Abstract
Study Objectives:
This study investigated the effects of fatty fish on sleep, daily functioning and biomarkers such as heart rate variability (HRV), vitamin D status (serum 25-hydroxyvitamin D (25OHD), and eicosapentaenoic acid (EPA, 20:5n-3) + docosahexaenoic acid (DHA, 22:6n-3) in red blood cells. Moreover the relationship among sleep, daily functioning, HRV, vitamin D status, and levels of EPA+DHA was investigated.
Methods:
Ninety-five male forensic patients from a secure forensic inpatient facility in the USA were randomly assigned into a Fish or a Control group. The Fish group received Atlantic salmon three times per week from September to February, and the Control group was provided an alternative meal (e.g., chicken, pork, beef), but with the same nutritional value as their habitual diet, three times per week during the same period. Sleep (sleep latency, sleep efficiency, actual sleep time, and actual wake time), self-perceived sleep quality and daily functioning, as well as vitamin D status, EPA+DHA, and HRV, were assessed pre- and post-intervention period.
Results:
There was a significant increase in sleep latency from pre- to post-test in the Control group. The Fish group reported better daily functioning than the Control group during post-test. Fish consumption throughout the wintertime had also an effect on resting HRV and EPA+DHA, but not on vitamin D status. However, at post-test, the vitamin D status in the Fish group was still closer to the level regarded as optimal compared to the Control group. Vitamin D status correlated negatively with actual wake time and positively with sleep efficiency during pre-test, as well as positively with daily functioning and sleep quality during post-test. Finally, HRV correlated negatively with sleep latency and positively with daily functioning.
Conclusions:
Fish consumption seemed to have a positive impact on sleep in general and also on daily functioning, which may be related to vitamin D status and HRV.
Citation:
Hansen AL, Dahl L, Olson G, Thornton D, Graff IE, Frøyland L, Thayer JF, Pallesen S. Fish consumption, sleep, daily functioning, and heart rate variability. J Clin Sleep Med 2014;10(5):567-575.
Keywords: fatty fish consumption, EPA, DHA, vitamin D, sleep, daily functioning, HRV
To date, very few studies have investigated the relationship between dietary habits, nutrient intake, and sleep quality in humans. Some studies have investigated how sleep may influence nutrition. For example, sleep deprivation has been found to alter levels of appetite regulating hormones, and short sleep duration has been associated with greater snack dominance over meals.1,2 Conversely, studies have also shown that nutrition may have an impact on sleep. For example weight restoration in anorexic patients seems to improve subjective sleep quality, and meals consumed very close to bedtime are associated with sleep disturbance.3,4 In terms of macronutrients and micronutrients, some studies have investigated the effects of protein, carbohydrate, vitamins, and minerals on different sleep parameters.5–10 However, knowledge about the effects of fatty fish on sleep is scarce, and it may have important preventive and clinical implications for different groups of people, e.g., institutionalized persons with restricted access to daylight, such as the elderly, inmates, and forensic patients.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Little is known about the relationship between fatty fish consumption and sleep. Fatty fish is the major dietary source of vitamin D and the marine omega-3 fatty acids. Fatty fish consumption has been shown to improve biological mechanisms involved in self-regulation, such as heart rate variability (HRV). Thus, fatty fish consumption may also influence sleep quality and daily functioning. The relationship between fatty fish consumption, sleep, and HRV remains to be investigated.
Study Impact: Fatty fish seemed to have an impact on sleep and daily functioning. Sufficient vitamin D status and high HRV seemed to be positively related to the beneficial effects.
Fatty fish (> 5% fat) is a major dietary source of vitamin D and the marine omega-3 fatty acids (eicosapentaenoic acid, EPA, 20:5n-3 and docosahexaenoic acid, DHA, 22:6n-3).11 These nutrients are suggested to play important roles for prevention of physical and mental health problems.12–14 For instance, Lansdowne and Provost demonstrated that 5 days of vitamin D supplementation during late winter resulted in an increase in positive affect and a decrease in negative affectivity, such as depression and anxiety.15 Moreover, the Tromsø study showed that low vitamin D status was significantly associated with depression.16 Using experimental performance tasks measuring both executive and non-executive functions, it was recently demonstrated in a group of Norwegian inmates that vitamin D status (serum 25-hydroxyvitamin D [25OHD] ≥ 50 nmol/L) may play an important role in executive functioning, an underlying mechanism important for emotional and self-regulation.17
It is well established that vitamin D status varies throughout the year in response to the seasonal changes in sunlight exposure, with its nadir in April on the Northern hemisphere.18,19 This is due to the impact of latitude, where the magnitude of UVB light necessary for skin vitamin D production decreases poleward,20 especially during fall and winter time.21 Even very close to equator, a decline in vitamin D status has been shown by increasing distance from the equator.22 In the absence of sufficient sun exposure for dermal synthesis, dietary vitamin D becomes increasingly important. There is an ongoing debate about the lower limit of adequacy of vitamin D status, although a growing body of evidence suggests that vitamin D status below 50 nmol/L may be associated with greater risk of a wide range of nonskeletal chronic diseases.23,24
Recently a pilot study demonstrated that fatty fish consumption for a period of six months (three times per week from the end of April until mid November) improved vitamin D status in a sample of Norwegian prison inmates with restricted access to daylight exposure.25 Moreover, the fish consumption improved heart rate variability (HRV), which is a measure of the interplay between the sympathetic and the parasympathetic branch of the autonomic nervous system, and regarded as an index of executive functioning, behavior, and self-regulation. Thus, the higher HRV, the more adaptive and flexible the organism is.26 Interestingly, a fish intervention study among American forensic patients found that fish consumption was also associated with better executive functioning.27 Studies focusing on self-reported fish consumption have also shown a relationship between fish consumption and better cognitive functioning as well as physical well-being.28,29
Recent studies have also investigated a possible relationship between vitamin D and sleep quality, sleep disturbances, and sleepiness during the day.30,31 There is evidence that vitamin D supplementation in a group of patients with different sleep disorders caused normal sleep after maintaining an optimal level of 25OHD (60-80 ng/mL) for several months.30 Seasonal variation in sleep patterns has also been reported. A recent study comparing students in Tromsø (69° northern latitude) and Accra (5° northern latitude) during winter and summer found overall poorer sleep and more dysphoric mood among the Tromsø students in winter compared to summer; seasonal effects on these parameters were absent among the students in Accra.32
If fatty fish consumption influences underlying mechanisms involved in self-regulation (e.g., HRV and executive functioning)25,27,28 and physical well-being,29 it remains to investigate if fatty fish consumption also influences sleep. Fatty fish is a source of vitamin D and marine omega-3 fatty acids (EPA and DHA), and both nutrients seem to be important for the regulation of serotonin,33,34 which is involved in the regulation of physiological functions, such as energy balance and sleep.35 Thus, since there seem to be seasonal variations in both vitamin D status and sleep, it is of importance to investigate if fatty fish consumption throughout the winter season has any effect on sleep.
