Abstract
Nasopharyngeal cancer (NPC) is a rare disease in most parts of the world, except for Southeast Asia, some parts of North Africa and the Arctic. It is mostly seen in people of Chinese origin. In India, NPC is also rare, except for the Hill States of Northeast India, particularly Nagaland, Manipur, and Mizoram. The striking feature of NPC in Northeast India is that the incidence ranges over the complete spectrum from the lowest (as 0.5/100 000 to 2.0/100 000 among Caucasoid) to the highest (as ∼20/100 000 among Cantonese/Zhongshan dialect Chinese). The age-adjusted rate of NPC in Kohima district of Nagaland State is 19.4/100 000, which is among the highest recorded rates. By contrast, in Assam, one of the so-called Hill States but not itself a hilly state, NPC is much less common. The Northeastern region is distinguished by a preponderance of the Tibeto-Burman languages and by variable mongoloid features among peoples of the region. The nature of the migratory populations who are presumed to be bearers of the mongoloid risk is unknown, but these NPC occurrence features provide an outstanding opportunity for NPC risk investigation, such as that of the hypothesis of Wee et al. for westward displacement of Chinese aborigines following the last glacial maximum.
Keywords: Nasopharyngeal neoplasm, Northeast India, age-adjusted rate
Nasopharyngeal carcinoma (NPC) is a rare malignancy in most regions of the world, with a remarkable racial and geographical distribution affecting South China, Southeast Asia, the Maghrebian Arabs in North America, and Eskimos in the Arctic[1]–[4]. It is common among the Chinese populations (especially Cantonese[2],[3], with an age-adjusted rate(AAR) of 30/100 000 for males and 13/100 000 for females[5]); among the Maghrebian Arabs in North Africa (3.4/100 000 for males and 1.1/100 000 for females in Algeria)[6]; and among the Eskimos in the Arctic (10/100 000 for males and 4/100 000 for females)[7]. Elsewhere, the incidence is low with an AAR of less than 1/100 000 reported in Europe and North America[8].
The disease is one of the most confusing, commonly misdiagnosed, and poorly understood entities because of the location of the involved area. The lesion is often situated in a relatively large and inert space where only air and mucus are in transit. NPC can be silent for a long time causing few primary symptoms.
Indians, comprising about one-sixth of the world population with large family sizes and high levels of endogamy, provide a unique resource for dissecting complex disease etiology and pathogenesis. Historically, the Indian population is a conglomeration of multiple cultures and races. The evolutionary history of India entails migrations from central Asia and South China, resulting in a rich tapestry of socio-cultural, linguistic, and biological diversity. Broadly, Indians belong to the Austro-Asiatic, Tibeto-Burman, Indo-European, and Dravidian language families. Linguistically, the Northeastern region is distinguished by a preponderance of the Tibeto-Burman languages, and the population here is thought to comprise migrating peoples from East and Southeast Asia, who are presumed to have brought with them the risk for NPC to this region.
Despite the high incidence of oral cancer in India, NPC is uncommon in most regions. For instance, in Mumbai, West India, the incidence is cited as 0.71% for all cancers[9]. These low rates are comparable to those commonly quoted for other Caucasoid populations of 0.5 to 2.0/100 000 [3],[4],[8],[10]. There are 23 Population-Based Cancer Registries (PBCR) in India under the network of National Cancer Registry Programme of the Indian Council of Medical Research; 9 PBCRs are in the Northeastern Region. Eight Northeastern States in India have high AARs of NPC (Figure 1), including Arunachal Pradesh (population: 1 098 000), Assam (26 656 000), Manipur (2 294 000), Meghalaya (2319000), Mizoram (889 000), Nagaland (1 990 000), Sikkim (541 000), and Tripura districts (3 199 000). Indeed, the cases shown elsewhere are said to include families who have migrated from the Northeastern states. A “slightly elevated percentage is observed in the northeastern parts of the country bordering China, viz. Assam, Manipur etc” [11], which might be the earliest report. The National Cancer Registry has reported the prevalence of NPC to be 1.82% among all cancers in this region, constituting the eighth most common cancer in the Northeastern states. Nagaland state has the highest incidence of about 4.3/100000. The types and distribution of cancer in Nagaland state show that more than 25% of the head and neck biopsies of suspected cancer cases are histopathologically positive for malignancies and of these about 60% are diagnosed as NPC. High incidences are also observed in Manipur, Mizoram, and Sikkim. However, in Assam, the proportion of NPC among all cancers is only about 0.6%.
