Abstract
Nasopharyngeal carcinoma (NPC) is a malignancy with remarkable ethnic and geographic distribution in southern China and Southeast Asia. Alternative to genetic changes, aberrant epigenetic events disrupt multiple genes involved in cell signaling pathways through DNA methylation of promoter CpG islands and/or histone modifications. These epigenetic alterations grant cell growth advantage and contribute to the initiation and progression of NPC. In this review, we summarize the epigenetic deregulation of cell signaling in NPC tumorigenesis and highlight the importance of identifying epigenetic cell signaling regulators in NPC research. Developing pharmacologic strategies to reverse the epigenetic-silencing of cell signaling regulators might thus be useful to NPC prevention and therapy.
Keywords: Epigenetic, cell signaling, nasopharyngeal neoplasm
Nasopharyngeal carcinoma (NPC) is rare in most part of the world but prevalent in southern China, including Guangdong and Hong Kong, and Southeast Asia, with an incidence rate of 20 to 30 per 100 000 people/year[1]. The unique ethnic and geographic distribution of NPC indicates its unusual etiology[2]. Three major etiologic factors, genetic, environmental, and viral factors, have been identified to lead to multiple genetic and epigenetic alterations during NPC pathogenesis by either acting alone or in synergy[3].
Carcinogenesis involves multiple genetic/epigenetic alterations including the activation of oncogenes and disruption of tumor suppressor genes (TSGs)[4]. Equally important as genetic mutations, epigenetic changes also drive tumor development[5]. Aberrant methylation of TSG-associated CpG islands is a characteristic epigenetic feature of tumor genomic DNA. Aberrant CpG island methylation can occur during the early stage of tumor pathogenesis by disrupting or over-activating key signaling pathways, even in pre-invasive lesions, predisposing tumor cells addictive to certain oncogenic pathways. In such status the cells are much more susceptible to genetic mutations, thus driving tumor progression[6].
In this review, we summarize the key genes in several important signaling pathways frequently disrupted by CpG methylation in NPC tumorigenesis, such as regulators for Ras and Rho GTPase signaling, p53 signaling, Wnt/β-catenin signaling, cell adhesion and apoptosis signaling, and cell cycle control-DNA damage signaling (Table 1). We also discuss the possibility of these genes served as biomarkers for risk assessment, early detection, and therapeutic targets for NPC treatment. The possible mechanisms of cell signaling disruption by CpG methylation in NPC are further summarized.
Table 1. List of methylated/silenced tumor suppressor genes (TSGs) involved in cell signaling in nasopharyngeal carcinoma (NPC).
| Classification | TSG | Full name | Other names | Location | Functions | Alterations in NPC | Refs |
| Ras GTPase signaling | DAB2 | Disabled homolog 2, mitogen-responsive phosphoprotein (Drosophila) | DOC2 | 5p13 | Mitogen responsive phosphoprotein, suppresses the mitogenic signaling via Ras pathway, cell differentiation, cell proliferation | Methylated | [15] |
| RASAL1 | RAS protein activator like 1 (GAP1 like) | RASAL | 12q23-q24 | Ras GTPase-activating protein, negatively regulates Ras signaling | Methylated | [9] | |
| RASSF1 | Ras association (RalGDS/AF-6) domain family 1 | RASSF1A | 3p21.3 | RAS effector protein, Ras signaling, cell cycle arrest, apoptosis, DNA repair, inhibits the accumulation of cyclin D1 | Methylated, mutated | [10]–[14] | |
| SCGB3A1 | Secretoglobin, family 3A, member 1 | HIN1 | 5q35-qter | AKT signaling pathway, cell communication | Methylated | [16] | |
| Rho GTPase signaling | DLC1 | Deleted in liver cancer 1 | ARHGAP7, STARD12, p122-RhoGAP | 8p22.3 | Cell cytoskeleton organization, GTPase activator, signal transduction, cell adhesion, invasion | Methylated, deleted (LOH) | [18] |
| p53 signaling | UCHL1 | Ubiquitin carboxyl-terminal esterase L1 (Ubiquitin thiolesterase) | PARK5, PGP9.5 | 4p14 | Apoptosis, binds p53/MDM2 complex and activates p53 signaling | Methylated | [23] |
| TP73 | Tumor protein p73 | p73 | 1p36.3 | Cell cycle, DNA damage response, apoptosis, transcription factor | Methylated | [10] | |
