ABSTRACT
Bone marrow stromal cell (BMSC) transplantation has been reported as treatments that promote functional recovery after spinal cord injury (SCI) in humans and animals. Polyethylene glycol (PEG) has been also reported as treatments that promote functional recovery after spinal cord injury (SCI) in humans and animals. Therefore, administration of PEG combined with BMSC transplantation may improve outcomes compared with BMSC transplantation only in SCI model mice. SCI mice were divided into a control-group, BMSC-group, PEG-group and BMSC+PEG-group. BMSC transplantation and PEG administration were performed immediately after surgery. Compared to the control-group, PEG- and BMSC+PEG-groups showed significant locomotor functional recovery 4 weeks after therapy. We observed no significant differences among the groups. In the BMSC- and BMSC+PEG-groups, immunohistochemistry showed that many neuronal cells aggressively migrated toward the glial scar from the region rostral of the lesion site. In the control- and PEG-groups, the boundary of the injured regions was covered with astrocytes, and a few neuronal cells were migrated toward the glial scar. We conclude that combined BMSC transplantation with PEG treatment showed no synergistic effects on locomotor functional recovery or beneficial cellular events. Further studies may improve the effect of the treatment, including modification of the timing of BMSC transplantation.
Keywords: bone marrow stromal cell, polyethylene glycol, spinal cord injury, synergistic effects
Despite the progress in treating spinal cord injury (SCI), recovery from severe paralysis remains difficult. Several cell types, including embryonic spinal cord stem cells [13], Schwann cells [21], olfactory ensheathing glia [19] and bone marrow stromal cells (BMSCs) [25], have been used in transplants aimed at spinal cord regeneration.
Among the various cell types used in treating SCI, embryonic neural stem cells have been very actively studied [22]. However, several difficulties, including ethical issues and clinical complications such as immune reactions and teratoma formation, make it impossible to use human fetal tissue as a practical and immediate cell source for therapeutic treatment [4, 25].
BMSCs are adherent, non-hematopoietic cells obtained from culturing bone marrow aspirates [25]. Canine BMSCs are easy to isolate and expand [16]. The most important practical advantages of using BMSCs are the capability of autologous transplantation, low cost of culturing and very low risk of teratoma formation [25]. Moreover, BMSCs can differentiate into bone [3, 16], cartilage [18], fat [3], muscle [29] and neurons [14]. Recently, spinal cord regenerative therapy using BMSCs has begun to be clinically applied, leading to positive results in human and veterinary medicine [1, 23, 26]. Transplanted BMSCs are believed to exert their effects by producing neurotrophins or by contacting host spinal tissues [6]. Other researchers, however, have shown only modest or inconsistent recovery [9], and transplantation does not improve repair or recovery in rats with thoracic contusion injuries [32]. These discrepancies among SCI studies will likely require additional studies before the inconsistencies can be resolved.
Administration of polyethylene glycol (PEG) is effective treatment for neurological disorders in rodents [5, 8, 20]. PEG has been shown to mechanically repair damaged cellular membranes and reduce secondary axotomy in the earlier stage of SCI [5, 8, 20]. Therefore, based on the findings in studies of human and rodent BMSCs and PEG application, we hypothesized that combined PEG treatment with BMSC transplantation would yield better clinical recovery than use of a single agent. No reports have been published describing the combined application both BMSCs and PEG in mice with SCI.
In the present study, we tested combination therapy with BMSC transplantation and PEG treatment during the acute phase of SCI in mice. We evaluated motor function and performed immunohistochemistry.
MATERIALS AND METHODS
All surgeries and handling procedures were carried out according to a protocol approved by the Animal Experimentation Committee at Yamaguchi University.
Bone marrow collection and culture of BMSCs: Bone marrow cells were harvested from a male ICR mouse (6 weeks old). An ICR mouse was anesthetized with pentobarbital (Somnopentyl, 50 mg/kg, i.p.), and bone marrow cells were harvested aseptically from tibias and femurs. BMSCs were cultured according to previously reported procedures [10] with modifications. Briefly, the harvested bone marrow cells were aseptically plated in a tissue culture flask in 10 ml Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), Pen/Strep (penicillin 50 U/ml and streptomycin 50 µg/ml) and 2.5 µg/ml amphotericin B. The BMSCs were grown at 37°C in a water-jacketed incubator with 5% CO2. After incubation for 72 hr, nonadherent cells were removed by replacing the medium, and the medium was replaced thereafter every 96 hr. The adherent cells were grown until semiconfluent, detached by incubation in a solution containing tryple Express (Gibco, Carlsbad, CA, U.S.A.) at 37°C for 10 min and subcultured twice in this manner. The surface antigens of cells cultured for three passages were CD44-positive, CD90-positive and CD45-negative. These cells that were cultured for three passages were considered BMSCs [7, 30].
