Abstract
Study Objectives:
Long-term use of hypnotics runs the risk of dependency, and subjects usually experience difficulties in withdrawal. The objective of this study was to investigate the success of withdrawal using pregabalin and its efficacy on sleep in patients with hypnotic-dependent insomnia.
Methods:
We enrolled patients with hypnotic-dependent insomnia who were 18 years or older. The starting dosage of pregabalin was 75 mg/day and was increased up to as much as 300 mg/day, depending on the individual patient's condition, while tapering off hypnotics. After 4 weeks of titration, the final dosage amount was maintained for at least another 4 weeks. Sleep and clinical variables were evaluated at baseline and after treatment, using the Korean versions of various sleep questionnaires as well as polysomnography.
Results:
Forty subjects were enrolled, with a mean age of 52.0 ± 8.5 years, of whom 28 (70.0%) were women. Twenty-one (52.5%) subjects successfully withdrew from hypnotics. The duration of withdrawal was 42.1 ± 16.0 days (range: 27.0∼84.0). The mean pregabalin dose was 121.4 ± 69.0 mg/day (range: 75.0∼300.0). After pregabalin treatment, there was a significant improvement in the total score of the Pittsburgh Sleep Quality Index (15.0 ± 2.1, 8.9 ± 3.0, p < 0.001), and insomnia severity index (20.9 ± 4.3, 9.6 ± 4.4, p < 0.001); however, most of the sleep variables of the PSG showed no differences. The main adverse effects of pregabalin were nausea and dizziness.
Conclusions:
Our results showed pregabalin may be a promising candidate for withdrawal from hypnotics and improved sleep in patients with hypnotic-dependent insomnia.
Citation:
Cho YW, Song ML. Effects of pregabalin in patients with hypnotic-dependent insomnia. J Clin Sleep Med 2014;10(5):545-550.
Keywords: pregabalin, hypnotic, dependent, insomnia
Patients with insomnia are prescribed a wide variety of sleep-promoting medications. The majority of patients take two or more forms of sleep-promoting medication concomitantly.1 The most common treatment for insomnia in recent years has been administration of the hypnotic benzodiazepine (BZD). However, long-term consumption of BZD is likely to induce poor sleep quality and carries a high risk of dependence.2–4 Abrupt discontinuation of BZD can produce severe withdrawal symptoms, such as anxiety, tinnitus, involuntary movement, and perceptual changes.5 Nonbenzodiazepines, such as zolpidem and zaleplon, have also been administered to insomnia patients as sleep-promoting measures.6 Although different in chemical structure, BZD and non-BZD drugs offer almost identical short-term benefits and long-term adverse effects.6
Pregabalin is a novel anticonvulsant drug and has also been reported effective in improving sleep quality in patients who suffer from a generalized anxiety disorder, fibromyalgia, neuralgia, or epilepsy.7–10 Functionally, pregabalin targets the alpha-2-delta subunit on neuronal voltage-gated calcium channels in the central nervous system, resulting in the slowed release of excitatory neurotransmitters.11 Pregabalin does not bind to plasma proteins or interact with other drugs once ingested and is completely eliminated through the kidneys. Furthermore, studies have proven its potency in curing anxiety and eliminating the potential for tolerance and rebound events.7,12–14 The benefits of pregabalin treatment are also durable when examining data from follow-up sessions with patients previously suffering from alcohol dependence.15
BRIEF SUMMARY
Current Knowledge/Study Rationale: Long-term use of hypnotics runs the risk of dependency, and the subject usually experiences difficulties in withdrawal or discontinuing treatment. Pregabalin is a novel anticonvulsant drug, and has also been reported effective in improving sleep quality in patients who suffer from other diseases.
Study Impact: We can consider pregabalin as a candidate for withdrawal from hypnotics and improved sleep in patients with hypnotic-dependent insomnia. The pregabalin-treated patients' discontinuation symptoms were mild.
Pregabalin qualifies as a viable substitute for BZD and other related hypnotics.12–14,16,17 The impact of pregabalin on sleep quality in insomnia patients has not been studied thoroughly. From observation of its benefits in sleep restoration in patients with other neurological diseases, such as fibromyalgia syndrome11 and epilepsy,18 further experimentation regarding the introduction of pregabalin to insomnia patients is appropriate. The purpose of this study was to evaluate the effects of pregabalin on hypnotic withdrawal and on sleep in patients with hypnotic-dependent insomnia.