| Wnt/β-catenin signaling | WIF1 | WNT inhibitory factor 1 | WIF-1 | 12q14.3 | Wnt-signaling pathway, binds and inhibits | Methylated | [26] |
| WNT proteins, protein-tyrosine kinase activity | |||||||
| Cell cycle signaling | BRD7 | Bromodomain containing 7 | 16q12 | Transcriptional regulation, cell cycle regulation | Methylated | [31] | |
| CDKN2A | Cyclin-dependent kinase inhibitor 2A (melanoma, p16, inhibits CDK4) | p16, INK4A, MTS1, CDK4I, CDKN2, p16INK4A | 9p21 | Cell cycle regulation p16: inhibits CDK4 kinase | p16: mutated, methylated, deleted | [10],[12],[13],[30] | |
| ARF | Alternate open reading frame | p14, p14ARF | ARF: stabilizes p53, interacts with MDM2 | ARF: methylated, deleted | |||
| CDKN2B | Cyclin-dependent kinase inhibitor 2B (p15, inhibits CDK4) | p15, MTS2, TP15, INK4B | 9p21 | Cyclin-dependent kinase inhibitor for CDK4 and CDK6, cell cycle regulation | Methylated | [10],[12],[13] | |
| CHFR | Checkpoint with forkhead and ring finger domains | RNF116, RNF196 | 12q24.33 | Mitotic checkpoint protein early in G2-M transition, cell cycle regulation | Methylated | [35] | |
| FHIT | Fragile histidine triad gene | FRA3B, AP3Aase | 3p14.2 | Cell cycle regulation, G1-S phase checkpoint, DNA-damage response, nucleotide and nucleic acid metabolism | Deleted, abnormal transcripts, methylated | [37] | |
| MIP0L1 | Mirror-image polydactyly 1 | 14q13.3 | Cell cycle (G1-S phase) regulation, up-regulates p21 and p27 protein | Methylated, deleted (LOH) | [32] | ||
| PTPRG | Protein tyrosine phosphatase, receptor type, G | PTPG, HPTPG, RPTPG | 3p21-p14 | Cell proliferation, cell cycle regulation, signal transduction | Methylated, deleted (LOH) | [38] | |
| DNA damage signaling | GADD45G | Growth arrest and DNA-damage-inducible, gamma | GADD45gamma, CR6, GRP17 | 9q22.1-q22.2 | DNA-damage response | Methylated, no mutation | [34] |
| MGMT | 0-6-methylguanine-DNA methyltransferase | 10q26 | DNA repair, senses and integrates DNA damage/repair-related signals with replication, cell cycle and genomic stability | Methylated | [10],[14] | ||
| MLH1 | Mut L homolog 1, colon cancer, nonpolyposis type 2 (E. coli) | hMLM, HNPCC, FCC2 | 3p21.3 | DNA mismatch repair protein, cell cycle G2/M arrest | Methylated | [10],[13] | |
| Cell adhesion signaling | CDH1 | Cadherin 1, type 1, E-cadherin (epithelial) | CDHE, ECAD, LCAM, CD324 | 16q22.1 | Classical cadherin, calcium dependent cell-cell adhesion, proliferation, invasion, metastasis | Methylated | [10],[13],[39] |
| CADM1 | Cell adhesion molecule 1 | IGSF4, TSLC1, NECL2, RA175, synCAM1, SglGSF | 11q23.2 | Ca2+/Mg2+-independent cell-cell adhesion, apoptosis | Methylated | [42] | |
| MMP19 | Matrix metallopeptidase 19 | MMP18, RASI-1 | 12q14 | Matrix metalloproteinase, anti-angiogenesis | Methylated, deleted (LOH) | [45] | |
| OPCML | Opioid binding protein/cell adhesion molecule-like | OPCM, OBCAM | 11q25 | IgLON immunoglobulin protein, cell adhension | Methylated | [46] | |
| PCDH10 | Protocadherin 10 | OL-PCDH,PCDH19 | 4q28.3 | Protocadherin, cell-cell adhesion, apoptosis, cell signaling | Methylated | [43] | |
| TFPI2 | Tissue factor pathway inhibitor 2 | PP5 | 7q22 | Serine protease inhibitor, metastasis | Methylated | [47] | |
| THBS1 | Thrombospondin 1 | TSP, THBS, TSP1 | 15q15 | Adhesive glycoprotein, cell-to-cell and cell-to-matrix interactions, angiogenesis, cell signaling, cell motility | Methylated | [10] | |
| Apoptosis signaling | CASP8 | Caspase 8, apoptosis-related cysteine peptidase | CAP4, MACH, MCH5, FLICE | 2q33-q34 | Apoptosis | Methylated | [10] |
| DAPK1 | Death-associated protein kinase 1 | DAPK | 9q34.1 | Positive mediator of gamma-interferon induced apoptosis | Methylated | [12]–[14] | |
| GSTP1 | Glutathione S-transferase pi 1 | DFN7, GST3 | 11q13 | Apoptosis, metabolism, energy pathways | Infrequently methylated | [14] |
Ras and Rho GTPase Signaling
Ras GTPase signaling
The Ras GTPases (H-RAS, N-RAS, and K-RAS), members of small GTPase superfamily, are aberrantly activated in most human tumors due to oncogenic mutations (10% to 90% of tumors) or deregulation of upstream or downstream signaling components, playing essential roles in tumor transformation[7]. Increasing evidences showing frequent Ras signaling deregulation in the setting of wild-type Ras in tumors indicate that epigenetic mechanism are also involved in Ras-mediated tumorigenesis.
