ABSTRACT
The olfactory and respiratory mucosae of the Corriedale sheep were examined using lectin histochemistry in order to clarify the histochemical and glycohistochemical differences between these two tissues. The olfactory epithelium was stained with 13 lectins out of 21 lectins examined, while the respiratory epithelium was positive to 16 lectins. The free border of both of the olfactory and respiratory epithelia was stained with 12 lectins: Wheat germ agglutinin (WGA), succinylated-wheat germ agglutinin (s-WGA), Lycopersicon esculentum lectin (LEL), Solanum tuberosum lectin (STL), Datura stramonium lectin (DSL), Soybean agglutinin (SBA), Bandeiraea simplicifolia lectin-I (BSL-I), Ricinus communis agglutinin-I (RCA-120), Erythrina cristagalli lectin (ECL), Concanavalin A (Con A), Phaseolus vulgaris agglutinin-E (PHA-E) and Phaseolus vulgaris agglutinin-L (PHA-L). The associated glands of the olfactory mucosa, Bowman’s glands, were stained with 13 lectins. While both the goblet cells and mucous nasal glands were stained with 8 lectins; five of them (WGA, s-WGA, STL, Vicia villosa agglutinin (VVA) and ECL) were mutually positive among the Bowman’s glands, mucous nasal glands and the goblet cells. These findings indicate that the glycohistochemical characteristics of the free borders of both olfactory and respiratory epithelia are similar to each other, suggesting that secretions from the Bowman’s glands and those of the goblet cells and mucous nasal glands are partially exchanged between the surface of two epithelia to contribute the functions of the respiratory epithelium and the olfactory receptor cells, respectively.
Keywords: glycoconjugates, olfactory system, ruminants
Lectins are proteins that bind to glycoconjugates non-immunologically. They are considered to be the major analytic tool for the study of both soluble and cellular glycoconjugates. They are mainly specific to the terminal carbohydrates of sugar chains [3, 4] and are extensively used for the differentiation of cells according to the glycoconjugate contents on the histological sections [5, 8, 11, 12, 14, 16,17,18,19].
The mammalian olfactory mucosa is located in the posterior roof of the nasal cavity, where it mainly lines the ethmoturbinates and carries the receptor cells responsible for the detection and discrimination among odors of different substances [1, 2, 6, 15, 23, 24]. The olfactory mucosa consists of the olfactory epithelium and an underlying lamina propria. The olfactory epithelium is comprised of olfactory receptor cells, supporting cells and basal cells. The respiratory mucosa is composed of both respiratory epithelium consisted of the ciliated, basal and goblet cells and the underlying connective tissue lamina propria.
In sheep, the olfactory mucosa is covered by pseudostratified sensory epithelium. The free border of this epithelium is predominantly made up of microvilli from the supporting cells together with the cilia from the rounded projecting rods of the olfactory receptor cells [13]. The histological and histochemical features of the sheep olfactory mucosa have been evaluated before by some authors: Kavoi et al. [9] compared the development and functional maturation of the olfactory mucosa in the domestic sheep and dog, and Scocco et al. [20] compared the glycohistochemical characterization of normal olfactory mucosa and enzootic nasal tumor of sheep, focusing on the lectin staining patterns of the free border and Bowman’s glands. However, the latter did not address the “lectin binding” affinities of the different cell types in the olfactory epithelium (receptor, supporting or basal cells).
The respiratory mucosa of sheep is covered with a typical respiratory epithelium, which is a pseudostratified ciliated columnar type with goblet cells, covering the major portion of the nasal septum as well as the recesses of the ethmoidal conchae [22]. Several reports evaluated histological and histochemical properties of the sheep respiratory mucosa: Khamas and Ghoshal [10] focused on the blood vessels supplying the respiratory mucosa, while Taher et al. [22] studied the anatomy and morphology of the respiratory epithelium and the underlying connective tissue containing thick coiled tubular glands. Finally, Scocco et al. [20] compared the lectin histochemical characteristics of the histologically normal respiratory mucosa and enzootic nasal tumor focusing on the lectin binding patterns of goblet cells and nasal glands without discussing the lectin stainings in the basal and ciliated cells of the respiratory epithelium or their free border.
In this study, the nasal mucosa of the adult Corriedale sheep was examined by lectin histochemistry to clarify the histochemical and glycohistochemical differences between the olfactory and respiratory mucosae by making a detailed description of the lectin binding patterns and to reveal the types and quantities of glycoconjugates in the different cellular components of both the olfactory and respiratory epithelia with special attention to the mucous covering the surface of these epithelia. By doing so, we aimed to find out the possible role played by each one of these tissues to contribute the function of the other one.
MATERIALS AND METHODS
Animals: Three adult, castrated male Corriedale sheep were studied. They were obtained from Kitasato University (Towada, Japan). Animals were euthanized with intravenous injection of sodium pentobarbital at a dose of 20 mg/kg body weight and then perfused with physiological saline through the common carotid arteries under deep anesthesia. All procedures were in accordance with the Principles for Animal Experiments of Iwate University and approved by the intramural committee for the care and use of animals. Animals were perfused with the Bouin’s solution without acetic acid via the common carotid arteries. After decapitation, the nasal parts were immersed in the same fixative for 2 days and then decalcified in 10% ethylenediaminetetraacetic acid for several months, routinely embedded in paraffin and cut transversally at 5 µm. Some sections were stained with hematoxylin-eosin (H&E), periodic acid/Schiff (PAS), alcian blue (pH 1.0 and 2.5) or Crossmon’s trichrome for histological examinations.