METHODS
Study Design
This was a prospective, open-label, single-arm interventional study. Subjects participated in 12- to 14-week trials and were seen on 4 scheduled visits. Subjects considered for participation in this trial were screened for eligibility fitness the first week of their visit. Pregabalin treatment started after screening. The treatment period was divided into 2 parts: the titration (hypnotic tapering) and the maintenance period. The starting dosage of pregabalin was 75 mg/day with a 6-8 week titration period. The speed of titration was based on clinical efficiency and the tolerability of each patient, according to the clinician's judgment, taking up to a maximum dosage of 300 mg. The maintenance period was ≥ 4 weeks of titration, and the maintenance dosage was 150 mg/day ∼ 300 mg/day, twice daily. Hypnotic tapering was initiated at a rate of 25% to 50% of the average dosage per week. The speed of the substitution process was adapted to the severity of each patient's withdrawal symptoms.
Study Subjects
The subjects were sequentially selected from a tertiary sleep center from September 2012 to January 2013. They were 18-65 years old had been diagnosed with hypnotic-dependent insomnia. The inclusion criteria of our study were as follows: (1) diagnosis of insomnia according to DSM-IV19; (2) complaints of poor sleep or excessive daytime sleepiness, despite nearly daily use of hypnotics ≥ 3 weeks, or those with adequate sleep using hypnotics; (3) patient concern about hypnotic dependence in spite of adequate sleep with hypnotics. The exclusion criteria were: (1) pregnant women and women who were trying to become pregnant or breastfeeding; (2) clinically significant abnormalities in laboratory parameters at screening at the investigator's discretion, or clinically significant medical illnesses (hepatic or renal disease at screening); (3) clinically significant cognitive decline; (4) shift work; (5) primary substance abuse disorder (alcohol, narcotics, or stimulants) or a history thereof.
For all participants, written informed consent was obtained prior to any procedures being performed, and all procedures were approved by the institutional review board of the regional university hospital in Korea (NO. 10-126-3).
Assessment and Outcome Measures
The primary variable to assess the effectiveness of pregabalin was the withdrawal rate. The secondary variables were sleep related parameters obtained from nocturnal polysomnography (PSG), which reflects objective data; subjective data were assessed by scales relating to sleep, such as the Korean version of the Pittsburgh sleep quality index (PSQI),20 insomnia severity index (ISI),21 Epworth Sleepiness Scale (ESS),22 and the hospital anxiety and depression scale (HADS).23 For assessing tapering, the Penn physician withdrawal checklist (PWC)24 was done at baseline and the third visit (6th week). The PWC is a 20-item instrument for assessing anxiolytic discontinuation symptoms (such as BZD-like treatment withdrawal symptoms), with a higher score meaning more severe withdrawal symptoms. The PSG and the questionnaires were repeated after withdrawal in the successful group.
Statistical Analysis
Data analysis was performed using the SPSS version 18.0, and p < 0.05 was considered statistically significant. Differences of clinical characteristics between the successfully withdrawn group and the unsuccessful group were analyzed by an independent t-test or χ2 test. Comparison of sleep related characteristics at baseline and after treatment for the successfully withdrawn group were analyzed by a paired t-test.
RESULTS
Demographic Characteristics of Subjects
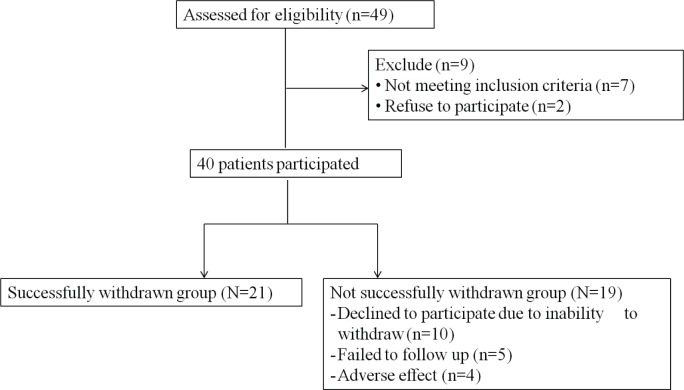
Forty-nine patients were assessed for eligibility for the study. Seven did not meet criteria, and 2 refused to participate. Thus, 40 subjects were enrolled; their data were utilized for further analysis (Figure 1). The mean age of the 40 subjects was 52.0 years; 70.0% were female. The mean duration of insomnia was 5.2 ± 5.4 years. The most commonly used hypnotic was benzodiazepine 20 (50%), and 52.5% of the subjects were taking one type of hypnotic (n = 21). The mean duration of those taking hypnotics previously was 2.6 ± 2.4 years. Comorbid disorders were: mood disorders 16 (40%), cardiovascular diseases 14 (35%), gastrointestinal problems 7 (17.5%), musculoskeletal diseases 3 (7.5%), type 2 diabetes mellitus (5%), thyroid diseases 2 (5%), and neurologic diseases 1 (2.5%) (Table 1).
