Abstract
Tumor-associated macrophages (TAMs) can elicit contrasting effects on tumor progression, depending on different tumor microenvironment. This study aimed to explore the correlation between TAM infiltration and clinicopathologic characteristics, metastasis, and prognosis of supraglottic laryngeal carcinoma. TAMs in intratumoral and peritumoral regions of 84 specimens of supraglottic laryngeal carcinoma tissues were detected by immunohistochemical staining with monoclonal CD68 antibody. The density of peritumoral CD68+ TAMs in recurrence cases (9/11) and in dead cases (17/23) were significantly higher than those in non-recurrence cases (33/73) and in survival cases (25/61), with significant differences (P = 0.024 and 0.007, respectively). The Kaplan-Meier survival analysis showed a significant relationship between the infiltration of both intratumoral and peritumoral CD68+ TAMs and the overall survival of patients. The 5-year survival rate was significantly lower in the group with a high density of intratumoral CD68+ TAMs than in the group with a low density (39.6% vs. 82.5%, P < 0.05). Similarly, the 5-year survival rate was significantly lower in the group with a high density of peritumoral CD68+ TAMs than in the group with a low density (50.6% vs. 73.1%, P < 0.05). Cox regression analysis revealed that T classification, distant metastasis, and intratumoral or peritumoral CD68+ TAMs were independent factors for disease-free survival, whereas T classification and intratumoral CD68+ TAMs were independent factors for overall survival. The results indicate that TAM infiltration in supraglottic laryngeal carcinoma can be used to predict metastasis and prognosis and is an independent factor for prognosis.
Keywords: Laryngeal neoplasm, squamous cell carcinoma, tumor-associated macrophages, prognostic factor
Tumor-associated macrophages (TAMs), macrophages in the tumor microenvironment, are the most common tumor interstitial inflammatory cells and account for 30% to 50% of the total inflammatory cell population. Macrophages are generally considered to play an important role in the process of anti-tumor immunological regulation. They can directly kill tumor cells by secreting a variety of cytokines, such as tumor necrosis factor-α (TNF-α), nitric oxide (NO), and interferon-γ (IFN-γ), which have antitumor activity[1], or clear tumor cells by presenting tumor-associated antigen to induce immune response. However, in recent years, TAMs have been found to promote tumor growth, angiogenesis, invasion, and metastasis by secreting a large amount of growth factors, cytokines, chemokines, and enzymes through complex autocrine and paracrine mechanisms[2]. A high degree of TAM infiltration in tumor tissues has been correlated with poor prognosis in many cancers, including such as lymphoma, cervical cancer, bladder cancer, and breast cancer. In recent years, many studies have also confirmed that a high degree of TAM infiltration is correlated with tumor metastasis[3]–[5]. Studies in genetically modified mice and other animal models, as well as cell culture show that low TAM infiltration can inhibit tumor growth and metastasis[6]. Hiraoka et al.[7] found that the incidence of bone and muscle metastasis was significantly decreased in the macrophage-deficient mouse model after injection of lung cancer HARA-B cells from the left ventricle. Nevertheless, some studies show opposite results. For example, increased TAMs indicated the favorite prognosis in gastric cancer, colorectal cancer, and melanoma. In addition, TAM level has no correlation with the prognosis of some tumors, such as lung cancer and colon cancer. This conflicting performance of TAMs in different tumors was previously attributed to functional polymorphism of TAMs in different tumor microenvironment. However, recent studies showed that the dual inhibitory and promotive functions of TAMs are due to unique sub-groups that exhibit different phenotypes and functional characteristics in distinct microenvironments[8].
The type and activity of activated macrophages depend on the stimulation signaling and the characteristics of the regulatory cytokines. Macrophages induced by IFN-γ, lipopolysaccharide, TNF-α, or granulocyte-macrophage colony stimulating factor are considered classically-activated M1 macrophages, which are the effector cells in type I immune responses that secrete inflammatory cytokines, present antigens, trigger immune response, and kill pathogens and tumor cells. Macrophages induced by interferon-4 (IL-4), IL-10, IL-13, transforming growth factor-β1 (TGF-β1), steroids, and prostaglandin E2 are considered alternatively-activated M2 macrophages. M2 macrophages have a weak ability to kill pathogens but a strong ability to remove body residue. M2 macrophages also promote tumor growth and metastasis by stimulating angiogenesis and extracellular matrix deposition, as well as inhibiting T cell proliferation. The distribution proportion of M1 and M2 macrophages determines the inhibitory or promotive effect of TAMs on tumors[9]. Previous studies suggest that a dominant proportion of M2 macrophages is the reason that TAMs predict poor prognosis for tumor patients [10]. Interestingly, Ohri et al.[11] found that a dominant proportion of M1 macrophages indicates a higher survival rate in non-small cell lung cancer.