Lectin histochemistry: The other sections were processed for histochemistry with the avidin-biotin complex (ABC) method using 21 types of biotinylated lectins (Table 1) in the lectin screening kits I-III (Vector Laboratories, Burlingame, CA, U.S.A.). After deparaffinization and rehydration, the sections were incubated with 0.3% H2O2 in methanol to eliminate endogenous peroxidase activity. The sections were rinsed in 0.01 M phosphate buffered saline (PBS, pH 7.4) and incubated with 1% bovine serum albumin to block nonspecific reactions. After rinsing, the sections were incubated with biotinylated lectins at 4°C overnight and reacted with ABC reagent (Vector Laboratories) at room temperature for 45 min. Thereafter, the sections were colorized with 0.05 M Tris-HCl (pH 7.4) containing 0.006% H2O2 and 0.02% 3–3′-diaminobenzidine tetrahydrochloride, dehydrated, cleared and mounted. Control stainings were made by using of PBS to replace lectins. No specific stainings were observed in the control stainings.
Table 1. Binding specifities of lectins used in this study.
| Lectins | Abbreviation | Concentration (mg/ml) | Binding specificity |
|---|---|---|---|
| Wheat germ agglutinin | WGA | 1.0 × 10ˉ2 | GlcNAc, NeuAc |
| Succinylated-wheat germ agglutinin | s-WGA | 1.0 × 10ˉ2 | (GlcNAc) n |
| Lycopersicon esculentum lectin | LEL | 2.0 × 10ˉ2 | (GlcNAc) 2–4 |
| Solanum tuberosum lectin | STL | 1.0 × 10ˉ2 | (GlcNAc) 2–4 |
| Datura stramonium lectin | DSL | 4.0 × 10ˉ3 | (GlcNAc) 2–4 |
| Bandeiraea simplicifolia lectin-II | BSL-II | 4.0 × 10ˉ3 | GlcNAc |
| Dolichos biflorus agglutinin | DBA | 1.0 × 10ˉ2 | Gal, GalNAc |
| Soybean agglutinin | SBA | 1.0 × 10ˉ2 | Gal, GalNAc |
| Bandeiraea simplicifolia lectin-I | BSL-I | 4.0 × 10ˉ3 | Gal, GalNAc |
| Vicia villosa agglutinin | VVA | 4.0 × 10ˉ3 | Gal, GalNAc |
| Sophora japonica agglutinin | SJA | 2.0 × 10ˉ2 | Gal, GalNAc |
| Ricinus communis agglutinin-I | RCA-120 | 2.0 × 10ˉ3 | Gal, GalNAc |
| Jacalin | 5.0 × 10ˉ4 | Gal, GalNAc | |
| Peanut agglutinin | PNA | 4.0 × 10ˉ3 | Gal |
| Erythrina cristagalli lectin | ECL | 2.0 × 10ˉ2 | Gal, GalNAc |
| Ulex europaeus agglutinin-I | UEA-I | 2.0 × 10ˉ2 | Fuc |
| Concanavalin A | ConA | 3.3 × 10ˉ3 | Man, Glc |
| Pisum sativum agglutinin | PSA | 4.0 × 10ˉ3 | Man, Glc |
| Lens culinaris agglutinin | LCA | 4.0 × 10ˉ3 | Man, Glc |
| Phaseolus vulgaris agglutinin-E | PHA-E | 5.0 × 10ˉ3 | Oligosaccharide |
| Phaseolus vulgaris agglutinin-L | PHA-L | 2.5 × 10ˉ3 | Oligosaccharide |
Fuc, fucose; Gal, galactose; GalNAc, N-acetylgalactosamine; Glc, glucose; GlcNAc, N-acetylglucosamine; Man, mannose; NeuAc, N-acetylneuraminic acid.
RESULTS
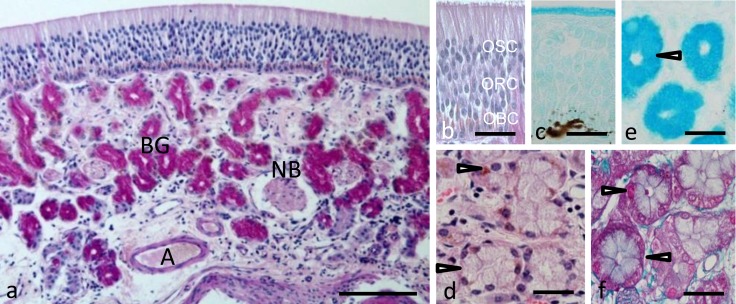
Topographical and histological features of the olfactory mucosa: The olfactory mucosa occupied only a small region at the caudal roof of the nasal cavity, where it entirely covered the ethmoturbinates and the caudal parts of the dorsal and middle nasal conchae. The olfactory mucosa consisted of the olfactory epithelium and the underlying lamina propria (Fig. 1a). The olfactory epithelium was composed of basal, receptor and supporting cells. Each type of cell had characteristics that have been already described for the cells of the olfactory epithelium in sheep [9, 13, 20, 22]. Briefly, nuclei of the olfactory basal, receptor and supporting cells were situated in the basal, middle and apical regions of the olfactory epithelium, respectively (Fig. 1b). The basal cells had scanty cytoplasm and an oval or somewhat round nucleus. Their nuclei were pale with distinct nucleolus and were smaller than those of the receptor and supporting cells. Cytoplasm of the basal cells reacted intensely positive to both eosin and PAS. The olfactory receptor cells were pear-shaped and extended their dendrites to the luminal surface and their axons toward the basal lamina. Their nuclei were arranged in three or four layers. The olfactory supporting cells had dark, oval-shaped nuclei arranged in three or four of the layers in the upper region of the olfactory epithelium. Some melanin pigments were found both in the basal region of the olfactory epithelium and the underlying lamina propria, but they were not equally distributed along the olfactory epithelium (Fig. 1c).
