ABSTRACT
The emergence of drug resistance and adverse side effects of current bovine babesiosis treatment suggest that the search of new drug targets and development of safer and effective compounds are required. This study focuses on dihydroorotate dehydrogenase (DHODH), the fourth enzyme of pyrimidine biosynthesis pathway as a potential drug target for bovine babesiosis. Recombinant Babesia bovis DHODH protein (rBboDHODH) was produced in Escherichia coli and used for characterization and measurement of enzymatic activity. Furthermore, the effects of DHODH inhibitors were evaluated in vitro. The recombinant B. bovis DHODH histidine fusion protein (rBboDHODH) had 42.4-kDa molecular weight and exhibited a specific activity of 475.7 ± 245 Unit/mg, a Km = 276.2 µM for L-dihydroorotate and a Km= 94.41 µM for decylubiquinone. A 44-kDa band of native BboDHODH was detected by Western blot analysis and found in parasites mitochondria using a confocal microscope. Among DHODH inhibitors, atovaquone (ATV) and leflunomide (LFN) significantly inhibited the activity of rBboDHODH as well as the growth of B. bovis in vitro. The half maximal inhibitory concentration (IC50) of ATV and LFN was 2.38 ± 0.53 nM and 52.41 ± 11.47 µM, respectively. These results suggest that BboDHODH might be a novel target for development of new drug for treatment of B. bovis infection.
Keywords: atovaquone, Babesia bovis, chemotherapeutic target agent, dihydroorotate dehydrogenase, leflunomide
Babesia bovis is an intra-erythrocytic apicomplexan parasite of Babesiidae family, which is considered as principal agent causing bovine babesiosis, the most economically important tick-borne disease affecting livestock industries worldwide [6]. Infection with Babesia bovis is fatal due to its pathogenesis and neurological symptoms [5, 12, 34]. The use of common chemotherapeutic drugs, namely imidazole diproprionate and diminazene aceturate, has been reported to predispose milk and meat to drug residues, cause adverse side effects and induce drug resistance [35]. Furthermore, the lack of commercial vaccine [16, 37] suggests that the intensive search for new drug targets and new chemotherapeutic compounds as strategy to combat bovine babesiosis is required.
The pyrimidine biosynthesis pathway is essential for RNA, DNA, glycoproteins and phospholipids biosynthesis, which are important for division and growth of cells [26, 32]. Six enzymes of de novo pyrimidine synthesis pathway have been identified from B. bovis homogenates, indicating self pyrimidines production ability [11]. Dihydroorotate dehydrogenase (DHODH) is the fourth enzyme in de novo pyrimidine biosynthesis pathway that catalyzes the oxidation of dihydroorotate to orotate [3]. Inhibition of DHODH results in reduced levels of uridine 5′ monophosphate (UMP), which is an essential pyrimidine precursor [36]. DHODHs have been identified as novel drug targets for malaria, toxoplasmosis and leishmaniasis [8, 15, 17]. In addition, atovaquone (ATV), an ubiquinone analog and approved antimalarial drug, leflunomide (LFN), an antirheumatic drug and brequinar (Breq), an immunosuppressive agent, have been identified as DHODH inhibitors [13, 19, 22]. Furthermore, triazolopyrimidine derivatives have been evaluated on Plasmodium falciparum and showed promising inhibitory effects on parasite growth by targeting DHODH enzyme [14]. Interruption of this enzyme by using chemotherapeutic compounds may affect the growth of Babesia parasites. Despite the availability of compounds that successfully inhibit DHODH in other apicomplexan parasites, to date, no study has been carried out on B. bovis DHODH (BboDHODH) as chemotherapeutic target. Therefore, this study aimed to characterize BboDHODH and assess its potential as a new drug target for bovine babesiosis by evaluating the effects of DHODH inhibitors on the growth of B. bovis.
MATERIALS AND METHODS
In vitro cultivation of Babesia parasites: B. bovis (Texas strain) was grown in bovine red blood cells (RBCs) using a continuous microaerophilous stationary phase culture system [18]. Cultivation of parasites was carried out using the GIT medium (Wako, Osaka, Japan) supplemented with 1% penicillin and streptomycin (Sigma, St. Louis, MO, U.S.A.). The overlaying medium was replaced daily. Culture plates of B. bovis were grown at 37°C in humidified CO2 (5%) and O2 (5%) incubator (BIO-LABO, Tokyo, Japan). The percentage of parasitized erythrocytes was estimated at day 4 by microscopic observation on Giemsa’s stained slide.
Cloning and bioinformatic analysis of BboDHODH: BboDHODH complete coding sequence gene available in GenBank database (accession no. XM001610187) was used for designing primers. The full-length BboDHODH gene was amplified from B. bovis cDNA template by PCR using primers with BamHI and SacI sites (Italic), DHODH1F (5′-CGCGGATCCATGTGCATTGCAGCAACCGGT-3′) and DHODH2R (5′-ACGGAGCTCTTACTTCTTTGTGGATTC-3′). Purified PCR product was cloned into pGEM-T easy vector (Promega, Madison, WI, U.S.A.), subsequently digested with BamHI and SacI and inserted into BamHI and SacI sites of pET-28a expression vector (Novagen, Billerica, MA, U.S.A.). The cloned BboDHODH was confirmed by sequencing using ABI PRISM 3100 sequencer (Applied Biosystems Inc., Carlsbad, CA, U.S.A.). Obtained nucleotide sequence was translated using Genetyx software (Genetyx Corporation, Tokyo, Japan), and the functional domain and enzyme active sites of its polypeptide were analyzed by the BLAST search tool. Comparison of B. bovis DHODH with bovine and other apicomplexan parasites DHODHs gene sequence available in the GenBank database was done using CLUSTAL X software. Phylogenetic analysis was generated using the neighbor-joining method incorporated into Mega 3.1 software.
