ABSTRACT
The study was to find patterns of progestagen (progesterone and its metabolite) and glucocorticoid and their metabolite concentrations in serum and feces of pregnant Asian elephants (Elephas maximus). The 5 female Asian domestic elephants were naturally mated until pregnancy. After that, blood and feces samples were collected monthly during pregnancy for progestagen, glucocorticoid and their metabolites analysis by enzyme immunoassay (EIA). The results showed the serum progestagen concentration during gestation was 2.11 ± 0.60 to 18.44 ± 2.28 ng/ml. Overall, serum progestagen concentration rose from the 1st month to reach peak in the 11th month, after which it declined to its lowest level in the 22nd month of pregnancy. Fecal progestagen concentration varied from 1.18 ± 0.54 to 3.35 ± 0.45 µg/g during pregnancy. In general, fecal progestagen concentration increased from the 1st month to its highest level in the 12th month. After this, it declined reaching its lowest point in the 22nd month of pregnancy. Glucocorticoid hormones and their metabolite concentrations both in serum and feces fluctuated from low to medium throughout almost the entire pregnancy period and then rapidly increased around the last week before calving. Our study suggests that this profile of progestagen and glucocorticoid hormones and their metabolite concentration levels in serum and feces can be used to assess the pregnancy status of Asian elephants. If serum and fecal progestagen concentrations were found in very low levels and glucocorticoid and their metabolite concentrations were found in very high levels, it was indicated that the cow elephant would calve within 7 days.
Keywords: Asian elephant, enzyme immunoassay, glucocorticoid, progestagen, serum and feces
The Asian elephant (Elephas maximus) is an endangered species and has been listed in Appendix 1 of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) since 1973. The captive elephant population has also declined along with that of the wild elephants [22]. Thus, it is necessary to encourage breeding management and to maintain the pregnancy of captive Asian elephants in order to increase the population and maintain genetic diversity. For the conservation of the captive elephant population, the assessment of female reproductive functioning is necessary [11], such as determining the pregnancy endocrine pattern. For pregnant elephants, determining the steroid level patterns in their serum and feces is an alternative method for assessing their pregnancy status, since there is no disruption of reproductive function during pregnancy. For free-ranging or wild elephants, measurement of fecal steroid metabolites is an especially useful and noninvasive method for monitoring reproductive cyclicity and pregnancy [10, 11]. Serum progesterone concentration during pregnancy in elephants varies from <50 to 2,100 pg/ml, mean values 400 to 600 pg/ml and significantly declines in the 2 to 3 days before parturition [5, 6, 17, 20, 21]. Previous studies have shown that progesterone is not found in significant amounts in the blood circulation of Asian and African elephants (Loxodonta Africana). Progesterone metabolite concentrations, however, are prevalent [13] and become about 10-fold higher during pregnancy [14]. Normally, the main progesterone hormones in feces are in the form of progesterone metabolites (5α-pregnane-3, 20-dione [5α-dihydroprogesterone: 5α-DHP] and 5α-pregnane-3-ol-20-one [5α-P-3OH]). However, the levels of fecal allopregnanolone (5α-P-3OH) predominate and are more useful for determining reproductive status, ranging from 1.41 to 7.38 µg/g [11] during pregnancy accompanied by a positive correlation with the level of serum progesterone [8, 10, 11, 15]. Serum glucocorticoid (its main forms are cortisol and corticosterone) is commonly measured for evaluating stress responsiveness in captive or restrained elephants [23]. The study of stress hormones is crucial to the understanding and evaluation of normal behavior, stress control, well-being, pregnancy status and efficiency regarding reproductive activity in elephants [2, 17]. During the gestation of Asian elephants, serum cortisol concentrations vary from 2.5 to 12.5 ng/ml and remain quite constant (on average 7.80 ± 2.36 ng/ml) throughout the whole gestation period [17]. Normally, before excretion in feces, the main glucocorticoid hormones are rapidly and extensively changed in form to glucocorticoid metabolites. Assays of fecal glucocorticoid or its metabolites in female elephants are validated for indicators of adrenal activity [2]. For example, overall fecal glucocorticoid metabolite concentrations rose after ACTH challenges in African elephants [9, 23]. But, fecal corticosterone metabolite concentrations increased dramatically in comparison to minor cortisol metabolite level changes [23]. Recently, fecal glucocorticoid assays have been approved and used for determining the reproductive status of elephants [1, 2, 23]. Measurement of fecal glucocorticoid is a practical, reliable and noninvasive method for this, because samples can be collected relatively easily in captive or semi-captive elephants without disturbing effects. Enzyme immunoassay (EIA) is the most common test for monitoring reproductive function, because it does not involve disposal of radioactive material or specialized and expensive equipment [4, 12]. Therefore, the aim of this study was to assess the pregnancy status of Asian elephants through the measurement of progestagen (progesterone and its metabolite), glucocorticoid (cortisol and corticosterone) and their metabolite level patterns and their correlations in serum and feces using EIA.
