Abstract
Unusual sites of metastases are recognized in patients with renal cell carcinoma (RCC). However, the prognostic implications of these sites are not well understood. We used the Memorial Sloan-Kettering Cancer Center (MSKCC) risk classification for metastatic RCC to evaluate 912 consecutive patients with RCC managed at the Singapore General Hospital between 1990 and 2009. Among these patients, 301 had metastases either at diagnosis or during the course of illness. Nasal metastases, all arising from clear cell RCC, were identified histologically in 4 patients (1.3% of those with metastasis). All 4 patients were classified as MSKCC poor prognosis by current risk criteria. Nasal metastases were significantly associated with lung and bone metastases. The frequency of nasal metastases in patients with metastatic RCC is about 1 %, occurring predominantly in patients with clear cell RCC. Nasal metastases are associated with poor prognosis as estimated by the MSKCC risk classification, with attendant implications for selection of targeted therapy, and are usually associated with multi -organ dissemination, including concurrent lung and bone involvement.
Keywords: Nasal metastasis, renal cell carcinoma, head and neck metastasis, sunitinib, metastatic cancer
The worldwide incidence of renal cell carcinoma (RCC) is about 209 000 new cases per year with a mortality of 102 000 deaths per year[1]. Synchronous metastasis occurs in 25%–30% of patients with RCC and metachronous metastasis may develop in up to 50% who have gone for a presumably curative radical or partial nephrectomy[2].
Common sites of metastasis are the lungs (75% of cases), regional lymph nodes (65%), bone (40%), liver (40%), and brain (5%)[3]. While head and neck metastases are unusual in general in all cancers, RCC is the most common infra-clavicular primary tumor to metastasize to the nose and paranasal sinus[4]. Despite this, nasal metastases are rare overall, and the frequency of nasal metastases is not well recognized. To our knowledge, there have been 27 reported cases to date[2]. It is uncertain whether nasal metastasis portends a good or poor prognosis, with conflicting outcomes in these case reports.
The Memorial Sloan-Kettering Cancer Center (MSKCC) risk stratification has been developed to estimate outcomes in patients with metastatic RCC, and has been modified for trials of targeted therapy[5]. Currently, it has been modified and used by the National Cancer Comprehensive Network (NCCN) in the USA in guiding the first-line treatment of metastatic RCC. In addition to its use for estimating survival, this initial risk stratification is extremely important from a clinical viewpoint in determining whether a patient should be treated by multi-targeted tyrosine kinase inhibitors such as sunitinib[6], or an mTOR inhibitor such as temsirolimus[7].
We reviewed our series of 912 consecutive patients with epithelial RCC seen over 20 years (from 1990 to 2009). Among them, 301 had metastatic disease either at the start or during the course of illness, including 4 with nasal metastasis.
Case report
At the end of follow-up, 224 (74.4%) of the 301 patients had lung metastasis, 124 (41.2%) had bone metastasis, 68 (22.6%) had liver metastasis, 57 (18.9%) had brain metastasis, and 4 (1.3%) had nasal metastasis. The clinical records of the 4 patients with nasal metastasis were reviewed.
Case 1
A 46-year-old Chinese male on health screening was found to have a left renal tumor, which was proved as RCC by biopsy. Three months later, he underwent a partial nephrectomy for localized stage III RCC. The 7.5 cm diameter lesion was confirmed as Fuhrman grade 4 clear cell renal cell carcinoma (CCRCC) by histology. A completion left radical nephrectomy was performed when he was admitted for severe gross hematuria 2 weeks later, but histology showed no residual tumor. At 19 months post nephrectomy, he noticed a painless lump over the nasal bridge, which was confirmed as metastatic RCC (Fuhrman grade 4) by a biopsy. Routine computed tomography (CT) done further revealed lung, bone, and adrenal gland metastases. He received a course of radiotherapy to the nasal metastasis (50 Gy by 20 fractions), but no clinical response was noted. The nasal metastasis was then resected. He died of metastatic RCC 2 years later.