GTPase-activating proteins (GAPs) are key regulators of the small GTPase GDP-GTP cycling. Alterative activities of GAPs may contribute to tumorigenesis by promoting tumor progression and growth[8]. Ras GTPase-activating-like protein (RASAL), a Ca2+-regulated Ras GAP that decodes the frequency of Ca2+ oscillations, was silenced by promoter CpG methylation in NPC. RASAL methylation was detected in ∼53% primary NPC but not in any nasopharyngitis or normal nasopharyngeal tissues. The malignant phenotype of NPC cells, harboring wild-type but not oncogenic forms of Ras, is dependent on loss of the Ras GAP activity of RASAL[9]. Therefore, epigenetic silencing of RASAL by promoter CpG methylation in NPC highlights the importance of deregulation of Ras GTPase signaling pathway in NPC transformation and progression.
Located at 3p21.3, a locus of particular relevance to NPC, RAS association family 1 gene (RASSF1A) is frequently inactivated by promoter CpG hypermethylation in NPC (75% cell lines and 40% to 85% primary tumors) without homozygous deletion, with no methylation detected in chronic nasopharyngitis tissues[10]–[14], suggesting that aberrant promoter methylation of RASSF1A is a critical event during NPC pathogenesis.
Remarkably, Ras GTPases exert their functions through multiple downstream effectors, such as mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3′-kinase/protein kinase B (PI3K/AM), regulating various cellular processes such as cell proliferation, survival, and differentiation [7]. Disabled-2 (DAB2), potentially suppressing mitogenic signaling via Ras pathway, is decreased or absent in most primary NPC due to promoter CpG methylation[15]. Acting as a functional tumor suppressor through inhibiting Akt signaling, high-in-normal-1 (HIN1) is hypermethylated in tumor tissues and body fluids from the patients with NPC, but not in normal samples including normal cultures, peripheral blood, nasal swabs, and throat-rinsing fluids[16]. The high specificity of HIN1 methylation in discriminating patients with NPC from normal individuals indicates its role as a tumor marker for NPC.
Rho GTPases signaling
As a member of “Ras-like” protein superfamily, Rho GTPases are deregulated during tumor progression, which promotes metastasis and cell cycle progression of tumor cells and is correlated with poor prognosis [17]. Sharing high sequence similarity (86%) to rat p122RhoGAP, Deleted in Liver Cancer 1 (DLC1) is frequently epigenetically inactivated in NPC cell lines and primary tumors from both endemic and sporadic areas, with no methylation detected in any normal nasopharyngeal tissues. Down-regulation of DLC1 contributes to NPC oncogenesis by disrupting Ras-mediated signaling pathways[18]. Epigenetic inactivation of DLC1 is common in NPC, indicating a role of aberrant Rho GTPase signaling in NPC tumorigenesis. More components in Ras and Rho GTPase signaling pathways inactivated by promoter CpG methylation and involved in NPC pathogenesis will be identified.
p53 Signaling
Unlike many other human tumors, virtually all NPC tumors with p53 accumulation are with wild-type TP53[19],[20]. EBV infection and p53 accumulation/dysfunction have been implicated in the multi-step Carcinogenesis of nasopharyngeal epithelial cells, and occur at the early stage of NPC development and associate with advanced disease stage, poor response to therapy as well[21],[22]. Although P53 protein has been shown to be regulated by various post-translational modifications, like phosphorylation and ubiquitination, modulated by EBV oncoproteins, the complexity of P53 function and regulation in NPC is still far from clear.
Located at a tumor susceptibility locus 4p11–p14, recently identified from genome-wide linkage analysis of familial NPC, Ubiquitin carboxyl-terminal hydrolase L1 (UCHL1) is expressed in normal upper respiratory tract tissues, but silenced in all NPC cell lines and 83% primary tumors by promoter CpG methylation, indicating that its methylation-mediated silencing is important in NPC pathogenesis[23]. As P53 protein is regulated through ubiquitin-dependent degradation in tumorigenesis, UCHL1 promotes p53 signaling by deubiquitinating p53 and p14ARF and ubiquitinating MDM2 for further MDM2 degradation and p53 stabilization, thus involved in NPC pathogenesis as a functional TSG[23]. The study also indicates that, for the first time, regulation of p53 stability through protein modification/degradation is involved in NPC pathogenesis.
Wnt/β-catenin Signaling
Abnormalities of Wnt/β-catenin pathway are frequently involved in multiple malignancies[24]. Epigenetic inactivation of negative Wnt/β-catenin signaling regulators leads to the aberrant activation of this signaling pathway in NPC tumorigenesis[25]. Wnt inhibitory factor-1 (WIF1), a secreted antagonist of the Wnt pathway, is frequently methylated in primary NPC tumors. With treatment of DNA demethylation reagent, WIF1 expression is restored, highlighting a direct role of epigenetic inactivation. Ectopic expression of WIF1 in NPC cells resulted in significant inhibition of tumor cell colony formation efficiency[26]. Therefore, epigenetic silencing of WIF1 contributes to the aberrant activation of Wnt/β-catenin pathway and is involved in NPC pathogenesis. Identification of more epigenetically silenced negative regulators of WNT/β-catenin signaling pathway will benefit the development of clinical strategies targeting NPC.