MATERIALS AND METHODS
Study area and experiment animals: This study was conducted on 5 pregnant Asian elephants which were domesticated in a semi-captive condition in the Ayuttaya Elephant Palace camp in Pranakornsri-Ayuttaya province, Thailand. All elephants in this study were chosen randomly from a mixed group. They varied in age between 21 and 37 years, had no reproductive problems and had not calved for at least 3 years previously. The animals were habituated to humans and used for tourist treks outside of the camp in the early morning or late afternoon. They were brought back to the camp for forage and supplementary feeding each evening. During their working period, they were given a variety of foods, such as banana, sugar cane and hay from tourists. In addition, they had access during this time to water ad libitum and natural grass. In camp, the elephants were divided into different groups according to age (calves, sub-adult, adult and old adult). The cows were separated from the bulls except during the breeding period. During this time, the bulls were walked to detect estrus for 30 to 60 min, 3 to 5 times per week in the early morning. When the cows were found to be in standing heat, they were allowed to mate until, they refused mounting. Pregnancy diagnosis was determined monthly by transrectal (linear transducer; 5.0 to 12.0 MHz) and transabdominal (convex transducer; 2.0 to 8.0 MHz) ultrasonography scanning (Model Medison portable ultrasound machine; Version SonoAce R3, Samsung Medison Co., Ltd., California, U.S.A.) between the 2nd and the 4th month after the last mating. Under their general management schedule, the elephants were dewormed and looked after by a veterinarian from the elephant camp. These experiments were approved by the Animal Care and Use Committee, Faculty of Veterinary Medicine, Mahanakorn University of Technology, Nong-chok, Bangkok, Thailand (Project: VET-MUT008/2553).
Sample collection: Blood and feces samples were collected monthly during the pregnancy period (beginning 30 days after the last mating) for routine progestagen (progesterone hormone and its metabolite) and glucocorticoid hormones and their metabolites analysis. The samples were collected twice a day (around 7.00 to 9.00 am and 16.00 to 18.00 pm), one day per month from each of the five pregnant elephants. Blood samples of around 10 ml were collected from the ear vein, while the cows were controlled by a mahout. Blood samples were kept for 8 to 16 hr at 4°C in a sealed box with ice packs, while the blood was transported to a laboratory. In the laboratory, the blood samples were centrifuged for 5 min at 1,500 g. The serum was collected and stored at −20°C until analysis. The fecal samples were collected from each elephant on the same day as the blood samples, 1 hr after blood sample collection. The fecal samples were taken from the rectum or chosen from the middle of a bolus of freshly dropped feces and were approximately 50 g. Fecal samples were packed in resealable plastic bags and kept for 8 to 16 hr at 4°C in a sealed box during transport and then stored at −20°C until analysis.