Figure 1. Flow chart.
Table 1.
Summary of patient characteristics
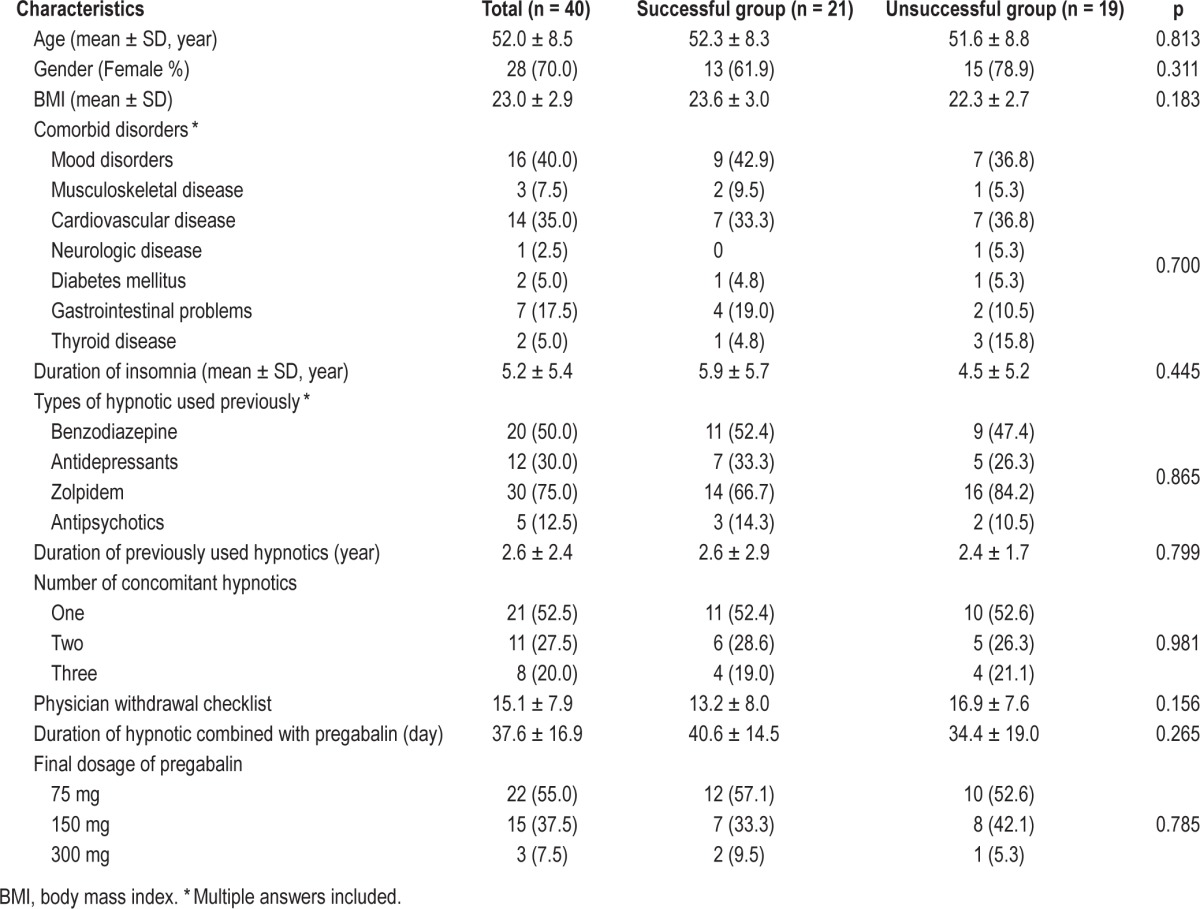
Twenty-one (52.5%) successfully withdrew from hypnotics after the 12-week pregabalin regimen—mean age was 52.3 years, and 61.9% were female. The remaining 19 (47.5%) did not successfully withdraw from hypnotics. No statistical significance could be observed in comparing the differences in patient characteristics between the successfully withdrawn group and the unsuccessful group.
The hypnotics that the successfully withdrawn group used prior to the study were benzodiazepines (n = 11, 52.4%), antidepressants (n = 7, 33.3%), zolpidem (n = 14, 66.7%), or antipsychotics (n = 3, 14.3%). And 52.4% of the subjects were taking one kind of hypnotics. The mean duration of withdrawal from hypnotics was 42.1 days (range 27.0-84.0, SD 16.0). The dosage of pregabalin administered throughout the treatment for most subjects (n = 12, 57.1%) remained the same as the starting dosage at 75 mg/day, while others logged final doses at 150 mg/day (n = 7, 33.3%) or 300 mg/day (n = 2, 9.5%) (Table 1).