Cell Cycle Control-DNA Damage Signaling
Cancer is marked by uncontrolled cell proliferation derived from multiple defects in cell cycle regulation by disruption of cyclin-dependent kinases and checkpoint controllers[27],[28]. Recent advances reveal how the fidelity of cell cycle regulation could be abrogated by epigenetic changes, further granting cancer cells proliferative advantages and susceptibility to the accumulation of additional genetic alterations.
At least three checkpoints of cell cycle are identified, G1-S checkpoint, G2-M checkpoint, and mitotic checkpoint. 9p, containing several cell cycle regulators, has a high frequency of loss of heterozygosity (LOH) (61% to 85%) in NPC[29]. The classical CDK inhibitors in G1-S checkpoint, p16/INK4A, p15/INK4A, and p14/ARF, located at 9p21, were identified to be tumor suppressors involved in NPC development. Promoter CpG methylation of p16/INK4A, p15/INK4A, and p14/ARF genes was detected in about 40%, 21%, and 18% of primary NPC tumors, respectively[10],[12],[13],[30]. As an NPC-associated bromodomain-containing gene, BRD7 is frequently methylated in NPC, functioning as a TSG via inhibiting cell growth and cell cycle progression from G1 to S phase by transcriptionally regulating key cell cycle-related genes[31]. Mirror-image POLydactyly 1 (MIPOL1), a G1-S transition negative regulator up-regulating p21 (WAF1/CIP1) and p27 (KIP1), is down-regulated in over 50% of NPC tumors via promoter CpG methylation and allelic loss[32].
DNA damage signaling acts as a guardian against activated Oncogene– and environmental stress–promoted tumor progression. Incompetent cell cycle checkpoint control finally leads to defective response to cellular DNA damage, while further deficiency of regulators in DNA repair pathways results in increased susceptibility to carcinogens and also reduces the effectiveness of cancer chemotherapy[33].
Epigenetic silencing of essential components of DNA repair pathways has been a common event in NPC tumorigenesis. O-6-methylguanine-DNA methyltransferase (MGMT) is a DNA repair gene epigenetically inactivated in 20% to 28% primary NPC[10]–[14]. Mismatch repair (MMR)-associated gene human mut L homolog 1 (MLH1) is methylated in ∼40% undifferentiated NPC, which may result in a mutator phenotype associated with microsatellite instability of NPC[10],[13].
Located in a commonly deleted region 9q22, GADD45 plays an important role in G2-M checkpoint control in response to DNA damage[34]. Unlike other GADD45 family members, GADD45G is transcriptionally silenced or down-regulated in NPC with rare genetic inactivation detected. Promoter methylation of GADD45G was frequently detected in 73% NPC cell lines, but less frequently in primary tumors and not in any immortalized normal epithelial cell line or normal tissue. GADD45G could be induced by heat shock or UV irradiation in unmethylated cell lines, thus acting as a functional tumor suppressor responsive to environmental stresses[35].
Impairment of mitotic checkpoint is causally associated with chromosomal instability [36]. CHFR, one of the mitotic checkpoint regulators, is significantly decreased or silenced in NPC cell lines as well as xenografts, but not in immortalized non-malignant nasopharyngeal cell lines. Epigenetic inactivation of CHFR through promoter methylation was detected in ∼60% of primary NPC tumors but not in non-malignant tissues [37], suggesting that CHFR down-regulation is a common event in NPC. Further investigation of other epigenetically disrupted cell cycle checkpoint regulators will expand our understanding of the genomic instability in NPC tumorigenesis[38],[39].
Apoptosis Signaling
Genetic and/or epigenetic defects of apoptotic signaling genes contribute to the development of multiple cancers, as well as the resistance to radiotherapy and chemotherapy. Detection of aberrant methylation of apoptotic signaling genes is useful in identifying predictive molecular marker for treatment efficacy and outcome of NPC. Although Caspase 8 (CASP8) is an initiation Caspase of the extrinsic apoptosis pathway, only ∼7% promoter methylation of CASP8 was detected in primary undifferentiated NPC [10]. Death-associated protein kinase (DAPK), a Ca2+/calmodulin-regulated serine/threonine kinase, plays a critical role in apoptotic signaling upon cytokine exposure. Methylation of the DAPK promoter was found in 76% of NPC, as well as plasma of patients with NPC[12]–[14].
Cell Adhesion Signaling
Invasion and metastasis of tumor cells are the primary cause of the fatal outcome of cancers. Cell adhesion signaling plays a vital role in controlling the development of recurrent, invasive, and distant metastasis[40]. A striking feature of metastatic tumor cells is the abnormalities of specific cell adhesion receptors, extracellular matrix molecules, and cell-dissociating cytokines in the metastatic cascade[41].