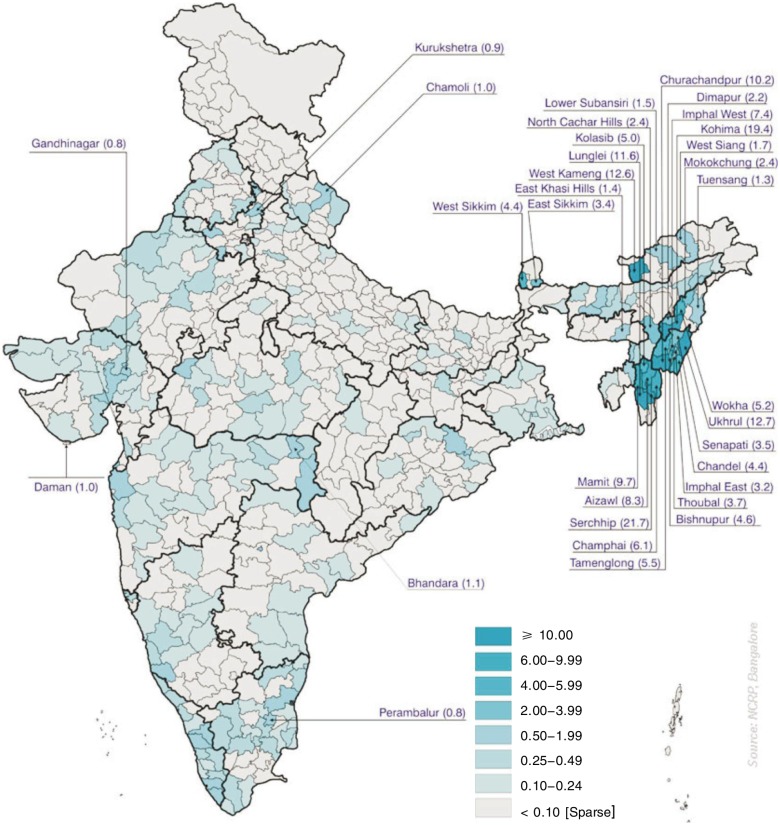
Figure 1. District-wise distribution of age-adjusted incidence rates of nasopharyngeal cancer (NPC) in males in different districts of India registered in 2002 in the Population-Based Cancer Registries (PBCRs) of the National Cancer Registry Programme of Indian Council of Medical Research. The figure was downloaded from the website of the National Cancer Registry Programme of Indian Council of Medical Research with publication permission. The rates are reported as per 100 000 population.
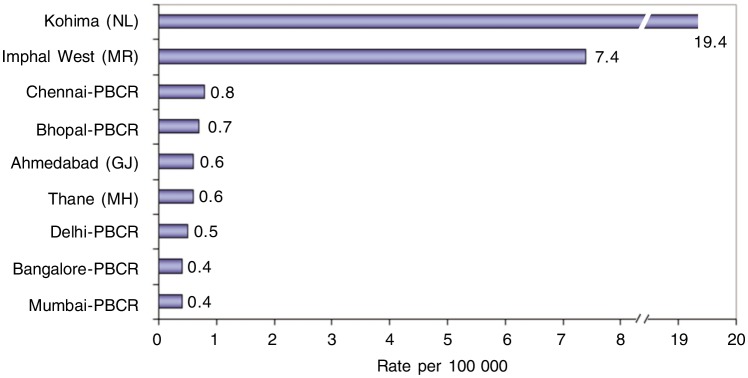
The AARs of NPC for males registered in Northeastern PBCRs and other PBCRs in India are shown in Figure 2. The district-wise distribution (population scattered over various districts within a State) of the AARs of NPC in Kohima district in Nagaland state is 19.4/100 000, among the highest AARs reported in the world; the Imphal West district in Manipur State followed with a high AAR of 7.4/100 000 (Figure 3). Several other districts in Mizoram and Manipur states recorded high AARs in both males and females, but this cannot be regarded as very significant because only less than 10 cases of cancer were recorded.
