant factor in local control and distant metastasis [J] Cancer. 1996;78(2):202–210. doi: 10.1002/(SICI)1097-0142(19960715)78:2<202::AID-CNCR3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 6.Cooper JS, Cohen R, Stevens RE. A comparison of staging systems for nasopharyngeal carcinoma [J] Cancer. 1998;83(2):213–219. [PubMed] [Google Scholar]
- 7.Ozyar E, Yildiz F, Akyol FH, et al. Comparison of AJCC 1988 and 1997 classifications for nasopharyngeal carcinoma. American Joint Committee on Cancer [J] Int J Radiat Oncol Biol Phys. 1999;44(5):1079–1087. doi: 10.1016/s0360-3016(99)00098-x. [DOI] [PubMed] [Google Scholar]
- 8.Hao SP TN. Nasopharyngeal carcinoma [M] In: GJP, editor. Practical head and neck oncology. San Diego, CA: 2008. pp. 175–190. [Google Scholar]
- 9.AI-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized intergroup study 0099 [J] J Clin Oncol. 1998;16(4):1310–1317. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 10.Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union Against Cancer stage III and IV nasopharyngeal cancer of the endemic variety [J] J Clin Oncol. 2005;23(27):6730–6738. doi: 10.1200/JCO.2005.16.790. [DOI] [PubMed] [Google Scholar]
- 11.Chan AT, Teo PM, Ngan RK, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial [J] J Clin Oncol. 2002;20(8):2038–2044. doi: 10.1200/JCO.2002.08.149. [DOI] [PubMed] [Google Scholar]
- 12.Cheng SH, Jian JJ, Tsai SY, et al. Long-term survival of nasopharyngeal carcinoma following concomitant radiotherapy and chemotherapy [J] Int J Radiat Oncol Biol Phys. 2000;48(5):1323–1330. doi: 10.1016/s0360-3016(00)00779-3. [DOI] [PubMed] [Google Scholar]
- 13.Baujat B, Audry H, Bourhis J, et al. Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients [J] Int J Radiat Oncol Biol Phys. 2006;64(1):47–56. doi: 10.1016/j.ijrobp.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 14.Guigay J, Temam S, Bourhis J, et al. Nasopharyngeal carcinoma and therapeutic management: the place of chemotherapy [J] Ann Oncol. 2006;17(Suppl 10):304–307. doi: 10.1093/annonc/mdl278. [DOI] [PubMed] [Google Scholar]
- 15.Ma BB, Chan AT. Recent perspectives in the role of chemotherapy in the management of advanced nasopharyngeal carcinoma [J] Cancer. 2005;103(1):22–31. doi: 10.1002/cncr.20768. [DOI] [PubMed] [Google Scholar]
- 16.Sanguineti G, Bossi P, Pou A, et al. Timing of chemoradiotherapy and patient selection for locally advanced nasopharyngeal carcinoma [J] Clin Oncol (R Coll Radiol) 2003;15(8):451–460. doi: 10.1016/s0936-6555(03)00201-2. [DOI] [PubMed] [Google Scholar]
- 17.Ayyoub M, Deknuydt F, Raimbaud I, et al. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t [J] Proc Natl Acad Sci USA. 2009;106(21):8635–8640. doi: 10.1073/pnas.0900621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Josting A, Wolf J, Diehl V. Hodgkin disease: prognostic factors and treatment strategies [J] Curr Opin Oncol. 2000;12(5):403–411. doi: 10.1097/00001622-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Liu BL, Robinson M, Han ZQ, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties [J] Gene Ther. 2003;10(4):292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- 20.Martuza RL, Malick A, Markert JM, et al. Experimental therapy of human glioma by means of a genetically engineered virus mutant [J] Science. 1991;252(5007):854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 21.Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial [J] Gene Ther. 2000;7(10):867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 22.MacKie RM, Stewart B, Brown SM. Intralesional injection of herpes simplex virus 1716 in metastatic melanoma [J] Lancet. 2001;357(9255):525–526. doi: 10.1016/S0140-6736(00)04048-4. [DOI] [PubMed] [Google Scholar]
- 23.Harrow S, Papanastassiou V, Harland J, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival [J] Gene Ther. 2004;11(22):1648–1658. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- 24.Hu JC, Coffin RS, Davis CJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor [J] Clin Cancer Res. 2006;12(22):6737–6747. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- 25.Kemeny N, Brown K, Covey A, et al. Phase I, open-label, dose-escalating study of a genetically engineered herpes simplex virus, NV1020, in subjects with metastatic colorectal carcinoma to the liver [J] Hum Gene Ther. 2006;17(12):1214–1224. doi: 10.1089/hum.2006.17.1214. [DOI] [PubMed] [Google Scholar]