Cell preparation and labeling: Before transplantation, cells were labeled using a carboxyfluorescein diacetate-succinimidyl ester (CFDA-SE) cell tracer kit (Invitrogen, Carlsbad, CA, U.S.A.). The culture medium was removed from BMSCs, the cells were washed with PBS, and cells were detached from the culture flask with trypsin. The cells were centrifuged, and the supernatant was removed. The cells were re-suspended in prepared reagent solution (90 µl of DMSO added to one vial of CFDA-SE and diluted with PBS is regarded as the prepared reagent solution: 1 µM) and incubated at 37°C for 15 min. The cells were centrifuged again, the supernatant was removed, and the cells were re-suspended in culture medium and maintained at 37°C for 30 min. This procedure was performed twice to label the cells. For transplantation, the labeled cells were suspended in phenol red-free culture medium at a density of 5 × 104 cells/µl. Two weeks and four weeks after cell transplantation, fluorescently labeled cells were observed using fluorescence microscopy.
Surgical procedures: The SCI model was performed using female ICR mice (n=36, body weight 30 g, 8 weeks old). Mice were anesthetized with pentobarbital (50 mg/kg, i.p.), and a dorsal laminectomy was performed at the T10 level. Then, exposed spinal cord was transected with a surgical knife. The animals were randomly divided into four groups of eight mice each: Control-group (infusion with 10 µl DMEM after SCI); BMSC-group (transplantation of BMSCs after SCI); PEG-group (administration of PEG after SCI); BMSC+PEG-group (BMSCs and PEG combined administration after SCI). Cell transplantation was performed immediately after SCI by infusing 1 × 105 cells/µl in 12 µl DMEM using a Hamilton syringe (Hamilton Co., Reno, NV, U.S.A.) into six points (2 µl per location for a total of 12 µl per animal) rostral and caudal to the injury site. PEG (Polyethlene glycol 4000, 50% w/v in PBS, Sigma Aldrich Co., St Louis, MO, U.S.A.) was instilled 10 µl into six points (10 µl was distributed among the six locations) rostral and caudal to the injury site. In the BMSC+PEG-group, BMSCs transplantation was performed by mentioned above, and PEG was been dropping of 10 µl into lesion site.
PCR detection of male-derived BMSCs: Two weeks and four weeks after transplantation, female ICR mice were anesthetized with pentobarbital (Somnopentyl, 50 mg/kg, i.p.). Cervical, thoracic and lumbar spinal cord weighing more than 25 mg was harvested on crushed ice, maintained at 4°C and placed in a 1.5-ml microcentrifuge tube at 4°C. Genomic DNA was prepared from spinal cord tissue homogenates from mice in each group using a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). The presence or absence of the sex determination region on the Y chromosome (Sry) gene in recipient female mice was assessed with PCR. Primers for Sry gene (sense primer; TGTCACAGAGGAGTGGCATT and antisense primer; CAGGCTGCCAATAAAAGCTTTG) were used to amplify a product of 162 bp. The PCR conditions were as follows: incubation at 94°C for 2 min; 38 cycles of incubation at 94°C for 30 min, 57°C for 30 min and 72°C for 30 min; with a final incubation at 4°C for 99 min. PCR products were separated using 2% agarose gel electrophoresis and stained with ethidium bromide. Positive (male mouse genomic DNA) and negative (female mouse genomic DNA) controls were included in each assay.
