Abstract
IMPORTANCE
Epileptic activity associated with Alzheimer disease (AD) deserves increased attention because it has a harmful impact on these patients, can easily go unrecognized and untreated, and may reflect pathogenic processes that also contribute to other aspects of the illness. We report key features of AD-related seizures and epileptiform activity that are instructive for clinical practice and highlight similarities between AD and transgenic animal models of the disease.
OBJECTIVE
To describe common clinical characteristics and treatment outcomes of patients with amnestic mild cognitive impairment (aMCI) or early AD who also have epilepsy or subclinical epileptiform activity.
DESIGN
Retrospective observational study from 2007 to 2012.
SETTING
Memory and Aging Center, University of California, San Francisco.
PATIENTS
We studied 54 patients with a diagnosis of aMCI plus epilepsy (n = 12), AD plus epilepsy (n = 35), and AD plus subclinical epileptiform activity (n = 7).
MAIN OUTCOMES AND MEASURES
Clinical and demographic data, electroencephalogram (EEG) readings, and treatment responses to antiepileptic medications.
RESULTS
Patients with aMCI who had epilepsy presented with symptoms of cognitive decline 6.8 years earlier than patients with aMCI who did not have epilepsy (64.3 vs 71.1 years; P = .02). Patients with AD who had epilepsy presented with cognitive decline 5.5 years earlier than patients with AD who did not have epilepsy (64.8 vs 70.3 years; P = .001). Patients with AD who had subclinical epileptiform activity also had an early onset of cognitive decline (58.9 years). The timing of seizure onset in patients with aMCI and AD was nonuniform (P < .001), clustering near the onset of cognitive decline. Epilepsies were most often complex partial seizures (47%) and more than half were nonconvulsive (55%). Serial or extended EEG monitoring appeared to be more effective than routine EEG at detecting interictal and subclinical epileptiform activity. Epileptic foci were predominantly unilateral and temporal. Of the most commonly prescribed antiepileptics, treatment outcomes appeared to be better for lamotrigine and levetiracetam than for phenytoin.
CONCLUSIONS AND RELEVANCE
Common clinical features of patients with aMCI- or AD-associated epilepsy at our center included early age at onset of cognitive decline, early incidence of seizures in the disease course, unilateral temporal epileptic foci detected by serial/extended EEG, transient cognitive dysfunction, and good seizure control and tolerability with lamotrigine and levetiracetam. Careful identification and treatment of epilepsy in such patients may improve their clinical course.
A lzheimer disease (AD) carries an increased risk of seizures.1 An estimated 10% to 22% of patients with AD develop unprovoked seizures, with higher rates in familial and early-onset cases.1-7 Patients with AD and seizure disorders have greater cognitive impairment,8 faster progression of symptoms,4 and more severe neuronal loss at autopsy9 than those without seizures. Because it is important to identify and treat these patients early, it is imperative to understand their characteristic clinical features.
In this group with AD and seizures, little is known about typical seizure semiology, diagnostic utility of electroencephalogram (EEG), common location of epileptogenic foci, seizure incidence relative to the onset of other neurodegenerative disease symptoms, and treatment responses to different antiepileptic medications. The goal of this report is to fill some of these knowledge gaps by describing epilepsies encountered in patients with mild AD and amnestic mild cognitive impairment (aMCI), a condition widely considered to be a harbinger or early stage of AD.10 Notably, more than half of the patients with aMCI and AD and comorbid epilepsy had only nonconvulsive seizures, which can easily go unrecognized.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
All research subjects provided written informed consent before participating in protocols from which data were derived and were not required to reconsent for this analysis. This study was approved by the University of California, San Francisco Committee on Human Research.
Patient Selection
We searched the database of the Memory and Aging Center at the University of California, San Francisco for all patients who presented with cognitive decline between 2007 and 2012 and met the International Working Group research criteria for aMCI (n = 233)11 or National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association research criteria for probable AD (n = 1024).12 Eighty-eight percent of patients were seen in clinic, 5% in research, and 6% in clinic and research. Among these patients, we searched for patients with a clinical diagnosis of epilepsy or subclinical epileptiform activity. An epilepsy diagnosis required 2 or more unprovoked seizures or a first unprovoked seizure in the setting of a corroborating EEG showing epileptiform activity.13 We identified 17 patients with aMCI-epilepsy, none with aMCI–subclinical epileptiform activity (0 of 16 with EEG), 55 with AD-epilepsy, and 8 with AD– subclinical epileptiform activity (8 of 98 with EEG). We then excluded 1 patient with aMCI and 7 patients with AD who had seizure onset during childhood or early adulthood (before age 30 years). Of the remaining patients, we excluded those with other possible epilepsy risk factors including cortical stroke(s) (2 patients with aMCI, 4 patients with AD), cavernous hemangioma (1 patient with aMCI), meningioma (1 patient with aMCI, 1 patient with AD), suspected brain tumor (1 patient with AD), subdural hematoma (3 patients with AD), history of alcohol abuse (3 patients with AD), amyloid angiopathy (1 patient with AD), and enrollment in clinical treatment trials (1 patient with AD). None of the remaining patients had focal cortical dysplasia, head trauma with loss of consciousness more than 30 minutes, hydrocephalus, or substance abuse. Diagnoses were made by a multidisciplinary team consisting of behavioral neurologists, epileptologists, neuropsychologists, and psychiatrists, who performed extensive behavioral, neuropsychological, neurophysiological, and neuroimaging assessments. Seizures were classified and described according to their clinical features following the revised guidelines of the International League Against Epilepsy.14 Patients who had at least 1 seizure that involved limb shaking were classified as having convulsive seizures. Generalized seizures were defined as seizures rapidly involving both hemispheres with no clear or consistent focus. Focal seizures were subdivided into simple partial seizures, which involved no alteration in cognition or consciousness, and complex partial seizures, which involved dyscognitive symptoms.