The first aim of the present study was to investigate the effects of fatty fish consumption on objective sleep parameters such as sleep latency, sleep efficiency, actual wake time, and total sleep time in a group of forensic patients in a secure treatment facility. Second, based on earlier investigation, it was also expected that fatty fish consumption would cause better sleep quality33–35 and daily functioning.27–30 The third aim was to investigate if fatty fish consumption had any effects on biological markers like HRV, vitamin D status, and marine omega-3 fatty acids (EPA+DHA), and whether intake of fatty fish during winter time could maintain the vitamin D status obtained during summer time. Finally, the different sleep parameters were investigated in relation to HRV, vitamin D status, and levels of EPA+DHA in red blood cells.
METHODS
Participants
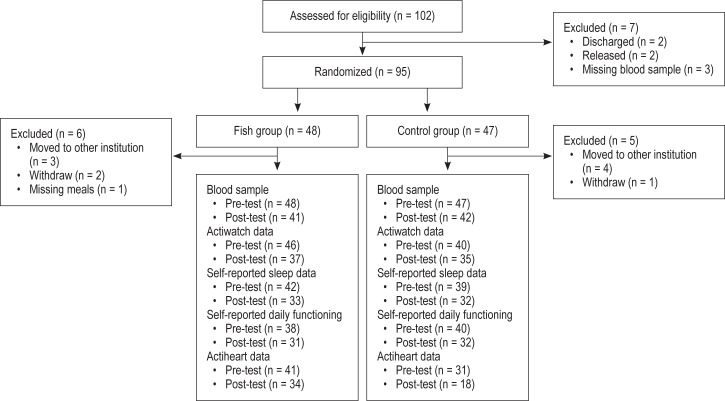
A total of 95 male sexual offenders (mean age 42 years, range 21-60) residing at a secure forensic inpatient facility in the US were randomly assigned into a Fish group or a Control group. The present study was part of a larger research project concerning the effects of fatty fish consumption. A total of 102 responded to an invitation to participate, and the flow diagram demonstrates in details the study progress (see Figure 1). All were American citizens: 78 were Caucasians, 18 were African Americans, 5 were Native Americans, and one was of Hispanic origin. Participants were matched on age, IQ (> 75 was used as an inclusion criterion) and Psychopathy Checklist List-Revised score and then randomly assigned into an intervention group (Fish group) or a Control group.
Figure 1. Flow diagram of the study progress.
Variations in number of participants related to the different measures and from pre-to post-test were due to reasons beyond our control (e.g., technical problems related to the Actiheart system or the Actiwatch Activity Monitoring Systems).
Apparatus
Actiwatch Activity Monitoring Systems
In order to collect data concerning sleep latency, sleep efficiency, actual sleep time, and actual wake time actigraphs from the Actiwatch Activity Monitoring Systems were used to measure changes in activity (Cambridge Neurotechnology, Cambridgeshire, UK). Actigraph is a compact, lightweight worn electronic device containing an accelerometer, a clock and a data storage unit that measures and records physical movement. Based on an algorithm, data are converted to sleep parameters.36
Subjective sleep parameters were assessed by sleep diaries. These were kept for 2 weeks (pre and post) and comprise daily morning assessment of sleep variables such as sleep quality (1 = poor to 5 = good) and daytime functioning (1 = very sleepy to 5 = very good).37
Actiheart System
Physiological activity was measured by recording heart rate (HR) and HRV using the Actiheart System (Cambridge Neurotechnology Ltd),38 a compact lightweight device that records HR and variability of R-R inter-beat intervals (IBI). The Actiheart clips onto a single ECG electrode (M-00-S/50 Blue Sensor) with a short ECG lead to another electrode that detects the ECG signal. The Actiheart was placed on the upper chest.
HR was defined as the average heart rate in beats per minute for the analysis epoch (1 minute). HRV was measured as high frequency power ([HF]; 0.15-0.4 Hz) derived by the fast Fourier transform (FFT) spectrum. The HF component is known to reflect primarily parasympathetic influences. The HF data were log transformed prior to analysis.39
Farmed Atlantic Salmon
The production of the Atlantic salmon (Salmo salar L.) was at Skretting Fish Trials Station (Stavanger, Norway). The fish were harvested at about 4.5 kg and processed into skin and boneless portions (150 g), vacuum packed, and frozen (Rex Star Seafood, Tysnes, Norway).
The total fat and protein content of the salmon was 13.5 ± 3.3 and 20.3 ± 0.5 g/100-g fillet, respectively. The level of vitamin D was 5 ± 3 μg/100-g fillet. The intake of EPA+DHA and vitamin D in a portion (300 g) of Atlantic salmon were 4.8 g and 15 μg, respectively. The standard portion was 300 g three times a week; however during the final four- weeks of the study, they were served portion sizes of 150 grams of salmon. The content of several undesirable substances was also determined in the Atlantic salmon. The level of mercury was 22 μg/kg, and the level of dioxins and dioxin-like PCBs was 0.48 ng TEQ/kg; both are far below the EUs upper limits of 500 μg/kg and 6,5 ng TEQ/kg in fish, respectively. Taking into account the amount of salmon consumed per week during the weeks with the highest salmon intake, the intake of dioxin and dioxin-like PCBs per week represents 31% of the tolerable weekly intake (TWI) in a person weighing 100 kg.40 Persons with higher body weight will have a correspondingly lower percentage of TWI.
