ABSTRACT
Inflammatory bowel disease (IBD) is a common cause of chronic gastrointestinal signs in dogs. In humans, T helper cells have important roles in the pathogenesis of IBD. In contrast, no specific involvement of a distinct T cell subset has been described in canine IBD. The present study evaluated the gene and protein expression of cytokines of T cell subsets in duodenal mucosa from dogs with IBD. Relative quantification of interleukin (IL)-17A, interferon (IFN)-γ, IL-4 and IL-10 mRNA transcription was performed using duodenal mucosa from 27 IBD dogs and 8 controls. Duodenal mucosal IL-17A, IFN-γ and IL-10 protein levels were determined by ELISA in 15 IBD dogs and 8 controls. There was no significant difference in each cytokines mRNA transcription level between groups. There was no significant difference in IL-17A, IFN-γ and IL-10 protein expression levels between groups. Thus, there is no clear evidence for the involvement of distinct Th cytokine in the pathogenesis of canine IBD.
Keywords: canine, cytokine, inflammatory bowel disease, T helper cell
Canine inflammatory bowel disease (IBD) is a common and often debilitating disease characterized by persistent or recurrent gastrointestinal signs, such as vomiting, diarrhea and weight loss. Diagnosis is based on elimination of other possible causes (eg, infection, food allergy, neoplasia, exocrine pancreatic insufficiency and hypoadrenocorticism) of these clinical signs and on histological evidence of mucosal inflammation on intestinal biopsy [6]. Lymphocytic-plasmacytic enteritis (LPE) represents the most common form of canine idiopathic IBD, characterized by the diffuse infiltration of lymphocytes and plasma cells into the enteric lamina propria. The definitive cause of canine IBD is unknown, although it is thought to involve loss of mucosal tolerance to endogenous microbial flora or other luminal dietary antigens in genetically predisposed individuals [8].
In human IBD, including Crohn’s disease (CD) and ulcerative colitis (UC), accumulating evidence suggests that dysfunction of the mucosal immune system plays important roles in IBD pathogenesis [9, 18]. Among a variety of inflammatory cells in the gut, mucosal CD4+ lymphocytes are thought to play central roles in both the induction and persistence of chronic inflammation. Traditionally, T helper (Th) cells have been classified into 2 distinct subsets, Th1 and Th2, according to their cytokine production profiles. CD had been thought to be associated with a Th1 cytokine profile and UC to be associated with a Th2 cytokine profile [5]. Recently, Th17 cells have been introduced as a third and new lineage of Th cells [19, 22]. Evidence from recent research suggests that CD is driven by Th1 cells producing interferon (IFN)-γ and Th17 cells producing interleukin (IL)-17A [9]. By contrast, the immunological basis for UC is much less clearly understood. Compared to the evidence of the roles for Th1 and Th17 in CD, support for Th2 pathogenesis in UC is much weaker [9]. Another important T cell subset is regulatory T (Treg) cells producing immunosuppressive cytokines, such as IL-10 and transforming growth factor (TGF)β. Impaired regulation of immune responses by Treg cells is also involved in the pathogenesis of human IBD [13].
In canine IBD, Peters and colleagues have shown that there is no significant difference in cytokine mRNA expression between dogs with and without chronic enteropathy, including IBD, and found no distinct cytokine profile of either a Th1 or Th2 pattern [17]. Recent meta-analysis of mucosal cytokines in dogs with IBD described that there was no predominant cytokine bias in inflamed duodenal mucosa [10]. In addition, a recent research paper demonstrated that IL-17A mRNA concentration was significantly lower in the duodenum of German Shepherd Dogs with IBD than in other breeds of dogs with IBD and healthy control dogs [20]. In contrast, IL-22, another Th17 cell cytokine, mRNA concentration was not different among the groups. In addition, IFN-γ, IL-10 and TGFβ mRNA levels were not different between dogs with IBD and healthy dogs. These studies found no clear evidence for the up-regulation of Th1, Th2, Th17 or Treg cytokine mRNA in dogs with IBD and indicated that immunopathogenesis of canine IBD could be very different from human IBD.