Case 2
A 56-year-old Chinese male was seen for epistaxis. A nasal mass was found and biopsy showed a clear cell carcinoma. He was staged, and found to have widespread metastases. Surgical resection of the nasal mass was performed, and histology proved clear cell cancer. A palliative radical nephrectomy was then performed, yielding an 8.4 cm lesion of Fuhrman grade 2 CCRCC. Both lesions were similar in morphology and grade. He declined all systemic therapy and died 19 months later.
Case 3
A 62-year-old Chinese female presented with flank pain and anemia. CT of the abdomen and pelvis revealed a large renal mass. Ultrasound-guided fine needle aspiration cytology was performed, confirming clear cell RCC. CT of the brain and thorax revealed lung and skull metastases. She declined palliative systemic therapy. A nasal lesion was found 4 months later. A biopsy was performed, confirming metastatic RCC. Within 8 months of her diagnosis, she died of metastatic RCC.
Case 4
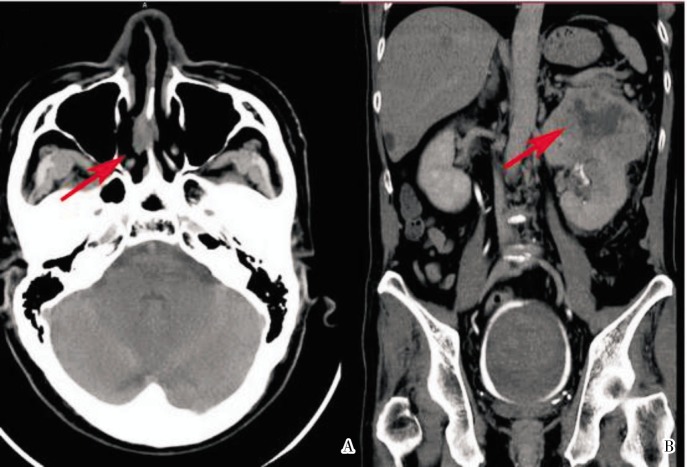
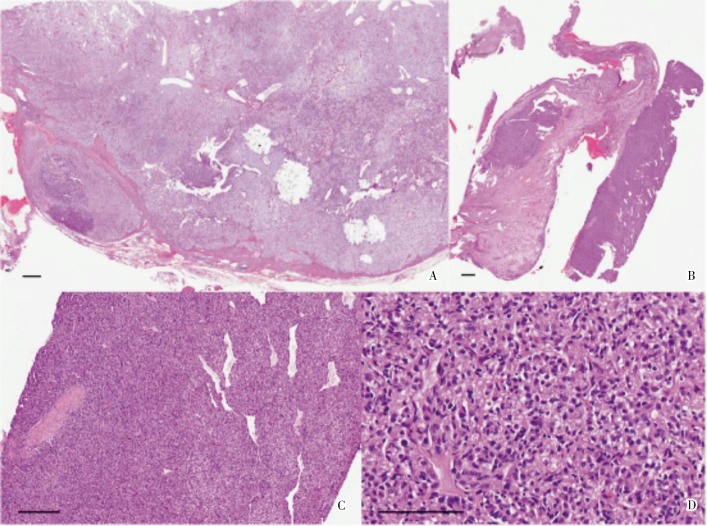
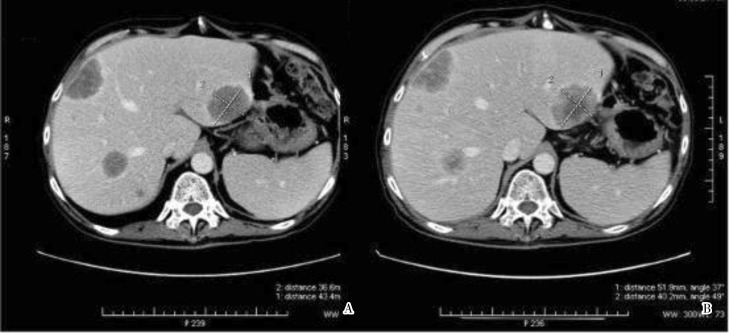
A 59-year-old Chinese male presented with a nasal septal lesion (Figure 1A). CT revealed a primary renal tumor (Figure 1B), with metastasis to the liver, lung, and bone. Palliative nephrectomy was performed, yielding an 11 cm × 10 cm × 10 cm tumor with several satellite tumor nodules. Histological examination showed Fuhrman grade 4 CCRCC, with focal sarcomatoid changes (Figure 2A). Surgical resection of the nasal tumor showed high grade clear cell adenocarcinoma (Figure 2B–D) morphologically resembling the renal tumor. He received sunitinib as first-line therapy because he preferred oral therapy for convenience, but his tumor continued to progress (Figure 3). He received everolimus subsequently. His visceral metastases responded after the first cycle, but he developed pneumonitis associated with mTOR inhibition.