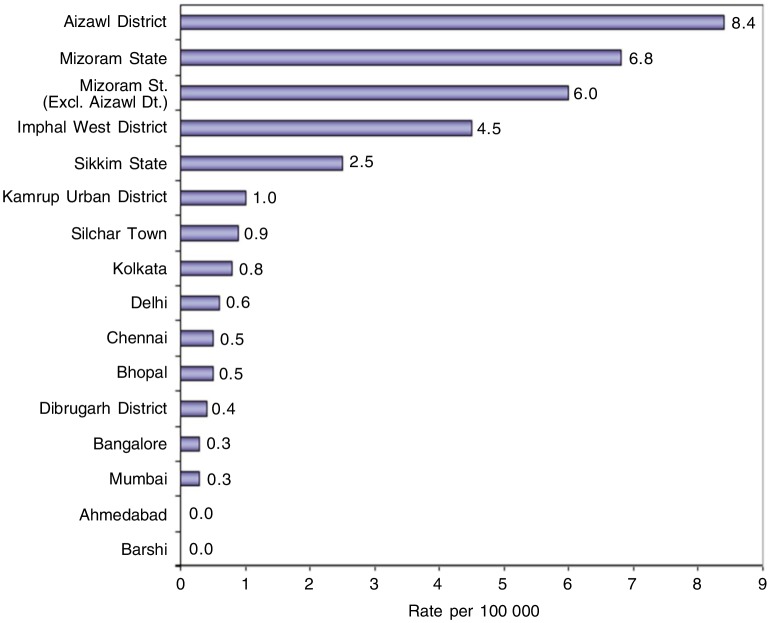
Figure 2. Age-adjusted incidence rates of NPC in males registered between 2004 and 2005 in all PBCRs. The data are from the PBCRs of National Cancer Registry Programme of Indian Council of Medical Research. Only 2 PBCRs, Mizoram and Sikkim PBCRs, cover an entire state. At the time of publication of the PBCR report of National Cancer Registry of ICMR, the data from new PBCRs are not available. The rates are reported as per 100 000 population. The Northeastern states of India (excluding Assam state) have higher incidence of NPC than the rest states of India.
Figure 3. Age-adjusted incidence rates of NPC in males registered in 2002 in some PBCRs. The data are from the PBCRs of National Cancer Registry Programme of Indian Council of Medical Research. The rates are reported as per 100 000 population. The Northeastern states of India (excluding Assam state) have higher incidence of NPC than the rest states of India.
The ten leading cancers registered between 2003 and 2004 in different Northeastern PBCRs are summarized in Tables 1–7. In Manipur (Table 1), Mizoram (Table 2), and Sikkhim (Table 3) states, NPC occurs commonly. In Dibrugarh district (Table 4), Urban Kamrup district (Tables 5), and Silchar town (Table 6) of Assam state, NPC does not find a place among the top ten cancers either for males or for females. In addition to Assam state, the data from the PBCRs of other Northeastern states indicate that NPC is rather uncommon among females in these states. However, noteworthy incidence of NPC in Assam state is indicated by cases referred to the Dr. B. Borooah Cancer Institute in Guahati, Urban Kamrup District, Assam state (Table 7).