Immunofluorescent analysis: To evaluate resident and regenerating neuronal cells, mice were deeply anesthetized with pentobarbital (50 mg/kg, i.p.) and perfused transcardially with Zamboni solution (Wako, Osaka, Japan) 4 weeks post surgery. The lesioned region, including adjacent intact areas of spinal cord, was excised, immersed in Zamboni solution overnight and cryoprotected by immersing in a series of sucrose solutions (10%, 15% and 20% sucrose in 0.1 M PBS) at 4°C. The tissues were then frozen, embedded in OCT compound (Sakura Finetek Co., Ltd., Tokyo, Japan), cut longitudinally at a thickness of 8 µm using a cryostat and mounted on Amino Silane (APS)-coated slides (Matsunami Glass Ind Ltd., Osaka, Japan) for use in immunohistochemical staining. To block nonspecific immune reactions, the sections were treated with 3% skim milk at room temperature for 30 min. The slides were incubated with primary antibodies against glial fibrillary acidic protein (GFAP; 1:50, Monoclonal mouse anti-GFAP, Progen, Heidelberg, Germany) and microtubule-associated protein-2 (MAP-2; 1:100, Polyclonal rabbit anti-MAP-2, Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) at 4°C overnight. Thereafter, the slides were incubated at room temperature for 1 hr with the appropriate secondary antibodies: goat anti-rabbit IgG-FITC (1:100, Santa Cruz Biotechnology) and donkey anti-rabbit IgG-Rhodamine (1:100, Santa Cruz Biotechnology). Subsequently, the slides were treated with Hoechst 33258 (1:1,000, Dojindo Molecular Technologies Inc., Kumamoto, Japan) at room temperature for 15 min. Goat anti-rabbit IgG-FITC (1:100) and goat anti-mouse IgG2a-Rhodamine (1:100) were used as secondary antibodies for double-staining. The slides were washed three times for 5 min with PBS-T (0.05% Tween20 in PBS) following each incubation. Immunofluorescence was observed with fluorescence microscopy (Eclipse TE2000-U, Nikon, Tokyo, Japan) using filters appropriate to each fluorochrome. To evaluate neuronal cells in lesion site, the positive cells were counted on 5 non-overlapping randomed fields in glial scar.
Motor functional evaluation: Motor functional evaluation was performed for each hindlimb at 1, 7, 14, 21 and 28 days post SCI, using the Basso-Beattie-Bresnahan (BBB) Locomotor Rating Scale [2] in eight mice of each group.
Statistical analysis: All data are shown as means ± SEM. The Kruskal-Wallis test was used to compare the four groups. Values of P<0.05 were considered statistically significant.
RESULTS
Kinetics of transplanted fluorescently labeled cells: Two weeks after fluorescently labeled cells were transplanted, the cells were detected around the lesion site of the injured spinal cord. Cells positive for GFAP were not located close to the fluorescently labeled transplanted cells (Fig. 1). Four weeks after transplantation, the positive cells were not observed.
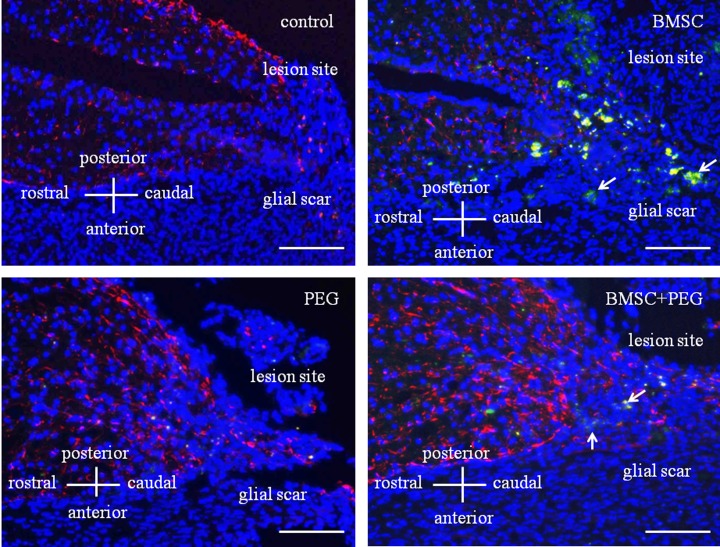
Fig. 1.
Kinetics of transplanted fluorescently labeled cells 2 weeks after surgery. Spinal cord section demonstrated immunofluorescence of GFAP (red) and cell nuclei (blue). Control- and PEG-groups did not show fluorescently labeled cells in the glial scar 2 weeks after surgery. BMSC- and BMSC+PEG-groups showed the transplanted fluorescently labeled cells (arrows) in the glial scar 2 weeks after transplantation. Bar=500 µm.
PCR detection of male-derived BMSCs: Two weeks after BMSC transplantation, canine Sry gene was detected at the lesion site of the thoracic cord and slightly observed in lumbar cord in recipient female mice. Four weeks after BMSC transplantation, Sry gene was only detected at the lesion site of the thoracic cord (Fig. 2).