Unfortunately, all of previous studies examined cytokine gene expression alone, but not the protein expression. To examine whether specific Th cytokines are increased in the duodenal mucosa of canine IBD, the present study quantified the mRNA transcription levels of IL-17A, IFN-γ, IL-4 and IL-10 concurrently with protein expression levels in the duodenal mucosa of dogs with IBD and healthy control dogs.
MATERIALS AND METHODS
IBD dogs: Dogs (n = 27) referred to Hokkaido University Veterinary Teaching Hospital for investigation of chronic gastrointestinal signs were recruited for this study (Table 1). The diagnosis of IBD was made based on appropriate clinical signs (vomiting and/or diarrhea, weight loss) of at least 3 weeks’ duration, exclusion of other causes of chronic gastrointestinal signs and the presence of lymphoplasmacytic inflammation on histopathological review of duodenal biopsies. All the dogs diagnosed with IBD were scored based on the Canine Chronic Enteropathy Clinical Activity Index (CCECAI) to assess the severity of the disease [1]. The total CCECAI score was considered clinically insignificant (score 0–3), mild (score 4–5), moderate (score 6–8), severe (score 9–11) or very severe (score ≥ 12). Gastroduodenoscopy was performed under general anesthesia using a VQ-8143A flexible videoendoscope (Olympus Medical Systems, Tokyo, Japan), and multiple mucosal biopsy specimens were taken using FB-53Q-1 biopsy forceps (Olympus Medical Systems). Each of the 6 mucosal biopsy specimens was obtained from the descending duodenum and caudal duodenal flexure for histopathological examination. The samples were placed in neutral-buffered 10% formalin and embedded in paraffin, and hematoxylin and eosin (HE)-stained sections were prepared. In addition, at least one mucosal biopsy was placed immediately in RNAlater (Qiagen, Hilden, Germany) and stored at −80°C until further use. Slides of biopsy samples were evaluated histologically by one pathologist (Y. S.) who was blinded to the diagnosis and scored based on histopathological standards established by the World Small Animal Veterinary Association (WSAVA) Gastrointestinal Standardization Group [3]. In this standard for the assessment of duodenal mucosa, 5 morphological features (villous stunting, epithelial injury, crypt distention, lacteal dilation and mucosal fibrosis) and 3 types of infiltrated leukocytes (intraepithelial lymphocytes, lamina propria lymphocytes and lamina propria neutrophils) were selected and scored from 0 to 3 according to the guidelines. The total composite score was classified as insignificant (score 0–4), mild (score 5–9), moderate (score 10–14), severe (score 15–19) or very severe (score ≥ 20). Written owner consent was obtained for all dogs included in the study.
Table 1. Dogs suffering from IBD used in this study.
| Breed | Number of cases | Number of cases |
|---|---|---|
| used for gene analysis | used for protein analysis | |
| Yorkshire Terrier | 5 | 2 |
| Miniature Dachshund | 3 | 3 |
| French Bulldog | 3 | 2 |
| Maltese | 2 | 2 |
| Border Collie | 2 | 1 |
| Jack Russell Terrier | 1 | 1 |
| Pomeranian | 1 | 1 |
| Beagle | 1 | 1 |
| Miniature Schnauzer | 1 | 1 |
| Bernese Mountain Dog | 1 | 1 |
| Shiba Inu | 1 | 0 |
| Shetland Sheepdog | 1 | 0 |
| Chihuahua | 1 | 0 |
| Boston terrier | 1 | 0 |
| Toy poodle | 1 | 0 |
| Italian greyhound | 1 | 0 |
| Mix breed dog | 1 | 0 |
Healthy dogs: Control samples were obtained endoscopically from 8 healthy dogs (6 Beagles and 2 mongrels) with no clinical signs of gastrointestinal disease, normal CBC, serum biochemistry, urinalysis and fecal examination and no previous drug treatment. All control dogs were 1 year old (21 to 23 months old). Use of dogs in this study was approved by the Laboratory Animal Experimental Committee, Graduate School of Veterinary Medicine, Hokkaido University (Approval no. 08–0332).