Procedure
The study protocol and all experimental procedures were approved by the Ethics Committee at the facility in Wisconsin, USA, and were in compliance with the Helsinki declaration for research ethics. Participants were recruited by both written and oral information about the study. Thus, the patients were invited to participate in a research project concerning nutrition and mental health. The participants were informed that the purpose of the study was to investigate if fatty oily fish/ nutrition would have any effects on mental health. They were also informed that they would be randomly assigned into two groups; one group that would have to eat fish (portion size 150-300 g) three times a week and one group that would have to eat meat (e.g., chicken, pork, beef) meals three times a week for a period for six months (September-February). Moreover, the instruction with regard to the sleep diary was exactly: “In order to investigate whether nutrition will have any effects on psychological processes we want to collect some information about your sleeping pattern before and after exposure to the food intervention.” The participants had to sign an informed consent form and they were informed about their rights to withdraw from the study at any time for any reason without penalty.
Before and at the end of the intervention period the participants went through a test procedure (pre-test in July and post-test in February while they still were eating the meals). Since the present study is part of a larger project concerning fatty fish consumption these pre- and post-test procedures involved collections of different kinds of data such as fasting blood sample, executive functioning, psychophysiology, and sleep data. For both the pre- and post-test, the psychophysiological activity was registered for 5 minutes of baseline, during exposure to the experimental tasks, and for 5 minutes of recovery. In order to investigate sleep in relation to resting HRV, both the baseline HF measure and the recovery HF measure were used in the present study. This was done since resting HRV before and after exposure to mild cognitive stress may differ from each other because of performance anxiety before the experimental procedure.41 All participants were tested individually.
With regard to collection of sleep data, the participants had to wear the actigraph one week before the food intervention period and one week by the end of the food intervention period. Two sum scores (one for the week before and one for the week by the end of the intervention period) were made for each variable: sleep latency, sleep efficiency, actual wake time, and total sleep time in order to do the data analyses.
Human blood samples for fatty acid composition in erythrocytes were collected in K2 EDTA vials, centrifuged (10 min, 2000 g, 20°C) immediately and stored at −80°C until analysis. Fatty acid composition of total lipids in erythrocytes was determined by a modification of a simplified gas liquid chromatographic (GLC) method using 19:0 methyl ester as internal standard as previously described.42 Some modifications were made: The methyl esters were separated using an Ultrafast Trace GC Ultra (5 min; Thermo Electron Corporation, MA, USA) equipped with a 5-m wax column (id: 0.1 mm, 0.2 μm film thickness; Thermo Electron Corporation), using split injection, with a temperature program of 100°C; 50°C/min up to 220°C; 80°C/min up to 250°C and flame ionization detector. The fatty acid composition was calculated using an integrator (Chromeleon 6.80, Dionex Corporation, CA USA), connected to the GLC and identification ascertained by standard mixtures of methyl esters (Nu-Chek, MN, USA). Limit of quantification (LOQ) was 10 μg fatty acid/g samples (wet weight).
Blood samples for vitamin D status measured as 25-hydroxyvitamin D (25OHD) in serum were collected in silica gel tubes, centrifuged (10 min, 2000 g, 20°C) within 2 h. Samples for 25OHD determination were kept in the refrigerator (4°C) until analysis within 48 hours. Direct competitive chemiluminescence immunoassay was performed at a routine clinical laboratory nearby the study site (Dynacare laboratories, Milwaukee, WI, USA).
The patients could go outdoor for recreation three times per day, with each session lasting 2 h (it was their choice to spend anywhere from 30 min to the full 2 h of that time). They also had “courtyards” attached to their units and could basically go outside any time during the day. We have not collected data on how much time the participants spent outside. Daylight hours at pre-test (in July) were between 14 h 36 min and 15 h 24 min, while daylight hours at post-test (in February) were between 9 h 55 min and 11 h 10 min.
Design and Statistics
Differences between the two groups on sleep latency, sleep efficiency, actual wake time, total sleep time, as well as HRV, vitamin D status and EPA+DHA, were analyzed by 2-way repeated measures ANOVAs ((Fish vs. Control group) × 2 (pre- vs. post-test conditions)). The analyses were followed up by Bonferroni correction. Pre-planned comparisons were used to test our hypotheses regardless of whether the omnibus tests were significant.43–45 Due to our specific expectations concerning sleep quality33–35 and daily functioning,27–30 one-tailed tests were used.43–45 In order to examine the magnitude of the significant differences between the independent means, we calculated the effect sizes as Cohen's d.46 Pearson product-moment correlation coefficients were calculated in order to investigate the different sleep parameters (sleep latency, sleep efficiency, actual wake time, total sleep time, sleep quality, and daily functioning) in relation to HRV, vitamin D status, and EPA+DHA.