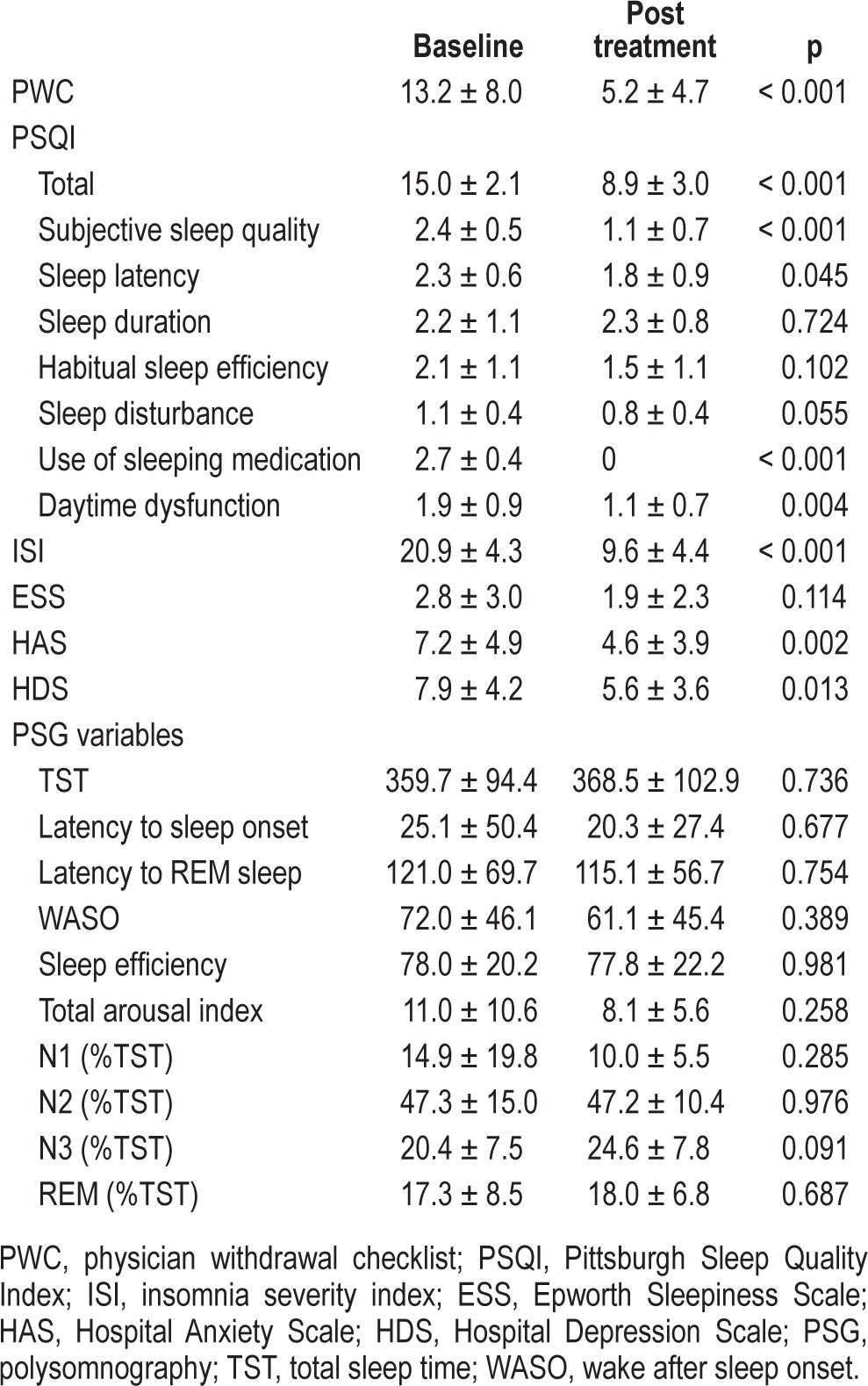
Nineteen (47.5%) did not successfully withdraw from hypnotics. The reasons were as follows: Declined to participate due to inability to withdraw (n = 10, 25.0%), experienced adverse effects (n = 4, 10.0%), failed to follow up (n = 5, 12.5%). The duration of taking pregabalin in the unsuccessful group was < 2 weeks for 5 subjects (26.3%), 2∼4 weeks for 3 subjects (15.8%), 4∼6 weeks for 4 subjects (21.1%), and > 6 weeks for 7 subjects (36.8%). For the successfully withdrawn group, the PWC score was significantly decreased from 13.2 at baseline to 5.2 after pregabalin treatment (p < 0.001). The adverse effects of pregabalin were dizziness and nausea.