Fig. 1.
Histological features of the sheep olfactory mucosa. (a) Transverse section of the olfactory mucosa stained with Periodic Acid Schiff (PAS). Bar=100 µm. Higher magnification of the olfactory epithelium stained with (b) PAS and (c) alcian blue (pH 2.5). Higher magnification of the Bowman’s glandular acini stained with Hematoxylin and Eosin (d), alcian blue (pH 2.5) (e) or Crossmon’s trichrome (f). Arrowheads indicate Bowman’s glandular acinar cells. A, artery; BG, Bowman’s glands; NB, nerve bundle; OBC, olfactory basal cells; ORC, olfactory receptor cells; OSC, olfactory supporting cells. Bar=30 µm in (b)–(f).
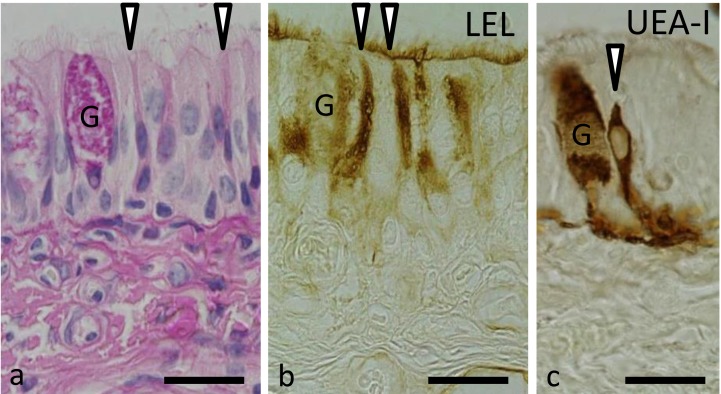
Within the lamina propria, Bowman’s glands were restricted to the superficial part of the propria. The secretory end-pieces of the Bowman’s glands were of the tubuloacinous type, and their ducts extended vertically through the olfactory epithelium to open into the free border of the olfactory epithelium. Acini of the Bowman’s glands consisted of pyramidal cells containing basally situated oval or round nuclei and surrounding a narrow lumen. Brownish granules were detected in the basal cytoplasm of the acinar cells as well as their duct cells (Fig. 1d). Both PAS (Fig. 1a) and alcian blue pH 2.5 (Fig. 1e) stained the apical cytoplasm of the acinar cells, while Crossmon’s trichrome (Fig. 1f) stained the basal portion of these cells.
Lectin binding patterns in the olfactory mucosa: The receptor cells of the olfactory epithelium were labeled, with varying intensity, among 12 of the 21 lectins used in this study: Wheat germ agglutinin (WGA), Succinylated-wheat germ agglutinin (s-WGA), Lycopersicon esculentum lectin (LEL), Solanum tuberosum lectin (STL), Datura stramonium lectin (DSL), Soybean agglutinin (SBA), Bandeiraea simplicifolia lectin-I (BSL-I), Vicia villosa agglutinin (VVA), Ricinus communis agglutinin-I (RCA-120), Concanavalin A (Con A), Pisum sativum agglutinin (PSA) and Phaseolus vulgaris agglutinin-E (PHA-E). Cell processes, cell membranes and perinuclear cytoplasm were intensely stained, but the nuclei were not stained (Fig. 2a, 2a’, 2b and 2b’). Three lectins, s-WGA, SBA and PSA, faintly stained the perinuclear cytoplasm of the receptor cells. Punctuated labeling of the receptor cells was made by VVA and BSL-I (Fig. 2c and 2c’). The olfactory receptor cells were not labeled by nine of the lectins used: Bandeiraea simplicifolia lectin-II (BSL-II), Dolichos biflorus agglutinin (DBA), Sophora japonica agglutinin (SJA), Jacalin, Peanut agglutinin (PNA), Erythrina cristagalli lectin (ECL), Ulex europaeus agglutinin-I (UEA-I), Lens culinaris agglutinin (LCA) and Phaseolus vulgaris agglutinin-L (PHA-L) (Fig. 2d–2f’).
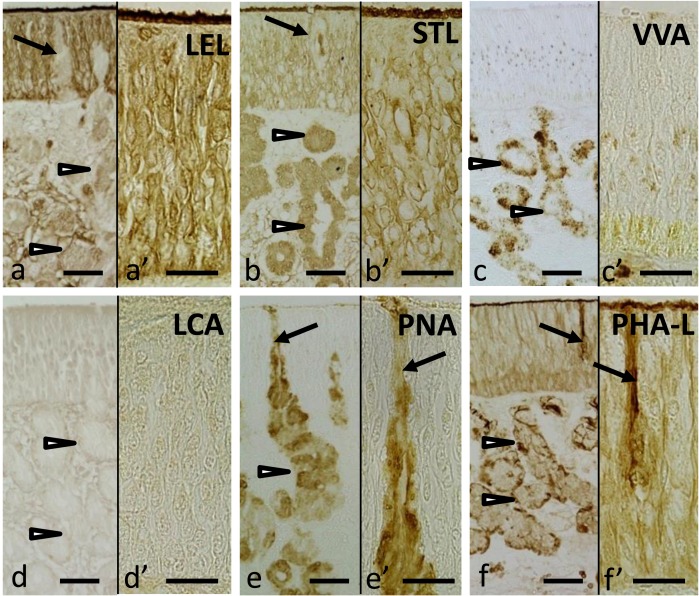
Fig. 2.