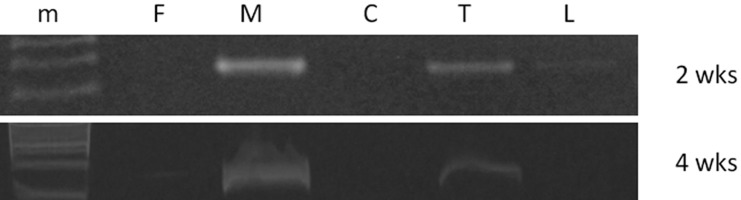
Fig. 2.
PCR analysis detected male-derived Sry gene in recipient female mice 2 weeks and 4 weeks after BMSC transplantation. (m) Marker, (F) Genomic DNA (female), (M) Genomic DNA (male), (C) Genomic DNA extracted from cervical cord, (T) Genomic DNA extracted from lesion site, (L) Genomic DNA extracted from lumber cord.
Immunohistochemical analysis: In the thoracic cord of normal mice, immunohistochemical analysis revealed that GFAP-positive cells were scattered throughout both the gray and white matter and were especially prominent surrounding the central canal. MAP-2-positive cells were not observed in the white matter, but were distributed throughout the gray matter.
In the control-group, GFAP-positive cells were scattered throughout both the gray and white matter. The number of GFAP-positive cells in the gray matter steadily increased from the rostral region to the lesion site. MAP-2-positive cells were not observed in the white matter, but were scattered throughout the gray matter. Few neuronal cells were observed in the glial scar. (Figs. 3 and 4). In the BMSC-group, there was a tendency that many neuronal cells migrated toward the glial scar from the region rostral of the lesion site, which was different from the control-group (Figs. 3 and 4). In the PEG-group, GFAP- and MAP-2-positive cells were similar in number and location to those of the control-group. However, a few neuronal cells were observed in the glial scar compared to the control-group (Figs. 3 and 4). In the BMSC+PEG-group, GFAP- and MAP-2-positive cells were also similar in number and location to those of the BMSC-group. Many neuronal cells had aggressively migrated toward the glial scar from the region rostral of the lesion site, which was significantly different from the control-group (Figs. 3 and 4).
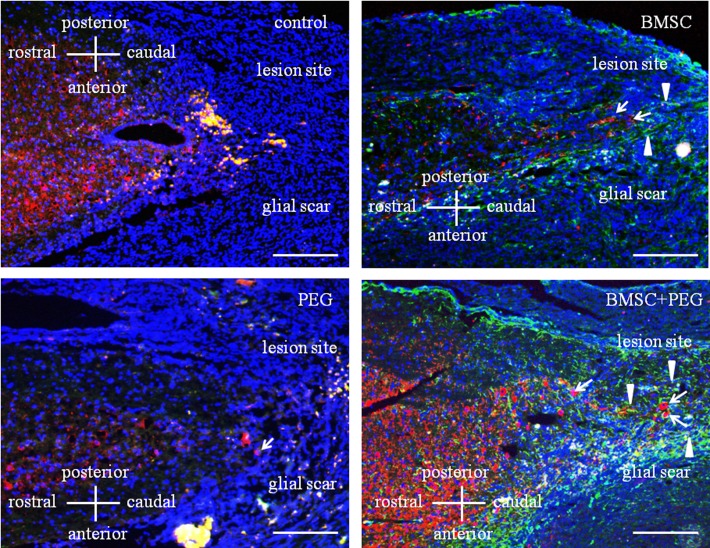
Fig. 3.
Immunohistochemical study of the thoracic cord from the control group 4 weeks after surgical procedures. Spinal cord section demonstrated double immunofluorescence of GFAP (green), MAP-2 (red) and cell nuclei (blue). In the control-group, few neuronal cells were observed in the glial scar. In the BMSC-group, neuronal cells migrated toward the glial scar from the region rostral of lesion site. MAP-2-positive cells (arrows). GFAP-positive cells (arrowheads). In the PEG-group, a few neuronal cells were observed in the glial scar compared to control-group. MAP-2-positive cell (arrow). In the BMSC+PEG-group, many neuronal cells had aggressively migrated toward the glial scar from the region rostral of the lesion site, which was significantly different from the control-group. MAP-2-positive cells (arrows). GFAP-positive cells (arrowheads). Bar=750 µm
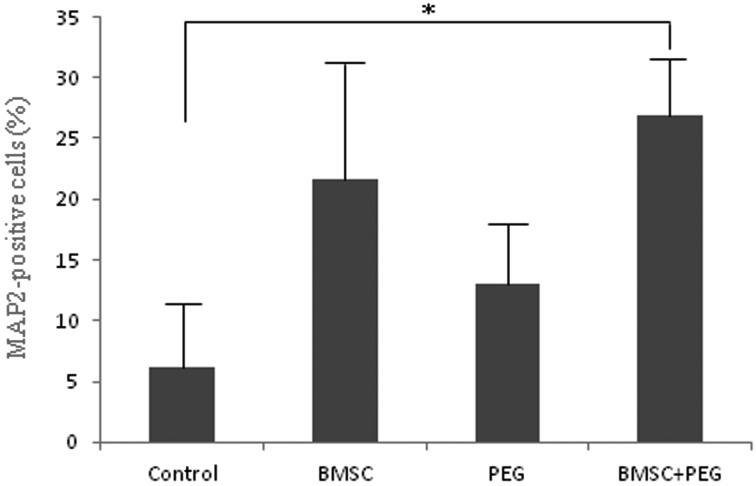
Fig. 4.