Comparison of Sleep and Mood Related Characteristics
At baseline, there was no difference in sleep and mood related characteristics between the successfully and non-successfully withdrawn groups (Table 2). After withdrawal from hypnotics for the successful group, the scores on the PSQI showed significant improvement: total score (15.0 ± 2.1, 8.9 ± 3.0, p < 0.001), subjective sleep quality (2.4 ± 0.5, 1.1 ± 0.7, p < 0.001), sleep latency (2.3 ± 0.6, 1.8 ± 0.9, p = 0.045), and daytime dysfunction (1.9 ± 0.9, 1.1 ± 0.7, p = 0.004). These subjects exhibited positive renewal of overall quality of sleep. The ISI yielded an average 11.3-point decrease (20.9 ± 4.3, 9.6 ± 4.4, p < 0.001) in insomnia severity. The HADS data also measured patients' enhancement of emotional well-being at the end of the study, which showed the anxiety score reduced from 7.2 ± 4.9 at baseline to 4.6 ± 3.9 (p = 0.002), and the depression score reduced from 7.9 ± 4.2 to 5.6 ± 3.6 (p = 0.013). However, there were no significant differences in the PSG variables, though there was a trend in the percentage of stage 3 sleep, increasing from an average of 20.4% to 24.6% (Table 3).
Table 2.
Comparison of sleep and mood related characteristics in the unsuccessful and successful groups prior to baseline
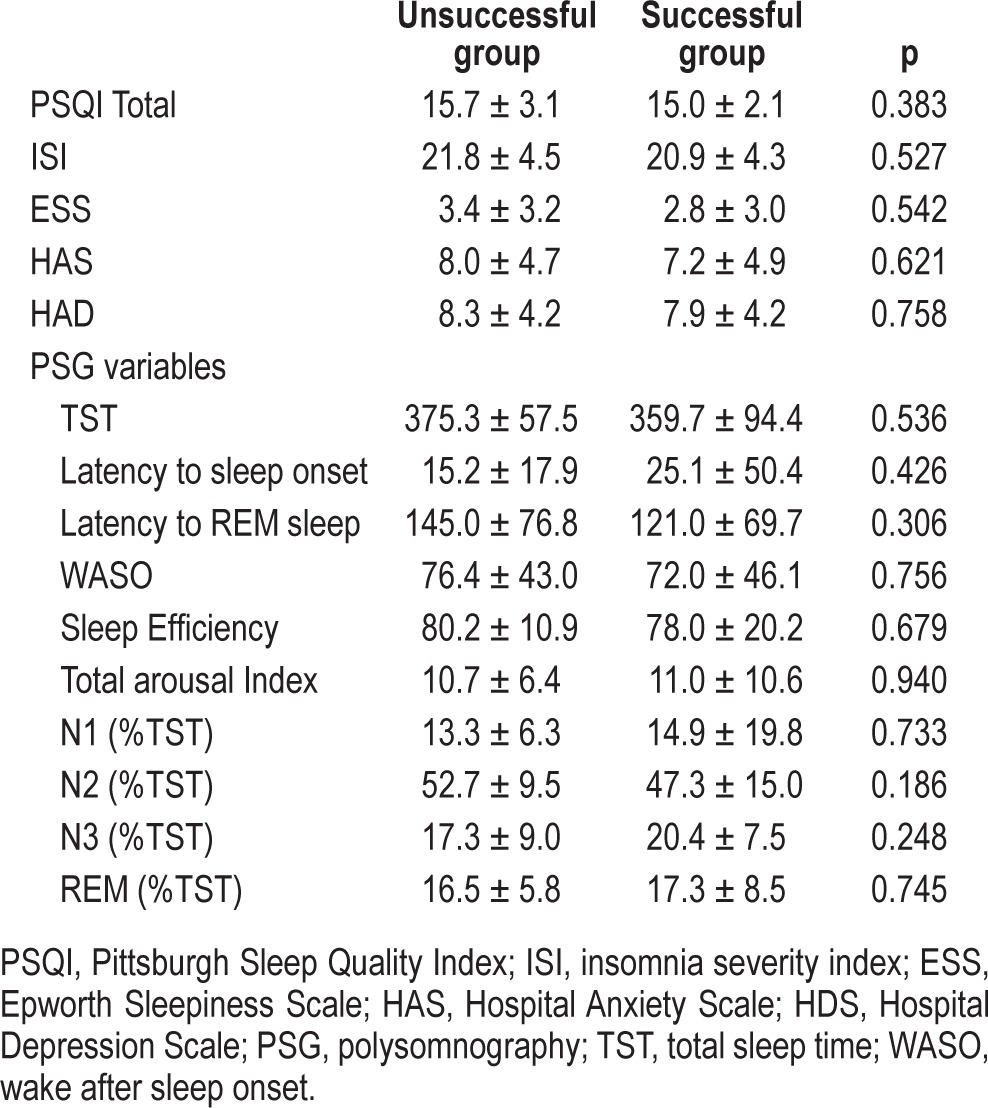
Table 3.