In the present study, we explored the clinicopathologic significance of TAMs and their effect on the prognosis of supraglottic laryngeal carcinoma, which could provide a theoretical basis for the clinical guide and intervention.
Materials and Methods
Clinical data
A total of 84 patients with supraglottic laryngeal carcinoma, 77 men and 7 women aged 43 to 95 years (median age: 67 years) who underwent surgical treatment in the Otolaryngology Department of Kurume University in Japan between January 1995 and January 2004, were enrolled in our study using a simple random sampling method. All cases were diagnosed as squamous cell carcinoma and confirmed by postoperative pathologic examination. According to the 2002 TNM staging standards, 4 cases were at stage T1, 22 at T2, 17 at T3, 41 at T4; 21 cases were poorly differentiated, 48 were moderately differentiated, and 15 were well differentiated; 34 patients had lymph node metastasis (6 cases at stage N1, 27 at N2, and 1 at N3), 50 had no lymph node metastasis; 13 patients had distant metastasis. Eleven patients had postoperative recurrence. Twenty-three patients died of supraglottic laryngeal carcinoma. The 5-year survival rate was 65.74%.
Immunohistochemistry
Main reagents
Mouse anti-human CD68 monoclonal antibody (1:100) and the Envision/HRP (DAB) kit were purchased from DAKO Co. (USA). Peroxidase blocking buffer, 0.01 mol/L (pH6.0) trisodium citrate antigen retrieval solution, and 0.01 mol/L (pH7.4) PBS were purchased from Maxim Biotech Co. (USA).
Sample preparation
The paraffin-embedded supraglottic laryngeal carcinoma tissues were sliced into 4-µm sections, dried at 42°C for 6 h, and deparaffinized. For each sample, one section was prepared for routine HE staining and others for immunohistochemical staining.
Immunohistochemical staining (Envision/HRP)
The sections were heated in 0.01 mol/L (pH6.0) trisodium citrate buffer at 100°C for 20 min for antigen retrieval. The sections were incubated with CD68 antibody for 2 h at room temperature. Then, the sections were incubated with Envision second antibody (1:100) at room temperature for 30 min. DAB was used to reveal specific binding, followed by hematoxylin counterstaining, dehydration, clearing, and neutral gum sealing. PBS replaced of the primary antibody served as the control. Under light microscope, the cells with brown CD68 staining in cytoplasm were TAMs.
TAM counting
According to the region dividing standard of Soeda et al.[12], the tumor tissue was divided into intratumoral and peritumoral regions. TAMs in these regions were counted. Macrophages in necrotic area were not counted. Three fields of vision with the highest densities of TAMs in the intratumoral and peritumoral regions were selected to count TAMs under the microscope with 10 × 40 magnification. The average value was recorded as the number of TAMs for each specimen.
Statistical analysis
Overall survival (OS) was the survival time from surgery to death due to any cause or to the last follow-up. Disease-free survival (DFS) was the survival time from surgery to recurrence, metastasis, or death due to supraglottic laryngeal carcinoma. The results were analyzed with t test and Chi-square test after a homogeneity test for variance. All data were analyzed with SPSS 13.0 software. A Kaplan-Meier survival curve was drawn to calculate OS and DFS. The intergroup difference was analyzed with log-rank test. The main prognostic factors affecting the survival rate of patients with supraglottic laryngeal carcinoma was screened using the multivariate Cox regression model. P < 0.05 was considered significant.
Results
TAM infiltration in supraglottic laryngeal carcinoma
TAMs had brown CD68 staining mainly in the cytoplasm, but also in some nuclei. In supraglottic laryngeal carcinoma, TAMs were mainly distributed in the peritumoral stroma (Figure 1A) and tumor tissues (Figure 1B). Intratumoral infiltration of TAMs was observed in 69.05% (58/84) of supraglottic laryngeal carcinoma cases, and peritumoral infiltration was observed in 80.95% (68/84) of cases, without significant difference (Figure 1). The number of TAMs ranged from 0 to 342 (median: 9.5) in the intratumoral region and from 0 to 361 (median: 23.5) in the peritumoral region. The TAMs in supraglottic laryngeal carcinoma showed a diffuse or aggregative distribution and oval or irregular shapes.