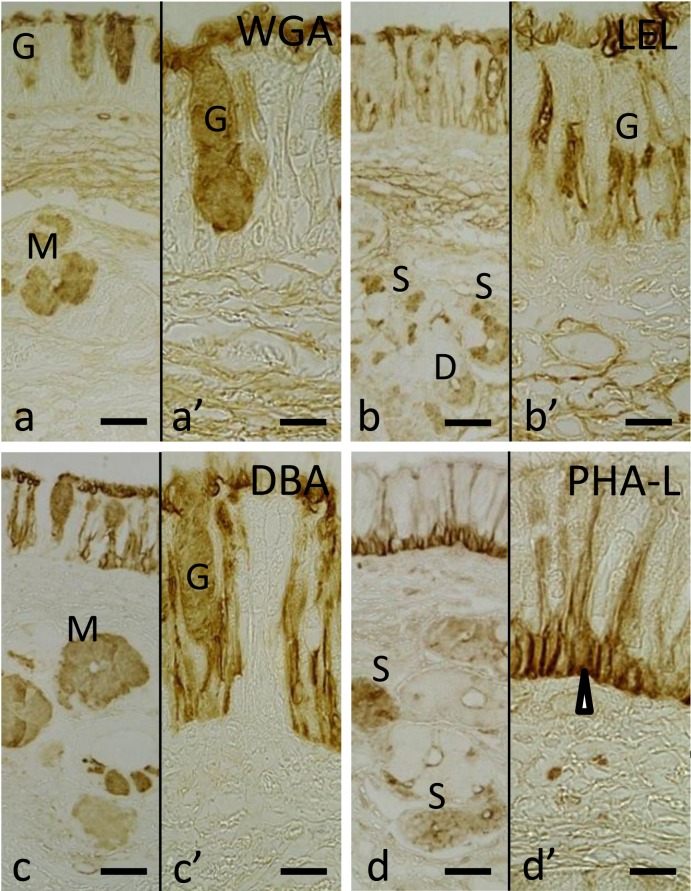
Olfactory mucosa reacted with six lectins: LEL (a), (a’), STL (b), (b’), VVA (c), (c’), LCA (d), (d’), PNA (e), (e’) and PHA-L (f) and (f’). Arrowheads indicate Bowman’s gland acini. Arrows indicate Bowman’s gland duct opening into the surface of olfactory epithelium. Bar=50 µm in (a)–(f) and 30 µm in (a’)–(f’).
Eight of the lectins used, WGA, LEL, STL, DSL, RCA-120, Con A, PHA-E and PHA-L, labeled both the olfactory supporting and basal cells with varying intensity. The localizations of staining reactions were similar to each other and were restricted to the cytoplasm of both the olfactory supporting and basal cells (Fig. 2f and 2f’). VVA labeled the cytoplasm of the basal cells (Fig. 2c and 2c’).
The free border of the olfactory epithelium was stained by 13 lectins: WGA, s-WGA, LEL, STL, DSL, SBA, BSL-I, RCA-120, PNA, ECL, Con A, PHA-E and PHA-L with varying intensity.
Among the 21 lectins used, 13 lectins labeled Bowman’s glands with varying intensity: WGA, s-WGA, STL, DSL, BSL-I, VVA, RCA-120, Jacalin, PNA, ECL, Con A, PHA-E and PHA-L. Seven lectins homogenously labeled the cytoplasm of Bowman’s gland cells: s-WGA, STL, DSL, RCA-120, Jacalin, PNA and ECL (Fig. 2b and 2e). BSL-I and VVA had punctuated staining pattern, and the staining reactions were scattered within the cytoplasm of the glandular cells (Fig. 2c). On the other hand, the staining patterns for WGA, Con A, PHA-E and PHA-L were similar to each other, where they intensely stained the basal part of the glandular cells (Fig. 2f).
The lectin binding patterns in the olfactory mucosa are summarized in Table 2.
Table 2. Lectin binding patterns of the sheep olfactory mucosa.
| Lectin | Free border | Receptor cells | Supporting cells | Basal cells | Bowman’s glands |
|---|---|---|---|---|---|
| WGA | ++ | + | + | + | + |
| s-WGA | + | +/– | – | – | +/– |
| LEL | ++ | ++ | ++ | ++ | – |
| STL | ++ | + | + | + | ++ |
| DSL | ++ | + | + | + | +/– |
| BSL-II | – | – | – | – | – |
| DBA | – | – | – | – | – |
| SBA | + | +/– | – | – | – |
| BSL-I | +/– | + | – | – | + |
| VVA | – | + | – | + | + |
| SJA | – | – | – | – | – |
| RCA-120 | ++ | + | + | + | + |
| Jacalin | – | – | – | – | +/– |
| PNA | +/– | – | – | – | + |
| ECL | + | – | – | – | +/– |
| UEA-I | – | – | – | – | – |
| Con A | ++ | + | + | + | + |
| PSA | – | +/– | – | – | – |
| LCA | – | – | – | - | – |
| PHA-E | ++ | + | + | + | + |
| PHA-L | ++ | – | + | ++ | + |
–: Negative staining, +/–: Faint staining, +: Moderate staining, ++: Intense staining.