RESULTS
Descriptive Data
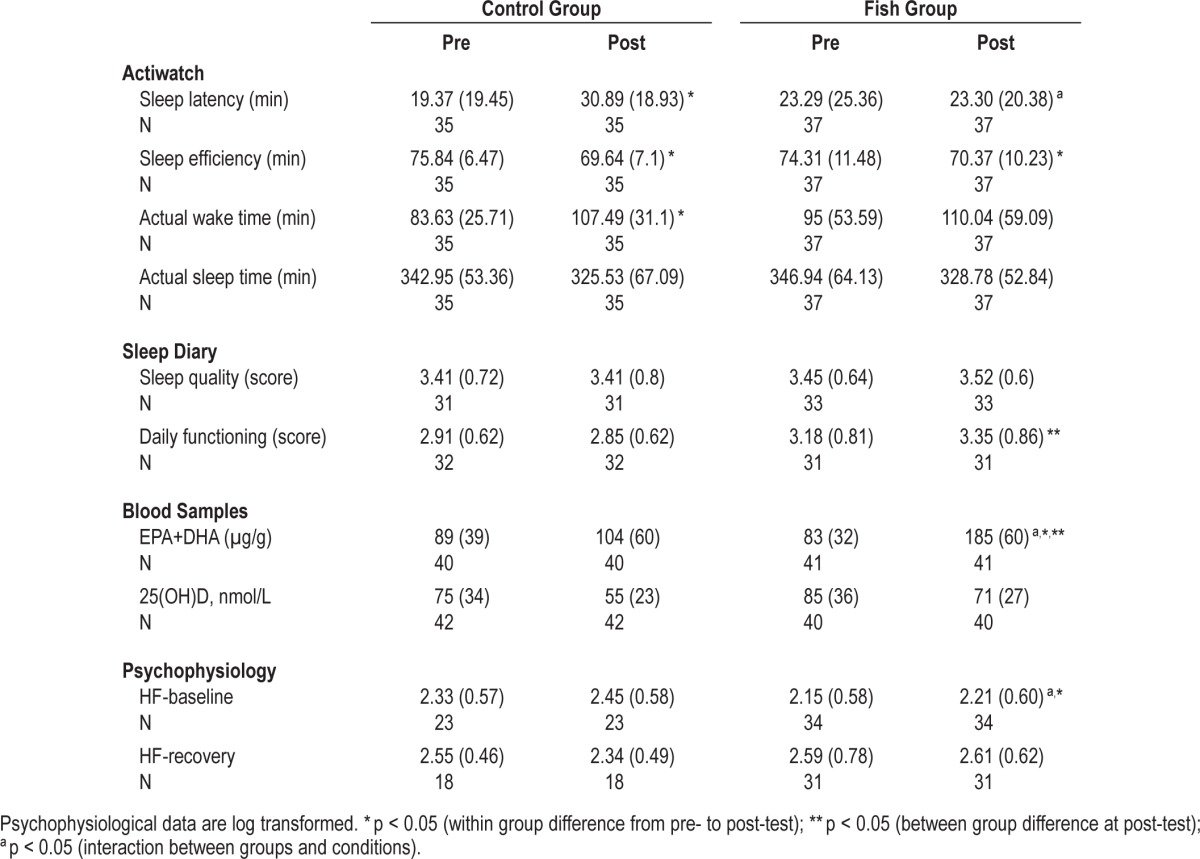
Means and standard deviations for sleep latency, sleep efficiency, actual wake time, total sleep time, sleep quality, daily functioning, vitamin D status, EPA+DHA, and HRV for both groups are shown in Table 1.
Table 1.
Sleep latency, sleep efficiency, actual sleep time, actual wake time, sleep quality, daily functioning, EPA+DHA (eicosapentaenoic acid+docosahexaenoic acid) in red blood cells, vitamin D status (serum 25OHD), and heart rate variability (high frequency; HF) baseline and HF-recovery presented as mean (standard deviations) during pre- and post-test for both groups.
Effects of Fatty Fish on Sleep
Actiwatch Data
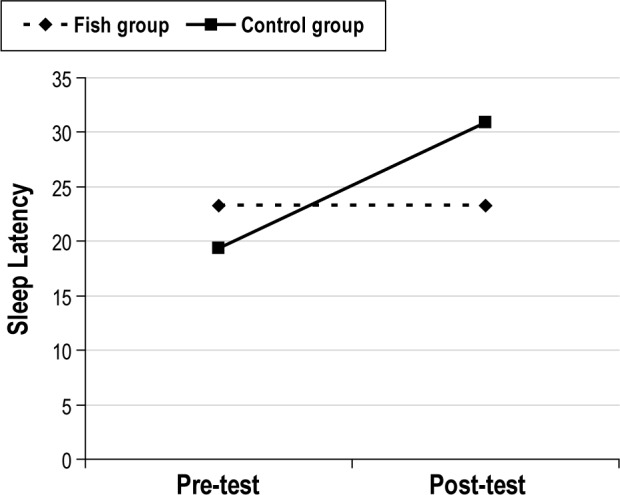
For the sleep latency data, the 2-way ANOVA showed no main effect of groups (F1,70 = 0.1980, p = 0.66). However, there was a significant effect of pre- and post-test conditions (F1,70 = 4.14, p = 0.05), showing a longer sleep latency during post- compared to pre-test, (p = 0.05). There was also a significant interaction effect between groups and conditions (F1,70 = 4.11, p = 0.05). Follow-up with Bonferroni demonstrated that there was a significant increase in sleep latency from pre- to post-test in the Control group (p = 0.04; d = 0.60; see Figure 2), which was not found in the Fish group.
Figure 2. Sleep latency (minutes) for both groups from preto post-test.
For the sleep efficiency there was no effect of groups. However, there was a main effect of pre- and post-test conditions (F1,70 = 32.84, p < 0.001). Follow-up test showed that there was a significant decrease in sleep efficiency from pre- to post-test (p < 0.001; d = 0.56). The interaction between groups and conditions was not significant (F1,70 = 1.63, p = 0.21). However, follow-up with Bonferroni correction the results revealed that there was a significant decrease in both groups (Control group p < 0.001; d = 0.91 and Fish group, p = 0.01; d = 0.36).
Looking at the actual wake time, again there was a significant effect of pre- and post-test conditions (F1,70 = 19.83, p < 0.001), with a significant increase in actual wake time from pre- to post-test, (p < 0.001; d = 0.43). Again the interaction between conditions and groups was not significant (F1,70 = 1.02, p = 0.32).
Follow-up with Bonferroni correction the results revealed that only the Control group had a significant increase in actual wake time from pre- to post-test (p = 0.002; d = 0.84).
For the actual sleep time there was a significant main effect of pre- and post-test conditions as well (F1,70 = 7.44, p = 0.008), showing a significant decrease in actual sleep time from pre- to post-test (p < 0.001; d = 0.30). The interaction between groups and conditions was not significant (F1,70 = 0.003, p > 0.95). No other significant results were found.