Figure. 1. Tumor-associated microphages (TAMs) in supraglottic laryngeal squamous cell carcinoma are detected by immunohistochemical staining for CD68. Tumor tissue was divided into intratumoral and peritumoral regions. The cells with brown CD68 staining in cytoplasm were identified as TAMs. A, peritumoral TAMs distribute in the interstitial area of supraglottic laryngeal squamous cell carcinoma. B, intratumoral TAMs distribute in the cancer cell nest area of supraglottic laryngeal squamous cell carcinoma.

Correlation of TAMs and the clinicopathologic features of supraglottic laryngeal carcinoma
Among 84 patients with supraglottic laryngeal carcinoma, the median number of intratumoral TAMs was 9.5. Values greater than or equal to 10 were recorded as high density of intratumoral TAMs, and values less than 10 were recorded as low density. The median number of peritumoral TAMs was 23.5. Values greater than or equal to 24 were recorded as high density of peritumoral TAMs, and values less than 24 were recorded as low density. The correlation between the presence of peritumoral or intratumoral TAMs and sex, tumor differentiation, T stage, lymph node metastasis, recurrence, and prognosis of patients were analyzed (Table 1). The density of peritumoral TAMs was significantly higher in the recurrence group than in the non-recurrence group (P = 0.024) and higher in the death group than in the survival group (P = 0.007). In contrast, the density of intratumoral TAMs was significantly higher in the lymph node metastasis group than in the non-lymph node metastasis group (P = 0.002).
Table 1. Association between clinicopathologic features of supraglottic laryngeal carcinoma and peritumoral and intratumoral tumor-associated microphage (TAM) infiltration.
| Variable | Cases | Peritumoral TAM count |
P value | Intratumoral TAM count |
P value | ||
| Low | High | Low | High | ||||
| Sex | 0.500 | 0.216 | |||||
| Male | 77 | 39 | 38 | 37 | 40 | ||
| Female | 7 | 3 | 4 | 5 | 2 | ||
| Differentiation | 0.190 | 0.336 | |||||
| Well | 15 | 7 | 8 | 5 | 10 | ||
| Moderate | 48 | 19 | 29 | 25 | 23 | ||
| Poor | 21 | 16 | 5 | 12 | 9 | ||
| T stage | 0.127 | 0.127 | |||||
| T1–2 | 30 | 18 | 12 | 18 | 12 | ||
| T3–4 | 54 | 24 | 30 | 24 | 30 | ||
| Lymph node metastasis | 0.412 | 0.002 | |||||
| No | 50 | 26 | 24 | 18 | 32 | ||
| Yes | 34 | 16 | 18 | 24 | 10 | ||
| Distant metastasis | 0.500 | 0.500 | |||||
| No | 71 | 36 | 35 | 36 | 35 | ||
| Yes | 13 | 6 | 7 | 6 | 7 | ||
| Recurrence | 0.024 | 0.289 | |||||
| No | 73 | 40 | 33 | 38 | 35 | ||
| Yes | 11 | 2 | 9 | 4 | 7 | ||
| Prognosis | 0.007 | 0.071 | |||||
| Live | 61 | 36 | 25 | 34 | 27 | ||
| Died | 23 | 6 | 17 | 8 | 15 | ||
Among 84 patients with supraglottic laryngeal carcinoma, the median number of intratumoral TAMs was 9.5. Values greater than or equal to 10 were recorded as high density of intratumoral TAMs, and values less than 10 were recorded as low density. The median number of peritumoral TAMs was 23.5. Values greater than or equal to 24 were recorded as high density of peritumoral TAMs, and values less than 24 were recorded as low density.