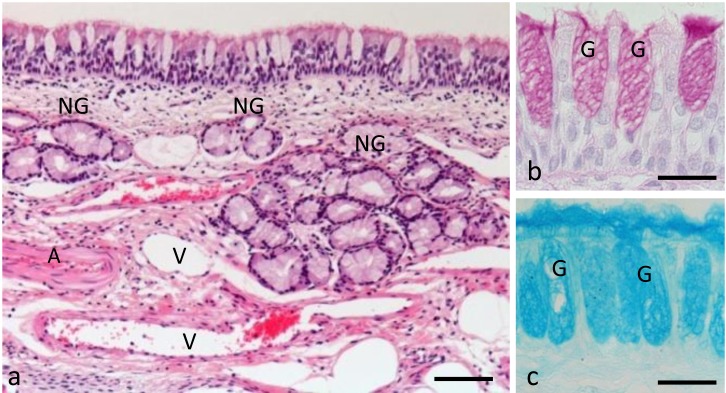
Topographical and histological features of the respiratory mucosa: The respiratory mucosa was located mostly in the middle part of the nasal cavity of the sheep. It lined the lateral wall of the nasal cavity and nasal septum and covered the vomeronasal organ laterally. The respiratory mucosa was composed of respiratory epithelium and connective tissue lamina propria (Fig. 3a). The respiratory epithelium was of a pseudostratified ciliated columnar type. It was composed of basal cells, ciliated cells and goblet cells. Each type of cell had properties that have been already described for the respiratory epithelial cells in sheep [20, 22]. The goblet cells consisted of an apical part containing mucopolysaccharide (mucin) granules and a basal part containing the nucleus. The goblet cells reacted positively with both PAS and alcian blue pH 2.5 with varying intensity according to their mucin content (Fig. 3b and 3c). The number of the goblet cells of the respiratory epithelium varied from one part to another in the nasal cavity. The basal cells had scanty cytoplasm and an oval or somewhat round nucleus. Their nuclei were darkly stained by hematoxylin and were smaller in size than those of the ciliated cells. The ciliated cells were located among the goblet cells of the respiratory epithelium. Their cytoplasm was only faintly stained by eosin, and their nuclei were also paler than those of the basal and goblet cells.
Fig. 3.
Histological features of the sheep respiratory mucosa. (a) Transverse section of the respiratory mucosa containing both the respiratory epithelium and the underlying lamina propria. Hematoxylin and Eosin. Bar=100 µm. (b) Periodic Acid Schiff (PAS). (c) Alcian blue (pH 2.5). Higher magnification of the respiratory epithelium. Bars=20 µm. A, muscular artery; G, goblet cell; NG, nasal glands; V, venous sinus.
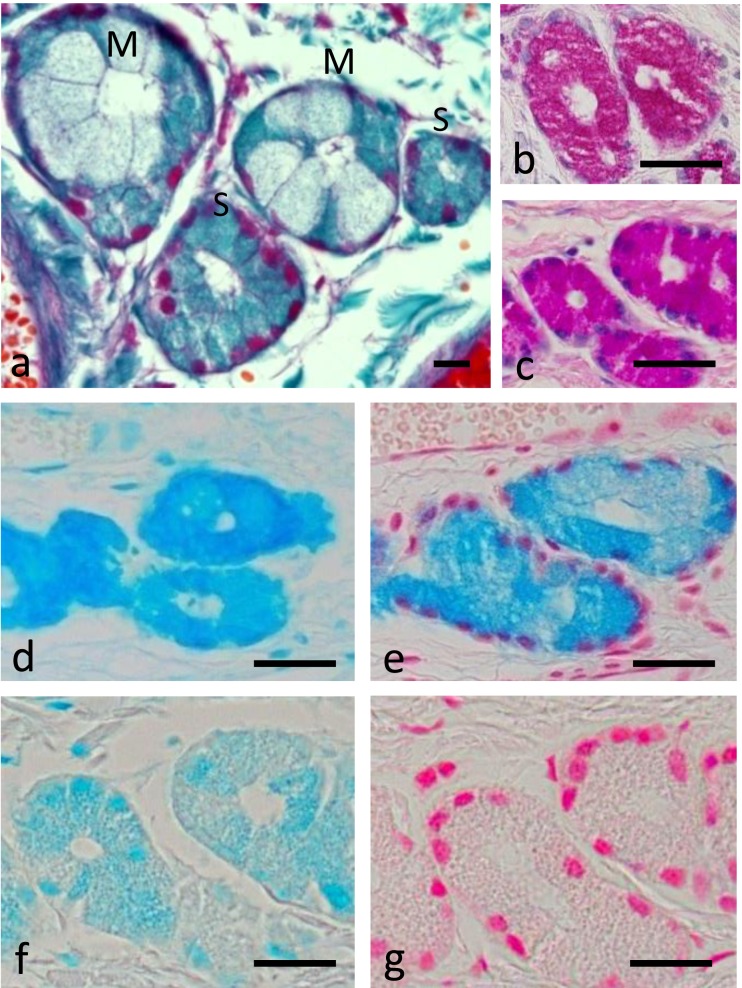
Within the propria, numerous glands with their ducts were found. Both serous and mixed glands were detected (Fig. 4a). The mixed end-pieces comprised of both mucous, high cuboidal cells (flattened nuclei) together with a few number of peripherally located serous cells surrounding a relatively wide lumen (Fig. 4b, 4d and 4e). On the other hand, the serous end-pieces consisted of pyramidal cells surrounding a narrow lumen. Their nuclei were rounded and located at the basal portion of the cell (Fig. 4c, 4f and 4g). Both glandular types reacted positively, but in a variable manner with PAS and alcian blue pH 2.5 (Fig. 4b, 4c, 4e and 4g). Mixed glands were stained with alcian blue pH 1.0, but the serous glands were not (Fig. 4d and 4f).
Fig. 4.
Histological features of the serous and mixed glands within the lamina propria of the respiratory mucosa. (a) Crossmon’s trichrome. Bar=50 µm. Mixed glands (b, d, e). Serous glands (c, f, g). PAS (b, c). Alcian blue (pH 2.5) (d, f). Alcian blue (pH 1.0) (e, g). M, mixed gland; S, serous gland. Bars=20 µm in (b)–(g).