We included 12 cases of aMCI-epilepsy, 35 cases of AD-epilepsy, and 7 cases of AD–subclinical epileptiform activity. Twelve patients with AD had an additional biomarker evaluation: 9 had fluorodeoxyglucose positron emission tomography, 3 of whom also had carbon 11–labeled Pittsburgh Compound B positron emission tomography amyloid imaging,15 and 3 had cerebrospinal fluid analysis for total tau, phosphorylated tau, and β-amyloid 1-42 peptide (Athena Diagnostics) levels. In all 12 cases, the biomarkers were supportive of an AD diagnosis. Apolipoprotein (apo) E genotyping was available for 11 patients with AD: 4 were homozygous E3/E3, 5 were E3/E4, and 2 were homozygous E4/E4. Two patients in the AD group came to autopsy and both met pathologic criteria for high-likelihood AD (National Institute on Aging–Reagan).16 A his tory of mild head trauma17,18 was reported in 21% of cases with epilepsy (2 patients with aMCI and 8 patients with AD) (eTable in Supplement): 6 patients had concussions before age 25 years, 3 had concussions in adulthood at least 4 years prior to seizure onset, and 1 had a concussion 7 years following seizure onset.
Clinical Measures
The date of onset of cognitive decline was obtained by asking the patient and caregivers when, in retrospect, they noticed the first cognitive or behavioral abnormality that was different from the patient's prior baseline and that developed into a feature of the fully apparent aMCI or AD.19 The date of onset of seizures was obtained by asking patients and caregivers when they noticed the first transient spells that were later characteristic of the patient's epilepsy. The date of diagnosis of neurodegenerative disease was the first visit when all research criteria were fulfilled to establish the diagnosis of aMCI11 or probable AD.12 General cognitive functioning was assessed using the Mini-Mental State Examination.20 Scores range from 0 to 30, with higher scores denoting better performance. Only Mini-Mental State Examination scores obtained within 5 years of seizure onset were included in the analysis.
Electroencephalogram
Clinical EEG recordings were obtained using a standard international 10-20 electrode placement. Recordings took place during routine 20-minute sessions unless otherwise stated in Table 1 or the eTable in Supplement. Epileptic foci were defined as the regions of maximum electronegativity, corresponding with scalp electrodes as follows: frontal (Fp1, Fp2, F3, F4), central frontal (Fz), central (C3, Cz, C4), central parietal (Pz), frontotemporal (F7, F8), temporal (T3, T4), posterior temporal (T5, T6), parietal (P3, P4), and occipital (O1, O2).
Table 1.
EEG and Demographic Features of Patients With aMCI and AD
| Patients With Epilepsy Diagnosis |
Patients Without Epilepsy Diagnosis |
|||||
|---|---|---|---|---|---|---|
| Measure | EEG Nonepileptiform (n = 15) | EEG Epileptiform (n = 24) | P Value | EEG Nonepileptiform (n = 106) | EEG Epileptiform (n = 7) | P Value |
| Routine EEG, No./total No. (%) | 10/15 (66.7) | 7/24 (29.2) | .049 | 99/106 (93.4) | 4/7 (57.1) | .01 |
| Sleep-deprived EEG, No./total No. (%) | 2/15 (13.3) | 2/24 (8.3) | .97 | 2/106 (1.9) | 0/7 | .27 |
| Serial EEG or LTM, No./total No. (%) | 3/15 (20.0) | 15/24 (62.5) | .02 | 5/106 (4.7) | 3/7 (42.9) | .002 |
| Age at onset of aMCI or AD, y, mean (SD) | 66.7 (9.8) | 63.2 (8.9) | .48 | 62.8 (11.8) | 58.7 (10.2) | .39 |
| Age at diagnosis of aMCI or AD, y, mean (SD) | 70.3 (9.5) | 67.6 (8.2) | .46 | 66.2 (13.5) | 62.9 (8.9) | .44 |
Abbreviations: AD, Alzheimer disease; aMCI, amnestic mild cognitive impairment; EEG, electroencephalogram; LTM, long-term video EEG monitoring.
Outcome Definition
Responses to antiepileptic drugs were assessed over a span of at least 3 months of continuous treatment and scored as follows: seizure free (no seizures at all), partial response (seizures reduced in severity or frequency), neutral (seizures were neither better nor worse), or paradoxical worsening (seizures increased in severity or frequency). These measurements were based on the observations of patients and their caregivers as recorded by physicians during clinic visits. Intolerance that necessitated discontinuation of a drug was reported with no restrictions on duration of treatment.
Statistical Analyses
Differences between groups within the aMCI and AD cohorts were evaluated with the χ2 test for sex, handedness, EEG type, and ratios of patients with early- (<65 years) vs late-onset (≥65 years) cognitive decline and the Mann-Whitney rank sum test for years of education and age at onset and age at diagnosis of aMCI or AD. The timing of seizure onset relative to the year of aMCI or AD onset was compared with a uniform distribution by the χ2 test. Our a priori hypothesis was that this distribution would be uniform.2 The tolerability and responder ratios (partial response or seizure freedom) for different antiepileptic medications were assessed with the Fisher exact test for pairwise comparisons. All pairwise comparisons were 2-tailed and P < .05 was required to reject the null hypothesis. Statistical analyses were performed with SigmaPlot version 12.0 (Systat Software Inc).
Results
Seizures and Epileptiform Activity Are Associated With an Earlier Age at Onset of Cognitive Decline
Clinical and demographic characteristics of the study groups are summarized in Table 2 and the eTable in Supplement. In the aMCI and AD cohorts, cognitive decline began roughly 5 to 7 years earlier in patients who developed epilepsy than in those without epilepsy (Table 2). Cognitive decline began before 65 years of age in 50% of aMCI-epilepsy cases compared with 21% of aMCI cases without epilepsy (P = .051) and in 51% of AD-epilepsy cases compared with 26% of AD cases without epilepsy (P = .002). Similarly, the diagnosis of aMCI or AD occurred significantly earlier in patients who developed epilepsy than those without epilepsy (Table 2), even though the AD-epilepsy cohort had more years of education than patients with AD and no epilepsy (16.1 vs 14.5 years; P = .007). Patients with AD who had subclinical epileptiform activity were analyzed separately from patients with AD-epilepsy and also showed an earlier cognitive decline than patients with AD who did not have epilepsy (age 58.9 vs 70.3 years; P = .009), although EEG evaluation for patients without epilepsy was more common in early–age-at-onset cases (Table 1).