Figure 1. CT images of a 59-year-old Chinese man with nasal metastasis and corresponding left renal cell carcinoma. A, axial CT image of the head shows a 2 cm × 1 cm lobulated ovoid soft tissue mass of heterogenous internal density arising from the nasal septum extending into the right nasal cavity (arrow). B, coronal CT image of the abdomen shows a 10 cm × 10.2 cm × 8.5 cm ovoid lobulated enhancing mass arising from the upper pole of the right kidney with associated necrotic hypodense center (arrow). A nonenhancing clot is seen in the bladder, lined by a peripheral rim of urine appearing hyperdense due to excreted contrast.
Figure 2. Histological examination of the renal cell carcinoma and corresponding nasal metastasis in a 59-year-old Chinese man. A, the renal cancer comprises solid sheets of pale to clear tumor cells and an arborizing vascularity, supporting the diagnosis of high grade clear cell RCC (scale bar = 25 µm); B, the fragments of nasal mucosa contains cellular tumor islands with sarcomatoid changes, a feature that is indicative of aggressive RCC (scale bar = 25 µm); C, interspersed narrow vessels (scale bar = 100 µm) are present in the tumor, reflecting a complex vascular network; D, the nasal metastasis presents as alveolate patterns of pale to clear cells (scale bar = 200 µm), resembling the renal tumor, supporting the diagnosis of metastatic RCC.
Figure 3. CT images show progression of MSKCC poor-prognosis metastatic RCC in a 59-year-old Chinese man after treatment with sunitinib. The lesion in the left hepatic lobe (segment 2) has enlarged from 4.3 cm × 3.7 cm (A) to 5.1 cm × 4.0 cm (B). Its central portion of lower density is stable in size, and the peripheral aspect is larger. Stable peripheral lesions in the right hepatic lobe (segment 8) and in the posterior aspect of the right hepatic lobe adjacent to the right hepatic vein (segment 7) show a similar heterogenous hypodensity. Subcentimeter hypodense lesions are seen in segment 8 in both images.
Fisher's exact test was performed to assess the associations between nasal metastasis and being classified as poor risk by a modified MSKCC classification[8] as well as between nasal metastasis and other organs of metastases. This MSKCC classification was performed for all the 92 patients treated with targeted therapy, among whom 55 belonged to the good/intermediate risk group and 37 patients to the poor risk group. Nasal metastasis was significantly associated with MSKCC classified poor risk (P = 0.030) and concurrent involvement of the lung and bone (P = 0.012).
Discussion
The frequency of nasal metastasis in RCC has never been evaluated, with only isolated single reports and small series in the literature to date. We estimated the frequency of nasal metastases in renal cell carcinoma as being about 1% of all patients with metastatic disease in a modern series.
All 4 of our cases in our series were classified as poor prognosis patients by MSKCC criteria and that nasal metastasis is associated with MSKCC-poor prognosis in RCC patients. Single case reports elsewhere have noted long survival in some patients with isolated nasal metastasis[9], and we noted that Case 1 had a survival of 24 months following diagnosis of metastasis while receiving several lines of systemic therapy.
The coincidence between nasal and widespread systemic metastasis can be readily explained by hematogenous spread of RCC through the lungs to arrive at the head and neck[10],[11]. The metastasis of RCC to the bone is explained by spread through the systemic circulation, venous circulation, or lymphatic circulation[12],[13]. How RCC may metastasize to the head and neck through the Batson's venous plexus or lymphatic spread through the thoracic duct has been discussed [10],[11],[14], whereas unusual head and neck metastases that do not seem to support normal flow patterns have been explained by hypotheses ranging from right-to-left heart shunt, to spontaneous regression of lung disease as well as microscopic lung seeding[11]. However, our data from a long series of consecutive patients suggests that, generally, multiple nasal metastases are the rule, and that single head and neck metastasis is very much the exception.