Self-reported Daily Functioning and Sleep Quality
There was a significant main effect of groups looking at the self-reported daily functioning (F1,61 = 54.63, p = 0.03), and the Fish group reported higher scores on daily functioning than the Control group. There was no effect of the pre- and post-test conditions (F1,61 = 0.49, p = 0.49). However, on follow-up of the nonsignificant interaction (F1,61 = 1.87, p = 0.17), the results revealed that the Fish group reported better daily functioning than the Control group only at post-test (p = 0.03; d = 0.65).
For sleep quality data, there was no effect of groups (F1,62 = 0.20, p = 0.66). Moreover, there was no effect of pre- and post-test conditions (F1,62 = 0.26, p = 0.61), nor was any significant interaction effect found (F1,62 = 0.26, p = 0.61). Follow-up of this nonsignificant interaction, the results revealed no significant effect of fish consumption on self-perceived sleep quality (p = 0.50).
Psychophysiology
For the HF-baseline, the 2-way ANOVA showed no main effect of groups (F1,55 = 0.044, p = 0.85). There was no significant effect of the conditions (F1,55 = 1.26, p = 0.29). However, there was a significant interaction effect between groups and conditions (F1,55 = 6.31, p = 0.01). Following up with Bonferroni correction, the result showed that the Fish group had a significant increase in HF power from pre- to post-test (p = 0.04; d = 0.54). With regard to the HF recovery data, there were no significant results.
Vitamin D Status
For vitamin D status, the 2-way ANOVA showed a significant main effect of groups, (F1,80 = 4.31, p = 0.04); the Fish group had significantly better vitamin D status than the Control group (p = 0.04; d = 0.43). Furthermore, there was a main effect of time (F1,80 = 48.65, p < 0.001), showing that there was a significant decrease in vitamin D status from pre-test in July to post-test in February (p < 0.001; d = 0.55). The interaction between pre- and post-test conditions and groups was not significant (F1,80 = 1.26, p = 0.26). Follow-up by Bonferroni correction the results revealed that there was a significant decrease in Vitamin D status from pre- to post-test in both groups (Control group, p < 0.001; d = 0.70 and Fish group, p < 0.001; d = 0.44). At post-test, the Fish group had a higher level of vitamin D than the Control group. However, the Bonferroni correction did not reveal a significant difference between the groups (p = 0.13), but the effect size was medium (d = 0.63).
EPA+DHA
For EPA+DHA, there was a main effect of groups (F1,79 = 17.87, p < 0.001), with higher levels of EPA+DHA in the Fish group than the Control group (p < 0.001; d = 0.76). Furthermore, there was a main effect of time (F1,79 = 68.09, p < 0.001), showing higher levels of EPA+DHA at post-test compared to pre-test (p < 0.001; d = 1.01). Finally the interaction between the groups and the pre- and post-test conditions was significant (F1,79 = 36.45, p < 0.001). Bonferroni correction revealed that the Fish group had a significant increase in EPA+DHA from pre- to post-test (p < 0.001; d = 2.05). This was not found in the Control group. Additionally, the Fish group revealed a higher level of EPA+DHA than the Control group at post-test (p < 0.001; d = 1.30).
Correlations
Sleep and Psychophysiology
No significant relationships were found for the pre-test. However, at the end of the intervention period, sleep latency correlated negatively with HF recovery (r = -0.25, p = 0.05), while daily functioning correlated positively with HF recovery (r = 0.28, p = 0.03).
Sleep and Vitamin D Status
Before the intervention period, there was a significant and negative correlation between actual wake time and vitamin D status (r = -0.24, p = 0.03). Moreover, there was a positive relationship between sleep efficiency and vitamin D status (r = 0.23, p = 0.03). At the end of the intervention period, the results revealed that there was a significant and positive relationship between daily functioning and vitamin D status (r = 0.33, p = 0.005), and there was a significant and positive relationship between sleep quality and vitamin D status (r = 0.24, p = 0.05).
Sleep and EPA+DHA
There was no significant relationship between any of the sleep parameters and the marine fatty acids at pre-test. However, at post-test there was a negative relationship between self-perceived sleep quality and EPA+DHA (r = -0.22, p = 0.05).
Correlations between Sleep Variables
Before intervention, sleep latency correlated negatively with actual sleep time (r = -0.22, p = 0.03) and sleep efficiency (r = -0.63, p < 0.001). Sleep efficiency correlated positively with actual sleep time (r = 0.52, p < 0.001) and negatively with actual wake time (r = -0.76, p < 0.001). At the end of the intervention period sleep latency correlated negatively with actual sleep time, (r = -0.32, p = 0.004), and sleep efficiency (r = -0.50, p < 0.001). Furthermore, there was a negative relationship between sleep efficiency and actual wake time (r = -0.57, p < 0.001), and a positive association between sleep efficiency and actual sleep time (r = 0.64, p < 0.001).
DISCUSSION
Overall, the present results revealed that there was a significant increase in sleep latency from pre- to post-test in the Control group. Moreover, there seemed to be a worsening of sleep for the whole sample from pre- to post-test. However, the Fish group reported better daily functioning than the Control group at post-test, and fatty fish consumption seemed to have an effect on biological markers such as HRV and omega-3. Both groups had a significantly decreased vitamin D status from July to February, but at the end of the intervention period the vitamin D status in the Fish group was still closer to the level regarded as optimal compared to the Control group. Interestingly, the results revealed that vitamin D status correlated negatively with actual wake time and positively with sleep efficiency during pre-test, as well as positively with daily functioning and sleep quality during post-test. Finally, HRV seemed to be negatively related to sleep latency and positively related to daily functioning after the intervention period.
Interestingly, the increase in sleep latency found in the Control group was not found in the Fish group. Several studies have shown that there is a relationship between sleep latency and heart rate, and high level of arousal is associated with elevated sleep latency. According to Bonnet and Arand, sleep latency should be regarded as a measure of more than just sleep latency, but also other factors such as underlying physiological activation.47 Thus, this argument is supported by the present results showing that sleep latency correlated negatively with the HF power, which is known to reflect primarily parasympathetic activity.39 Importantly the current results also demonstrated that fatty fish consumption caused an increase in resting HF, and HF power is a more powerful way to measure the relationship between psychological and physiological processes than HR.48 Thus, the increased sleep latency found in the Control group might be related to levels of general arousal and lower parasympathetic activity.