Table 2.
Main Demographic and Clinical Characteristics of the Study Population
| Characteristic | aMCI-No Epilepsy (n = 216) | aMCI-Epilepsy (n = 12) | P Value | AD-No Epilepsy (n = 969) | AD-Epilepsy (n = 35) | P Value |
|---|---|---|---|---|---|---|
| Female, % | 55.1 | 41.7 | .54 | 57.6 | 51.4 | .58 |
| Left-handed, % | 5.6a | 8.3 | .81 | 5.9b | 2.9 | .70 |
| Education, y, mean (SD) | 15.5c (4.7) | 15.8 (4.0) | .67 | 14.5d (4.3) | 16.1 (3.4) | .007 |
| Age at onset of cognitive decline, y, mean (SD) | 71.1e (9.7) | 64.3 (8.1) | .02 | 70.3f (10.5) | 64.8 (9.7) | .001 |
| Age at diagnosis of aMCI or AD, y, mean (SD) | 74.6 (9.5) | 68.0 (7.8) | .01 | 74.5g (10.3) | 69.1 (9.0) | <.001 |
Abbreviations: AD, Alzheimer disease; aMCI, amnestic mild cognitive impairment.
Obtained using available data from 198 patients.
Obtained using available data from 915 patients.
Obtained using available data from 212 patients.
Obtained using available data from 954 patients.
Obtained using available data from 199 patients.
Obtained using available data from 917 patients.
Obtained using available data from 926 patients.
Seizures Occur Early in Relation to Cognitive Decline
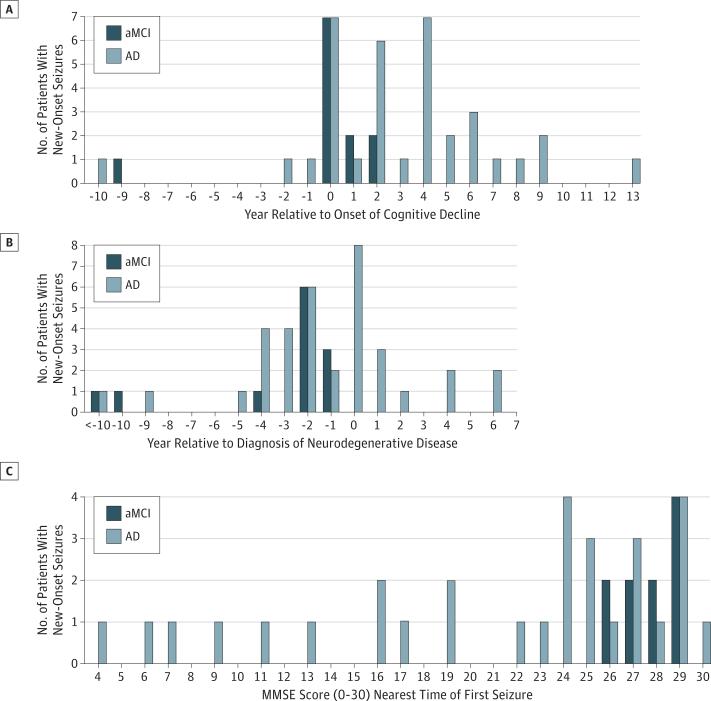
Seizures in aMCI and AD generally began early in the disease course when patients had mild impairments on cognitive testing. The timing of new-onset seizures was nonuniform (P < .001) with clustering near the onset of cognitive decline (Figure 1A). Seizure onset preceded or coincided with the diagnosis of aMCI or AD in 83% (39 of 47) of patients (Figure 1B), and an epilepsy diagnosis preceded or coincided with the diagnosis of aMCI or AD in 51% (24 of 47) of patients. Scores on the Mini-Mental State Examination, which is a global test of cognition, nearest to the date of the first seizure were grouped near the upper end of the scale, indicating mild impairments (Figure 1C).
Figure 1. Seizure Onset in Relation to Disease Course.
A, Seizures were coincident with, or followed the onset of, nonepileptic symptoms of Alzheimer disease (AD) in all but 4 cases. The yearly distribution of new-onset seizures relative to the year of amnestic mild cognitive impairment (aMCI) or AD onset was nonuniform (P < .001, χ2 test) and clustered near the onset of cognitive decline. B, Seizure onset generally occurred prior to neurodegenerative diagnosis or early into the disease course. C, Mini-Mental State Examination (MMSE) scores obtained nearest the time of the first seizure were clustered toward the upper end of the range (2 missing data points for patients with aMCI and 4 missing data points for patients with AD).
Seizure Semiology
The seizure type and semiology for each epilepsy case, along with the first nonepileptic symptoms of AD, are presented in Table 3 and the eTable in Supplement. The most common seizure type, occurring in 47% of cases (22 of 47), started locally and was associated with dyscognitive symptoms (complex partial seizures). Of the 22 cases with complex partial seizures, 7 developed bilateral convulsive seizures. Among the 25 remaining cases, 17 had apparent generalized seizures without clear focal or lateralizing symptoms and 8 presented with simple partial seizures.
Table 3.