Expression and purification of recombinant BboDHODH protein: The BboDHODH cloned in pET-28a vector was transformed into Escherichia coli RosettaTM 2 (DE3) competent cells (Novagen, Darmstadt, Germany). Protein expression was performed according to the procedure previously described with some modifications [3]. The rBboDHODH-Histidine fusion protein was purified by a Ni-NTA affinity chromatography, according to manufacturer’s instructions (Qiagen, Hilden, Germany). The recombinant protein was analyzed by sodium dodecyl polyacrylamide gel electrophoresis (SDS-PAGE), and its molecular weight was calculated using Gel Pro Analysis software. Protein concentration was measured using bicinchoninic acid (BCA) protein assay (Pierce Biotechnology Inc., Rockford, IL, U.S.A.) with bovine serum albumin (BSA) as a standard.
Production of mouse anti-rBboDHODH sera and characterization of native enzyme: Anti-rBboDHODH serum was produced in 6-week-old female ICR mice (Clea, Tokyo, Japan) following standard immunization regime. Briefly, three mice were intra-peritoneally immunized with 50 µg of purified rBboDHODH protein emulsified with an equal volume of Freund’s complete adjuvant (Sigma). Thereafter, the same amount of antigen was emulsified with Freund’s incomplete adjuvant (Sigma) and administered to each mouse via the same route at days 14 and 28. Then, antiserum was collected from each mouse 14 days after the last booster [7]. To identify the native BboDHODH enzyme, B. bovis lysate was separated using a 12% SDS-PAGE and then probed with anti-rBboDHODH serum by Western blot analysis. In addition, an indirect fluorescence antibody test (IFAT) and confocal microscopy were performed with the same antiserum after labeling parasite’s mitochondria with MitoTracker® probes (Invitrogen, Paisley, U.K.).
Enzymatic activity of recombinant protein: The enzymatic activity of purified rBboDHODH was measured by monitoring 2,6-dichlorophenol-indophenol (DCIP) reduction [17]. The reaction contains 0.1 mM of L-dihydroorotic acid (L-DHO), 0.1 mM of decylubiquinone (QD) and 0.1 mM DCIP in DCIP buffer (50 mM Tris HCl, pH 8.0, KCl 150 mM, 0.1% Triton X-100 and 10% glycerol). After being separately incubated at 30°C for 30 min, rBboDHODH-His and reaction buffer were mixed, and the DCIP reduction was measured at 600 nm (ɛ = 18,800 M−1 cm−1). The reaction without recombinant protein was used as a control. The specific activity of the enzyme was measured in 96-well plate (Nunc, Roskilde, Denmark). Each well was filled with 200 µl of reaction mix containing 0.1 mM DCIP, 1 mM L-DHO, 0.1 mM QD and 0.205 µg of recombinant protein. To further evaluate the enzymatic activity of the rBboDHODH, 0.1 mM coenzyme Q10 and 0.1 mM sodium fumarate dibasic (FMN) were used as an alternative electron acceptor in the DCIP reduction assay. The kinetic constants of L-DHO and QD were determined by varying L-DHO concentration (0.01 to 8.0 mM) with QD fixed at 0.1 mM or by varying QD concentrations (0.02 to 0.8 mM) at a fixed L-DHO concentration of 1 mM. Each reaction was repeated at least 3 times, and then, the Michealis-Menten equation was used to calculate Km (GraphPad, La Jolla, CA, U.S.A.). Furthermore, the relative activity of rBboDHODH was evaluated in presence of 1 µM atovaquone (ATV), 1 mM leflunomide (LFN), 0.1 mM brequinar (Breq) and 1 mM 7-hydroxy-5- [1,2,4] triazolo [1,5,a] pyrimidine (TAZ) using DCIP reduction assay, in order to determine their inhibitory effect on the recombinant protein.
Effect of DHODH inhibitors on B. bovis growth in vitro: The effects of DHODH inhibitors on B. bovis growth were evaluated using 96-well plate (Nunc), according to the procedure previously described [31]. Atovaquone (ATV), brequinar (Breq), leflunomide (LFN) and 7-hydroxy-5- [1, 2, 4] triazolo [1, 5, a] pyrimidine (TAZ) were dissolved in dimethyl sulfoxide (DMSO) (Wako) as stock solutions, while diminazene aceturate (Di), the control drug, was prepared in distilled water. These compounds were individually added to the parasite cultures at the following concentrations, 0.04 to 10,903.94 nM of ATV, 19.66 to 20,133.88 nM of Breq, 0.26 to 1,000 µM of LFN, 1.95 to 2,000 µM of TAZ and 0.03 to 2,000 µM of Di. B. bovis cultures containing only 0.2% DMSO, 1% DMSO and 0.2% distilled water were used as controls. The inhibition assay was conducted for four days, and the overlaying medium was replaced daily with fresh medium containing the indicated concentration of each compound. Level of parasitemia and morphological changes of parasites were monitored daily by microscopic examination of Giemsa-stained thin blood smear. The half maximal inhibitory concentration (IC50) value for each compound was calculated (GraphPad) based on parasitemia level recorded on the 3rd day of in vitro culture. Next, the viability of parasites after treatment with each compound was also evaluated. Briefly, 4 µl from treated parasites and controls were sub-cultured in GIT medium with bovine RBCs without inhibitors for 10 days. Parasite recrudescence was determined under a light microscope daily in order to assess the parasite viability. To further elucidate the action mode of inhibitor on growth of B. bovis, orotic acid (ORA) or uridine 5′ monophosphate (UMP) and the inhibitor were simultaneously added to parasite cultures. DHODH inhibitor treated parasites were supplemented with 25, 50 and 100 µM of ORA or UMP. Untreated parasites and DHODH inhibitor treated but unsupplemented parasite cultures were used as controls. The level of parasitemia was determined using Giemsa-stained thin blood smear at 48 hr after supplementation.
