Abstract
Histone lysine methyltransferase EZH2 has been reported to be frequently overexpressed in hepatocellular carcinoma (HCC) tissues and associated with hepatocarcinogenesis. However, the exact mechanism of EZH2 up-regulation in HCC has not been determined. In this study, we used murine hepatocyte AML12 cells to investigate the role of hepatitis B virus X protein (HBx) in regulating the expression of mEZH2. Western blot analysis demonstrated that the expression level of mEZH2 protein in AML12 cells was up-regulated by HBx in a dose-dependent manner. To further investigate the mechanism of mEZH2 overexpression, the 2500 bp regulatory sequence upstream from the first exon of the mEZH2 gene was amplified from AML12 genomic DNA and constructed into a luciferase reporter plasmid. The luciferase activity of the mEZH2 promoter significantly increased in AML12 cells co-transfected with HBx plasmid, and deleting the −486/−214 promoter region decreased HBx-induced mEZH2 promoter activation by nearly 50%. The −486/−214 region was then analyzed in the TRANSFAC 6.0 database and a typical E2F1-binding site was found. Mutation of this E2F1-binding site or knockdown of E2F1 expression by RNAi led to a dramatic decrease in HBx-induced activation of the mEZH2 promoter and mEZH2 overexpression in AML12 cells. These results provide evidence that HBx up-regulates mEZH2 expression by transactivating the mEZH2 promoter through E2F1 transcription factor, thereby providing new epigenetic evidence for the carcinogenic effect of HBx.
Keywords: Hepatitis B virus, hepatitis B virus X protein, enhancer of zeste homolog 2 of mouse, promoter activity, E2F1
Hepatocellular carcinoma (HCC), one of the most common malignancies in the world, imperils people's health and quality of life and leads to a high mortality[1]. Chronic infection of hepatitis B virus (HBV) is the leading cause of HCC in China, and the encoded protein of the HBV X gene (HBx) plays a critical role in the tumorigenesis of HBV-related HCC[2]. HBx protein, which is encoded by HBV after infecting cells, has a wide range of biological activities, including activating cellular and viral gene transcription, inhibiting DNA damage repair, regulating cell apoptosis, and activating signal transduction. These activities affect viral replication and cell transformation[3]. However, its mechanism is still unclear.
Enhancer of zeste homolog 2 (EZH2), one component of the polycomb-repressive complex 2 (PRC2), plays a crucial role in gene silencing by trimethylating lysine 27 of histone H3 (H3K27) and has been associated with the regulation of tumorigenesis. Several studies have shown that elevated EZH2 protein expression has been found in prostate cancer, breast cancer, lymphoma, and other cancers, and it is also closely related to tumor progression and prognosis [4]. Recently, it is also reported that EZH2 is highly expressed in HCC, especially in the tumor tissues with vascular infiltration, suggesting that high expression of EZH2 is closely related to the progression of hepatoma[5]–[7].
HBx significantly increased EZH2 expression levels in hepatoma cells in our earlier experiments, thereby affecting cell migration and invasion (unpublished results). However, the mechanism has not been elucidated. In the present study, we investigated the molecular mechanisms of mEZH2 regulation by HBx and found that HBx could up-regulate mEZH2 expression by transactivating the mEZH2 promoter through E2F1 transcription factor.
Materials and Methods
Bacterial strains, cell lines, and plasmids
E. coli DH5α competent cells were purchased from TransGen Biotech. The mouse hepatocyte AML12 cell line was purchased from ATCC. AML12 cells were cultured in DMEM-F12 medium containing 10% fetal bovine serum, 0.005 mg/mL insulin, 0.005 mg/mL transferrin, 5 ng/mL selenium, and 40 ng/mL dexamethasone. The pGEM®-T vector and pGL3-Basic vector were purchased from Promega. The small hairpin RNA (shRNA) interference vector pRS-puro was purchased from ORIGENE. The enhanced green fluorescent protein (EGFP) and HBx eukaryotic expression vectors, pcDNA4.0/TO/myc-His-EGFP and pcDNA4.0/TO/myc-His-EGFP-HBx, respectively, were from our lab.