Seizure Subtype, Semiology, and Nonepileptic Symptoms of Cognitive Decline for All Patients With aMCI-Epilepsy or AD-Epilepsy
| Epilepsy Type | Sample Size | Semiology (No. of Patients)a | First Nonepileptic Symptoms of Cognitive Decline (No. of Patients) |
|---|---|---|---|
| Simple partial | |||
| NC | 7 | Jamais vu/déjà vu (4), psychic phenomena (2), sensory phenomena (4) | Apathy (1), memory (6) |
| C | 1 | Single-limb shaking (1) | Language (1) |
| Complex partial | |||
| NC | 11 | Altered consciousness (6), amnestic spells (1), confusion (2), psychic phenomena (4), sensory phenomena (7), speech arrest (2) | Language (1), memory (10) |
| Cb | 11 | Altered consciousness (3), amnestic spells (2), bilateral limb shaking (5), confusion (4), single-limb shaking (7), speech arrest (3) | Apathy (1), executive (1), language (1), memory (8) |
| Generalized | |||
| NC | 7 | Atonic/tonic (2), behavioral arrest (4), staring spells (1) | Language (2), memory (5) |
| C | 10 | Atony (1), behavioral arrest (1), epileptic myoclonus (3), generalized tonic-clonic (8), staring spells (1) | Language (1), memory (9) |
Abbreviations: C, convulsive; NC, nonconvulsive.
More than 1 semiology component may occur in the same patient.
Includes seizures with secondary generalization.
Notably, 55% of the patients with aMCI or AD who had epilepsy (26 of 47) had only nonconvulsive seizures. Nonconvulsive symptoms included jamais vu, déjà vu, sensory phenomena (eg, metallic taste, burning smell, epigastric rising sensation, prickling, or chest warmth), psychic phenomena (eg, intense fear or dread), speech/behavioral arrest, aphasia, and amnestic spells. Five patients with epilepsy (3 with aMCI and 2 with AD) had pacemaker implantation after ictal bradycardia or asystole.
Epileptiform Activity Detection and Location
The EEG revealed epileptiform activity in 62% (24 of 39) of patients with aMCI or AD who were evaluated for seizures and in 6% (7 of 113) of those without known seizures. The EEGs in nonepilepsy cases were obtained as part of a routine workup to rule out other causes of cognitive decline, which in some cases included vague reports of clinical fluctuations. None of the patients in this group were diagnosed with epilepsy. Across all patients, serial EEGs (≥2) or long-term video EEG monitoring (≥24 hours) appeared to be more effective at detecting epileptiform activity than routine EEG (Table 1). Sleep-deprived EEG was rarely performed.
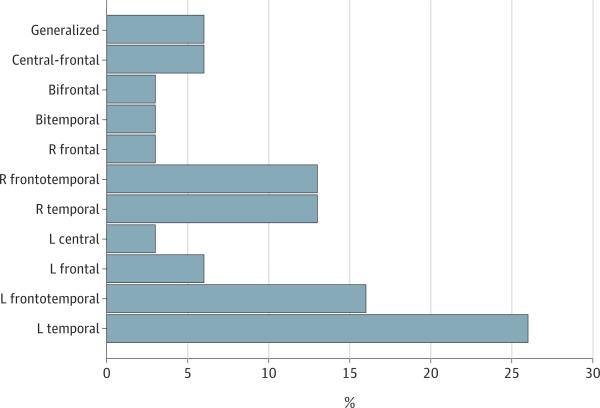
Epileptic foci were predominantly unilateral and most commonly temporal, followed by frontotemporal, frontal, central, and generalized (Figure 2). Seventy-eight percent (7 of 9) of epileptiform EEGs in patients with aMCI or AD who had generalized seizures revealed a focal pattern. Epileptiform activity often occurred in brain regions supporting functions that were impaired clinically. Eighty-two percent (18 of 22) of cases with temporal/frontotemporal epileptic foci presented initially with memory impairments. Two patients with left frontal epileptic foci presented initially with aphasia; 1 of them is described in Figure 3.
Figure 2. Distribution of Electroencephalogram Epileptiform Activity.
Of the 31 cases who had spikes or sharp waves on electroencephalogram, epileptiform activity was predominantly unilateral and most commonly temporal (8 left, 4 right, and 1 bitemporal), followed by frontotemporal (5 left and 4 right), frontal (2 left, 1 right, and 1 bifrontal), central (1 left central and 2 central frontal), and generalized (2). L indicates left, and R, right.
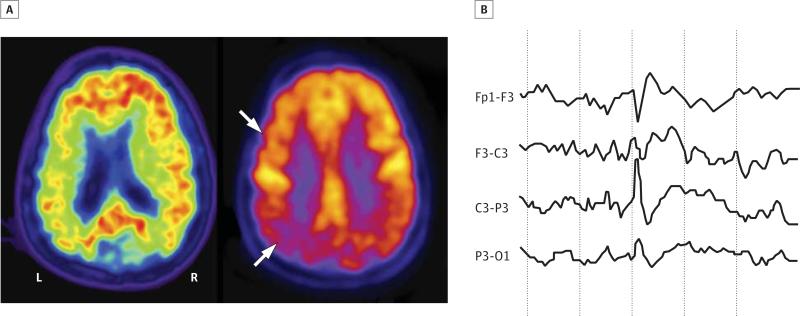
Figure 3. Right-Handed, 53-Year-Old Woman Who Presented With 3.5 Years of Progressive Expressive Language Difficulties and Executive Dysfunction.