The ratio of neuronal MAP2-positive cells in glial scar 4 weeks post surgery in each group. Bars indicate means ± SEM. *=Significantly (P<0.05) different from control value.
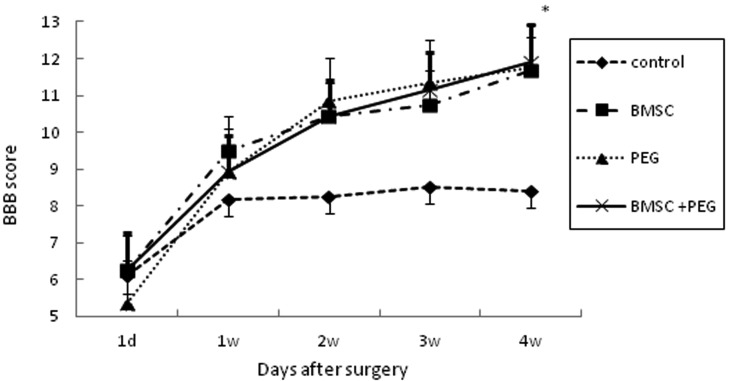
Motor functional evaluation: The BBB locomotor rating scores were evaluated over a 28-day period following SCI. Hind limb function recovered significantly in the PEG- and BMSC+PEG-groups at 28 days post-lesion compared with the control-group. There was a tendency that the BMSC-group recovered hind limb function compared to the control-group, although it was not significant. No significant differences in BBB scores were observed in the BMSC+PEG-group compared with the BMSC- and PEG-groups (Fig. 5).
Fig. 5.
Comparison of BBB locomotor rating scores at 1 day, 1 week, 2 weeks, 3 weeks and 4 weeks after surgery in the control-group, BMSC-group, PEG-group and BMSC+PEG-group. Bars indicate means ± SEM. *=Significantly (P<0.05) different PEG and BMSC+PEG value from control value.
DISCUSSION
In this study, administration of BMSCs, PEG and BMSCs+PEG was effective in functional recovery and reconstruction during the acute phase of SCI in mice. However, combined PEG treatment with BMSC transplantation showed no significant difference in locomotor functional recovery or beneficial cellular events compared with the BMSC-group.
The role of transplanted BMSCs remains to be elucidated. Several studies have reported that BMSCs have indirect neuroprotective effects due to secretion of neurotrophic or growth factors, including basic fibroblast growth factor, nerve growth factor, brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF) and insulin-like growth factor 1 [6, 11]. A recent study reported that BDNF and GDNF induce extensive axonal sprouting in the injured CNS [11]. On the other hand, other studies have reported that transplanted BMSCs are integrated into the host spinal cord and contribute to rebuilding of axons and axonal function [6]. Moreover, about 30% of the BMSCs acquire a neuronal phenotype without evidence of cell fusion, when co-cultured with neurons [11]. BMSCs can also acquire electrophysiological functions similar to neurons in vitro [17] and express receptors specific to neurons [31]. Therefore, the transplanted BMSCs may promote functional restoration through multiple mechanisms [11]. In the BMSC- and BMSC+PEG-groups in our current study, many neuronal cells aggressively migrated toward the glial scar from the region rostral of the lesion site, indicating that neuronal regeneration is an advantageous effect of transplanted BMSCs. Indeed, male-derived BMSCs were detected at lesion site of thoracic cord in recipient female mice at 4 weeks after BMSC transplantation. These results are congruent with those reported [15]. The engrafted BMSCs also degrade the extracellular matrix in the glial scar by secreting several proteases, such as matrix metalloproteases, to promote neurite outgrowth from spinal cord neurons [28]. Thus, transplanted BMSCs may play an indirect neuroprotective role [27] and may not differentiate into cells with neural phenotypes as suggested by the location of the fluorescently labeled transplanted cells, which were not close to neuronal cells.