Enzymes, reagents, and antibodies
Prime STAR™ HS DNA polymerase was purchased from TaKaRa. T4 ligase and restriction enzymes (Xho I, BamH I, and Hind III) were purchased from New England Biolabs. The DNA extraction kit NucleoSpin® Extract II was purchased from Macherey-Nagel. FuGENE® HD cell transfection reagent was purchased from Roche. The genomic DNA extraction kit and Luciferase Assay System with Reporter Lysis Buffer were purchased from Promega Corporation. Polyvinylidene fluoride (PVDF) membrane, ECL Plus Western Blotting Detection System, and the protein quantification kit (2-D Quant Kit) were purchased from GE Healthcare. Anti-EZH2 mouse monoclonal antibody (Clone BD43) was purchased from Millipore Corporation, anti–β-actin mouse monoclonal antibody (Clone AC-15) was purchased from SIGMA, and HRP-labeled goat anti-mouse IgG was purchased from Zhongshan Golden Bridge Biotechnology Corporation. Other reagents were analytical grade reagents made in China.
Construction of the mEZH2 promoter vector
Genomic DNA extracted from AML12 cells and purified using Promega Wizard Genomic DNA purification kit was used as the template for polymerase chain reaction (PCR). Promoter sequence upstream of the first exon of mEZH2 was amplified by PCR using primers listed in Table 1. Briefly, four different PCR fragments were linked together to make full length 2500 bp mEZH2 promoter using overlap extension PCR. A single adenosine residue was added to the 3′-end of the full-length promoter sequence, which was subsequently cloned into the pGEM-T vector to make the pGEM-T–mEZH2 promoter vector.
Table 1. Primer sequences used in the amplification of mEZH2 promoter.
| Primer name | Primer sequence | Amplified fragment |
| P1F | 5′-TTCTGCCCATCACTATACCCTA-3′ | −2406/−1517 |
| P1R | 5′-GCACAAATGCCAATAAAGGA-3′ | |
| P2F | 5′-CTAAGGGTACACCCACGATG-3′ | −1650/−885 |
| P2R | 5′-CCCGAGGAATAGAAATGAGC-3′ | |
| P3F | 5′-CCATTATTCACCTCATTGCCAGAT-3′ | −917/−215 |
| P3R | 5′-CAGTCGCTGTCTTTGTTCTTTT-3′ | |
| P4F | 5′-CTCCCCGCCTCCTGCCCATA-3′ | −333/+22 |
| P4R | 5′-ACCGGACCGAGCGCCAAAC-3′ |
Construction of luciferase reporter vectors
The pGEM-T–mEZH2 promoter vector was used as a PCR template to amplify the −2406/+22 full-length mEZH2 promoter (transcription starts from the first exon of mEZHZ), and deletion mutants −486/+22, −214/+22, and -214/-50 with primers listed in Table 2. Additional point mutant −486/+22M was generated using primers E2F1-MF and E2F1-MR based on deletion mutant -486/+22. Xho I and Hind III restriction sites were introduced into the ends of all the amplified fragments, and the fragments were cloned into pGL3–basic vector to obtain luciferase reporter vectors under control of mEZH2 promoter or its mutants. The reporter constructs included pGL3-pmEZH2 (−2406/+22), pGL3-pmEZH2 (−486/+22), pGL3-pmEZH2 (−214/+22), pGL3-pmEZH2 (−214/−50), and the E2F1 binding site mutant pGL3-pmEZH2 (−486/+22M).