Statistical analysis: Effects of DHODH inhibitors and ORA or UMP supplementation were analyzed by comparing treated and supplemented parasites to controls using ANOVA and Chi-square (χ2) test (GraphPad). Data were considered statistically significant when the P value was<0.05.
RESULTS
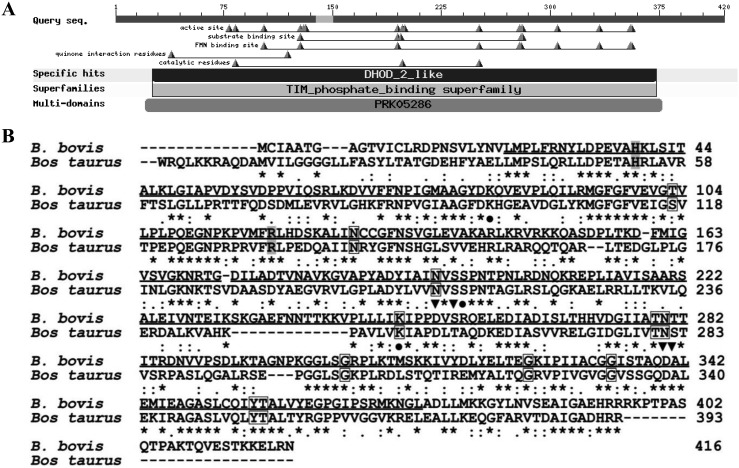
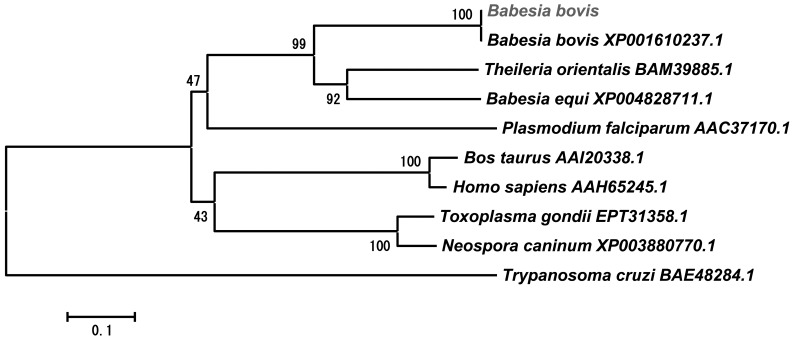
Bioinformatic analysis of BboDHODH: The amplified and sequenced BboDHODH gene has an open reading frame of 1,248 bp encoding 416 amino acids with a predicted 44-kDa molecular weight. The predicted amino acid residues were predominantly hydrophobic (52.64%), although 25.96% and 21.15% were hydrophilic and neutral, respectively. Computational analysis of BboDHODH revealed the presence of DHOD 2-like region, known as functional domain containing active sites, substrate binding sites, quinone interaction sites and FMN binding sites (Fig. 1A). There was no signal peptide in the BboDHODH sequence as predicted by signalP 4.1 server (http://www.cbs.dtu.dk/services/SignalP/). Comparison of BboDHODH with Bos Taurus DHODH (BosDHODH) amino acid sequence showed 42% similarity. Interestingly, the Thr103 (FMN binding site) and Thr281 (substrate binding site) observed in the parasite enzyme (BboDHODH) were replaced by Ser117 and Ser282 in the host enzyme (BosDHODH) (Fig. 1B). In addition, the comparison of B. bovis DHODH with other apicomplexan DHODH 2-like enzymes revealed 42%, 40%, 57% and 59% homology with Plasmodium falcifarum (accession no. AAC37170.1), Toxoplasma gondii (accession no. EPT31358.1), Theileria orientalis (accession no. BAM39885.1) and T. equi (accession no. XP004828711.1), respectively. Moreover, the phylogeneic analysis showed that B. bovis, T. equi and T. orientalis DHODHs belong to the same cluster, which was distinct from mammalian DHODHs (Fig. 2).
Fig. 1.
Bioinformatics analysis of translated BboDHODH polypeptides. (A) The predicted functional domains of BboDHODH as shown by BLASTp. (B) Alignment of B. bovis DHODH with bovine DHODH enzyme. Completely conserved residues are highlighted as asterisk, DHODH 2 like region is shown as underline, quinone binding sites are shown in grey, fumarate binding sites are shown in box, active sites are shown as (●) and substrate binding sites are shown as (▼).
Fig. 2.
A phylogenetic tree based on the DHODH amino acid sequences of apicomplexa protozoan and mammalian. The tree was generated using the neighbor-joining method incorporated into the MEGA 3 program.