Quantification of cytokine mRNA transcription by real-time RT-PCR: Total RNA was extracted from duodenal samples from 27 dogs with IBD and 8 controls using a RNeasy Protect Mini Kit (Qiagen), according to the manufacturer’s instructions. Genomic DNA was removed from the samples with a commercially available kit (RNase-Free DNase set; Qiagen). cDNA was synthesized from 0.5 µg total RNA using High Capacity RNA-to-cDNA Master Mix containing the Oligo dT primer and random primer (Applied Biosystems, Foster City, CA, U.S.A.). Controls did not undergo reverse transcription. None of the samples showed evidence of amplifiable genomic DNA with this assay.
Primers used for IL-17A, IFN-γ, IL-4 and IL-10 qPCR were designed using Primer3 software based on the canine GenBank sequences. For accurate quantification, three reference genes: TBP, GAPDH and SDHA, were considered to be appropriate by GeNorm [21] among five candidate reference genes (GAPDH, TBP, SDHA, YWHAZ and RPL13A) [16]. However, appropriate standard curve for TBP could not be obtained. Thus, GAPDH and SDHA were chosen as reference genes. Sequences of primer pairs used for qPCR are shown in Table 2. The specificities of these primers were confirmed to amplify each target mRNA by sequential analysis of PCR products using a CEQ 8800 DNA analysis system (Beckman Coulter, Fullerton, CA, U.S.A.). Each qPCR reaction was performed in 25 µl, containing 200 nM of each primer and 1 µl cDNA in addition to POWER SYBER Green PCR Master Mix (Applied Biosystems) using a 7300 Real-Time PCR System (Applied Biosystems). The amplification conditions were 95°C for 10 min and 40 cycles PCR (95°C for 15 sec and 60°C for 1 min), followed by dissociation (95°C for 15 sec, 60°C for 30 sec and 95°C for 15 sec). Reaction efficiency was determined for each primer set using 10-fold dilutions (107 molecules µl−1 to 101 molecules µl−1) of plasmids ligated with each cytokine and reference gene and ranged from 92.6% to 100%. CT (threshold cycle) values that indicated the point where the threshold intersected with amplification curves of PCR reaction were determined using software (7300 SDS Software; Applied Biosystems). Melting curve analysis did not show misprinting in any of the reactions.
Table 2. Sequences of oligonucleotide primers used for quantitative real-time PCR.
| Target gene | Forward primer (5′-3′) | Reverse primer (5′-3′) | Product size (bp) | Accession number |
|---|---|---|---|---|
| IL-17A | GGAATCTGCACCGCAATGAGGAC | CGCAGAACCAGGATCTCTTGCTGG | 142 | AB514445 |
| IFN-γ | GCGCAAGGCGATAAATGAAC | CTGACTCCTTTTCCGCTTCC | 82 | NM_001003174 |
| IL-4 | TCACCAGCACCTTTGTCCAC | CGCTTGTGTTCTTTGGAGCA | 144 | NM_001003159 |
| IL-10 | CGGGAGGGTGAAGACTTTCT | GGCATCACCTCCTCCAAGTA | 144 | NM_001003077 |
| GAPDH | ATCACTGCCACCCAGAAGAC | TCAGCTCAGGGATGACCTTG | 133 | NM_001003142 |
| SDHA | GCCTTGGATCTCTTGATGGA | TAACCTCCGGTAGCCACAAC | 116 | XM535807 |
Gene expression was quantified by averaging the duplicate measurements for each biological sample for all genes, following normalization of the expression ratio of each target gene to the geometric mean of the two reference genes, as described recently [21].
Quantification of cytokine protein expression by ELISA: Duodenal biopsy specimens for cytokine protein quantification were available in 15/27 dogs with IBD and 8 control dogs (Table 1). One mucosal biopsy for ELISA was placed in empty Eppendorf tubes and stored at −80°C. Samples were homogenized for 30 sec in 500 µl homogenization buffer (PBS containing 0.05% Tween 20) with protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany) using a mechanical tissue homogenizer [12]. Tissue homogenates were centrifuged briefly, and the supernatants were frozen at −20°C. Protein levels were determined using commercial ELISA kits specific for canine IL-17A (USCN Life Science, Wuhan, China, 100 µl of supernatants/well), IFN-γ and IL-10 (R&D systems, Minneapolis, MN, U.S.A., 50 µl supernatant/well). Absorbance was read at 450 nm using a microplate reader (Bio-Rad Laboratories, Hercules, CA, U.S.A.). All samples were examined in duplicate, and the mean OD value was calculated. Total protein concentrations were determined by the Bradford method (Protein assay kit; Bio-Rad Laboratories), and cytokine levels were normalized. IL-4 protein expression level could not be measured owing to the limited availability of duodenal samples.