Serum and fecal hormone analysis: Serum and fecal progestagens, cortisol, corticosterone hormones and their metabolites were analyzed by enzyme immunoassay (EIA) at Conservation Research and Animal Health, Khao-Kheow open zoo, Cholburi province, Thailand. Serum, feces extraction and EIA analysis were conducted following the standardized protocol used by Brown et al. [4]. The concentrations of progestagen were determined using monoclonal antibody (dilution 1:10,800; Quidel clone no. 425; supplied by Coralie Munro (CM), University of California (UC) Davis, CA, U.S.A.) against 100% progesterone hormone and cross-reacted with various progesterone metabolites [12]. Horseradish peroxidase (HRP) conjugated antibody was used on already treated and visualized progestagens (dilution 1:60,000; supplied by CM, UC Davis) which were examined with a ELISA plate reader (TECANTM sunrise absorbance reader; Tecan Austria GmbH, Grödig, Austria) for quantitative progesterone detection by comparison with progesterone standard levels. The sensitivity of this assay was 0.02 ng/ml, and the inter-assay coefficients of variation (CV) for high and low concentration controls were 9.39% and 10.22%, respectively. The intra-assay CV for high and low concentration controls were 5.12% and 4.57%, respectively. Cortisol hormone and its metabolite assays used polyclonal cortisol antiserum (dilution 1:8,500; Quidel clone no. R4866; supplied by CM, UC Davis), against cortisol-3-carboxymethyloxime, linked to bovine serum albumin and cross-reacted with cortisol (100%), prednisolone (9.9%), prednisone (6.3%), cortisone (5%) and <0.1% with androstenedione, androsterone, corticosterone, desoxycorticosterone, 11-desoxycortisol, 21-desoxycortisone and testosterone [18]. The horseradish peroxidase (HRP) conjugated antibody was labeled cortisol (dilution 1:20,000; supplied by CM, UC Davis), and the use of the ELISA plate reader was for quantitative cortisol detection by comparison with cortisol standard levels. The sensitivity of this assay was 0.08 ng/ml. The inter-assay CV for high and low concentration controls and the intra-assay CV for high and low concentration controls were 8.23, 8.28, 4.75 and 4.93%, respectively. Corticosterone hormone and its metabolite assays were conducted by using polyclonal corticosterone antiserum (dilution 1:30,000; Quidel clone no. CJM06; supplied by CM, UC Davis), 100% binding with corticosterone hormone and cross-reacted with 14.25% desoxycorticosterone and 0.9% tetrahydrocorticosterone (CM, UC Davis). The horseradish peroxidase-conjugated antibody was labeled corticosterone (dilution 1:40,000; supplied by CM, UC Davis) and used with the ELISA plate reader for quantitative corticosterone detection. The sensitivity of this assay was 0.08 ng/ml. The inter- and intra-assay CV for high and low concentration controls were 8.56, 9.82, 5.81 and 6.08%, respectively.
Statistical analysis: Correlations between serum and fecal progestagens, cortisol and their metabolites and corticosterone and their metabolites were analyzed using Spearman’s correlation test, using SPSS (SPSS 10.0 for Windows, SPSS Inc. Chicago, IL, U.S.A.). The significant differences were preset at P<0.05.
RESULTS
Two of the pregnant elephants calved at about 21.5 months, and the other 3 pregnant elephants gave birth after approximately 22 months within 2 to 7 days after the last blood and feces samples collection (Last sample collections were at day 21 of the 22nd month).