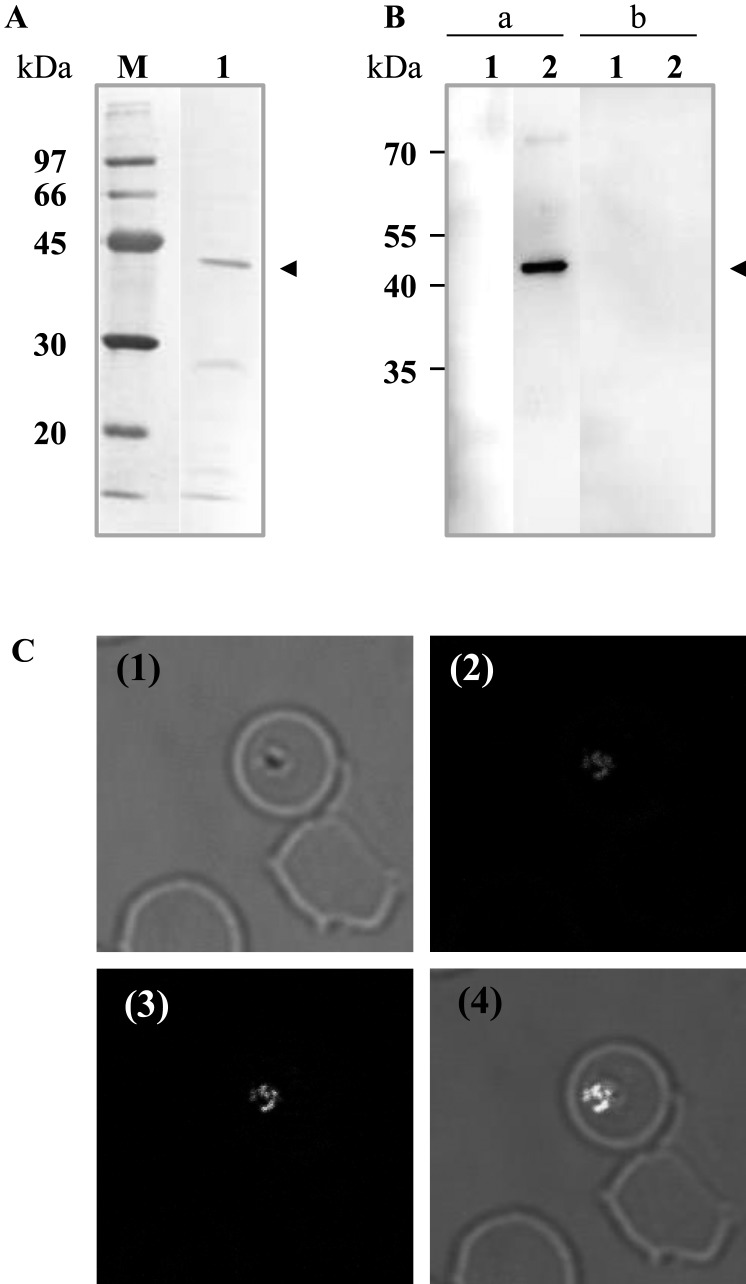
Expression and characterization of BboDHODH: The recombinant BboDHODH was successfully expressed as soluble protein with 42.4-kDa on 12% SDS-PAGE (Fig. 3A). However, the apparent molecular weight of the recombinant (42.4-kDa) was smaller than the expected as calculated from amino acid including His tag (47-kDa=44-kDa + 3-kDa). This difference of molecular weight can be explained by the interaction of SDS with hydrophobic residues, the ionic strength of denatured protein and conformation of denatured proteins which altogether may affect the migration behavior on SDS-PAGE [29]. Mouse anti-rBboDHODH reacted with B. bovis lysate yielding a specific band of approximately 44-kDa, but did not react with bovine RBC lysate. Additionally, non-immunized mice sera neither reacted with the parasite nor host’s RBC lysates (Fig. 3B). Confocal laser microscopy revealed that the mice anti-serum reacted with B. bovis DHODH yielding the specific green fluorescence overlaid with a red fluorescence representing parasite’s mitochondria (Fig. 3C). This result suggests that BboDHODH is expressed in merozoites stage and may localize in parasite mitochondria.
Fig. 3.
Characterization of native BboDHODH. (A) SDS-PAGE showing purified BboDHODH-His stained with amide black. M: Low molecular marker; lane 1: purified rBboDHODH-His. (B) Western blot analysis showing reaction of anti rBboDHODH mice sera with parasite lysates. Lane 1: non infected bovine RBC lysates; lane 2: B. bovis lysates; lane a: anti-rBboDHODH mice sera; lane b: non-immunize mice sera. The arrowhead shows BboDHODH protein. (C) Localization of BboDHODH by IFAT. (1) Phase-contrast; (2) MitoTracker (red); (3) Anti-rBboDHODH (green), (4) The overlaid of fluorescence reactivity.
Enzymatic activity of recombinant protein: The BboDHODH (EC 1.3.3.1)-catalyzed oxidation of dihydroorotate was measured in the presence of decylubiquinone as electron acceptor using the DCIP reduction assay. Recombinant BboDHODH exhibited enzymatic properties with specific activity of 475.7 ± 245 Unit/mg, and the kinetic constant for this enzyme revealed Km values of 276.2 µM and 94.41 µM for L-DHO and QD, respectively (Table 1). Histidine fusion protein did not affect the enzymatic activity as previously described [3]. DCIP reduction assay with alternative electron acceptors showed that BboDHODH could oxidize dihydrootate to orotate via Q10 or FMN with relative activity of 64.1% and 97.7%, respectively (data not shown). In addition, rBboDHODH relative activity in the presence of 1 µM ATV, 1 mM LFN, 0.1 mM Breq and 1 mM TAZ was 19.1%, 51.5%, 106.5% and 101.0%, respectively, compared to the control (Table 2).
Table 1. Enzymatic properties of BboDHODH recombinant protein.
| Substrate | Km (µM) | Vmax (nM min−1) | Specific activity (Unit/mg) |
|---|---|---|---|
| L-DHO | 276.2 | 50.69 | 475.7 ± 245 |
| QD | 94.41 | 65.49 |
Table 2. Relative activity of rBboDHODH protein in presence of different DHODH Inhibitors.
| Inhibitor | Relative activity (%) |
|---|---|
| ATV (1 µM) | 19.10 |
| LFN (1 mM) | 51.58 |
| Breq (0.1 mM) | 106.50 |
| TAZ (1 mM) | 101.00 |
| Without inhibitor | 100.00 |
The reaction was measured using DCIP reduction assay, 200 µl-reactions contained 0.1 mM DCIP, 1 mM L-DHO, 0.1 mM QD and 0.205 µg of the recombinant protein. Relative activity of rBboDHODH in presence of inhibitor was compared to reaction of the enzyme without inhibitor which is taken as 100%.