Comparison of clinical characteristics at baseline and after taking pregabalin in the successful group
DISCUSSION
Over half the subjects taking pregabalin were successful withdrawing from hypnotics, and most subjective sleep parameters improved for those who successfully withdrew in our study.
The harmful effects of long-term usage of hypnotics are well-known.2,3,25,26 In pharmacologic treatment of insomnia, general precautions should be taken when using sedative-hypnotics; for instance, usage for more than 2-4 weeks should be avoided if possible, due to dependence, tolerance, addiction, and abuse through long-term use.4,27 However, abuse of hypnotics is still a problem in sleep clinics. Pregabalin has shown a lack of dependency in previous studies.7,15,28 Thus, our study shows clinical significance in giving patients with hypnotic-dependent insomnia a chance for withdrawal.
Using pregabalin, the withdrawal rate from hypnotic dependence for insomnia patients was similar to that of a previous report pertaining to alcohol-dependent patients.29 After using pregabalin, the discontinuation symptoms were mild. However, we could not find any predictive factors indicating who would be successful withdrawing from hypnotics, as the baseline characteristics showed no difference between the successful and the unsuccessful withdrawal groups. The reported adverse effects of pregabalin in previous studies were dizziness, somnolence, nausea, weight increase, peripheral edema, headache, dry mouth, and constipation.30,31 In our study, 4 patients dropped out during the period of the study, due to similar adverse effects, though mild or moderate.
The dosage of pregabalin was from 75 mg to the 300 mg, 57.1% of the subjects took 75 mg, and 33.3% of the subjects took 150 mg. These dosages were relatively lower than those of western studies, usually 300∼600 mg.32 This may be related to racial differences as well as the relatively smaller physical bodies of Asians.
In the successfully withdrawn group using pregabalin, there was a measured improvement in sleep. The severity of insomnia decreased and sleep quality improved significantly. In the PSQI component scores, subjective sleep quality improved by over 50%. Sleep latency showed significant reduction, and daytime function showed marked improvements. However, objective measures of sleep in the PSG study did not show significant improvements in sleep architecture, though there was a positive trend. The previous pregabalin studies using a PSG in target epilepsy patients and health volunteers showed positive effects on objective sleep, such as significant enhancement in slow wave sleep.10,17,33 Also, a previous study with cognitive behavior therapy in hypnotic-dependent insomnia reported participants accrued incremental self-reported, but not PSG, sleep benefits.34 Therefore we need a further study with a larger sample size to clarify this aspect and to compare the effect of pregabalin to that of cognitive behavior therapy for insomnia.
Depression and anxiety symptoms also improved with pregabalin use. In previous studies for fibromyalgia and anxiety disorder patients, pregabalin users showed improved sleep quality due to decreased pain35 and decreased anxiety levels,7 through a benzodiazepine withdrawal program.14 Therefore, alleviating insomnia with pregabalin may be related to decreasing anxiety and depressive symptoms, directly or indirectly.
The limitations of our research were as follow: First, due to the relatively small sample size, no comparison could be made between each type of hypnotic and the individual effect that pregabalin treatment may have had. However, it is encouraging that more than fifty percent of the patients with hypnotic-dependent insomnia could withdraw from hypnotics using pregabalin. Second, we did not conduct follow-ups on patients who were not successful in the discontinuation of hypnotics, so we could not report the effect factors for them. Therefore, further research should be done to clarify it. Third, in previous research, it was reported that in patients with persistent insomnia, long-term outcome was optimized when medication was discontinued during maintenance CBT.36 Therefore, it would be interesting to study the long-term effects of pregabalin and its combined effect with cognitive behavior therapy in a large sample size in the future.
In conclusion, pregabalin is effective in the successful discontinuation of hypnotics, as well as providing improvement of sleep quality in patients with insomnia.
DISCLOSURE STATEMENT
This study was supported by funding, including the use of Pregabalin, from Pfizer, Inc. Pfizer was not involved in the accrual or analysis of the data or the preparation of the manuscript for publication beyond the participation of Drs. Cho and Song.
ACKNOWLEDGMENTS
The authors thank Max HoJung Lee, a student at the University of Washington in Seattle, for his assistance in this study.