Promoter CpG methylation silences E-cadherin (CDM), a key cell-adhesion molecule gene located at 16q22, contributing to NPC invasion and metastasis (methylated in 52% to 60% of tumors). Interestingly, a high frequency of CDH1 methylation was detected in the peripheral blood of patients with NPC (∼45%), suggesting its potential clinical application as a diagnostic marker for NPC[10],[13],[41]. Cell adhesion molecule 1 (CADM1/IGSF4/TSLC1), a member of the immunoglobulin superfamily, is another synaptic cell adhesion molecule. Promoter methylation of CADM1 was responsible for its absence or low expression in NPC, indicating its potential role in inhibiting cell proliferation and metastasis of NPC[42].
Protocadherins are a subfamily of the cadherins that encode cadherin-related neuronal receptors, playing important roles in the establishment and function of cell-cell connections. PCDH10 is the first protocadherin gene identified to be frequently silenced by promoter methylation in a tumor-specific manner in over 80% primary NPC, whereas ectopic expression of PCDH10 in silenced NPC cells dramatically inhibits their proliferation, colony formation, migration, and invasion, suggesting that PCDH10 inactivation is important in NPC tumorigenesis[43].
Matrix metalloproteinases (MMPs), zinc-dependent endopeptidases, are responsible for the degradation of various components of the extracellular matrix (ECM), involved in multiple physiological activities including the regulation of cell cycle, apoptosis, and angiogenesis [44]. MMP19 expression was down-regulated in ∼70% of primary NPC due to allelic deletion and promoter CpG methylation, whereas the catalytic activity of MMP-19 showed anti-tumor and anti-angiogenesis activities in NPC through decreasing vascular endothelial growth factor (VEGF) level[45]. Thus, disturbance of cell adhesion signaling molecules by aberrant methylation promotes tumor invasion and metastasis during NPC tumorigenesis [10],[46],[47].
Molecular Mechanisms of Aberrant Methylation Contributing to Abnormal Cell Signaling in NPC
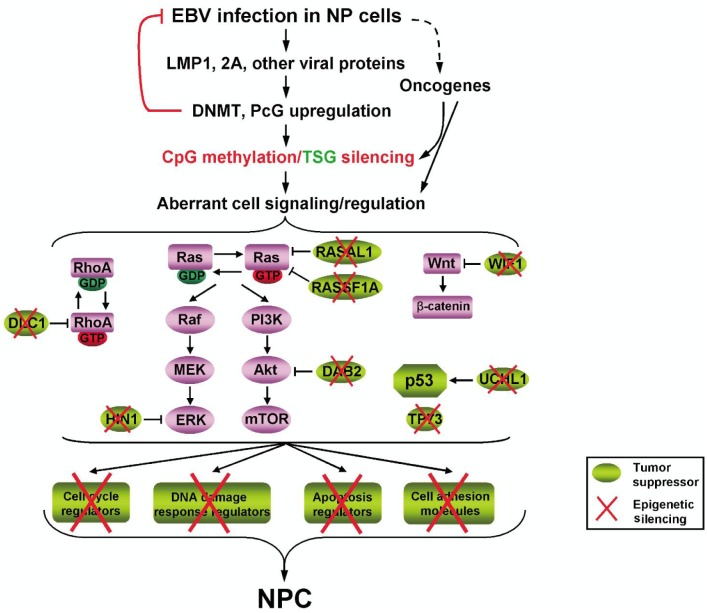
Virtually all NPC is Epstein-Barr virus (EBV) –associated. Epigenetic disruption of key host genes mediated by EBV genes is an essential event during NPC pathogenesis[48]. EBV-encoded proteins, like latent membrane protein 1 (LMP1), are key players in disrupting cell signaling in NPC through aberrant promoter methylation[49]. Elevated expression of epigenetic modifiers, such as DNA methyltransferases (DNMTs) and polycomb repressive complexes (PRCs), has been observed in various tumors including NPC[50]. Loss of TSG functions through promoter CpG methylation mediated by DNMTs and PRCs occurs frequently during tumor development. Elevated DNMT levels mediated by EBV oncoproteins were reported in NPC. EBV proteins could positively regulate both maintenance and de novo methylation [51],[52]. In addition to DNMTs, activated PRCs through EBV proteins could also modulate multiple cellular signaling pathways[53]. Thus, EBV-encoded proteins could function as initiators or cofactors in inducing epigenetic alterations of cell signaling (Figure 1).