PEG has been shown to mechanically repair damaged cellular membranes by sealing the membranes and reducing secondary axotomy after traumatic brain injury and SCI [5, 20]. These actions result in improved behavioral recovery after SCI in rodents and in clinical cases of paraplegia in dogs [5]. Moreover, immediate PEG treatment significantly increases the volume of the intact spinal parenchyma and reduces the volume of cystic cavitation [5, 8]. In this study, although motor functional recovery was observed, recovery may be related to membrane repair. In other words, motor functional recovery may be related to reducing secondary axotomy in spinal cord. Because a few neuronal cells migrate toward the glial scar, suggesting that neuronal cells may be sealed by PEG in the PEG-group.
The reason we did not observe significant differences in locomotor functional recovery between the BMSC+PEG- and BMSC-groups may be related to nerve regeneration and neuroprotective mechanisms of BMSCs and PEG. Transplanted BMSCs may play an indirect neuroprotective role, such as secret of neurotrophic or growth factors and nerve regeneration [6, 11, 28]. PEG has been shown to be immediately mechanically repair damaged cellular membranes by sealing the membranes and reducing secondary axotomy after SCI [5, 20]. After all, transplanted BMSCs may not play an indirect neuroprotective role, such as secret of neurotrophic or growth factors and nerve regeneration, because the cell membranes of transplanted BMSCs were sealed by PEG. Our result suggests that PEG treatment in the early stage of SCI does not exert sufficient effects on BMSC transplantation. Moreover, previous studies have shown that BMSC transplantation is effective in the acute, subacute and chronic stages of SCI in rats [12, 25, 33]. Therefore, use of PEG in the earlier stage of SCI and delayed transplantation of BMSCs after the sealing by PEG is broken may lead to additional effects in the functional recovery of SCI mice. Delaying cell transplantation for 7–14 days after SCI promotes functional recovery of the SCI mice and rat, whereas little recovery is observed with transplantation acute and chronic phases of SCI [12, 24].
In conclusion, this study demonstrates that BMSCs, PEG and combined application of both BMSCs and PEG provide significant effects on the locomotor functional recovery during the acute phase of the SCI mice, but synergistic effects were not observed. These effects of BMSCs do not preclude the use of PEG in the acute stage of SCI. To our knowledge, this is the first report to evaluate the effects of PEG and BMSC transplantation therapy during the acute phase of SCI in mice. Further investigation into the timing of the effect of BMSC transplantation and long-term examination are necessary. The present findings may help establish scientifically verified strategies of cell transplantation therapy for SCI in the clinical situation.
REFERENCES
- 1.Azari M. F., Mathias L., Ozturk E., Cram D. S., Boyd R. L., Petratos S.2010. Mesenchymal stem cells for treatment of CNS injury. Curr. Neuropharmacol. 8: 316–323. doi: 10.2174/157015910793358204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basso D. M., Beattie M. S., Bresnahan J. C.1995. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 12: 1–21. doi: 10.1089/neu.1995.12.1 [DOI] [PubMed] [Google Scholar]
- 3.Beresford J. N., Bennett J. H., Devlin C., Leboy P. S., Owen M. E.1992. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J. Cell Sci. 102:(Pt 2): 341–351 [DOI] [PubMed] [Google Scholar]
- 4.Björklund A., Lindvall O.2000. Cell replacement therapies for central nervous system disorders. Nat. Neurosci. 3: 537–544. doi: 10.1038/75705 [DOI] [PubMed] [Google Scholar]
- 5.Borgens R. B., Shi R., Bohnert D.2002. Behavioral recovery from spinal cord injury following delayed application of polyethylene glycol. J. Exp. Biol. 205: 1–12 [DOI] [PubMed] [Google Scholar]