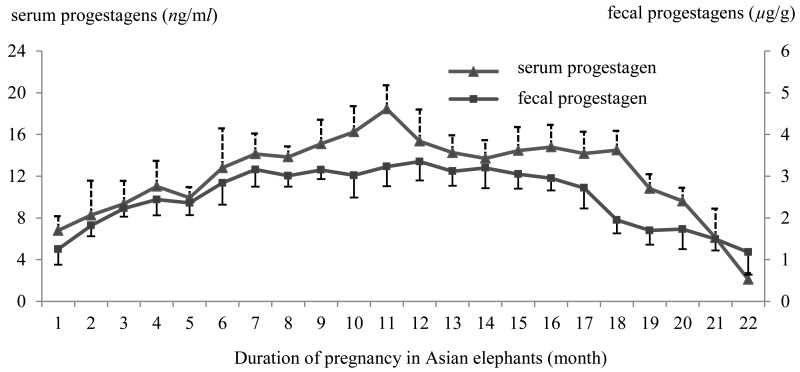
The average serum progestagen concentration profile during gestation in all pregnant elephants was 12.08 ± 3.87 ng/ml. Overall, mean serum progestagen gradually rose from the 1st month to the 4th month and then slightly decreased in the 5th month of pregnancy (Fig. 1). After this, it increased rapidly from over 12 ng/ml in the 6th month to a peak concentration of 18.44 ± 2.28 ng/ml in the 11th month (mid-gestation). It remained >12 ng/ml until the 18th month and then began to decline to its lowest level (2.11 ± 0.60 ng/ml) in the 22nd month shortly before parturition (2 to 7 days). Fecal progestagen concentration varied from 1.18 ± 0.54 to 3.35 ± 0.45 µg/g with an average of 2.50 ± 0.71 µg/g during pregnancy until the prepartum period and was highest during the 12th month (mid-gestation) of pregnancy (Fig. 1). Fecal progestagen levels showed a significant positive correlation with serum progestagen in all five Asian pregnant elephants (r=0.88, P<0.001, Table 1). In a pattern similar to serum progestagen, fecal progestagen rose slowly from the 1st month to the 4th month and then decreased a little in the 5th month of pregnancy. Fecal progestagen remained higher than 3 µg/g from the 7th until the 15th month and then declined rapidly to reach its lowest level in the 22nd month of pregnancy (on average 2 to 7 days before calving).
Fig. 1.
The mean (± SD) of serum and fecal progestagens concentration level profiles in Asian elephants during pregnancy (22 months).
Table 1. Correlations between serum and fecal progestagens, cortisol and their metabolites and corticosterone and their metabolites analyzed using Spearman’s correlation test.
| Correlation between hormone sorts | Correlation coefficient (r) | P-value |
|---|---|---|
| Serum and fecal progestagens | 0.880 | <0.001 |
| Serum corisol and fecal cortisol metabolite | 0.179 | 0.425 |
| Serum corticosterone and fecal corticosterone metabolite | 0.487 | 0.021 |
| Serum progestagen and serum cortisol | –0.719 | <0.001 |
| Serum progestagen and serum corticosterone | –0.206 | 0.357 |
| Serum cortisol and serum corticosterone | 0.438 | 0.042 |
| Fecal progestagen and fecal cortisol metabolite | 0.091 | 0.687 |
| Fecal progestagen and fecal corticosterone metabolite | 0.057 | 0.801 |
| Fecal cortisol metabolite and fecal corticosterone metabolite | 0.605 | 0.003 |
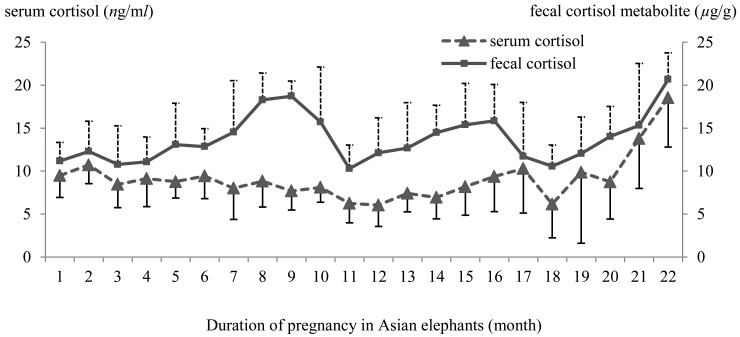
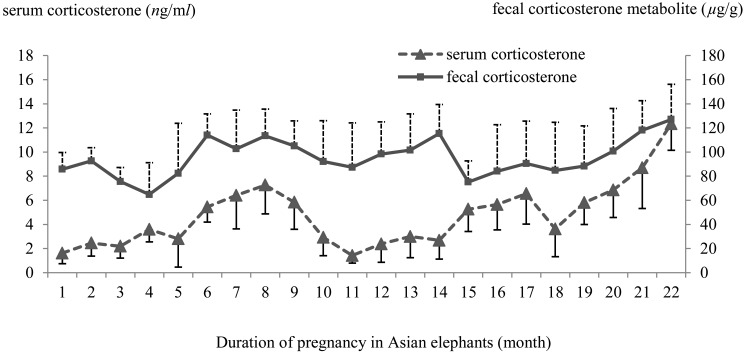
The serum cortisol concentration profile during gestation in all five pregnant elephants was 6.05 ± 2.48 to 18.54 ± 5.73 ng/ml(averaging 9.10 ± 2.72 ng/ml). Overall, serum cortisol concentrations remained relatively stable almost the whole period from the 1st month to the 20th month of pregnancy and then rapidly increased until parturition (Fig. 2). The fecal cortisol metabolite concentration level ranged from 10.3 ± 2.74 to 20.68 ± 3.09 µg/g (averaging 13.81 ± 2.82 µg/g). In general, fecal cortisol metabolite concentration levels fluctuated from the 1st to the 21st months of pregnancy and then rose to reach a peak in the 22nd month of pregnancy (Fig. 2). Serum cortisol concentrations showed no significant correlation with fecal cortisol metabolite levels in all five Asian pregnant elephants (Table 1). Serum corticosterone concentration levels during gestation in all 5 pregnant elephants ranged from 1.42 ± 0.63 to 12.35 ± 2.21 ng/ml (averaging 4.77 ± 2.67 ng/ml), and fecal corticosterone metabolite concentrations varied from 64.81 ± 26.45 to 127.07 ± 29.04 µg/g (averaging 95.44 ± 15.76 µg/g). Generally, both serum corticosterone and fecal corticosterone metabolite concentrations fluctuated almost the whole period from the 1st month to the 20th month of pregnancy and then reached a peak in the last month of the gestation period (Fig. 3). Serum corticosterone and fecal corticosterone metabolites concentration levels showed a significant positive correlation (Table 1).