Data analysis: Statistical analyses were performed using the computer program JMP 8 (SAS Institute Inc., Cary, NC, U.S.A.). Nonparametric analysis (Wilcoxon rank sum test) was used to compare the transcription level of each gene between the 2 groups in the present study. Unpaired t-test was used to compare the expression level of each protein between the 2 groups. Values of P<0.05 were considered significant.
RESULTS
Clinical and pathological findings: Twenty-seven dogs diagnosed with IBD were included in the gene expression study. All the dogs had evidence of inflammation within intestinal mucosa and histopathological diagnosis of LPE. Breeds of dogs with IBD are listed in Table 1. The median age of these dogs was 6 years (range, 1–10 years) with 18 females (5 intact and 13 neutered) and 9 males (6 intact and 3 neutered). The median body weight was 6 kg (range, 1.7–33.1 kg). CCECAI scores of dogs with IBD were 4/27 clinically insignificant, 7/27 mild, 9/27 moderate, 5/27 severe and 2/27 very severe. The median CCECAI of IBD dogs was 6 (range, 1–17). The median total WSAVA score of dogs with IBD was 4 (range 1–8). All dogs with IBD received prednisolone (2 mg/kg daily) after endoscopy, and 2 dogs were also treated with cyclosporine (5 mg/kg daily) in combination with prednisolone. Fifteen of 27 dogs with IBD were included in the protein expression analysis. The median age of these dogs was 7 years (range, 1–10 years) with 10 females (10 neutered) and 5 males (4 intact and 1 neutered). The median body weight was 6 kg (range, 2–33.1 kg). CCECAI scores of 15 dogs with IBD were 3/15 clinically insignificant, 2/15 mild, 5/15 moderate, 4/15 severe and 1/15 very severe. The median CCECAI of 15 dogs with IBD was 6 (range, 2–17). The median total WSAVA score of 15 dogs with IBD was 4 (range 1–8).
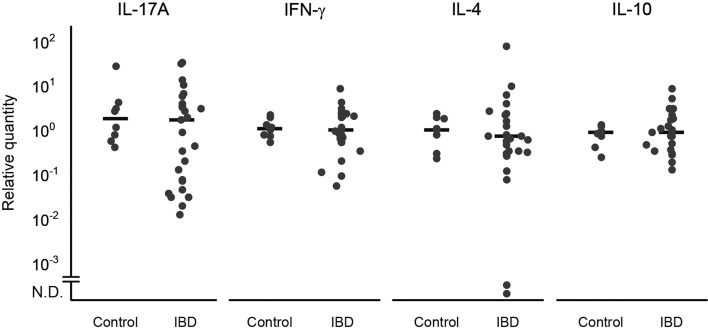
Quantification of cytokine mRNA expression in duodenal mucosa: There was no significant difference in IL-17A, IFN-γ, IL-4 and IL-10 mRNA expression between the dogs with IBD and the control dogs (Fig. 1).
Fig. 1.
Relative IL-17A, IFN-γ, IL-4 and IL-10 mRNA transcription levels in the duodenal mucosa of dogs with inflammatory bowel disease (IBD; n = 27) and healthy control dogs (Control; n = 8). Horizontal lines correspond to the median of the relative level in each group. N.D., not detected.