- 6.Chiba Y., Kuroda S., Shichinohe H., Hokari M., Osanai T., Maruichi K., Yano S., Hida K., Iwasaki Y.2010. Synergistic effects of bone marrow stromal cells and a Rho kinase (ROCK) inhibitor, fasudil on axon regeneration in rat spinal cord injury. Neuropathology 30: 241–250. doi: 10.1111/j.1440-1789.2009.01077.x [DOI] [PubMed] [Google Scholar]
- 7.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E.2006. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317. doi: 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]
- 8.Duerstock B. S., Borgens R. B.2002. Three-dimensional morphometry of spinal cord injury following polyethylene glycol treatment. J. Exp. Biol. 205: 13–24 [DOI] [PubMed] [Google Scholar]
- 9.Himes B. T., Neuhuber B., Coleman C., Kushner R., Swanger S. A., Kopen G. C., Wagner J., Shumsky J. S., Fischer I.2006. Recovery of function following grafting of human bone marrow-derived stromal cells into the injured spinal cord. Neurorehabil. Neural Repair 20: 278–296. doi: 10.1177/1545968306286976 [DOI] [PubMed] [Google Scholar]
- 10.Hiyama A., Mochida J., Iwashina T., Omi H., Watanabe T., Serigano K., Tamura F., Sakai D.2008. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J. Orthop. Res. 26: 589–600. doi: 10.1002/jor.20584 [DOI] [PubMed] [Google Scholar]
- 11.Hokari M., Kuroda S., Shichinohe H., Yano S., Hida K., Iwasaki Y.2008. Bone marrow stromal cells protect and repair damaged neurons through multiple mechanisms. J. Neurosci. Res. 86: 1024–1035. doi: 10.1002/jnr.21572 [DOI] [PubMed] [Google Scholar]
- 12.Ide C., Nakai Y., Nakano N., Seo T. B., Yamada Y., Endo K., Noda T., Saito F., Suzuki Y., Fukushima M., Nakatani T.2010. Bone marrow stromal cell transplantation for treatment of sub-acute spinal cord injury in the rat. Brain Res. 1332: 32–47. doi: 10.1016/j.brainres.2010.03.043 [DOI] [PubMed] [Google Scholar]
- 13.Iwashita Y., Kawaguchi S., Murata M.1994. Restoration of function by replacement of spinal cord segments in the rat. Nature 367: 167–170. doi: 10.1038/367167a0 [DOI] [PubMed] [Google Scholar]
- 14.Jiang J., Lv Z., Gu Y., Li J., Xu L., Xu W., Lu J., Xu J.2010. Adult rat mesenchymal stem cells differentiate into neuronal-like phenotype and express a variety of neuro-regulatory molecules in vitro. Neurosci. Res. 66: 46–52. doi: 10.1016/j.neures.2009.09.1711 [DOI] [PubMed] [Google Scholar]
- 15.Jung D. I., Ha J., Kang B. T., Kim J. W., Quan F. S., Lee J. H., Woo E. J., Park H. M.2009. A comparison of autologous and allogenic bone marrow-derived mesenchymal stem cell transplantation in canine spinal cord injury. J. Neurol. Sci. 285: 67–77. doi: 10.1016/j.jns.2009.05.027 [DOI] [PubMed] [Google Scholar]
- 16.Kadiyala S., Young R. G., Thiede M. A., Bruder S. P.1997. Culture expanded canine mesenchymal stem cells possess osteochondrogenic potential in vivo and in vitro. Cell Transplant 6: 125–134. doi: 10.1016/S0963-6897(96)00279-5 [DOI] [PubMed] [Google Scholar]
- 17.Kohyama J., Abe H., Shimazaki T., Koizumi A., Nakashima K., Gojo S., Taga T., Okano H., Hata J., Umezawa A.2001. Brain from bone: efficient “meta-differentiation” of marrow stroma-derived mature osteoblasts to neurons with Noggin or a demethylating agent. Differentiation 68: 235–244. doi: 10.1046/j.1432-0436.2001.680411.x [DOI] [PubMed] [Google Scholar]
- 18.Lennon D. P., Haynesworth S. E., Young R. G., Dennis J. E., Caplan A. I.1995. A chemically defined medium supports in vitro proliferation and maintains the osteochondral potential of rat marrow-derived mesenchymal stem cells. Exp. Cell Res. 219: 211–222. doi: 10.1006/excr.1995.1221 [DOI] [PubMed] [Google Scholar]
- 19.Li Y., Field P. M., Raisman G.1997. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science 277: 2000–2002. doi: 10.1126/science.277.5334.2000 [DOI] [PubMed] [Google Scholar]