Lectin binding patterns in the respiratory mucosa: Twelve out of the 21 lectins used in this study: WGA, s-WGA, LEL, STL, BSL-II, DBA, SBA, BSL-I, VVA, RCA-120, ECL and UEA-I stained the goblet cells of the respiratory epithelium with varying intensity. The staining patterns were similar to each other, and the reactions were localized to the mucin granules in the apical cytoplasm. The intensity of staining was dependent on the amount of mucin granules in each goblet cell (Fig. 5a, 5a’, 5b and 5b’). No lectins labeled the nuclei of the goblet cells.
Fig. 5.
Respiratory mucosa reacted with 4 lectins: WGA (a), (a’) LEL (b), (b’), DBA (c), (c’) and PHA-L (d), (d’). Arrowhead indicates basal cells. D, Glandular duct; G, goblet cell; M, mucous cell; S, serous cell. Bar=50 µm in (a)–(d) and 30 µm in (a’)–(d’).
Staining patterns for DBA, SBA and VVA in the respiratory epithelium were similar to each other. There were alternative intervals of stained and unstained regions in the basal and ciliated cells of the respiratory epithelium (Fig. 5c and 5c’).
Eight lectins: DSL, DBA, SBA, VVA, RCA-120, UEA-I, Con A and PHA-L, labeled the ciliated cells with varying intensity (Fig. 5c, 5c’, 5d and 5d’). The staining patterns were similar to each other; these lectins labeled both their cell membranes and cytoplasm.
The basal cells were stained by 11 lectins: LEL, STL, DSL, DBA, SBA, BSL-I, VVA, UEA-I, Con A, PHA-E and PHA-L with varying intensity. Cell membranes and cytoplasm of the basal cells were stained, but their nuclei were not stained (Fig. 5c, 5c’, 5d and 5d’).
The free border of the respiratory epithelium was labeled by 15 lectins: WGA, s-WGA, LEL, STL, DSL, DBA, SBA, BSL-I, VVA, RCA-120, ECL, UEA-I, Con A, PHA-E and PHA-L with varying intensity.
Four lectins out of the 21 lectins used: SJA, Jacalin, PNA and LCA were negative in neither epithelium.
Several spindle-shaped cells of unknown origin with dark nuclei and scanty cytoplasm were detected within the respiratory epithelium mostly located close to the goblet cells (Fig. 6a). These cells were labeled with varying intensity by 7 lectins: LEL, DBA, SBA, BSL-I, VVA, UEA-I and PHA-L. The staining reactions were similar to each other, including both their cytoplasm and nuclei (Fig. 6b). Only UEA-I distinguished their cytoplasm from nuclei by staining the cytoplasm intensely with the nuclei remaining unstained (Fig. 6c).
Fig. 6.
Unidentified, spindle-shaped cells within the respiratory epithelium (arrowheads). (a) Periodic Acid Schiff (PAS). (b) LEL. (c) UEA-I. G, goblet cell. Bar=20 µm in (a)-(c).
The lectin binding patterns in the respiratory epithelium are summarized in Table 3.
Table 3. Lectin binding patterns of the sheep respiratory epithelium.
| Lectin | Free border | Goblet cells | Basal cells | Ciliated cells | Unrecognized type of cells |
|---|---|---|---|---|---|
| WGA | ++ | + | – | – | – |
| s-WGA | + | ++ | – | – | – |
| LEL | ++ | +/– | +/– | – | ++ |
| STL | + | + | +/– | – | – |
| DSL | ++ | – | + | + | – |
| BSL-II | – | + | – | – | – |
| DBA | +* | +/++ | ++* | + | + |
| SBA | +* | ++ | ++ | + | + |
| BSL-I | +/– | + | + | – | + |
| VVA | +* | + | + | + | + |
| SJA | – | – | – | – | – |
| RCA-120 | ++ | +/– | – | + | – |
| Jacalin | – | – | – | – | – |
| PNA | – | – | – | – | – |
| ECL | + | + | – | – | – |
| UEA-I | +/– | ++ | + | +/– | ++ |
| Con A | + | – | + | +/– | – |
| PSA | – | – | – | – | – |
| LCA | – | – | – | – | – |
| PHA-E | + | – | +/– | – | – |
| PHA-L | + | – | ++ | +/– | + |
–: Negative staining, +/–: Faint staining, +: Moderate staining, ++: Intense staining. *: Remarkable staining (binding reaction is not equally distributed along the cell membrane).
In the lamina propria, the mucous cells were stained intensely by 9 among the 21 lectins used in this study: WGA, s-WGA, STL, DBA, SBA, VVA, ECL, UEA-I and PHA-E (Fig. 5a and 5c). The serous glands as well as the serous cells of the mixed glands were labeled by six lectins: LEL, DSL, BSL-II, Con A, PHA-E and PHA-L with varying intensity (Fig. 5b and 5d).
The lectin binding patterns of the nasal glands are summarized in Table 4.
Table 4. Lectin binding patterns of the glands in the lamina propria of the respiratory epithelium.
| Lectin | Serous glands | Mixed glands |
|
|---|---|---|---|
| Serous cells | Mucous cells | ||
| WGA | – | – | ++ |
| s-WGA | – | – | ++ |
| LEL | ++ | ++ | – |
| STL | – | – | + |
| DSL | + | + | – |
| BSL-II | +/– | +/– | – |
| DBA | – | – | ++ |
| SBA | – | – | + |
| BSL-I | – | – | – |
| VVA | – | – | ++ |
| SJA | – | – | – |
| RCA-120 | – | – | – |
| Jacalin | – | – | – |
| PNA | – | – | – |
| ECL | – | – | ++ |
| UEA-I | – | – | ++ |
| Con A | + | + | – |
| PSA | – | – | – |
| LCA | – | – | – |
| PHA-E | + | + | + |
| PHA-L | ++ | ++ | – |
–: Negative staining, +/–: Faint staining, +: Moderate staining, ++: Intense staining.