Further, there is reason to believe that the results concerning sleep latency could be caused by the vitamin D status in both groups. Both groups showed a significant decrease in vitamin D status. However, the Control group showed a drop in vitamin D status below the optimal level (for US population: > 75 nmol/L) at post-test (55 nmol/L), while the vitamin D status in the Fish group was still close to the optimal level at post-test (71 nmol/L). The stable sleep latency from pre- to post-test observed in the Fish group might then be related to the optimal 25OHD level.30 This hypothesis is strengthened by the suggestion that vitamin D status is important for seasonal changes in serotonin regulation.33 This explanation is further corroborated by the fact that fish is a source of tryptophan, the precursor for serotonin.49 Thus, the present results are consistent with work by Hartmann, who found that tryptophan increased sleepiness.50 Based on the sleep latency results from the present study and the executive performance results from Hansen et al.,27 a plausible explanation could be that the fish consumption induced feelings of sleepiness during the evening, which in turn resulted in better sleep and executive functioning during the day. In the present study, sleep latency correlated negatively with both sleep efficiency and actual sleep time.
Overall for the whole sample, there also seemed to be significant changes towards worse sleep from pre- to post-test. This was true for sleep efficiency, actual wake time, and total sleep time, looking at the effect of time for both groups pooled together. Follow-up for the nonsignificant interactions both groups showed a significant decrease in sleep efficiency. However, only the Control group showed a significant increase in actual wake time from pre- to post-test. The change in sleep efficiency found in both groups may indicate poorer sleep quality during winter than summer. This can be interpreted in line with the results found by Friborg et al. who found that students in Tromsø (69° northern latitude) had overall poorer sleep and more dysphoric mood in winter than in summer.32 Interestingly this seasonal effect was not found in students in Accra (5° northern latitude).32 Due to the reduction in vitamin D status during winter in both groups, but especially in the Control group which showed a vitamin D status below the optimal level, insufficient vitamin D status might be the underlying cause of the poorer sleep quality during this season. This emphasizes the impact of diet on the nutritional status, and that it even may have an impact on sleep. However, it is worth noting that the vitamin D contribution from salmon was not sufficient to maintain the vitamin D status in the Fish group at the same level as during summer time. It is also important to note that even if there was not a significant difference between the groups in vitamin D status at pre-test, the Fish group had a higher level of vitamin D before the intervention. However, independent of the levels of vitamin D status in the groups, the correlation analyses suggested that vitamin D status might be related to sleep quality.
Moreover, it was expected that fish consumption would improve the subjective parameter, sleep quality, and daily functioning. Contrary to our expectations, we did not find any improvement in self-reported sleep quality in the Fish group. However, we found support for the specific expectation concerning daily functioning. The Fish group reported better perceived daily functioning at post-test compared to the Control group. In addition the current results demonstrated a positive relationship between subjective reports of daily functioning and parasympathetic activity, an index of good self-regulation and adaptation. Thus, the self-perceived feeling of daily functioning is in line with the executive experimental performance results found in Hansen et al.27 The results are also consistent with those of Markus et al.51 concerning morning alertness and better performance on a vigilance task after α-lactalbumin (A-LAC) consumption, which is a rich source of tryptophan.49
Finally, the relationship between sleep variables as well as daily functioning and specific nutrients such as vitamin D and EPA+DHA were investigated. Thus, even if the current study demonstrated a significant decrease in vitamin D status from pre-test to post-test in both groups, we found, in line with previous results,30 that vitamin D status seemed to play a key role in relation to sleep. First, during pre-test there was a negative correlation between vitamin D status and actual wake time. Additionally, there was a positive correlation between vitamin D status and sleep efficiency during pre-test. By the end of the intervention, vitamin D status correlated positively with both sleep quality and daily functioning. Thus, the question is whether the beneficial effects associated with fatty fish consumption (e.g., improved executive functioning)27 are actually caused by better sleep quality. This issue needs further investigation. Moreover, the Fish group showed a significant increase in EPA+DHA from pre- to post-test. At pre-test, EPA+DHA did not correlate with any of the dependent variables. However, there was a negative relationship between EPA+DHA and self-perceived sleep quality at post-test. To our knowledge, this is the first time EPA+DHA has been investigated in relation to sleep, and one should draw conclusions with caution. There are contradictory findings in the literature concerning the positive effects of EPA+DHA in relation to psychological functioning.34,52 Thus, the effect of omega-3 is not clearly understood.
The present study has some limitations that should be noted. It is a challenge to find a control group or placebo for the fish group. Therefore, to minimize the effect of participation in an intervention study as such, the diet was also changed for the control group compared to their habitual diet, and the instructions to the participants focused on nutritional effects, not only for the fatty fish group. The subjects eating fish and the subjects eating meat will of course know this as blinding is hardly possible in interventions with food. Nevertheless, this kind of study is valuable to identify whether there is an effect of diet, although animal studies are required to elucidate the mechanisms. However, even if the participants could have specific expectations regarding fish consumption due to general knowledge about the health benefit from fish consumption, it is not easy to manipulate objective assessment methods such as the actigraphic sleep variables and the Actiheart data. It should also be acknowledged that the results from this relatively small sample of male forensic patients are not necessarily able to be generalized to other groups. However, since the reduced vitamin D status during winter time is not limited to the study population, it is our expectation that the same effect of fish consumption would be found on similar outcome variables in different kinds of groups such as children and adolescents. It might also be found in groups with and without different kinds of impaired self-regulation and behavioral problems. Furthermore, the correlations between sleep parameters and psycho-physiology as well as sleep parameters and vitamin D are not strong. Results of the present study should be interpreted cautiously, and further studies are of importance in order to validate the results.