- 20.Liu-Snyder P., Logan M. P., Shi R., Smith D. T., Borgens R. B.2007. Neuroprotection from secondary injury by polyethylene glycol requires its internalization. J. Exp. Biol. 210: 1455–1462. doi: 10.1242/jeb.02756 [DOI] [PubMed] [Google Scholar]
- 21.Martin D., Robe P., Franzen R., Delrée P., Schoenen J., Stevenaert A., Moonen G.1996. Effects of Schwann cell transplantation in a contusion model of rat spinal cord injury. J. Neurosci. Res. 45: 588–597. doi: [DOI] [PubMed] [Google Scholar]
- 22.McDonald J. W., Liu X. Z., Qu Y., Liu S., Mickey S. K., Turetsky D., Gottlieb D. I., Choi D. W.1999. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat. Med. 5: 1410–1412. doi: 10.1038/70986 [DOI] [PubMed] [Google Scholar]
- 23.Nishida H., Nakayama M., Tanaka H., Kitamura M., Hatoya S., Sugiura K., Suzuki Y., Ide C., Inaba T.2011. Evaluation of transplantation of autologous bone marrow stromal cells into the cerebrospinal fluid for treatment of chronic spinal cord injury in dogs. Am. J. Vet. Res. 72: 1118–1123. doi: 10.2460/ajvr.72.8.1118 [DOI] [PubMed] [Google Scholar]
- 24.Nishimura S., Yasuda A., Iwai H., Takano M., Kobayashi Y., Nori S., Tsuji O., Fujiyoshi K., Ebise H., Toyama Y., Okano H., Nakamura M.2013. Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol. Brain 6: 3. doi: 10.1186/1756-6606-6-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohta M., Suzuki Y., Noda T., Ejiri Y., Dezawa M., Kataoka K., Chou H., Ishikawa N., Matsumoto N., Iwashita Y., Mizuta E., Kuno S., Ide C.2004. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp. Neurol. 187: 266–278. doi: 10.1016/j.expneurol.2004.01.021 [DOI] [PubMed] [Google Scholar]
- 26.Saito F., Nakatani T., Iwase M., Maeda Y., Murao Y., Suzuki Y., Fukushima M., Ide C.2012. Administration of cultured autologous bone marrow stromal cells into cerebrospinal fluid in spinal injury patients: a pilot study. Restor. Neurol. Neurosci. 30: 127–136 [DOI] [PubMed] [Google Scholar]
- 27.Sarnowska A., Braun H., Sauerzweig S., Reymann K. G.2009. The neuroprotective effect of bone marrow stem cells is not dependent on direct cell contact with hypoxic injured tissue. Exp. Neurol. 215: 317–327. doi: 10.1016/j.expneurol.2008.10.023 [DOI] [PubMed] [Google Scholar]
- 28.Shichinohe H., Kuroda S., Tsuji S., Yamaguchi S., Yano S., Lee J. B., Kobayashi H., Kikuchi S., Hida K., Iwasaki Y.2008. Bone marrow stromal cells promote neurite extension in organotypic spinal cord slice: significance for cell transplantation therapy. Neurorehabil. Neural Repair 22: 447–457. doi: 10.1177/1545968308315596 [DOI] [PubMed] [Google Scholar]
- 29.Wakitani S., Saito T., Caplan A. I.1995. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve 18: 1417–1426. doi: 10.1002/mus.880181212 [DOI] [PubMed] [Google Scholar]
- 30.Woodbury D., Schwarz E. J., Prockop D. J., Black I. B.2000. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 61: 364–370. doi: [DOI] [PubMed] [Google Scholar]
- 31.Yano S., Kuroda S., Shichinohe H., Seki T., Ohnishi T., Tamagami H., Hida K., Iwasaki Y.2006. Bone marrow stromal cell transplantation preserves gammaaminobutyric acid receptor function in the injured spinal cord. J. Neurotrauma 23: 1682–1692. doi: 10.1089/neu.2006.23.1682 [DOI] [PubMed] [Google Scholar]
- 32.Yoshihara H., Shumsky J. S., Neuhuber B., Otsuka T., Fischer I., Murray M.2006. Combining motor training with transplantation of rat bone marrow stromal cells does not improve repair or recovery in rats with thoracic contusion injuries. Brain Res. 1119: 65–75. doi: 10.1016/j.brainres.2006.08.080 [DOI] [PubMed] [Google Scholar]
- 33.Zurita M., Vaquero J.2004. Functional recovery in chronic paraplegia after bone marrow stromal cells transplantation. Neuroreport 15: 1105–1108. doi: 10.1097/00001756-200405190-00004 [DOI] [PubMed] [Google Scholar]