Her Mini-Mental State Examination score was 23 of 30. Her speech was effortful with word-finding pauses, and she had difficulty repeating. Her comprehension was relatively preserved. She also had ocular apraxia and optic ataxia. Brain magnetic resonance imaging showed diffuse cortical atrophy, greatest in the left parietal lobe. Her apoE genotype was E3/E3. Carbon 11–labeled Pittsburgh compound B positron emission tomography (A, left image) showed amyloid deposits throughout the neocortex, as indicated by the red signal, and fluorodeoxyglucose positron emission tomography (A, right image) revealed reduced glucose metabolism in the temporoparietal lobes (left > right) and left frontal lobe, as indicated by reduction in the yellow signal and white arrows. Both findings support a diagnosis of Alzheimer disease. At age 54 years, she drove into a parked car that she did not see on her right side. She was not injured. The accident prompted a routine electroencephalogram (EEG) evaluation, which showed intermittent left frontotemporal slowing. Long-term video EEG monitoring (B) revealed epileptiform spikes, occurring as frequently as every few minutes, centered in the left frontal lobe with phase reversal at F3, corresponding neuroanatomically to a language region that was clinically impaired and hypometabolic on fluorodeoxyglucose positron emission tomography. She started treatment with levetiracetam, with a final dose of 1000 mg twice daily. Her Mini-Mental State Examination score at age 54 years was 20 of 30. Routine EEG during levetiracetam treatment showed diffuse theta slowing with no asymmetry and no epileptiform activity. At the age of 55 years, she developed occasional arm myoclonus but remained otherwise seizure free.
Response to Antiepileptic Treatment
Clinical responses to commonly prescribed antiepileptic medications are provided in Table 4 and Table 5. The 4 medications prescribed most frequently as monotherapy were lamotrigine (n = 25), levetiracetam (n = 23), phenytoin (n = 9), and valproic acid (n = 11). Lamotrigine, levetiracetam, and valproic acid were all well tolerated, whereas phenytoin was poorly tolerated (22% tolerability; P < .05 vs lamotrigine, levetiracetam, and valproic acid). The antiepileptic efficacy of lamotrigine (53% seizure free; 41% partial responders) and levetiracetam (44% seizure free; 50% partial responders) was higher than that of phenytoin (17% seizure free; 33% partial responders) (P < .05). Valproic acid administration provided an intermediate level of seizure control (11% seizure free; 67% partial responders).
Table 4.
Efficacy of the Most Common AEDs Prescribed as Monotherapy
| AED | Sample Size | Dose Range, mg/d | Antiepileptic Efficacy, No./Total No. (%)a | Total Responders, No./Total No. (%) | P Valueb | |||
|---|---|---|---|---|---|---|---|---|
| Worse | Neutral | Partial Response | Seizure Free | |||||
| Lamotrigine | 17 | 50-600 | 0 | 1/17 (5.9) | 7/17 (41.2) | 9/17 (52.9) | 16/17 (94.1) | .04 vs Phenytoin |
| Levetiracetam | 16 | 250-3000 | 0 | 1/16 (6.3) | 8/16 (50.0) | 7/16 (43.8) | 15/16 (93.8) | .046 vs Phenytoin |
| Phenytoin | 6 | 100-600 | 0 | 3/6 (50.0) | 2/6 (33.3) | 1/6 (16.7) | 3/6 (50.0) | .04 vs Lamotrigine; .046 vs levetiracetam |
| Valproic acid | 9 | 250-1500 | 0 | 2/9 (22.2) | 6/9 (66.7) | 1/9 (11.1) | 7/9 (77.8) | .33 vs Phenytoin |
Abbreviation: AED, antiepileptic drug.
Not including patients with only subclinical epileptiform activity or those who did not tolerate the AED for more than 3 months.
Fisher exact test for each pairwise comparison of total responder ratios.
Table 5.
Tolerability of the Most Common AEDs Prescribed as Monotherapy
| AED | Sample Size | Dose Range, mg/d | Tolerability, No./Total No. (%) | Adverse Effects (No. of Patients)a | P Valueb |
|---|---|---|---|---|---|
| Lamotrigine | 25 | 50-600 | 18/25 (72.0) | Aggressiveness (1), allergic rash (2), blurry vision (1), confusion (1), dizziness (1), fatigue (2), imbalance (2), tremor (2) | .02 vs Phenytoin |
| Levetiracetam | 23 | 250-3000 | 18/23 (78.3) | Anxiety (1), confusion (2), diarrhea (1), dizziness (1), headache (1), incoordination (1), irritability (3), nausea (1) | .006 vs Phenytoin |
| Phenytoin | 9 | 100-600 | 2/9 (22.2) | Ataxia (3), cognitive worsening (3), delirium (1), dizziness (1), lethargy (2), low appetite (1), myoclonus (1), nausea (1), sedation (1), weakness (1) | .02 vs Lamotrigine; .006 vs levetiracetam; .02 vs valproic acid |
| Valproic acid | 11 | 250-1500 | 9/11 (81.8) | Cognitive worsening (1), delirium (1), hypertension (1), sedation (1), weight gain (1) | .02 vs Phenytoin |
Abbreviation: AED, antiepileptic drug.
More than 1 adverse effect was experienced by some patients.
Fisher exact test for each pairwise comparison.
Discussion
Our findings indicate that epileptic activity may be more prevalent in the early stages of AD than was previously recognized. Several features of our study enabled us to capture patients who are often excluded from this type of investigation: (1) inclusion of patients with aMCI, (2) allowing seizures to precede an aMCI/AD diagnosis, (3) reporting of nonconvulsive seizures, and (4) use of nonroutine EEG. Epileptiform activity occurred most often in the temporal lobes, which harbor memory centers that are affected early and severely by AD, raising the possibility that it contributes to memory decline in these patients.