The classification of these patients using the MSKCC criteria is useful as there are implications for systemic therapy. Currently, the standard approach to first-line targeted therapy in patients with metastatic RCC depends on initial risk stratification by the MSKCC criteria. Patients with good- or intermediate-risk RCC benefit from sunitinib[6], whereas patients with poor-risk RCC benefit from mTOR inhibitors such as temsirolimus[7]. Given that our study evaluated patients over 20 years, only 1 patient received multi-targeted tyrosine kinase inhibitors developed in the last five years. It should be noted that this patient initially chose to receive sunitinib and he subsequently exhibited cancer progression. A characteristic consistent with MSKCC poor-prognosis RCC. He was then switched to everolimus, an mTOR inhibitor[15], and his liver metastases underwent subsequent response, also consistent with MSKCC poor-prognosis RCC. Certainly, appropriate MSKCC prognostic classification of patients for selection of treatment is illustrated here, but our series does not suggest that nasal metastases independently add predictive value.
Conclusions
In summary, nasal metastasis occurs in about 1% of all patients with metastatic RCC, and is predominantly associated with the clear cell subtype. We report an association between nasal metastasis with lung and bone metastases, and with poor prognosis estimated by the MSKCC classification.
Acknowledgments
We thank Dr. Choon Hua Thng (Diagnostic Imaging, National Cancer Center Singapore) and Dr. Yu Unn Gan (Asia HealthPartners, Singapore) for their kind review of the radiological images.
References
- 1.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma [J] Lancet. 2009;373(9669):1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 2.Brener ZZ, Zhuravenko I, Jacob CE, et al. An unusual presentation of renal cell carcinoma with late metastases to the small intestine, thyroid gland, nose and skull base [J] Nephrol Dial Transplant. 2007;22(3):930–932. doi: 10.1093/ndt/gfl772. [DOI] [PubMed] [Google Scholar]
- 3.Ziari M, Shen S, Amato RJ, et al. Metastatic renal cell carcinoma to the nose and ethmoid sinus [J] Urology. 2006;67(1):199. doi: 10.1016/j.urology.2005.07.052. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein JM, Montgomery WW, Balogh K., Jr Metastatic tumors to the maxilla, nose and paranasal sinuses [J] Laryngoscope. 1966;76(4):621–650. doi: 10.1288/00005537-196604000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma [J] J Clin Oncol. 2004;22(3):454–463. doi: 10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 6.Motzer R, Hutson T, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma [J] N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 7.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma [J] N Engl J Med. 2007;356(22):2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 8.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma [J] N Engl J Med. 2007;356(22):2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 9.Simo R, Sykes AJ, Hargreaves SP, et al. Metastatic renal cell carcinoma to the nose and paranasal sinuses [J] Head Neck. 2000;22(7):722–727. doi: 10.1002/1097-0347(200010)22:7<722::aid-hed13>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Boles R, Cerny J. Head and neck metastases from renal carcinomas [J] Mich Med. 1971;70(16):616–618. [PubMed] [Google Scholar]
- 11.Gottlieb MD, Roland JT., Jr Paradoxical spread of renal cell carcinoma to the head and neck [J] Laryngoscope. 1998;108(9):1301–1305. doi: 10.1097/00005537-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma [J] N Engl J Med. 1996;335(12):865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 13.Russo P. Renal cell carcinoma: presentation, staging, and surgical treatment [J] Semin Oncol. 2000;27:160–176. [PubMed] [Google Scholar]
- 14.Nahum AM, Bailey BJ. Malignant tumors metastatic to the paranasal sinuses: Case report and review of the literature [J] Laryngoscope. 1963;73:942–953. doi: 10.1288/00005537-196307000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Motzer R, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. [J] Lancet. 2008;372(9637):449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]