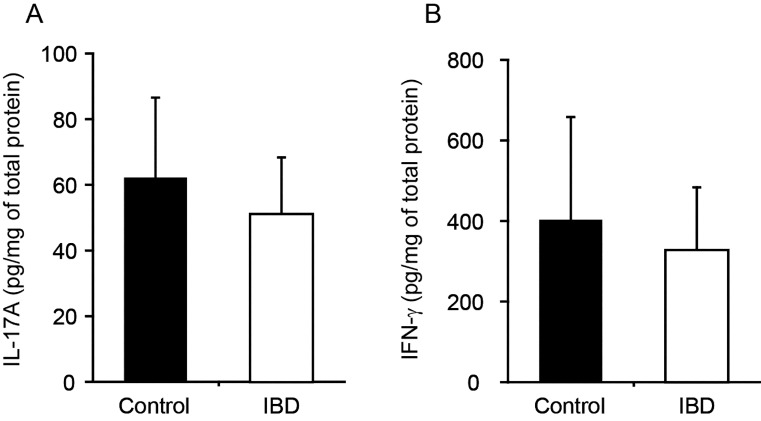
Quantification of cytokine protein expression in duodenal mucosa: IL-17A and IFN-γ protein expression levels were not different between dogs with IBD and control dogs (Fig. 2). In addition, IL-10 protein expression was not detected in any dogs (data not shown).
Fig. 2.
Levels of expression of IL-17A (A) and IFN-γ (B) protein in the duodenal mucosa of dogs with inflammatory bowel disease (IBD; n = 15) and healthy control dogs (Control; n = 8). The levels of protein were normalized to the total protein concentration in each dog and are expressed as pg/mg of total protein. The data are the mean ± standard deviation of the mean.
We performed statistical analysis including only 6 Beagle dogs as healthy control, and again there was no significant difference in each cytokine mRNA and protein expression between the dogs with IBD and the control dogs.
DISCUSSION
In the present study, we aimed to elucidate whether specific Th cytokines are increased in the duodenal mucosa of canine IBD by evaluating cytokine mRNA and protein expression in the duodenal mucosa of dogs with IBD. The present study demonstrated that duodenal samples from dogs with IBD used in this study did not show up-regulation of the IL-17A, IFN-γ, IL-4 and IL-10 mRNA compared to healthy controls. These results were in agreement with previous report in canine IBD [10, 17, 20]. In addition, there was no significant difference in duodenal mucosal IL-17A, IFN-γ and IL-10 protein expression levels between groups.
Although pathological feature of canine IBD is different from human IBD, comparisons have been made to the immunopathogenesis of human IBD [10, 17, 20]. Among various inflammatory cells in the gut, T helper cells are thought to play central roles in the pathogenesis of human IBD. It has been thought to be true for canine IBD, because mucosal populations of CD4+ T cells are increased in dogs with IBD [7]. In human IBD, IL-17A mRNA was found to be increased in inflamed mucosa from both UC and CD [4]. Tissue from the intestinal mucosa of patients with CD contains abundant transcripts for IFN-γ, and isolated mucosal T cells secrete large amounts of IFN-γ [9, 11]. However, in contrast to the situation in human IBD, IL-17A and IFN-γ mRNA expression was not increased in duodenal mucosa of dogs with IBD compared to healthy dogs, as shown by a recent study [20]. The present study also demonstrated that not only IL-17A and IFN-γ mRNA expression but also their protein expression were not increased in duodenal mucosa of dogs with IBD compared to healthy dogs. Therefore, at present, there is no clear evidence of the involvement of a typical Th17 and Th1-type response in the pathogenesis of canine IBD.
Recently, we reported that IL-17A mRNA expression was markedly increased in colorectal polyps from dogs with inflammatory colorectal polyps (ICRPs) [15]. In that study, we used the same real-time PCR analysis method used in the current study. ICRPs in miniature dachshunds are characterized by the formation of polyps in the colorectal area and characterized histopathologically by severe neutrophils infiltration [14]. In contrast, severe neutrophil infiltration in the duodenal mucosa is rare in canine LPE. IL-17A has been shown to induce neutrophils infiltration, and therefore, IL-17A might be not of particular importance in the recruitment of inflammatory cells into the duodenal mucosa of dogs with LPE.
In human IBD, dysregulated secretion of regulatory cytokines, such as IL-10, could play a role in the pathogenesis of IBD. Local production of IL-10 by mucosal mononuclear cells in IBD is insufficient to down-regulate pro-inflammatory cytokines [2]. Thus, lack of evidence of the up-regulation of IL-10 mRNA and undetectable levels of IL-10 protein in inflamed duodenal mucosa may indicate the loss of counter-regulatory mechanisms in canine IBD. It is possible that failure to up-regulate IL-10 in the duodenal mucosa could enhance the inflammatory response in canine IBD.