Our observation that seizures in patients with AD are associated with an earlier onset of cognitive decline is consistent with the literature6,21,22; our study extends these findings to patients with aMCI. Seizures in AD are commonly reported in advanced cases3,5 and in such cases have been in terpreted as a consequence of end-stage neuronal loss and gliosis. Although this supposition may or may not be true, seizure incidence appears to be independent of disease stage,2 and it can occur early,23,24 or even coincide with the onset of cognitive decline, as our cohorts illustrate. Additionally, nonconvulsive seizures, which are more difficult to recognize than convulsions, are likely to be underreported in a cognitively impaired population, as others have speculated.2,25 Interestingly, the patients in our study with AD-epilepsy had a higher level of education than the patients with AD who did not have epilepsy, raising the possibility that patients who are better informed about nonconvulsive epileptic features, such as sensory or psychic phenomena, may be more likely to call attention to such symptoms during a clinic visit. We also identified 7 cases who had subclinical epileptiform activity but no clinically obvious seizures, 3 of whom were discovered by serial EEG or long-term video EEG monitoring. Such activity is rarely observed on a single routine awake EEG in patients with dementia26 but might be captured by serial or extended EEGs and provocative maneuvers, which are seldom used in these patients. Indeed, others have shown that hyperventilation can increase the likelihood of detecting epileptiform sharp waves in apoE4 carriers, who are at increased risk for AD.27
The extent to which subclinical epileptiform activity occurs in patients with AD in general is unknown. Larger prospective studies are needed to address this important issue and to confirm or refute several of the other possibilities raised by our study, which had several limitations. First, our sample sizes were relatively small and patient selection was biased toward those seen at a single tertiary referral center that specializes in mildly impaired, early-onset dementia cases. Second, the cohorts were highly educated and would be expected to have better medical knowledge and access to medical services than the general population. Third, our data relied on clinic notes and observations of patients’ caregivers rather than the standardized methods of a prospective trial. Having a neurological diagnosis such as epilepsy might expedite workup and diagnosis of a coexisting neurological disorder such as AD, and we cannot rule out potential reporting bias that may occur in such patients. Fourth, epilepsy adds to the complexity of diagnosing neurodegenerative disease. For example, patients with aMCI who have seizures can be difficult to distinguish from patients who solely have transient epileptic amnesia. To address this concern, we required consensus diagnoses and selected patients with objective memory impairments on neuropsychological testing, a feature that distinguishes neurode-generative cases from isolated transient epileptic amnesia.28 We also realize that patients with aMCI/AD and epilepsy can exhibit a spectrum of putative epileptic and nonepileptic cognitive symptoms that can be difficult or impossible to differentiate. In our cohorts, we attempted to distinguish “epileptic” cognitive symptoms from “nonepileptic” symptoms of AD by their abrupt onset and stereotyped nature (Table 3). In reality, though, some of the “nonepileptic” symptoms might result, at least in part, from chronic alterations caused by inter mittent epileptic activity, such as remodeling of hippocampal circuits.25,29 Fifth, patients with long-standing epilepsy may develop mesial temporal sclerosis, and we cannot rule out co-morbid mesial temporal sclerosis in our cohorts. However, pure mesial temporal sclerosis is a very rare cause of dementia,30 and magnetic resonance imaging suspicion for mesial temporal sclerosis was lacking in all but 3 patients included in this study. Sixth, we had limited apoE genotyping in our cohorts, and we were unable to assess potential interactions between apoE, mild head trauma (present in 21% of epilepsy cases), epileptic activity, and AD, which others have reported.17,27,31
Within the limitations of a retrospective study, our findings are clinically instructive when placed in context of the current literature. Consistent with our observations, others have reported that treatment with levetiracetam or lamotrigine resulted in good seizure control and tolerability in patients with AD and epilepsy.32,33 In contrast, the sodium channel blockers phenytoinandcarbamazepine,aswellasphenobarbitalandbenzodiazepines, can worsen cognitive function in patients with AD.5,33 Of the 9 patients who were treated with phenytoin in our study, 6 reported worsening of cognitive or motor symptoms and only 3 had a reduction in seizure frequency (Table 4 and Table 5 and eTable in Supplement). Phenytoin also worsened cognition in patients with Down syndrome who had AD and seizures.34 Despite these findings, phenytoin may still be the antiepileptic drug most commonly used for patients with AD and epilepsy in clinical practice.5,35 Valproic acid has been investigated in AD clinical trials because of its possible neuroprotective properties and beneficial effects on agitation. However, patients treated with valproic acid (10-12 mg/kg/d) had a more rapid decline in brain atrophy36,37 and Mini-Mental State Examination scores37 than those treated with placebo, making this drug less appealing for patients with AD and epilepsy.
Our observations also underscore similarities between AD and transgenic animal models of the disease. Human amyloid precursor protein (hAPP) transgenic mice, which simulate key aspects of AD, have epileptiform activity and nonconvulsive seizures.29 Epileptogenesis in this model appears to depend on the presence of tau and pathologically elevated levels of β-amyloid peptides in the brain and involve mechanisms that are at least partly distinct from those causing common seizure disorders.1,29,38-40 Phenytoin paradoxically exacerbated epileptiform activity, seizures, and cognitive deficits in hAPP mice.39 These detrimental effects of phenytoin and other sodium channel blockers may be due, at least in part, to an exacerbation of voltage-gated sodium channel deficits in inhibitory interneurons that were recently identified in both hAPP mice and humans with AD.39 In contrast, levetiracetam suppressed epileptiform activity in hAPP mice and improved learning and memory in this AD model41 as well as in age-impaired rats.42 Low-dose levetiracetam also suppressed hippocampal hyperactivation and improved cognitive performance in a hippocampus-dependent task in patients with aMCI.43 Although more randomized comparative clinical trials are needed, these previous studies, together with our observations, support the use of levetiracetam over phenytoin in the treatment of seizures in patients with AD, when clinically appropriate.Lamotrigineappearstobeanotherreasonablechoice. In our cohorts, both levetiracetam and lamotrigine were often effective at suppressing seizures, even at low doses. Al though lamotrigine inhibits sodium channels, it preferentially inhibits activity-driven glutamate release from presynaptic terminals of excitatory neurons,44 which is reminiscent of levetiracetam effects.45
Conclusions
Our findings add to the mounting evidence that AD-related neural network hypersynchrony is an early and potentially treatable component of the disease.29,38-41,43,46,47 Features that should raise clinical suspicion for seizures in patients with aMCI or AD include cognitive decline at a relatively early age, transient stereotyped cognitive symptoms such as aphasia, amnestic spells, or déjà vu, and possibly a history of head trauma. Whether antiepileptic medications such as levetiracetam can improve cognition or disease course in these patients, and what doses are optimal, are critical questions that require further investigation.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by National Institutes of Health grants K23 AG038357 (Dr Vossel), K23 AG031861 (Dr Rabinovici), and P50 AG023501 and P01 AG19724 (Dr Miller), the John Douglas French Alzheimer's Foundation (Drs Vossel and Rabinovici), a University of California, San Francisco Alzheimer's Disease Research Center pilot project grant (Dr Vossel), and a gift from the S. D. Bechtel Jr Foundation (Dr Mucke).