Table 1. Age-adjusted incidence rates of leading cancers in males in Imphal West District of Manipur State registered between 2004 and 2005 in the Population-Based Cancer Registries (PBCRs).
| Serial No. | Leading cancer | No. of cases | % | MR |
| 1 | Lung cancer | 65 | 20.50 | 19.2 |
| 2 | Gastric cancer | 26 | 8.20 | 8.2 |
| 3 | Esophageal cancer | 23 | 7.26 | 6.7 |
| 4 | Nasopharyngeal cancer | 18 | 5.68 | 5.4 |
| 5 | Non–Hodgkin's lymphoma | 16 | 5.05 | 3.6 |
| 6 | Colon cancer | 13 | 4.10 | 3.5 |
| 7 | Hypopharyngeal cancer | 10 | 3.15 | 3.4 |
| 8 | Laryngeal cancer | 11 | 3.47 | 2.8 |
| 9 | Myeloid leukemia | 11 | 3.47 | 2.6 |
| 10 | Tongue cancer | 9 | 2.84 | 2.7 |
| All cancers | 317 | 100.00 | 88.05 |
The data are from the PBCRs of National Cancer Registry Programme of Indian Council of Medical Research. The total population in Imphal West District of Manipur State are 444 381, including 221 781 males.
Table 7. NPC cases referred to the Dr B Borooah Cancer Institute at Guahati region of Assam state since 2004.
| Period | Total [cases (%)] | Males [cases (%)] | Females [cases (%)] |
| April 2004 to March 2005 | 72 (1.75) | 51 (1.95) | 21 (1.40) |
| April 2005 to March 2006 | 65 (1.61) | 51 (2.07) | 14 (0.90) |
| April 2006 to March 2007 | 59 (1.40) | 44 (1.67) | 15 (0.95) |
| April 2007 to March 2008 | 38 (0.89) | 26(1.00) | 12 (0.72) |
| April 2008 to March 2009 | 41 (0.90) | 35 (1.28) | 6 (0.33) |
The data are from the 2004–2008 annual report of Dr. B. Borooah Cancer Institute at Guwahati region.
Table 2. Age-adjusted incidence rates of leading cancers in Mizoram State registered between 2004 and 2005 in the PBCR.
| Serial No. | Leading cancer | No. of cases | % | AAR |
| Males | ||||
| 1 | Gastric cancer | 298 | 24.65 | 50.64 |
| 2 | Lung cancer | 136 | 11.25 | 24.85 |
| 3 | Esophageal cancer | 132 | 10.92 | 19.73 |
| 4 | Hypopharyngeal cancer | 70 | 5.79 | 10.31 |
| 5 | Liver cancer | 42 | 3.47 | 6.58 |
| 6 | Cancer of Rectum | 29 | 2.40 | 4.61 |
| 7 | Non–Hodgkin's lymphoma | 27 | 2.23 | 4.24 |
| 8 | Nasopharyngeal cancer | 23 | 1.90 | 3.47 |
| 9 | Oral cancer (except tongue cancer) | 22 | 1.82 | 3.54 |
| 10 | Prostate cancer | 20 | 1.65 | 3.67 |
| All sites | 1209 | 100.00 | 194.53 | |
| Females | ||||
| 1 | Cervical cancer | 142 | 14.96 | 19.88 |
| 2 | Lung cancer | 132 | 13.91 | 24.72 |
| 3 | Gastric cancer | 124 | 13.07 | 23.29 |
| 4 | Breast cancer | 113 | 11.91 | 16.72 |
| 5 | Ovarian cancer | 25 | 2.63 | 3.59 |
| 6 | Liver cancer | 24 | 2.53 | 4.35 |
| 7 | Gall Bladder cancer | 22 | 2.32 | 4.06 |
| 8 | Esophageal cancer | 21 | 2.21 | 3.65 |
| 9 | Nasopharyngeal cancer | 21 | 2.21 | 3.48 |
| 10 | Cancer of Rectum | 20 | 2.11 | 3.70 |
| All sites | 949 | 100.00 | 155.73 |
The data are from the PBCRs of National Cancer Registry Programme of Indian Council of Medical Research. The total population in Mizoram State are 888 573 (459 109 males and 429 464 females).