Table 2. Primer sequences used in the construction of luciferase reporter vectors.
| Primer name | Primer sequence (5′→3′) |
| −2406 XhoIF | 5′-CCGCTCGAGTTCTGCCCATCACTATAC-3′ |
| −486 XhoIF | 5′-CCGCTCGAGGGCGGTTAAGACCGTTA-3′ |
| −214 XhoIF | 5′-CCGCTCGAGTGGTGCTTCCACCCAGCAAGCCGCG-3′ |
| +22 HindIIIR | 5′-CCCAAGCTTACCGGACCGAGCGCCAAAC-3′ |
| −50 HindIIIR | 5′-CCCAAGCTTGCCTCTCCGATTGGAGGATG-3′ |
| E2F1-MF | 5′-AGAACCACTCAGCGCCCATGATCGCCAAGAGCTGGCCCGCC-3′ |
| E2F1-MR | 5′-GGCGGGCCAGCTCTTGGCGATCATGGGCGCTGAGTGGTTCT-3′ |
Construction of shRNA vectors
RNAi sequences targeting the mouse E2F1 gene were selected based on the literature[8]. The following two oligonucleotides were designed and synthesized: 5′-GATCGACGGAGGCTGGATCTGGAGTTCAAGAGAC-TCCAGATCCAGCCTCCGTTTTTTTGGAAA-3′ for mE2-F1-1 and 5′-AGCTTTTCCAAAAAAACGGAGGCTGGAT-CTGGAGTCTCTTGAACTCCAGATCCAGCCTCCGTC-3′ for mE2F1-2. The two fragments were annealed and ligated into the pRS-puro vector, which was linearized by BamH I and Hind III.
Detection of luciferase activity
Equal amounts of luciferase reporter plasmid under control of mEZH2 promoter and other plasmids (the total amount of plasmids was 0.8 µg) were co-transfected into AML12 cells. Cells were harvested after 36 h and fluorescence intensity indicating luciferase activity was determined using a single luciferase activity kit. Triplicates of each experimental group were repeated independently 3 times according to the manufacturer's instructions.
Western blotting
HBx expression plasmid pcDNA4.0/TO/myc-His-EGFP-HBx and control plasmid pcDNA4.0/TO/myc-His-EGFP were mixed in different proportions to a total amount of 1.0 µg and co-transfected into AML12 cells. After 48 h, total proteins were extracted by directly adding 1× SDS sample buffer. Protein concentration was quantified using 2-D Quant Kit. Protein samples (25 µg) were resolved by SDS-PAGE and then transferred onto PVDF membranes. EZH2 monoclonal antibody was used to detect mEZH2 expression levels in the cells. β-actin was used as an internal reference. Bands were scanned using a Typhoon 9410 scanner.
Results
HBx up-regulates protein levels of mEZH2 in AML12 cells
In the previous experiments, we found that HBx significantly up-regulated the expression level of mEZH2 in human hepatocarcinoma cell line HepG2. To further confirm this observation, we transfected HBx expression plasmid into mouse hepatocyte AML12 cells and examined its effect on mEZH2 expression. We found that increasing the amount of HBx plasmid up to 1.0 µg resulted in a gradual increase in mEZH2 expression level in AML12 cells (Figure 1A), indicating that HBx significantly up-regulated the expression level of mEZH2 in AML12 cells.
Figure 1. HBx up-regulates the mEZH2 expression by activating the mEZH2 promoter. A, the expression of mEZH2 is up-regulated by HBx. The HBx expression plasmid (0–1 µg) was transfected into AML12 cells seeded in 24-well plates. When less than 1 µg of the HBx-expressing plasmid was used for transfection, the control vector was added to bring the amount of transfected DNA up to 1 µg. The protein expression level was analyzed by Western blotting 48 h after transfection. B, amplification of mEZH2 promoter sequences. The amplified mEZH2 promoter fragments were separated by agarose electrophoresis. Lane M, Marker III; lane 1, amplified mEZH2 promoter sequence from −2406 to −1517; lane 2, mEZH2 promoter sequence from −1650 to −885; lane 3, mEZH2 promoter sequence from −971 to −215; lane 4, mEZH2 promoter sequence from −333 to +22; lane 5, the whole length of mEZH2 promoter sequence amplified from −2406 to +22. C and D, the activity of mEZH2 promoter is up-regulated by HBx in a dose-dependent manner using luciferase reporter assays. The mEZH2 promoter luciferase reporter vector pGL3-pmEZH2 (−2406/+22) was co-transfected with HBx expression vector pcDNA4.0-EGFP-HBx or control vector pcDNA4.0-EGFP. The pGL3-Basic vector without any promoter sequence was also transfected as a negative control (C). AML12 cells were co-transfected with 0.5 µg of pGL3-pmEZH2 (−2406/+22) and 0 to 1 µg of pcDNA4.0-EGFP-HBx. When less than 1 µg of the pcDNA4.0-EGFP-HBx plasmid was used for transfection, the pcDNA4.0-EGFP vector was added to bring the amount of transfected DNA up to 1 µg (D). The data shown was one result of two independent experiments.