Footnotes
Author Contributions: Study concept and design: Vossel, Miller, Mucke.
Acquisition of data: Vossel, Beagle, Rabinovici, Shu, Lee, Naasan, Hegde, Cornes, Henry, Nelson, Seeley, Geschwind, Gorno-Tempini, Shih, Miller.
Analysis and interpretation of data: Vossel, Beagle, Rabinovici, Shu, Lee, Naasan, Hegde, Henry, Nelson, Geschwind, Gorno-Tempini, Shih, Kirsch, Garcia, Miller, Mucke.
Drafting of the manuscript: Vossel, Beagle, Miller, Mucke.
Critical revision of the manuscript for important intellectual content: Vossel, Beagle, Rabinovici, Shu, Lee, Naasan, Hegde, Cornes, Henry, Nelson, Seeley, Geschwind, Gorno-Tempini, Shih, Kirsch, Garcia, Miller, Mucke.
Statistical analysis: Vossel.
Obtained funding: Vossel, Seeley, Miller, Mucke. Administrative, technical, and material support: Beagle.
Study supervision: Miller, Mucke.
Additional Contributions: We thank Jorge Palop, PhD, and Pascal Sanchez, PhD, for helpful comments on the manuscript; Kamalini Ranasinghe, MBBS, PhD, for assistance with medical record review; William Jagust, MD, for carbon 11–labeled Pittsburgh compound B positron emission tomography imaging; Anna Karydas, BA, for apoE genotyping; Anna Lisa Lucido, PhD, for editorial review; John C. W. Carroll for graphics support; and Monica Dela Cruz, BS, for administrative assistance.
Conflict of Interest Disclosures: Dr Garcia receives research support from UCB Pharma and Medtronics Inc. Dr Mucke receives sponsored research support from Bristol-Myers Squibb and Takeda Pharmaceuticals.
REFERENCES
- 1.Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66(4):435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hauser WA, Morris ML, Heston LL, Anderson VE. Seizures and myoclonus in patients with Alzheimer's disease. Neurology. 1986;36(9):1226–1230. doi: 10.1212/wnl.36.9.1226. [DOI] [PubMed] [Google Scholar]
- 3.Romanelli MF, Morris JC, Ashkin K, Coben LA. Advanced Alzheimer's disease is a risk factor for late-onset seizures. Arch Neurol. 1990;47(8):847–850. doi: 10.1001/archneur.1990.00530080029006. [DOI] [PubMed] [Google Scholar]
- 4.Volicer L, Smith S, Volicer BJ. Effect of seizures on progression of dementia of the Alzheimer type. Dementia. 1995;6(5):258–263. doi: 10.1159/000106956. [DOI] [PubMed] [Google Scholar]
- 5.Mendez M, Lim G. Seizures in elderly patients with dementia: epidemiology and management. Drugs Aging. 2003;20(11):791–803. doi: 10.2165/00002512-200320110-00001. [DOI] [PubMed] [Google Scholar]
- 6.Amatniek JC, Hauser WA, DelCastillo-Castaneda C, et al. Incidence and predictors of seizures in patients with Alzheimer's disease. Epilepsia. 2006;47(5):867–872. doi: 10.1111/j.1528-1167.2006.00554.x. [DOI] [PubMed] [Google Scholar]
- 7.Cabrejo L, Guyant-Maréchal L, Laquerrière A, et al. Phenotype associated with APP duplication in five families. Brain. 2006;129(pt 11):2966–2976. doi: 10.1093/brain/awl237. [DOI] [PubMed] [Google Scholar]
- 8.McAreavey MJ, Ballinger BR, Fenton GW. Epileptic seizures in elderly patients with dementia. Epilepsia. 1992;33(4):657–660. doi: 10.1111/j.1528-1157.1992.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 9.Förstl H, Burns A, Levy R, Cairns N, Luthert P, Lantos P. Neurologic signs in Alzheimer's disease: results of a prospective clinical and neuropathologic study. Arch Neurol. 1992;49(10):1038–1042. doi: 10.1001/archneur.1992.00530340054018. [DOI] [PubMed] [Google Scholar]
- 10.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58(3):397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 11.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 12.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 13.Fisher RS, van Emde Boas W, Blume W, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 2005;46(4):470–472. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 14.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51(4):676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 15.Rabinovici GD, Furst AJ, O'Neil JP, et al. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2007;68(15):1205–1212. doi: 10.1212/01.wnl.0000259035.98480.ed. [DOI] [PubMed] [Google Scholar]
- 16.National Institute on Aging. Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol Aging. 1997;18(4)(suppl)):S1–S2. [PubMed] [Google Scholar]
- 17.Annegers JF, Hauser WA, Coan SP, Rocca WA. A population-based study of seizures after traumatic brain injuries. N Engl J Med. 1998;338(1):20–24. doi: 10.1056/NEJM199801013380104. [DOI] [PubMed] [Google Scholar]
- 18.Plassman BL, Havlik RJ, Steffens DC, et al. Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology. 2000;55(8):1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 19.Roberson ED, Hesse JH, Rose KD, et al. Frontotemporal dementia progresses to death faster than Alzheimer disease. Neurology. 2005;65(5):719–725. doi: 10.1212/01.wnl.0000173837.82820.9f. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Irizarry MC, Jin S, He F, et al. Incidence of new-onset seizures in mild to moderate Alzheimer disease. Arch Neurol. 2012;69(3):368–372. doi: 10.1001/archneurol.2011.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scarmeas N, Honig LS, Choi H, et al. Seizures in Alzheimer disease: who, when, and how common? Arch Neurol. 2009;66(8):992–997. doi: 10.1001/archneurol.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hesdorffer DC, Hauser WA, Annegers JF, Kokmen E, Rocca WA. Dementia and adult-onset unprovoked seizures. Neurology. 1996;46(3):727–730. doi: 10.1212/wnl.46.3.727. [DOI] [PubMed] [Google Scholar]