Correlation of intratumoral and peritumoral TAM infiltration and the prognosis of supraglottic laryngeal carcinoma
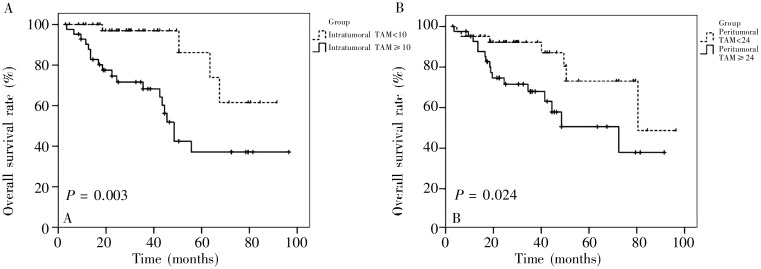
A Kaplan-Meier survival analysis was used to assess the correlation between prognosis and intratumoral or peritumoral TAM infiltration in supraglottic laryngeal carcinoma. The 5-year survival rate was significantly lower in the patients with a high density of intratumoral TAMs than in those with a low density of intratumoral TAMs (39.6% vs. 82.5%, P = 0.003) (Figure 2A). Similarly, the 5-year survival rate was significantly lower in the patients with a high density of peritumoral TAMs than in those with a low density of peritumoral TAMs (50.6% vs. 73.1%, P = 0.024) (Figure 2B). The patients with a high TAM count showed significantly poorer prognosis than those with a low TAM count.
Figure 2. Survival curves of 84 supraglottic laryngeal carcinoma patients with a high or low density of TAMs. The patients' survival was analyzed by Kaplan-Meier method. The intergroup differences were determined by log-rank test. A, the 5-year survival rate of the patients with high intratumoral TAM infiltration is significantly lower than that of the patients with low infiltration (P = 0.003). B, the 5-year survival rate of the patients with high peritumoral TAM infiltration is significantly lower than that of the patients with low infiltration (P = 0.024).
Prognostic factor analysis of supraglottic laryngeal carcinoma
Univariate analysis showed that TNM stage, tumor differentiation, distant metastasis, and intratumoral and peritumoral TAM density were the risk factors for OS, whereas tumor differentiation, distant metastasis, and intratumoral and peritumoral TAM density were the risk factors for DFS (P < 0.05). The significant factors from the univariate analysis were analyzed using the Cox regression model. The results showed that T stage, distant metastasis, intratumoral and peritumoral TAM density were independent prognostic factors for DFS (P < 0.05), and T stage and intratumoral TAM density were independent prognostic factors for OS (P < 0.05) (Table 2).
Table 2. Cox regression analysis of prognosis of supraglottic laryngeal carcinoma.
| Variable | DFS |
OS |
||
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| T stage | ||||
| T1–2 vs. T3–4 | 3.266(1.316–8.103) | 0.007 | 2.432 (0.826–7.163) | 0.036 |
| N stage | ||||
| No vs. yes | 1.459(0.442–4.820) | 0.533 | 1.436(0.632–3.264) | 0.385 |
| Differentiation | ||||
| Well vs. moderate vs. poor | 0.934 (0.364–2.398) | 0.887 | 0.881 (0.465–1.671) | 0.699 |
| M stage | ||||
| No vs. yes | 6.004 (1.627–22.156) | 0.002 | 2.043 (0.744–5.613) | 0.157 |
| TAM count | ||||
| Peritumoral (low vs. high) | 5.455 (1.168–25.470) | 0.016 | 1.409(0.551–3.603) | 0.472 |
| Intratumoral (low vs. high) | 4.204 (0.906–19.497) | 0.046 | 1.006 (1.001–1.011) | 0.018 |
CI, confidence interval.
Discussion
Macrophages are derived from CD34+ bone marrow precursor immature monocytes[13], which are the key component of inflammatory cells in the tumor interstitial substance. Under the influence of TNF-α and some chemotatic factors, such as monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory proteins-1 (MIP-1), and Rantes, monocytes migrate to the tumor tissue and differentiate into tumor-associated macrophages, which play a role in tumor growth, progression, invasion, metastasis, adaptive immunity, mesenchymal remodeling, and blood vessel and lymphoduct formation. The specific mechanisms driving their roles in tumors include the following: (1) TAMs secrete growth factors, such as epidermal growth factor (EGF), platelet-derived growth factor (PDGF) and hepatocyte growth factor (HGF), which directly promote tumor cell growth; (2) TAMs secrete vascular growth factors, such as vascular endothelial growth factor A (VEGF-A) and basic fibroblast growth factor (bFGF), which promote tumor angiogenesis; (3) TAMs secrete VEGF-C and stimulate tumor cells to secrete VEGF-C and VEGF-D, which promote the formation of lymphoducts; (4) TAMs secrete proteolytic enzymes, such as matrix metalloproteinase-9 (MMP-9), fibrinolysin, and urokinase-type plasminogen activator (uPA) and its receptor, which destroy the basement membrane, dissolve the extracellular matrix, and enhance the invasiveness and metastasis of tumor cells; (5) TAMs produce chemokines, such as CCL17, CCL22, and AMAC-1; and (6) TAMs suppress the immune system.