Amplification of the mEZH2 promoter sequence and construction of reporter vectors
HBx can activate the transcriptional regions of various homologous or heterologous viruses and cells, including the HBV enhancer/core promoter, cytomegalovirus (CMV) promoter, herpes simplex virus thymidine kinase (HSV-TK) promoter, and simian virus 40 (SV40) promoter[3]. To further elucidate the mechanism by which HBx increases mEZH2 expression in AML12 cells, we amplified the 2500 bp sequence upstream of the first exon of mEZH2 gene from AML12 cell genomic DNA (Figure 1B), and then constructed the luciferase reporter vector pGL3-pmEZH2 (−2406/+22) under control of the mEZH2 promoter.
HBx up-regulates mEZH2 promoter activity
Luciferase reporter vector pGL3-pmEZH2 (−2406/+22), which was under the control of the mEZH2 promoter, and HBx expression vector pcDNA4.0-EGFP-HBx were co-transfected into AML12 cells. The pcDNA4.0-EGFP plasmid was used as a negative control, and pGL3-basic vector was also transfected as an empty control for endogenous luciferase activity. Luciferase activity was detected 36 h after transfection. The mEZH2 promoter sequence (−2406/+22) upstream of the first exon had transcriptional activity compared to the control, and the expression of HBx in cells significantly increased its activity (Figure 1C).
To further determine the effect of HBx on mEZH2 promoter activity, 0 to 1 µg of HBx expression plasmid was co-transfected with 0.5 µg mEZH2 promoter-controlled luciferase reporter vector pGL3-pmEZH2 (−2406/+22) into AML12 cells. The total amount of HBx expression plasmid and pcDNA4.0-EGFP vector was 1 µg to ensure transfection consistency and efficiency. We found that the luciferase activity of the mEZH2 promoter (−2406/+22) gradually increased as the amount of HBx increased, indicating that HBx regulates mEZH2 promoter activity in AML12 cells in a dose-dependent manner (Figure 1D).
HBx primarily regulates mEZH2 promoter activity through the −486/−214 promoter region
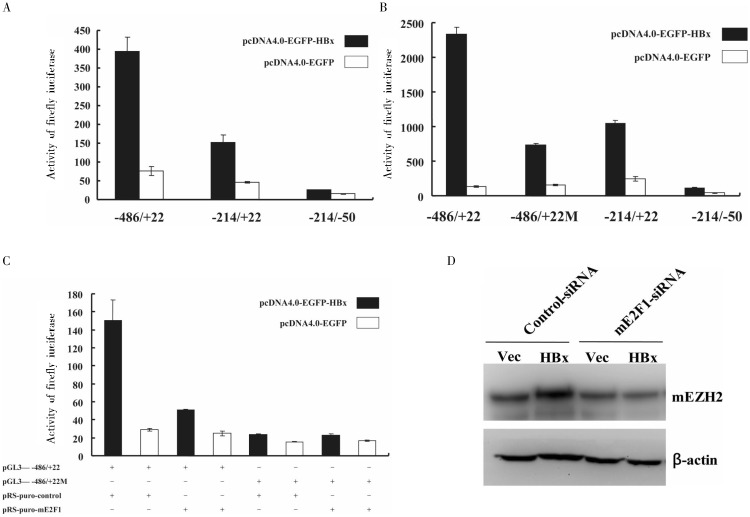
To further identify the region of the mEZH2 promoter that is critical for the HBx effect, we truncated the mEZH2 promoter region −2406/+22 to create fragments of differing lengths. The truncated construct vectors, pGL3-pmEZH2 (−486/+22), pGL3-pmEZH2 (−214/+22), and pGL3-pmEZH2 (−214/−50), were co-transfected with HBx expression plasmid into AML12 cells, and the effects of HBx on the truncated mEZH2 promoters were detected. When the mEZH2 promoter fragment −486/+22 was truncated to −214/+22, the effect of HBx dropped by nearly a half compared to the -486/+22 fragment (Figure 2A), suggesting that a critical element for HBx-induced mEHZ2 promoter regulation lies within the −486/−214 region of the mEHZ2 promoter. The promoter mutant −214/−50, in which the transcription start site had been lost, exhibited only minimal transcriptional activity and was used as a negative control.