Fig. 2.
The mean (± SD) of serum cortisol and fecal cortisol metabolite concentration level profiles in Asian elephants during pregnancy (22 months).
Fig. 3.
The mean (± SD) of serum corticosterone and fecal corticosterone metabolite concentration level profiles in Asian elephants during pregnancy (22 months).
DISCUSSION
The present study obviously demonstrated that serum glucocorticoid and fecal glucocorticoid metabolite concentrations in the last month of pregnancy (the 22nd month) archived their peak levels, while progestagens were in their lowest levels. These results could be specifically explained by the fact that serum and feces collections in the last period of pregnancy were done shortly (2 to 7 days) prior to parturition. This period was during which the elephant cows have a higher stress response. Our findings were similar to a previous report [17], in that the dramatically increased serum cortisol and the sharply decreased progestagens levels around 5 days before calving and the highest serum cortisol and the lowest serum progestagens levels were observed on the day of parturition. Brown and Lehnhardt [3] reported a gradual decline of serum progesterone and an increase of cortisol concentrations during the last month of gestation in Asian elephant. Thereafter, 2 days before parturition, the serum progesterone was obviously decreased to non-detectable levels, while the serum cortisol concentrations increased to reach the peak on the day of parturition. For fecal glucocorticoid metabolites and progestagens, our results did not agree with the other study by Foley et al. [9] who presented that the fecal corticoid metabolites pursued fecal progestagens during periparturition until postpartum if a dietary effect of seasonal changes was responded in free-ranging African elephants. In our study, the elephants were domesticated in semi-captive condition which they were fed with optimal diets. Therefore, the diet should not affect the outcome presented in this study. In addition, the high glucocorticoid concentrations could be due to a calving stress response resulting in increased concentration levels in serum and feces shortly before parturition. This means that measuring serum or fecal glucocorticoid concentration levels as an indicator of stress could be used to monitor the progestagen profile indirectly in the short period of periparturition. However, the prediction of parturition required more frequent sample collections during the last month of gestation period.
Although the number of elephants (5 pregnant elephants) and the frequency of sampling collections (1 day per month) were limited, the present study could demonstrate a gestation length of 21.5 to 22 months, similar to that found by other studies [2, 16, 17]. There would be only a minimal disruption of the pregnant elephant’s habitat and working routines. The monthly samplings, however, were carried out twice a day in order to increase the sample number and reduce the possibility of error in the hormone concentration levels readings.