Overall, the present results indicated that fish consumption seemed to have a positive impact on both sleep and daily functioning. Interestingly, the participants with the highest vitamin D status in the present study had shorter actual wake time, better sleep efficiency, better daily functioning, and better sleep quality. Thus, due to the fact that there were some relationships between vitamin D and different sleep parameters, one could speculate that the beneficial effect of fatty fish consumption was due to vitamin D. However, fatty fish also contains other important nutrients (e.g., selenium, iodine, proteins), and further investigation is therefore needed in order to gain more knowledge about the specific underlying mechanisms responsible for the beneficial effects of fatty fish consumption.
DISCLOSURE STATEMENT
This was not an industry supported study. Financial support was provided by the Program board Nutrition, University of Bergen, Norway and the Centre for Research and Education in Forensic Psychiatry, Haukeland University Hospital, Bergen, Norway. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful to Skretting ARC for providing the farmed Atlantic salmon for this study. The authors thank all participants for their cooperation. We also thank the kitchen staff for preparing all the meals and the health department for collecting blood samples at the secure forensic inpatient facility in USA. Thanks to the laboratories at NIFES for skilful analytical help.
REFERENCES
- 1.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 2.Kim S, DeRoo LA, Sandler DP. Eating patterns and nutritional characteristics associated with sleep duration. Public Health Nutr. 2011;14:889–95. doi: 10.1017/S136898001000296X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pieters G, Theys P, Vandereycken W, Leroy B, Peusken J. Sleep variables in anorexia nervosa: Evolution with weight restoration. Int J Eat Disorder. 2004;35:342–7. doi: 10.1002/eat.10256. [DOI] [PubMed] [Google Scholar]
- 4.Porter JM, Horne JA. Bed-time food supplements and sleep. Effects of different carbohydrate levels. Electroen Clinl Neuro. 1981;51:426–33. doi: 10.1016/0013-4694(81)90106-1. [DOI] [PubMed] [Google Scholar]
- 5.Spring B, Maller O, Wurtman J, Digman L, Cozolino L. Effects of protein and carbohydrate meals on mood and performance - interactions with sex and age. J Psychiatr Res. 1983;17:155–67. doi: 10.1016/0022-3956(82)90017-6. [DOI] [PubMed] [Google Scholar]
- 6.Peuhkuri K, Sihvola N, Korpela R. Diet promotes sleep duration and quality. Nutr Res. 2012;32:309–19. doi: 10.1016/j.nutres.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Ebben M, Lequerica A, Spielman A. Effects of pyridoxine on dreaming: A preliminary study. Percept Motor Skill. 2002;94:135–40. doi: 10.2466/pms.2002.94.1.135. [DOI] [PubMed] [Google Scholar]
- 8.Ohta T, Ando K, Iwata T, et al. Treatment of persistent sleep-wake shcedule disorders in adolescents with methylcobalamin (vitamin-B12) Sleep. 1991;14:414–8. [PubMed] [Google Scholar]
- 9.Kordas K, Siegel EH, Olney DK, et al. The effects of iron and/or zinc supplementation on maternal reports of sleep in infants from Nepal and Zanzibar. J Dev Behav Pediatr. 2009;30:131–9. doi: 10.1097/DBP.0b013e31819e6a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Held K, Antonijevic IA, Künzel H, et al. Oral Mg(2+) supplementation reverses age-related neuroendocrine and sleep EEG changes in humans. Pharmacopsychiatry. 2002;35:135–43. doi: 10.1055/s-2002-33195. [DOI] [PubMed] [Google Scholar]
- 11.Alexander J, Frøyland L, Hemre GI, et al. A comprehensive assessment of fish and other seafood in the Norwegian diet. Norwegian Scientific Committee for Food Safety. 2006. Available from: http://www.vkm.no/eway/default.aspx?pid=278&trg=Content_6615&Main_6359=6582:0:31,2567&Content_6582=6615:0:31,2669&Content_6615=6393:1812541::0:6450:7:::0:0.
- 12.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin D and 1, 25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–9. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 13.Holick MF. Vitamin D: a millennium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 14.McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction. FASEB J. 2008;22:982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- 15.Landsdowne AT, Provost SC. Vitamin D3 enhances mood in healthy subjects during winter subjects during winter. Psychopharmacology. 1998;135:319–23. doi: 10.1007/s002130050517. [DOI] [PubMed] [Google Scholar]
- 16.Jorde R, Waterloo K, Saleh F, Haug E, Svartberg J. Neuropsychological function in relation to serum parathyroid hormone and serum 25-hydroxyvitamin D levels The Tromsø study. J Neurol. 2006;253:464–70. doi: 10.1007/s00415-005-0027-5. [DOI] [PubMed] [Google Scholar]
- 17.Hansen AL, Dahl L, Bakke L, Thayer JF. Vitamin D and executive function: a preliminary report. Percept Motor Skill. 2011;113:677–85. doi: 10.2466/02.09.13.15.16.PMS.113.5.677-685. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF. Environmental factors that influence the cutaneous production of vitamin D. Am J Clin Nutr. 1995;49:211–8. doi: 10.1093/ajcn/61.3.638S. [DOI] [PubMed] [Google Scholar]
- 19.Christensen MH, Lien EA, Hustad S, Almås B. Seasonal and age-related differences in serum 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D and parathyroid hormone in patients from Western Norway. Scand J Clin Lab Invest. 2010;70:281–6. doi: 10.3109/00365511003797172. [DOI] [PubMed] [Google Scholar]
- 20.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–8. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 21.Holick MF, Chen TC, Lu Z, Sauter E. Vitamin D and skin physiology: a D-lightful story. J Bone Miner Res. 2007;22(Suppl 2):V28–V33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- 22.Arantes HP, Kulak CAM, Fernandes CE, et al. Correlation between 25-hydroxyvitamin D levels and latitude in Brazilian postmenopausal women: from the Arzoxifene Generations Trial. Osteoporos Int. 2003;24:2707–12. doi: 10.1007/s00198-013-2366-x. [DOI] [PubMed] [Google Scholar]