Figure 1. Overview of the role of epigenetic disruption of cell signaling regulators mediated by EBV infection during NPC tumorigenesis. DAB2, disabled-2; DLC1, deleted in liver cancer 1; DNMT, DNA methyltransferase; EBV, Epstein-Barr virus; HIN1, high-in-normal 1; LMP1, latent membrane protein 1; LMP2A, latent membrane protein 2A; NPC, nasopharyngeal carcinoma; PcG, Polycomb protein; RASAL1, Ras GAP-activating-like protein 1; RASSF1A, Ras association domain family 1A; TSG, tumor suppressor gene; UCHL1, Ubiquitin carboxyl-terminal hydrolase L1; WIF1, Wnt inhibitory factor-1. Ras GTPase signaling negative regulators (e.g., RASAL1, RASSF1A, HIN1, and DAB2), Rho GTPase signaling negative regulators (e.g., DLC1), p53 signaling positive regulators (e.g., UCHL1 and TP73), Wnt/β-catenin signaling negative regulators (e.g., WIF1), cell cycle control-DNA damage signaling regulators, cell adhesion regulators, and apoptosis regulators play important roles in the initiation and progression of NPC. Epigenetic silencing of these antagonists or activators through promoter CpG methylation or histone modifications, initiated or mediated by EBV-encoded viral proteins, disrupts multiple cell signaling pathways during NPC tumorigenesis.
Conclusions
Aber rant “epigenetic code” of cell signaling facilitates the subsequent selection of genetic mutations of certain signaling pathways in the initiation and progression of NPC. As more epigenetic alterations of cell signaling genes are found, we will obtain systematic understanding of the molecular features of NPC. Study of epigenetically silenced cell signaling regulators in NPC will lead to the further development of clinical strategies of NPC prevention and therapy. Moreover, promoter methylation of cell signaling regulators could serve as diagnostic biomarkers for NPC risk assessment, early detection, and prognosis.
Acknowledgments
This study was supported by grants from Hong Kong RGC (GRF #473908 and #475009) and National Natural Science Foundation of China (No. 81071634).
References
- 1.Tao Q, Chan AT. Nasopharyngeal carcinoma: molecular pathogenesis and therapeutic developments [J] Expert Rev Mol Med. 2007;9(12):1–24. doi: 10.1017/S1462399407000312. [DOI] [PubMed] [Google Scholar]
- 2.Lo KW, To KF, Huang DP. Focus on nasopharyngeal carcinoma [J] Cancer Cell. 2004;5(5):423–428. doi: 10.1016/s1535-6108(04)00119-9. [DOI] [PubMed] [Google Scholar]
- 3.Lo KW, Huang DP. Genetic and epigenetic changes in nasopharyngeal carcinoma [J] Semin Cancer Biol. 2002;12(6):451–462. doi: 10.1016/s1044579x02000883. [DOI] [PubMed] [Google Scholar]
- 4.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer [J] Nat Rev Genet. 2002;3(6):415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 5.Jones PA, Baylin SB. The epigenomics of cancer [J] Cell. 2007;128(4):683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer—a mechanism for early oncogenic pathway addiction? [J] Nat Rev Cancer. 2006;6(2):107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 7.Downward J. Targeting RAS signalling pathways in cancer therapy [J] Nat Rev Cancer. 2003;3(1):11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 8.Vigil D, Cherfils J, Rossman KL, et al. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? [J] Nat Rev Cancer. 2010;10(12):842–857. doi: 10.1038/nrc2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin H, Wang X, Ying J, et al. Epigenetic silencing of a Ca(2+)- regulated Ras GTPase-activating protein RASAL defines a new mechanism of Ras activation in human cancers [J] Proc Natl Acad Sci USA. 2007;104(30):12353–12358. doi: 10.1073/pnas.0700153104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong TS, Tang KC, Kwong DL, et al. Differential gene methylation in undifferentiated nasopharyngeal carcinoma [J] Int J Oncol. 2003;22(4):869–874. [PubMed] [Google Scholar]
- 11.Lo KW, Kwong J, Hui AB, et al. High frequency of promoter hypermethylation of RASSF1A in nasopharyngeal carcinoma [J] Cancer Res. 2001;61(10):3877–3881. [PubMed] [Google Scholar]
- 12.Chang HW, Chan A, Kwong DL, et al. Evaluation of hypermethylated tumor suppressor genes as tumor markers in mouth and throat rinsing fluid, nasopharyngeal swab and peripheral blood of nasopharyngeal carcinoma patient [J] Int J Cancer. 2003;105(6):851–855. doi: 10.1002/ijc.11162. [DOI] [PubMed] [Google Scholar]
- 13.Wong TS, Kwong DL, Sham JS, et al. Quantitative plasma hypermethylated DNA markers of undifferentiated nasopharyngeal carcinoma [J] Clin Cancer Res. 2004;10(7):2401–2406. doi: 10.1158/1078-0432.ccr-03-0139. [DOI] [PubMed] [Google Scholar]