DISCUSSION
In the present study, we have revealed the details of the glycoconjugate localizations in sheep. Our histological and histochemical studies using H&E, PAS and alcian blue had good agreement with previous reports by Kavoi et al. [9], Khamas and Ghoshal [10] and Taher et al. [22]. On the contrary, some of our findings using lectin histochemistry were inconsistent with the previous report in the olfactory epithelium by Scocco et al. [20]. Our study showed eight lectins labeled both the supporting and basal cells with varying intensity: WGA, LEL, STL, DSL, RCA-120, Con A, PHA-E and PHA-L. On the other hand, Scocco et al. [20] labeled these cells using nine lectins and found that six lectins: WGA, Con A, LTA, UEA-I, SBA and GSA-IB4 were positive in these cells. Only 2 lectins, WGA and Con A, were positive in both of our work and Scocco’s work. In the respiratory epithelium, the case was the same as in the olfactory epithelium. Our results and Scocco’s results showed only partial consistency; 5 of mutually examined lectins (Con A, DBA, SBA, UEA-I and RCA) were positive by both studies, but one lectin (WGA) was positive only in Scocco’s study. The discrepancies in the results between Scocco et al. [20] and our own study may at least be partially due to the differences in the fixatives employed (Carnoy’s fluid for 24 hr at room temperature and postfixed in 2% calcium acetate-4% paraformaldehyde solution 1:1 by Scocco et al.). But, it could also be attributed to the differences between the Corriedale and Lacaune sheep breeds.
Twelve lectins stained both the free border of the olfactory epithelium and that of the respiratory epithelium: WGA, s-WGA, LEL, STL, DSL, SBA, BSL-I, RCA-120, ECL, Con A, PHA-E and PHA-L, indicating that the expression pattern of glycoconjugate was similar between the free border of these two epithelia. The lectin stainings in the free border may come partially from the glycoconjugates in the mucus covering the surface of the epithelium and partially from those expressed in the cell membrane of the cilia or the microvilli of the goblet, ciliated, receptor or supporting cells. The mucus at the surface of the olfactory mucosa constitutes the milieu in which perireceptor events associated with olfactory signal transduction occur [7]. It is tempting to speculate, firstly, that the goblet cells and mucous nasal glands in the respiratory epithelium may contribute to the function of the olfactory epithelium in the perception of odorants in the olfactory receptor cells. The secretory products of the respiratory epithelium may distribute caudally on the free border of the olfactory epithelium [21] by the movement of cilia and gravity and thus accelerating the movement of various odorants towards their targeted olfactory receptor cells, resulting in enhanced perception of odorants in the olfactory epithelium. Another possibility is that the secretory products of the Bowman’s glands may distribute cranially on the free border of the respiratory epithelium and thus help in discarding of dust particles and foreign organisms from the respiratory epithelium, accelerating the function of the goblet cells as a part of the nasal cavity immune system. The 2nd idea is that there are similarities in the carbohydrate expressions of the cell membranes of the two epithelia. Although it is possible that the secretion from Bowman’s and nasal glands affects the lectin-binding patterns on the free border, the patterns may be attributed to the sugar residues expressed on the olfactory knobs, cilia or microvilli of the free borders, since some of lectins binding to the free borders did not bind to the associated glands. For example, LEL stained the free borders of both the olfactory and respiratory epithelia, but its staining intensity in the Bowman’s glands and goblet cells is faint. This may indicate that the sugar residues that were labeled by LEL may originate from the cell processes and not secreted or expressed either in the Bowman’s glands or goblet cells.
Unrecognized spindle-shaped cells were detected in the respiratory epithelium. These cells were labeled by seven out of the 21 lectins used in this study. Exclusively, UEA-I stained the cytoplasm of these cells, but not the nuclei. As far as we know, this is the first report detecting this type of cell in the respiratory epithelium of sheep. We speculate that the presence of these cells can be attributed to one of the different cell cycle phases of the goblet or ciliated cells, as immature differentiated cells.
Acknowledgments
This work was supported by Grant-in-Aid for Graduate Students from The United Graduate School of Veterinary Science, Gifu University (D.I.). We thank Prof. Kitamura of Obihiro University of Agriculture and Veterinary Medicine for his appropriate advice.