Because many studies suggest that TAMs directly promote tumor progression, we hypothesized that reducing TAMs in the supraglottic laryngeal carcinoma may be an effective treatment for controlling tumor progression. Recent studies show that IL-10 and Linomide can effectively reduce the number of TAMs. More specifically, TAM infiltration in tumor-bearing mice with IL-10 transfection was significantly lower than in those without IL-10 transfection[14], whereas Linomide could significantly reduce intratumoral TAM infiltration and the amount of TNF-α secreted by TAMs[15]. Further studies are needed to confirm whether these treatments can effectively reduce TAM infiltration in head and neck tumor.
Correlation between TAM infiltration and prognosis differs according to tumor type. TAM infiltration predicts poor prognosis in breast cancer[16], uterine cervix cancer[17], and bladder cancer[18], whereas it predicts good prognosis in prostate cancer[19], lung cancer[20], and brain tumors[21]. These inconsistencies may be related to the number of clinical cases, tumor grade, tumor stage, tumor type, TAM type, invasive site, and the method used to assess TAM infiltration (systemic counting or hot spot counting, counting peritumoral TAMs or intratumoral TAMs, or both).
To date, only a few studies on the correlation between TAM infiltration and prognosis in head and neck cancers have been conducted. These studies have mainly focused on oral and thyroid cancers, with only a few focused on laryngeal cancer. Marcus et al.[22] found that TAM infiltration in oral cancer was significantly associated with lymph node metastasis. In the present study, we divided the tumor into intratumoral and peritumoral regions according to the method of Soeda et al.[12] and counted TAMs in these areas, excluding TAMs in necrotic region. The results showed that the number of peritumoral TAMs was significantly associated with tumor differentiation and recurrence. In addition, the density of peritumoral TAMs was higher in samples from patients who had tumor recurrence or died due to the tumor. The intratumoral TAM number was significantly related with N stage, and a high density of intratumoral TAMs was only found in samples from patients with lymph node metastasis. The Kaplan-Meier survival analysis showed that a high density of peritumoral and intratumoral TAMs was related with a low overall survival rate, which was consistent with the results of Marcus et al.[22]. In addition, the degree of TAM infiltration was significantly related with tumor recurrence and prognosis, and intratumoral and peritumoral TAM count was a key prognostic factor for supraglottic laryngeal carcinoma.
Cox multivariate analysis showed that the number of intratumoral TAMs was an independent prognostic factor for OS, and the number of both intratumoral and peritumoral TAMs were independent prognostic factors for DFS. These results suggest that intratumoral and peritumoral TAM infiltration can be used to well evaluate the patient's condition and predict the prognosis of supraglottic laryngeal carcinoma. Currently, there is still dissension regarding cervical treatment of supraglottic laryngeal carcinoma, especially for how to identify and handle occult neck metastasis. We found that the supraglottic laryngeal carcinoma patients with high intratumoral and peritumoral TAM infiltration were prone to metastasis and had poor prognosis and low survival rate. Therefore, we hypothesize that TAM infiltration may be an early, sensitive prognostic indicator. Expanded radical surgery or appropriate expansion of surgical range should be considered even for patients with early stage, well-differentiated, low TN M stage supraglottic laryngeal carcinoma once they are identified to have high intratumoral and peritumoral TAM infiltration. However, further tests with large-scale samples are needed to determine how to apply this predictive indicator into clinical work.
Acknowledgments
This work was supported by grant from Pujiang Talent Project of the Shanghai Science and Technology Committee (No. 07pj14064).