Traditionally, human UC was thought to be a Th2 cytokine-mediated disease, characterized by IL-4 mediated immune response. In canine IBD, recent meta-analysis [10] and the current study demonstrated that IL-4 mRNA transcript was not increased in duodenal mucosa of dogs with IBD, although IL-4 protein expression level was not evaluated. The lack of increased expression of IL-4 mRNA in IBD dogs suggested that the typical Th2 response is less important in the pathogenesis of canine IBD.
There are several limitations of the current study. First, protein concentration was quantified using total protein extracted from whole duodenal biopsies rather than solely from isolated lamina propria lymphocytes; therefore, in future studies, the expression of Th cytokines on duodenal lamina propria lymphocytes should be analyzed by flow cytometry when canine-specific monoclonal antibodies are available. Second, the total WSAVA score of dogs with IBD used in this study was relatively low, although clinically severe cases were included. Finally, the healthy control dogs used in this study were not age matched with IBD dogs, because of the limited availability of healthy control dogs.
In conclusion, no clear Th1/Th17 or Th2 cytokine pattern was found in canine IBD at the level of mRNA and protein expression in the duodenal samples included in this study. There is no clear evidence of the contribution of specific T cell cytokines to the pathogenesis of canine IBD. Our data indicate that the canine IBD bears little resemblance immunopathologically to human IBD.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research (KAKENHI No. 23780315) from the Japan Society for the Promotion of Science.
REFERENCES
- 1.Allenspach K., Wieland B., Grone A., Gaschen F.2007. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J. Vet. Intern. Med. 21: 700–708. doi: 10.1111/j.1939-1676.2007.tb03011.x [DOI] [PubMed] [Google Scholar]
- 2.Autschbach F., Braunstein J., Helmke B., Zuna I., Schürmann G., Niemir Z. I., Wallich R., Otto H. F., Meuer S. C.1998. In situ expression of interleukin-10 in noninflamed human gut and in inflammatory bowel disease. Am. J. Pathol. 153: 121–130. doi: 10.1016/S0002-9440(10)65552-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Day M. J., Bilzer T., Mansell J., Wilcock B., Hall E. J., Jergens A., Minami T., Willard M., Washabau R.2008. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: a report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J. Comp. Pathol. 138: (Suppl. 1): S1–S43. doi: 10.1016/j.jcpa.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 4.Fujino S., Andoh A., Bamba S., Ogawa A., Hata K., Araki Y., Bamba T., Fujiyama Y.2003. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 52: 65–70. doi: 10.1136/gut.52.1.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuss I. J.2008. Is the Th1/Th2 paradigm of immune regulation applicable to IBD? Inflamm. Bowel Dis. 14:(Suppl. 2): S110–S112 [DOI] [PubMed] [Google Scholar]
- 6.German A. J., Hall E. J.2008. Inflammatory bowel disease. pp. 312–329. In: Small Animal Gastroenterology (Steiner, J. M. ed.), Schlütersche, Hannover. [Google Scholar]
- 7.German A. J., Hall E. J., Day M. J.2001. Immune cell populations within the duodenal mucosa of dogs with enteropathies. J. Vet. Intern. Med. 15: 14–25. doi: 10.1111/j.1939-1676.2001.tb02292.x [DOI] [PubMed] [Google Scholar]
- 8.German A. J., Hall E. J., Day M. J.2003. Chronic intestinal inflammation and intestinal disease in dogs. J. Vet. Intern. Med. 17: 8–20. doi: 10.1111/j.1939-1676.2003.tb01318.x [DOI] [PubMed] [Google Scholar]