- 24.Cretin B, Blanc F, Gaultier C, Sellal F. Epileptic amnesic syndrome revealing Alzheimer's disease. Epilepsy Res. 2012;102(3):206–209. doi: 10.1016/j.eplepsyres.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Scharfman HE. Alzheimer's disease and epilepsy: insight from animal models. Future Neurol. 2012;7(2):177–192. doi: 10.2217/fnl.12.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liedorp M, Stam CJ, van der Flier WM, Pijnenburg YA, Scheltens P. Prevalence and clinical significance of epileptiform EEG discharges in a large memory clinic cohort. Dement Geriatr Cogn Disord. 2010;29(5):432–437. doi: 10.1159/000278620. [DOI] [PubMed] [Google Scholar]
- 27.Ponomareva NV, Korovaitseva GI, Rogaev EI. EEG alterations in non-demented individuals related to apolipoprotein E genotype and to risk of Alzheimer disease. Neurobiol Aging. 2008;29(6):819–827. doi: 10.1016/j.neurobiolaging.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 28.Butler CR, Graham KS, Hodges JR, Kapur N, Wardlaw JM, Zeman AZ. The syndrome of transient epileptic amnesia. Ann Neurol. 2007;61(6):587–598. doi: 10.1002/ana.21111. [DOI] [PubMed] [Google Scholar]
- 29.Palop JJ, Chin J, Roberson ED, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55(5):697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ala TA, Beh GO, Frey WH II. Pure hippocampal sclerosis: a rare cause of dementia mimicking Alzheimer's disease. Neurology. 2000;54(4):843–848. doi: 10.1212/wnl.54.4.843. [DOI] [PubMed] [Google Scholar]
- 31.Mayeux R, Ottman R, Maestre G, et al. Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer's disease. Neurology. 1995;45(3)(pt 1):555–557. doi: 10.1212/wnl.45.3.555. [DOI] [PubMed] [Google Scholar]
- 32.Belcastro V, Costa C, Galletti F, Pisani F, Calabresi P, Parnetti L. Levetiracetam monotherapy in Alzheimer patients with late-onset seizures: a prospective observational study. Eur J Neurol. 2007;14(10):1176–1178. doi: 10.1111/j.1468-1331.2007.01907.x. [DOI] [PubMed] [Google Scholar]
- 33.Cumbo E, Ligori LD. Levetiracetam, lamotrigine, and phenobarbital in patients with epileptic seizures and Alzheimer's disease. Epilepsy Behav. 2010;17(4):461–466. doi: 10.1016/j.yebeh.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Tsiouris JA, Patti PJ, Tipu O, Raguthu S. Adverse effects of phenytoin given for late-onset seizures in adults with Down syndrome. Neurology. 2002;59(5):779–780. doi: 10.1212/wnl.59.5.779. [DOI] [PubMed] [Google Scholar]
- 35.Rao SC, Dove G, Cascino GD, Petersen RC. Recurrent seizures in patients with dementia: frequency, seizure types, and treatment outcome. Epilepsy Behav. 2009;14(1):118–120. doi: 10.1016/j.yebeh.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tariot PN, Schneider LS, Cummings J, et al. Alzheimer's Disease Cooperative Study Group. Chronic divalproex sodium to attenuate agitation and clinical progression of Alzheimer disease. Arch Gen Psychiatry. 2011;68(8):853–861. doi: 10.1001/archgenpsychiatry.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleisher AS, Truran D, Mai JT, et al. Alzheimer's Disease Cooperative Study. Chronic divalproex sodium use and brain atrophy in Alzheimer disease. Neurology. 2011;77(13):1263–1271. doi: 10.1212/WNL.0b013e318230a16c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks. Nat Neurosci. 2010;13(7):812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verret L, Mann EO, Hang GB, et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149(3):708–721. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Busche MA, Chen X, Henning HA, et al. Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2012;109(22):8740–8745. doi: 10.1073/pnas.1206171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez PE, Zhu L, Verret L, et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer's disease model. Proc Natl Acad Sci U S A. 2012;109(42):2895–2903. doi: 10.1073/pnas.1121081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koh MT, Haberman RP, Foti S, McCown TJ, Gallagher M. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology. 2010;35(4):1016–1025. doi: 10.1038/npp.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bakker A, Krauss GL, Albert MS, et al. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74(3):467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leach MJ, Marden CM, Miller AA. Pharmacological studies on lamotrigine—a novel potential antiepileptic drug, II: neurochemical studies on the mechanism of action. Epilepsia. 1986;27(5):490–497. doi: 10.1111/j.1528-1157.1986.tb03573.x. [DOI] [PubMed] [Google Scholar]
- 45.Meehan AL, Yang X, McAdams BD, Yuan L, Rothman SM. A new mechanism for antiepileptic drug action: vesicular entry may mediate the effects of levetiracetam. J Neurophysiol. 2011;106(3):1227–1239. doi: 10.1152/jn.00279.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Busche MA, Eichhoff G, Adelsberger H, et al. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science. 2008;321(5896):1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- 47.Putcha D, Brickhouse M, O'Keefe K, et al. Hippocampal hyperactivation associated with cortical thinning in Alzheimer's disease signature regions in non-demented elderly adults. J Neurosci. 2011;31(48):17680–17688. doi: 10.1523/JNEUROSCI.4740-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.