REFERENCES
- 1.Badawi H., Fateh El-Bab M. R.1974. Anatomical and histological studies on the nasal cavity of the camel, Camelus dromedaries. Assuit Vet. Med. J. 1: 1–14 [Google Scholar]
- 2.Buck L., Axel R. A.1991. Novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65: 175–187. doi: 10.1016/0092-8674(91)90418-X [DOI] [PubMed] [Google Scholar]
- 3.Cook G. M.1986. Cell surface carbohydrates: molecules in search of a function? J. Cell Sci. Suppl. 4: 45–70. doi: 10.1242/jcs.1986.Supplement_4.4 [DOI] [PubMed] [Google Scholar]
- 4.Damjanov I.1987. Lectin cytochemistry and histochemistry. Lab. Invest. 57: 5–20 [PubMed] [Google Scholar]
- 5.Endo D., Yamamoto Y., Nakamuta N., Taniguchi K.2011. Developmental changes in lectin-binding patterns of three nasal sensory epithelia in Xenopus laevis. Anat. Rec. (Hoboken) 294: 839–846. doi: 10.1002/ar.21377 [DOI] [PubMed] [Google Scholar]
- 6.Endo D., Yamamoto Y., Yamada M., Nakamuta N., Taniguchi K.2011. Localization of eNOS in the olfactory epithelium of the rat. J. Vet. Med. Sci. 73: 423–430. doi: 10.1292/jvms.10-0353 [DOI] [PubMed] [Google Scholar]
- 7.Getchell M. L., Getchell T. V.1992. Fine structural aspects of secretion and extrinsic innervation in the olfactory mucosa. Microsc. Res. Tech. 23: 111–127. doi: 10.1002/jemt.1070230203 [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim D., Nakamuta N., Taniguchi K., Taniguchi K.2013. Lectin histochemical studies on the vomeronasal organ of the sheep. J. Vet. Med. Sci. 75: 1131–1137. doi: 10.1292/jvms.12-0532 [DOI] [PubMed] [Google Scholar]
- 9.Kavoi B., Makanya A., Hassanali J., Carlsson H. E., Kiama S.2010. Comparative functional structure of the olfactory mucosa in the domestic dog and sheep. Ann. Anat. 192: 329–337. doi: 10.1016/j.aanat.2010.07.004 [DOI] [PubMed] [Google Scholar]
- 10.Khamas W. A., Ghoshal N. G.1982. Histomorphologic studies of the nasal cavity of sheep, Ovis aries, and its significance in temperature regulation of the brain. Acta Anat. 113: 340–351. doi: 10.1159/000145568 [DOI] [PubMed] [Google Scholar]
- 11.Kondoh D., Nashimoto M., Kanayama S., Nakamuta N., Taniguchi K.2011. Ultrastructural and histochemical properties of the olfactory system in the Japanese jungle crow, Corvus macrorhynchos. J. Vet. Med. Sci. 73: 1007–1014. doi: 10.1292/jvms.10-0574 [DOI] [PubMed] [Google Scholar]
- 12.Kondoh D., Yamamoto Y., Nakamuta N., Taniguchi K., Taniguchi K.2010. Lectin histochemical studies on the olfactory epithelium and vomeronasal organ in the Japanese striped snake, Elaphe quadrivirgata. J. Morphol. 271: 1197–1203. doi: 10.1002/jmor.10864 [DOI] [PubMed] [Google Scholar]
- 13.Kratzing J.1970. The olfactory mucosa of the sheep. Aust. J. Biol. Sci. 108: 447–458 [Google Scholar]
- 14.Leathem A., Atkins N.1983. Lectin binding to formalin-fixed paraffin sections. J. Clin. Pathol. 36: 747–750. doi: 10.1136/jcp.36.7.747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendoza A. S.1993. Morphological studies on the rodent main and accessory olfactory systems: the regio olfactoria and vomeronasal organ. Ann. Anat. 175: 425–446. doi: 10.1016/S0940-9602(11)80110-X [DOI] [PubMed] [Google Scholar]
- 16.Nakajima T., Shiratori K., Ogawa K., Tanioka Y., Taniguchi K.1998. Lectin-binding patterns in the olfactory epithelium and vomeronasal organ of the common marmoset. J. Vet. Med. Sci 60: 1005–1011. doi: 10.1292/jvms.60.1005 [DOI] [PubMed] [Google Scholar]
- 17.Nakamuta S., Nakamuta N., Taniguchi K., Taniguchi K.2012. Histological and ultrastructural characteristics of the primordial vomeronasal organ in lungfish. Anat. Rec.(Hoboken) 295: 481–491. doi: 10.1002/ar.22415 [DOI] [PubMed] [Google Scholar]
- 18.Nakamuta N., Yokoyama N., Yamamoto Y., Taniguchi K., Taniguchi K.2010. Lectin histochemical analysis of the olfactory bulbs in the barfin flounder (Verasper moseri). Anat. Histol. Embryol. 39: 67–73. doi: 10.1111/j.1439-0264.2009.00979.x [DOI] [PubMed] [Google Scholar]
- 19.Saito S., Matsui T., Kobayashi N., Wakisaka H., Mominoki K., Matsuda S., Taniguchi K.2003. Lectin histochemical study on the olfactory organ of the newt, Cynops pyrrhogaster, revealed heterogenous mucous environments in a single nasal cavity. Anat. Embryol. (Berl) 206: 349–356 [DOI] [PubMed] [Google Scholar]
- 20.Scocco P., Lepri E., Mercati F., Vitellozzi G., Mechelli L., Ceccarelli P.2012. Glycohistochemical characterization of histologically normal nasal mucosa and enzootic nasal tumor of sheep. Am. J. Vet. Res. 73: 1128–1136. doi: 10.2460/ajvr.73.8.1128 [DOI] [PubMed] [Google Scholar]
- 21.Sung Y. K., Moon C., Yoo J.Y., Moon C., Pearse D., Pevsner J., Ronnett G. V.2002. Plunc, a member of the secretory gland protein family, is up-regulated in nasal respiratory epithelium after olfactory bulbectomy. J. Biol. Chem. 277: 12762–12769. doi: 10.1074/jbc.M106208200 [DOI] [PubMed] [Google Scholar]
- 22.Taher E. M., Ali A. M., Saad A. H., Gaily S. H., Ahmed A. K.1989. Histological and carbohydrate histochemical studies of the nasal mucosa of sheep in Saudi Arabia with special reference to its glandular tissue. Z. Mikrosk. Anat. Forsch. 103: 993–1003 [PubMed] [Google Scholar]
- 23.Taniguchi K., Saito S., Taniguchi K.2011. Phylogenic outline of the olfactory system in vertebrates. J. Vet. Med. Sci. 73: 139–147. doi: 10.1292/jvms.10-0316 [DOI] [PubMed] [Google Scholar]
- 24.Taniguchi K., Taniguchi K., Arai T., Ogawa K.1992. Enzyme histochemistry of the olfactory and vomeronasal sensory epithelia in the golden hamster. J. Vet. Med. Sci. 54: 1007–1016. doi: 10.1292/jvms.54.1007 [DOI] [PubMed] [Google Scholar]