Table 3. Age-adjusted incidence rates of leading cancers in Sikkim State registered between 2004 and 2005 in the PBCR.
| Serial No. | Leading cancer | No. of cases | % | AAR |
| Males | ||||
| 1 | Gastric cancer | 57 | 18.15 | 14.20 |
| 2 | Esophageal cancer | 32 | 10.19 | 7.73 |
| 3 | Liver cancer | 25 | 7.96 | 6.02 |
| 4 | Laryngeal cancer | 22 | 7.01 | 4.98 |
| 5 | Lung cancer | 21 | 6.69 | 5.18 |
| 6 | Nasopharyngeal cancer | 19 | 6.05 | 4.06 |
| 7 | Tongue cancer | 8 | 2.55 | 2.11 |
| 8 | Hypopharyngeal cancer | 7 | 2.23 | 1.97 |
| 9 | Brain cancer | 7 | 2.23 | 1.16 |
| 10 | Oral cancer (except tongue cancer) | 6 | 1.91 | 1.33 |
| All sites | 314 | 100.00 | 73.61 | |
| Females | ||||
| 1 | Breast cancer | 46 | 14.24 | 13.32 |
| 2 | Cervical cancer | 39 | 12.07 | 9.35 |
| 3 | Esophageal cancer | 33 | 10.22 | 6.78 |
| 4 | Lung cancer | 17 | 5.26 | 6.22 |
| 5 | Gastric cancer | 14 | 4.33 | 3.90 |
| 6 | Liver cancer | 13 | 4.02 | 2.79 |
| 7 | Laryngeal | 13 | 4.02 | 3.43 |
| 8 | Nasopharyngeal cancer | 10 | 3.10 | 1.81 |
| 9 | Myeloid leukemia | 9 | 2.79 | 2.61 |
| 10 | Skin cancer | 9 | 2.79 | 3.12 |
| All sites | 323 | 100.00 | 88.16 |
The data are from the PBCRs of National Cancer Registry Programme of Indian Council of Medical Research. The total population in Sikkim State are 540 851 (288 484 males and 252 367 females).
Table 4. Age-adjusted incidence rates of leading cancers in males in Dibrugarh District of Assam State registered between 2004 and 2005 in the PBCR.
| Serial No. | Leading cancer | No. of cases | % | MR |
| 1 | Esophageal cancer | 134 | 17.52 | 15.70 |
| 2 | Hypopharyngeal cancer | 90 | 11.76 | 10.99 |
| 3 | Gastric cancer | 60 | 7.97 | 7.48 |
| 4 | Oral cancer (except tongue cancer) | 53 | 6.93 | 6.30 |
| 5 | Lung cancer | 42 | 5.49 | 5.45 |
| 6 | Tongue cancer | 41 | 5.36 | 4.69 |
| 7 | Laryngeal cancer | 26 | 3.40 | 2.99 |
| 8 | Tonsil cancer | 22 | 2.88 | 2.53 |
| 9 | Gallbladder cancer | 20 | 2.61 | 2.44 |
| 10 | Colon cancer | 16 | 2.09 | 1.76 |
| All sites | 764 | 100.00 | 89.44 |
The data are from the PBCRs of National Cancer Registry Programme of Indian Council of Medical Research. The total population in Dibrugarh District of Assam State are 1 185 072, including 613 555 males.