Figure 2. HBx up-regulates the activity of mEZH2 promoter and mEZH2 expression through E2F1 transcription factor. A, HBx up-regulates the activity of mEZH2 promoter through −486/−214 region. The truncated mEZH2 promoter vector pGL3-pmEZH2 (−486/+22) or pGL3-pmEZH2 (−214/+22) was co-transfected with HBx expression vector pcDNA4.0-EGFP-HBx, and the luciferase activity was detected 36 h after tranfection. The mEZH2 promoter vector pGL3-pmEZH2 (−214/−50), of which candidate transcriptional start site deleted, was used as the negative control. B, mutation of E2F1-binding site leads to noticeable decline of HBx activation to mEZH2 promoter. The mutated mEZH2 promoter vector pGL3-pmEZH2 (−486/+22M) or pGL3-pmEZH2 (−486/+22) was co-transfected with HBx expression vector pcDNA4.0-EGFP-HBx, the luciferase activity was detected 36 h after tranfection. The mEZH2 promoter vector pGL3-pmEZH2 (−214/−50), in which the candidate transcription start site was deleted, and the pGL3-pmEZH2 (−214/+22), in which the candidate E2F1-binding site was deleted, were used as the negative and positive controls, respectively. C, HBx up-regulates the activity of mEZH2 promoter through E2F1 transcription factor. The mEZH2 promoter pGL3-pmEZH2 (−486/+22) was co-transfected with E2F1 shRNA expression vector pRS-puro-mE2F1 and HBx expression vector pcDNA4.0-EGFP-HBx, and the luciferase activity was detected 36 h after tranfection. The mutated mEZH2 promoter vector pGL3-pmEZH2 (−486/+22M) co-transfected with pRS-puro-mE2F1 and pcDNA4.0-EGFP-HBx was used as negative control. D, HBx up-regulates the expression level of mEZH2 through E2F1 transcription factor. The E2F1 shRNA expression vector pRS-puro-mE2F1 was co-transfected with HBx expression vector pcDNA4.0-EGFP-HBx (HBx) or empty vector pcDNA4.0-EGFP (Vec), and the protein expression level was analyzed by Western blotting 48 h after tranfection. The control shRNA expression vector pRS-puro-control co-transfected with pcDNA4.0-EGFP-HBx (HBx) or pcDNA4.0-EGFP (Vec) was used as negative control.
HBx reg ulates mEZH2 promoter activity and protein expression through E2Fl-binding sites
We analyzed the cis-elements in the mEZH2 promoter region −2406/+22 using the TRANSFAC 6.0 database and previous literature[9] and found a canonical E2F1 transcription factor-binding site (CCCATTCCCGC) in the promoter region −486/−214. To test whether HBx increases mEZH2 promoter activity through the E2F1-binding site, we mutated the E2F1-binding site to CCCATGATCGC in the promoter fragment −486/+22, thereby disrupting E2F1-binding ability. HBx-induced luciferase activity of this E2F1 mutant (−486/+22M) was significantly decreased compared to the fragment with the wild-type E2F1-binding site (−486/+22) and exhibited similar activity as the −214/+22 fragment (Figure 2B). These results show that the E2F1-binding site plays an important role on HBx-regulated promoter activity of mEZH2.