REFERENCES
- 1.Mendelson WB, Roth T, Cassella J, et al. The treatment of chronic insomnia: drug indications, chronic use and abuse liability. Summary of a 2001 New Clinical Drug Evaluation Unit meeting symposium. Sleep Med Rev. 2004;8:7–17. doi: 10.1016/S1087-0792(03)00042-X. [DOI] [PubMed] [Google Scholar]
- 2.Beland SG, Preville M, Dubois MF, et al. Benzodiazepine use and quality of sleep in the community-dwelling elderly population. Aging Ment Health. 2010;14:843–50. doi: 10.1080/13607861003781833. [DOI] [PubMed] [Google Scholar]
- 3.Béland SG, Préville M, Dubois MF, et al. The association between length of benzodiazepine use and sleep quality in older population. Int J Geriatr Psychiatry. 2011;26:908–15. doi: 10.1002/gps.2623. [DOI] [PubMed] [Google Scholar]
- 4.Lader M, Tylee A, Donoghue J. Withdrawing benzodiazepines in primary care. CNS Drugs. 2009;23:19–34. doi: 10.2165/0023210-200923010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Busto U, Sellers EM, Naranjo CA, Cappell H, Sanchez-Craig M, Sykora K. Withdrawal reaction after long-term therapeutic use of benzodiazepines. N Engl J Med. 1986;315:854–59. doi: 10.1056/NEJM198610023151403. [DOI] [PubMed] [Google Scholar]
- 6.Holm KJ, Goa KL. Zolpidem. Drugs. 2000;59:865–89. doi: 10.2165/00003495-200059040-00014. [DOI] [PubMed] [Google Scholar]
- 7.Holsboer-Trachsler E, Prieto R. Effects of pregabalin on sleep in generalized anxiety disorder. Int J Neuropharmacol. 2013;16:925–36. doi: 10.1017/S1461145712000922. [DOI] [PubMed] [Google Scholar]
- 8.Russell IJ, Crofford LJ, Leon T, et al. The effects of pregabalin on sleep disturbance symptoms among individuals with fibromyalgia syndrome. Sleep Med. 2009;10:604–10. doi: 10.1016/j.sleep.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Sabatowski R, Gálvez R, Cherry DA, et al. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trial. Pain. 2004;109:26–35. doi: 10.1016/j.pain.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Bazil CW, Dave J, Cole J, Stalvey J, Drake E. Pregabalin increases slow-wave sleep and may improve attention in patients with partial epilepsy and insomnia. Epilepsy Behav. 2012;23:422–5. doi: 10.1016/j.yebeh.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Fink K, Dooley DJ, Meder WP, et al. Inhibition of neuronal Ca (2+) influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002;42:229. doi: 10.1016/s0028-3908(01)00172-1. [DOI] [PubMed] [Google Scholar]
- 12.Rickels K, Shiovitz TM, Ramey TS, Weaver JJ, Knapp LE, Miceli JJ. Adjunctive therapy with pregabalin in generalized anxiety disorder patients with partial response to SSRI or SNRI treatment. Int Clin Psychopharmacol. 2012;27:142–50. doi: 10.1097/YIC.0b013e328350b133. [DOI] [PubMed] [Google Scholar]
- 13.Oulis P, Nakkas G, Masdrakis VG. Pregabalin in zolpidem dependence and withdrawal. Clin Neuropharmacol. 2011;34:90–91. doi: 10.1097/WNF.0b013e31820a3b5a. [DOI] [PubMed] [Google Scholar]
- 14.Rubio G, Bobes J, Cervera G, et al. Effects of pregabalin on subjective sleep disturbance symptoms during withdrawal from long-term benzodiazepine use. Eur Addict Res. 2011;17:262–70. doi: 10.1159/000324850. [DOI] [PubMed] [Google Scholar]
- 15.Oulis P, Konstantakopoulos G. Pregabalin in the treatment of alcohol and benzodiazepines dependence. CNS Neurosci Ther. 2010;16:45–50. doi: 10.1111/j.1755-5949.2009.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mańas A, Ciria JP, Fernández MC, et al. Post hoc analysis of pregabalin vs. non-pregabalin treatment in patients with cancer-related neuropathic pain: better pain relief, sleep and physical health. Clin Transl Oncol. 2011;13:656–63. doi: 10.1007/s12094-011-0711-0. [DOI] [PubMed] [Google Scholar]
- 17.Hindmarch I, Dawson J, Stanley N. A double-blind study in healthy volunteers to assess the effects on sleep of pregabalin compared with alprazolam and placebo. Sleep. 2005;28:187–93. doi: 10.1093/sleep/28.2.187. [DOI] [PubMed] [Google Scholar]