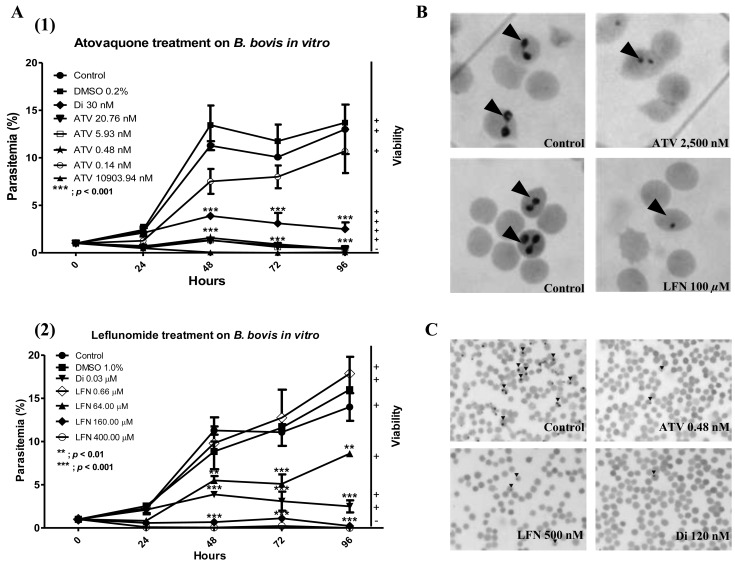
Effect of DHODH inhibitors on B. bovis growth: In order to assess the effect of DHODH inhibitors on the growth of parasites, B. bovis cultures were treated by ATV, LFN, Breq, TAZ and Di at different concentrations, and the parasites were monitored for 4 days. The concentration of DHODH inhibitors used in this study has no effect on red blood cells, according to the hemolytic activity assay carried out (data not show). Strikingly, treatment with ATV and LFN significantly inhibited parasites growth from 48 hr post treatment (P<0.001). ATV at 20.76 nM decreased the growth of parasites by 92.2 ± 5.5% (Fig. 4A-1), while at 64 µM, LFN decreased parasite growth by 49.3 ± 15.9% (Fig. 4A-2). On the other hand, the inhibitory effect by Di at 30 nM was 64.2% (P<0.001), observed from 24 hr post treatment. However, B. bovis growth was not affected by Breq and TAZ treatment. The IC50 values of ATV, LFN and Di were 2.38 ± 0.53 nM, 52.41 ± 11.47 µM and 0.50 µM, respectively. The morphological changes were observed 48 hr post treatment with ATV and LFN, as the parasites were small and shrank (Fig. 4B). Similar shapes were also were observed in parasite cultures treated with Di (data not show). Viability test after drug withdrawal showed that there was no re-growth of parasite in cultures previously treated with ATV, LFN and Di. At concentration of 0.48 nM ATV, 500 nM LFN and 120 nM Di, the parasites exhibited shrink and dot-like appearance after 10 days of culture (Fig. 4C).
Fig. 4.
In vitro inhibition assay. (A-1) Growth of B. bovis parasites was inhibited by treatment by atovaquone (ATV) and (A-2) leflunomide (LFN) from 48 hr post treatment. (B) Morphological change of parasites after treatment with ATV and LFN, respectively. (C) Viability of parasite after treatment by ATV, LFN and Di, respectively, at the significant inhibitory concentration (observed at day 10 after drug withdrawing).
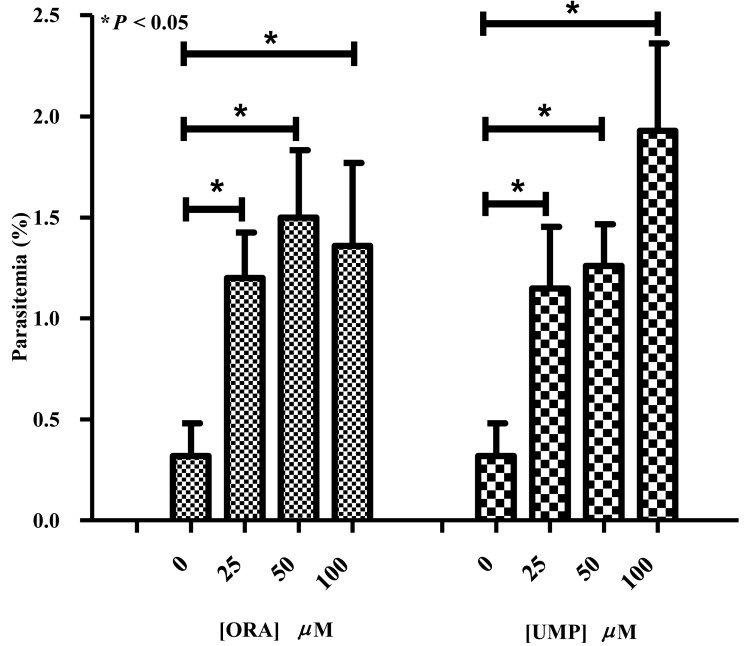
Effect of ORA and UMP supplementation on the growth of DHODH inhibited parasites: To confirm that the effects of DHODH inhibitors on B. bovis growth were directly related to an orotate or UMP deficiency caused by BboDHODH inhibition, treated parasites were supplemented with ORA and UMP. Inhibitory assay results suggested that ATV was the most effective inhibitor against B. bovis growth. Therefore, only ATV at 100 nM was used to totally inhibit the growth of Babesia bovis in supplementation assay. Supplemented parasite cultures exhibited significantly higher percentage of parasitemia (>0.30%) compared to non-supplemented (control) (P<0.05) (Fig. 5). Supplementation of ORA at 25, 50 and 100 µM in ATV treated parasite cultures led to 1.20 ± 0.2%, 1.50 ± 0.3% and 1.36 ± 0.4% parasitemia, respectively. The slight decrease of parasitemia at 100 µM ORA supplementation might be related to the toxicity of ORA at high concentration. On the other hand, supplementation of UMP at 25, 50 and 100 µM in ATV treated parasite cultures led to 1.15 ± 0.3%, 1.26 ± 0.2% and 1.93 ± 0.4% parasitemia, respectively. These results suggest that supplementation with ORA or UMP prevents the parasite starvation by probably providing pyrimidine precursors needed for B. bovis survival.