- 23.Zitterman A. Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr. 2003;89:552–72. doi: 10.1079/BJN2003837. [DOI] [PubMed] [Google Scholar]
- 24.Cashman KD, Hill TR, Lucwy AJ, et al. Estimation of the dietary requirement for vitamin D in healthy adults. Am J Clin Nutr. 2008;88:1535–42. doi: 10.3945/ajcn.2008.26594. [DOI] [PubMed] [Google Scholar]
- 25.Hansen AL, Dahl L, Bakke L, Frøyland L, Thayer JF. Fish consumption and heart rate variability: preliminary results. J Psychophysiol. 2010;24:41–7. [Google Scholar]
- 26.Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disorders. 2000;61:201–16. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- 27.Hansen AL, Dahl L, Olson G, et al. Fish consumption and underlying mechanisms in self-regulation. Psychophysiology. 2012;49:S121. [Google Scholar]
- 28.Eskelinen MH, Ngandu T, Helkala E-L, et al. Fat intake at midlife and cognitive impairment later in life: a population-based CAIDE study. Int J Geriatr Psych. 2008;23:741–7. doi: 10.1002/gps.1969. [DOI] [PubMed] [Google Scholar]
- 29.Schiepers OJ, de Groot RH, Jolles J, van Boxtel MP. Fish consumption, not fatty acid status, is related to quality of life in a healthy population. Prostag Leukotr Ess. 2010;83:31–5. doi: 10.1016/j.plefa.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 30.Gominak SC, Stumpf WE. The world epidemic of sleep disorders is linked to vitamin D deficiency. Med Hypotheses. 2012;79:132–5. doi: 10.1016/j.mehy.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 31.McCarty DE, Reddy A, Keigley Q, Kim PY, Marino AA. Vitamin D, race, and excessive daytime sleepiness. J Clin Sleep Med. 2012;8:693–7. doi: 10.5664/jcsm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friborg O, Bjorvatn B, Amponsah B, Pallesen S. Associations between seasonal variations in day length (photoperiod), sleep timing, sleep quality and mood: a comparison between Ghana (5 degrees) and Norway (69 degrees) J Sleep Res. 2012;21:176–84. doi: 10.1111/j.1365-2869.2011.00982.x. [DOI] [PubMed] [Google Scholar]
- 33.Stumpf WE, Privette TH. Light, vitamin D and psychiatry: Role of 1,25-dihydroxyvitamin D3 (soltriol) in etiology and therapy of seasonal affective disorder and other mental processes. Psychopharmacology. 1989;97:285–94. doi: 10.1007/BF00439440. [DOI] [PubMed] [Google Scholar]
- 34.Hibbeln JR, Ferguson TA, Blasbalg TL. Omega-3 fatty acid deficiencies in neurodevelopment, aggression, and autonomic dysregulation: Opportunities for intervention. Int Rev Psychiatr. 2006;18:107–18. doi: 10.1080/09540260600582967. [DOI] [PubMed] [Google Scholar]
- 35.Praschak-Rieder N, Willeit M, Wilson AA, Houle S, Meyer JH. Seasonal variation in human brain serotonin transporter binding. Arch Gen Psychiatry. 2008;65:1072–8. doi: 10.1001/archpsyc.65.9.1072. [DOI] [PubMed] [Google Scholar]
- 36.Sadeh A. The role and validity of actigraphy in sleep medicine: An update. Sleep Med Rev. 2011;15:259–67. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Lichstein KL, Riedel BW. Behavioral assessment of insomnia. A review with emphasis on clinical application. Behav Ther. 1994;25:659–88. [Google Scholar]
- 38.Brage S, Brage N, Franks PW, Ekelund U, Wareham NJ. Reliability and validity of the combined heart rate and movement sensor Actiheart. Eur J Clin Nutr. 2005;59:561–70. doi: 10.1038/sj.ejcn.1602118. [DOI] [PubMed] [Google Scholar]
- 39.Malik M Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 40.Van den Berg M, Birnbaum L, Denison M, et al. The 2005 World Health Organization Reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93:223–41. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. Int J Psychophysiol. 2003;48:263–74. doi: 10.1016/s0167-8760(03)00073-4. [DOI] [PubMed] [Google Scholar]
- 42.Araujo P, Nguyen T, Frøyland L, Wang J, Kang JX. Evaluation of a rapid method for the quantitative analysis of fatty acids in various matrices. J Chromatorg A. 2008;1212:106–13. doi: 10.1016/j.chroma.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilcox RR. New designs in analysis of variance. Annu Rev Psychol. 1987;38:29–60. [Google Scholar]
- 44.Rosnow RL, Rosenthal R. Effect sizes. Why, when, and how to use them, J Psychol. 2009;217:6–14. [Google Scholar]
- 45.Voght WP. London: Sage Publications; 1999. Dictionary of Statistics & Methodology: A Nontechnical Guide for the Social Sciences. [Google Scholar]
- 46.Cohen J. A power primer. Psychol Bull. 1992;112:155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 47.Bonnet MH, Arand DL. Activity, arousal and the MSLT in patients with insomnia. Sleep. 2000;23:1–7. [PubMed] [Google Scholar]
- 48.Berntson GG, Bigger JT, Eckberg DL, et al. Heart rate variability: origins, methods, and interpretative caveats. Psychophysiology. 1997;34:623–48. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 49.Halson S. Nutrition, sleep and recovery. Eur J Sport Sci. 2008;8:119–26. [Google Scholar]
- 50.Hartmann E. Effects of l-Tryptophan on sleepiness and on sleep. J Psychiat Res. 1982;17:107–13. doi: 10.1016/0022-3956(82)90012-7. [DOI] [PubMed] [Google Scholar]
- 51.Markus CR, Jonkman LM, Lammers JH, Deutz NE, Messer MH, Rigtering N. Evening intake α-lactalbumin increases plasma tryptophan availability and improves morning alertness and brain measures of attention. Am J Clin Nutr. 2005;81:1026–33. doi: 10.1093/ajcn/81.5.1026. [DOI] [PubMed] [Google Scholar]
- 52.Antypa N, Van der Does AJW, Smelt AHM, et al. Omega-3 fatty acids (fish oil) and depression-related cognition in healthy volunteers. J Psychopharmacol. 2009;23:831–40. doi: 10.1177/0269881108092120. [DOI] [PubMed] [Google Scholar]