- 14.Kwong J, Lo KW, To KF, et al. Promoter hypermethylation of multiple genes in nasopharyngeal carcinoma [J] Clin Cancer Res. 2002;8(1):131–137. [PubMed] [Google Scholar]
- 15.Tong JH, Ng DC, Chau SL, et al. Putative tumour-suppressor gene DAB2 is frequently down regulated by promoter hypermethylation in nasopharyngeal carcinoma [J] BMC Cancer. 2010;10:253. doi: 10.1186/1471-2407-10-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong TS, Kwong DL, Sham JS, et al. Promoter hypermethylation of high-in-normal 1 gene in primary nasopharyngeal carcinoma [J] Clin Cancer Res. 2003;9(8):3042–3046. [PubMed] [Google Scholar]
- 17.Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins [J] Biol Cell. 2007;99(2):67–86. doi: 10.1042/BC20060086. [DOI] [PubMed] [Google Scholar]
- 18.Seng TJ, Low JS, Li H, et al. The major 8p22 tumor suppressor DLC1 is frequently silenced by methylation in both endemic and sporadic nasopharyngeal, esophageal, and cervical carcinomas, and inhibits tumor cell colony formation [J] Oncogene. 2007;26(6):934–944. doi: 10.1038/sj.onc.1209839. [DOI] [PubMed] [Google Scholar]
- 19.Kunimi K, Amano T, Uchibayashi T. Point mutation of the p53 gene is an infrequent event in untreated prostate cancer [J] Cancer Detect Prev. 1996;20(3):218–222. [PubMed] [Google Scholar]
- 20.Spruck CH, 3rd, Tsai YC, Huang DP, et al. Absence of p53 gene mutations in primary nasopharyngeal carcinomas [J] Cancer Res. 1992;52(17):4787–4790. [PubMed] [Google Scholar]
- 21.Li L, Zhou S, Chen X, et al. The activation of p53 mediated by Epstein-Barr virus latent membrane protein 1 in SV40 large T-antigen transformed cells [J] FEBS Lett. 2008;582(5):755–762. doi: 10.1016/j.febslet.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Guo L, Tao Y, et al. Latent membrane protein 1 of Epstein-Barr virus regulates p53 phosphorylation through MAP kinases [J] Cancer Lett. 2007;255(2):219–231. doi: 10.1016/j.canlet.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Tao Q, Jin H, et al. The tumor suppressor UCHL1 forms a complex with p53/MDM2/ARF to promote p53 signaling and is frequently silenced in nasopharyngeal carcinoma [J] Clin Cancer Res. 2010;16(11):2949–2958. doi: 10.1158/1078-0432.CCR-09-3178. [DOI] [PubMed] [Google Scholar]
- 24.Clevers H. Wnt/beta-catenin signaling in development and disease [J] Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Ying Y, Tao Q. Epigenetic disruption of the WNT/beta-catenin signaling pathway in human cancers [J] Epigenetics. 2009;4(5):307–312. doi: 10.4161/epi.4.5.9371. [DOI] [PubMed] [Google Scholar]
- 26.Chan SL, Cui Y, van Hasselt A, et al. The tumor suppressor Wnt inhibitory factor 1 is frequently methylated in nasopharyngeal and esophageal carcinomas [J] Lab Invest. 2007;87(7):644–650. doi: 10.1038/labinvest.3700547. [DOI] [PubMed] [Google Scholar]
- 27.Hartwell LH, Kastan MB. Cell cycle control and cancer [J] Science. 1994;266(5192):1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 28.Collins K, Jacks T, Pavletich NP. The cell cycle and cancer [J] Proc Natl Acad Sci USA. 1997;94(7):2776–2778. doi: 10.1073/pnas.94.7.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang DP, Lo KW, van Hasselt CA, et al. A region of homozygous deletion on chromosome 9p21–22 in primary nasopharyngeal carcinoma [J] Cancer Res. 1994;54(15):4003–4006. [PubMed] [Google Scholar]
- 30.Lo KW, Cheung ST, Leung SF, et al. Hypermethylation of the p16 gene in nasopharyngeal carcinoma [J] Cancer Res. 1996;56(12):2721–2725. [PubMed] [Google Scholar]
- 31.Liu H, Zhang L, Niu Z, et al. Promoter methylation inhibits BRD7 expression in human nasopharyngeal carcinoma cells [J] BMC Cancer. 2008;8:253. doi: 10.1186/1471-2407-8-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung AK, Lung HL, Ko JM, et al. Chromosome 14 transfer and functional studies identify a candidate tumor suppressor gene, mirror image polydactyly 1, in nasopharyngeal carcinoma [J] Proc Natl Acad Sci USA. 2009;106(34):14478–14483. doi: 10.1073/pnas.0900198106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hegi ME, Sciuscio D, Murat A, et al. Epigenetic deregulation of DNA repair and its potential for therapy [J] Clin Cancer Res. 2009;15(16):5026–5031. doi: 10.1158/1078-0432.CCR-08-1169. [DOI] [PubMed] [Google Scholar]
- 34.Amanullah A, Azam N, Balliet A, et al. Cell signalling: Cell survival and a Gadd45-factor deficiency [J] Nature. 2003;424(6950):741–742. doi: 10.1038/424741b. [DOI] [PubMed] [Google Scholar]