Table 5. Age-adjusted incidence rates of leading cancers in males in Urban Kamrup District of Assam State registered between 2004 and 2005 in the PBCR.
| Serial No. | Leading cancer | No. of cases | % | MR |
| 1 | Esophageal cancer | 239 | 18.83 | 32.55 |
| 2 | Hypopharyngeal cancer | 161 | 12.77 | 22.34 |
| 3 | Lung cancer | 94 | 7.41 | 14.78 |
| 4 | Tongue cancer | 83 | 6.54 | 12.16 |
| 5 | Oral cancer (except tongue cancer) | 68 | 5.36 | 8.73 |
| 6 | Tonsil cancer | 62 | 4.89 | 8.20 |
| 7 | Laryngeal cancer | 58 | 4.57 | 8.18 |
| 8 | Gastric cancer | 56 | 4.41 | 7.50 |
| 9 | Prostrate cancer | 36 | 2.84 | 6.69 |
| 10 | Non–Hodgkin's lymphoma | 31 | 2.44 | 3.45 |
| All sites | 1269 | 100.00 | 172.23 |
The data are from the PBCRs of National Cancer Registry Programme of Indian Council of Medical Research. The total population in Urban Kamrup District of Assam State are 908 217, including 493 543 males.
Table 6. Age-adjusted incidence rates of leading cancers in males in Silchar Town of Assam State registered between 2004 and 2005 in the PBCR.
| Serial No. | Leading cancer | No. of cases | % | MR |
| 1 | Laryngeal cancer | 15 | 8.57 | 10.68 |
| 2 | Lung cancer | 14 | 8.00 | 10.39 |
| 3 | Esophageal cancer | 14 | 8.00 | 8.81 |
| 4 | Tongue cancer | 13 | 7.43 | 8.27 |
| 5 | Hypopharyngeal cancer | 10 | 5.71 | 6.70 |
| 6 | Gastric cancer | 9 | 5.14 | 6.59 |
| 7 | Rectal cancer | 8 | 4.57 | 3.99 |
| 8 | Oral cancer (except tongue cancer) | 8 | 4.57 | 5.41 |
| 9 | Colon cancer | 7 | 4.00 | 3.67 |
| 10 | Liver cancer | 5 | 2.86 | 3.43 |
| All sites | 175 | 100.00 | 113.77 |
The data are from the PBCRs of National Cancer Registry Programme of Indian Council of Medical Research. The total population in Mizoram State are 201 387, including 18 654 males.
The decrease of patients with NPC at Dr. B. Borooah Cancer Institute is due to the increase of cancer treatment centers in the Northeastern states in recent years. Now, five cancer treatment centers in Assam state have radiotherapy facility, therefore, more patients are referred to these cancer centers than before. Radiotherapy facilities are also available in Tripura, Manipur, Meghalaya, and Mizoram states, whereas no radiotherapy facilities are available in Sikkim, Nagaland, and Arunachal Pradesh state where the incidence of NPC is high.
From the environmental aspect, Northeast India experiences predominantly humid sub-tropical climate with hot, humid summers, severe monsoons, and mild winters. The west coast of India has some of the Indian sub-continent's last remaining rain forests. People from Nagaland and neighboring hill states have the habit of eating smoked fish and meat. The houses are not well-ventilated. A possible correlation between the consumption of smoked meat by the tribal people and high susceptibility to NPC has been postulated[12],[13]. The infection of type A Epstein-Barr virus (EBV) is far more prevalent in West India; whereas in East India, particularly in Assam state, the infection of type B EBV is more prevalent, indicating a significant variation in the type of EBV infection in different ethnic populations in India[14].
The spectrum of incidence from < 1 % to > 20% in the Northeastern states is not easily accessible elsewhere in the world and provides an outstanding opportunity for investigating NPC risk. Despite the relatively low incidence of NPC in West India, two reports from the Tata Memorial Hospital, Bombay cover cases seen from 1941 to the 1970's which reveal clear bi-modal distributions[15],[16]. This institution is a major comprehensive cancer center in India and receives patients from all over India and neighbor countries. About 80% of the patients are from two western states, Maharashtra and Gujarat, suggesting that the bimodality occurred among low incidence populations[15]. The teenage peak occurred earlier in females (10 to 14 years) than in males (15 to 19 years)[15]. Most cases of NPC diagnosed histopathologically as lymphoepithelioma occurred in young patients; the incidence declines sharply from the population of 15 to 20 years old to that of 30–35 years old, with a slower decline thereafter[16]. A relative high incidence of NPC was observed by 1970 in the world, including East and North Africa[17],[18]. Young-age modality is now well-established in intermediate incidence countries of the Maghreb region[19]. By contrast, from the earliest reports of NPC in China[20], the low incidence in young patients was not associated with a modal peak.