The serum progestagen concentration profile in our study was similar to the serum concentration profiles of progesterone, 5α-P-3OH and 5α-DHP (progesterone metabolites) in pregnant African elephants that were reported previously [15, 16, 24, 25]. Moreover, the serum progestagen concentration profile in our research could indicate that luteal activity was at its highest level in mid-gestation (in order to maintain pregnancy) and then declined to its lowest level from 7 to 2 days prior to calving, which is supported by the serum progesterone concentration profiles of pregnant elephants found in other studies [5, 6, 16, 17, 19,20,21, 24, 25]. The average fecal progestagen concentration in this study was lower than the 5α-P-3OH (major progesterone metabolite in elephant feces) concentration that was found previously [11]. That study used a 100% specific antibody, while our study used a 64% specific antibody with 5α-P-3OH (Monoclonal antibody Quidel clone no. 425). However, the fecal progestagen concentration profile in this study was quite similar to the fecal progesterone, 5α-P-3OH and 5α-DHP concentration profiles found in a previous study of pregnant African elephants [8]. A significant positive correlation with serum progestagen concentrations could mean that high concentration levels of progesterone in serum during pregnancy are excreted mainly in the form of progesterone metabolites in feces. If so, assays of fecal progesterone metabolites could be useful as an indicator of luteal activity for assessing the pregnancy status of Asian elephants according to other studies [8, 10, 11, 15].
Glucocorticoid hormones (cortisol and corticosterone) were measured to evaluate stress responsiveness in the elephants in this study. It was found that the serum cortisol concentration profile was relatively similar to a previous study [17, 20]. Fecal cortisol metabolite concentration levels during pregnancy in our study, however, were lower than the levels in the pregnant African elephants reported previously [9]. Normally, serum glucocorticoid (cortisol and corticosterone) hormone is rapidly and extensively changed into the form of metabolites before excretion in feces [2]. Therefore, the correlation between serum cortisol and fecal cortisol metabolites should be positive. But, serum cortisol and fecal cortisol metabolite concentration patterns in this study showed no significant correlation (Table 1). This could be because the polyclonal antiserum used in this study (R4866) had a limited cross-reactivity with <10% fecal cortisol metabolites. This could cause a reduction in the detection of fecal cortisol metabolites and help account for the lack of correlation between these concentration levels and those of serum cortisol. In this study, however, there were significant positive correlations between serum corticosterone and fecal corticosterone metabolites and serum cortisol and corticosterone and fecal cortisol metabolites and corticosterone metabolites (Table 1). The average fecal corticosterone metabolite concentration was about 7 times higher than the fecal cortisol metabolite concentration during gestation in all five pregnant elephants. An increase of corticosterone metabolites concentrations in feces during pregnancy was also reported previously [23]. Our research found that serum progestagen was negatively correlated with serum cortisol and an inclination (non significant) with serum corticosterone according to Meyer et al. [17]. Furthermore, in our study, fecal progestagen did not correlate with fecal glucocorticoid that it was different from a previous study which showed a positive correlation in African elephants [9]. One explanation could be that our study had a relatively low sampling frequency (twice a day and one day a month). A second explanation could be that glucocorticoid metabolite concentrations are significantly correlated with season. They are highest in the dry season [9], but our research did not control for dry season influences. Moreover, excited or stressful behavior of elephants during pregnancy could lead to an increase in glucocorticoid levels which, in turn, could cause abortion or parturition according to Dobson and Smith [7].
In conclusion of our study, it was found that during pregnancy, the progestagen level increased and only returned to low levels in the short period before calving. The glucocorticoid level, in contrast, fluctuated from low to medium throughout the pregnancy up until the last month shortly before calving when it increased dramatically. These findings may help for evaluating and managing the pregnancy status and stress condition in order to decrease the rate of fetal loss and predicting parturition in Asian elephants.
Acknowledgments
Authors are thankful to Mr. Chainarong Punkong for hormone analysis, Dr. Somkiat Huaijantug for ultrasonography, Dr. Jamlong Mitchaothai for statistical analysis and Assoc. Prof. Dr. Theera Rukkwamsuk for very good suggestion.