Fig. 5.
Supplementation of orotic acid (ORA) and uridine 5′ monophosphate (UMP) to ATV treated B. bovis parasites. The difference between parasitemia level of non-supplemented and supplemented groups was considered statistically significant when P<0.05.
DISCUSSION
DHODH has been studied as chemotherapeutic target in certain apicomplexan parasites, but not in B. bovis. Therefore, we validated B. bovis DHODH as a novel chemotherapeutic target. Bioinformatics analyses revealed that amino acid sequence of B. bovis DHODH mostly contained hydrophobic residues. The N-terminal region was highly divergent and shorter than those of T. gondii, P. falciparum and B. taurus DHODHs. BboDHODH contains both quinone and FMN binding sites, which support the ability of BboDHODH enzyme to use either quinone or FMN as an electron acceptor. Generally, two forms of DHODHs have been documented; the cytosolic (class 1), which utilizes fumarate or NAD+ as an electron acceptor and the membrane-bound (class 2), found in eukaryotes which use quinone as an electron acceptor [4, 33]. Our results suggest that BboDHODH is a membrane-bound (class 2) DHODH, the same as some other apicomplexa DHODHs, which have been studied earlier as a chemotherapeutic target [3, 27]. Moreover, the differences of substrate binding sites of B. bovis versus the host DHODH, suggest that BboDHODH might have different biochemical properties from their host and highlight the possibility of this enzyme as novel drug target against bovine babesiosis. In addition, BboDHODH enzyme was found as a 44-kDa expressed in erythrocytic stage and was detected in parasite mitochondria. Localization result was consistent with previous finding [30], which indicates that the DHODH protein is located within mitochondrial intermembrane space. This location may allow free diffusion of the DHODH substrate (dihydroorotate) and product (orotate) through the outer mitochondrial membrane from and to the proceeding and subsequent steps of de novo pyrimidine biosynthesis [9]. Nevertheless, further studies using electron microscopy might be necessary to precisely confirm BboDHODH localization within parasite mitochondria.
Enzymatic assay revealed that the recombinant BboDHODH was highly active with a specific activity 95,000 fold higher than the native BboDHODH [11] which might be explained by the lower purity and yield of protein obtained from the parasite extracts. In addition, the specific activity of rBboDHODH was six fold higher than recombinant TgDHODH which suggests that the enzymatic activity of rBboDHODH observed in the present study was similar to the previous report on apicomplexan DHODH [17]. The enzymatic activity of rBboDHODH could be specifically inhibited by ATV and LFN, but not by Breq and TAZ. According to previous reports, Breq is effective on human DHODH enzyme, but not on rat, T. gondii and Ustilago maydis DHODH enzymes, which suggests its species related inhibitory effect [17, 19, 38]. Similarly, the in vitro inhibition assay showed that ATV and LFN significantly inhibited B. bovis growth, while Breq and TAZ did not affect the parasites. ATV was the most effective drug in this study, as its IC50 value was lower than those of LFN and Di (control drug). Moreover, ATV IC50 was lower than those of Epoxomicin [1], EGCG [2], Fusidic acid [31] and Trisubstitute pyrrole [21] which were recently evaluated as anti-B. bovis compounds. However, ATV inhibitory effect was similar to Apicidin (IC50 = 7.1 ng/ml) [24]. Our results are consistent with previous reports on the in vitro inhibitory effect of ATV on T. gondii [10] and B. gibsoni [23]. Interestingly, ATV has already been successfully used to treat P. falciparum, T. gondii, B. microti, B. gibsoni and B. divergens infections [10, 20, 23, 25, 28].
To understand the mechanism of ATV inhibitor on growth of parasites, we supplemented the ATV-treated parasites with ORA or UMP. The result showed that supplementation improved their growth. This finding agrees with a previous report [38] that suggests the supplementation compensates the growth after disruption of DHODH. Our result emphasized that ATV affects the growth of parasites by interrupting orotic acid production.
In conclusion, molecular characterization of BboDHODH as a chemotherapeutic target was elucidated for the first time. Our findings suggest that BboDHODH might be a potential chemotherapeutic target for bovine babesiosis. Atovaquone specifically inhibited BboDHODH and consequently inhibited the growth of the parasites by disrupting pyrimidine biosynthesis. Taken together, these findings confirm that BboDHODH is a novel chemotherapeutic target and ATV could be a beneficial drug for controlling B. bovis infection.