- 18.De Haas S, Otte A, De Weerd A, Van Erp G, Cohen A, Van Gerven J. Exploratory polysomnographic evaluation of pregabalin on sleep disturbance in patients with epilepsy. J Clin Sleep Med. 2007;3:473–8. [PMC free article] [PubMed] [Google Scholar]
- 19.First MB, Frances A, Pincus HA. American Psychiatric Pubishing; 2002. DSM-IV-TR Handbook of Differential Diagnosis. [Google Scholar]
- 20.Sohn SI, Kim do H, Lee MY, Cho YW. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. 2012;16:803–12. doi: 10.1007/s11325-011-0579-9. [DOI] [PubMed] [Google Scholar]
- 21.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 22.Cho YW, Lee JH, Son HK, Lee SH, Shin C, Johns MW. The reliability and validity of the Korean version of the Epworth sleepiness scale. Sleep Breath. 2011;15:377–84. doi: 10.1007/s11325-010-0343-6. [DOI] [PubMed] [Google Scholar]
- 23.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand Suppl. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 24.Rickels K, Garcia-Espana F, Mandos LA, Case GW. Physician Withdrawal Checklist (PWC-20) J Clin Psychopharmacol. 2008;28:447–51. doi: 10.1097/JCP.0b013e31817efbac. [DOI] [PubMed] [Google Scholar]
- 25.Orriols L, Philip P, Moore N, et al. Benzodiazepine-like hypnotics and the associated risk of road traffic accidents. Clin Pharmacol Ther. 2011;89:595–601. doi: 10.1038/clpt.2011.3. [DOI] [PubMed] [Google Scholar]
- 26.Vermeeren A, Coenen AM. Effects of the use of hypnotics on cognition. Prog Brain Res. 2011;190:89–103. doi: 10.1016/B978-0-444-53817-8.00005-0. [DOI] [PubMed] [Google Scholar]
- 27.Ashton H. Guidelines for the rational use of benzodiazepines. When and what to use. Drugs. 1994;48:25–40. doi: 10.2165/00003495-199448010-00004. [DOI] [PubMed] [Google Scholar]
- 28.Oulis P, Konstantakopoulos G, Kouzoupis AV, et al. Pregabalin in the discontinuation of long-term benzodiazepines' use. Hum Psychopharmacol. 2008;23:337–40. doi: 10.1002/hup.937. [DOI] [PubMed] [Google Scholar]
- 29.Martinotti G, Di Nicola M, Tedeschi D, Mazza M, Janiri L, Bria P. Efficacy and safety of pregabalin in alcohol dependence. Adv Ther. 2008;25:608–18. doi: 10.1007/s12325-008-0066-2. [DOI] [PubMed] [Google Scholar]
- 30.Arnold LM, Emir B, Murphy TK, et al. Safety profile and tolerability of up to 1 year of pregabalin treatment in 3 open-label extension studies in patients with fibromyalgia. Clin Ther. 2012;34:1092–102. doi: 10.1016/j.clinthera.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Kamel JT, D'Souza WJ, Cook MJ. Severe and disabling constipation: an adverse effect of pregabalin. Epilepsia. 2010;51:1094–96. doi: 10.1111/j.1528-1167.2009.02381.x. [DOI] [PubMed] [Google Scholar]
- 32.Montgomery SA, Herman BK, Schweizer E, Mandel FS. The efficacy of pregabalin and benzodiazepines in generalized anxiety disorder presenting with high levels of insomnia. Int Clin Psychopharmacol. 2009;24:214–22. doi: 10.1097/YIC.0b013e32832dceb9. [DOI] [PubMed] [Google Scholar]
- 33.Romigi A, Izzi F, Marciani MG, et al. Pregabalin as add-on therapy induces REM sleep enhancement in partial epilepsy: a polysomnographic study. Eur J Neurol. 2009;16:70–75. doi: 10.1111/j.1468-1331.2008.02347.x. [DOI] [PubMed] [Google Scholar]
- 34.Lichstein KL, Nau SD, Wilson NM, et al. Psychological treatment of hypnotic-dependent insomnia in a primarily older adult sample. Behav Res Ther. 2013;51:787–96. doi: 10.1016/j.brat.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roth T, Lankford DA, Bhadra P, Whalen E, Resnick EM. Effect of pregabalin on sleep in patients with fibromyalgia and sleep maintenance disturbance: a randomized, placebo-controlled, 2-way crossover polysomnography study. Arthritis Care Res. 2012;64:597–606. doi: 10.1002/acr.21595. [DOI] [PubMed] [Google Scholar]
- 36.Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301:2005–15. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]