REFERENCES
- 1.Bayazit V.2009. Evaluation of cortisol and stress in captive animals. Aust. J. Basic Appl. Sci. 3: 1022–1031 [Google Scholar]
- 2.Brown J. L.2000. Reproductive endocrine monitoring of elephants: an essential tool for assisting captive management. Zoo Biol. 19: 347–367. doi: [DOI] [Google Scholar]
- 3.Brown J. L., Lehnhardt J.1995. Serum and urinary hormones during pregnancy and the peri- and postpartum period in an Asian elephant (Elephas maximus). Zoo Biol. 14: 555–564. doi: 10.1002/zoo.1430140608 [DOI] [Google Scholar]
- 4.Brown J. L., Walker S., Steinman K.2005. Endocrine manual for the reproductive assessment of domestic and non-domestic species. In: Endocrine Laboratory. Conversation and Research Center Smithsonian’s National Zoological Park, Virginia. [Google Scholar]
- 5.Brown J. L., Citino S. B., Bush M., Lehnhardt J., Phillips L. G.1991. Cyclic patterns of luteinizing hormone, follicle-stimulating hormone, inhibin and progesterone secretion in the Asian elephant (Elephas maximus). J. Zoo Wildlife Med. 22: 49–57 [Google Scholar]
- 6.Carden M., Schmitt D., Tomasi T., Bradford J., Moll D., Brown J. L.1998. Utility of serum progesterone and prolactin analysis for assessing reproductive status in the Asian elephant (Elephas maximus). Anim. Reprod. Sci. 53: 133–142. doi: 10.1016/S0378-4320(98)00109-2 [DOI] [PubMed] [Google Scholar]
- 7.Dobson H., Smith R. F.1995. Stress and reproduction in farm animals. J. Reprod. Fertil. Suppl. 49: 451–461 [PubMed] [Google Scholar]
- 8.Fiess M., Heistermann M., Hodges J. K.1999. Patterns of urinary and fecal steroid excretion during the ovarian cycle and pregnancy in the African elephant (Loxodonta africana). Gen. Comp. Endocrinol. 115: 76–89. doi: 10.1006/gcen.1999.7287 [DOI] [PubMed] [Google Scholar]
- 9.Foley C. A. H., Papageorge S., Wasser S. K.2001. Noninvasive stress and reproductive measures of social and ecological pressures in free-ranging African elephants. Conservation Biol. 15: 1134–1142. doi: 10.1046/j.1523-1739.2001.0150041134.x [DOI] [Google Scholar]
- 10.Ghosal R., Sukumar R., Seshagiri P. B.2010. Prediction of estrus cyclicity in Asian elephant (Elephas maximus) through estimation of fecal progesterone metabolite: development of an enzyme-linked immuno-sorbent assay. Theriogenology 73: 1051–1060. doi: 10.1016/j.theriogenology.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 11.Ghosal R., Kalaivanan N., Sukumar R., Seshagiri P. B.2012. Assessment of estrus cyclicity in the Asian elephant (Elephas maximus) by measurement of fecal progesterone metabolite 5α-P-3OH, using a non-invasive assay. Gen. Comp. Endocrinol. 175: 100–108. doi: 10.1016/j.ygcen.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 12.Graham L. H., Schwarzenberger F., Moestl E., Galama W., Savage A.2001. A versatile enzyme immunoassay for the determination of progestogens in feces and serum. Zoo Biol. 20: 227–236. doi: 10.1002/zoo.1022 [DOI] [Google Scholar]
- 13.Heistermann M., Trohorsch B., Hodges J. K.1997. Assessment of ovarian function in the African elephant (Loxodonta africana) by measurement of 5α-reduced progesterone metabolites in serum and urine. Zoo Biol. 16: 273–284. doi: [DOI] [Google Scholar]
- 14.Hodges J. K.1998. Endocrinology of the ovarian cycle and pregnancy in the Asian (Elephas maximus) and African (Loxodonta africana) elephant. Anim. Reprod. Sci. 53: 3–18. doi: 10.1016/S0378-4320(98)00123-7 [DOI] [PubMed] [Google Scholar]