Acknowledgments
This study was supported by grants from the Global COE Program and Grants-in-Aid for Scientific Research, both from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Aboulaila M., Nakamura K., Govind Y., Yokoyama N., Igarashi I.2010. a. Evaluation of the in vitro growth-inhibitory effect of epoxomicin on Babesia parasites. Vet. Parasitol. 167: 19–27. doi: 10.1016/j.vetpar.2009.09.049 [DOI] [PubMed] [Google Scholar]
- 2.Aboulaila M., Yokoyama N., Igarashi I.2010. b. Inhibitory effects of (−)- Epigallocatecgin-3-gallate from green tea on the growth of Babesia parasites. Parasitology 137: 785–791. doi: 10.1017/S0031182009991594 [DOI] [PubMed] [Google Scholar]
- 3.Baldwin J., Farajallah A. M., Malmquist N. A., Rathod P. K., Phillips M. A.2002. Malarial dihydroorotate dehydrogenase. J. Biol. Chem. 277: 41827–41834. doi: 10.1074/jbc.M206854200 [DOI] [PubMed] [Google Scholar]
- 4.Björnberg O., Rowland P., Larsen S., Jensen K. F.1997. Active site of dihydroorotate dehydrogenase A from Lactococcus lactis investigated by chemical modification and mutagenesis. Biochemistry 36: 16197–16205. doi: 10.1021/bi971628y [DOI] [PubMed] [Google Scholar]
- 5.Bock R., Jackson L., de Vos A., Jorgensen W.2004. Babesiosis of cattle. Parasitology 129: S247–269. doi: 10.1017/S0031182004005190 [DOI] [PubMed] [Google Scholar]
- 6.Brown W. C., Palmer G. H.1999. Designing blood-stage vaccines against Babesia bovis and B. bigemina. Parasitol. Today 15: 275–281. doi: 10.1016/S0169-4758(99)01471-4 [DOI] [PubMed] [Google Scholar]
- 7.Cao S., Aboge G. O., Terkawi M. A., Zhou M., Luo Y., Yu L., Li Y., Goo Y. K., Kamyingkird K., Masatani T., Suzuki H., Igarashi I., Nishikawa Y., Xuan X.2013. Cloning, characterization and validation of inosine 5′- monophosphate dehydrogenase of Babesia gibsoni as molecular drug target. Parasitol. Int. 62: 87–94. doi: 10.1016/j.parint.2012.10.005 [DOI] [PubMed] [Google Scholar]
- 8.Cheleski J., Rocha J. R., Pinheiro M. P., Wiggers H. J., da Silva A. B. F., Nonato M. C., Montanari C. A.2010. Novel insights for dihydroorotate dehydrogenase class 1A inhibitors discovery. Eur. J. Med. Chem. 45: 5899–5909. doi: 10.1016/j.ejmech.2010.09.055 [DOI] [PubMed] [Google Scholar]
- 9.Chen J. J., Jones M. E.1976. The cellular localization of dihydroorotate dehydrogenase: relation to de novo biosynthesis of pyrimidines. Arch. Biochem. Biophys. 176: 82–90. doi: 10.1016/0003-9861(76)90143-0 [DOI] [PubMed] [Google Scholar]
- 10.Ferreira R. A., de Oliveira A. B., Gualberto S. A., Corral J. M. M. D., Fujiwara R. T., Guimarães P. H. G., de Almeida Vitor R. W.2012. New naphthoquinones and an alkaloid with in vitro activity against Toxoplasma gondii RH and EGS strains. Exp. Parasitol. 132: 450–457. doi: 10.1016/j.exppara.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 11.Gero A. M., O’Sullivan W. J., Wright I. G., Mahoney D. F.1983. The enzymes of pyrimidine biosynthesis in Babesia bovis and Babesia bigemina. Aust. J. Exp. Biol. Med. Sci. 61: 239–243. doi: 10.1038/icb.1983.22 [DOI] [PubMed] [Google Scholar]
- 12.Gohil S., Herrmann S., Günther S., Cooke B. M.2013. Bovine babesiosis in the 21st century: advances in biology and functional genomics. Int. J. Parasitol. 43: 125–132. doi: 10.1016/j.ijpara.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 13.Greene S., Watanabe K., Braatz-Trulson J., Lou L.1995. Inhibition of dihydroorotate dehydrogenase by the immunosuppressive agent leflunomide. Biochem. Pharmacol. 50: 861–867. doi: 10.1016/0006-2952(95)00255-X [DOI] [PubMed] [Google Scholar]
- 14.Gujjar R., Mazouni F. E., White K. L., White J., Creason S., Shackleford D. M.,, Deng, X.,, Charman W. N., Bathurst I, Burrows J., Floyd D. M., Matthews D., Buckner F. S., Charman S. A., Phillips M. A., Rathod P. K.2011. Lead-optimization of aryl and aralkyl amine based triazolopyrimidine inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase with antimalarial activity in mice. J. Med. Chem. 54: 3935–3949. doi: 10.1021/jm200265b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heikkilä T., Thirumalairajan S., Davies M., Parsons M. R., McConkey A. G., Fishwick C. W. G., Johnson P.2006. The first de novo designed inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase. Bioorg. Med. Chem. Lett. 16: 88–92. doi: 10.1016/j.bmcl.2005.09.045 [DOI] [PubMed] [Google Scholar]
- 16.Homer M. J., Aguilar-Delfin I., Telford S. R., III, Krause P. J., Persing D. H.2000. Babesiosis. Clin. Microbiol. 13: 451–469. doi: 10.1128/CMR.13.3.451-469.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hortua Triana M. A., Huynh M. H., Garavito M. F., Fox B. A., Bzik D. J., Carruthers V. B., Löffler M., Zimmermann B. H.2012. Biochemical and molecular characterization of the pyrimidine biosynthetic enzyme dihydroorotate dehydrogenase from Toxoplasma gondii. Mol. Biochem. Parasitol. 184: 71–81. doi: 10.1016/j.molbiopara.2012.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Igarashi I., Njonge F. K., Kaneko Y., Nakamura Y.1998. Babesia bigemina: in vitro and in vivo effects of curdlan sulfate on growth of parasites. Exp. Parasitol. 90: 290–293. doi: 10.1006/expr.1998.4331 [DOI] [PubMed] [Google Scholar]