References
- 1.Macmicking J, Xie QW, Nathan C. Nitric oxide and macrophage function [J] Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 2.Bingle L, Brown NJ, Lewis CE. The role of tumor-associated macrophages in tumor progression: implications for new anticancer therapies [J] J Pathol. 2002;196(3):254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 3.Tsutsui S, Yasuda K, Suzuki K, et al. Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density [J] Oncol Rep. 2005;14(2):425–431. [PubMed] [Google Scholar]
- 4.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis [J] Cell. 2006;124(2):263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Pollard JW. Macrophages define the invasive microenvironment in breast cancer [J] J Leukoc Biol. 2008;84(3):623–630. doi: 10.1189/jlb.1107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin EY, Li JF, Gnatovskiy L, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer [J] Cancer Res. 2006;66(23):11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 7.Hiraoka K, Zenmyo M, Watari K, et al. Inhibition of bone and muscle metastases of lung cancer cells by a decrease in the number of monocytes/macrophages [J] Cancer Sci. 2008;99(8):1595–1602. doi: 10.1111/j.1349-7006.2008.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective [J] Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 9.Mantovani A, Bottazzi B, Colotta F, et al. The origin and function of tumor-associated macrophages [J] Immunol Today. 1992;13(7):265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- 10.Allavena P, Sica A, Solinas G, et al. The inflammatory microenvironment in tumor progression: The role of tumor-associated macrophages [J] Crit Rev Oncol Hematol. 2008;66(1):1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Ohri CM, Shikotra A, Green RH, et al. Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival [J] Eur Respir J. 2009;33(1):118–126. doi: 10.1183/09031936.00065708. [DOI] [PubMed] [Google Scholar]
- 12.Soeda S, Nakamura N, Ozeki T, et al. Tumor-associated macrophages correlate with vascular space invasion and myometrial invasion in endometrial carcinoma [J] Gynecol Oncol. 2008;109(1):122–l28. doi: 10.1016/j.ygyno.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 13.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments [J] Cancer Res. 2006;66(2):605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 14.Richter G, Kruger KS, Hem G, et al. Interleukin 10 transfected into Chinese hamster ovary cells prevents tumor growth and macrophage infiltration [J] Cancer Res. 1993;53(18):4134–4137. [PubMed] [Google Scholar]
- 15.Vukanovic J, Isaacs JT. Linomide inhibits angiogenesis, growth, metastasis, and macrophage infiltration within rat prostatic cancers [J] Cancer Res. 1995;55(7):1499–1504. [PubMed] [Google Scholar]
- 16.Leek RD, Lewis CE, Whitehouse R, et al. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma [J] Cancer Res. 1996;56(20):4625–4629. [PubMed] [Google Scholar]
- 17.Salvesen HB, Akslen LA. Significance of tumour-associated macrophages, vascular endothelial growth factor and thrombospondin-1 expression for tumour angiogenesis and prognosis in endometrial carcinomas [J] Int J Cancer. 1999;84(5):539–543. doi: 10.1002/(sici)1097-0215(19991022)84:5<538::aid-ijc17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Hanada T, Nakagawa M, Emoto A, et al. Prognostic value of tumour-associated macrophage count in human bladder cancer [J] Int J Urol. 2000;7(7):263–269. doi: 10.1046/j.1442-2042.2000.00190.x. [DOI] [PubMed] [Google Scholar]
- 19.Lissbrant IF, Stattin P, Wikstrom P, et al. Tumour associated macrophages in human prostate cancer: relation to Clinicopathological variables and survival [J] Int J Oncol. 2000;17(3):445–451. doi: 10.3892/ijo.17.3.445. [DOI] [PubMed] [Google Scholar]
- 20.Koukourakis Ml, Giatromanolaki A, Kakolyris S, et al. Different patterns of stromal and cancer cell thymidine phosphorylase reactivity in non-small-cell lung cancer: impact on tumour neo-angiogenesis and survival [J] Br J Cancer. 1998;77(10):1696–1703. doi: 10.1038/bjc.1998.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badie B, Schartner JM. Flow cytometric characterization of tumor-associated macrophages in experimental gliomas [J] Neurosurgery. 2000;46(4):957–961. doi: 10.1097/00006123-200004000-00035. [DOI] [PubMed] [Google Scholar]
- 22.Marcus B, Arenberg D, Lee J, et al. Prognostic factors in oral cavity and oropharyngeal squamous cell carcinoma [J] Cancer. 2004;101(12):2779–2787. doi: 10.1002/cncr.20701. [DOI] [PubMed] [Google Scholar]