The main genetic risk is the admixed Mongoloid elements. In a study from Manipur state in India, 275 (83.3%) of the 330 were Mongoloids, and 55 were not obviously mongoloid[21]. They also found that in Manipur state, the incidence of NPC was the highest among the Tangkhul tribe (a Naga sub tribe) and most patients were from Ukhrul district where 60% of the population are Tangkhuls[21].
The Indian Council of Medical Research Bulletin released a statement in September 2003 that the Mongoloid population, particularly Nagas, have a high risk of NPC[22]. A 3-year project entitled “Immunogenetic profile of nasopharyngeal cancer in a high-prevalence region of Northeast India” has been approved by the Department of Biotechnology, Ministry of Science & Technology, Government of India. The project was commenced in July 2010 by Dr. B. Borooah Cancer Institute; Institute of Pathology, Indian Council of Medical Research, New Delhi; and Regional Institute of Medical Sciences, Imphal (Manipur state).
The incidence data from Northeastern states reveal two main, consistent features: (1) non-random prevalence of NPC even in limited geographical regions; (2) higher incidence in males. The high incidence of NPC in Northeastern states bordering West China was firstly reported in 1976[11]. Since then, “tribal” groups exhibiting mongoloid socio-cultural features was noted[1]. However, the nature of these migratory populations who are presumed to be bearers of the mongoloid risk remains unknown, current populations provide an opportunity to test the hypothesis for westward displacement of Chinese aborigines after the last glacial maximum [1]. Detailed patient socio-cultural / epidemiological description and NPC type stratification (age of onset, histopathologic type, response to therapy, and so on), as well as application of new archeohaplomic developments for whole-genome di-haploid definition of both mongoloid features and NPC risk will help to test the hypothesis[23].
Acknowledgments
We are grateful to National Cancer Registry Programme of Indian Council of Medical Research for permission to use the data from the Population-Based Cancer Registries. We thank Dr. Chao-Nan Qian, Van Andel Research Institute, Grand Rapids, Michigan, for critical reading of the manuscript.
References
- 1.Wee JT, Ha TC, Loong SL, Qian CN. Is nasopharyngeal carcinoma really a “Cantonese Cancer”? [J] Chin J Cancer. 2010;29(5):517–526. doi: 10.5732/cjc.009.10329. [DOI] [PubMed] [Google Scholar]
- 2.Zheng YM, Tuppin P, Hubert A, et al. Environmental and dietary risk factors for nasopharyngeal carcinoma: a case-control study in Zangwu County, Guangxi, China [J] Br J Cancer. 1994;69(3):508–514. doi: 10.1038/bjc.1994.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma [J] Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 4.Hirayama T. Descriptive and analytic epidemiology of nasopharyngeal carcinoma [M] In: De The G, Ito Y, editors. Nasopharyngeal carcinoma: etiology and control. Lyon: IARC Scientific Publications; 1978. pp. 167–189. [PubMed] [Google Scholar]
- 5.Muir C, Waterhouse J, Mack T, editors. Lyon: IARC Scientific Publications; 1987. Cancer incidence in five continents [M] pp. 787–789. [Google Scholar]
- 6.Parkin DM, editor. Lyon: IARC Scientific Publications; 1986. Cancer occurrence in developing countries [M] pp. 208–209. [PubMed] [Google Scholar]