We further determined the function of transcription factor E2F1 on HBx-regulated mEZH2 expression using RNAi to knockdown E2F1 expression. E2F1 knockdown significantly decreased the luciferase activity of the mEZH2 promoter region −486/+22 by HBx compared to the control, but had no effect on the luciferase activity of the mutant −486/+22M (Figure 2C). Furthermore, Western blotting showed that E2F1 knockdown significantly reduced HBx-induced expression of mEZH2 (Figure 2D). These results further confirm that HBx can activate mEZH2 promoter activity to increase mEZH2 protein through the transcription factor E2F1.
Discussion
Numerous studies suggest that HBx transactivates several cancer-related genes is an important mechanism leading to malignant transformation and tumorigenesis, therefore, HBx is considered to play an important role during HBV chronic infection-induced HCC progression [3],[10]. Several oncogenes, such as c-myc, c-fos, c-jun and c-met, are found to be transactivated by HBx, leading to malignant transformation of hepatocytes and tumorigenesis.
Polycomb gene EZH2 is a newly identified Oncogene with histone methyltransferase activity and involved in regulation of cell differentiation and of embryonic development. In recent years, EZH2 has been reported to be highly expressed in many tumors and is closely related to tumor formation and growth and facilitates cell proliferation, tumor invasion, and metastasis[4],[11]. In HCC studies, the Sasaki and Sudo laboratories reported that the expression level of EZH2 protein in cancer tissues was significantly higher than that in the adjacent tissues and was closely related to tumor malignancy[5],[7]. Gong et al.[12] also reported similar observations. We further found that nearly 90% of the tumor specimens that Gong and colleagues used were HBsAg-positive[12], EZH2 protein was also highly expressed in 10 out of 14 HBsAg-positive HCC specimens used by Sudo et al.[7] and in 16 out of 21 HBsAg-positive HCC specimens used by Yonemitsu et al.[6]. These results suggest that high levels of EZH2 expression in HCC may be closely related to HBV infection.
The sequence of the upstream regulatory region of the EZH2 promoter and the EZH2 protein are highly conserved between humans and mice, indicating that murine mEZH2 plays a similar role to human EZH2 in cell differentiation, individual development, and tumorigenesis[4],[13],[14]. In the present study, we investigated the effect of HBx on mEZH2 expression in the murine hepatocyte cell line AML12. We found that HBx increased mEZH2 expression level by transactivating mEZH2 promoter activity, suggesting that the expression of EZH2 may be regulated by HBV in human hepatocarcinoma. Bracken et al.[15] confirmed that EZH2 is located in downstream of the pRB-E2F signaling pathway and is directly regulated by the E2F transcription factors. Similarly, we found in our experiments that the mEZH2 promoter region contains an E2F1-binding site, which is very important for HBx-enhanced promoter activity. E2F1 knockdown greatly reduced the HBx-induced mEZH2 promoter activation and increase in mEZH2 protein expression level. HBx has been shown to activate the pRb-E2F1 signaling pathway by inducing pRb phosphorylation[16],[17] and enhance E2F1 transcriptional activity by interacting to E2F1[18]. These results suggest that HBx may activate the pRb-E2F1 signaling pathway, up-regulate mEZH2 expression, and thus contribute to malignant transformation and tumorigenesis.
In summary, we found that HBx transactivates the promoter of histone methyltransferase mEZH2 through the transcription factor E2F1-binding site, resulting in increased mEZH2 expression in liver cells. Our results provide new evidence of the role of HBx protein in tumorigenesis and lay the molecular foundation for HCC diagnosis and treatment in the future.