- 9.Hibi T., Ogata H.2006. Novel pathophysiological concepts of inflammatory bowel disease. J. Gastroenterol. 41: 10–16. doi: 10.1007/s00535-005-1744-3 [DOI] [PubMed] [Google Scholar]
- 10.Jergens A. E., Sonea I. M., O’Connor A. M., Kauffman L. K., Grozdanic S. D., Ackermann M. R., Evans R. B.2009. Intestinal cytokine mRNA expression in canine inflammatory bowel disease: a meta-analysis with critical appraisal. Comp. Med. 59: 153–162 [PMC free article] [PubMed] [Google Scholar]
- 11.MacDonald T. T., Monteleone G., Pender S. L.2000. Recent developments in the immunology of inflammatory bowel disease. Scand. J. Immunol. 51: 2–9. doi: 10.1046/j.1365-3083.2000.00658.x [DOI] [PubMed] [Google Scholar]
- 12.Maeda S., Ohno K., Nakamura K., Uchida K., Nakashima K., Fukushima K., Tsukamoto A., Goto-Koshino Y., Fujino Y., Tsujimoto H.2012. Mucosal imbalance of interleukin-1β and interleukin-1 receptor antagonist in canine inflammatory bowel disease. Vet. J. 194: 66–70. doi: 10.1016/j.tvjl.2012.02.026 [DOI] [PubMed] [Google Scholar]
- 13.Maul J., Loddenkemper C., Mundt P., Berg E., Giese T., Stallmach A., Zeitz M., Duchmann R.2005. Peripheral and intestinal regulatory CD4+CD25(high) T cells in inflammatory bowel disease. Gastroenterology 128: 1868–1878. doi: 10.1053/j.gastro.2005.03.043 [DOI] [PubMed] [Google Scholar]
- 14.Ohmi A., Tsukamoto A., Ohno K., Uchida K., Nishimura R., Fukushima K., Takahashi M., Nakashima K., Fujino Y., Tsujimoto H.2012. A retrospective study of inflammatory colorectal polyps in miniature dachshunds. J. Vet. Med. Sci. 74: 59–64. doi: 10.1292/jvms.11-0352 [DOI] [PubMed] [Google Scholar]
- 15.Ohta H., Takada K., Torisu S., Yuki M., Tamura Y., Yokoyama N., Osuga T., Lim S. Y., Murakami M., Sasaki N., Nakamura K., Yamasaki M., Takiguchi M.2013. Expression of CD4+ T cell cytokine genes in the colorectal mucosa of inflammatory colorectal polyps in miniature dachshunds. Available online at: http://dx.doi.org/10.1016/j.vetimm.2013.07.006 [DOI] [PubMed]
- 16.Peters I. R., Peeters D., Helps C. R., Day M. J.2007. Development and application of multiple internal reference (housekeeper) gene assays for accurate normalisation of canine gene expression studies. Vet. Immunol. Immunopathol 117: 55–66. doi: 10.1016/j.vetimm.2007.01.011 [DOI] [PubMed] [Google Scholar]
- 17.Peters I. R., Helps C. R., Calvert E. L., Hall E. J., Day M. J.2005. Cytokine mRNA quantification in duodenal mucosa from dogs with chronic enteropathies by real-time reverse transcriptase polymerase chain reaction. J. Vet. Intern. Med. 19: 644–653. doi: 10.1111/j.1939-1676.2005.tb02742.x [DOI] [PubMed] [Google Scholar]
- 18.Podolsky D. K.2002. Inflammatory bowel disease. N. Engl. J. Med. 347: 417–429. doi: 10.1056/NEJMra020831 [DOI] [PubMed] [Google Scholar]
- 19.Rouvier E., Luciani M. F., Mattei M. G., Denizot F., Golstein P.1993. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J. Immunol. 150: 5445–5456 [PubMed] [Google Scholar]
- 20.Schmitz S., Garden O. A., Werling D., Allenspach K.2012. Gene expression of selected signature cytokines of T cell subsets in duodenal tissues of dogs with and without inflammatory bowel disease. Vet. Immunol. Immunopathol. 146: 87–91. doi: 10.1016/j.vetimm.2012.01.013 [DOI] [PubMed] [Google Scholar]
- 21.Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De paepe A, Speleman F.2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3: H0034. doi: 10.1186/gb-2002-3-7-research0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao Z., Painter S. L., Fanslow W. C., Ulrich D., Macduff B. M., Spriggs M. K., Armitage R. J.1995. Human IL-17: a novel cytokine derived from T cells. J. Immunol. 155: 5483–5486 [PubMed] [Google Scholar]