- 35.Ying J, Srivastava G, Hsieh WS, et al. The stress-responsive gene GADD45G is a functional tumor suppressor, with its response to environmental stresses frequently disrupted epigenetically in multiple tumors [J] Clin Cancer Res. 2005;11(18):6442–6449. doi: 10.1158/1078-0432.CCR-05-0267. [DOI] [PubMed] [Google Scholar]
- 36.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers [J] Nature. 1998;396(6712):643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 37.Cheung HW, Ching YP, Nicholls JM, et al. Epigenetic inactivation of CHFR in nasopharyngeal carcinoma through promoter methylation [J] Mol Carcinog. 2005;43(4):237–245. doi: 10.1002/mc.20106. [DOI] [PubMed] [Google Scholar]
- 38.Loyo M, Brait M, Kim MS, et al. A survey of methylated candidate tumor suppressor genes in nasopharyngeal carcinoma [J] Int J Cancer. 2011;128(6):1393–1403. doi: 10.1002/ijc.25443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheung AK, Lung HL, Hung SC, et al. Functional analysis of a cell cycle-associated, tumor-suppressive gene, protein tyrosine phosphatase receptor type G, in nasopharyngeal carcinoma [J] Cancer Res. 2008;68(19):8137–8145. doi: 10.1158/0008-5472.CAN-08-0904. [DOI] [PubMed] [Google Scholar]
- 40.Birchmeier W. Cell adhesion and signal transduction in cancer. Conference on cadherins, catenins and cancer [J] EMBO Rep. 2005;6(5):413–417. doi: 10.1038/sj.embor.7400408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okegawa T, Pong RC, Li Y, et al. The role of cell adhesion molecule in cancer progression and its application in cancer therapy [J] Acta Biochim Pol. 2004;51(2):445–457. [PubMed] [Google Scholar]
- 42.Lung HL, Cheng Y, Kumaran MK, et al. Fine mapping of the 11q22–23 tumor suppressive region and involvement of TSLC1 in nasopharyngeal carcinoma [J] Int J Cancer. 2004;112(4):628–635. doi: 10.1002/ijc.20454. [DOI] [PubMed] [Google Scholar]
- 43.Ying J, Li H, Seng TJ, et al. Functional epigenetics identifies a protocadherin PCDH10 as a candidate tumor suppressor for nasopharyngeal, esophageal and multiple other carcinomas with frequent methylation [J] Oncogene. 2006;25(7):1070–1080. doi: 10.1038/sj.onc.1209154. [DOI] [PubMed] [Google Scholar]
- 44.George SJ, Dwivedi A. MMPs, cadherins, and cell proliferation [J] Trends Cardiovasc Med. 2004;14(3):100–105. doi: 10.1016/j.tcm.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 45.Chan KC, Ko JM, Lung HL, et al. Catalytic activity of matrix metalloproteinase-19 is essential for tumor suppressor and anti-angiogenic activities in nasopharyngeal carcinoma [J] Int J Cancer. 2010 Dec 16; doi: 10.1002/ijc.25855. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Cui Y, Ying Y, van Hasselt A, et al. OPCML is a broad tumor suppressor for multiple carcinomas and lymphomas with frequently epigenetic inactivation [J] PLoS One. 2008;3(8):e2990. doi: 10.1371/journal.pone.0002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang S, Xiao X, Zhou X, et al. TFPI-2 is a putative tumor suppressor gene frequently inactivated by promoter hypermethylation in nasopharyngeal carcinoma [J] BMC Cancer. 2010;10:617. doi: 10.1186/1471-2407-10-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li HP, Leu YW, Chang YS. Epigenetic changes in virus-associated human cancers [J] Cell Res. 2005;15(4):262–271. doi: 10.1038/sj.cr.7290295. [DOI] [PubMed] [Google Scholar]
- 49.Niller HH, Wolf H, Minarovits J. Epigenetic dysregulation of the host cell genome in Epstein-Barr virus–associated neoplasia [J] Semin Cancer Biol. 2009;19(3):158–164. doi: 10.1016/j.semcancer.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 50.Mohammad HP, Baylin SB. Linking cell signaling and the epigenetic machinery [J] Nat Biotechnol. 2010;28(10):1033–1038. doi: 10.1038/nbt1010-1033. [DOI] [PubMed] [Google Scholar]
- 51.Tsai CN, Tsai CL, Tse KP, et al. The Epstein-Barr virus Oncogene product, latent membrane protein 1, induces the downregulation of E-cadherin gene expression via activation of DNA methyltransferases [J] Proc Natl Acad Sci USA. 2002;99(15):10084–10089. doi: 10.1073/pnas.152059399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsai CL, Li HP, Lu YJ, et al. Activation of DNA methyltransferase 1 by EBV LMP1 involves c-Jun NH(2)-terminal kinase signaling [J] Cancer Res. 2006;66(24):11668–11676. doi: 10.1158/0008-5472.CAN-06-2194. [DOI] [PubMed] [Google Scholar]
- 53.Song LB, Li J, Liao WT, et al. The Polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells [J] J Clin Invest. 2009;119(12):3626–3636. doi: 10.1172/JCI39374. [DOI] [PMC free article] [PubMed] [Google Scholar]