References
- 1.Aravalli RN, Steer CJ, Cressman EN. Molecular mechanisms of hepatocellular carcinoma [J] Hepatology. 2008;48(6):2047–2063. doi: 10.1002/hep.22580. [DOI] [PubMed] [Google Scholar]
- 2.Liu CJ, Kao JH. Hepatitis B virus-related hepatocellular carcinoma: epidemiology and pathogenic role of viral factors [J] J Chin Med Assoc. 2007;70(4):141–145. doi: 10.1016/S1726-4901(09)70346-6. [DOI] [PubMed] [Google Scholar]
- 3.Tang H, Oishi N, Kaneko S, et al. Molecular functions and biological roles of hepatitis B virus x protein [J] Cancer Sci. 2006;97(10):977–983. doi: 10.1111/j.1349-7006.2006.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics [J] Mutat Res. 2008;647(1–2):21–29. doi: 10.1016/j.mrfmmm.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki M, Ikeda H, Itatsu K, et al. The overexpression of Polycomb group proteins Bmi1 and EZH2 is associated with the progression and aggressive biological behavior of hepatocellular carcinoma [J] Lab Invest. 2008;88(8):873–882. doi: 10.1038/labinvest.2008.52. [DOI] [PubMed] [Google Scholar]
- 6.Yonemitsu Y, Imazeki F, Chiba T, et al. Distinct expression of Polycomb group proteins EZH2 and BMI1 in hepatocellular carcinoma [J] Hum Pathol. 2009;40(9):1304–1311. doi: 10.1016/j.humpath.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Sudo T, Utsunomiya T, Mimori K, et al. Clinicopathological significance of EZH2 mRNA expression in patients with hepatocellular carcinoma [J] Br J Cancer. 2005;92(9):1754–1758. doi: 10.1038/sj.bjc.6602531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang WH, Hullinger RL, Andrisani OM. Hepatitis B virus X protein via the p38MAPK pathway induces E2F1 release and ATR kinase activation mediating p53 apoptosis [J] J Biol Chem. 2008;283(37):25455–25467. doi: 10.1074/jbc.M801934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matys V, Fricke E, Geffers R, et al. Transfac?: transcriptional regulation, from patterns to profiles [J] Nucl Acids Res. 2003;31(1):374–378. doi: 10.1093/nar/gkg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lupberger J, Hildt E. Hepatitis B virus-induced oncogenesis [J] World J Gastroenterol. 2007;13(1):74–81. doi: 10.3748/wjg.v13.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tonini T, D'Andrilli G, Fucito A, et al. Importance of Ezh2 Polycomb protein in tumorigenesis process interfering with the pathway of growth suppressive key elements [J] J Cell Physiol. 2008;214(2):295–300. doi: 10.1002/jcp.21241. [DOI] [PubMed] [Google Scholar]
- 12.Gong ZB, Li YM, Yu CH. Polycomb group protein EZH2 overexpresses in the hepatocellular carcinoma [J] J Prac Oncol. 2006;21(6):523–526. [in Chinese] [Google Scholar]
- 13.Laible G, Wolf A, Dom R, et al. Mammalian homologues of the Polycomb-group gene Enhancer of zeste mediate gene silencing in Drosophila heterochromatin and at S. cerevisiae telomeres [J] Embo J. 1997;16(11):3219–3232. doi: 10.1093/emboj/16.11.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Carroll D, Erhardt S, Pagani M, et al. The polycomb-group gene Ezh2 is required for early mouse development [J] Mol Cell Biol. 2001;21(13):4330–4336. doi: 10.1128/MCB.21.13.4330-4336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bracken AP, Pasini D, Capra M, et al. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer [J] Embo J. 2003;22(20):5323–5335. doi: 10.1093/emboj/cdg542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung JK, Arora P, Pagano JS, et al. Expression of DNA methyltransferase 1 is activated by hepatitis B virus X protein via a regulatory circuit involving the p16INK4a-cyclin D1-CDK 4/6-pRb-E2F1 pathway [J] Cancer Res. 2007;67(12):5771–5778. doi: 10.1158/0008-5472.CAN-07-0529. [DOI] [PubMed] [Google Scholar]
- 17.Choi BH, Choi M, Jeon HY, et al. Hepatitis B viral X protein overcomes inhibition of E2F1 activity by pRb on the human Rb gene promoter [J] DNA Cell Biol. 2001;20(2):75–80. doi: 10.1089/104454901750070274. [DOI] [PubMed] [Google Scholar]
- 18.Choi M, Lee H, Rho HM. E2F1 activates the human p53 promoter and overcomes the repressive effect of hepatitis B viral X protein (Hbx) on the p53 promoter [J] IUBMB Life. 2002;53(6):309–317. doi: 10.1080/15216540213466. [DOI] [PubMed] [Google Scholar]