- 26.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress [J] Nat Clin Pract Oncol. 2007;4(2):101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 27.Chou J, Kern ER, Whitley RJ, et al. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture [J] Science. 1990;250(4985):1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 28.Mohr I, Gluzman Y. A herpesvirus genetic element which affects translation in the absence of the viral gadd34 function [J] EMBO J. 1996;15(17):4759–4766. [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein DJ, Weller SK. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant [J] J Virol. 1988;62(1):196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mineta T, Rabkin SD, Yazaki T, et al. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas [J] Nat Med. 1995;1(9):938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 31.Carroll NM, Chiocca EA, Takahashi K, et al. Enhancement of gene therapy specificity for diffuse colon carcinoma liver metastases with recombinant herpes simplex virus [J] Ann Surg. 1996;224(3):323–329; discussion 329–330. doi: 10.1097/00000658-199609000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mineta T, Rabkin SD, Martuza RL. Treatment of malignant gliomas using ganciclovir-hypersensitive, ribonucleotide reductase-deficient herpes simplex viral mutant [J] Cancer Res. 1994;54(15):3963–3966. [PubMed] [Google Scholar]
- 33.York IA, Roop C, Andrews DW, et al. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes [J] Cell. 1994;77(4):525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 34.Huard B, Fruh K. A role for MHC class I down-regulation in NK cell lysis of herpes virus-infected cells [J] Eur J Immunol. 2000;30(2):509–515. doi: 10.1002/1521-4141(200002)30:2<509::AID-IMMU509>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 35.Todo T, Martuza RL, Rabkin SD, et al. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing [J] Proc Natl Acad Sci USA. 2001;98(11):6396–6401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song LB, Zeng MS, Liao WT, et al. Bmi-1 is a novel molecular marker of nasopharyngeal carcinoma progression and immortalizes primary human nasopharyngeal epithelial cells [J] Cancer Res. 2006;66(12):6225–6232. doi: 10.1158/0008-5472.CAN-06-0094. [DOI] [PubMed] [Google Scholar]
- 37.Liu MT, Hsieh CY, Chang TH, et al. Prognostic factors affecting the outcome of nasopharyngeal carcinoma [J] Jpn J Clin Oncol. 2003;33(10):501–508. doi: 10.1093/jjco/hyg092. [DOI] [PubMed] [Google Scholar]
- 38.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002 [J] CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 39.Chan AT, Gregoire V, Lefebvre JL, et al. Nasopharyngeal cancer: Ehns-esmo-estro clinical practice guidelines for diagnosis, treatment and follow-up [J] Ann Oncol. 2010;(21 Suppl 5):v187–v189. doi: 10.1093/annonc/mdq186. [DOI] [PubMed] [Google Scholar]
- 40.Aghi M, Martuza RL. Oncolytic viral therapies—the clinical experience [J] Oncogene. 2005;24(52):7802–7816. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 41.Alajez NM, Mocanu JD, Shi W, et al. Efficacy of systemically administered mutant vesicular stomatitis virus (VSVdelta51) combined with radiation for nasopharyngeal carcinoma [J] Clin Cancer Res. 2008;14(15):4891–4897. doi: 10.1158/1078-0432.CCR-07-4134. [DOI] [PubMed] [Google Scholar]
- 42.Xu RH, Yuan ZY, Guan ZZ, et al. Reverse effect of genetically modified adenovirus H101 on drug-resistance of A549/DDP cells to cisplatin [J] Ai Zheng. 2005;24(8):975–979. [in Chinese] [PubMed] [Google Scholar]
- 43.Lu W, Zheng S, Li XF, et al. Intra-tumor injection of H101, a recombinant adenovirus, in combination with chemotherapy in patients with advanced cancers: a pilot phase II clinical trial [J] World J Gastroenterol. 2004;10(24):3634–3638. doi: 10.3748/wjg.v10.i24.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu RH, Yuan ZY, Guan ZZ, et al. Phase II clinical study of intratumoral H101, an E1b deleted adenovirus, in combination with chemotherapy in patients with cancer [J] Ai Zheng. 2003;22(12):1307–1310. [in Chinese] [PubMed] [Google Scholar]
- 45.Xia ZJ, Chang JH, Zhang L, et al. Phase III randomized clinical trial of intratumoral injection of e1b gene-deleted adenovirus (h101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus [J] Ai Zheng. 2004;23(12):1666–1670. [in Chinese] [PubMed] [Google Scholar]
- 46.Kanai R, Wakimoto H, Martuza RL, et al. A novel oncolytic herpes simplex virus that synergizes with phosphoinositide 3-kinase/Akt pathway inhibitors to target glioblastoma stem cells [J] Clin Cancer Res. 2010;17(11):3686–3696. doi: 10.1158/1078-0432.CCR-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prabhakar S, Messerli SM, Stemmer-Rachamimov AO, et al. Treatment of implantable NF2 schwannoma tumor models with oncolytic herpes simplex virus G47delta [J] Cancer Gene Ther. 2007;14(5):460–467. doi: 10.1038/sj.cgt.7701037. [DOI] [PubMed] [Google Scholar]
- 48.Castelo-Branco P, Passer BJ, Buhrman JS, et al. Oncolytic herpes simplex virus armed with xenogeneic homologue of prostatic acid phosphatase enhances antitumor efficacy in prostate cancer [J] Gene Ther. 2010;17(6):805–810. doi: 10.1038/gt.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fukuhara H, Martuza RL, Rabkin SD, et al. Oncolytic herpes simplex virus vector G47delta in combination with androgen ablation for the treatment of human prostate adenocarcinoma [J] Clin Cancer Res. 2005;11(21):7886–7890. doi: 10.1158/1078-0432.CCR-05-1090. [DOI] [PubMed] [Google Scholar]
- 50.Liu RB, Rabkin SD. Oncolytic herpes simplex virus vectors for the treatment of human breast cancer [J] Chin Med J (Engl) 2005;118(4):307–312. [PubMed] [Google Scholar]