- 15.Hodges J. K., Heistermann M., Beard A., Aarde R. J.1997. Concentrations of progesterone and the 5α-reduced progestins, 5α-pregnane-3, 20-dione and 3α-hydroxy-5α-pregnan-20-one, in luteal tissue and circulating blood and their relationship to luteal function in the African elephant. Biol. Reprod. 56: 640–646. doi: 10.1095/biolreprod56.3.640 [DOI] [PubMed] [Google Scholar]
- 16.Lueders I., Niemuller C., Rich P., Gray C., Hermes R., Goeritz F., Hildebrandt T. B.2012. Gestating for 22 months: The mechanism of luteal development and pregnancy maintenance in elephants. Proc. R. Soc. B 279: 3687–3696. doi: 10.1098/rspb.2012.1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer J. M., Walker S. L., Freeman E. W., Steinetz B. G., Brown J. L.2004. Species and fetal gender effects on the endocrinology of pregnancy in elephants. Gen. Comp. Endocrinol. 138: 263–270. doi: 10.1016/j.ygcen.2004.06.010 [DOI] [PubMed] [Google Scholar]
- 18.Munro C. J., Lasley B. L.1988. Non-radiometric methods for immunoassay of steroid hormones. Prog. Clin. Biol. Res. 285: 289–329 [PubMed] [Google Scholar]
- 19.Niemuller C. A., Gray C., Cummings E., Liptrap R. M.1998. Plasma concentrations of immunoreactive relaxin activity and progesterone in the pregnant Asian elephant (Elephas maximus). Anim. Reprod. Sci. 53: 119–131. doi: 10.1016/S0378-4320(98)00131-6 [DOI] [PubMed] [Google Scholar]
- 20.Oliveira C. A., Felippe E. C. G., Chelini M. O. M.2008. Serum cortisol and progestin concentrations in pregnant and non-pregnant Asian elephants. Res. Vet. Sci. 84: 361–363. doi: 10.1016/j.rvsc.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 21.Olsen J. H., Chen C. L., Boules M. M., Morris L. S., Coville B. R.1994. Determination of reproductive cyclicity and pregnancy in Asian elephants (Elephas maximus) by rapid radioimmunoassay of serum progesterone. J. Zoo Wildlife Med. 25: 349–354 [Google Scholar]
- 22.Thongtip N., Mahasawangkul S., Thitaram C., Pongsopavijitr P., Kornkaewrat K., Pinyopummin A., Angkawanish T., Jansittiwate S., Rungsri R., Boonprasert K., Wongkalasin W., Homkong P., Dejchaisri S., Wajjwalku W., Saikhun K.2009. Successful artificial insemination in the Asian elephant (Elephas maximus) using chilled and frozen-thawed semen. Reprod. Biol. Endocrinol. 7: 75. doi: 10.1186/1477-7827-7-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wasser S. K., Hunt K. E., Brown J. L., Cooper K., Crockett C. M., Bechert U., Millspaugh J. J., Larson S., Monfort S. L.2000. A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen. Comp. Endocrinol. 120: 260–275. doi: 10.1006/gcen.2000.7557 [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto Y., Yuto N., Yamamoto T., Kaewmanee S., Shiina O., Mouri Y., Narushima E., Katayanagi M., Sugimura K., Nagaoka K., Watanabe G., Taya K.2012. Secretory pattern of inhibin during estrous cycle and pregnancy in African (Loxodonta africana) and Asian (Elephas maximus) elephants. Zoo Biol. 31: 511–522. doi: 10.1002/zoo.20415 [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto Y., Yamamoto T., Yuto N., Hildebrandt T. B., Lueders I., Wibbelt G., Shiina O., Mouri Y., Sugimura K., Sakamoto S., Kaewmanee S., Nagaoka K., Watanabe G., Taya K.2012. The secretory pattern and source of immunoreactive prolactin in pregnant African (Loxodonta africana) and Asian (Elephas maximus) elephants. J. Reprod. Dev. 58: 105–111. doi: 10.1262/jrd.11-117S [DOI] [PubMed] [Google Scholar]