- 19.Knecht W., Löffler M.1998. Species-related inhibition of human and rat dihydroorotate dehydrogenase by immunosuppressive Isoxazol and Cinchoninic acid derivatives. Biochem.Pharmacol. 56: 1259–1264. doi: 10.1016/S0006-2952(98)00145-2 [DOI] [PubMed] [Google Scholar]
- 20.Korsinczky M., Chen N., Ketecka B., Saul A., Rieckmann K., Cheng Q.2000. Mutation in Plasmodium falciparum cytochrome B that are associated with atovaquone resistance are located at a putative drug-binding site. Antimicrob. Agents Chemother. 44: 2100–2108. doi: 10.1128/AAC.44.8.2100-2108.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau A. O. T., Pedroni M. J., Bhanot P.2013. Target specific-trisubstituted pyrrole inhibits Babesia bovis erythrocytic growth. Exp. Parasitol. 133: 365–368. doi: 10.1016/j.exppara.2012.12.013 [DOI] [PubMed] [Google Scholar]
- 22.Löffler M., Grein K., Knecht W., Klein A., Bergjohann U.1998. Dihydroorotate dehydrogenase: profile of a novel target for antiproliferative and immunosuppressive drugs. Adv. Exp. Med. Biol. 431: 507–513. doi: 10.1007/978-1-4615-5381-6_99 [DOI] [PubMed] [Google Scholar]
- 23.Matsuu A., Koshida Y., Kawahara M., Inoue K., Ikadai H., Hikasa Y., Okano S., Higuchi S.2004. Efficacy of atovaquone against Babesia gibsoni in vivo and in vitro. Vet. Parasitol. 124: 9–18. doi: 10.1016/j.vetpar.2004.07.005 [DOI] [PubMed] [Google Scholar]
- 24.Munkhjargal T., Aboulaila M. R. A., Sivakumar T., Yokoyama N., Igarashi I.2009. Inhibitory effect of apicidin on in vitro and in vivo growth of Babesia parasites. J. Protozool. Res. 19: 42–49 [Google Scholar]
- 25.Oz H. S., Tobin T.2012. Atovaquone ameliorate gastrointestinal Toxoplasmosis complications in a pregnancy model. Med. Sci. Monit. 18: 337–345. doi: 10.12659/MSM.883342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips M. A., Gujjar R., Malmquist N. A., White J., Mazouni F. E., Baldwin J., Rathod P. K.2008. Triazolopyrimidine-based dihydroorotate dehydrogenase inhibitors with potent and selective activity against the malaria parasite Plasmodium falciparum. J. Med. Chem. 51: 3649–3653. doi: 10.1021/jm8001026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips M. A., Rathod P. K.2010. Plasmodium dihydroorotate dehydrogenase: a promising target for novel anti-malarial chemotherapy. Infect. Disord. Drug Targets 10: 226–239. doi: 10.2174/187152610791163336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pudney M., Gray J. S.1997. Therapeutic efficacy of atovaquone against the bovine intraerythrocytic parasite, Babesia divergens. J. Parasitol. 83: 307–310. doi: 10.2307/3284461 [DOI] [PubMed] [Google Scholar]
- 29.Rath A., Glibowicka M., Nadeau V. G., Chen G., Deber C. M.2009. Detergent binding explains anomalous SDS-PAGE migration of membrane proteins. Proc. Natl. Acad. Sci. U.S.A. 106: 1760–1765. doi: 10.1073/pnas.0813167106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rawls J., Knecht W., Diekert K., Lill R., Löffler M.2000. Requirements for the mitochondrial import and localization of dihydroorotate dehydrogenase. Eur. J. Biochem. 267: 2079–2087. doi: 10.1046/j.1432-1327.2000.01213.x [DOI] [PubMed] [Google Scholar]
- 31.Salama A. A., AbouLaila M., Moussa A. A., Nayel M. A., El-Sify A., Terkawi M. A., Hassan H. Y., Yokoyama N., Igarashi I.2013. Evaluation of in vitro and in vivo inhibitory effects of fusidic acid on Babesia and Theileria parasites. Vet. Parasitol. 191: 1–10. doi: 10.1016/j.vetpar.2012.08.022 [DOI] [PubMed] [Google Scholar]
- 32.Shambaugh G. E.1979. Pyrimidine biosynthesis. Am. J. Clin. Nutr. 32: 1290–1297 [DOI] [PubMed] [Google Scholar]
- 33.Sierra Pagan M. L., Zimmermann B. H.2003. Cloning and expression of the dihydroorotate dehydrogenase from Toxoplasma gondii. Biochim. Biophys. Acta. 1637: 178–181. doi: 10.1016/S0925-4439(02)00226-0 [DOI] [PubMed] [Google Scholar]
- 34.Uilenberg G.2006. Babesia-a historical overview. Vet. Parasitol. 138: 3–10. doi: 10.1016/j.vetpar.2006.01.035 [DOI] [PubMed] [Google Scholar]
- 35.Vial H. J., Gorenflot A.2006. Chemotherapy against babesiosis. Vet. Parasitol. 138: 147–160. doi: 10.1016/j.vetpar.2006.01.048 [DOI] [PubMed] [Google Scholar]
- 36.Walse B., Dufe V. T., Svensson B., Fritzson I., Dahlberg L., Khairoullina A., Wellmar U., Al-Karadaghi S.2008. The structures of human dihydroorotate dehydrogenase with and without inhibitor reveal conformational flexibility in the inhibitor and substrate binding sites. Biochemistry 47: 8929–8936. doi: 10.1021/bi8003318 [DOI] [PubMed] [Google Scholar]
- 37.World Organization for Animal Health (OIE) Manual of diagnostic tests and vaccines (online). Paris 2008. (Bovine babesiosis: http://www.oie.int/fr/nomes/mmanual/A_00059.htm).
- 38.Zameitat E., Fraymark G., Dietz C. D., Löffler M., Bölker M.2007. Functional expression of human dihydroorotate dehydrogenase (DHODH) in pry4 mutants of Ustilago maydis allows target validation of DHODH inhibitors in vivo. Appl. Environ. Microbiol. 73: 3371–3379. doi: 10.1128/AEM.02569-06 [DOI] [PMC free article] [PubMed] [Google Scholar]