- 7.Lanier AP, Bender TR, Blot WJ, et al. Cancer incidence in Alaska natives [J] Int J Cancer. 1976;18:409–412. doi: 10.1002/ijc.2910180403. [DOI] [PubMed] [Google Scholar]
- 8.Waterhouse J, Muir C, Shanmugaratnam K, et al. Lyon: IARC Scientific Publications; 1982. Cancer incidence in five continents [M] pp. 210–213. [Google Scholar]
- 9.Bhatia PL, Singh LS. Evaluation of contrast radiography in nasopharyngeal malignancy [J] Indian J Cancer. 1981;18:141–146. [PubMed] [Google Scholar]
- 10.Ferlay J, Bray F, Pisani P, et al. GLOBOCAN 2002: cancer incidence, mortality and prevalence worldwide. Lyon: IARC Press; 2004. Available at: http://www-dep.iarc.fr. [Google Scholar]
- 11.Balakrishnan V, Gangadharan P, Nagaraj RD. Some epidemiological aspects of nasopharyngeal cancer [M] In: Shanmugaratnam K, Nambiar R, Tan KK, et al., editors. Liver cancer: cancer problems in Asian countries. Singapore: Singapore Cancer Society; 1976. pp. 268–274. [Google Scholar]
- 12.Sarkar S, Nagabhushan M, Soman CS, et al. Mutagenicity and carcinogenicity of smoked meat from Nagaland, a region of India prone to a high incidence of nasopharyngeal cancer [J] Carcinogenesis. 1989;10(4):733–736. doi: 10.1093/carcin/10.4.733. [DOI] [PubMed] [Google Scholar]
- 13.Chelleng PK, Narain K, Das HK, et al. Risk factors for cancer nasopharynx: a case-control study from Nagaland, India [J] Natl Med J India. 2000;13(1):6–8. [PubMed] [Google Scholar]
- 14.Rathaur RG, Chitale AR, Banerjee K. Epstein-Barr virus in nasopharyngeal carcinoma in Indian patients [J] Indian J Cancer. 1999;36(2–4):80–90. [PubMed] [Google Scholar]
- 15.Balakrishnan U. An additional younger-age peak for cancer of the nasopharynx [J] Int J Cancer. 1975;15(4):651–657. doi: 10.1002/ijc.2910150414. [DOI] [PubMed] [Google Scholar]
- 16.Sawai MM, Talwalkar GV, Gangadharan P. Cancer of the nasopharynx—a review of 1036 cases seen at the Tata Memorial Hospital, Bombay, India [J] Indian J Cancer. 1983;20:89–96. [PubMed] [Google Scholar]
- 17.Clifford P. A review of the Epidemiology of nasopharyngeal carcinoma [J] Int J Cancer. 1970;5(3):287–309. doi: 10.1002/ijc.2910050302. [DOI] [PubMed] [Google Scholar]
- 18.Cammoun M, Moerner VG, Mourali N. Tumours of the nasopharynx in Tunisia [J] Cancer. 1974;33(1):184–192. doi: 10.1002/1097-0142(197401)33:1<184::aid-cncr2820330127>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Ghandri N, Piancatelli D, et al. Associations between HLA class I alleles and the prevalence of nasopharyngeal carcinoma (NPC) among Tunisians [J] J Transl Med. 2007;5:22. doi: 10.1186/1479-5876-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh CK, Li CC, Min HC, et al. Clinical analysis of 1000 cases of nasopharyngeal carcinoma [J] Chin Med J. 1965;84(12):767–780. [PubMed] [Google Scholar]
- 21.Singh I, Lyngdoh NC. Nasopharyngeal carcinoma—our experience in Regional Institute of Medical Sciences, Manipur [J] Ann J Otolaryngol Head Neck Surg. 2009–2010;18:10–15. [Google Scholar]
- 22.Kumar S. Epidemiological and etiological factors associated with nasopharyngeal carcinoma [J] ICMR Bulletin. 2003;33(9):1–9. [Google Scholar]
- 23.Turnbull D. On the trails of markers and proxies: the socio-cognitive technologies of human movement, knowledge assemblage, and their relevance to the etiology of nasopharyngeal cancer [J] Chin J Cancer. 2011;30(2):85–95. doi: 10.5732/cjc.010.10597. [DOI] [PMC free article] [PubMed] [Google Scholar]